Abstract
Patients with multiple serious conditions account for a high proportion of health care spending. Such spending is projected to continue to grow substantially because of increased insurance eligibility, the ever-rising cost of care, the continued use of nonbeneficial high-intensity treatments at the end of life, and demographic changes. We evaluated the impact of palliative care consultation on hospital costs for adults with advanced cancer, excluding those with dementia. We found that compared to usual care, the receipt of a palliative care consultation within two days of admission was associated with 22 percent lower costs for patients with a comorbidity score of 2–3 and with 32 percent lower costs for those with a score of 4 or higher. Earlier consultation was also found to be systematically associated with a larger cost-saving effect for all subsamples defined by multimorbidity. Given ongoing workforce shortages, targeting early specialist palliative care to hospitalized patients with advanced cancer and higher numbers of serious concurrent conditions could improve care while complementing strategies to curb the growth of health spending.
Improving care for people with cancer is a US health care priority. Forty percent of Americans will develop cancer in their lifetime, and cancer remains the second leading cause of death in the United States—accounting for almost 600,000 deaths annually.[1] Multimorbidity (the presence of more than one chronic condition) is common in cancer patients because key risk factors for cancer, including aging and unhealthy behaviors such as alcohol and tobacco use, are also major risk factors for other serious chronic conditions.[2]
Patients with multimorbidity are a well-established policy priority in the United States and other high-income countries.[3, 4] Ten-year projections estimate that annual Medicare expenditures will have increased 98 percent by 2024, reaching $1.2 trillion, and that total annual national health spending will have grown 76 percent, reaching $5.4 trillion.[5] These estimates are strongly driven by the cost of treating patients with multiple chronic conditions.
Two-thirds of Medicare beneficiaries have multimorbidity, and there is a strong association between the number of co-occurring conditions and cost: The 16 percent of beneficiaries with six or more chronic conditions account for 47 percent of total program expenditures.[6] The economic burden of treatment for patients with serious illnesses such as cancer and multimorbidity is projected to grow because of expanding insurance eligibility through the Affordable Care Act, demographic changes, and the limited capacity of health systems originally designed to provide acute and episodic care.[3, 7–9]
Moreover, expenditure often does not equate to value: Patients with serious illness continue to receive fragmented care of poor quality,[10] and end-of-life care is becoming more aggressive—with more use of the intensive care unit, more transitions between sites of care, and shorter hospice stays.[11] Multiple chronic conditions act synergistically to increase difficulties in finding appropriate medications and treatment regimens that work for all conditions.[12] Among patients with advanced cancer and other serious illnesses, aggressive treatments often are inconsistent with patients’ preferences,[13, 14] have limited efficacy,[15, 16] and are associated with worse quality of life, compared to other treatments.[17]
Studies have demonstrated the beneficial effects on patient and family outcomes when palliative care is introduced into routine cancer care. The effects include improvements in pain and other symptoms; improved family outcomes; reduced hospital costs and readmissions; increased hospice use; and enhanced survival.[18] Palliative care is a team-based specialty (incorporating medicine, nursing, social work, and chaplaincy) focused on improving quality of life for people with serious illness such as cancer by adding a layer of support for patients, their families, and health care providers. Palliative interventions are not focused only on people at end of life and are increasingly available earlier in the care trajectory with observable benefits, and the American Society of Clinical Oncology has recommended the integration of palliative care into standard oncology care.[19]
Although multiple studies have shown that palliative care reduces average costs of care,[20] little is known about how the treatment effect of palliative care on costs varies according to diagnosis or comorbidity.[21] The effect of palliative care is likely not homogeneous and may vary according to a multifaceted interaction of individual and service factors.[22, 23]
In this article we report the effect of palliative care consultation teams—the dominant model of palliative care delivery in US hospitals—on direct hospital costs for advanced cancer patients with multiple comorbidities. Evidence on the relationship among palliative care, multimorbidity, and cost will inform decision making as policy makers seek to improve care for patients with serious illness while curbing cost growth.
Study Data And Methods
Study Population
Clinical and hospital cost data were collected between 2007 and 2011 using a prospective and observational multisite study design to evaluate the effect of palliative care on patients with an advanced cancer diagnosis. Patients were recruited from six hospitals—two tertiary care academic medical centers (Mount Sinai Medical Center, in New York City; and Froedtert Hospital, in Milwaukee, Wisconsin), a specialty cancer center (Virginia Commonwealth University Massey Cancer Center, in Richmond), and three community teaching hospitals (Mount Carmel East, Mount Carmel West, and Mount Carmel St. Ann’s, all part of the Mount Carmel Health System, in Columbus, Ohio). Study sites were geographically and structurally diverse and represented ethnically and socioeconomically diverse patient populations. The study was approved by each facility’s Institutional Review Board. All participants provided written informed consent at enrollment.
Patients were eligible to participate in the study if they were older than eighteen years and fluent in English and had been admitted to a hospital with an advanced cancer diagnosis. Eligible diagnoses were the following: stage 3 or 4 laryngeal, throat, nasopharyngeal, mouth, or head and neck cancer; non-small-cell lung cancer; mesothelioma, esophageal, stomach or gastric, pancreatic, gallbladder, bile duct, cholangio, ampullary, liver, hepatic, hepatocellular, or ovarian cancer; stage 4 breast, kidney, renal cell, endometrial, uterine, cervical, sarcoma, prostate, or melanoma cancer; Dukes’ stage D colon cancer; extensive stage small-cell lung cancer; transplant-ineligible multiple myeloma; relapsed or transplant-ineligible lymphoma; and glioblastoma multiforme.
Patients were excluded if their primary physicians refused to approve their participation in the study or if they were unresponsive or nonverbal, had a diagnosis of dementia, or had previously received a hospital palliative care consultation.
Methodology
Patients were not randomly assigned to the treatment or comparison group: Those who were seen by a palliative care consultation team were placed in the treatment group, and those who received usual care only were placed in the comparison group. We controlled for differences using propensity score weights.[24] Treatment and comparison groups were matched based upon multiple potential confounders (Exhibit 1). Full details of the propensity score generation and balancing are provided in the online Appendix.[25]
Exhibit 1.
Baseline Characteristics Of Matched Groups Of 906 Patients, Treatment (Patients Seen By A Palliative Care Consultation Team [PCCT]) And Comparison (Usual Care Only) Between 2007 And 2011
| Characteristic | Compari son group (n = 713) |
Treatme nt group (n = 193) |
Standar dized differenc e (%) |
|---|---|---|---|
| Age (years) | |||
| 55–75 | 53% | 55% | 5.3 |
| More than 75 | 13 | 11 | −6.7 |
| Female | 53 | 52 | −1.7 |
| Race | |||
| White | 60 | 62 | 4.7 |
| Black | 35 | 35 | <0.1 |
| Had advance directive | 49 | 47 | −3.4 |
| Insurance | |||
| Medicare only | 23 | 24 | 2.6 |
| Medicaid and Medicare | 26 | 24 | −4.3 |
| Highest level of education | |||
| High school | 55 | 56 | 1.2 |
| College | 37 | 35 | −2.8 |
| Used visiting nurse services 2 weeks before hospitalization | 15 | 14 | −1.9 |
| Hours of home health aide use 2 weeks before hospitalization |
1.0 | 0.9 | −2.7 |
| Primary diagnosis of lymphoma or myeloma | 5% | 5% | 1.1 |
| Patient had a complicationb | 1% | 1% | −1.0 |
| Mean Elixhauser comorbidity score | 3.4 | 3.3 | −6.8 |
| Needed partial or complete help with ADLs | |||
| Bathing (partial) | 39% | 39% | 0.8 |
| Transferring from chair (partial) | 34 | 35 | 2.7 |
| One or more ADL (complete) | 13 | 13 | 1.3 |
| Mean ESAS score | |||
| Physical at admission | 2 | 2 | −0.4 |
| Psychological at admission | 1.6 | 1.6 | 3.8 |
| Physical on the reference day | 1.8 | 1.8 | −1.8 |
| Psychological on the reference day | 1.5 | 1.4 | −3.8 |
| Mean CMSAS scorec | |||
| Number at admission | 8.9 | 8.9 | 0.2 |
| Number on the reference day | 7.8 | 7.7 | −4.1 |
| Severity at admission | 15.8 | 15.8 | 0.4 |
| Severity on the reference day | 12.5 | 12.4 | −1.2 |
| Equivalent dose of morphine (mg),d | 21.7 | 22.2 | 1.3 |
| In paine | |||
| Somewhat | 9% | 10% | 3.0 |
| Quite a bit | 29 | 30 | 1.2 |
| Very much | 35 | 35 | 0.5 |
| Fatiguede | |||
| A little, somewhat, or quite a bit | 38 | 37 | −2.7 |
| Very much | 29 | 29 | 0.3 |
SOURCE Authors’ analysis. NOTES There were 910 patients in the final sample who were eligible for the primary analysis in this article. In matching stratified subsamples, one palliative care consultation team (PCCT) patient with 2–3 comorbidities and three PCCT patients with 4 or more comorbidities were lost to matching. No patients in the comparison group were lost to matching in any subsample. There are therefore 906 patients in the primary analysis in this article. For patients in the treatment group, the reference day was the day of consultation; for patients in the comparison group, it was the day their symptom severity was most similar to that of palliative care patients. ADLs are activities of daily living. ESAS is the Edmonton Symptom Assessment Scale [45], which evaluates six physical and three psychologic symptoms on a scale of 0 to 10 (0=absence of symptom; 10=most severe presence): Pain, tiredness, nausea, drowsiness, appetite and shortness of breath; and depression, anxiety and wellbeing. CMSAS is the Condensed Memorial Symptom Assessment Scale [46], which evaluates 14 symptoms on a scale of 0 to 4 (0=absent; 4=Very much): Lack of energy, lack of appetite, pain, dry mouth, weight loss, feeling drowsy, shortness of breath, constipation, difficulty sleeping, difficulty concentrating, nausea, worrying, feeling sas and feeling nervous. Reference categories are as follows: for binary variables, no; age, younger than fifty-five; race, other; insurance, neither Medicare nor Medicaid; education, elementary school; pain and fatigue, none.
Major or minor complication on the reference day.
Number is the number of physical symptoms on the CMSAS; severity is the number of physical symptoms multiplied by the mean severity of physical symptoms on the CMSAS.
Average daily dose of opioids in milligrams of morphine sulfate equivalents in week prior to hospitalization.
On the reference day. Standardized differences measure the imbalance between treatment and comparison groups on baseline characteristics, taking into account both means and variances.
To examine the cost effect of palliative care for patients by multimorbidity, we stratified our sample using the Elixhauser comorbidity index.[26] This is an additive index that counts the presence of thirty-one serious conditions, including the following three cancer diagnoses: lymphoma, metastatic cancer, and solid tumor without metastasis. It was therefore possible for a patient to have an advanced cancer diagnosis (for example, myeloma) that made him or her eligible for the study but whose diagnosis was not reflected in his or her comorbidity score. We created three subsamples according to multimorbidity at hospital admission: patients with scores on the Elixhauser comorbidity index of 0–1, 2–3, and 4 or higher (Exhibit 2).
Exhibit 2.
Prevalence Of Specific Comorbidities Within Subsamples, By Elixhauser Comorbidity Score At Admission
| Elixhauser comorbidity score | ||||||
|---|---|---|---|---|---|---|
| 0–1 (n = 235) |
2–3 (n = 362) |
4 or higher (n = 309) |
||||
| Comorbidity | No. | % | No. | % | No. | % |
| Congestive heart failure | 0 | 0 | 5 | 1 | 54 | 17 |
| Cardiac arrhythmia | 0 | 0 | 20 | 6 | 93 | 30 |
| Valvular disease | 0 | 0 | 3 | 1 | 26 | 8 |
| Pulmonary circulation | 1 | 0 | 6 | 2 | 33 | 11 |
| Peripheral vascular disorders |
0 | 0 | 7 | 2 | 18 | 6 |
| Hypertension (uncomplicated) |
6 | 3 | 166 | 46 | 169 | 55 |
| Hypertension (complicated) |
0 | 0 | 1 | 0 | 47 | 15 |
| Paralysis | 0 | 0 | 7 | 2 | 15 | 5 |
| Neurologic disorders other than paralysis |
0 | 0 | 18 | 5 | 25 | 8 |
| Chronic pulmonary disease |
1 | 0 | 36 | 10 | 106 | 34 |
| Diabetes (uncomplicated) | 0 | 0 | 11 | 3 | 26 | 8 |
| Diabetes (complicated) | 0 | 0 | 0 | 0 | 12 | 4 |
| Hypothyroidism | 1 | 0 | 26 | 7 | 50 | 16 |
| Renal failure | 1 | 0 | 7 | 2 | 55 | 18 |
| Liver disease | 0 | 0 | 10 | 3 | 17 | 6 |
| Peptic ulcer disease | 0 | 0 | 1 | 0 | 6 | 2 |
| AIDS or HIV | 0 | 0 | 2 | 1 | 1 | 0 |
| Lymphoma | 9 | 4 | 22 | 6 | 30 | 10 |
| Metastatic cancer | 107 | 46 | 248 | 69 | 207 | 67 |
| Solid tumor without metastasis |
116 | 49 | 251 | 69 | 217 | 70 |
| Rheumatoid arthritis | 0 | 0 | 2 | 1 | 11 | 4 |
| Coagulopathy | 0 | 0 | 8 | 2 | 36 | 12 |
| Obesity | 0 | 0 | 3 | 1 | 16 | 5 |
| Weight loss | 0 | 0 | 59 | 16 | 100 | 32 |
| Fluid or electrolyte disorders |
6 | 3 | 108 | 30 | 178 | 58 |
| Anemia (blood loss) | 0 | 0 | 3 | 1 | 1 | 0 |
| Anemia (deficiency) | 0 | 0 | 5 | 1 | 15 | 5 |
| Alcohol abuse | 0 | 0 | 4 | 1 | 18 | 6 |
| Drug abuse | 0 | 0 | 10 | 3 | 10 | 3 |
| Psychoses | 0 | 0 | 2 | 1 | 9 | 3 |
| Depression | 0 | 0 | 47 | 13 | 95 | 31 |
SOURCE Authors’ analysis. NOTES Of the 906 matched patients, 98 had an advanced cancer that was not lymphoma, metastatic cancer, or a solid tumor. Of these, 49 (50 percent) had no comorbidity on the Elixhauser comorbidity scale and thus had an Elixhauser comorbidity score of 0, as explained in the text. The other 49 had an Elixhauser comorbidity score of 1–8 (median: 2), with their cancer diagnosis unaccounted for in the score.
We separated patients discharged alive from those who died in the hospital to reduce the amount of unobserved heterogeneity in clinical status and underlying treatment decisions and preferences.[27] The number of patients with multimorbidity who died during the hospitalization (n = 54; survival data missing for 3 patients) was too small to support a separate weighted analysis. Therefore, we performed our primary analysis on those discharged alive only and conducted sensitivity analyses with patients pooled irrespective of discharge status. Our treatment variable was the receipt of a consultation by a palliative care team within two days of hospital admission. Such a timing-sensitive specification of treatment (as opposed to receiving a consultation at any time) reduces the risk of a type 2 error and improves model performance (details available from the authors). Patients seen by a palliative care team after more than two days in hospital were excluded from our primary analyses and incorporated into our secondary and sensitivity analyses.[22]
We examined several different approaches to propensity score matching (for example, one-to-one matching, one-to-many matching within specified calipers, and inverse probability matching). Kernel weights achieved the best balance across observed confounders with the least amount of bias and were selected for analyses. Treated patients received a weight of one. Individuals in the comparison group with a propensity score within a bandwidth of 0.06 of a treated individual’s propensity score were weighted based on their distance from the treated individual. A detailed description of the sample construction, matching methodology selection, and propensity score weight calculation for this study has been published previously.[24] Separate weights were calculated within each subsample.[28]
The primary outcome of interest was total direct hospital costs for the index hospitalization—specifically, the estimated mean treatment effect, or the mean estimated change in total direct hospital costs if a patient in the comparison group was moved to the treatment group, with all other covariates held constant at their original values. Direct costs are those attributable to a specific utilization during hospital stay. Variable direct costs are those that are dictated wholly by treatment of the specific patient, such as those for medical supplies and pharmaceuticals and imaging and laboratory expenditures. Fixed direct costs are those that do not vary with a specific patient’s utilization but that nonetheless can be identified with the treatment of that patient (for example, staff salaries and equipment expense).[29] Cost data were standardized to 2011 dollars, since that year was the end point of data collection.
Generalized linear model regression (gamma distribution, log link) was performed on total direct costs against a binary intervention variable, the independent variables listed in Exhibit 1, and fixed-effects variables to control for hospital site.
Limitations
Our study had several limitations. First, propensity score weights ensured balance between treatment and comparison arms on observed covariates but did not control for unobserved confounders. An instrumental variable, which would have helped control for unobserved confounding, was not available within our data set.
However, a strength of our data set and what sets our study apart from previous ones[21] is its inclusion of rich patient-reported information on many important potential confounders, including demographic and socioeconomic factors, psychological and physical symptoms, functional status, and formal health care use before hospitalization or at hospital admission (Exhibit 1). Because we hypothesized the hospital site to be a weaker potential confounder than the patient characteristics included in our propensity score model, we chose to account for site differences in costs via fixed effects in our regression model instead of including them in the propensity score models.
A second limitation of our study is that patients who received palliative care consultations may have been more inclined to elect less aggressive (and less expensive) care, even without the involvement of palliative care. However, previous reports that demonstrate that per diem hospital costs decline after palliative care consultations suggest that palliative care consultation teams have a causal impact on goals of care and treatment decisions.[30, 31]
Our models included data on advance directives—whether or not a patient had completed a living will and designated a proxy at baseline. Although we did not have data on specific patient preferences, people who wish to restrict life-prolonging treatment are more likely to complete an advance directive than those who do not wish to restrict such treatment (because the default treatment option is usually to intervene). Although patient preferences should drive care, studies have consistently demonstrated that the effect of such preferences on treatments received is small compared to the other variables included in our analyses.[32]
A third limitation is that the impact of palliative care consultation teams on patient and family outcomes has not yet been evaluated with the study data analyzed in this article; this impact will be addressed in future articles. Therefore, the cost savings reported here represent only evidence that the intervention is cost-effective, based on a “noninferiority” assumption—that is, the assumption that outcomes were at least no worse for patients in the intervention group than for those in the comparison group. This assumption is well supported by reports that hospital inpatient palliative care teams improve symptom control, quality of life, emotional burden, and caregiver and patient satisfaction.[33–36]
Our results were derived from studying data from hospitals with established palliative care teams that met both the current standards for the Joint Commission’s Advanced Certification Program for Palliative Care[37] and the guidelines established by the National Consensus Project for Quality Palliative Care.[38] Thus, our results likely reflect savings that can be expected from programs of acceptable quality and provide a target for programs that are being developed. As access to high-quality palliative care teams increases, the generalizability of our results to hospitals with substandard programs will become less of a concern.
A fourth limitation is that inclusion in our study reflected patients’ ability to participate throughout their hospitalization. This means that the very sickest enrolled patients may have disproportionately been omitted as a result of incomplete data. The final limitation is that our data did not include professional fees or the costs of postacute care, costs from the payer’s perspective, or costs from the patient’s or family’s perspective, which may include an impact on family wages or savings.[21] Hospital costs reflect one portion of all costs of hospitalization.
Study Results
Patient Attributes
There were 906 patients with advanced cancer matched for analysis, 193 (21 percent) of whom were seen by a palliative care consultation team within two days of admission during the index hospitalization. Baseline characteristics treated as covariates are reported in Exhibit 1. The prevalence of each of the thirty-one comorbidities in the Elixhauser index for each multimorbidity-defined subsample is provided in Exhibit 2.
Treatment Effect
Receipt of a consultation by a palliative care team within two days of admission was significantly associated with lower total direct hospital costs for advanced cancer patients with multimorbidity, and the effect size grew larger as the number of comorbidities increased (Exhibit 3). For patients with a comorbidity score of 0–1, the estimated mean treatment effect was not significant. For patients with a score of 2–3, the estimated effect was a reduction in costs of $2,321 (22 percent). For patients with a score of 4 or higher, the reduction was $3,515 (32 percent).
Exhibit 3.
Estimated Effect Of A Consultation With A Palliative Care Team Within Two Days Of Admission On Total Direct Hospital Costs For Patients With Advanced Cancer, By Elixhauser Comorbidity Score At Admission
| Sample | Utilization summary | Primary results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comorbidity score | Compariso n group |
Treatme nt group |
All patie nts |
Proporti on in treatmen t group |
Mean direct costs per patient |
Mean LOS per patient (days) |
Estimated mean treatment effecta |
95% CI | Implied mean savingb |
| 0–1 | 207 | 28 | 235 | 12% | $8,440 | 7.4 | −$1,775 | −5,093, 1,544 | 18% |
| 2–3 | 276 | 86 | 362 | 24 | 8,528 | 7.0 | −2,321* | −3,869, −773 | 22 |
| 4 or higher | 230 | 79 | 309 | 26 | 10,030 | 8.2 | −3,515* | −5,949, −1,081 | 32 |
SOURCE Authors’ analysis. NOTES We regressed total direct costs against a binary intervention variable, the independent variables listed in Exhibit 1, and fixed-effects variables to control for hospital site, applying subsample-specific propensity score weights in all cases. Further details are available in the Appendix (see Note 25 in text). Of the 906 matched patients, 98 had an advanced cancer that was not lymphoma, metastatic cancer, or a solid tumor. Of these, 49 (50 percent) had an advanced cancer diagnosis but no comorbidity on the Elixhauser comorbidity scale and thus had an Elixhauser comorbidity score of 0, as explained in the text. The other 49 had an Elixhauser comorbidity score of 1–8 (median: 2), with their cancer diagnosis unaccounted for in the score. Costs are in 2011 dollars. Significance refers to the estimated mean treatment effect.LOS is length-of-stay. CI is confidence interval.
The estimated effect on total direct hospital cost of moving a patient from the comparison arm to the treatment arm, holding all other values constant.
Savings in total direct hospital costs resulting from the consultation. Further details on how these savings were calculated are available in the Appendix (see Note 25 in text).
p < 0.01
Secondary Analyses
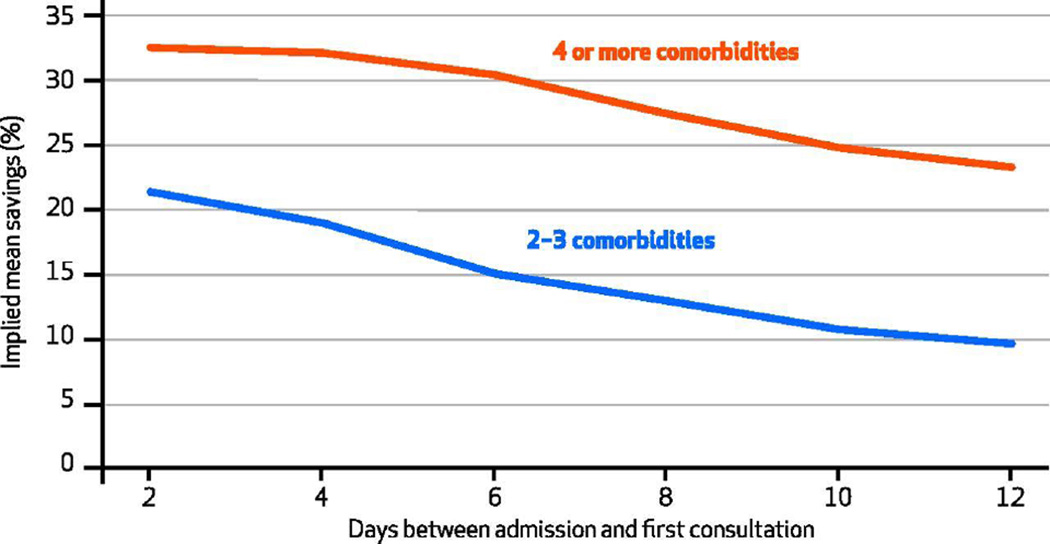
Elsewhere we have demonstrated a systematic relationship between time to consult and the palliative care consultation’s effect on cost.[22] To examine whether earlier palliative team treatment was associated with lower hospital costs among patients with differing levels of multimorbidity, we combined the two analytical approaches. We created subsamples defined both by number of comorbidities and by definitions of treatment according to time to consult. The results demonstrate a consistent pattern: Associations between higher patient comorbidity scores and cost-saving effect of the treatment and between earlier time to consultation and cost-saving effect were both robust (Exhibit 4). For any given definition of treatment according to timing, the cost-saving effect was larger for the group with higher comorbidity scores. And for either subsample defined by comorbidity score, the cost-saving effect was larger for earlier interventions.
Exhibit 4.
Estimated Effect Of Consultation With Palliative Care Consultation Team On Total Direct Hospital Cost For Patients With Advanced Cancer, By Elixhauser Cormorbidity Score At Admission And Timing Of Consultation
SOURCE Authors’ analysis. NOTES We regressed total direct costs as explained in Exhibit 3 Notes. Of the 906 matched patients, 98 had an advanced cancer that was not lymphoma, metastatic cancer, or a solid tumor. Of these, 49 (50 percent) had no comorbidity on the Elixhauser comorbidity scale and thus had an Elixhauser comorbidity score of 0, as explained in the text. The other 49 had an Elixhauser comorbidity score of 1–8 (median: 2), with their cancer diagnosis unaccounted for in the score.
To examine the underlying source of the observed cost-saving effect, we examined the treatment effect on hospital length-of-stay and major utilization categories. The results showed that the intervention was significantly associated with a reduction in laboratory costs, which was inferred to result from palliative care consultation’s reducing the number of patient tests, and shorter length-of-stay, which was inferred to result from patient discharge being expedited by discussions of goals of care. Both effects were larger for subsamples with higher comorbidity scores (see the Appendix).[25]
Confirmatory Analyses
Our results were robust to multiple sensitivity analyses: pooling of decedents with those discharged alive; removing high-cost utilization outliers; alternative approaches to intervention definition by timing; modeling outcomes with and without propensity score weights; and using length-of-stay to control for unobserved confounding (data not shown).
Discussion
Our results demonstrate that palliative care consultation is significantly associated with reduced direct hospital costs for advanced cancer patients with multimorbidity, and the average effect is larger for patients with higher comorbidity scores. Previous studies have estimated that the cost-saving effect of palliative care consultation for patients discharged from the hospital alive is in the range of 5–14 percent.[21] Thus, the magnitude of cost savings for patients with multimorbidity – 22% for a comorbidity score of two or three, 32% for a score of four or more - appears much larger than previously suggested. These savings result from a combination of reduced utilization during hospital stay and reduced length-of-stay.
This is the first study we are aware of to examine if the treatment effect of palliative care consultation varied by level of patient comorbidities. Our results have a number of potentially important implications in the policy context of care for patients with advanced cancer and multimorbidity.
Reforming Care For Patients With Multimorbidity
The long-term viability not only of government-funded health programs such as Medicaid and Medicare but also of the national health system overall depends on reforming the provision of care to patients with serious illness in a way that reduces costs without compromising quality and access.[39] One piece of this jigsaw puzzle is timely access to palliative care. Patterns of improved quality and reduced costs through coordinated patient-centered palliative care are already evident in the literature.[20, 35]
Our results demonstrate for the first time that the cost–effects of palliative care consultation teams are on average larger for patients with advanced cancer and higher comorbidity scores, compared to those with advanced cancer and lower comorbidity scores. This indicates that the cost-saving scope of hospital-based palliative care programs for patients with multimorbidity, who account for a disproportionate share of health care costs, may be larger than previously realized. Early palliative consultations on the sickest patients may help reverse two trends: the increasing use of unwanted aggressive end-of-life care observed in Medicare patients with advanced cancer and the increasing percentage of patients who use hospice for less than seven days.[11] While the cost-saving effect of palliative care consultation teams appears greatest for patients with higher comorbidity scores, the intervention may also be beneficial for both patient outcomes and in-hospital utilization among people with a single serious illness. The nonsignificant result for patients with a comorbidity score of 0–1 in Exhibit 3 may arise from a sample size issue within this subsample. Only twenty-eight patients in the treatment group had such low comorbidity scores, which may have contributed to the absence of a significant association in our analysis.
Workforce Allocation
There are demonstrable short- and long-term gaps in the hospice and palliative care workforce.[40] The projected level of future need is such that not all patients will be seen by specialists—who are already and will remain a scarce resource to be allocated in the most effective way. Our results strongly suggest that palliative care consultation teams are most likely to have an impact with patients who have higher numbers of co-occurring conditions. On the evidence-based assumption that patient and family outcomes are at least as good for advanced cancer patients with multimorbidity who receive palliative care as they are for those who do not, specialist palliative care would be most cost-effective with patients who have more comorbidities, and they should thus be prioritized.
Case For Increased Access To Palliative Care
Programs using palliative care consultation teams have rapidly expanded in recent years, and over 90 percent of medium-size to large hospitals in the United States now have a palliative team.[41] Yet in our primary analysis 25 percent of patients with an advanced cancer diagnosis and multimorbidity admitted to hospitals with well-established palliative care programs received a consultation with a palliative team within two days of admission (Exhibit 3). There is demonstrable scope for reducing costs and improving care through increased access to specialist palliative care for patients with advanced cancer and complex multimorbidity needs. Currently 35 percent of direct medical cancer costs in the United States are attributable to inpatient hospital stays, and these costs are expected to increase.[1]
Importance Of Screening At Admission And Early Intervention
Palliative care is increasingly available both earlier in the care trajectory than it was in the past and concurrent with curative care, with observable benefits.[33, 34, 42] We have shown elsewhere that delivering palliative care consultations earlier to patients with advanced cancer also brings economic benefits.[22]
There was no formal system for identifying or prioritizing potential palliative care patients on the basis of comorbidity in the consultation model we studied. Screening patients with advanced cancer for palliative care needs at hospital admission may facilitate early intervention, which our results suggest would maximize the cost impact.
Policy Implications
Translating a growing body of evidence on palliative care programs into improved care for seriously ill patients requires changes to policy. In addition to workforce allocation, areas requiring urgent attention include scaling up and disseminating successful models of provision of high-quality palliative care and the design of regulatory, accreditation, payment, and financing mechanisms that strengthen access.[43]
Our results relate only to the intervention’s impact on hospital costs. It is not clear how the use of palliative care consultation teams affects insurance expenditures, since the extent to which reduced hospital costs are passed on to payers varies by reimbursement system.[44] Palliative care provided early in a hospitalization may change the procedures performed and thus the Medicare Severity Diagnosis-Related Group, which could result in lower expenditures by Medicare (or other case-rate payers) for that case than would otherwise have been required.
Future Research
The fact that we found greater cost savings for cancer patients with more comorbidities than for those with fewer comorbidities raises the question of whether similar results would be observed in patients with other serious illnesses and multimorbidity. Further important extensions of this work will be to identify how costs and palliative care’s effects on costs vary for specific combinations of comorbidities and diagnoses, and to determine when in the course of illness specialist palliative care is most cost-effective. Finally, future studies should examine whether and how the cost-saving effect of inpatient palliative care consultation teams has an impact on payer expenditures.
Conclusion
Patients with multiple serious conditions account for a high proportion of US health care spending, and substantial health spending growth is projected over the next decade. Previous studies have established the clinical and financial benefits of palliative care, and our results supplement these studies by demonstrating that among patients with advanced cancer, the cost effect is greater for those with higher numbers of serious coexisting conditions. Increasing access to palliative care during hospitalization for patients with advanced cancer and multiple chronic conditions could improve care while complementing strategies to curb cost growth.
Acknowledgments
An early version of these results was presented at the Annual Assembly of the American Academy of Hospice and Palliative Medicine and the Hospice and Palliative Nurses Association , Philadelphia, Pennsylvania, February 26th, 2015. The study was funded by the National Cancer Institute (NCI) and the National Institute of Nursing Research (Project No. 5R01CA116227-04). The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All authors are independent of the study sponsors. Peter May is sponsored by a health economics fellowship from the Health Research Board of Ireland and by the NCI. Mellisa Garrido is supported by a Veterans Affairs Health Services Research & Development career development award (Award No. CDA 11-201/CDP 12-255). Amy Kelley was supported by the National Institute on Aging (NIA; Grant No. 1K23AG040774-01A1). Thomas Smith is supported by a grant from the NCI to the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University (Grant No. P30 CA 006973). Sean Morrison received a Midcareer Investigator Award in Patient-Oriented Research from the National Institutes of Health (Award No. 5K24AG022345) during the course of this work. This work was supported by the NIA, the Claude D. Pepper Older Americans Independence Center at the Icahn School of Medicine at Mount Sinai (Grant No. 5P30AG028741), and the National Palliative Care Research Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The authors thank Robert Arnold, Phil Santa Emma, Mary Beth Happ, Tim Smith, and David Weissman for their contributions to the ‘Palliative Care for Cancer’ project.
Notes
- 1.American Cancer Society. Cancer facts and figures 2015 [Internet] Atlanta (GA): ACS; [cited 2015 Nov 18]. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2.Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. The state of aging and health in America 2013 [Internet] Atlanta (GA): CDC; 2013. [cited 2015 Nov 18]. Available from: http://www.cdc.gov/features/agingandhealth/state_of_aging_and_health_in_america_2013.pdf. [Google Scholar]
- 4.Busse R, Blümel M, Scheller-Kreinsen D, Zentner A. Tackling chronic disease in Europe : strategies, interventions and challenges [Internet] Copenhagen: European Observatory on Health Systems and Policies; 2010. [cited 2015 Nov 18]. (Observatory Studies Series No. 20). Available from: http://www.euro.who.int/__data/assets/pdf_file/0008/96632/E93736.pdf. [Google Scholar]
- 5.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2014–2024 [Internet] Baltimore (MD): CMS; [cited 2015 Nov 18]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/proj2014.pdf. [Google Scholar]
- 6.Lochner KA, Goodman RA, Posner S, Parekh A. Multiple chronic conditions among Medicare beneficiaries: state-level variations in prevalence, utilization, and cost, 2011. Medicare Medicaid Res Rev. 2013;3(3) doi: 10.5600/mmrr.003.03.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisko AM, Keehan SP, Cuckler GA, Madison AJ, Smith SD, Wolfe CJ, et al. National health expenditure projections, 2013–23: faster growth expected with expanded coverage and improving economy. Health Aff (Millwood) 2014;33(10):1841–1850. doi: 10.1377/hlthaff.2014.0560. [DOI] [PubMed] [Google Scholar]
- 8.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, et al. Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387–420. doi: 10.1177/1077558711399580. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Dying in America: improving quality and honoring individual preferences near the end of life. Washington (DC): National Academies Press; 2014. [PubMed] [Google Scholar]
- 11.Teno JM, Gozalo PL, Bynum JP, Leland NE, Miller SC, Morden NE, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie CS, Zulman DM. Research priorities in geriatric palliative care: multimorbidity. J Palliat Med. 2013;16(8):843–847. doi: 10.1089/jpm.2013.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downey L, Au DH, Curtis JR, Engelberg RA. Life-sustaining treatment preferences: matches and mismatches between patients’ preferences and clinicians’ perceptions. J Pain Symptom Manage. 2013;46(1):9–19. doi: 10.1016/j.jpainsymman.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh TN, Kleerup EC, Wiley JF, Savitsky TD, Guse D, Garber BJ, et al. The frequency and cost of treatment perceived to be futile in critical care. JAMA Intern Med. 2013;173(20):1887–1894. doi: 10.1001/jamainternmed.2013.10261. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay J, Dooley M, Martin J, Fay M, Kearney A, Barras M. Reducing potentially inappropriate medications in palliative cancer patients: evidence to support deprescribing approaches. Support Care Cancer. 2014;22(4):1113–1119. doi: 10.1007/s00520-013-2098-7. [DOI] [PubMed] [Google Scholar]
- 17.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755. doi: 10.1056/NEJMra1404684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 20.Smith S, Brick A, O’Hara S, Normand C. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med. 2014;28(2):130–150. doi: 10.1177/0269216313493466. [DOI] [PubMed] [Google Scholar]
- 21.May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med. 2014;17(9):1054–1063. doi: 10.1089/jpm.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745–2752. doi: 10.1200/JCO.2014.60.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy IM, Robinson C, Huq S, Philastre M, Fine RL. Cost savings from palliative care teams and guidance for a financially viable palliative care program. Health Serv Res. 2015;50(1):217–236. doi: 10.1111/1475-6773.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701–1720. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To access the Appendix, click on the Appendix link in the box to the right of the article online
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital length of stay. J Palliat Med. 2010;13(6):761–767. doi: 10.1089/jpm.2009.0379. [DOI] [PubMed] [Google Scholar]
- 28.Green KM, Stuart EA. Examining moderation analyses in propensity score methods: application to depression and substance use. J Consult Clin Psychol. 2014;82(5):773–783. doi: 10.1037/a0036515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taheri PA, Butz D, Griffes LC, Morlock DR, Greenfield LJ. Physician impact on the total cost of care. Ann Surg. 2000;231(3):432–435. doi: 10.1097/00000658-200003000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood) 2011;30(3):454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 31.Morrison RS, Penrod JD, Cassel JB, Caust-Ellenbogen M, Litke A, Spragens L, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 32.Pritchard RS, Fisher ES, Teno JM, Sharp SM, Reding DJ, Knaus WA, et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46(10):1242–1250. doi: 10.1111/j.1532-5415.1998.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 33.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 35.Luckett T, Phillips J, Agar M, Virdun C, Green A, Davidson PM. Elements of effective palliative care models: a rapid review. BMC Health Serv Res. 2014;14:136. doi: 10.1186/1472-6963-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casarett D, Pickard A, Bailey FA, Ritchie C, Furman C, Rosenfeld K, et al. Do palliative consultations improve patient outcomes? J Am Geriatr Soc. 2008;56(4):593–599. doi: 10.1111/j.1532-5415.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 37.Joint Commission. Facts about the Advanced Certification Program for Palliative Care [Internet] Oakbrook Terrace (IL): Joint Commission; 2015. Apr 17, [cited 2015 Nov 18]. Available from: http://www.jointcommission.org/certification/certification_main.aspx. [Google Scholar]
- 38.National Consensus Project for Quality Palliative Care [home page on the Internet] Pittsburgh (PA): The Project; [cited 2015 Nov 18]. Available from: http://www.nationalconsensusproject.org/Default.aspx. [Google Scholar]
- 39.Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenker Y, Arnold R. The next era of palliative care. JAMA. 2015;314(15):1565–1566. doi: 10.1001/jama.2015.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med. 2015 Sep 29; doi: 10.1089/jpm.2015.0351. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakitas M, Tosteson T, Li Z, Lyons K, Hull J, Li Z, et al. The ENABLE III randomized controlled trial of concurrent palliative oncology care. Paper presented at: American Society of Clinical Oncology Annual Meeting; 2014 May 30–Jun 3; Chicago, IL. [Google Scholar]
- 43.Unroe KT, Meier DE. Research priorities in geriatric palliative care: policy initiatives. J Palliat Med. 2013;16(12):1503–1508. doi: 10.1089/jpm.2013.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassel JB, Kerr KM, Kalman NS, Smith TJ. The business case for palliative care: translating research Into program development in the U.S. J Pain Symptom Manage. 2015 Aug 20; doi: 10.1016/j.jpainsymman.2015.06.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 46.Chang VT, Hwang SS, Kasimis B, Thaler HT. Shorter symptom assessment instruments: The Condensed Memorial Symptom Assessment Scale (CMSAS) Cancer Invest. 2004;22:526–536. doi: 10.1081/cnv-200026487. [DOI] [PubMed] [Google Scholar]