Abstract
Background. Appropriate management, including pulmonary rehabilitation, associated with correct diagnosis of chronic obstructive pulmonary disease (COPD) in patients can contribute to improving clinical conditions of these patients. Physical activity is recommended for COPD patients. Whole-body vibration (WBV) is a modality of physical activity. Putting together the biological effects and safe use of WBV, it may be a potentially feasible intervention to add to pulmonary rehabilitation. The purpose of this investigation was to systematically review studies regarding the effects of WBV, as a component of the pulmonary rehabilitation, in patients with COPD. Results. A total of six publications met inclusion for review. There was evidence to support the beneficial use of WBV to improve functional performance of the lower limbs and quality of life. However, the appropriateness of and descriptors of WBV methods were poorly described. Conclusions. The results of this review support the use of WBV as a component of pulmonary rehabilitation to assist management of patients with COPD. However, future research should examine the dose-response curve and optimal dosing regimen of WBV according to standard reporting recommendations for people with COPD. Such an approach will allow comparison among studies and the potential of meta-analysis of randomized controlled trials.
1. Background
A disease of the lungs, chronic obstructive pulmonary disease (COPD), is a preventable and/or treatable respiratory disease. It can be characterized by progressive airflow limitation [1] and chronic inflammatory response to noxious particles or gases [2]. However, the progressive and incurable nature of COPD remains a major public health problem across the developing and developed world [2]. The disease is a significant contributor to global morbidity and mortality, accounting for more than 3 million deaths in 2012 [3]. In the United States of America alone, COPD was the third-ranked cause of mortality, responsible for more than 120,000 annual deaths [4]. Furthermore, COPD is associated with comorbid conditions such as skeletal muscle dysfunction and cardiovascular diseases [5]. In addition, the severity of the disease and associated exacerbations may require repeated clinical evaluations, treatments, and inpatient admission [1].
Pulmonary rehabilitation can be a nonpharmacological intervention that is part of clinical management of patients with chronic respiratory disease who remain symptomatic or continue to have decreased function despite standard medical therapy [2, 6–8]. Among people with COPD, pulmonary rehabilitation can reduce dyspnoea and fatigue [6] and improve peripheral skeletal muscle function [2, 7] and quality of life [9, 10] and may prolong survival [11]. As a component of pulmonary rehabilitation, physical activity is recommended to all patients with a diagnosis of COPD and may improve exercise tolerance and performance of activities of daily living [2]. However, given that aerobic conditioning [12] and resistance training [13] are associated with high levels of perceived dyspnoea, fear of breathlessness can lead to reduced participation in physical activity [14, 15] and the so called dyspnoea spiral [16]. As such, the use of peripheral muscle training in the management of patients with COPD involves careful consideration given the potential for exacerbations, the risk of acute dyspnoea or hypoxemia during resistance training [13, 17], and/or aerobic conditioning [18–20]. Due to the necessity of physical activity to the COPD patient and the potential limitations of both aerobic conditioning and resistance training, other modalities of exercise should be examined.
As a component of pulmonary rehabilitation, whole-body vibration (WBV) is emerging as a potentially beneficial modality of physical activity for people with COPD [21–27]. Whole-body vibration has also emerged among other populations with suboptimal health such as fibromyalgia [28], cystic fibrosis [29–31], and multiple sclerosis [32, 33]. As a mode of physical activity, WBV may be performed when a person either (a) stands stationary on a base of the platform or (b) performs movements while standing, sitting, or lying on an oscillating/vibratory platform such as flexion and extension of the lower limbs [34–37]. It is speculated that the effect of WBV on the musculoskeletal system is to produce changes in the length-tension relationship moderated within the muscle spindle and may elicit a tonic vibration reflex [36, 38, 39] and subsequently improve the performance of skeletal muscles of the lower limbs [21].
A recent systematic review of COPD and WBV aimed to report on outcome measures of functional performance: the 6-minute walk test, the sit-to-stand test, peak knee extension force, and quality of life [40]. Despite a small number of randomized controlled trials, preliminary evidence emerged to support the use of WBV to improve the aforementioned measures of functional performance of people with COPD [40]. However, evidences about the specific frequency, intensity, type, duration, and gravitational properties of the vibration platform and WBV are yet to be described that may enhance peripheral muscle training for people with a diagnosis of COPD. Some recommendations by the International Society of Musculoskeletal and Neuronal Interactions (ISMNI) about WBV interventions include reporting [41] (1) the vibration device and brand, (2) direction, frequency, peak-to-peak displacement, gravitational forces, and accuracy, and (3) evaluation of skidding and foot position.
As evidence is supporting the beneficial use of WBV for people with COPD [40], clinicians should be able to prescribe WBV as a mode of peripheral muscle training based on best available evidence. However, there is a total absence of how WBV should be prescribed to improve peripheral muscles of the lower limbs of people with COPD. Therefore, the aim of this narrative review was to advance the seminal work of Gloeckl et al. (2015) [40] by describing the methods of WBV exercise for people with COPD using ISMNI recommendations and describe evidence based on National Health and Medical Research Council hierarchy of evidence (NHMRC, 2003–2007) [42].
2. Methods
2.1. Search Strategy
Three reviewers independently accessed bibliographical databases through the Universidade do Estado do Rio de Janeiro. Searches were performed in the PubMed (MEDLINE), Scopus, Science Direct, and PEDro databases on February 9, 2015, each with the keywords “Chronic obstructive pulmonary disease” OR “COPD” AND “whole-body vibration” OR “WBV”. The review was performed with PRISMA guidelines [43].
2.2. Inclusion and Exclusion Criteria
To be included for review, all studies investigating effects of WBV in persons with COPD needed to be conducted as a randomized controlled trial or single group experimental studies with crossover designs. Only studies published in English were considered for inclusion. Studies were reviewed if they included participants with COPD and who performed static or dynamic exercises on a WBV platform and the method of WBV was clearly described. Inclusion for review was based on consensus among three reviewers. Data were independently abstracted by the same three reviewers and disagreements were resolved by majority consensus. Studies/papers were excluded if they were review articles, replies, editorials, trial protocols, books, or chapters.
2.3. Level of Evidence of the Selected Papers
The included studies were classified according to the National Health and Medical Research Council hierarchy of evidence (NHMRC, 2003–2007) [42] (see Table 1). Each article was assigned to one reviewer and cross-checked by a second reviewer and where there was disagreement a third party was consulted and the issue discussed until consensus was reached.
Table 1.
NHMRC levels of evidence relevant to the review inclusion criteria.
| Level | Intervention | Diagnostic accuracy |
|---|---|---|
| I | A systematic review/meta-analysis of Level II studies | A systematic review of Level II studies |
|
| ||
| II | A randomized controlled trial | A study of test accuracy with an independent, blinded comparison with a valid reference standard, among consecutive persons with a defined clinical presentation |
|
| ||
| III-1 | A pseudorandomized controlled trial | A study of test accuracy with an independent, blinded comparison with a valid reference standard, among nonconsecutive persons with a defined clinical presentation |
3. Results
Of thousands of papers identified (see Table 2), a total of 100 were screened for review. A total of six papers met the inclusion criteria. A meta-analysis was inappropriate due to a vast difference of intervention group and control group interventions among the level of evidence II studies (see Figure 1). The descriptions and level of evidences of six reviewed studies are shown in Table 3. The level of evidence was Level II [23, 26, 27] and Level III-1 [22, 24, 25]. The degree to which the six reviewed papers met ISMNI recommendations is shown in Table 4.
Table 2.
Number of publications identified.
| Search | Keywords | PubMed | Scopus | PEDro | Science Direct |
|---|---|---|---|---|---|
| 1 | “Chronic obstructive pulmonary disease” OR “COPD” |
97,248 | 70,969 | 1559 | 108,463 |
|
| |||||
| 2 | “Whole body vibration” OR “WBV” | 1,257 | 2,626 | 167 | 2,738 |
|
| |||||
| Combined 1 and 2 | 16 | 25 | 5 | 54 | |
Figure 1.
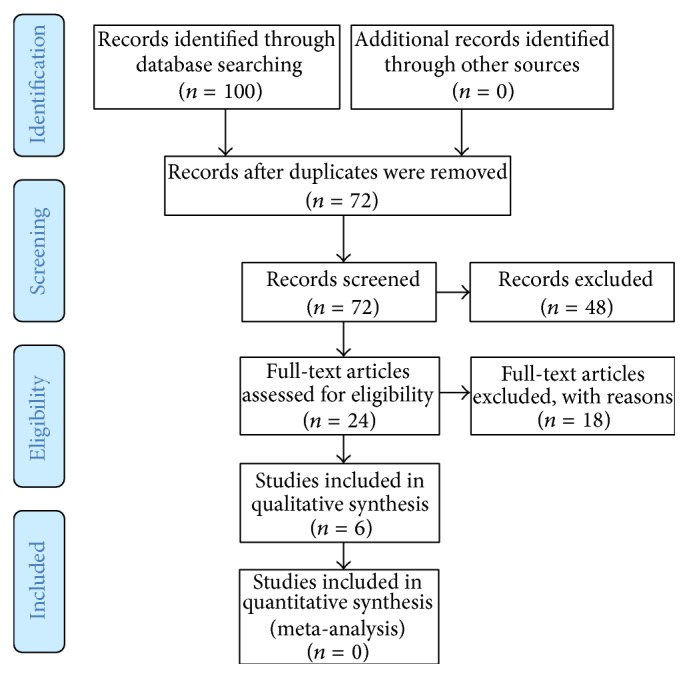
PRISMA flowchart.
Table 3.
Level of evidence and outcomes of reviewed papers.
| Level of evidence | Authors | Sample | Protocols and outcome measures | WBV findings |
|---|---|---|---|---|
| II | Greulich et al., 2014 [23] |
N = 40 (26 males and 14 females) IG n = 20 IG age = 66 ± 10 years CG n = 20 CG age = 70 ± 10 years |
IG: standard physiotherapy with WBV CG: standard physiotherapy Duration: during inpatient admission Outcomes: CRT, 6MWT, QoL, and serum markers |
Improved CRT, 6MWT, and QoL Increased expression of the TFPP receptor gamma coactivator-1-α and levels of irisin Decreased serum interleukin-8 |
|
| ||||
| II | Pleguezuelos et al., 2013 [26] |
N = 51 males IG n = 26 IG age = 73 ± 14 years CG n = 25 CG age = 74 ± 10 years |
IG: WBV CG: lifestyle education Duration: 6 weeks Outcomes: IKFET lower limb performance, 6MWT, and pulmonary muscular assessment with MIP and MEP |
No differences for IKFET Improved 6MWT, MIP, and MEP |
|
| ||||
| II | Gloeckl et al., 2012 [27] |
N = 72 (37 males and 35 females) IG n = 36 IG age = 64 ± 11 years CG n = 36 CG age = 65 ± 7 years |
IG: WBV with dynamic squats CG: dynamic squats Duration: 3 weeks Outcomes: 6MWT, sit-to-stand test, and QoL |
Improvement in 6MWT, sit-to-stand test No difference between groups for QoL |
|
| ||||
| III-1 | Braz Júnior et al., 2015 [22] |
N = 11 (8 males and 3 females) Age: 63 ± 9 years Design: crossover with washout |
IG: WBV CG: No intervention Duration: 12 weeks Outcomes: 6MWT, IPE, and QoL |
Improved 6MWT and QoL No difference among groups for IPE |
|
| ||||
| III-1 | Furness et al., 2014 [24] |
N = 16 (12 males and 4 females) Age: 72 ± 9 years Design: crossover with washout |
IG: WBV CG: SWBV Duration: 6 weeks Outcomes: Borg CR-10, heart rate, saturation of oxygen, TUG test, 5-chair stand test, and gait velocity |
No exacerbations were reported during the WBV or SWBV interventions. After improved TUG test, 5-chair stand test and gait velocity No meaningful difference among groups for Borg CR-10, heart rate, and saturation of oxygen |
|
| ||||
| III-1 | Furness et al., 2013 [25] |
N = 17 Age: 69 ± 8 years Design: crossover with washout |
IG: WBV CG: SWBV Duration: 1 session Outcomes: Borg CR-10, heart rate, and saturation of oxygen |
No meaningful differences among groups |
IG: intervention group; CG: control group; CRT: chair rising test; 6MWT: 6-minute walk test; QoL: quality of life; IKFET: isokinetic knee flexor/extensor testing; MIP: maximum inspiratory pressure; MEP: maximum expiratory pressure; IPE: index of perceived exertion; SWBV: sham whole-body vibration.
Table 4.
Descriptors of WBV based on ISMNI recommendations.
| Authors | Vibration device | Vibration direction | Vibration frequency | Peak-to-peak displacement | Gravitational force | Accuracy | Skidding | Foot position |
|---|---|---|---|---|---|---|---|---|
| Braz Júnior et al., 2015 [22] | Power Plate | Not stated | 35 Hz 35 Hz |
1 mm 2 mm |
2.46 g 4.92 g |
Not assessed | Not assessed | 200 mm apart |
| Greulich et al., 2014 [23] | Galileo | Side alternating | 12 Hz 26 Hz 26 Hz |
3 mm 4 mm 6 mm |
0.86 g 5.43 g 8.15 g |
Not assessed | Not assessed | Not stated |
| Furness et al., 2014 [24] | Amazing Super Health | Side alternating | 25 Hz | 2 mm | 2.52 g | Assessed | Assessed | 200 mm from axis of rotation |
| Furness et al., 2013 [25] | Amazing Super Health | Side alternating | 25 Hz | 2 mm | 2.52 g | Assessed | Assessed | 200 mm from axis of rotation |
| Pleguezuelos et al., 2013 [26] | Fitybe excel pro | Vertical | 35 Hz | 4 mm | 9.85 g | Not assessed | Not assessed | Not stated |
| Gloeckl et al., 2012 [27] | Galileo | Side alternating | 24 Hz 25 Hz 26 Hz |
6 mm∗ | Cannot be calculated | Not assessed | Not assessed | Not stated |
∗Peak-to-peak amplitude reported.
3.1. Level of Evidence II and ISMNI Recommendations for WBV Interventions
At this level of evidence, varying WBV interventions improved QoL and functional performance of the lower limbs of people with COPD [23, 26, 27]. However, the interventions varied relative to the direction of the vibration platform, the vibration frequency, peak-to-peak displacement, and gravitational forces (see Table 4). The validity of each vibration platform was not established (i.e., frequency and displacement) nor was skidding or the position of the feet upon the vibration platform.
3.2. Level of Evidence III-1 and ISMNI Recommendations for WBV Interventions
At this level of evidence, WBV improved functional performance of the lower limbs [22, 24, 25] and QoL [22] of people with COPD (see Table 4). Two studies met the ISMNI recommendations [24, 25]. One study did not report vibration direction or test for accuracy and skidding.
4. Discussion
The major finding of this study was the benefit of WBV, as a component of pulmonary rehabilitation, for people with COPD. Specifically, evidence was found to support the use of WBV to improve functional performance of the lower limbs and QoL (see Table 5). The results of the current study support the seminal work of Gloeckl et al. (2015) [40]. However, despite evidence to support the use of WBV for people with COPD, the results of the current review revealed that reporting WBV methods are poorly disclosed.
Table 5.
Statement of evidence for WBV interventions among people with COPD.
| Statement | Level of evidence |
|---|---|
| WBV improves performance of field tests that simulate activities of daily living (e.g., 6MWT and sit-to-stand test) | II and III-1 |
| WBV improves serum markers associated with COPD | II |
| WBV may improve quality of life | II and III-1 |
| WBV does not add clinically meaningful stress on the cardiorespiratory system | III-1 |
By accepting manufacture claims of vibration frequency and peak-to-peak displacement rather than ascertaining vibration parameters by using accelerometers, gravitational forces cannot be confidently described [41]. Furthermore, if skidding is not assessed, the parameters of the vibration study that the participant is subjected to can no longer be defined [Rauch]. Skidding occurs when the feet lose contact with the vibration platform with increasing gravitational forces [34]. Only two studies of lower evidence [24, 25] could confirm the vibration parameters for the participants with COPD. As such, despite levels of evidence II and III-1 supporting the use of WBV for people with COPD, clinicians remain without a clear method of how WBV should be prescribed.
Whole-body vibration has been used without exacerbating people with COPD. The lowest frequency of the vibrations generated in the platforms was about 20 Hz [23]. Although all the authors of the investigations selected in this narrative revision [22–27] have suggested that the conditions of the protocols are without problems to the COPD patient, it would be interesting to use protocols with frequencies lower than 20 Hz. Lower vibration frequency has been beneficially used among patients with Parkinson's disease with 3 Hz, 6 Hz, and 9 Hz [44] and with fibromyalgia (12.5 Hz) [28]. The benefit of lower vibration frequency for people with COPD is that the risk of damaging fragile bone of older adults may be mitigated [45]. Subsequently, the appropriateness of prescribed doses of WBV should be at the forefront of clinicians as people with COPD are known to have lower bone mineral density compared with healthy matched controls [46, 47].
In general, the studies included in the current review reported benefits without important clinical complication to the patient with COPD. These findings are highly desirable and support the use of WBV as a component of the pulmonary rehabilitation. Whole-body vibration was reported as effective and feasible interventions [27] which does not exacerbate perceived dyspnoea [24, 25] and may safely improve clinical parameters of the patient with COPD [22].
5. Conclusions
The results of this review support the use of WBV as a potentially important component of pulmonary rehabilitation to assist management of patients with a diagnosis of COPD. Furthermore, WBV has been feasible and safely completed by patients with COPD. However, future research should examine the dose-response curve and optimal dosing regimen of WBV according to ISMNI recommendations for people with COPD. Such an approach will allow comparison among studies and the potential of meta-analysis of randomized controlled trials.
Acknowledgments
The authors thank the Brazilian Government agencies (CNPq and FAPERJ) and UERJ for the support.
Abbreviations
- CG:
Control group
- COPD:
Chronic obstructive pulmonary disease
- GOLD:
Global Initiative for Chronic Obstructive Lung Disease
- IG:
Intervention group
- IKFET:
Isokinetic knee flexor/extensor testing
- IPE:
Index of perceived exertion
- ISMNI:
International Society of Musculoskeletal and Neuronal Interactions
- MEP:
Maximum expiratory pressure
- MIP:
Maximum inspiratory pressure
- 6MWT:
Six-minute walk test
- NHMRC:
National Health and Medical Research Council
- PEDro:
Physiotherapy Evidence Database
- QoL:
Quality of life
- SWBV:
Sham whole-body vibration
- TFPP:
Transcription factor peroxisome proliferator
- TUG:
Timed up and go test
- WBV:
Whole-body vibration.
Competing Interests
The authors declare that there are no financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial, or any other) related to this paper.
Authors' Contributions
Danubia Sá-Caputo, Cintia Renata Gonçalves, Danielle Soares Morel, Eloá Moreira Marconi, and Patrícia Fróes participated in the conception and design of the study as well as preparing the paper. Rogério Rufino, Cláudia Henrique Costa, Agnaldo José Lopes, Pedro Jesus Marin, and Trentham Furness coordinated the clinical approaches of the study. Danubia Sá-Caputo, Adriano Arnóbio, and Nasser Ribeiro Asad did the searches in the databases and aided in the selection of the papers to be discussed in the paper. Cintia Renata Gonçalves, Danielle Soares Morel, and Pedro Jesus Marin aided in the corrections of the tables. Mario Bernardo-Filho has done the final version of the paper. Mario Bernardo-Filho conceived the protocol, obtained funding, and oversaw the study. All the authors read and approved the final paper.
References
- 1.Buist A. S., McBurnie M. A., Vollmer W. M., et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. The Lancet. 2007;370(9589):741–750. doi: 10.1016/s0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 2.GOLD. Global Strategy for the Diagnosis, Management, Prevention of COPD (Updated 2015) 2015. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf. [Google Scholar]
- 3. March 2015, http://www.who.int/mediacentre/factsheets/fs315/en/
- 4.Miniño A. M., Murphy S. L., Xu J., Kochanek K. D. Deaths: final data for 2008. National Vital Statistics Reports. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 5.Barnes P. J., Celli B. R. Systemic manifestations and comorbidities of COPD. The European Respiratory Journal. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy B., Casey D., Devane D., Murphy K., Murphy E., Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2015;(2) doi: 10.1002/14651858.cd003793.pub3.CD003793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spruit M. A., Singh S. J., Garvey C., et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634st. [DOI] [PubMed] [Google Scholar]
- 8.Postolache P., Cojocaru D. C. Pulmonary rehabilitation—from guidelines to practice. Revista Medico-Chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2013;117(2):380–387. [PubMed] [Google Scholar]
- 9.Bernard S., Whittom F., Leblanc P., et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;159(3):896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- 10.Karpman C., Benzo R. Gait speed as a measure of functional status in COPD patients. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:1315–1320. doi: 10.2147/copd.s54481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpman C., DePew Z. S., LeBrasseur N. K., Novotny P. J., Benzo R. P. Determinants of gait speed in COPD. Chest. 2014;146(1):104–110. doi: 10.1378/chest.13-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark C. J., Cochrane L. M., Mackay E., et al. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. The European Respiratory Journal. 2000;15:92–97. doi: 10.1183/09031936.00.15109200. [DOI] [PubMed] [Google Scholar]
- 13.Panton L. B., Golden J., Broeder C. E., Browder K. D., Cestaro-Seifer D. J., Seifer F. D. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. European Journal of Applied Physiology. 2004;91(4):443–449. doi: 10.1007/s00421-003-1008-y. [DOI] [PubMed] [Google Scholar]
- 14.Normandin E. A., McCusker C., Connors M., Vale F., Gerardi D., ZuWallack R. L. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest. 2002;121(4):1085–1091. doi: 10.1378/chest.121.4.1085. [DOI] [PubMed] [Google Scholar]
- 15.Vogiatzis I. Strategies of muscle training in very severe COPD patients. The European Respiratory Journal. 2011;38(4):971–975. doi: 10.1183/09031936.00075011. [DOI] [PubMed] [Google Scholar]
- 16.Prefaut C., Varray A., Vallet G. Pathophysiological basis of exercise training in patients with chronic obstructive lung disease. European Respiratory Review. 1995;5(25):27–32. [Google Scholar]
- 17.Kongsgaard M., Backer V., Jørgensen K., Kjær M., Beyer N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients—a pilot study. Respiratory Medicine. 2004;98(10):1000–1007. doi: 10.1016/j.rmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira C. C., Carrascosa C. R., Borghi-Silva A., et al. Influence of respiratory pressure support on hemodynamics and exercise tolerance in patients with COPD. European Journal of Applied Physiology. 2010;109(4):681–689. doi: 10.1007/s00421-010-1408-8. [DOI] [PubMed] [Google Scholar]
- 19.O'Driscoll B. R., Neill J., Pulakal S., Turkington P. M. A crossover study of short burst oxygen therapy (SBOT) for the relief of exercise-induced breathlessness in severe COPD. BMC Pulmonary Medicine. 2011;11, article 23 doi: 10.1186/1471-2466-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin A. D., Davenport P. W. Extrinsic threshold PEEP reduces post-exercise Dyspnea in COPD patients: a placebo-controlled, double-blind cross-over study. Cardiopulmonary Physical Therapy Journal. 2011;22:5–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Furness T., Bate N., Welsh L., Naughton G., Lorenzen C. Efficacy of a whole-body vibration intervention to effect exercise tolerance and functional performance of the lower limbs of people with chronic obstructive pulmonary disease. BMC Pulmonary Medicine. 2012;12, article 71 doi: 10.1186/1471-2466-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braz Júnior D. S., Dornelas de Andrade A., Teixeira A. S., Cavalcanti C. A., Morais A. B., Marinho P. E. M. Whole-body vibration improves functional capacity and quality of life in patients with severe chronic obstructive pulmonary disease (COPD): a pilot study. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10:125–132. doi: 10.2147/copd.s73751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greulich T., Nell C., Koepke J., et al. Benefits of whole body vibration training in patients hospitalised for COPD exacerbations—a randomized clinical trial. BMC Pulmonary Medicine. 2014;14, article 60 doi: 10.1186/1471-2466-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furness T., Joseph C., Naughton G., Welsh L., Lorenzen C. Benefits of whole-body vibration to people with COPD: a community-based efficacy trial. BMC Pulmonary Medicine. 2014;14, article 38 doi: 10.1186/1471-2466-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furness T., Joseph C., Welsh L., Naughton G., Lorenzen C. Whole-body vibration as a mode of dyspnoea free physical activity: a community-based proof-of-concept trial. BMC Research Notes. 2013;6, article 452 doi: 10.1186/1756-0500-6-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pleguezuelos E., Pérez M. E., Guirao L., et al. Effects of whole body vibration training in patients with severe chronic obstructive pulmonary disease. Respirology. 2013;18(6):1028–1034. doi: 10.1111/resp.12122. [DOI] [PubMed] [Google Scholar]
- 27.Gloeckl R., Heinzelmann I., Baeuerle S., et al. Effects of whole body vibration in patients with chronic obstructive pulmonary disease—a randomized controlled trial. Respiratory Medicine. 2012;106(1):75–83. doi: 10.1016/j.rmed.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Sañudo B., De Hoyo M., Carrasco L., et al. The effect of a 6-week exercise programme and whole body vibration on strength and quality of life in women with fibromyalgia: a randomised study. Clinical and Experimental Rheumatology. 2010;28(supplement 6):S40–S45. [PubMed] [Google Scholar]
- 29.Roth J., Wust M., Rawer R., et al. Whole body vibration in cystic fibrosis—a pilot study. Journal of Musculoskeletal Neuronal Interactions. 2008;8(2):179–187. [PubMed] [Google Scholar]
- 30.Rietschel E., van Koningsbruggen S., Fricke O., Semler O., Schoenau E. Whole body vibration: a new therapeutic approach to improve muscle function in cystic fibrosis? International Journal of Rehabilitation Research. 2008;31(3):253–256. doi: 10.1097/mrr.0b013e3282fb783d. [DOI] [PubMed] [Google Scholar]
- 31.Maiworm A. I., Monteiro M. B., Santos-Filho S. D., et al. Cystic fibrosis and the relevance of the whole-body vibration exercises in oscillating platforms: a short review. Health. 2011;03(10):656–662. doi: 10.4236/health.2011.310110. [DOI] [Google Scholar]
- 32.Jackson K. J., Merriman H. L., Vanderburgh P. M., Brahler C. J. Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis. Journal of Neurologic Physical Therapy. 2008;32(4):171–176. doi: 10.1097/npt.0b013e31818ee760. [DOI] [PubMed] [Google Scholar]
- 33.Santos-Filho S. D., Cameron M. H., Bernardo-Filho M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis: a systematic review. Multiple Sclerosis International. 2012;2012:6. doi: 10.1155/2012/274728.274728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. European Journal of Applied Physiology. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 35.Machado A., García-López D., González-Gallego J., Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scandinavian Journal of Medicine and Science in Sports. 2010;20(2):200–207. doi: 10.1111/j.1600-0838.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 36.Cochrane D. J. Vibration exercise: the potential benefits. International Journal of Sports Medicine. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 37.Cochrane D. J., Stannard S. R., Firth E. C., Rittweger J. Comparing muscle temperature during static and dynamic squatting with and without whole-body vibration. Clinical Physiology and Functional Imaging. 2010;30(4):223–229. doi: 10.1111/j.1475-097x.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 38.Cardinale M., Bosco C. The use of vibration as an exercise intervention. Exercise and Sport Sciences Reviews. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Rittweger J., Mutschelknauss M., Felsenberg D. Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise. Clinical Physiology and Functional Imaging. 2003;23(2):81–86. doi: 10.1046/j.1475-097x.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 40.Gloeckl R., Heinzelmann I., Kenn K. Whole body vibration training in patients with COPD: a systematic review. Chronic Respiratory Disease. 2015;12(3):212–221. doi: 10.1177/1479972315583049. [DOI] [PubMed] [Google Scholar]
- 41.Rauch F., Sievanen H., Boonen S., et al. Reporting whole-body vibration interventions studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. Journal of Musculoskeletal and Neuronal Interactions. 2010;10:193–198. [PubMed] [Google Scholar]
- 42.NHMRC levels of evidence and grades for recommendations for developers of guidelines. 2009, https://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf.
- 43.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100.e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chouza M., Arias P., Viñas S., Cudeiro J. Acute effects of whole-body vibration at 3, 6, and 9 hz on balance and gait in patients with Parkinson's disease. Movement Disorders. 2011;26(5):920–921. doi: 10.1002/mds.23582. [DOI] [PubMed] [Google Scholar]
- 45.Kiiski J., Heinonen A., Järvinen T. L., Kannus P., Sievänen H. Transmission of vertical whole body vibration to the human body. Journal of Bone and Mineral Research. 2008;23(8):1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 46.Dam T.-T., Harrison S., Fink H. A., Ramsdell J., Barrett-Connor E. Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporosis International. 2010;21(8):1341–1349. doi: 10.1007/s00198-009-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duckers J. M., Evans B. A. J., Fraser W. D., Stone M. D., Bolton C. E., Shale D. J. Low bone mineral density in men with chronic obstructive pulmonary disease. Respiratory Research. 2011;12, article 101 doi: 10.1186/1465-9921-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]


