Abstract
BACKGROUND
Positive allosteric modulators (PAMs) facilitate endogenous neurotransmission and/or enhance the efficacy of agonists without directly acting on the orthosteric binding sites. In this regard, selective α7 nicotinic acetylcholine receptor type II PAMs display antinociceptive activity in rodent chronic inflammatory and neuropathic pain models. This study investigates whether 3-furan-2-yl-N-p-tolyl-acrylamide (PAM-2), a new putative α7-selective type II PAM, attenuates experimental inflammatory and neuropathic pains in mice.
METHODS
We tested the activity of PAM-2 after intraperitoneal administration in 3 pain assays: the carrageenan-induced inflammatory pain, the complete Freund adjuvant induced inflammatory pain, and the chronic constriction injury–induced neuropathic pain in mice. We also tested whether PAM-2 enhanced the effects of the selective α7 agonist choline in the mouse carrageenan test given intrathecally. Because the experience of pain has both sensory and affective dimensions, we also evaluated the effects of PAM-2 on acetic acid–induced aversion by using the conditioned place aversion test.
RESULTS
We observed that systemic administration of PAM-2 significantly reversed mechanical allodynia and thermal hyperalgesia in inflammatory and neuropathic pain models in a dose- and time-dependent manner without motor impairment. In addition, by attenuating the paw edema in inflammatory models, PAM-2 showed antiinflammatory properties. The antinociceptive effect of PAM-2 was inhibited by the selective competitive antagonist methyllycaconitine, indicating that the effect is mediated by α7 nicotinic acetylcholine receptors. Furthermore, PAM-2 enhanced the antiallodynic and antiinflammatory effects of choline, a selective α7 agonist, in the mouse carrageenan test. PAM-2 was also effective in reducing acetic acid induced aversion in the conditioned place aversion assay.
CONCLUSIONS
These findings suggest that the administration of PAM-2, a new α7-selective type II PAM, reduces the neuropathic and inflammatory pain sensory and affective behaviors in the mouse. Thus, this drug may have therapeutic applications in the treatment and management of chronic pain.
Chronic pain remains one of the most challenging of all neurologic diseases and, currently approved drugs have only a modest efficacy in several patient groups and numerous side effects. In recent years, a variety of structurally distinct α7 nicotinic acetylcholine receptor (nAChR) agonists have been developed and profiled for a variety of neurologic diseases. In particular, some of these agonists have been shown to have therapeutic significance in reducing inflammation and nociception, as well as in providing neuroprotection in animal models.1-8 The α7 nAChRs were targeted because they are expressed on both supraspinal and spinal pain transmission pathways.9-14 These receptors are also found on immune and nonimmune cytokine-producing cells, such as macrophages and keratinocytes.15 However, there are still a number of uncertainties in the development of α7 nAChR agonists for the treatment of pain, including receptor selectivity (namely cross-reactivity with 5-HT3 receptors, which have high homology with α7 nAChRs) and possible adverse effects.16,17
In addition, although α7 nAChR agonists have shown beneficial effects in chronic pain models in some studies, this effect was not consistently seen in other studies.18 An alternative therapeutic approach has been the development of positive allosteric modulators (PAMs), which can synergize and augment orthosteric-site–mediated signaling of endogenous neurotransmitters, including acetylcholine and choline, without, in most cases, directly activating or desensitizing the receptor. Several selective α7 nAChR PAMs have been reported and classified as type I and type II. Type I PAMs increase peak agonist-evoked responses, but have little or no effect on the decay rate of macroscopic currents or the equilibrium desensitization of α7 nAChRs, whereas type II PAMs both increase peak currents and slow down the apparent desensitization profile of the agonist response and/or recover nAChRs from the desensitized state.19,20 Both PAM types have been recently tested in vivo for their efficacy in animal models of inflammation and neuropathic pain; however, type II but not type I PAMs were shown to be effective in neuropathic pain models.21,22 Most studies with α7 nAChR PAMs in chronic pain models were conducted with PNU-120596,21-24 and more recently with the newly reported 2,4,20,50-tetrahydroxychalcone.25 Hence, additional studies are needed to fully explore and investigate the analgesic-like properties of α7 nAChR PAMs in animal models of chronic pain.
Therefore, in this study, we sought to evaluate the anti-nociceptive and antiinflammatory effects of 3-furan-2-yl-N-p-tolyl-acrylamide (PAM-2), a novel and putative selective α7 nAChR type II PAM,26,27 using different mouse models of chronic pain. PAM-2 has recently been found to enhance α7 nAChR activity in vitro26 and to produce antidepressant activity in mice in vivo.27 The first aim of this study was to evaluate whether PAM-2 produces antiallodynic or antihyperalgesic activities in several mouse inflammatory and neuropathic pain models. We also assessed the effects of PAM-2 on aversion, an important affective component of pain.
METHODS
Animals
Male adult Institute of Cancer Research (ICR) mice obtained from Harlan Laboratories (Indianapolis, IN) were used throughout the study. Mice were housed in a 21°C humidity-controlled animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. They were housed in groups of 4 and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7:00 AM). Mice were weighed approximately 25 to 35 g at the start of all experiments. All experiments were performed during the light cycle (between 7:00 AM and 7:00 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Chemical Synthesis, Purification, and Chemical Characterization of PAM-2
The synthesis of PAM-2 was performed by the coupling between (E)-3-(furan-2-yl)acrylic acid and p-toluidine using N,N′-2-dicyclohexylcarbodiimide and 4-(dimethylamino) pyridine (Appendix 1). (E)-3-(Furan-2-yl)acrylic acid (10.1 mmol), N,N′-2-dicyclohexylcarbodiimide (10.1 mmol), and 4-(dimethylamino)pyridine (10.1 mmol) were dissolved in anhydrous dichloromethane (50 mL), and the solution was cooled to 0°C and stirred for 30 minutes. Then, a solution of p-toluidine (1.07 g, 10.0 mmol) in dichloromethane (50 mL) was added dropwise over 15 minutes. When the addition was complete, the mixture was warmed to room temperature and stirred for 12 hours. The reactions were monitored by thin-layer chromatography using precoated silica gel 60 plates (Merck 60 F254 0.2 mm, Merck KGaA, Darmstadt, Germany). Thin-layer chromatography spots were visualized by ultraviolet light at 254 nm, and final compounds were purified by using silica gel column chromatography and mixtures of dichloromethane-methanol as eluent. The product showed a purity of approximately 98%.
1H and 13C NMR spectra for PAM-2 were determined in deuterated chloroform (CDCl3) using a Bruker Advance 400 at 400 and 100 MHz, respectively. Chemical shifts are given in parts per million (δ), and coupling constant values (J) are given in Hertz (Hz). Signal multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quadruplet), m (multiplet), and br (broad signal). The obtained NMR data are as follows: 1H NMR (CDCl3, 400 MHz): δ: 2.32 (s, 3H), 6.45 (d, 1H), 6.51 (s, 1H), 6.55 (d, 1H), 7.12 (d, 2H), 7.52 (d, 2H), 7.43 (s, 1H), 7.47 (m, 1H), 7.63 (s, 1H); 13C NMR (CDCl3, 400 MHz): δ δ: 166.31, 153.14, 145.86, 135.58, 131.14, 130.13, 121.83, 120.67, 115.78, 113.93, 22.53.
Melting points were determined on a Reichert Galen III hot plate microscope apparatus and are uncorrected. Infrared spectra were obtained with a Bruker Vector 22-FT spectrophotometer (Bruker, Billerica, MA) using potassium bromide plates. The activity of PAM-2 was further tested by Ca2+ influx assays. The method to determine Ca2+ influx in CH3-hα7 cells was the same as described previously.26
Other Drugs
PAM-2 was dissolved in a mixture of ethanol/Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ)/distilled water (1:1:18, by volume) and administered intraperitoneally (IP). Mecamylamine hydrochloride, Complete Freund Adjuvant (CFA), and λ-carrageenan were purchased from Sigma-Aldrich (St. Louis, MO). Methyllycaconitine (MLA) citrate and choline were obtained from RBI (Natick, MA). Other drugs were dissolved in physiologic saline (0.9% sodium chloride) and injected subcutaneously (SC) at a total volume of 1 mL/100 g body weight, unless noted otherwise. All doses are expressed as the free base of the drug. For control injections in the behavioral tests, 1:1:18 was used as a vehicle of PAM-2, whereas saline was used for the other drugs. In the sham studies of inflammatory models, distillated water was used as a vehicle of carrageenan, whereas mineral oil was used as a vehicle of CFA.
BEHAVIORAL ASSESSMENTS
Carrageenan-Induced Inflammatory Pain Model
Mice were injected with 20 μL of (0.5%) of λ-carrageenan in the intraplantar region of the right hind paw. Paw diameter and mechanical allodynia were measured before and 6 hours after carrageenan injection. PAM-2 or vehicle was injected IP 6 hours after the carrageenan injection, and the animals were tested for mechanical allodynia for possible efficacy. In the sham group of carrageenan, mice were injected with distilled water as the vehicle of λ-carrageenan under the same conditions. Mechanical stimuli thresholds were determined for each animal 15, 30, 60, and 120 minutes after the PAM-2 injection.
To determine whether mecamylamine or MLA inhibits the effects of PAM-2, mecamylamine (2 mg/kg SC), MLA (10 mg/kg SC), or its vehicle was injected 15 minutes before PAM-2 (8 mg/kg IP) or its vehicle injection, and animals were then tested for mechanical allodynia. Mechanical thresholds were determined for each animal 30 minutes after the last injection.
To assess the interaction between PAM-2 and choline in the carrageenan-induced inflammatory pain model, separate groups of mice were administrated PAM-2 (2 mg/kg IP) or vehicle and 30 minutes later were given intrathecal (IT) injections of vehicle or choline (10 μg/mouse in 5 μL volume). After drug treatment, mechanical stimuli thresholds were tested for each animal 10, 20, 30, 60, 120, and 240 minutes after the last injection.
CFA-Induced Chronic Inflammation
Mice were injected with 20 μL of CFA (50%) in the intraplantar region of the right hind paw. In the sham group of CFA, mice were injected with mineral oil as the vehicle of CFA. Paw diameter and thermal hyperalgesia were measured before and 3 days after the CFA injection. PAM-2 (2, 6, and 8 mg/kg) or vehicle were injected IP 3 days after the CFA injection, and the animals were tested for mechanical allodynia for possible efficacy. Mechanical stimuli thresholds were determined for each animal 15, 30, 60, and 120 minutes after the PAM-2 injection.
Measurement of Paw Edema
The thickness of the carrageenan or CFA-treated and control paws were measured both before and after injections at the time points indicated earlier, by using a digital caliper (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ±0.01 mm and expressed as change in paw thickness (ΔPD = difference in the ipsilateral paw diameter before and after injection paw thickness).
Chronic Constrictive Nerve Injury Induced Neuropathic Pain Model
This chronic constriction injury (CCI) procedure was conducted as described previously.22 PAM-2 (2, 6, and 8 mg/kg) or vehicle was injected IP 2 weeks after CCI surgery, and the animals tested for mechanical allodynia for possible efficacy. Mechanical stimuli thresholds were determined for each animal 15, 30, 60, and 120 minutes after the PAM-2 injection.
To determine whether mecamylamine or MLA inhibits the effects of PAM-2, mecamylamine (2 mg/kg SC), MLA (10 mg/kg SC) or its vehicle was injected 15 minutes before PAM-2 (8 mg/kg IP) or its vehicle injection, and animals were then tested for mechanical allodynia. Mechanical thresholds were determined for each animal 30 minutes after the last injection.
Evaluation of Thermal Hyperalgesia and Mechanical Allodynia
Thermal hyperalgesia was measured by the Hargreaves test as described previously.22 Results were expressed either as paw withdrawal latency (PWL) for each paw or as ΔPWL (in seconds) = contralateral latency – ipsilateral latency. Mechanical allodynia thresholds were determined using the Von Frey filaments according to the method suggested by Chaplan et al.,28 as described in our previous reports.22 The mechanical threshold was expressed as log10(10 £ force in [mg]). All behavioral testing on animals was performed in a blinded manner.
Acetic Acid Induced Conditioned Place Aversion
To evaluate the negative affective component of pain, the conditioned place aversion (CPA) test was performed.29,30 In brief, separate groups of mice were handled for 3 days before the initiation of CPA testing. The CPA apparatus consisted of a 3-chambered box with a white compartment, a black compartment, and a center gray compartment. The black and white compartments also had different floor textures to help the mice further differentiate between the 2 environments. On day 1, mice were placed in the gray center compartment for a 5-minute habituation period, followed by a 15-minute test period of freely exploring all compartments to determine baseline responses. A baseline score was recorded and used to randomly pair each mouse with either the black or white compartment. Drug-paired sides were randomized so that an even number of mice received drug on the black and white side. On day 2 (conditioning session), conditioning was performed as follows: the mice were given an IP injection of saline (10 mL/kg) as a control nonnoxious stimulus or 1% acetic acid (AA; 10 mL/kg) as a noxious stimulus and then immediately confined in the drug-paired compartment for 40 minutes. In addition, mice were pretreated with saline as a vehicle (IP) or PAM-2 (2 and 8 mg/kg IP) 15 minutes before AA or saline injection. On the test day (day 3), mice were allowed to freely explore all compartments, and the day 1 procedure was repeated. Data were expressed as time spent on the drug-paired side postconditioning minus time spent on the drug-paired side preconditioning. A positive number indicates preference for the drug-paired side, whereas a negative number indicates aversion to the drug-paired side. A number at or near 0 indicates no preference for either side.
Motor Coordination
The effects of drugs on motor coordination were measured using the rotarod test (IITC Life Science, Woodland Hills, CA) as described previously.21 The percentage of impairment was calculated as follows: % impairment = (180 – test time)/(180 × 100). Mice were pretreated with either IP vehicle or PAM-2 (8 or 20 mg/kg IP) 15 minutes before the test.
Intrathecal Injections
Injections were performed free hand between the fifth and sixth lumbar vertebra in unanesthetized male mice according to the method suggested by Hylden and Wilcox.31
Statistical Analysis
The data obtained were analyzed using the GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± SEM. We selected sample size based on our previous studies.21-23 Dose-response curves of PAM-2 in each pain model were analyzed by using 2-way repeated measure analysis of variance (ANOVA) and followed by the post hoc Tukey test. The antiedema effect of PAM-2 was analyzed using an unpaired t test. The involvement of α7 AChRs in the effect of PAM-2 was analyzed by 1-way ANOVA using pharmacologic antagonists and followed by the post hoc Tukey test. Before ANOVA, the data were first assessed for the normality of the residuals (using Shapiro-Wilk test for n > 6 or Kolmogorov-Smirnov test with Dallal-Wilkinson-Lilliefor test for n ≤ 6) and equal variance (F test). The homogeneity of variance was evaluated by the Brown-Forsythe test. Because some data did not pass these tests, we used strict statistic method to treat P < 0.01 as significant. The exact values for 0.01 < P < 0.05 were given in figures.
RESULTS
PAM-2 was first synthesized by a new chemical strategy on a 10-mmol scale (Appendix 1), and its structure characterized by spectroscopic methods. This new strategy gave a product with higher purity (~98%) than that obtained using the previously published method (~70%).26 The activity of PAM-2 was further tested by Ca2+ influx assays as described previously.26 The Ca2+ influx results indicated that 10 μM PAM-2 enhances (±)-epibatidine-induced α7 AChR activity, increasing the potency of (±)-epibatidine from 52 ± 4 to 17 ± 5 nM, with efficacy (Emax = 190%) in the same range as that determined previously (204% ± 13%).26
The antiallodynic effects of PAM-2 (2, 6, and 8 mg/kg IP) were explored in the carrageenan-induced inflammatory pain model. Mice were given an intraplantary injection of carrageenan (0.5%) and then tested for allodynia 6 hours later. PAM-2 induced a significant dose by time interaction for the measure of allodynia (F dosextime(15,75) = 4.818, P < 0.001, Fig. 1A). The antiallodynic effects of PAM-2 were evident from 15 to 60 minutes after injection and returned to baseline by 120 minutes. In addition, treatment with the highest dose of PAM-2 (8 mg/kg IP) showed a trend toward attenuation of carrageenan-induced paw edema (Fig. 1B). The antiallodynic effect of PAM-2 (8 mg/kg IP) in the carrageenan test was blocked by pretreatment with either antagonist mecamylamine (nonselective) or MLA (relatively α7-selective; F(2,17) = 48.963, P < 0.001; Fig. 1C). Interestingly, the highest dose of PAM-2 (8 mg/kg IP) failed to show an antinociceptive effect in sham mice that received vehicle of carrageenan (F dose(1,5) = 0.6984, P = 0.4414; Ftime(5,25) = 0.6893, P = 0.6361 and Fdose × time(5,25) = 0.3174, P = 0.8978; Fig. 1D).
Figure 1.
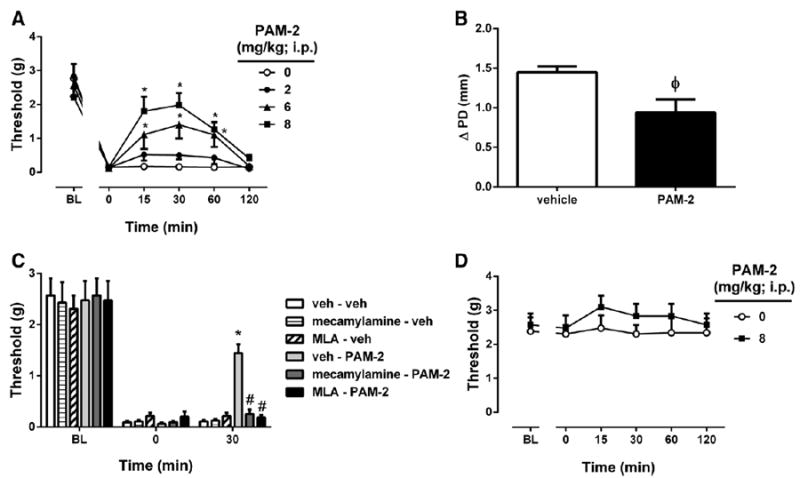
The antiallodynic and antiinflammatory effects of 3-furan-2-yl-N-p-tolyl-acrylamide (PAM-2) using the carrageenan-induced inflammatory pain model in mice. A, The antiallodynic effects after intraperitoneal (IP) administration of various doses of PAM-2 (2, 6, and 8 mg/kg). B, Antiinflammatory effect of PAM-2, as measured by the difference in the ipsilateral paw diameter before and after carrageenan injection (ΔPD), 1 hour after IP injection of PAM-2 (8 mg/kg). C, Blockade of the antinociceptive effects of PAM-2 by subcutaneous administration of either mecamylamine or methyllycaconitine (MLA). Mecamylamine (2 mg/kg) or MLA (10 mg/kg) was given 15 minutes before an active dose of 8 mg/kg of PAM-2 or vehicle. D, The antinociceptive effects of PAM-2 (8 mg/kg IP) in vehicle of carrageenan-injected*mice. Pain sensitivity was measured by von Frey filament thresholds. Data are given as the mean ± SEM of 5 to 7 animals for each group. *P < 0.01, significantly different from its vehicle group; #P < 0.01, significantly different from PAM-2–treated group. φP = 0.022, t= 2.815 for ΔPD difference between groups.
We then determined the effects of PAM-2 on the choline-evoked possible antiallodynic effects using the carrageenan test. A 2-way (time × treatment) repeated measures ANOVA for antiallodynia was significant for treatment (Ftreatment(3,15) = 26.21, P < 0.001), time (Ftime(7,35) = 16.36, P < 0.001), and their interaction (Ftreatment × time(21,105) = 11.05, P < 0.001). Pretreatment with a low dose of PAM-2 (2 mg/kg IP) or choline (10 μg/5 μL IT) showed slight but significant antiallodynic effects in early evaluation times when tested alone (F(2,10) = 7.709, P = 0.0094, Fig. 2A). However, combination of choline and PAM-2 markedly induced antiallodynic effects. The combination showed greater and more prolonged activity when compared with choline or PAM-2 alone (F(2,10) = 27.47, P < 0.001 and F(2,10) = 27.15, P < 0.001, respectively). The combination induced antiallodynic activity started after drug injection and peaked 30 minutes later and then slowly reduced, but was still evident after 4 hours (F(3,20) = 6.001, P = 0.0044). Similarly, the combination of PAM-2 and choline significantly reversed paw edema (F(1,11) = 48.5, P < 0.001, Fig. 2B).
Figure 2.
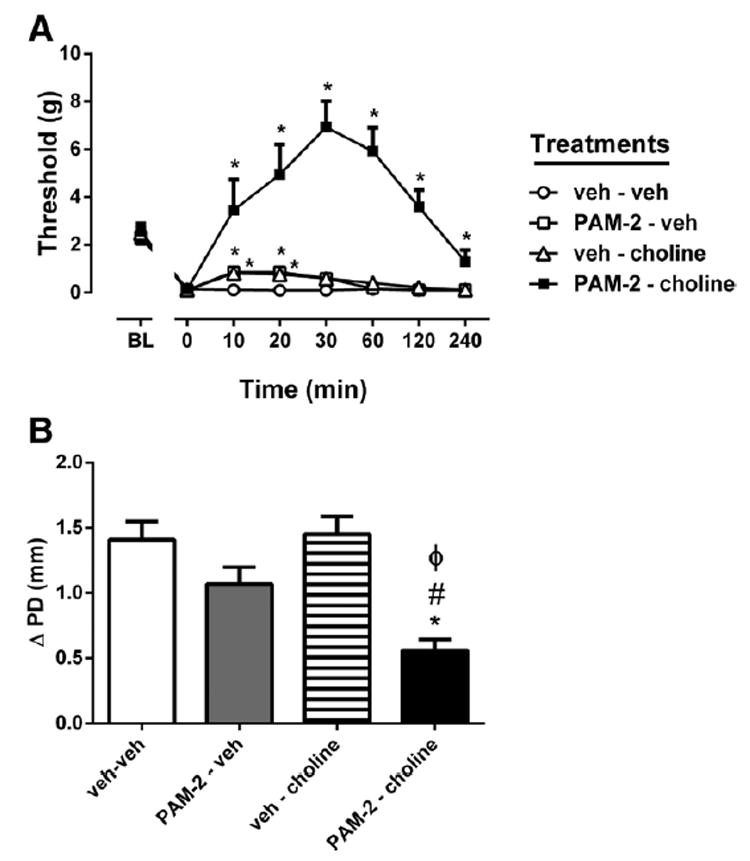
Effects of PAM-2 and choline on carrageenan-induced inflammatory pain behaviors (A) and paw edema (B) in mice. Mice received PAM-2 (2 mg/kg intraperitoneally) or vehicle, and 30 minutes later mice were given an intrathecal injection of choline (10 μg/mouse) or vehicle. Paw edema, measured by the difference in the ipsilateral paw diameter before and after carrageenan injection (ΔPD), was assessed 1 hour after the last injection. Pain sensitivity was measured by von Frey filament thresholds. Data are given as the mean ± SEM of 6 animals for each group. * P < 0.01, significantly different from vehicle group; #P < 0.01, significantly different from PAM-2 treated group; φP < 0.01, significantly different from cholinetreated group. PAM-2 = 3-furan-2-yl-N-p-tolyl-acrylamide.
We next evaluated possible antihyperalgesic and antiinflammatory effects of PAM-2 in the CFA model. Interestingly, PAM-2 dose dependently reduced CFA-induced hyperalgesia (F dose(3,15) = 10.06, P < 0.001; F time(5,25) = 49.33, P < 0.001 and F dose × time(15,75) = 2.356, P = 0.0079, respectively; Fig. 3A), and there was a trend toward attenuation of carrageenan-induced paw edema by the highest dose (8 mg/kg IP) of PAM-2 (Fig. 3B). The antihyperalgesic effects of PAM-2 peaked between 15 and 30 minutes after the injection and lasted for 2 hours. We also evaluated the possible antinociceptive effects of the highest dose of PAM-2 (8 mg/kg IP) in sham group of CFA. PAM-2 did not show significant effect on thermal sensitivity (F dose(1,5) = 0.01253, P = 0.9152; Ftime(5,25) = 0.6925, P = 0.6339; and Fdose × time (5,25) = 0.356, P = 0.87735, Fig. 3C).
Figure 3.
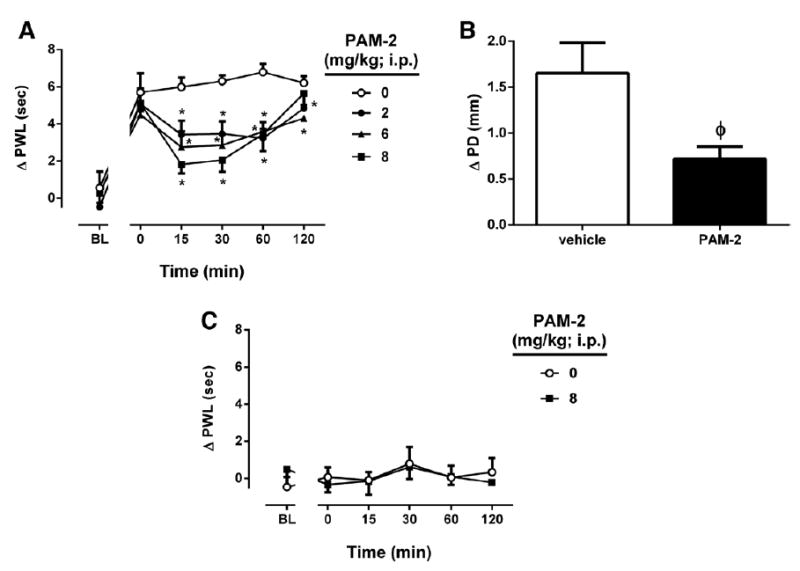
The antihyperalgesic and antiinflammatory effects of 3-furan-2-yl-N-p-tolyl-acrylamide (PAM-2) using the complete Freund adjuvant (CFA)-induced chronic inflammatory pain model in mice. A, The antihyperalgesic effects after intraperitoneal (IP) administration of various doses of PAM-2 (2, 6, and 8 mg/kg). B, Antiinflammatory effect of PAM-2, measured by the difference in the ipsilateral paw diameter before and after carrageenan injection (ΔPD), was assessed 1 hour after the injection of PAM-2 (8 mg/kg). C, The antinociceptive effects of PAM-2 (8 mg/kg IP) on vehicle of CFA-injected mice. Pain sensitivity was measured by paw withdrawal latency (PWL) with radiant heat beam. Data were given as the mean ± SEM of 5 to 6 animals for each group. *P < 0.01, significantly different from its vehicle group; φP = 0.0257, t = 2.617 for ΔPD difference between groups.
The antiallodynic effect of systemic PAM-2 administration in mice was then tested after 2 weeks in the CCI or sham mice. PAM-2 (2, 6, and 8 mg/kg IP) significantly reversed the allodynia in a dose-, time-, and dose time-dependent manner (Fdose(3,15) = 7.235, P = 0.0032; F time(5,25) = 109.3, P < 0.001; and Fdose × time(15,75) = 5.020, P < 0.001, respectively; Fig. 4A). Similar to the previous test, the antiallodynic effects of PAM-2 peaked between 15 and 30 minutes after injection and lasted for 120 minutes. In addition, PAM-2, at the highest dose (8 mg/kg IP), failed to show any significant antinociceptive effect in sham mice (Fdose(1,5) = 0.09956, P = 0.7651; Ftime(5,25) = 0.2312, P = 0.9453; and Fdose × time (5,25) = 1.798, P = 0.1498, Fig. 4B). The antiallodynic effect of PAM-2 (t = 5.678, P < 0.001; vehicle-PAM-2 versus vehicle-vehicle treatments) in the CCI model was totally inhibited by pretreatment with mecamylamine or MLA (F(2,17) = 22.341, P < 0.001; Fig. 4C). By themselves, the 2 nicotinic antagonists did not significantly affect the mechanical thresholds of the CCI mice (F(2,15) = 0.068, P = 0.9345).
Figure 4.
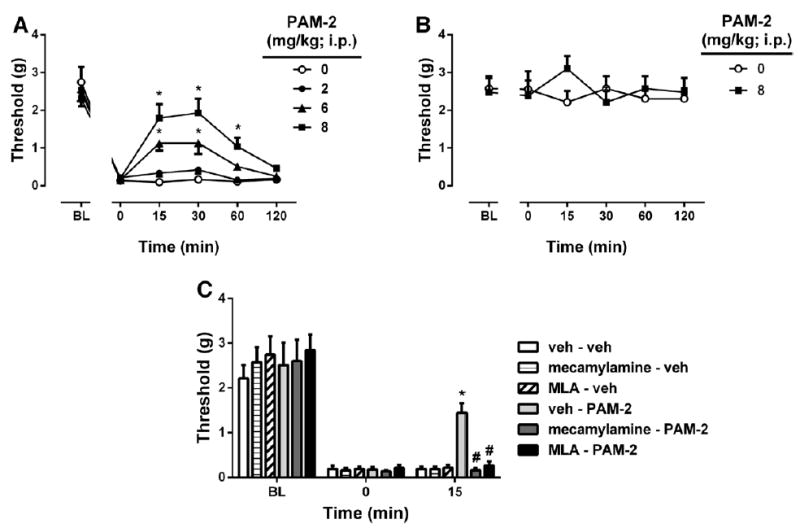
The antiallodynic effects of PAM-2 using the chronic constriction injury (CCI)-induced neuropathic pain model in mice. A, The antiallodynic effects after intraperitoneal (IP) administration of various doses of PAM-2 (2, 6, and 8 mg/kg) in CCI mice. B, The effects of PAM-2 (8 mg/kg IP) on mechanical sensitivity in sham mice. C, Blockade of the antiallodynic effects of PAM-2 by subcutaneous administration of mecamylamine or MLA. Mecamylamine (2 mg/kg) or MLA (10 mg/kg) was given 15 minutes before an active dose of 8 mg/kg of PAM-2 or vehicle. Pain sensitivity was measured by von Frey filament thresholds. Data are given as the mean ± SEM of 6 animals for each group. *P < 0.01, significantly different from its vehicle group; #P < 0.01, significantly different from PAM-2– treated group. MLA = methyllycaconitine; PAM-2 = 3-furan-2-yl-N-p-tolyl-acrylamide.
Finally, AA administration induced place aversion in the CPA test (F(1,14) = 5.874, P = 0.0077; Fig. 5B) in mice. Pretreatment with PAM-2 (2 or 8 mg/kg IP) significantly attenuated AA-induced aversion in a dose-related manner (F(5,37) = 5.607, P = 0.0015; Fig. 5C). At 8 mg/kg, PAM-2 totally blocked the aversion in the CPA test. At the highest dose (8 mg/kg), PAM-2 did not induce a place preference or aversion on its own in mice (t = 0.2612, P = 0.2612; PAM-2-vehicle versus vehicle-vehicle treatments).
Figure 5.
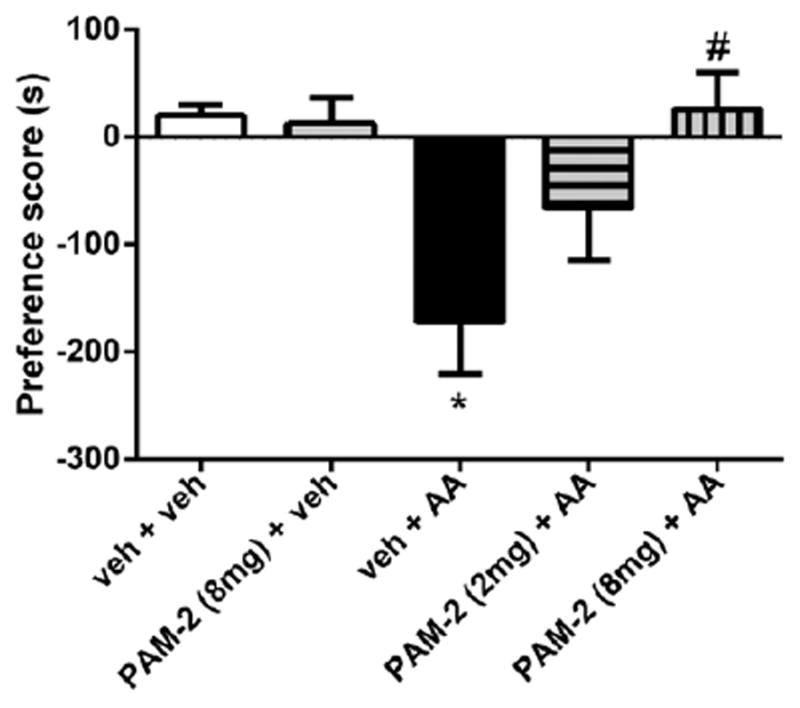
Effect of 3-furan-2-yl-N-p-tolyl-acrylamide (PAM-2) on acetic acid (AA)-induced conditioned place aversion. PAM-2 (2 or 8 mg/kg) or vehicle was injected intraperitoneally 15 minutes before administration of AA (1%). Data are given as the mean ± SEM of 6 to 9 ani-mals for each group. *P < 0.01, compared with the vehicle-injected mice; #P < 0.01, compared with the AA-injected mice.
At the highest effective dose of PAM-2 (8 mg/kg IP) in the pain models, naïve mice treated with PAM-2 did not show significant changes in motor coordination using the rotarod test (t = 1.0, P = 0.3409; Table 1). Furthermore, when mice were treated with a much higher dose of PAM-2 (20 mg/kg IP), no disruption of motor coordination of the animals were observed (t = 0.9045, P = 0.3893; Table 1). In addition, we measured the possible motor coordination in carrageenan-treated mice using the highest dose of PAM-2 in the behavioral experiments (Table 2). PAM-2 (8 mg/kg IP) did not significantly alter motor coordination (t = 1.0, P = 0.3466).
Table 1.
Effects of PAM-2 on Motor Coordination of Mice
| PAM-2 (mg/kg IP) | % Impairment |
|---|---|
| 0 | 0.6 ± 0.6 |
| 8 | 0.0 ± 0.0 |
| 20 | 0.0 ± 0.0 |
The effect of PAM-2 on motor coordination was evaluated using the rotarod test in mice 15 min after injection of either PAM-2 or vehicle. Mice were placed on the rotarod for 3 min. Data were presented as mean ± SEM. % impairment of 5 to 6 animals for each group.
IP = intraperitoneal; PAM-2 = 3-furan-2-yl-N-p-tolyl-acrylamide.
Table 2.
Effects of PAM-2 on Motor Coordination in Carrageenan-Treated Mice
| PAM-2 (mg/kg; IP) | % Impairment |
|---|---|
| 0 | 0 ± 0 |
| 8 | 1 ± 1 |
Mice were injected with 20 μL of (0.5%) of -carrageenan in the intraplantar region of the right hind paw. PAM-2 or vehicle was injected IP 6 h after the carrageenan injection, and the animals were tested for possible motor impairment. The motor coordination was evaluated using the rotarod test in mice 15 min after injection of either PAM-2 or vehicle. Mice were placed on the rotarod for 3 min. Data were presented as mean ± SEM. % impairment of 5 animals for each group.
IP = intraperitoneal; PAM-2 = 3-furan-2-yl-N-p-tolyl-acrylamide.
DISCUSSION
The identification of PNU-120596, an α7 nAChR-selective type II PAM,32 has stimulated the development of other α7 nAChR-selective PAMs. PNU-120596 has been reported to have several bioactivities, such as cognitive enhancement and neuroprotective effects.32-34 PNU-120596, by itself, showed relevant antinociceptive activities in rodent pain models.21-24 This study establishes the antinociceptive efficacy of PAM-2, a new putative α7 nAChR-selective type II PAM,26,27 in mice. PAM-2 attenuated the allodynic pain behavior in the carrageenan test of short-term inflammation. Furthermore, it was also able to reverse hyperalgesia in the CFA test of long-term infiammation. In addition, treatment with the highest dose of PAM-2 (8 mg/kg IP) showed a trend toward attenuation of carrageenan- and CFA-induced inflammation as seen in paw edema. In the CCI-induced neuropathic pain model, PAM-2 was effective in reversing mechanical allodynia in mice. However, PAM-2 did not change the mechanical sensitivity of sham mice. A potential explanation for this may be that PAM-2 has no effect on acute mechanical sensitivity and that it works only after neuropathic and inflammatory conditions. Furthermore, our results with MLA, an α7-selective antagonist, suggest that α7 nAChRs play a critical role in its action, confirming its receptor selectivity.26 Importantly, the antinociceptive effects of PAM-2 occurred at doses that had no effect on motor coordination in mice. Because α7 nAChR PAMs presumably have the capability of enhancing α7 nAChR agonists response,32,35 we investigated the interaction between PAM-2 and the selective α7 nAChR agonist choline. As predicted, the combination of PAM-2 and choline produced enhanced antiallodynic and antiinflammatory effects in the carrageenan model compared with the effects of each drug given separately. Therefore, these results suggest that PAM-2 potentiates the effect mediated by choline given exogenously and support the idea that the activity of PAM-2 (when administered alone) can be mediated by enhancement of the endogenous cholinergic tone.
The exact mechanisms involved in the antinociceptive effects of PAM-2 in the chronic pain models are unknown. Our results with IT choline suggest that PAM-2 could be enhancing endogenous cholinergic tone through α7 nAChRs in the spinal cord.36 In addition, PAM-2 could be enhancing endogenous cholinergic tone through α7 nAChRs in dorsal root ganglia neurons, reducing pain-related behaviors. Indeed, up-regulation of α7 nAChRs in dorsal root ganglia was observed in CCI-induced neuropathic pain in rodents.4,37-39 Furthermore, it is also possible that the effects of PAM-2 could be mediated by an α7-dependent regulation of antiinflammatory chemokines such as tumor necrosis factor-a through a nuclear factor-κB pathway.40 In line with this suggestive mechanism, Munro et al.24 recently showed that antiinflammatory effects of PNU-120596 in rats are possibly mediated through a decrease in tumor necrosis factor-α and interleukin-6 levels. Furthermore, Ca2+ influx results indicated that PAM-2 potentiates hα7 nAChRs with apparent EC50 approximately 5 μM.26
Overall, our results in chronic pain models with PAM-2 are consistent with those found using PNU-120596. Indeed, PNU-120596 at equivalent doses (3 8 mg/kg) was shown to have pronounced antiallodynic and antihyperalgesic activities in CCI-induced neuropathic and carrageenan-induced inflammatory pain models in mice22 and rats.24 However, the antinociceptive effects of PAM-2 in these chronic pain models were shorter than those observed with PNU-120596 (between 3 and 6 hours) in the mouse.22 Similarly, both PAM-2 and PNU-120596 enhanced the antinociceptive properties of choline after IT administration in a synergistic fashion.23
Because the experience of pain has both sensory and affective dimensions, we also evaluated the effects of PAM-2 on AA-induced aversion, an important affective component of pain, by using the CPA test.41,42 In this study, we show for the first time that PAM-2, a putative type II α7 nAChR-selective PAM, was effective in blocking the development of AA-induced pain-related depressive behavior as seen in CPA. The PAM-2 induced inhibition of CPA was not the result of its intrinsic reward properties, because the drug alone did not induce conditioned place preference.
Because α7 nAChR PAMs presumably have the capability of enhancing agonist-induced α7 nAChR responses,32,35 we investigated the interaction between PAM-2 and choline, an endogenous α7 nAChR agonist. The antiallodynic effects of PAM-2 were investigated in the presence of choline to address potential drug interactions (i.e., synergism) by using low or inactive doses of both compounds. The drug combination produced greatly enhanced antiallodynic and antiedema effects in the carrageenan-induced inflammatory pain model compared with the effects of each drug given separately. The current results are in line with those using a combination of PNU-120596 and other α7 nAChR agonists, such as choline and PHA-543613.22,23 These results also support the concept that the antiallodynic effects are mediated by α7 nAChRs.
In conclusion, this study suggests that PAM-2 and other α7 nAChR type II PAMs may represent novel therapeutic agents in the treatment of inflammatory and neuropathic pain symptoms.
Acknowledgments
This work was supported by a Pilot Project Award from the Massey Cancer Center at VCU (to MID), by grants from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT, Chile, Grant 1130079), Millennium Scientific Initiative (Ministerio de Economía, Fomento y Turismo, Chile. ICM, Grant P10-035-F; to EGP), from the Polish National Science Center (Sonata funding, UMO-2013/09/D/NZ7/04549; to KMT-D [PI] and HRA [Co-PI], and Scientific and Technical Research Council of Turkey (TUBITAK) for postdoctoral research scholarship (2219-2013; to DB).
APPENDIX
Scheme 1.
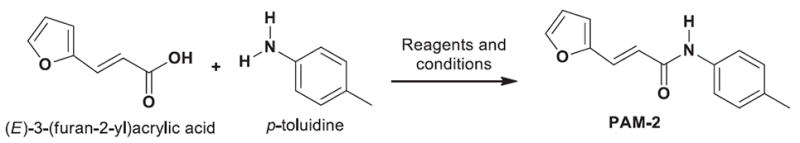
New strategy for the synthesis of 3-furan-2-yl-N-p-tolyl-acrylamide. Reagents and conditions: (E)-3-(furan-2-yl)acrylic acid (10.1 mmol, 1.01 Eq), p-toluidine (10 mmol, 1.0 equiv.), N,N-dicyclohexylcarbodiimide (DCC) (10.1 mmol, 1.01 Eq), 4-(dimethylamino)pyridine (10.1 mmol, 1.01 Eq), dichloromethane, 0°C to RT, 12 hours (see Methods for more details).
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Deniz Bagdas, DVM, PhD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Deniz Bagdas approved the final manuscript, and is the author responsible for archiving the study files.
Name: Katarzyna M. Targowska-Duda, PhD.
Contribution: This author performed the locomotor activity experiments.
Attestation: Katarzyna M. Targowska-Duda approved the final manuscript.
Name: Jhon J. López, MSc.
Contribution: This author synthesized, purified, and chemically characterized PAM-2.
Attestation: Jhon J. López approved the final manuscript.
Name: Edwin G. Perez, PhD.
Contribution: This author designed the chemical method for the synthesis of PAM-2.
Attestation: Edwin G. Perez approved the final manuscript.
Name: Hugo R. Arias, PhD.
Contribution: This author helped write the article.
Attestation: Hugo R. Arias approved the final manuscript.
Name: M. Imad Damaj, PhD.
Contribution: This author conducted the study design, reviewed the analysis of the data, and helped write the manuscript
Attestation: M. Imad Damaj has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Jianren Mao, MD, PhD.
References
- 1.Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–91. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;9:580–7. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- 4.Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–63. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201–7. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–43. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagdas D, Sonat FA, Hamurtekin E, Sonal S, Gurun MS. The antihyperalgesic effect of cytidine-5’-diphosphate-choline in neuropathic and inflammatory pain models. Behav Pharmacol. 2011;22:589–98. doi: 10.1097/FBP.0b013e32834a1efb. [DOI] [PubMed] [Google Scholar]
- 9.Gillberg PG, Aquilonius SM. Cholinergic, opioid and glycine receptor binding sites localized in human spinal cord by in vitro autoradiography. Changes in amyotrophic lateral sclerosis. Acta Neurol Scand. 1985;72:299–306. doi: 10.1111/j.1600-0404.1985.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 10.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–35. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 11.Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan IM, Taylor P, Yaksh TL. Stimulatory pathways and sites of action of intrathecally administered nicotinic agents. J Pharmacol Exp Ther. 1994;271:1550–7. [PubMed] [Google Scholar]
- 13.Cordero-Erausquin M, Changeux JP. Tonic nicotinic modulation of serotoninergic transmission in the spinal cord. Proc Natl Acad Sci USA. 2001;98:2803–7. doi: 10.1073/pnas.041600698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordero-Erausquin M, Pons S, Faure P, Changeux JP. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain. 2004;109:308–18. doi: 10.1016/j.pain.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–31. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papke RL, Porter Papke JK, Rose GM. Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett. 2004;14:1849–53. doi: 10.1016/j.bmcl.2003.09.104. [DOI] [PubMed] [Google Scholar]
- 18.Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk Mv, Deng H, Guo W, Lehto SG, Matson D, McDermott JS, Knop J, Gaida K, Cao L, Waldon D, Albrecht BK, Boezio AA, Copeland KW, Harmange JC, Springer SK, Malmberg AB, McDonough SI. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149:33–49. doi: 10.1016/j.pain.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Arias HR. Positive and negative modulation of nicotinic receptors. Adv Protein Chem Struct Biol. 2010;80:153–203. doi: 10.1016/B978-0-12-381264-3.00005-9. [DOI] [PubMed] [Google Scholar]
- 20.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011;82:915–30. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freitas K, Carroll FI, Damaj MI. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013;344:264–75. doi: 10.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013;65:156–64. doi: 10.1016/j.neuropharm.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitas K, Negus SS, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013;169:567–79. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro G, Hansen R, Erichsen H, Timmermann D, Christensen J, Hansen H. The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol. 2012;167:421–35. doi: 10.1111/j.1476-5381.2012.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balsera B, Mulet J, Fernández-Carvajal A, de la Torre-Martínez R, Ferrer-Montiel A, Hernández-Jiménez JG, Estévez-Herrera J, Borges R, Freitas AE, López MG, García-López MT, González-Muñiz R, Pérez de Vega MJ, Valor LM, Svobodová L, Sala S, Sala F, Criado M. Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: a new target for a privileged structure. Eur J Med Chem. 2014;86:724–39. doi: 10.1016/j.ejmech.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Arias HR, Gu RX, Feuerbach D, Guo BB, Ye Y, Wei DQ. Novel positive allosteric modulators of the human α7 nicotinic acetylcholine receptor. Biochemistry. 2011;50:5263–78. doi: 10.1021/bi102001m. [DOI] [PubMed] [Google Scholar]
- 27.Targowska-Duda KM, Feuerbach D, Biala G, Jozwiak K, Arias HR. Antidepressant activity in mice elicited by 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic acetylcholine receptor. Neurosci Lett. 2014;569:126–30. doi: 10.1016/j.neulet.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 29.Deyama S, Nakagawa T, Kaneko S, Uehara T, Minami M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behav Brain Res. 2007;176:367–71. doi: 10.1016/j.bbr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology. 2014;39:614–24. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 32.Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alphα7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalappa BI, Sun F, Johnson SR, Jin K, Uteshev VV. A positive allosteric modulator of α7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br J Pharmacol. 2013;169:1862–78. doi: 10.1111/bph.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun F, Jin K, Uteshev VV. A type-II positive allosteric modulator of α7 nAChRs reduces brain injury and improves neurological function after focal cerebral ischemia in rats. PLoS One. 2013;8:e73581. doi: 10.1371/journal.pone.0073581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–24. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 36.Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2005;1031:229–37. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 37.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99:8360–5. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fucile S, Sucapane A, Eusebi F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol. 2005;565:219–28. doi: 10.1113/jphysiol.2005.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnevale D, De Simone R, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6:388–97. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- 40.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–8. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–4. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 42.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2001;98:8077–82. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]


