Abstract
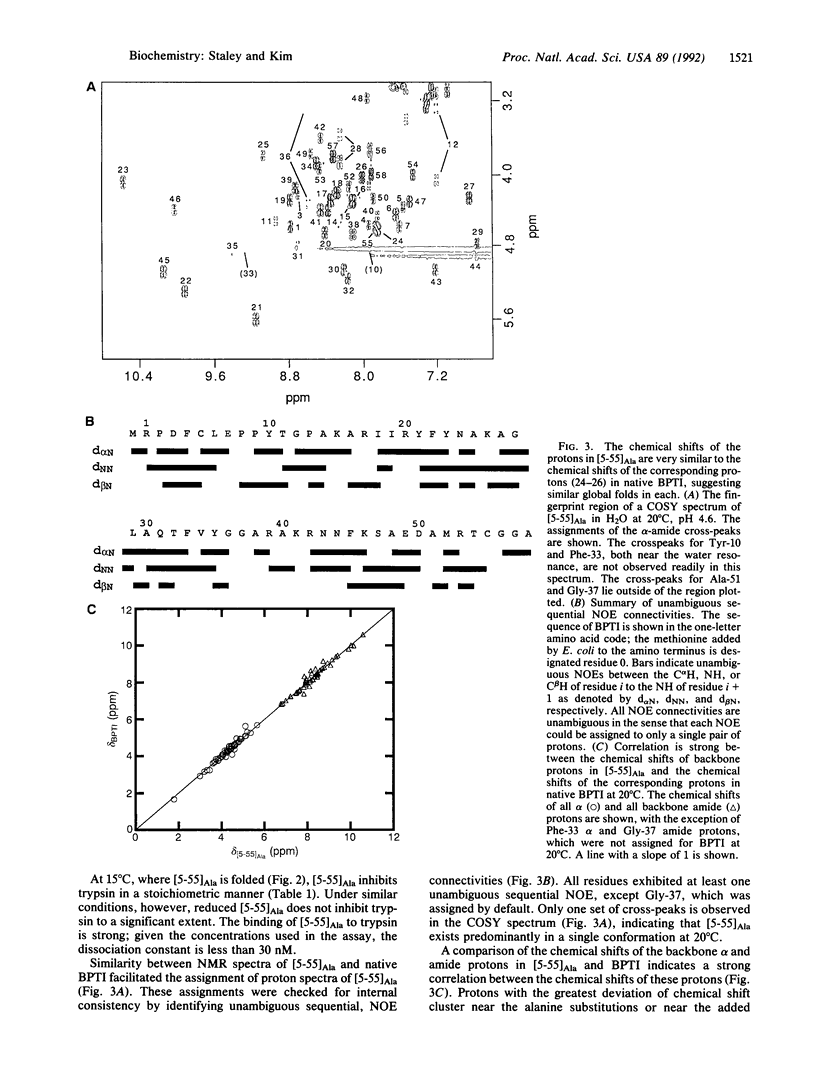
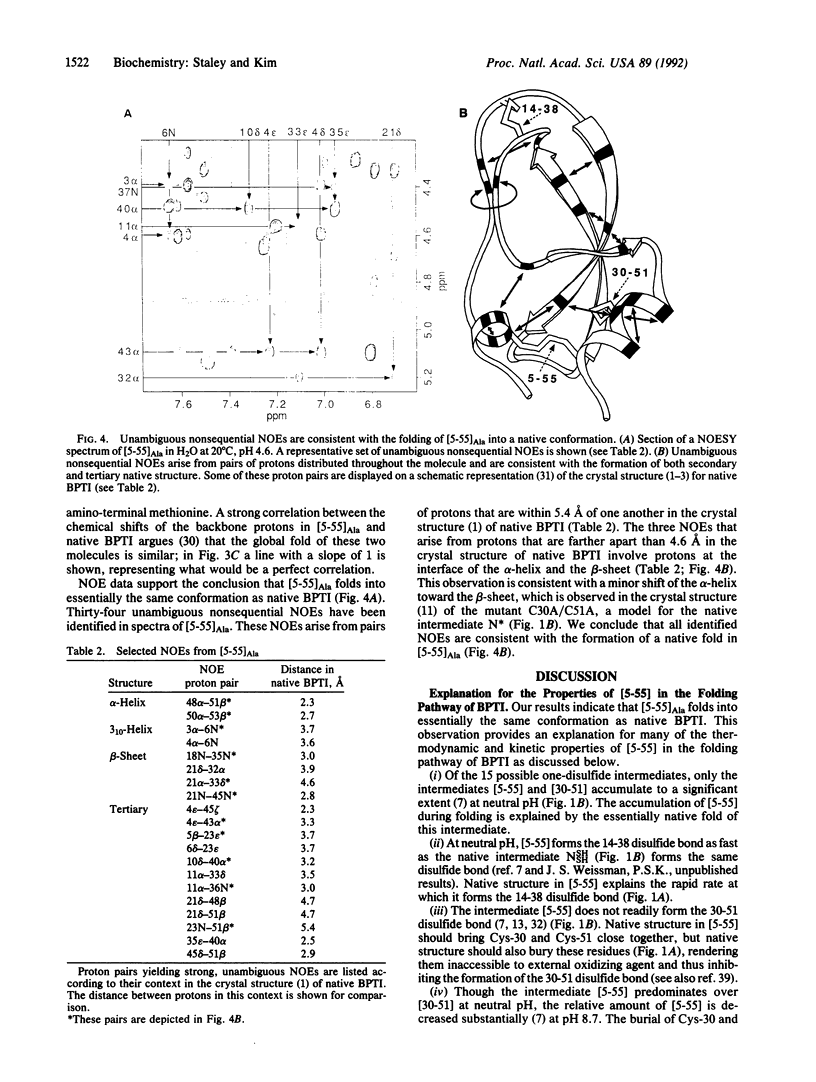
In the oxidative folding of bovine pancreatic trypsin inhibitor (BPTI) at neutral pH, only two one-disulfide intermediates accumulate to a significant extent, namely [5-55] and [30-51]. In this paper we describe a recombinant model of [5-55], designated [5-55]Ala, which was made by replacing the cysteine residues not involved in the disulfide bond with alanine. As judged by two-dimensional NMR, [5-55]Ala folds into essentially the same conformation as native BPTI. Moreover, like native BPTI, [5-55]Ala inhibits trypsin stoichiometrically. Thus, the disulfide-bonded intermediate [5-55] corresponds not to a partially folded protein folding intermediate but rather to an essentially completely folded protein. This conclusion provides an explanation for many of the thermodynamic and kinetic properties of [5-55] in the folding pathway of BPTI.
Full text
PDF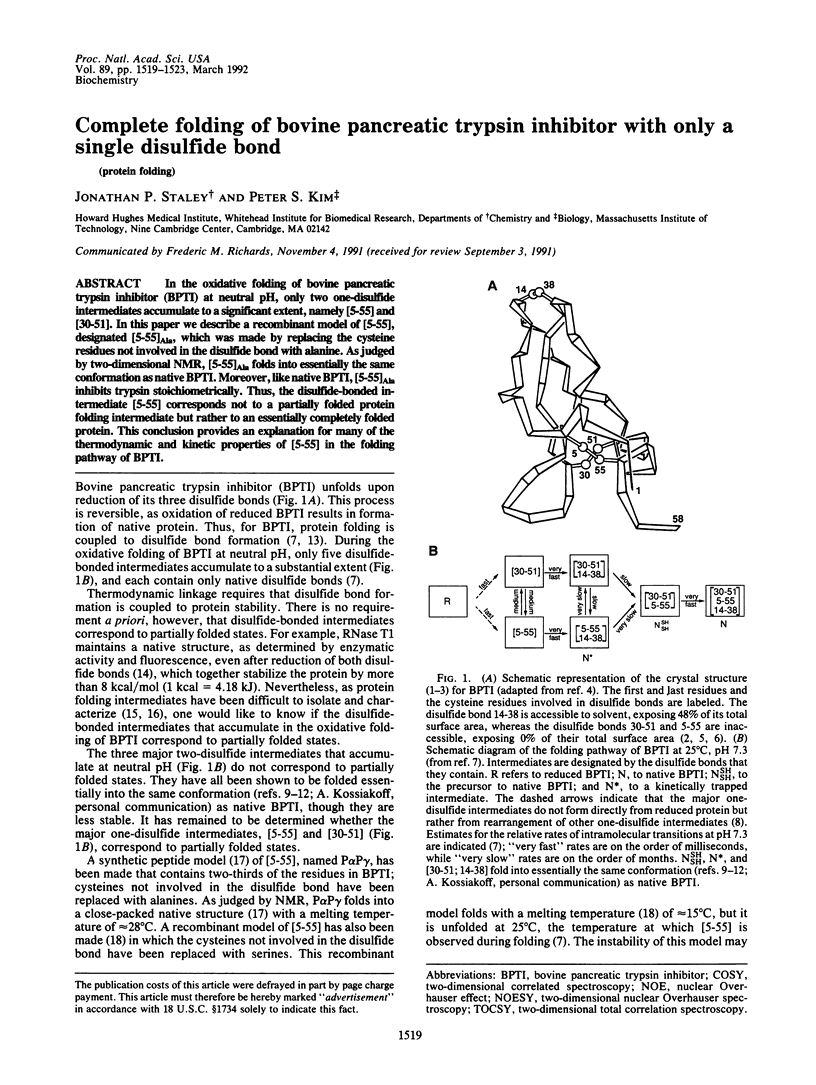


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creighton T. E. Effects of urea and guanidine-HCl on the folding and unfolding of pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):313–328. doi: 10.1016/0022-2836(77)90144-9. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Goldenberg D. P. Kinetic role of a meta-stable native-like two-disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J Mol Biol. 1984 Nov 5;179(3):497–526. doi: 10.1016/0022-2836(84)90077-9. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Protein folding. Biochem J. 1990 Aug 15;270(1):1–16. doi: 10.1042/bj2700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Renaturation of the reduced bovine pancreatic trypsin inhibitor. J Mol Biol. 1974 Aug 15;87(3):563–577. doi: 10.1016/0022-2836(74)90104-1. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. The two-disulphide intermediates and the folding pathway of reduced pancreatic trypsin inhibitor. J Mol Biol. 1975 Jun 25;95(2):167–199. doi: 10.1016/0022-2836(75)90389-7. [DOI] [PubMed] [Google Scholar]
- Darby N. J., van Mierlo C. P., Creighton T. E. The 5-55 single-disulphide intermediate in folding of bovine pancreatic trypsin inhibitor. FEBS Lett. 1991 Feb 11;279(1):61–64. doi: 10.1016/0014-5793(91)80251-w. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C., Randal M., Kossiakoff A. A. Structural effects induced by removal of a disulfide-bridge: the X-ray structure of the C30A/C51A mutant of basic pancreatic trypsin inhibitor at 1.6 A. Protein Eng. 1990 Jul;3(7):591–598. doi: 10.1093/protein/3.7.591. [DOI] [PubMed] [Google Scholar]
- Goto Y., Hamaguchi K. Formation of the intrachain disulfide bond in the constant fragment of the immunoglobulin light chain. J Mol Biol. 1981 Mar 5;146(3):321–340. doi: 10.1016/0022-2836(81)90391-0. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lin T. Y., Kim P. S. Urea dependence of thiol-disulfide equilibria in thioredoxin: confirmation of the linkage relationship and a sensitive assay for structure. Biochemistry. 1989 Jun 13;28(12):5282–5287. doi: 10.1021/bi00438a054. [DOI] [PubMed] [Google Scholar]
- Oas T. G., Kim P. S. A peptide model of a protein folding intermediate. Nature. 1988 Nov 3;336(6194):42–48. doi: 10.1038/336042a0. [DOI] [PubMed] [Google Scholar]
- Pace C. N., Grimsley G. R., Thomson J. A., Barnett B. J. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem. 1988 Aug 25;263(24):11820–11825. [PubMed] [Google Scholar]
- Pardi A., Wagner G., Wüthrich K. Protein conformation and proton nuclear-magnetic-resonance chemical shifts. Eur J Biochem. 1983 Dec 15;137(3):445–454. doi: 10.1111/j.1432-1033.1983.tb07848.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. Schematic drawings of protein structures. Methods Enzymol. 1985;115:359–380. doi: 10.1016/0076-6879(85)15026-3. [DOI] [PubMed] [Google Scholar]
- Roder H., Elöve G. A., Englander S. W. Structural characterization of folding intermediates in cytochrome c by H-exchange labelling and proton NMR. Nature. 1988 Oct 20;335(6192):700–704. doi: 10.1038/335700a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. P., Kim P. S. Role of a subdomain in the folding of bovine pancreatic trypsin inhibitor. Nature. 1990 Apr 12;344(6267):685–688. doi: 10.1038/344685a0. [DOI] [PubMed] [Google Scholar]
- Stassinopoulou C. I., Wagner G., Wüthrich K. Two-dimensional 1H NMR of two chemically modified analogs of the basic pancreatic trypsin inhibitor. Sequence-specific resonance assignments and sequence location of conformation changes relative to the native protein. Eur J Biochem. 1984 Dec 3;145(2):423–430. doi: 10.1111/j.1432-1033.1984.tb08571.x. [DOI] [PubMed] [Google Scholar]
- States D. J., Creighton T. E., Dobson C. M., Karplus M. Conformations of intermediates in the folding of the pancreatic trypsin inhibitor. J Mol Biol. 1987 Jun 5;195(3):731–739. doi: 10.1016/0022-2836(87)90192-6. [DOI] [PubMed] [Google Scholar]
- States D. J., Dobson C. M., Karplus M. A new two-disulphide intermediate in the refolding of reduced bovine pancreatic trypsin inhibitor. J Mol Biol. 1984 Apr 5;174(2):411–418. doi: 10.1016/0022-2836(84)90345-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tüchsen E., Woodward C. Assignment of asparagine-44 side-chain primary amide 1H NMR resonances and the peptide amide N1H resonance of glycine-37 in basic pancreatic trypsin inhibitor. Biochemistry. 1987 Apr 7;26(7):1918–1925. doi: 10.1021/bi00381a020. [DOI] [PubMed] [Google Scholar]
- Udgaonkar J. B., Baldwin R. L. NMR evidence for an early framework intermediate on the folding pathway of ribonuclease A. Nature. 1988 Oct 20;335(6192):694–699. doi: 10.1038/335694a0. [DOI] [PubMed] [Google Scholar]
- Wagner G., Braun W., Havel T. F., Schaumann T., Go N., Wüthrich K. Protein structures in solution by nuclear magnetic resonance and distance geometry. The polypeptide fold of the basic pancreatic trypsin inhibitor determined using two different algorithms, DISGEO and DISMAN. J Mol Biol. 1987 Aug 5;196(3):611–639. doi: 10.1016/0022-2836(87)90037-4. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Amide protein exchange and surface conformation of the basic pancreatic trypsin inhibitor in solution. Studies with two-dimensional nuclear magnetic resonance. J Mol Biol. 1982 Sep 15;160(2):343–361. doi: 10.1016/0022-2836(82)90180-2. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Sequential resonance assignments in protein 1H nuclear magnetic resonance spectra. Basic pancreatic trypsin inhibitor. J Mol Biol. 1982 Mar 5;155(3):347–366. doi: 10.1016/0022-2836(82)90009-2. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. Reexamination of the folding of BPTI: predominance of native intermediates. Science. 1991 Sep 20;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Nachman J., Gilliland G. L., Gallagher W., Woodward C. Structure of form III crystals of bovine pancreatic trypsin inhibitor. J Mol Biol. 1987 Dec 5;198(3):469–480. doi: 10.1016/0022-2836(87)90294-4. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Walter J., Huber R., Sjölin L. Structure of bovine pancreatic trypsin inhibitor. Results of joint neutron and X-ray refinement of crystal form II. J Mol Biol. 1984 Dec 5;180(2):301–329. doi: 10.1016/s0022-2836(84)80006-6. [DOI] [PubMed] [Google Scholar]
- van Mierlo C. P., Darby N. J., Neuhaus D., Creighton T. E. Two-dimensional 1H nuclear magnetic resonance study of the (5-55) single-disulphide folding intermediate of bovine pancreatic trypsin inhibitor. J Mol Biol. 1991 Nov 20;222(2):373–390. doi: 10.1016/0022-2836(91)90217-t. [DOI] [PubMed] [Google Scholar]




