Abstract
The interactive association of age and dopaminergic polymorphisms on cognitive function has been studied extensively. However, there is limited research on whether age interacts with the association between genetic polymorphisms and motor learning. We examined a group of young and older adults’ performance in three motor tasks: explicit sequence learning, visuomotor adaptation, and grooved pegboard. We assessed whether individuals’ motor learning and performance were associated with their age and genotypes. We selected three genetic polymorphisms: Catechol-O-Methyl Transferase (COMT val158met) and Dopamine D2 Receptor (DRD2 G > T), which are involved with dopaminergic regulation, and Brain Derived Neurotrophic Factor (BDNF val66met) that modulates neuroplasticity and has been shown to interact with dopaminergic genes. Although the underlying mechanisms of the function of these three genotypes are different, the high performance alleles of each have been linked to better learning and performance. We created a composite polygene score based on the Number of High Performance Alleles (NHPA) that each individual carried. We found several associations between genetic profile, motor performance, and sensorimotor adaptation. More importantly, we found that this association varies with age, task type, and engagement of implicit versus explicit learning processes.
Keywords: Aging, COMT, DRD2, BDNF, Sequence learning, Visuomotor adaptation
1. Introduction
Aging is associated with a variety of motor and cognitive declines, many of which have been linked to changes in corticostriatal function and dopaminergic transmission (Bäckman et al., 2006, 2000; Volkow et al., 1998). Several genetic polymorphisms have been identified which affect the dopaminergic metabolism pathway, including Catechol-O-Methyl Transferase (COMT), Dopamine D2 Receptor (DRD2), and Brain Derived Neurotrophic Factor (BDNF) polymorphisms (Savitz et al., 2006). COMT and DRD2 are directly linked to dopamine signaling (Jönsson et al., 1999; Meyer-Lindenberg et al., 2005; Stelzel et al., 2009). BDNF on the other hand, plays an important role in neuroplasticity and is indirectly involved in dopamine function (Egan et al., 2003; Guillin et al., 2001; Hünnerkopf et al., 2007; Hyman et al., 1991).
Previous work has shown that dopaminergic genotypes modulate the availability of dopamine in prefrontal and striatal regions, and are associated with varying levels of motor learning and performance. Joundi et al. (2012) investigated the association of BDNF with visuomotor processes and they found that carriers of the val-met genotype showed reduced rates of visuomotor adaptation during learning and the long term retention phase. However, their performance did not differ from the val-val genotype group at a retention test following a short delay. They also found more pronounced differences between the two genotype groups when they adapted to a larger deviation from the target, suggesting that BDNF genotype is associated with explicit processes of adaptation. Their findings further suggest that the association of BDNF with visuomotor adaptation is influenced by the task design (complexity and learning phase). Moreover, McHughen et al. (2011) provided evidence that the well-established effects of the BDNF val-met polymorphism in the early phase of motor learning, where val-met individuals show reduced learning, disappear with long term intense training. Their findings also support the notion that genotype associations with motor learning are influenced by task design (amount of practice).
We have recently reported that COMT val-val and DRD2 TT genotypes were associated with poorer performance in motor sequence learning for young adults (Noohi et al., 2014). We also observed that COMT val-val individuals exhibited slower visuomotor adaptation, however, there was no association of the DRD2 TT polymorphism with visuomotor adaptation. Thus, our findings are in line with previous reports of task specific associations between genetic polymorphisms and differing forms of motor learning and adaptation.
Several studies have shown declines in motor learning and performance with healthy aging (Gage et al., 1989; Kluger et al., 1997; Seidler et al., 2010). However, some older adults sustain learning and performance patterns equivalent to those of young adults (Albert et al., 1995; Dennis et al., 2007; Kattenstroth et al., 2010; Lustig et al., 2009); it is unclear what underlies this “successful aging” in some individuals. Flöel et al. (2005) provided evidence that dopamine levels modulate the rate of motor learning in healthy young and older adults. They showed that older adults with diminished motor memory improved significantly after receiving a single dose of oral l-Dopa. They suggested that individual differences in dopamine transmission could be used as an index of successful aging. These individual differences can be partly captured by an individual's genotype, and more specifically, genotypes that regulate dopamine function.
The role of dopaminergic genetic polymorphisms on performance in older adults has been studied extensively, although previous work has predominantly investigated cognitive rather than motor function. For example, Nagel et al. (2008b) showed that age magnifies associations between genotype and memory, and suggested that carriers of low-dopamine alleles have more pronounced deficits in learning and memory in older age. However, other studies have failed to replicate these age by gene interactions (see Appendix A for a summarized selection of studies that were/were not able to replicate these findings). Thus it is difficult to predict whether there might be age by gene interactions for associations between dopaminergic polymorphisms and motor skill learning. The studies reporting increasing genotype associations with behavior in advancing age (Lindenberger et al., 2008) suggest that young adults might engage some compensatory mechanisms to overcome the effects of “low dopamine” alleles, whereas for older adults general declines reduce the effectiveness of compensation (Cabeza et al., 2002; Collier et al., 2007; Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Cappell, 2008). However, this hypothesis remains to be critically evaluated.
Apart from previous animal studies (Boger et al., 2011; Chen et al., 2005; Li et al., 2010a, 2010b; Markowska and Breckler, 1999; Watanabe et al., 1991), only a few studies have investigated whether there is an age by genotype interaction in the motor domain for human subjects (Alcalay et al., 2014; Schuck et al., 2013). Schuck and colleagues showed that the interactive effect of dopaminergic genotypes (DAT VNTR and DARPP-32) and age is more pronounced in explicit components of motor sequence learning than implicit. Alcalay et al. (2014) assessed the association of a Parkinson's risk gene with motor and cognitive performance, and found that CH/H PARKIN carriers exhibited slower progression of Parkinson's disease and less motor and cognitive impairments than non-carriers. These suggest that motor learning and performance are influenced by age and genotype, however, it is not clear whether the results would hold across differing dopaminergic polymorphisms and a range of motor learning paradigms.
Given the evidence that motor sequence learning and sensorimotor adaptation rely on dopaminergic processes (Carbon et al., 2004; Joundi et al., 2012; Marinelli et al., 2009; Noohi et al., 2014; Tremblay et al., 2010), we investigated the association of COMT, DRD2, and BDNF polymorphisms with motor learning and performance in healthy young and older adults. To more robustly explain variations in dopamine modulation that links to an individual's behavior, we employed the polygene approach (David et al., 2013; De Quervain and Papassotiropoulos, 2006; Hamrefors et al., 2010; Lluís-Ganella et al., 2010; Nikolova et al., 2011; Noohi et al., 2014; Papenberg et al., 2013; Pearson-Fuhrhop et al., 2013); that is, we created a count score of the number of purportedly high performance alleles (i.e. alleles that have been previously linked to better performance in cognitive and motor tasks) that an individual carries across COMT, DRD2, and BDNF. We hypothesized that age-related declines in motor learning and performance would be associated with the presence of fewer “high performance alleles” in our three genes of interest. Given our findings with young adults (Noohi et al., 2014), we also predicted that these associations would vary between motor sequence learning and sensorimotor adaptation for both young and older adults.
2. Methods
2.1. Participants
Subjects were recruited from the University of Michigan student population and the National Institutes of Health Claude D. Pepper Older Americans Independence Center. Considering the effect of gender and ethnicity on genotype modulations (Barnett et al., 2007; Farrer et al., 1997; Garte, 1998; Kates et al., 2006; Laing et al., 2012), we limited our recruitment to females with Caucasian ethnicity. From a total of 142 individuals (72 YA, 70 OA) who participated in our study, those with a score of < 27 in the Mini Mental State Examination (MMSE), history of neurological disorders, contaminated DNA samples, or incomplete/missing data were excluded. The final sample size consisted of 68 young (21±1.9 yrs) and 63 older adults (71±4.9 yrs). We included “Estrogen therapy” as a covariate in our analyses as it has been shown to improve dopamine function in post-menopausal women (Duff and Hampson, 2000; Tsang et al., 2000; Yaffe et al., 1998). All participants signed a written informed consent form that was approved by the University of Michigan Institutional Review Board.
2.2. Genotyping
As described in our previous report (Noohi et al., 2014), we collected participants’ saliva samples with Oragene DNA self-collection kits. We identified the single nucleotide polymorphisms (SNPs) of COMT (rs4680), DRD2 (rs1076560), and BDNF (rs6265) genes for the provided DNA samples. Table 1 presents that the distribution of alleles for each gene within the two age groups was in accordance with Hardy-Weinberg equilibrium. Taking the COMT-met, DRD2-G, and BDNF-val alleles as the “high performance” alleles, we defined a polygene index representing the Number of High Performance Alleles (NHPA) that each individual carries. As depicted in Table 2, the total number of high performance alleles (i.e. NHPA score) can vary between 0 and 6. None of the subjects in our sample of young adults were carriers of 0 or 1 NHPA; only two older adult subjects were carriers of 0 (n=1) and 1 (n=1) NHPA. To keep the consistency of comparisons across the two age groups, and to avoid the confounding effects of outliers, we did not include these two subjects in the final analyses.
Table 1.
Frequency of allelic distribution for COMT, DRD2, and BDNF genes. The observed values represent the percentage of allelic distribution in our sample of young and older adults combined. The expected values represent the Hardy Weinberg equilibrium for the percentage of allelic distribution in a mixed gender/age population of European ethnicity.
| Gene | Allele | %Observed | %Expected | χ 2 | p-Value |
|---|---|---|---|---|---|
| COMT | MM | 26.7 | 30 | .316 | .574 |
| VM | 51.9 | 40 | 3.24 | .071 | |
| VV | 21.5 | 30 | 2.217 | .136 | |
| DRD2 | GG | 66.2 | 75 | 2.145 | .143 |
| GT | 28.8 | 23 | .999 | .317 | |
| TT | 5 | 2 | 1.409 | .223 | |
| BDNF | VV | 64.3 | 62 | .131 | .717 |
| VM | 27.1 | 35 | 1.701 | .192 | |
| MM | 8.6 | 3 | 3.09 | .078 |
Table 2.
Frequency of NHPA score in our sample of young adults, older adults, and young and old combined.
| NHPA | Frequency (%) YA | Frequency (%) OA | Frequency (%) YA+OA |
|---|---|---|---|
| 0 | 0 | 1.5 | .7 |
| 1 | 0 | 1.5 | .7 |
| 2 | 5.8 | 7.7 | 6.7 |
| 3 | 18.8 | 20.0 | 19.4 |
| 4 | 18.8 | 30.8 | 24.6 |
| 5 | 37.7 | 27.7 | 32.8 |
| 6 | 18.8 | 10.8 | 14.9 |
2.3. Sensorimotor tasks
2.3.1. Explicit sequence learning
Participants were in a seated position in front of the computer screen. We instructed the participants to use the manufactured key-box for responding bimanually (with right index and middle fingers, and left index and middle fingers) to the visual stimuli presented on the computer screen. The stimulus, letter X, popped up on the screen in a random (R) or sequential (S) pattern. We asked the participants to press one of the four keys on the key-box that corresponded to the position of letter X on the screen. For example, if letter X appeared on the far left side, the participants pressed the farthest left key. Prior to the beginning of each block, a notification message appeared on the screen that explicitly informed the participant whether the upcoming block would be random or sequential. A sequential trial consisted of a pattern of eight elements, with no repeat of the same element in a row (i.e. 2,2,2...), no runs (i.e. 1,2,3...) and no trills (i.e. 1,2,1,2...). Throughout the experiment, participants performed 6 sequence blocks within which there were 96 trials of the same pattern of the eight elements. Therefore, they were able to gradually anticipate the upcoming position of the letter X in sequence blocks and respond faster. The random trials were structured similarly, except that there was no pattern to learn, and the letter X popped up in an arbitrary fashion. The random and sequential blocks appeared in the following order: R1, R2, S3, S4, R5, S6, S7, R8, S9, S10, R11. A learning index was calculated as the difference between median reaction time in each sequence block and its subsequent random block. Therefore, the extent of learning in early, middle, and late phase was calculated based on the difference of median reaction time for “S4&R5”, “S7&R8”, “S10&R11”; respectively. Similarly, the error-learning extent (i.e. the difference in accuracy between random and sequence blocks) in early, middle, and late phase was calculated based on the difference in accuracy level (i.e. average number of errors in each block) for “S4&R5”, “S7&R8”, “S10&R11”; respectively.
2.3.2. Visuomotor adaptation
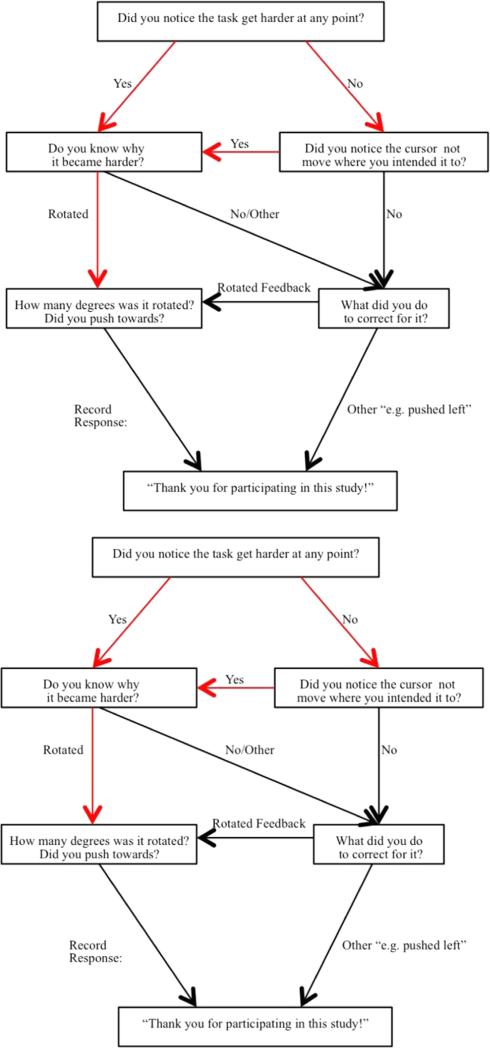
As described at length in our previous works (Anguera et al., 2009; Benson et al., 2011) participants were instructed to respond to the visual stimuli appearing on the computer screen by moving a Logitech Extreme 3D joystick. The goal of the task was to hit the target (i.e. a red circle with .8 cm diameter) as quickly and accurately as possible. The participants controlled the joystick with their dominant hand and moved the cursor from the start point (i.e. center of the screen) to hit the target that appeared randomly in four different positions: above, below, left, or right of the start point. After hitting the target, they held the joystick until the target disappeared and the cursor automatically returned to the start point. This task consisted of 14 blocks, within each there were 24 trials. At the beginning of block 3, we applied a 30° clockwise rotation to the cursor feedback (Mazzoni and Krakauer, 2006; Seidler, 2004). Participants received no notification about this distortion in the visual feedback. Therefore, they gradually adapted their trajectory to hit the target. We calculated the Direction Error (DE) based on the angle between two lines: (1) the line representing the participants’ movement trajectory at the maximum velocity, and (2) the line connecting the start point to the target. The feedback distortion existed only in blocks 3–12. The last two blocks, 13 and 14, served as washout blocks when the visual feedback returned to veridical (same as blocks 1 and 2). We calculated the rate of visuomotor adjustment as the exponential decay of DE across adaptation blocks. Moreover, we polled participants regarding their explicit awareness of the visual distortion to evaluate differences in strategies (Benson et al., 2011); as depicted in Appendix B, after the completion of the task subjects responded to the questionnaire that indicated whether they had explicit awareness of the feedback rotation. We further assessed the possible correlation between subject's strategy and performance with regard to age and NHPA scores.
2.3.3. Grooved peg board test
The Grooved pegboard test is designed to provide a basic measure of individual's motor speed (Merker and Podell, 2011). Participants were instructed to pick up one peg at a time from the peg pool and place it in the holes as fast as possible. Pegs needed to be rotated between the thumb and the index finger to fit the alignment of the holes. The proper placement of the pegs required skillful eye-hand coordination and fine motor control. Participants performed this task once with the right hand and once with the left hand. We evaluated participants’ performance based on the time they needed to complete the task (i.e. place all the pegs in the holes).
3. Results
The pattern of results for single genotypes was generally consistent with previously reported effects for COMT and DRD2 polymorphisms. Given this, and the fact that we (Noohi et al., 2014) and others (Pearson-Fuhrhop et al., 2013) have combined across these genes to compute a “number of high performance alleles” score (NHPA), we do not present the single polymorphism results here, but only focus on NHPA effects.
Similar to some previous reports (Barton et al., 2014; Erickson et al., 2008; Gajewski et al., 2011; Getzmann et al., 2013), we found paradoxical results for the BDNF met allele in relation to behavior. That is, we found that BDNF met homozygotes performed superior to the val allele carriers in the sequence learning task. Therefore, in addition to the conventional analyses, we performed an exploratory post hoc analysis for the sequence-learning task (results are presented in Appendix C) with the BDNF met allele coded as the high performance allele (i.e. reverse coding).
Unless otherwise specified, we conducted a series of non-parametric mixed model ANOVAs to find the amount of variance that the NHPA factor contributes to motor learning for young and older adults. Bonferroni correction was applied to adjust for multiple comparisons. The Mann-Whitney U non-parametric test was used for Post-hoc pairwise comparisons.
3.1. Explicit sequence learning
3.1.1. Reaction time
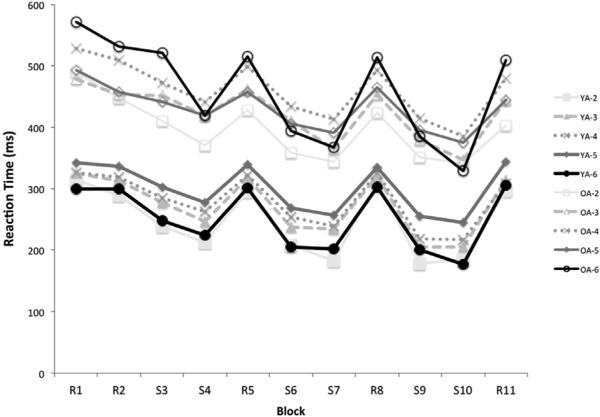
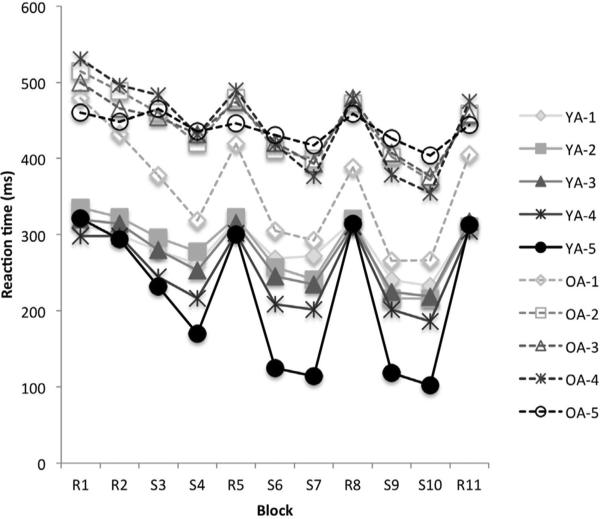
The results (Fig. 1) showed that there was a main effect of age (F1,1320=732.98, p=.0001), block (F10,1320=7.21, p=.0001), NHPA score (F5,1320=5.01, p=.0001), and a significant interaction between age and NHPA score (F4,1320=3.02, p=.017) on reaction time. The post hoc analyses revealed that the main effect of age was caused by overall significantly slower reaction time of OA compared to YA (MWU=283, Z= −8.56, p=.0001).
Fig. 1.
Mean Reaction Time across 11 blocks based on the NHPA score in young and older adult groups. R: random blocks, S: sequence blocks.
Also, the age magnification effect on genotype association was evident, as there was no significant effect of NHPA level on performance for YA; the NHPA-related differential performance emerged only in the OA group (resulting in an age*NHPA interaction): Carriers of NHPA=4 showed significantly slower reaction time than carriers of NHPA=2 (MWU=3831, z= −4.2, p=.0001), NHPA=3 (MWU=11694, z= −4.13, p=.0001); and NHPA=5 (MWU=18352, z= −2.7, p=.005). Carriers of NHPA=6 were also significantly slower than NHPA=2 (MWU=1225, z= −3.06, p=.002). The other pairwise comparisons showed no significant difference across NHPA levels (p's > .05).
3.1.2. Learning extent
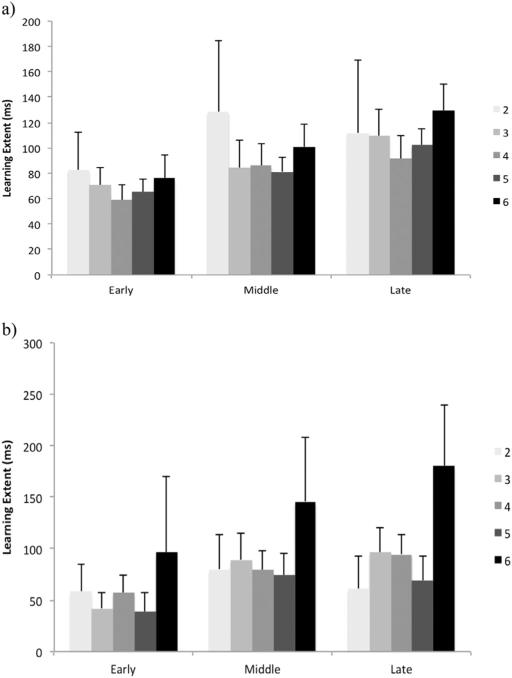
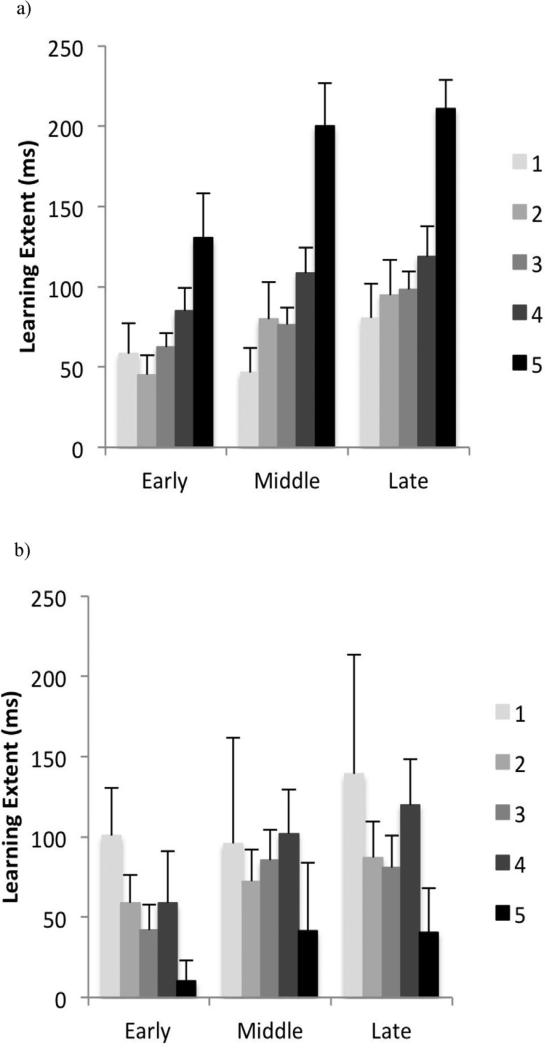
There was no significant main effect of age or NHPA score on learning extent at each stage. Also we found no significant interaction between these factors (Fig. 2).
Fig. 2.
Learning extent in early, middle and late phases based on the NHPA score in young (a) and older (b) adults groups. The error bars represent the standard error of the mean.
3.1.3. Accuracy
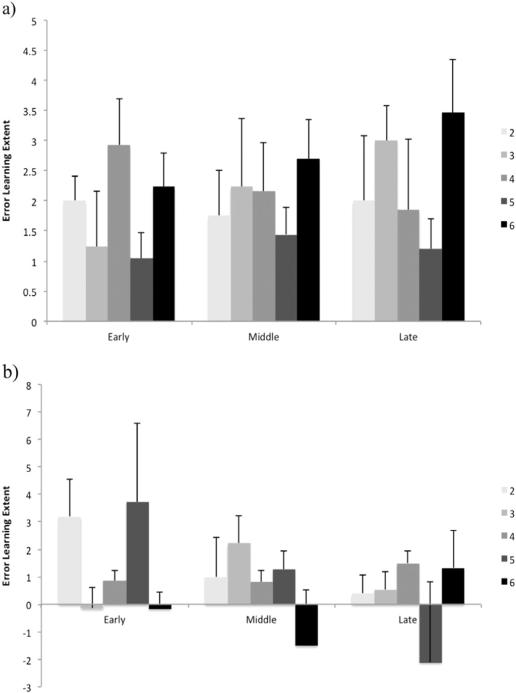
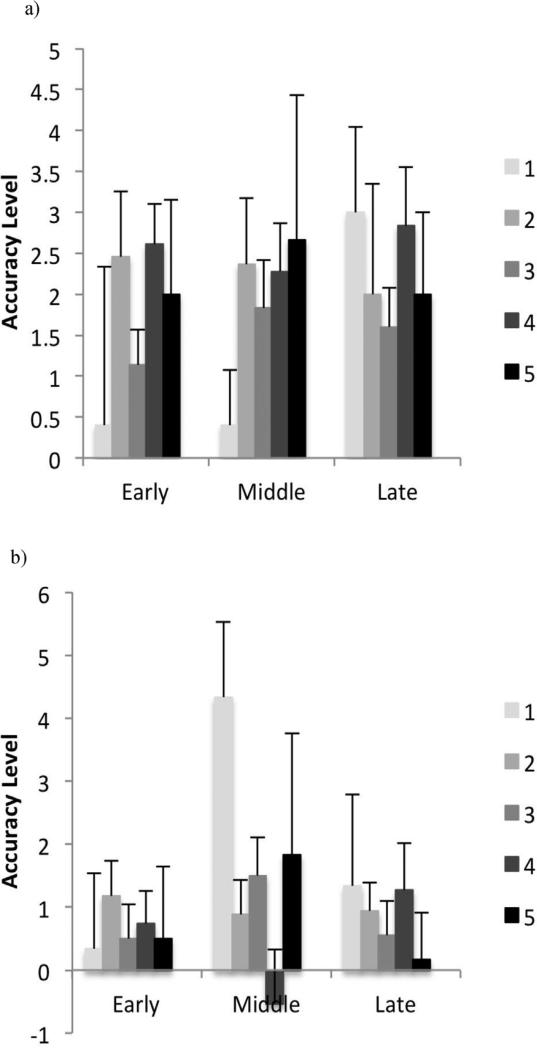
Fig. 3 shows the error learning extent in early, middle, and late phase, for young and older adult groups separately. There was only a main effect of age in the middle stage (F1,120=4.54, p=.035), suggesting a higher rate of learning in young adults (MWU=1691, z= −1.96, p=.50).
Fig. 3.
Extent of error learning based on the NHPA score in young (a) and older (b) adult groups. The error learning extent represents the difference in number of errors made in sequence blocks and their subsequent random blocks (i.e. a positive score reflects fewer errors in sequence blocks compared to random blocks, indicating a higher magnitude of learning). Error bars show the standard error of the mean.
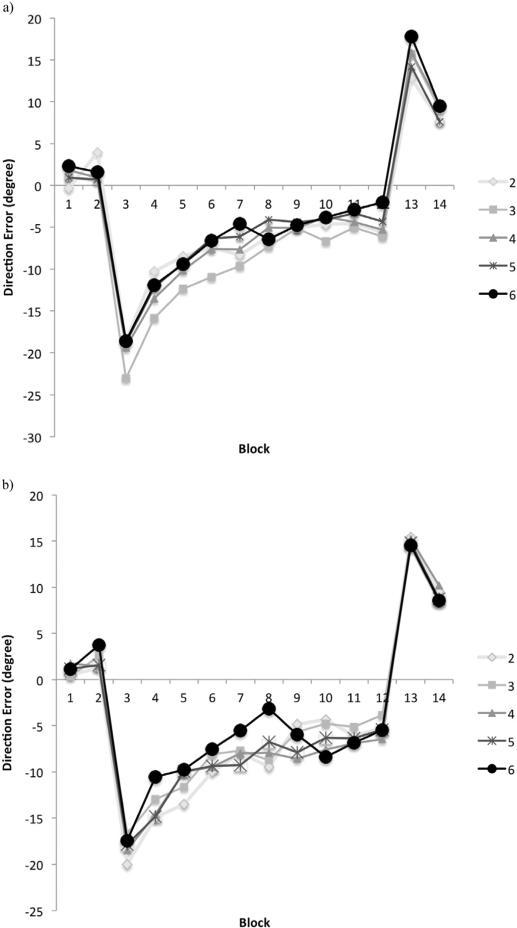
3.2. Visuomotor adaptation
As depicted in Fig. 4a and b, there was a general decay in the average direction error in adaptation blocks (blocks 3–12) for both young and older adult groups with practice, regardless of their NHPA score. This indicated that all participants showed some level of adaptation over time and their performance improved towards the end of the task. Moreover, there was a main effect of age (F1,1210=13.03, p=.0001), block (F9,1210=42.98, p=.0001), NHPA score (F61210=24.22, p=.0001), and an NHPA by age interaction (F4,1210=9.79, p=.0001). The post hoc analyses revealed that the average direction error of YA with an NHPA score of 3 was significantly higher than for YA with an NHPA score of 5 (MWU=70, Z= −2.94, p=.003) and 6 (MWU=30, Z= −2.79, p=.005) (Fig. 4a). The other pairwise comparisons showed no significant difference across NHPA levels (p's > .05).
Fig. 4.
Mean direction error across 14 blocks, based on the NHPA score for young (a) and older (b) adult groups.
The average direction error between different NHPA groups was not significantly different in OA (Fig. 4b). Therefore, the age by NHPA interaction was driven by YA who showed benefits of higher NHPA scores. Moreover, the post hoc analysis showed that the main effect of age was caused by overall better performance of YA compared to OA (MWU=1596, Z= −2.5, p=.012).
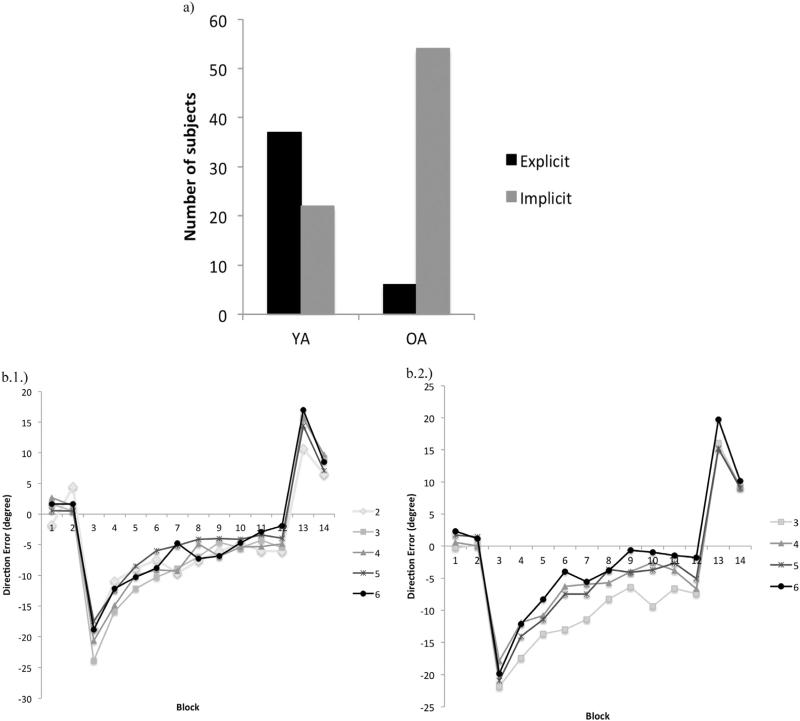
Further, we assessed whether subjects’ strategies in the adaptation blocks were associated with their age and/or NHPA score. As depicted in Fig. 5a the results revealed a significant effect of age (F1=39.25, p=.0001) on subjects’ strategy, but no main effect of NHPA score was found. The young adults were more likely to exhibit explicit awareness (i.e. noticing the change in visual feedback) in the debriefing questionnaire (see Appendix B) than older adults.
Fig. 5.
Number of subjects who exhibited explicit versus implicit awareness on the debriefing questionnaire after the completion of the task, based on age (a). NHPA association with performance in visuomotor adaptation task in young adults with explicit (b1) and implicit (b2) awareness. None of the subjects with NHPA=2 exhibited implicit awareness; therefore the comparison for implicit awareness is shown for NHPA scores=3, 4, 5, and 6. Note: Subjects were considered to have some explicit awareness if their answers followed the red path showed in Appendix B.
Next, we assessed the influence of strategy on performance with regard to age and NHPA. The results (Fig. 5b1 and 2) showed that there was a significant NHPA effect on average direction error in YA who relied on implicit strategies, suggesting that YA with NHPA scores of 6 performed significantly better than YA with NHPA scores of 3 (MWU=1, Z= −2.02, p=.04); these differences were not significant in YA who relied more on explicit strategies for adapting to the visual distortion. The other pairwise comparisons showed no significant difference across NHPA levels (p's > .05).
The same analyses for the OA group did not reveal any significant effect of strategies on average direction error, and the results did not vary across NHPA groups.
3.3. Grooved pegboard
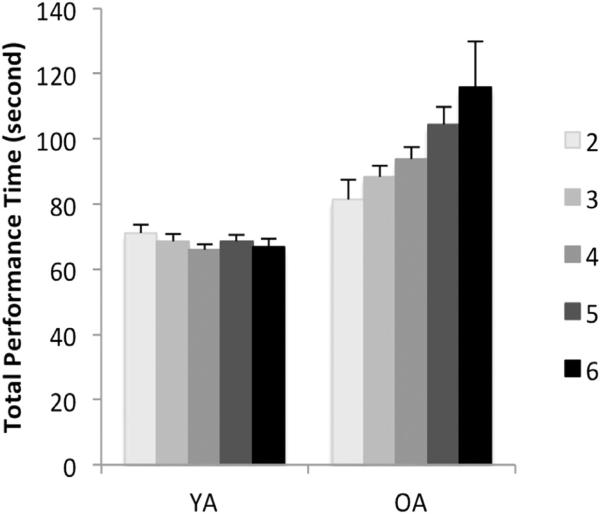
We found a main effect of age (right: F1=82.19, p=.0001; left: F1=88.16, p=.0001), and NHPA score (right: F6=2.32, p=.03; left: F6=2.28, p=.04) in both right and left hand performance. A significant interaction between age and NHPA score was only evident in left hand performance (F4=2.52, p=.04) (Fig. 6). The post hoc analyses revealed that the main effect of age was driven by the OA group performing significantly slower than the YA group (right: MWU=367.5, Z= −8.67, p=.0001; left: MWU=320.5, Z= −8.76, p=.0001). Also, the interaction of age with NHPA score was caused by significantly slower performance of those with NHPA score of 5 compared to 2 (left: MWU=16, Z= −2.07, p=.039) only in OA group. The other pairwise comparisons showed no significant difference across NHPA levels (p's > .05).
Fig. 6.
Left hand performance in Grooved pegboard test, categorized based on NHPA score for young and older adult groups. Error bars reflect the standard error of the mean.
The young adults’ performance was not influenced by their NHPA score (F4=.40, p=.8), which supports that the NHPA*age interaction was driven by the older adults’ performance.
Considering the general pattern of results (summarized in Table 3), we additionally assessed whether the best performers in our sample of older adults are the youngest individuals in this group. We analyzed the age distribution in the older adult group with regard to the allelic distribution of COMT, DRD2 and BDNF genotypes, as well as the NHPA score. We found no significant age distribution differences among older adults across different genotype groups and NHPA scores (Table 4).
Table 3.
Summary of results. N.S: non significant.
| Motor learning paradigm | Age effect | NHPA effect | Age by NHPA interactive effect |
|---|---|---|---|
| Sequence learning | |||
| Reaction time | OA slower than YA | Only in OA | OA with NHPA=4 slower than NHPA=2,3,5 and OA with NHPA=6 slower than NHPA=2 |
| Learning extent | N.S | N.S | N.S |
| Error learning extent | OA lower magnitude of error learning | N.S | N.S |
| Visuomotor adaptation | |||
| Direction error | OA higher rate of direction error | Only in YA | YA with NHPA= 3 higher direction error than NHPA= 5,6 |
| Explicit versus implicit strategy | OA: more implicit/YA: more explicit | Only in YA with implicit strategy | YA with NHPA= 3 higher direction error than NHPA= 6 |
| Grooved pegboard | |||
| Performance time | OA slower performance (both hands) | Only in OA left hand performance | OA with NHPA=5 slower than NHPA=2 |
Table 4.
Mean age in older adult group based on allelic distribution of COMT, DRD2 and BDNF genotypes, and NHPA score. There was only one subject with NHPA=0 and one subject with NHPA=1. Therefore, they were not included in the analyses.
| Allele | Mean age in OA group | Standard deviation | |
|---|---|---|---|
| COMT | mm | 71 | 4.43 |
| vm | 72 | 5.33 | |
| vv | 70 | 4.38 | |
| DRD2 | GG | 72 | 4.30 |
| GT | 71 | 6.10 | |
| TT | 68 | 3.26 | |
| BDNF | vv | 71 | 4.23 |
| vm | 73 | 5.22 | |
| mm | 69 | 5.51 | |
| NHPA | 2 | 70 | 3.98 |
| 3 | 72 | 5.88 | |
| 4 | 71 | 4.98 | |
| 5 | 73 | 4.63 | |
| 6 | 73 | 0 |
4. Discussion
We hypothesized that genetic polymorphisms of COMT, DRD2 and BDNF genes would be associated with motor learning and performance in healthy young and older adults. We modeled individuals’ polygene scores based on the number of high performance alleles (NHPA) across these genes, and hypothesized that carriers of fewer NHPAs would show systematically poorer motor learning and performance, potentially depending on age and task structure.
We found that NHPA scores are predictive of motor performance and adaptation in a manner that is influenced by both age and task structure. Motor performance in a sequence learning task and for the grooved pegboard test was influenced by NHPA score only in OA; where higher NHPA scores were associated with poorer performance. In contrast, performance in the visuomotor adaptation task was affected by NHPA score only in YA; where higher NHPA scores were associated with faster visuomotor adaptation, particularly for YA who remained unaware of the perturbation. Although the results of the sequence learning and grooved pegboard tests indicated that genotype associations were amplified by age, the results of the visuomotor adaptation task did not support this notion.
Lindenberger et al. (2008) have proposed that age changes in cognitive function can be related to the inverted U shaped relationship between performance and dopamine levels. Our findings in the sequence learning task and grooved pegboard test are partially in line with this premise, in that age amplified the association of genotype and motor performance (i.e. an NHPA effect was only evident for OA). However, the non-linear pattern of NHPA effects that we observed did not fit the inverted U relationship of dopamine level and performance in older adults; that is, lower NHPA scores were associated with better performance. Similar inconsistencies are reported in a recent review by Floresco (2013), suggesting a “family of functions” that influence the relationship between dopamine level and performance. The author reviewed the results of a battery of cognitive tasks (e.g. working memory, set shifting, risk/reward decision making) in animal and human studies, across which low and high mesocortical dopamine levels showed different relationships with performance (e.g. sigmoidal, exponential, biphasic, etc.). This “family of functions” varied based on the specific dopamine receptors and targeted neural pathways across different studies. The age-related reversal effects of NHPA in our results also varied based on the specific dopaminergic pathways engaged for the particular tasks (only observed in explicit sequence learning task and grooved pegboard test, not in the visuomotor adaptation task). Moreover, considering the collective effect of the three genes that comprise the NHPA factor on dopamine regulation (i.e. interactive effects of COMT, DRD2, and BDNF across different task structures that rely on different neural networks, which also vary by age), our findings support the complex model of “family of functions”, but in the motor domain.
Other studies have demonstrated age differences in task performance strategies, which may have contributed to our results. For example, Schuck and colleagues assessed the performance of young and older adults in a sequence learning task and showed that age declines in performance were related to the explicit component of the task, and not the implicit. Moreover, they showed that dopaminergic genotypes (DAT and DARPP-32) were associated with this age-related decline in explicit sequence learning, but not the implicit component (Schuck et al., 2013). We have also previously reported age differences in strategies and mechanisms of visuomotor adaptation; young adults engage spatial working memory processes and the right dorsolateral pre-frontal cortex, while older adults do not (Anguera et al., 2010, 2011). These age differences in learning processes may have contributed to the age by NHPA interactions that we observed here. Interestingly, the association between NHPA and adaptation rate for young adults was only observed for participants that remained implicit regarding the visual perturbation. This suggests that the slow, implicit processes of adaptation are at least partially mediated by dopaminergic transmission.
There is a literature on compensatory processes in older adults (Reuter-Lorenz and Lustig, 2005; Seidler et al., 2010), but it is not clear whether similar forms of compensation are in place for lower NHPAs. For example, Rieckmann and Bäckman (2009) have suggested that older adults rely on medial temporal brain regions during implicit learning to overcome age declines in striatal function. In a study of young adults, Jaspar et al. (2014) found that those homozygous for the COMT val allele exhibited greater frontal and temporal lobe activity and connectivity during performance of the Stroop interference resolution task. The authors suggest that this pattern of activity/connectivity allows these individuals to compensate for lower dopamine availability. Interestingly, a recent study found that older adults, and COMT val allele carriers (regardless of age), exhibit reduced efficiency of the dorsolateral prefrontal cortex (DLPFC) during a working memory task (Nyberg et al., 2014). That is, older adults and COMT val allele carriers both exhibited greater DLPFC activation at lower task demands, and then had a reduced range of activity with increasing task demands. It is difficult to draw a direct parallel between that study and the current one however, because Nyberg et al. (2014) did not observe age by genotype interactions in either working memory performance or DLPFC activation levels. Nevertheless, our finding that NHPAs interact with sensorimotor adaptation differentially in young adults who gain explicit awareness versus those that do not supports the notion of specific compensatory mechanisms. That is, young adults who reported explicit awareness of the visuomotor distortion did not exhibit varying performance based on NHPA status.
5. Potential caveats
Although we controlled for the potential confounds of estrogen supplementation in the older adults sample, it was behind the scope of this study to take into account many other contributing factors to dopaminergic modulation, such as serotonergic geno-types (Olvera-Cortés et al., 2008). In addition to our small sample size, this study is also limited in that the findings cannot be generalized to males and other ethnicities.
6. Conclusion
Our findings suggest that an individual's genetic profile predicts their motor performance and sensorimotor adaptation. More importantly, we found that this prediction varies based on age, and the specific implicit and explicit mechanisms involved. The significance of our findings can be further perceived considering the recent review by Howard and Howard, (2013): While the evidence for spared implicit processes in healthy aging is emerging throughout the literature, they reported implicit probabilistic sequence learning to be an exception. Their report highlights the role of individual differences (and in particular dopaminergic genotypes) and task structure as the main contributing factors that need to be taken into account for studying implicit and explicit learning processes in older adults. Here, we showed that the number of high performance alleles (NHPA) across COMT, DRD2, and BDNF genes differentially predicts the performance of young and older adults in implicit and explicit processes. Further, our findings raise additional questions about the relationship between dopamine levels and performance in older adults, supporting the previous reports on process-specific relationships instead of a universal inverted U shaped model (Floresco, 2013). A greater understanding of these relationships may further elucidate the underlying causes of age declines in sensorimotor function, and could highlight new avenues for interventions.
Acknowledgments
We thank Dr. David T. Burke and Joshua West for their assistance in genotype analysis and Katie Handely for assistance in literature review. This work was supported by a pilot grant (to R.D. Seidler) from the University of Michigan, National Institutes of Health, and Claude D. Pepper Older Americans Independence Center Grant AG-024824.
Appendix A
Selection of articles that provided evidence in support of (Table A1) or in contrast with (Table A2) the age-magnifying theory.
Table A1.
Selection of articles that are inline with previously reported age-magnifying effect of genotypes.
| Title | Authors/Year | Journal | Genotypes | Tasks/cognitive measures | Main finding | Sample size |
|---|---|---|---|---|---|---|
| Aging magnifies the effects of dopamine transporter and D2 receptor genes on backward serial memory | Li et al. (2013) | Neurobiology of Aging | DAT, DRD2 | Backward serial recall | Aging magnifies effects of DAT and DRD2 genes, and gene*gene interaction in the frontostriatal circuitry | 1288 (545 M, 743 F) |
| Human aging magnifies genetic effects on executive functioning and working memory | Nagel et al. (2008a) | Frontiers in Human Neuroscience | COMT, BDNF | Spatial working memory; Wisconsin card sorting | Effects on cognitive performance of the COMT genotype were magnified by age | 318 (188 M, 130 F) |
| The genetic impact (C957T-DRD2) on inhibitory control is magnified by aging | Colzato et al. (2013) | Neuropyschologia | DRD2 | Stop signal, Raven Standard Progressive Matrices | Aging magnifies effect of DRD2 gene on inhibitory control | 170 (62 M, 108 F) |
| COMT genotype and cognitive function: an 8-year longitudinal study in white and black elders | Fiocco et al. (2010) | Neurology | COMT | The Modified Mini-mental State Examination, The Digit Symbol Substitution Test | Aging magnifies the effect of COMT gene | 2858 (gender not specified) |
| Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults | De Frias et al. (2005) | Journal of Cognitive Neuroscience | COMT | Verbal fluency, working memory, Tower of Hanoi | Aging magnifies the effects of COMT gene, but only in val/val group | 292 (all M) |
| Functional COMT polymorphism, Val158Met, is associated with Logical Memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds | Harris et al. (2005) | Neuroscience Letters | COMT | Goldberg's IPIP 50-item personality trait questionnaire, Logical Memory Test | Age magnifies the COMT genotype effect in favor of Val/Met heterozygotes | 460 (272 F, 188 M) |
| Effects of age, genes, and pulse pressure on executive functions in healthy adults | Raz et al. (2011) | Neurobiology of Aging | COMT | Wisconsin Card Sorting Test; Working memory: n-back test, Size Judgment Span Test | Aging magnifies the effect of COMT, more prominently in men | 158 (45 M, 113 F) |
| Dopaminergic gene polymorphisms affect long-term forgetting in old age: further support for the Magnification Hypothesis | Papenberg et al. (2013) | Journal of Cognitive Neuroscience | DRD2, DRD3, DAT1 | Recognition Memory Task | Aging magnifies the effect of the DRD2, DRD3, DAT1 | 1228 (518 M, 710 F) |
| Effects of aging and dopamine genotypes on the emergence of explicit memory during sequence learning | Schuck et al. (2013) | Neuropsychologia | DARPP-32, DAT | Implicit and explicit sequence learning task | Aging magnifies the effect of the DARPP-32 and DAT | 150 (77 M, 73 F) |
| Ebbinghaus Revisited: influences of the BDNF Val66Met polymorphism on backward serial recall are modulated by human Aging | Li et al. (2010a, 2010b) | Journal of Cognitive Neuroscience | BDNF | Backward serial-recall task | Age magnifies the effect of BDNF | 948 (481 M, 467 F) |
Table A2.
Selection of articles that are NOT inline with previously reported age-magnifying effect of genotypes (or found no genotype effect in elderly).
| Title | Authors/Year | Journal | Genotypes | Tasks/cognitive measures | Main finding | Sample size |
|---|---|---|---|---|---|---|
| Meta-analysis of the cognitive effects of the catechol-O-Methyltransferase gene Val158/108Met polymorphism | Barnett et al. (2008) | Biological Psychiatry | COMT | Trail Making task, verbal recall, verbal fluency, Wisconsin card Sorting Test | No association between COMT genotype and the cognitive function | 46 studies |
| Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms | Erickson et al. (2008) | Frontiers in Human Neuroscience | COMT, BDNF | Task Switching, Modified Mini-mental State Examination | Aging does not change the COMT effect, while it magnifies the BDNF effect (only in val allele carriers) | 53 (14 M, 39 F) |
| COMT genotype, gender and cognition in community-dwelling, older adults | O'Hara et al. (2006) | Neuroscience Letters | COMT | Symbol-Digit Modalities Test, Wechsler Memory Scale-Revised, Stroop, backwards digit span, Boston Naming Test, logical memory test | COMT effect on cognition only emerged in interaction with gender (not age) | 163 (62 M, 101 F) |
| APOE genotype and cognitive functioning in a large age-stratified population sample. | Jorm et al. (2007) | Neuropsychology | APOE E4 carriers | Episodic memory, working memory, mental speed, reaction time, and reading vocabulary | Age did not change the effect of APOE E4 on cognition | 7485 (3675 M, 3810 F) |
| COMT Val 108/158Met genotype affects neural but not cognitive processing in healthy individuals | Dennis et al. (2010) | Cerebral Cortex | COMT, BDNF | Cantab battery, Attix battery | Age did not change the COMT association with cognition | 3536 (1614 M, 1922 F) |
| Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936 | Houlihan et al. (2009) | Genes, Brain, Behavior | BDNF, COMT, DISC1, KL, NCSTN, PPP1R1B, PRNP, SHANK3, SORL1, WRN | Verbal reasoning, backward digit span, spatial span, verbal paired associates from the Wechsler memory scale | Age did not change the genotypes association with cognition | 1031 (519 M, 512 F) |
| The COMT Val158Met polymorphism and cognition in depressed and nondepressed older adults | Potter et al. (2009) | International Journal of Geriatric Psychiatry | COMT | Trail Making Test, Symbol-Digit Modalities Test, forward and backward Digit Span, Controlled Oral Word Association Test, immediate and delayed recall from Wechsler Memory Scale | No COMT effect on cognitive performance in healthy elderly | 105 healthy, 126 patients |
Appendix B
Debriefing questionnaire for visuomotor adaptation task. Subjects responded to this questionnaire after the completion of the task. The lines indicate the path of increasingly specific questions about the task. Subjects were considered to have some explicit awareness if their answers followed the red path.
Appendix C
The additional exploratory post-hoc analyses for sequence learning task, with reverse coded BDNF in NHPA score.
C.1. Reaction time
The results (Fig. C1) showed that there was a main effect of age (F1,1320=345.92, p=.0001), block (F10,1320=11.57, p=.0001), reverse coded NHPA score (F5,1320=3.77, p=.002), and a significant interaction between age and reverse coded NHPA score (F5,1320=6.31, p=.0001) on reaction time. The post hoc analyses revealed that the main effect of age was caused by overall significantly slower reaction time for OA versus YA (MWU=318, Z= −8.78, p=.0001). Further analyses showed that the age*reverse coded NHPA interaction was driven by the significantly slower reaction time of YA with reverse coded NHPA=1 compared to reverse coded NHPA=5 (MWU=.0001, Z= −2.23, p=.025) in sequence learning blocks. The same analysis showed that there was a strong trend in the 0A group, suggesting that those with reverse coded NHPA scores of 5 performed slower than reverse coded NHPA scores of 1 (MWU=2, Z= −1.80, p=.07).
C.2. Learning extent
As depicted in Fig. C2, the extent of learning in all three phases didn't vary based on age or reverse coded NHPA score.
C3. Accuracy
As shown in Fig. C3, the error learning extent in early, middle, and late phase did not vary based on age and reverse coded NHPA score.
Fig. C1.
Mean Reaction Time across 11 blocks based on the NHPA score (reverse coded for BDNF) in young and older adult groups.
Fig. C2.
Learning extent in early, middle and late phases based on the reverse coded NHPA score (1–5) in young (a) and older (b) adults groups. The error bars represent the standard error of the mean.
Fig. C3.
Extent of error learning based on the reverse coded NHPA score in young (a) and older (b) adult groups. The accuracy level represents the difference in number of errors made in sequence blocks and their subsequent random blocks. Error bars show the standard error of the mean.
References
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol. Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, Ruiz D, Louis ED, Comella CL, Nance MA, Bressman SB, Scott WK, Tanner CM, Mickel SF, Waters CH, Fahn S, Cote LJ, Frucht SJ, Ford B, Rezak M, Novak KE, Friedman JH, Pfeiffer RF, Marsh L, Hiner B, Payami H, Molho E, Factor SA, Nutt JG, Serrano C, Arroyo M, Ottman R, Pauciulo MW, Nichols WC, Clark LN, Marder KS. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol. 2014;71:62–67. doi: 10.1001/jamaneurol.2013.4498. http://dx.doi.org/10.1001/jamaneurol.2013.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Seidler RD, Gehring WJ. Changes in performance monitoring during sensorimotor adaptation. J. Neurophysiol. 2009;102:1868–1879. doi: 10.1152/jn.00063.2009. http://dx.doi.org/10.1152/jn.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J. Cogn. Neurosci. 2010;22:1917–1930. doi: 10.1162/jocn.2009.21351. http://dx.doi.org/10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J. Cogn. Neurosci. 2011;23:11–25. doi: 10.1162/jocn.2010.21451. http://dx.doi.org/10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci. Biobehav. Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. http://dx.doi.org/10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am. J. Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. http://dx.doi.org/10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Ring SM, Golding J, Goldman D, Xu K, Jones PB. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am. J. Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. http://dx.doi.org/10.1176/appi.ajp.164.1.142. [DOI] [PubMed] [Google Scholar]
- Barton B, Treister A, Humphrey M, Abedi G, Cramer SC, Brewer AA. Paradoxical visuomotor adaptation to reversed visual input is predicted by BDNF Val66Met polymorphism. J. Vis. 2014;14 doi: 10.1167/14.9.4. http://dx.doi.org/10.1167/14.9.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J. Neurophysiol. 2011;105:2843–2851. doi: 10.1152/jn.00002.2011. http://dx.doi.org/10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Mannangatti P, Samuvel DJ, Saylor AJ, Bender TS, McGinty JF, Fortress AM, Zaman V, Huang P, Middaugh LD, Randall PK, Jayanthi LD, Rohrer B, Helke KL, Granholm A-C, Ramamoorthy S. Effects of brain-derived neurotrophic factor on dopaminergic function and motor behavior during aging. Genes Brain Behav. 2011;10:186–198. doi: 10.1111/j.1601-183X.2010.00654.x. http://dx.doi.org/10.1111/j.1601-183X.2010.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004;21:1497–1507. doi: 10.1016/j.neuroimage.2003.12.014. http://dx.doi.org/10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J. Biol. Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. http://dx.doi. org/10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RAE, Sladek JR, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman Primates: diminished compensatory mechanisms as a prelude to Parkinsonism. Neurobiol. Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. http://dx.doi.org/10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B. The genetic impact (C957T-DRD2) on inhibitory control is magnified by aging. Neuropsychologia. 2013;51:1377–1381. doi: 10.1016/j.neuropsychologia.2013.01.014. http://dx.doi.org/10.1016/j.neuropsychologia.2013.01.014. [DOI] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, Bergen AW, Swan GE, Benowitz NL, Tyndale RF, Conti DV, Brown RA, Lerman C, Niaura R. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction. 2013;108:2202–2211. doi: 10.1111/add.12325. http://dx.doi.org/10.1111/add.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson L-G. Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J. Cogn. Neurosci. 2005;17:1018–1025. doi: 10.1162/0898929054475136. http://dx.doi.org/10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ-F, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc. Natl. Acad. Sci. USA. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. http://dx.doi.org/10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging. 2007;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. http://dx.doi.org/10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters-Metenier S, Cirulli ET, Kragel J, Goldstein DB, Cabeza R. COMT val108/158 Met genotype affects neural but not cognitive processing in healthy individuals. Cereb. Cortex. 2010;20:672–683. doi: 10.1093/cercor/bhp132. http://dx.doi.org/10.1093/cercor/bhp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm. Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. http://dx.doi.org/10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function : a 10-year longitudinal study of COMT and BDNF polymorphisms. Front. Hum. Neurosci. 2008;2:1–9. doi: 10.3389/neuro.09.011.2008. http://dx.doi.org/10.3389/neuro.09.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease Meta analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fiocco AJ, Lindquist K, Ferrell R, Li R, Simonsick EM, Nalls M, Harris TB, Yaffe K. COMT genotype and cognitive function: an 8-year longitudinal study in white and black elders. Neurology. 2010;74:1296–1302. doi: 10.1212/WNL.0b013e3181d9edba. http://dx.doi.org/10.1212/WNL.0b013e3181d9edba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann. Neurol. 2005;58:121–130. doi: 10.1002/ana.20536. http://dx.doi.org/10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front. Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. http://dx.doi. org/10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol. Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The Met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiol. Aging. 2011;32 doi: 10.1016/j.neurobiolaging.2011.06.010. http://dx.doi.org/10.1016/j.neurobiolaging.2011.06.010 2327.e7-19. [DOI] [PubMed] [Google Scholar]
- Garte S. The role of ethnicity in cancer susceptibility gene polymorphisms: the example of CYP1A1. Carcinogenesis. 1998;19:1329–1332. doi: 10.1093/carcin/19.8.1329. [DOI] [PubMed] [Google Scholar]
- Getzmann S, Gajewski PD, Hengstler JG, Falkenstein M, Beste C. BDNF Val66Met polymorphism and goal-directed behavior in healthy elderly-evidence from auditory distraction. Neuroimage. 2013;64:290–298. doi: 10.1016/j.neuroimage.2012.08.079. http://dx.doi.org/10.1016/j.neuroimage.2012.08.079. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. http://dx.doi.org/10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hünnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP. Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–2560. doi: 10.1038/sj.npp.1301383. http://dx.doi.org/10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- Hamrefors V, Orho-Melander M, Krauss RM, Hedblad B, Almgren P, Berglund G, Melander O. A gene score of nine LDL and HDL regulating genes is associated with fluvastatin-induced cholesterol changes in women. J. Lipid Res. 2010;51:625–634. doi: 10.1194/jlr.P001792. http://dx.doi.org/10.1194/jlr.P001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The functional COMT polymorphism, Val 158 Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci. Lett. 2005;385:1–6. doi: 10.1016/j.neulet.2005.04.104. http://dx.doi.org/10.1016/j.neulet.2005.04.104. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Harris SE, Luciano M, Gow AJ, Starr JM, Visscher PM, Deary IJ. Replication study of candidate genes for cognitive abilities: the Lothian birth cohort 1936. Genes Brain Behav. 2009;8:238–247. doi: 10.1111/j.1601-183X.2008.00470.x. http://dx.doi.org/10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- Howard JH, Howard DV. Aging mind and brain: is implicit learning spared in healthy aging? Front. Psychol. 2013;4:817. doi: 10.3389/fpsyg.2013.00817. http://dx.doi.org/10.3389/fpsyg.2013.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. http://dx.doi.org/10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Jaspar M, Genon S, Muto V, Meyer C, Manard M, Dideberg V, Bours V, Salmon E, Maquet P, Collette F. Modulating effect of COMT genotype on the brain regions underlying proactive control process during inhibition. Cortex. 2014;50:148–161. doi: 10.1016/j.cortex.2013.06.003. http://dx.doi.org/10.1016/j.cortex.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. http://dx.doi.org/10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Joundi RA, Lopez-Alonso V, Lago A, Brittain J-S, Fernandez-Del-Olmo M, Gomez-Garre P, Mir P, Jenkinson N, Cheeran B, Brown P. The effect of BDNF val66met polymorphism on visuomotor adaptation. Exp. Brain Res. 2012;223:43–50. doi: 10.1007/s00221-012-3239-9. http://dx.doi.org/10.1007/s00221-012-3239-9. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, Higgins AM, Kucherlapati R, Shprintzen RJ. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome). Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:274–280. doi: 10.1002/ajmg.b.30284. http://dx.doi.org/10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenstroth J-C, Kolankowska I, Kalisch T, Dinse HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front. Aging Neurosci. 2010;2:31. doi: 10.3389/fnagi.2010.00031. http://dx.doi.org/10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, Ferris SH, George AE, Franssen E, Reisberg B. Patterns of motor impairement in normal aging, mild cognitive decline, and early Alzheimer's disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 1997;52B:P28–P39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- Laing KR, Mitchell D, Wersching H, Czira ME, Berger K, Baune BT. Brain-derived neurotrophic factor (BDNF) gene: a gender-specific role in cognitive function during normal cognitive aging of the MEMO-study? Age (Dordr.) 2012;34:1011–1022. doi: 10.1007/s11357-011-9275-8. http://dx.doi.org/10.1007/s11357-011-9275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Chicherio C, Nyberg L, von Oertzen T, Nagel IE, Papenberg G, Sander T, Heekeren HR, Lindenberger U, Bäckman L. Ebbinghaus revisited: influences of the BDNF Val66Met polymorphism on backward serial recall are modulated by human aging. J. Cogn. Neurosci. 2010a;22:2164–2173. doi: 10.1162/jocn.2009.21374. http://dx.doi.org/10.1162/jocn.2009.21374. [DOI] [PubMed] [Google Scholar]
- Li S-C, Papenberg G, Nagel IE, Preuschhof C, Schröder J, Nietfeld W, Bertram L, Heekeren HR, Lindenberger U, Bäckman L. Aging magnifies the effects of dopamine transporter and D2 receptor genes on backward serial memory. Neurobiol. Aging. 2013;34 doi: 10.1016/j.neurobiolaging.2012.08.001. http://dx.doi.org/10.1016/j.neurobiolaging.2012.08.001 358.e1-10. [DOI] [PubMed] [Google Scholar]
- Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. J. Neurosci. 2010b;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. http://dx.doi.org/10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li S-C, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front. Neurosci. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. http://dx.doi.org/10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluís-Ganella C, Lucas G, Subirana I, Sentí M, Jimenez-Conde J, Marrugat J, Tomás M, Elosua R. Additive effects of multiple genetic variants on the risk of coronary artery disease. Rev. Esp. Cardiol. 2010;63:925–933. doi: 10.1016/s1885-5857(10)70186-9. http://dx.doi.org/10.1016/S1885-5857(10)70186-9. [DOI] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler RD, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychol. Rev. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. http://dx.doi.org/10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Parkinsonism Relat. Disord. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. http://dx.doi.org/10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Breckler SJ. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B549–B566. doi: 10.1093/gerona/54.12.b549. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J. Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Exp. Brain Res. 2011;213:415–422. doi: 10.1007/s00221-011-2791-z. http://dx.doi.org/10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- Merker B, Podell K. Grooved Pegboard Test. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. Springer; New York, NY: 2011. pp. 1176–1178. http://dx.doi.org/10.1007/978-0-387-79948-3. [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat. Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. http://dx.doi.org/10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S, Von Oertzen T, Sander T. Human aging magnifi es genetic effects on executive functioning and working memory. Front. Hum. Neurosci. 2008a;2:1–8. doi: 10.3389/neuro.09.001.2008. http://dx.doi.org/10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S-C, von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Front. Hum. Neurosci. 2008b;2:1. doi: 10.3389/neuro.09.001.2008. http://dx.doi.org/10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsycho-pharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. http://dx.doi.org/10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noohi F, Boyden NB, Kwak Y, Humfleet J, Burke DT, Müller MLTM, Bohnen NI, Seidler RD. Association of COMT val158met and DRD2 G > T genetic polymorphisms with individual differences in motor learning and performance in female young adults. J. Neurophysiol. 2014;111:628–640. doi: 10.1152/jn.00457.2013. http://dx.doi.org/10.1152/jn.00457.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Andersson M, Kauppi K, Lundquist A, Persson J, Pudas S, Nilsson L-G. Age-related and genetic modulation of frontal cortex efficiency. J. Cogn. Neurosci. 2014;26:746–754. doi: 10.1162/jocn_a_00521. http://dx.doi.org/10.1162/jocn_a_00521. [DOI] [PubMed] [Google Scholar]
- O'Hara R, Miller E, Liao C-P, Way N, Lin X, Hallmayer J. COMT genotype, gender and cognition in community-dwelling, older adults. Neurosci. Lett. 2006;409:205–209. doi: 10.1016/j.neulet.2006.09.047. http://dx.doi.org/10.1016/j.neulet.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera-Cortés ME, Anguiano-Rodríguez P, López-Vázquez MA, Alfaro JMC. Serotonin/dopamine interaction in learning. Prog. Brain Res. 2008;172:567–602. doi: 10.1016/S0079-6123(08)00927-8. http://dx.doi.org/10.1016/S0079-6123(08)00927-8. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schröder J, Bertram L, Heekeren HR, Lindenberger U, Li S-C. Dopaminergic gene polymorphisms affect long-term forgetting in old age: further support for the magnification hypothesis. J. Cogn. Neurosci. 2013;25:571–579. doi: 10.1162/jocn_a_00359. http://dx.doi.org/10.1162/jocn_a_00359. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. http://dx.doi.org/10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Minton B, Acevedo D, Shahbaba B, Cramer SC. Genetic variation in the human brain dopamine system influences motor learning and its modulation by l-dopa. PLoS One. 2013;8:e61197. doi: 10.1371/journal.pone.0061197. http://dx.doi.org/10.1371/journal.pone.0061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Taylor WD, McQuoid DR, Steffens DC, Welsh-Bohmer KA, Krishnan KRR. The COMT Val158Met polymorphism and cognition in depressed and nondepressed older adults. Int. J. Geriatr. Psychiatry. 2009;24:1127–1133. doi: 10.1002/gps.2235. http://dx.doi.org/10.1002/gps.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiol. Aging. 2011;32:1124–1137. doi: 10.1016/j.neurobiolaging.2009.05.015. http://dx.doi.org/10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. http://dx.doi.org/10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17:177–182. http://dx.doi.org/10.1111/j.1467-8721.2008.00570.x. [Google Scholar]
- Rieckmann A, Bäckman L. Implicit learning in aging: extant patterns and new directions. Neuropsychol. Rev. 2009;19:490–503. doi: 10.1007/s11065-009-9117-y. http://dx.doi.org/10.1007/s11065-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. http://dx.doi.org/10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Schuck NW, Frensch PA, Schjeide B-MM, Schröder J, Bertram L, Li S-C. Effects of aging and dopamine genotypes on the emergence of explicit memory during sequence learning. Neuropsychologia. 2013;51:2757–2769. doi: 10.1016/j.neuropsychologia.2013.09.009. http://dx.doi.org/10.1016/j.neuropsychologia.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Seidler RD. Multiple motor learning experiences enhance motor adaptability. J. Cogn. Neurosci. 2004;16:65–73. doi: 10.1162/089892904322755566. http://dx.doi.org/10.1162/089892904322755566. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. http://dx.doi.org/10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Effects of dopamine-related gene-gene interactions on working memory component processes. Eur. J. Neurosci. 2009;29:1056–1063. doi: 10.1111/j.1460-9568.2009.06647.x. http://dx.doi.org/10.1111/j.1460-9568.2009.06647.x. [DOI] [PubMed] [Google Scholar]
- Tremblay P-L, Bedard M-A, Langlois D, Blanchet PJ, Lemay M, Parent M. Movement chunking during sequence learning is a dopamine-dependant process: a study conducted in Parkinson's disease. Exp. Brain Res. 2010;205:375–385. doi: 10.1007/s00221-010-2372-6. http://dx.doi.org/10.1007/s00221-010-2372-6. [DOI] [PubMed] [Google Scholar]
- Tsang K-L, Ho S-L, Lo S-K. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology. 2000;54:2292–2298. doi: 10.1212/wnl.54.12.2292. http://dx.doi.org/10.1212/WNL.54.12.2292. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohta H, Imamura L, Asakura W, Matoba Y, Matsumoto K. Effect of Panax ginseng on age-related Changes in the spontaneous motor activity and dopaminergic nervous system in the rat. Jpn. J. Pharmacol. 1991;55:51–56. doi: 10.1254/jjp.55.51. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women. JAMA. 1998;279:688. doi: 10.1001/jama.279.9.688. http://dx.doi.org/10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]