Abstract
Pancreatic progenitor cell research has been in the spotlight, as these cells have the potential to replace pancreatic β‐cells for the treatment of type 1 and 2 diabetic patients with the absence or reduction of pancreatic β‐cells. During the past few decades, the successful treatment of diabetes through transplantation of the whole pancreas or isolated islets has nearly been achieved. However, novel sources of pancreatic islets or insulin‐producing cells are required to provide sufficient amounts of donor tissues. To overcome this limitation, the use of pancreatic progenitor cells is gaining more attention. In particular, pancreatic exocrine cells, such as duct epithelial cells and acinar cells, are attractive candidates for β‐cell regeneration because of their differentiation potential and pancreatic lineage characteristics. It has been assumed that β‐cell neogenesis from pancreatic progenitor cells could occur in pancreatic ducts in the postnatal stage. Several studies have shown that insulin‐producing cells can arise in the duct tissue of the adult pancreas. Acinar cells also might have the potential to differentiate into insulin‐producing cells. The present review summarizes recent progress in research on the transdifferentiation of pancreatic exocrine cells into insulin‐producing cells, especially duct and acinar cells.
Keywords: β‐Cell regeneration, Pancreatic progenitor cells, Transdifferentiation
Introduction
Diabetes mellitus is increasing in prevalence worldwide. Defects in pancreatic β‐cell function and loss of β‐cell mass are the major characteristics of both type 1 and type 2 diabetes. This phenomenon is prevalent in all patients with type 1 diabetes and in patients with late‐stage type 2 diabetes. Complications of diabetes, such as diabetic neuropathy, nephropathy, retinopathy, heart disease and stroke could develop during the course of the disease. For the treatment of intractable diabetes, exogenous insulin injection has been widely used, and this method has improved the quality of life of diabetic patients, especially those with type 1 diabetes. After the first successful extraction of insulin, the use of exogenous insulin became a mainstay of diabetes treatment. Biosynthetic recombinant human insulin can be provided for effective treatment through the use of continuous subcutaneous insulin infusion therapy, such as the insulin pump1, 2, 3, 4, 5, 6.
In 2000, a breakthrough was recorded in human pancreatic islet transplantation. Pancreatic islets were isolated from human cadavers and transplanted into the portal veins of type 1 diabetic patients. The advantage of islet transplantation is that it is a minimally‐invasive method compared with traditional pancreas–kidney transplantation. Furthermore, the Edmonton and its related protocols offer proof of concept on the use of cell replacement therapy for diabetes mellitus7, 8, 9, 10. However, the shortage of donor organs and side‐effects of immunosuppressants limit the clinical applications of islet transplantation6. Limitations of islet transplantation has led to research on alternative transplantable β‐cell sources, such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs; Figure 1). Human ESCs derived from the inner cell mass of a blastocyst were first cultured by Thompson et al.11 However, the ethical problem is the main issue in utilization of ESCs. To overcome this limitation, iPSCs were developed by nucleus reprogramming of somatic cells. iPSCs have highly similar properties to that of ESCs, and were generated by transfection of four transcription factors, such as OCT3/4, KLF4, SOX2 and c‐MYC, or OCT4, NANOG, SOX2 and LIN2812, 13, 14. iPSCs can also be generated by other factors, small molecules and ribonucleic acids15, 16, 17, 18, 19, 20, 21, 22, 23, 24. iPSCs show a slightly different possibility compared with ESCs, because the use of iPSCs could make patient‐specific cell replacement therapy possible. iPSCs are able to match with patients genetically, thus avoiding the issue of immune rejection. These alternative β‐cell sources have unlimited expansion capacity and pluripotency to differentiate into all other types of germ layer cells. We have also generated iPSCs derived from human pancreatic duct cells by using lenti‐OCT3/4, ‐SOX2, ‐KLF4 and c‐MYC, named HD‐iPSCs. These HD‐iPSCs present similar levels of specific markers of ESCs, and spontaneously differentiate into three‐germ layers when HD‐iPSCs forms an embryoid body. Furthermore, teratomas were formed in immunodeficient mice with subcutaneous injection of HD‐iPSCs. Interestingly, HD‐iPSCs had superior differentiation potential to iPSCs derived from human skin fibroblasts when these cells were induced with differentiation protocol developed by Zhang et al.25. This different efficiency in differentiation of each iPSC was caused by its own epigenetic memory (Kim HS, Lee MK, unpublished data). However, the differentiation of ESCs and iPSCs requires numerous stages and various kinds of factors. Furthermore, clinical trials of these differentiation protocols take a long time, and undifferentiated ESCs and iPSCs pose the risk of tumorigenesis. These disadvantages limit the utilization of ESCs and iPSCs for the treatment of diabetes. Pancreatic progenitor cells (PPCs) could overcome limitations of ESCs and iPSCs, because these cells already have pancreatic lineage. PPCs also could be the targets of drug development aimed at regenerating β‐cells and forming transplantable β‐cell sources (Figure 1). Although PPCs have been well studied, it is still unclear whether PPCs actually reside in the adult pancreas and can be differentiated into functional insulin‐secreting cells. The present article reviews the current progress of research on the development of β‐cell sources using the transdifferentiation of PPCs, and discusses whether these cells are useful for the treatment of diabetes mellitus.
Figure 1.
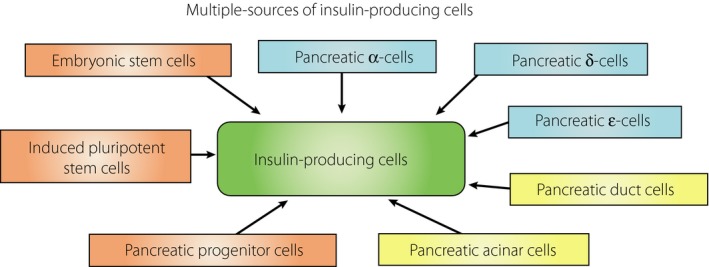
Embryonic stem cells and induced pluripotent stem cells are considered as having potency to differentiate into insulin‐producing cells. Likewise, pancreatic progenitor cells express various markers and are able to differentiate into insulin‐producing cells. Pancreatic α‐, δ‐, ε‐, duct and acinar cells also have differentiation potency.
Transdifferentiation of Pancreatic Cells
Transdifferentiation is a process whereby a differentiated cell is converted into another type of cell. Recently, transdifferentiation has become the most attractive method of developing β‐cell sources for cell replacement therapy. This process depends on cellular reprogramming, such as β‐cell neogenesis, the regeneration of new β‐cells from alternative PPCs in the adult pancreas. A representative example of transdifferentiation in the pancreas is acino‐ductal transdifferentiation (acinar ductal metaplasia). Acinar ductal metaplasia is the process by which acinar cells differentiate into duct cells (Figure 2). In addition, acinar cells are able to differentiate into hepatocyte‐like cells and adipocytes, depending on the microenvironment26, 27, 28. Another example of transdifferentiation in the pancreas has been reported, namely, that glucagon‐secreting α‐cells are able to differentiate into β‐cells. In that study, more than 99% of β‐cells were genetically removed when diphtheria toxin was used as a treatment on an insulin promoter that was conjugated with the diphtheria toxin receptor. After the ablation of the β‐cells, genetic lineage tracing with the glucagon‐TetO system showed that the α‐cells had converted into β‐cells29. This result was confirmed in PAX4‐overexpressing α‐cells30. Recently, Ye et al.31 has provided direct evidence that α‐cells are able to convert into β‐cells by using β‐cell ablation model in zebra fish. Glucagon and glucagon‐like peptide‐1 (GLP‐1) have a potent effect on α‐ to β‐cell transdifferentiation; β‐ to α‐cell transdifferentiation is possible in the case of opposition. Overexpression of aristaless‐related homeobox, suppression of pancreatic and duodenal homeobox‐1 (PDX‐1), NK2 homeobox 2 and forkhead box O1 induced the dedifferentiation of β‐cells, and transdifferentiated into α‐cells32, 33, 34, 35, 36, 37, 38. Interestingly, pancreatic δ‐cells are also able to transdifferentiate into insulin‐producing cells. This ‘somatostatin‐to‐insulin’ δ‐cell conversion only occurs in juveniles, and the forkhead box O1 network is associated with this juvenile adaptability (Figure 1)39.
Figure 2.

Pancreatic ducts consist of duct cells, and are connected with complexes of acinar cells that secrete the enzyme through the pancreatic duct into the duodenum. These pancreatic exocrine cells are able to transdifferentiate into insulin‐producing cells under various conditions. In addition, it is known that acinar cells are differentiated into duct cells through acinar‐ductal metaplasia. EGF, epidermal growth factor; GLP‐1, glucagon‐like peptide‐1; HGF, hepatocyte growth factor; INGAP, islet neogenesis associated protein; LIF, leukemia inhibitory factor; MafA, musculoaponeurotic fibrosarcoma oncogene family protein A, MAPK, mitogen‐activated protein kinase; Ngn3, neurogenin 3; Pdx‐1, pancreatic and duodenal homeobox‐1; STAT3, signal transducer and activator of transcription 3.
Pancreatic Progenitor Cells in the Adult Pancreas
Tissue‐specific adult stem cells reside in every organ, and have a regenerative potential. Based on tissue origin, these cells can be classified into skin stem cells, hematopoietic stem cells, neural stem cells, mesenchymal stem cells and gut stem cells. PPCs were relatively recently studied, and able to be distinguished by the expressions of hepatocyte growth factor, c‐Met receptor, and the absence of CD45, TER119, c‐Kit and Flk‐140, 41. The pancreas consists of two types of cells, endocrine and exocrine cells. The endocrine cells are composed of the insulin‐secreting β‐cells, glucagon‐secreting α‐cells, somatostatin‐secreting δ‐cells and pancreatic polypeptide‐secreting cells. The exocrine cells are composed of acinar cells and duct epithelial cells (duct cells). Acinar cells are the major constituents of the pancreatic tissue, and secrete digestive enzymes. Duct cells organize the epithelial linings of branched tubes that are connected to the duodenum (Figure 2). These cells are considered as PPCs and sources for β‐cell neogenesis. The specific differentiation of endocrine cell types begins earlier than E9.5 in the mouse, and the cells are specialized between E12.5 and E15.5. Endocrine‐specific transcription factors are associated with the maturation and differentiation of PPCs from the duct cords in the developmental stage42. A mechanism of recovering β‐cell mass and function similar to that which occurs in the developmental stage has been observed in injured pancreases or intractable diabetes models. Pancreas regeneration in adult rodents has been reported in many studies. We have also observed pancreas regeneration in 8‐week‐old mice after 70% pancreatectomy. The pancreas weight and β‐cell mass gradually increased over time, and the fasting blood glucose levels were normalized (Kim MH, Lim SB, Kim HS, Lee MK, unpublished data).
It is still under debate whether pancreas regeneration in humans is possible. Spontaneous pancreas regeneration has been reported in type 1 diabetic patients, which suggests that pancreas regeneration might be possible, despite continuous β‐cell destruction through autoimmunity and glucotoxicity43, 44, 45, 46. To determine whether regenerated β‐cells originate from pre‐existing β‐cells or from β‐cell neogenesis and transdifferentiation, a number of studies have been carried out. An insulin promoter‐driven Cre‐Loxp labeling system dependent on tamoxifen treatment was used as a genetic lineage method, through which β‐cell neogenesis was identified in adult mice. Lineage‐tracing is an essential tool of developmental biology, which involves labeling target cells and tracing their lineage over time. The authors monitored whether the number of labeled β‐cells remained constant or decreased as a result of β‐cell neogenesis from progenitor cells47. They concluded that pre‐existing β‐cells are the major sources of new β‐cells, rather than β‐cell neogenesis after birth, and that terminally differentiated β‐cells have the capacity to proliferate. Another study was carried out with similar results in normal, pregnant, 50% pancreatectomized, and the GLP‐1 analog, exendin‐4 (EX‐4)‐treated mice with genetically‐removed β‐cells, using a serial thymidine analog labeling method48, 49. After transforming growth factor‐α treatment in vivo, insulin‐positive cells arose from pre‐existing insulin‐positive cells near the duct epithelium50. These results suggested that β‐cells are derived only from pre‐existing β‐cells after birth, rather than from progenitor cells. However, it is possible that progenitor cells are β‐cell sources for the expansion of β‐cells in β‐cell neogenesis.
The basic helix‐loop‐helix transcription factor, neurogenin 3 (Ngn3), expression is important in PPCs. Ngn3 knockout mice lose all of the endocrine hormone‐expressing cells. In addition, the expression level of Ngn3 is important for the fate of endocrine and exocrine cells derived from PPCs. These data about Ngn3 show that specific transcription factors are able to induce the differentiation of endocrine and exocrine cells in the pancreas51, 52, 53, 54, 55, 56. In addition, Ngn3 expression was observed in a pancreatic duct ligation (PDL) animal model. After duct ligation, the pancreatic β‐cell mass had expanded, and the expanded β‐cells strongly expressed Ngn3. Furthermore, the increase in the number of β‐cells was reduced by Ngn3 small interfering ribonucleic acid. Wang et al.57 observed the expression of Ngn3 in duct‐neighboring pancreatic islets. It has been suggested that Ngn3‐positive cells are true progenitor cells located near or within the adult pancreas. In the PDL model, it is possible that the remaining β‐cells stimulated Ngn3 expression, but the results suggested that the cells were not derived from β‐cells. In other study, cross‐breeding of an insulin‐causes recombination (Cre) mouse expressing Cre recombinase under the control of the insulin promoter with an Ngn3‐reporter mouse showed that adult acinar cells were able to upregulate the expression of Ngn3. The authors suggested that Ngn3‐positive cells were derived from acinar cells in the PDL animal model30. In contrast, Desai et al.58 reported that acinar cells did not differentiate into β‐cells in an elastase‐tracing mouse. In another study using a transgenic mouse with telomerase reverse transcription‐driven green fluorescent protein (GFP), GFP‐positive cells were detected in non‐islet tissue after treatment with EX‐459, 60. These findings provide clues that PPCs exist in the mouse pancreas.
Pdx‐1 is a transcription factor for insulin expression, and has important role in expansion and differentiation of pancreatic cells34, 61, 62. In addition, Pdx‐1 is a representative marker in differentiation of ESCs and iPSCs. In the developmental stage, Pdx‐1‐positive progenitor cells are located at the tip of pancreatic branches, and can be detected with bromodeoxyuridine and thymidine analogs63. By transfection of PDX‐1, adipose tissue‐derived stem cells were able to differentiate into insulin‐producing cells. These differentiated cells reduced hyperglycemia in diabetic animals64. Recently, it has been reported that progenitor‐like cells isolated from the adult pancreas have formed duct‐like ‘ring/dense’ colonies, and these ring/dense colonies expressed Pdx‐1 and Sox9, and differentiated into ‘endocrine/acinar’ colonies. Most endocrine/acinar colonies contained β‐like cells that secreted insulin and C‐peptide65.
Nestin‐positive cells have been suggested as one of the PPC types in adult rat islets, and these cells have the ability to differentiate into insulin‐producing cells. Nestin is an intermediate filament protein expressed in neural cells and the pancreas, although some groups have suggested that nestin is expressed only during human pancreatic epithelial development66, 67, 68. In addition, other groups using the lineage‐tracing method have suggested that nestin‐positive cells contribute to the vascular and acinar lineages, but not to the endocrine lineage69, 70, 71. In addition, proliferative human islet precursor cells in adult human islets were able to proliferate in vitro 72. These cells can show a mesenchymal phenotype through a process known as epithelial‐to‐mesenchymal transition, and are derived from insulin‐expressing cells. In immunodeficient mice, human islet precursor cells were shown to express and secrete insulin, and to redifferentiate into islet‐like cell aggregates (ICAs). However, some have claimed that islet‐derived fibroblast‐like cells are not generated through epithelial‐to‐mesenchymal transition from pancreatic β‐cells in the mouse pancreas, and that human islet precursor cells are pancreatic mesenchymal stromal cells73, 74, 75, 76.
Ghrelin‐secreting ε‐cells are considered as a candidate for PPCs. Ghrelin is a 28‐amino acid polypeptide hormone, and regulates appetite and insulin secretion. Ghrelin‐secreting cells originate from the stomach, and are detectable in the gestational developmental stage77, 78, 79. The lineage‐tracing method showed that ghrelin‐secreting ε‐cells are not a terminally differentiated endocrine population, and ghrelin cells give rise to α‐ and PP cells, and rarely β‐cells in the adult mouse islets. Interestingly, ghrelin‐secreting ε‐cells contribute to cells within the duct and exocrine compartments80. It is still unclear and requires more studies whether ε‐cells are substantial PPCs in islets (Figure 1).
Pancreatic Duct Epithelial Cells
There is substantial evidence supporting the possibility that PPCs exist in the adult pancreatic duct81. Exocrine duct and acinar cells are able to transdifferentiate into pancreatic β‐cells82. Histological evidence of β‐cell regeneration through the transdifferentiation of duct cells has existed for many years. Islet renewal and pancreas regeneration are thought to be achieved by β‐cell replication and/or Ngn‐3 expressing progenitor cells near or within the pancreatic duct47, 83. These cells have also been detected with cytokeratin (CK)‐19, and are capable of self‐renewal and differentiation84, 85, 86. New evidence of duct cells’ origin was reported by Wang et al.87. They classified duct cells into two types, peribiliary glands and pancreatic duct glands duct cells. Peribiliary glands are located nearby in bile duct walls and expressed pluripotency markers, such as NANOG, OCT4 and SOX2, whereas pancreatic duct glands expressed endocrine markers. Authors suggested that these biliary tree‐derived stem cells and their network contribute to PPC constitution in the pancreas. According to these results, pancreatic duct cells might be ancestors derived from peribiliary glands duct cells87.
However, it is still controversial whether duct cells are true PPCs. Various genetic lineage‐tracing models have been used to examine the nature of duct cells. Human carbonic anhydrase‐II (CA‐II)‐positive cell tracing, in which the CA‐II promoter is conjugated with the Cre‐Loxp system, showed that CA‐II‐positive cells merged with β‐cells in the adult pancreas and ligated duct. This experiment showed that CA‐II‐expressing cells might be progenitor cells and have the potential to generate new islets. In contrast, the differentiation potency of duct cells was found to be restricted to the end of gestation in an experiment using the mouse hepatocyte nuclear factor 1β (Hnf1β) promoter conjugated with the Cre‐Loxp system. The investigators found that mouse hepatocyte nuclear factor 1β‐positive cells from embryonic days 11.5–13.5 differentiated into acinar, duct and endocrine cells. They identified the transition of the duct epithelium to duct and endocrine cells, but not acinar cells. In that study, the authors suggested that the duct cells were multipotent progenitor cells only in the embryonic stage, but were not associated with β‐cell regeneration after birth88, 89. In addition, the mucin‐1 gene tracing system was used to verify that duct cells and acinar cells are PPCs. The results suggested that mucin‐1‐positive cells were associated with an increase in β‐cell mass. However, mucin‐1‐labeled cells were not detected in adult islets. It appeared that exocrine duct cells did not contribute to β‐cell regeneration during pancreas injury or after birth90. Nevertheless, there is evidence that postnatal pancreatic duct cells might be the main source of progenitor cells for β‐cell regeneration, and many in vitro studies have reported the differentiation of adult duct cells into insulin‐producing cells41, 91, 92, 93, 94, 95. Isolated CA‐19‐9 (+), CD133 (+), CD34 (−), CD45 (−) and TER 199 (−) cells were identified as pancreatic duct cells that could differentiate into insulin‐producing cells.
Bonner‐Weir et al.96, 97 showed that human duct cells could be cultured in matrigel and form islet‐like buds. These cells are detected by CK‐19 and insulin antibodies. Noguchi et al.98 confirmed this result by the same protocol, and showed that CK‐19‐positive cells could be differentiated into endocrine cells with nicotinamide treatment. A combination of other differentiation factors, such as epidermal growth factor and gastrin, can stimulate the proliferation of β‐cells, and upregulate genes associated with regeneration (Figure 2)99. These factors led to the expansion and proliferation of the β‐cell mass. They carried out lineage‐tracing experiments, and found that new β‐cells were budding from a new lobe of the duct during normal postnatal development through β‐cell regeneration. In that experiment, duct‐specific CAII‐Cre R26R was constitutively expressed in islets, which suggested that CAII‐expressing duct cells had transdifferentiated into acinar cells and new islets. In addition, they carried out other lineage‐tracing experiments in which CAII‐CreERTM was used to detect β‐cell regeneration after PDL88. In these experiments, mature duct cells retained the abilities of pancreatic progenitor cells in adults. Furthermore, PDL promotes the Wnt target gene, leucine‐rich repeat‐containing G‐protein‐coupled receptor 5 expression in mouse duct cells. In that study, isolated duct cells could be cultured into pancreatic organoids, which contained leucine‐rich repeat‐containing G‐protein‐coupled receptor 5‐positive progenitor cells with Wnt agonist; R‐spondin contained expansion media (N‐acetylcysteine, gastrin, epidermal growth factor, noggin, FGF10, nicotinamide and B27 in Dulbecco's modified Eagle's medium F‐12 media). R‐spondin allowed continuous self‐renewal of pancreatic organoid up to 9 months100. This research provided a possibility of efficient utilization of duct cells by Wnt activation. However, these experiments were only carried out in rodents, and the insulin secretion capacity was gradually lost. In addition, direct effects of Wnt signal in the pancreas are still unclear.
Insulin‐producing cells were generated from duct cells with GLP‐1 treatment. GLP‐1 induced proliferation, and reduced apoptosis of pancreatic β‐cells. Furthermore, the GLP‐1 receptor was activated during β‐cell regeneration by upregulation of Pdx‐1. It was suggested that these Pdx‐1‐positive progenitor cells were the source of β‐cell regeneration101, 102. Considerable evidence has suggested that duct cells can be differentiated into β‐cells in vitro with GLP‐1 and EX‐4 treatment103, 104. Thus, GLP‐1 could regulate the generation of new β‐cells from pancreatic duct cells. Another factor, islet neogenesis associated protein, also induced duct cells to differentiate into insulin‐producing cells105. In addition, Smad2 (an activator of the transforming growth factor‐β superfamily), activin A (ActA) and hepatocyte growth factor also affect the differentiation of duct cells when cotreated with β‐cellulin or Pdx‐1 (Figure 2)106, 107.
We have also shown the existence of rat and human pancreatic progenitor cells in the duct, and the differentiation potential of these cells108, 109. We isolated CK‐19‐positive human duct cells from remnant cells after islet isolation. Cells were treated with ActA, EX‐4 and a high concentration (11 mmol/L) of glucose for 30 days, and we observed that cotreatment of ActA and EX‐4 induced the expression of β‐cell specific markers, such as Ngn3, Pdx‐1 and insulin, and promoted glucose‐stimulated insulin secretion. In addition, transplantation of differentiated human duct cells normalized hyperglycemia in type 1 diabetic immunodeficient mice. After human duct cell transplantation, the fasting blood glucose levels of the mice gradually declined for 60 days (Figure 3a), and the transplanted cells expressed insulin and GFP, which had been delivered by an adenovirus to trace transplanted duct cells (Figure 3b). We concluded that human CK‐19‐positive PPCs exist in the adult pancreatic duct, and that these cells are able to differentiate into insulin‐secreting cells on cotreatment with ActA and EX‐4109. Human CK‐19‐positive duct cells could be considered as PPCs, and these cells also express CA‐19 and E‐cadherin. Isolated human CD133‐positive cells formed multicellular epithelial spheres in matrigel, and were merged with CK‐19, and did not express acinar and endocrine markers. Then, investigators introduced Pdx‐1, Ngn3 and musculoaponeurotic fibrosarcoma oncogene family protein A (MafA) with adenoviral vector into duct cells. These cells expressed β‐cell‐specific markers, and also stored and secreted insulin110.
Figure 3.
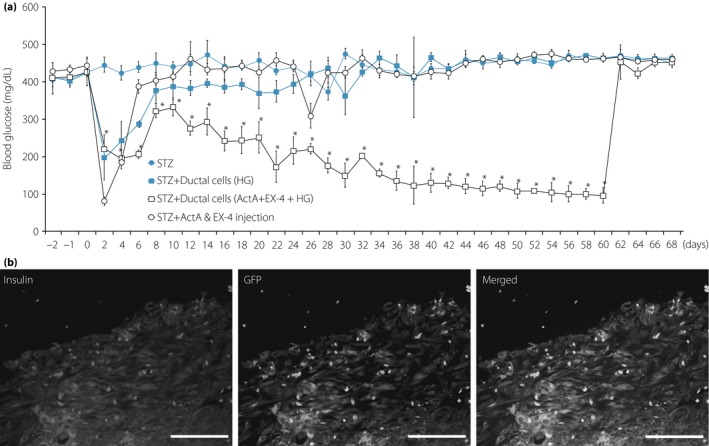
(a) After differentiation by cotreatment with activin A (ActA) and exendin‐4 (Ex‐4) for 30 days, differentiated human duct cells were transplanted into streptozotocin (STZ)‐induced diabetic animals. Hyperglycemia (HG) was gradually reduced after transplantation of differentiated human duct cells (P < 0.05 vs STZ). (b) For the tracing of transplanted duct cells, adenovirus‐green fluorescent protein (GFP) infection was carried out before transplantation. Transplanted duct cells were detected with insulin (red) and GFP (green) 60 days after transplantation (magnification: ×400; scale bar, 100 μm).
Recently, it was confirmed that adult pancreatic duct cells were able to differentiate into insulin‐producing cells after transduction of adenoviral vectors encoding Pdx1, Ngn3 and MafA. The authors used adult pancreatic duct cells isolated from the mouse insulin I gene promoter ‐GFP transgenic mouse to detect the activation of the insulin 1 gene promoter as a transdifferentiation reporter, and found that transduction of the three transcription factors increased the expression of β‐cell‐specific markers, such as insulin 1 and 2. Furthermore, treatment with EX‐4 enhanced the expression of NeuroD and the GLP‐1 receptor (Figure 2). However, these transdifferentiated duct cells were not fully differentiated, because they did not secrete insulin in response to glucose111. The Edmonton group has attempted to improve the efficiency of islet transplantation, including the long‐term survival of islets after transplantation. They identified the correlations between the success of the transplanted islets and the composition of the cotransplanted cells. When they cotransplanted islets with duct cells, a significant positive effect of duct cells was observed. Islets cotransplanted with duct cells had long‐term metabolic success, as determined by an intravenous glucose tolerance test 2 years post‐transplantation9, 112. It could be possible that islet transplantation might be improved when cotransplanted with the duct cells.
Pancreatic Acinar Cells
Large populations of acinar cells are discarded after islet isolation from donors, and in fact, have been shown to transdifferentiate into β‐cells in vivo and in vitro, with the generation of duct cells as an intermediate step. Thus, verification of the potential value of acinar cells is attractive as a member of PPC candidates. Lemoine et al.113 showed that acinar cells isolated from the human pancreas were able to transdifferentiate into CK‐19‐expressing duct cells. In another study, rat pancreatic exocrine cells (including acinar cells) transdifferentiated into insulin‐producing cells on treatment with epidermal growth factor and leukemia inhibitory factor114, 115. Furthermore, cotransplantation with fetal pancreatic cells and acinar cells into immunodeficient mice showed that acinar cells gave rise to endocrine cells. They named these cells highly purified population of non‐endocrine pancreatic epithelial cells. Non‐endocrine pancreatic epithelial cells were capable of endocrine differentiation, and support the existence of PPCs within the epithelial compartment of the adult pancreas116. The Melton group117 showed the adenoviral Ngn3‐, Pdx‐1‐ and MafA‐induced reprogramming of acinar cells. These reprogrammed acinar cells secreted insulin and reduced hyperglycemia in diabetic animals. These direct phenotype shifts of acinar cells were regarded as evidence that acinar cells are pancreatic progenitor cells. Furthermore, the reprogramming of acinar cells proceeded without activation of the cell cycle or dedifferentiation. For this reason, Melton's reprogramming protocol might have a low risk of tumorigenesis. However, a safer delivery system will be required if reprogramming using adenoviral vectors is to be applied clinically117.
Recently, Melton et al.118 also provided new evidence of exocrine cell reprogramming. The expression of Ngn3, Pdx‐1 and MafA were forced through the use of elastase 2A as a specific inducer of acinar cells. Then, these genes were introduced separately and sequentially into exocrine cells. The investigators verified the role of each gene in the transdifferentiation of exocrine cells. In that study, they suggested that the expression of Ngn3 and MafA suppresses the acinar cell fate. Ngn3 activated pancreas endocrine cell differentiation, and the differentiation of the three islet endocrine subtypes, α‐, β‐ and δ‐cells. MafA also suppressed the fates of acinar and δ‐cells, and activated α‐ and β‐cell fates. In addition, Pdx‐1 suppressed δ‐cell differentiation and induced β‐cell differentiation. In that study, the authors suggested that the three major cell subtypes in the islet are derived from the transdifferentiation of acinar cells (Figure 2)118.
Another study reported that in vitro differentiation of mouse acinar cells was achieved by treatment with epidermal growth factor and nicotinamide. In that study, acinar cells were specifically labeled with lectin, which suggested that new β‐cells had originated from acinar cells by this method119. Baeyens et al.120 suggested that the JAK/STAT signal pathway is associated with Ngn3 expression during β‐cell neogenesis. Recently, they showed that constitutive overexpression of mitogen‐activated protein kinase and signal transducer and activator of transcription 3 induced transdifferentiation of pancreatic acinar cells. After 7 days of lentiviral gene delivery into acinar cells, the expression of β‐cell specific markers including insulin and Pdx‐1 had increased. Interestingly, a free‐floating and 3‐D culture method increased the transdifferentiation efficiency of acinar cells120. In addition, a lineage‐tracing method using the adenovirus‐recombinase elastase 2A promoter showed that human acinar cells are the source of Ngn3‐ and insulin‐expressing cells. This result showed that human exocrine cells could transdifferentiate into insulin‐producing cells, and raised the possibility of obtaining a large number of exocrine cells from a donor for cell replacement therapy (Figure 2)121. All these studies demonstrated that acinar cells have multiplasticity in vitro and in vivo, and that these cells could serve as a pool of pancreatic progenitor cells for the treatment of diabetes.
Conclusion
β‐Cells were once considered to be quiescent after birth. However, β‐cells are able to expand under some conditions, such as pregnancy or obesity122. Although the debate over β‐cells and pancreas regeneration is still in progress, β‐cell regeneration through transdifferentiation could be possible, and pancreatic duct and acinar cells are considered as the potential candidates for β‐cell replacement therapy. The final number of endocrine cells is limited by the size of the progenitor cell pool in the pancreatic bud123 . Pancreatic duct and acinar cells could be members of the postnatal progenitor cell pool.
A major problem with several studies on the existence of pancreatic progenitor cells and β‐cell regeneration by transdifferentiation is that the results have not been reproducible. This problem might be the reason that we cannot make a clear conclusion in this research field124. In addition, the experimental results in various animal models might be misleading, because the mechanism of β‐cell maintenance, the capacity for regeneration and the size of the progenitor cell pool might vary among species125. Although human physiology is similar to those of rodents and monkeys, there are certain differences among them. Rodents are more frequently studied at <3 months‐of‐age, when there is a high capacity for β‐cell replication. However, studies in the human pancreas are carried out in adulthood. Therefore, we should recognize the differences in the species‐specific or injury‐specific conditions. Knowledge about the mechanisms of β‐cell regeneration and transdifferentiation in pathophysiological conditions is very important for the success of clinical applications. Thus, these efforts will provide new strategies for the treatment of diabetes, and also promote the development and utilization of other β‐cell candidates.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the Ministry of Health, Welfare & Family Affairs (A084065) and Samsung Biomedical Research Institute (SBRI) (SMX1132001), Korea.
J Diabetes Investig 2016; 7: 286–296
References
- 1. Shapiro AM, Lakey JR, Ryan EA, et al Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 2. Noguchi H, Iwanaga Y, Okitsu T, et al Evaluation of islet transplantation from non‐heart beating donors. Am J Transplant 2006; 6: 2476–2482. [DOI] [PubMed] [Google Scholar]
- 3. Noguchi H, Ikemoto T, Naziruddin B, et al Iodixanol controlled density gradient during islet purification improves recovery rate in human islet isolation. Transplantation 2009; 87: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 4. Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immuunol 2004; 4: 259–268. [DOI] [PubMed] [Google Scholar]
- 5. Hering BJ, Kandaswamy R, Ansite JD, et al Single donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA 2005; 293: 830–835. [DOI] [PubMed] [Google Scholar]
- 6. Robertson RP. Islet transplantation as a treatment for diabetes – a work in progress. N Engl J Med 2004; 350: 694–705. [DOI] [PubMed] [Google Scholar]
- 7. Keymeulen B, Ling Z, Gorus FK, et al Implantation of standardized beta‐cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin‐independence under maintenance immunosuppression for prior kidney graft. Diabetologia 1998; 41: 452–459. [DOI] [PubMed] [Google Scholar]
- 8. Ryan EA, Lakey JR, Rajotte RV, et al Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001; 50: 710–719. [DOI] [PubMed] [Google Scholar]
- 9. Street CN, Lakey JR, Shapiro AM, et al Islet graft assessment in the Edmonton Protocol: implications for predicting long‐term clinical outcome. Diabetes 2004; 53: 3107–3114. [DOI] [PubMed] [Google Scholar]
- 10. Warnock GL, Kneteman NM, Ryan EA, et al Long‐term follow‐up after transplantation of insulin‐producing pancreatic islets into patients with type1 (insulin‐dependent) diabetes mellitus. Diabetologia 1992; 35: 89–95. [DOI] [PubMed] [Google Scholar]
- 11. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi K, Tanabe K, Ohnuki M, et al Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 14. Yu J, Hu K, Smuga‐Otto K, et al Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009; 324: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warren L, Manos PD, Ahfeldt T, et al Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010; 7: 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subramanyam D, Lamouille S, Judson RL, et al Multiple targets of miR‐302 and miR‐372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol 2011; 29: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anokye‐Danso F, Trivedi CM, Juhr D, et al Highly efficient miRNA‐mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 2011; 8: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyoshi N, Ishii H, Nagano H, et al Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011; 8: 633–638. [DOI] [PubMed] [Google Scholar]
- 19. Mali P, Chou BK, Yen J, et al Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency‐associated genes. Stem Cells 2010; 28: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huangfu D, Osafune K, Maehr R, et al Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 2008; 26: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 21. Lin T, Ambasudhan R, Yuan X, et al A chemical platform for improved induction of human iPSCs. Nat Methods 2009; 6: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichida JK, Blanchard J, Lam K, et al A small‐molecule inhibitor of TGF‐β signaling replaces Sox2 in reprogramming by inducing nanog. Cell Stem Cell 2009; 5: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esteban MA, Wang T, Qin B, et al Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010; 6: 71–79. [DOI] [PubMed] [Google Scholar]
- 24. Zhu S, Li W, Zhou H, et al Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010; 7: 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang D, Jiang W, Liu M, et al Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin‐producing cells. Cell Res 2009; 19: 429–438. [DOI] [PubMed] [Google Scholar]
- 26. Lardon J, De Breuck S, Rooman I, et al Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte‐like cells in primary culture. Hepatology 2004; 39: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 27. Wu SY, Hsieh CC, Wu RR, et al Differentiation of pancreatic acinar cells to hepatocytes requires an intermediate cell type. Gastroenterology 2010; 138: 2519–2530. [DOI] [PubMed] [Google Scholar]
- 28. Bonal C, Thorel F, Ait‐Lounis A, et al Pancreatic inactivation of c‐Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology 2009; 136: 309–319. [DOI] [PubMed] [Google Scholar]
- 29. Thorel F, Népote V, Avril I, et al Conversion of adult pancreatic alpha‐cells to beta‐cells after extreme beta‐cell loss. Nature 2010; 464: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collombat P, Xu X, Ravassard P, et al The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009; 138: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye L, Robertson MA, Hesselson D, et al Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development 2015; 143: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera PL. Adult insulin‐ and glucagon‐producing cells differentiate from two independent cell lineages. Development 2000; 127: 2317–2322. [DOI] [PubMed] [Google Scholar]
- 33. Collombat P, Hecksher‐Sørensen J, Krull J, et al Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arxmisexpression. J Clin Invest 2007; 117: 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao T, McKenna B, Li C, et al Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab 2014; 19: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sussel L, Kalamaras J, Hartigan‐O'Connor DJ, et al Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 1998; 125: 2213–2221. [DOI] [PubMed] [Google Scholar]
- 36. Papizan JB, Singer RA, Tschen SI, et al Nkx2.2 repressor complex regulates islet beta‐cell specification and prevents beta‐to‐alpha‐cell reprogramming. Genes Dev 2011; 25: 2291–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhawan S, Georgia S, Tschen SI, et al Pancreatic beta cell identity is maintained by DNA methylation‐mediated repression of Arx. Dev Cell 2011; 20: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Talchai C, Xuan S, Lin HV, et al Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 2012; 150: 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chera S, Baronnier D, Ghila L, et al Diabetes recovery by age‐dependent conversion of pancreatic δ‐cells into insulin producers. Nature 2014; 514: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramiya VK, Maraist M, Arfors KR, et al Reversal of insulin‐dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000; 6: 278–282. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow‐cytometric cell sorting. Diabetes 2004; 53: 2143–2152. [DOI] [PubMed] [Google Scholar]
- 42. Herrera PL, Huarte J, Sanvito F, et al Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development 1991; 113: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 43. Karges B, Durinovic‐Belló I, Heinze E, et al Complete long‐term recovery of beta‐cell function in autoimmune type 1 diabetes after insulin treatment. Diabetes Care 2004; 27: 1207–1208. [DOI] [PubMed] [Google Scholar]
- 44. Karges B, Muche R, Riegger I, et al Injection of acidic or neutral insulin and pain: a single‐center, prospective, controlled, non interventional study in pediatric patients with type 1 diabetes mellitus. Clin Ther 2006; 28: 2094–2101. [DOI] [PubMed] [Google Scholar]
- 45. Butler AE, Galasso R, Meier JJ, et al Modestly increased beta cell apoptosis but no increased beta cell replication in recent‐onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 2007; 50: 2323–2331. [DOI] [PubMed] [Google Scholar]
- 46. Meier JJ, Bhushan A, Butler AE, et al Sustained beta cell apoptosis in patients with long‐standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005; 48: 2221–2228. [DOI] [PubMed] [Google Scholar]
- 47. Dor Y, Brown J, Martinez OI, et al Adult pancreatic beta‐cells are formed by self‐duplication rather than stem‐cell differentiation. Nature 2004; 429: 41–46. [DOI] [PubMed] [Google Scholar]
- 48. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117: 2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teta M, Rankin MM, Long SY, et al Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007; 12: 817–826. [DOI] [PubMed] [Google Scholar]
- 50. Blaine SA, Ray KC, Anunobi R, et al Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 2010; 137: 2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gradwohl G, Dierich A, LeMeur M, et al Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 2000; 97: 1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002; 129: 2447–2457. [DOI] [PubMed] [Google Scholar]
- 53. Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 2003; 120: 35–43. [DOI] [PubMed] [Google Scholar]
- 54. Johansson KA, Dursun U, Jordan N, et al Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007; 12: 457–465. [DOI] [PubMed] [Google Scholar]
- 55. Wang S, Yan J, Anderson DA, et al Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol 2010; 339: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gouzi M, Kim YH, Katsumoto K, et al Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn 2011; 240: 589–604. [DOI] [PubMed] [Google Scholar]
- 57. Wang S, Jensen JN, Seymour PA, et al Sustained Neurog3 expression in hormone‐expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci USA 2009; 106: 9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desai BM, Oliver‐Krasinski J, De Leon DD, et al Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 2007; 117: 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Breault DT, Min IM, Carlone DL, et al Generation of mTert‐GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA 2008; 105: 10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aguayo‐Mazzucato C, Bonner‐Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 2010; 6: 139–148. [DOI] [PubMed] [Google Scholar]
- 61. Jonsson J, Carlsson L, Edlund T, et al Insulin‐promoter‐factor 1 is required for pancreas development in mice. Nature 1994; 371: 606–609. [DOI] [PubMed] [Google Scholar]
- 62. Ohneda K, Mirmira RG, Wang J, et al The homeodomain of PDX‐1 mediates multiple protein‐protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol 2000; 20: 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seymour PA, Freude KK, Tran MN, et al SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA 2007; 104: 1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kajiyama H, Hamazaki TS, Tokuhara M, et al Pdx1‐transfected adipose tissue‐derived stem cells differentiate into insulin‐producing cells in vivo and reduce hyperglycemia in diabetic mice. Int J Dev Biol 2010; 54: 699–705. [DOI] [PubMed] [Google Scholar]
- 65. Jin L, Feng T, Shih HP, et al Colony‐forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci USA 2013; 110: 3907–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zulewski H, Abraham EJ, Gerlach MJ, et al Multipotential nestin‐positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001; 50: 521–533. [DOI] [PubMed] [Google Scholar]
- 67. Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev 2002; 113: 189–192. [DOI] [PubMed] [Google Scholar]
- 68. Piper K, Ball SG, Turnpenny LW, et al Beta‐cell differentiation during human development does not rely on nestin‐positive precursors: implications for stem cell‐derived replacement therapy. Diabetologia 2002; 45: 1045–1047. [DOI] [PubMed] [Google Scholar]
- 69. Treutelaar MK, Skidmore JM, Dias‐Leme CL, et al Nestin‐lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes 2003; 52: 2503–2512. [DOI] [PubMed] [Google Scholar]
- 70. Esni F, Stoffers DA, Takeuchi T, et al Origin of exocrine pancreatic cells from nestin‐positive precursors in developing mouse pancreas. Mech Dev 2004; 121: 15–25. [DOI] [PubMed] [Google Scholar]
- 71. Delacour A, Nepote V, Trumpp A, et al Nestin expression in pancreatic exocrine cell lineages. Mech Dev 2004; 121: 3–14. [DOI] [PubMed] [Google Scholar]
- 72. Gershengorn MC, Hardikar AA, Wei C, et al Epithelial‐to‐mesenchymal transition generates proliferative human islet precursor cells. Science 2004; 306: 2261–2264. [DOI] [PubMed] [Google Scholar]
- 73. Chase LG, Ulloa‐Montoya F, Kidder BL, et al Islet‐derived fibroblast‐like cells are not derived via epithelial‐mesenchymal transition from Pdx‐1 or insulin‐positive cells. Diabetes 2007; 56: 3–7. [DOI] [PubMed] [Google Scholar]
- 74. Atouf F, Park CH, Pechhold K, et al No evidence for mouse pancreatic beta‐cell epithelial‐mesenchymal transition in vitro. Diabetes 2007; 56: 699–702. [DOI] [PubMed] [Google Scholar]
- 75. Davani B, Ikonomou L, Raaka BM, et al Human islet‐derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone‐expressing cells in vivo. Stem Cells 2007; 25: 3215–3222. [DOI] [PubMed] [Google Scholar]
- 76. Russ HA, Bar Y, Ravassard P, et al In vitro proliferation of cells derived from adult human beta‐cells revealed by cell‐lineage tracing. Diabetes 2008; 57: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 77. Dezaki K. Ghrelin function in insulin release and glucose metabolism. Endocr Dev 2013; 25: 135–143. [DOI] [PubMed] [Google Scholar]
- 78. Kojima M, Hosoda H, Date Y, et al Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999; 402: 656–660. [DOI] [PubMed] [Google Scholar]
- 79. Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol 2014; 52: R35–R49. [DOI] [PubMed] [Google Scholar]
- 80. Arnes L, Hill JT, Gross S, et al Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS ONE 2012; 7: e52026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bonner‐Weir S. Perspective: postnatal pancreatic beta cell growth. Endocrinology 2000; 141: 1926–1929. [DOI] [PubMed] [Google Scholar]
- 82. Bouwens L. Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta‐cells in the pancreas. Microsc Res Tech 1998; 43: 332–336. [DOI] [PubMed] [Google Scholar]
- 83. Xu X, D'Hoker J, Stange G, et al Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008; 132: 197–207. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto T, Yamato E, Taniguchi H, et al Stimulation of cAMP signaling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia 2006; 49: 2359–2367. [DOI] [PubMed] [Google Scholar]
- 85. Noguchi H, Oishi K, Ueda M, et al Establishment of mouse pancreatic stem cell line. Cell Transplant 2009; 18: 563–571. [DOI] [PubMed] [Google Scholar]
- 86. Noguchi H, Naziruddin B, Jackson A, et al Characterization of human pancreatic progenitor cells. Cell Transplant 2010; 19: 879–886. [DOI] [PubMed] [Google Scholar]
- 87. Wang Y, Lanzoni G, Carpino G, et al Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life‐long pancreatic organogenesis. Stem Cells 2013; 31: 1966–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Inada A, Nienaber C, Katsuta H, et al Carbonic anhydrase II‐positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 2008; 105: 19915–19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Solar M, Cardalda C, Houbracken I, et al Pancreatic exocrine duct cells give rise to insulin‐producing beta cells during embryogenesis but not after birth. Dev Cell 2009; 17: 849–860. [DOI] [PubMed] [Google Scholar]
- 90. Kopinke D, Murtaugh LC. Exocrine‐to‐endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 2010; 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heremans Y, Van De Casteele M, In't Veld P, et al Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin3. J Cell Biol 2002; 159: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Noguchi H, Kaneto H, Weir GC, et al PDX‐1 protein containing its own antennapedia‐like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes 2003; 52: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 93. Gao R, Ustinov J, Pulkkinen MA, et al Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 2003; 52: 2007–2015. [DOI] [PubMed] [Google Scholar]
- 94. Oshima Y, Suzuki A, Kawashimo K, et al Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c‐Met by flowcytometric cell sorting. Gastroenterology 2007; 132: 720–732. [DOI] [PubMed] [Google Scholar]
- 95. Yatoh S, Dodge R, Akashi T, et al Differentiation of affinity purified human pancreatic duct cells to beta‐cells. Diabetes 2007; 56: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 96. Bonner‐Weir S, Taneja M, Weir GC, et al In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 2000; 97: 7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bonner‐Weir S, Inada A, Yatoh S, et al Transdifferentiation of pancreatic ductal cells to endocrine beta‐cells. Biochem Soc Trans 2008; 36: 353–356. [DOI] [PubMed] [Google Scholar]
- 98. Noguchi H, Naziruddin B, Shimoda M, et al Induction of insulin‐producing cells from human pancreatic progenitor cells. Transplant Proc 2010; 42: 2081–2083. [DOI] [PubMed] [Google Scholar]
- 99. Suarez‐Pinzon WL, Lakey JR, Brand SJ, et al Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet beta‐cells from pancreatic duct cells and an increase in functional beta‐cell mass. J Clin Endocrinol Metab 2005; 90: 3401–3409. [DOI] [PubMed] [Google Scholar]
- 100. Huch M, Bonfanti P, Boj SF, et al Unlimited in vitro expansion of adult bi‐potent pancreas progenitors through the Lgr5/R‐spondin axis. EMBO J 2013; 32: 2708–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Abraham EJ, Leech CA, Lin JC, et al Insulinotropic hormone glucagon‐like peptide‐1 differentiation of human pancreatic islet derived progenitor cells into insulin‐producing cells. Endocrinology 2002; 143: 3152–3161. [DOI] [PubMed] [Google Scholar]
- 102. Zhou J, Pineyro MA, Wang X, et al Exendin‐4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX‐1 and HNF3β transcription factors. J Cell Physiol 2002; 192: 304–314. [DOI] [PubMed] [Google Scholar]
- 103. Zhou J, Wan X, Pineyro MA, et al Glucagon‐like peptide 1 and exendin‐4 convert pancreatic AR42J cells into glucagon and insulin‐producing cells. Diabetes 1999; 48: 2358–2366. [DOI] [PubMed] [Google Scholar]
- 104. Bulotta A, Hui H, Anastasi E, et al Cultured pancreatic ductal cells undergo cell cycle re‐distribution and β‐cell‐like differentiation in response to glucagon‐like peptide‐1. J Mol Endocrinol 2002; 29: 347–360. [DOI] [PubMed] [Google Scholar]
- 105. Li J1, Wang Y, Yu X, et al Islet neogenesis‐associated protein‐related pentadecapeptide enhances the differentiation of islet‐like clusters from human pancreatic duct cells. Peptide 2009; 30: 2242–2249. [DOI] [PubMed] [Google Scholar]
- 106. Mashima H, Ohnishi H, Wakabayashi K, et al Betacellulin and activinA coordinately convert amylase‐secreting pancreatic AR42J cells into insulin‐secreting cells. J Clin Invest 1996; 97: 1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mashima H, Shibata H, Mine T, et al Formation of insulin producing cells from pancreatic acinar AR42J cells by hepatocyte growth factor. Endocrinology 1996; 137: 3969–3976. [DOI] [PubMed] [Google Scholar]
- 108. Park MK, Han C, Lee KH, et al Effects of activin A on pancreatic ductal cells in streptozotocin‐induced diabetic rats. Transplantation 2007; 83: 925–930. [DOI] [PubMed] [Google Scholar]
- 109. Kim HS, Hong SH, Oh SH, et al Activin A, exendin‐4, and glucose stimulate differentiation of human pancreatic ductal cells. J Endocrinol 2013; 217: 241–252. [DOI] [PubMed] [Google Scholar]
- 110. Lee J, Sugiyama T, Liu Y, et al Expansion and conversion of human pancreatic ductal cells into insulin‐secreting endocrine cells. Elife 2013; 2: e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yamada T, Cavelti‐Weder C, Caballero F, et al Reprogramming mouse cells with a pancreatic duct phenotype to insulin‐producing beta‐like cells. Endocrinology 2015; 156: 2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Street CN, Lakey JR, Rajotte RV, et al Enriched human pancreatic ductal cultures obtained from selective death of acinar cells express pancreatic and duodenal homeoboxgene‐1 age‐dependently. Rev Diabet Stud 2004; 1: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol 1992; 166: 97–103. [DOI] [PubMed] [Google Scholar]
- 114. Baeyens L, De Breuck S, Lardon J, et al In vitro generation of insulin producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005; 48: 49–57. [DOI] [PubMed] [Google Scholar]
- 115. Minami K, Okuno M, Miyawaki K, et al Lineage tracing and characterization of insulin‐secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA 2005; 102: 15116–15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hao E, Tyrberg B, Itkin‐Ansari P, et al Beta‐cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 2006; 12: 310–316. [DOI] [PubMed] [Google Scholar]
- 117. Zhou Q, Brown J, Kanarek A, et al In vivo reprogramming of adult pancreatic exocrine cells to beta‐cells. Nature 2008; 455: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li W, Nakanishi M, Zumsteg A, et al In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife 2014; 3: e01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baeyens L, Bonne S, Bos T, et al Notch signaling as gatekeeper of rat acinar‐to‐beta‐cell conversion in vitro. Gastroenterology 2009; 136: 1750–1760. [DOI] [PubMed] [Google Scholar]
- 120. Baeyens L, Bonne S, German MS, et al Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ 2006; 13: 1892–1899. [DOI] [PubMed] [Google Scholar]
- 121. Lemper M, Leuckx G, Heremans Y, et al Reprogramming of human pancreatic exocrine cells to beta‐like cells. Cell Death Differ 2015; 22: 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bernard‐Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes 2001; 50(Suppl. 1): S30–S35. [DOI] [PubMed] [Google Scholar]
- 123. Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007; 445: 886–891. [DOI] [PubMed] [Google Scholar]
- 124. Check E. Stem cells: the hard copy. Nature 2007; 446: 485–486. [DOI] [PubMed] [Google Scholar]
- 125. Hanley NA, Hanley KP, Miettinen PJ, et al Weighing up beta‐cell mass in mice and humans: self‐renewal, progenitors or stem cells? Mol Cell Endocrinol 2008; 288: 79–85. [DOI] [PubMed] [Google Scholar]


