Abstract
Background:
National Surgical Adjuvant Breast and Bowel Project R-04 was designed to determine whether the oral fluoropyrimidine capecitabine could be substituted for continuous infusion 5-FU in the curative setting of stage II/III rectal cancer during neoadjuvant radiation therapy and whether the addition of oxaliplatin could further enhance the activity of fluoropyrimidine-sensitized radiation.
Methods:
Patients with clinical stage II or III rectal cancer undergoing preoperative radiation were randomly assigned to one of four chemotherapy regimens in a 2x2 design: CVI 5-FU or oral capecitabine with or without oxaliplatin. The primary endpoint was local-regional tumor control. Time-to-event endpoint distributions were estimated using the Kaplan-Meier method. Hazard ratios were estimated from Cox proportional hazard models. All statistical tests were two-sided.
Results:
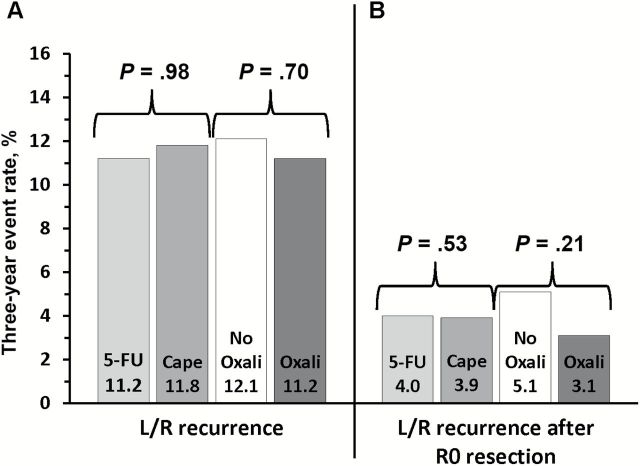
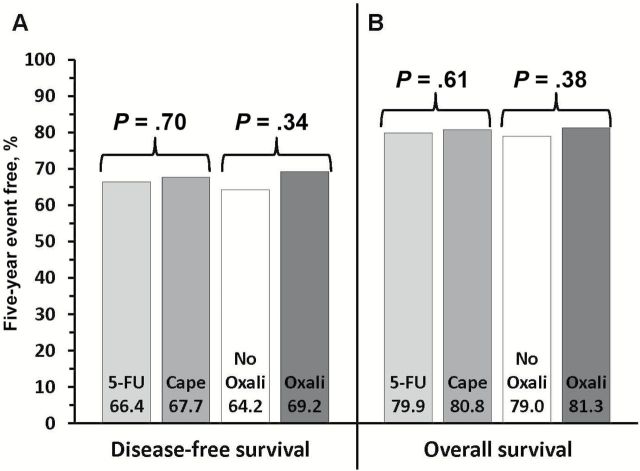
Among 1608 randomized patients there were no statistically significant differences between regimens using 5-FU vs capecitabine in three-year local-regional tumor event rates (11.2% vs 11.8%), 5-year DFS (66.4% vs 67.7%), or 5-year OS (79.9% vs 80.8%); or for oxaliplatin vs no oxaliplatin for the three endpoints of local-regional events, DFS, and OS (11.2% vs 12.1%, 69.2% vs 64.2%, and 81.3% vs 79.0%). The addition of oxaliplatin was associated with statistically significantly more overall and grade 3–4 diarrhea (P < .0001). Three-year rates of local-regional recurrence among patients who underwent R0 resection ranged from 3.1 to 5.1% depending on the study arm.
Conclusions:
Continuous infusion 5-FU produced outcomes for local-regional control, DFS, and OS similar to those obtained with oral capecitabine combined with radiation. This study establishes capecitabine as a standard of care in the pre-operative rectal setting. Oxaliplatin did not improve the local-regional failure rate, DFS, or OS for any patient risk group but did add considerable toxicity.
Rectal cancer accounts for almost one-third of all large bowel cancers and occurs in approximately 40 000 patients annually in the United States (1). For patients with stage II and III rectal cancer, the present standard of care is neoadjuvant chemo-radiotherapy followed by surgical intervention and adjuvant chemotherapy. Continuous infusion 5-fluorouracil (5-FU) was shown to be superior to bolus 5-FU in a study published by O’Connell and colleagues in 1994, and the use of infusion 5-FU has since remained the gold standard for radiation sensitization in the neoadjuvant rectal setting (2). Presumably, continuous infusion 5-FU allows for optimal intracellular accumulation and maintenance of the key anabolites of 5-FU responsible for radiation sensitization.
Capecitabine is an oral 5-FU prodrug that was found to have activity similar to that of intravenous 5-FU in patients with metastatic colorectal cancer and was approved for this indication by the FDA in 2001 (3,4). It was subsequently approved in 2005 for the adjuvant treatment of patients with stage III colon cancer based on the X-ACT trial, which demonstrated noninferiority compared with the bolus intravenous administration of 5-FU in this patient population (5). In addition to its more convenient oral route of administration, capecitabine is activated initially through hepatic metabolism and finally to 5-FU at the level of the cancer cell through the action of thymidine phosphorylase, also known as platelet-derived growth factor, which is expressed at higher levels in cancer cells than in the surrounding normal tissues (6,7). Twice-daily administration assures continuous exposure to 5-FU at the level of the cancer cell, with potentially greater radiation sensitization compared with the normal surrounding tissues by virtue of the differences in the levels of the thymidine phosphorylase-activating enzyme. It has also been found that radiation itself upregulates expression levels of thymidine phosphorylase, suggesting a further mechanism for a greater-than-additive interaction between radiation and capecitabine (8). The National Surgical Adjuvant Breast and Bowel Project (NSABP) R-04 trial was designed to address whether oral capecitabine, when given along with radiation therapy, could be substituted for the continuous infusion of intravenous 5-FU in patients with stage II and III rectal cancer in the neoadjuvant setting.
R-04 also addressed whether the addition of oxaliplatin could further enhance the activity of fluoropyrimidine-sensitized radiation therapy in the neoadjuvant rectal setting. Despite the advantages of neoadjuvant chemoradiation, local-regional recurrence rates reported in recent rectal investigations remain in the 5% to 11% range (9–12). Oxaliplatin has been shown to have radiation-sensitizing properties in preclinical model systems and has been found to enhance the activity of 5-FU in both the palliative setting in chemotherapy-naïve and previously treated patients with colon or rectal cancer and in the adjuvant setting in patients with lymph node–positive colon cancer (13–20). Both NSABP C-07 and the European MOSAIC trials showed that the addition of oxaliplatin to leucovorin-modulated 5-FU resulted in significant clinical benefit in patients with colon cancer treated in the adjuvant setting (19,20).
Methods
Patient Eligibility
NSABP R-04 (NCT00058474) (21) was approved by local human investigations committees or institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services. Written informed patient consent was required.
This was a multi-institution clinical trial with participating institutions located predominantly in the United States. Patients were required to be at least 18 years old with an ECOG performance score of 0–1 and a life expectancy of five years, excluding their rectal cancer diagnosis. The diagnosis of adenocarcinoma of the rectum must have been established by a biopsy technique that left the major portion of the tumor intact within 42 days before random assignment. The distal border of the tumor was required to be fewer than 12cm from the anal verge, and the tumor had to be palpable by digital rectal exam or accessible via proctoscope or sigmoidoscope. It also had to be clinically (by transrectal ultrasonography and CT scan or MRI) stage II (T3-4N0) or stage III (T1-4N1-2), with a positive node defined as being at least 1.0cm in diameter on imaging). There must have been no evidence of metastatic disease on physical examination, chest x-ray, or CT scan of the abdomen and pelvis. If technically feasible, a complete colonoscopic examination was performed; otherwise a proctoscopic or sigmoidoscopic examination was performed. Following surgery, MRI or CT scans were required every 12 months for two years, and proctoscopy or sigmoidoscopy annually for five years. Satisfactory hematologic parameters, liver function tests, and renal function tests were required. Patients with nonmalignant systemic disease, which would preclude safe administration of therapy or prescribed follow-up, were excluded, as were patients with a recent myocardial infarction (6 months), active inflammatory bowel disease, preexisting peripheral neuropathy, or previous pelvic radiation for any reason. The tumor had to be considered amenable to curative resection by the surgeon, and there could be no evidence of pelvic sidewall involvement on imaging studies. Before random assignment, the investigator had to specify whether a sphincter-sparing operation was feasible or whether non-sphincter-sparing surgery would be required.
Random Assignment and Treatment
Patients were stratified by institution, sex, intended operative procedure (sphincter-saving surgery or non-sphincter-saving surgery), and clinical tumor stage (stage II [T 3-4 N0] or stage III [T 1-4 N 1-2]). They were randomly assigned to treatment groups using the NSABP biased-coin minimization algorithm (22).
Radiotherapy
Radiotherapy was delivered at 180 cGy per day, five days per week, for a total of 25 fractions over five weeks, a total dose of 4500 cGy to the large pelvic field. A minimum boost of 540 cGy (given over 3 days in 180 cGY fractions) was required for patients with T3 nonfixed cancer and nondistal tumors (total cumulative dose, 5040 cGy, including large pelvic fields). For patients with T4-fixed cancer and/or distal rectal tumors, a boost dose of 1080 cGy (given over 3 days in 360 cGy fractions) was required (total cumulative dose of 5580 cGy, including large pelvic fields). We performed central review for the first two patients entered from each institution as a quality assurance measure.
Chemotherapy + Radiotherapy
NSABP R-04 was activated in July 2004, and patients were randomly assigned to receive radiation therapy + 5-FU (Group 1) or radiation therapy + capecitabine (Group 2). For Group 1, 225mg/m2 per day 5-FU was delivered by continuous intravenous infusion, seven days a week beginning the day of the start of radiation therapy and ending the evening of the last dose of radiation therapy. Patients in Group 2 received capecitabine 825mg/m2 po bid throughout the course of radiation therapy, seven days a week beginning the day of the start of radiation therapy and ending with the last dose of radiation therapy.
Protocol Amendment
In October 2005, the protocol was amended to add oxaliplatin (50mg/m2 IV weekly x 5 during radiation therapy), creating a 2 x 2 factorial design with four treatment groups: radiation therapy + 5-FU (Group 3), radiation therapy + 5-FU + oxaliplatin (Group 4), radiation therapy + capecitabine (Group 5), and radiation therapy + capecitabine + oxaliplatin (Group 6). The daily dose of chemotherapy remained the same, but the number of days of capecitabine and 5-FU treatment was reduced on all four arms from seven days a week to five, with administration of chemotherapy only on days of planned radiation therapy to reduce the incidence of severe diarrhea.
Surgery
The protocol required surgery to be performed within six to eight weeks after the completion of radiation therapy. Adequacy of surgery was based on pathologic review of each patient. The pathologic examination followed the January 2005 College of American Pathologists (CAP) protocol for invasive carcinomas of the colon and rectum.
Endpoints
The primary endpoint of this study was local-regional tumor control at three years. Events for local-regional control include local and regional recurrence subsequent to surgery. Local recurrence was defined as an anastomotic or pelvic recurrence, and regional recurrence included pelvic or retroperitoneal lymph nodes at or below the L5 level.
Additionally, incidents of inadequate resection (unclear margin or gross residual disease) were considered events at the time of surgery; failing to have surgery at the time one should have had surgery with a normal course of treatment was considered an event, except that those who withdrew consent early were censored; patients with documented clinical complete response were followed for recurrence even if they had no surgery. A patient was considered an early consent withdrawal if he or she withdrew consent fewer than 56 days after the end of protocol therapy or within 91 days of random assignment if he or she had not begun protocol therapy.
The secondary endpoints of this study were overall survival (OS), disease-free survival (DFS), and time to loco-regional recurrence (TLRR). Events for OS included death from any cause. Events for DFS included all events for the primary endpoint of local-regional control, plus death and second primary cancer. TLRR was only evaluated in patients who had an R0 resection. Events for TLRR included local and regional recurrence. Time for all endpoints was measured from random assignment except for TLRR, which was measured from surgery.
Statistical Methods
This trial used a two-by-two factorial design with patients randomly assigned to fluoropyrimidine therapy of 5-FU or capecitabine while concurrently randomly assigned to receive oxaliplatin or not. Analyses were intent-to-treat, with patients allocated to their randomly assigned therapy regardless of therapy actually received. As prespecified in the protocol, ineligible patients were excluded from analysis. The primary analysis used the stratified log-rank test (23) to compare local-regional control between the fluoropyrimidine treatments, stratifying for sex, stage, intended surgery, and oxaliplatin group (yes, no, prior to amendment). Similarly, the primary oxaliplatin-to-none comparison was stratified for type of fluoropyrimidine as well as for sex, stage, and intended surgery, although patients randomly assigned before the amendment introducing the oxaliplatin were omitted. Similar techniques were used for the other time-to-event endpoints.
The distributions of time-to-event endpoints were estimated using the Kaplan-Meier method (24). Hazard ratios (HRs) were estimated from Cox proportional hazard models (25) that included stratification factors. All P values are two-sided, and a cutoff of .05 was used for statistical significance for all tests except the primary comparison of the fluoropyrimidine, which was prespecified to conclude equivalence if the Z-score associated with the stratified log-rank test fell between +/- 0.842 (roughly equivalent to HRs of 0.89-1.12 or P values > .40 two-sided). This design had a 5% probability (alpha) of concluding equivalence if the true hazard ratio for fluoropyrimidine was 0.7 or 1.43, based on the observed 192 events. The power to conclude equivalence for a true hazard ratio of 1.0 was 60%. The power to conclude superiority of oxaliplatin vs no oxaliplatin was 80% for a true hazard ratio of 0.64, based on the observed 157 events.
Results
Patient Demographics
From July 2004 to August 2010, a total 1608 patients were accrued to R-04, and 1595 (99.2%) were eligible for analysis; 13 ineligible patients were excluded (Figure 1 [CONSORT]). Age, sex, clinical stage, and surgical intent were well balanced across the four arms of the study (Table 1). Of note, fewer patients were randomly assigned to the oxaliplatin-containing arms as a result of the amendment adding the oxaliplatin question, which occurred after the trial had been open for accrual to the other arms for more than one year.
Figure 1.
CONSORT diagram: National Surgical Adjuvant Breast and Bowel Project protocol R-04. *All patients were assigned radiation therapy. †Includes pre- and post-amendment patients. ‡Post-amendment patients. FU = 5-fluorouracil; CAPE = capecitabine; OX = oxaliplatin.
Table 1.
Clinical characteristics and patient assignment: National Surgical Adjuvant Breast and Bowel Project R-04*
| Variable | Pre-amendment | Post-amendment | ||||
|---|---|---|---|---|---|---|
| 5-FU (2 Arm) Grp 1 |
CAPE (2 Arm) Grp 2 |
5-FU (4 Arm) Grp 3 |
5-FU + OX (4 Arm) Grp 4 |
CAPE (4 Arm) Grp 5 |
CAPE + OX (4 Arm) Grp 6 |
|
| No. patients | ||||||
| Randomly assigned | 147 | 146 | 330 | 329 | 326 | 330 |
| Ineligible | 1 | 5 | 2 | 2 | 1 | 2 |
| All patients, % | ||||||
| Age, y | ||||||
| ≤59 | 59.2 | 52.7 | 56.1 | 61.4 | 57.1 | 61.2 |
| ≥60 | 40.8 | 47.3 | 43.9 | 38.6 | 42.9 | 38.8 |
| Sex | ||||||
| Male | 68.0 | 67.8 | 67.0 | 68.1 | 67.8 | 67.6 |
| Female | 32.0 | 32.2 | 33.0 | 31.9 | 32.2 | 32.4 |
| Clinical stage† | ||||||
| II | 49.7 | 46.6 | 61.5 | 61.7 | 62.3 | 61.5 |
| III | 50.3 | 53.4 | 38.5 | 38.3 | 37.7 | 38.5 |
| Surgical intent† | ||||||
| Sphincter sparing | 74.8 | 71.9 | 73.6 | 73.6 | 74.2 | 73.3 |
| Nonsphincter sparing | 25.2 | 28.1 | 26.4 | 26.4 | 25.8 | 26.7 |
* 5-FU = 5-flourouracil; CAPE = capecitabine; OX = oxaliplatin.
† As reported at the time of random assignment.
Efficacy
The early endpoints of pathological complete response (pCR) and sphincter-sparing surgery and surgical downstaging have been previously published (26). The primary endpoint of local-regional control at three years showed nearly identical outcomes with 5-FU (11.2%) or capecitabine (11.8%) (HR = 1.0, P = .98) (Figures 2 and 3), thus we conclude equivalence according to our prespecified criteria. Similarly, there was no statistically significant difference between the use or nonuse of oxaliplatin (12.1% vs 11.2%, respectively) (HR = 0.94, P = .70) and no evidence of oxaliplatin-treatment-by-fluoropyrimidine-treatment interaction (P = .46). The three-year local-regional recurrence rate (ranging from 3.1% to 5.1% depending on the study arm) restricted to those patients who successfully underwent an R0 surgical resection is illustrated in Figure 2. Again, no statistically significant differences were noted between 5-FU and capecitabine (HR = 0.86, P = .53) or with the use or nonuse of oxaliplatin, although the recurrence rate with the use of oxaliplatin was numerically lower than that in patients treated without oxaliplatin (3.1% and 5.1%, respectively) (HR = 0.71, P = .21). Further unplanned analyses that included only patients at high risk for recurrence (lymph node–positive and clinical stage TIII/IV disease) also demonstrated a lack of statistically significant difference in local-regional control with the use of oxaliplatin (HR = 1.27, P = .38). The three-year rate of local events in the oxaliplatin group was 24.1% compared with 19.2% in the nonoxaliplatin group.
Figure 2.
Three-year event rate for patients treated with either continuous infusion 5-FU or capecitabine with or without oxaliplatin for (A) local-regional recurrence for all patients and (B) local-regional recurrence rates for only those patients achieving an R0 resection in National Surgical Adjuvant Breast and Bowel Project R-04. Note: No significant fluoropyrimidine by oxaliplatin interaction. L/R = local-regional.
Figure 3.
Kaplan-Meier plots of local-regional recurrence for patients treated with (A) either continuous infusion 5-FU or capecitabine (B) with or without oxaliplatin in National Surgical Adjuvant Breast and Bowel Project R-04. L/R = local-regional.
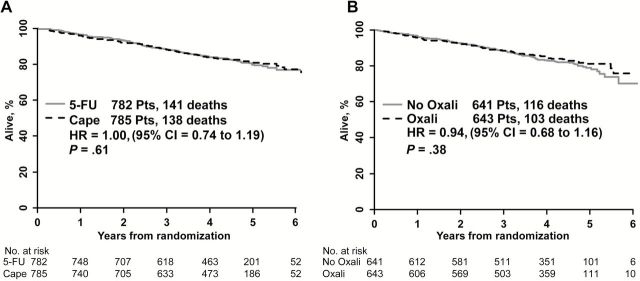
The five-year disease-free and overall survival data are shown in Figures 4 and 5. There was no difference in DFS or OS between the use of 5-FU and capecitabine for either endpoint, with five-year event-free rates for DFS of 66.4% and 67.7% (HR = 0.97, P = .70) and for OS of 79.9% and 80.8% (HR = 0.94, P = .61), respectively. Similarly, there was no statistically significant difference in DFS (64.2% vs 69.2%; HR = 0.91, P = .34) or OS (79% vs 81.3%; HR = 0.89, P = .38) between the nonuse or use of oxaliplatin, respectively.
Figure 4.
Five-year event rates for (A) disease-free survival and (B) overall survival for patients treated with either continuous infusion 5-FU or capecitabine with or without oxaliplatin in National Surgical Adjuvant Breast and Bowel Project R-04. Note: No significant fluoropyrimidine-by-oxaliplatin interaction.
Figure 5.
Kaplan-Meier plots of overall survival for patients treated with (A) either continuous infusion 5-FU or capecitabine (B) with or without oxaliplatin in National Surgical Adjuvant Breast and Bowel Project R-04.
Treatment Compliance
Treatment compliance was defined as having completed at least 80% of protocol-prescribed therapy and was as follows: for 5-FU without oxaliplatin, patients achieved a 90% compliance rate; for 5-FU with oxaliplatin they achieved 84% (P = .016). For capecitabine, 97% achieved protocol compliance without oxaliplatin and 96% with (P = .55). For oxaliplatin, compliance was 69% with 5-FU and 62% with capecitabine (P = .069). Ninety-six percent to 98% achieved radiation therapy compliance; this did not differ statistically significantly by arm (P = .42).
Toxicity
Overall grade 3+ toxicities were substantially greater in the oxaliplatin-containing arms (Table 2). This was primarily the result of an increase in grade 3 and 4 diarrhea (P < .0001) with the addition of this agent. Although there were numerically more deaths (on treatment and within 45 days of completing neoadjuvant treatment) in the capecitabine arms (4 and 5 vs 1 each for the capecitabine vs 5-FU arms, respectively), these differences did not reach statistical significance (P = .064 Fisher’s exact test, postamendment, 2 vs 9 deaths) (0.091, pre- and postamendment, 3 vs 10 deaths). The data also demonstrate that reducing 5-FU and capecitabine from seven to five days of radiation a week decreased the incidence of grade 3 to 5 diarrhea from 15.6%-17.1% to 6.9% (P < .0001).
Table 2.
Patient toxicity in the National Surgical Adjuvant Breast and Bowel Project R-04 study*
| Toxicity | Pre-amendment | Post-amendment | ||||
|---|---|---|---|---|---|---|
| 5-FU, % (n=141) |
CAPE, % (n=146) |
5-FU, % (n=317) |
5-FU + OX, % (n=322) |
CAPE, % (n=319) |
CAPE + OX, % (n=322) |
|
| Greatest toxicity: Grade 3 | 28.4 | 35.6 | 25.6 | 37.0 | 26.6 | 36.6 |
| Greatest toxicity: Grade 4 | 2.8 | 2.7 | 0.6 | 2.8 | 2.2 | 3.7 |
| Greatest toxicity: Grade 5 | 0.7 | 0.7 | 0.3 | 0.3 | 1.3 | 1.6 |
| Greatest toxicity: Grades 3–5 | 31.9 | 39.0 | 26.5 | 40.1 | 30.1 | 41.9 |
| Toxicity, % of grades observed | ||||||
| Diarrhea: Grades 3-5 | 15.6 | 17.1 | 6.9 | 16.5 | 6.9 | 16.5 |
| Nausea : Grade 3 | 1.4 | 2.7 | 0.3 | 0.6 | 1.3 | 2.2 |
| Vomiting: Grade 3 | 0 | 3.4 | 0.3 | 1.6 | 0 | 1.2 |
| Fatigue: Grade 3 | 3.5 | 6.8 | 1.3 | 4.0 | 2.2 | 5.9 |
| Abdominal pain: Grade 3 | 2.1 | 3.4 | 1.6 | 2.8 | 0.3 | 1.9 |
| Anal pain: Grade 3 | 1.4 | 5.5 | 3.2 | 4.0 | 3.4 | 3.1 |
| Radiation dermatitis: Grades 3–5 | 2.1 | 7.5 | 2.5 | 2.2 | 2.5 | 1.2 |
| Dehydration: Grade 3 | 5.0 | 8.2 | 0.3 | 2.8 | 2.2 | 4.0 |
| Hand-foot syndrome: Grade 3 | 1.4 | 3.4 | 0.3 | 0 | 0.3 | 0.3 |
| Periph. sens. neurop.:Grades 2–4 | 2.1 | 2.1 | 0.6 | 5.6 | 2.2 | 6.5 |
*5-FU = 5-flourouracil; CAPE = capecitabine; OX = oxaliplatin.
Discussion
NSABP R-04 was designed to study the comparability of capecitabine to 5-FU and the potential benefit of adding oxaliplatin to either of the fluoropyrimidines in the neoadjuvant rectal setting. This study clearly demonstrated that patients with stage II and III rectal cancer treated in the neoadjuvant setting have nearly identical outcomes when treated with either continuous infusion 5-FU or oral twice-daily capecitabine as the radiation sensitizer for both the primary endpoint of local-regional control and the five-year rates of DFS and OS. Furthermore, the addition of oxaliplatin to either fluoropyrimidine did not result in statistically significant benefit relative to either agent used singly. The neoadjuvant approach for patients with stage II and III rectal cancers is supported by several recently reported investigations demonstrating an enhanced rate of sphincter-sparing operations and local control with the use of this therapy (9–12). Based on these reports, neoadjuvant therapy has become the worldwide preferred approach for patients with stage II or III rectal cancers. The use of constant infusion 5-FU rather than bolus intravenous administration is based primarily on a US Intergroup study demonstrating superior outcomes with the infusion regimen (2). Capecitabine was found to be associated with outcomes similar to those with 5-FU in patients with metastatic colorectal cancer (3,4) and noninferior to intravenous 5-FU in the treatment of patients with stage III colon cancer (5).
A recent publication of the early endpoints associated with R-04 showed that 5-FU and capecitabine use resulted in similar rates of PCR and of sphincter-sparing surgery and surgical downstaging (26). The rates of PCR reported in that paper of 17.8% to 20.7% compare favorably with other recently published reports using neoadjuvant chemoradiation therapy, which recorded rates of 9% to 15% (9–12). In addition, the primary endpoint of local-regional failure rates at three years reported in the present study in the 11% to 12% range are similar to the 5 % to 11% rates reported in other recently completed neoadjuvant rectal investigations, particularly considering that these studies did not include patients who either had not undergone surgery or who had an R2 resection as local-regional failures at the time of surgery, in contrast to patients in NSABP R-03 and the NSABP R-04 study (9–12).
These data support the use of either capecitabine or infusional 5-FU as a standard of care in the neoadjuvant rectal setting. Despite the theoretical advantage of capecitabine in terms of greater tumor vs normal tissue activation, particularly in the setting of concomitant radiation exposure, which has been shown to increase thymidine phosphorylase levels in preclinical models, our study did not show either a marked enhancement in efficacy or decrement in toxicity with the use of capecitabine relative to 5-FU. Several small retrospective reports lend further support to the comparability of 5-FU and capecitabine in the rectal neoadjuvant setting (27,28). Although we did observe a numerically greater number of deaths while on or within 45 days of active treatment in the capecitabine-containing arms relative to the 5-FU arms (0.3% in the 5-FU arms and 1.3%-1.6% in the capecitabine arms), this increase did not reach statistical significance and the overall survival among the arms was identical (HR = 1.0). Thus, the difference in deaths did not translate into a worse outcome for the patients treated with capecitabine. However, the increase in deaths is a concern that should be further investigated in studies using this agent. The primary advantage of capecitabine is its oral route of administration, which obviates the need for an indwelling catheter or an infusion pump, along with their attendant costs and health risks. A quality-of-life assessment associated with R-04 was presented in preliminary form with data from year 1 of follow-up and showed essentially no difference in the Functional Assessment of Cancer Therapy–Colorectal version 4 questionnaire (FACT-C) with the use of either fluoropyrimidine (29,30). We await data from the five-year follow-up questionnaires.
Oxaliplatin has been found to sensitize human cancer cells to the effects of radiation in vitro and to enhance the outcomes of patients with metastatic colorectal and locally advanced colon cancers treated in the adjuvant setting following curative-intent surgery when added to fluoropyrimidine-based therapy (5,13–20). DFS was enhanced by 23% and 26% in two large randomized investigations testing the addition of oxaliplatin to either biweekly infusion 5-FU or weekly bolus 5-FU, respectively (19, 20). Because of the potential for sensitizing cancer cells to the effects of radiation and direct cytotoxic effect on colorectal cancer cells, we investigated the impact of adding oxaliplatin to either capecitabine or 5-FU in the neoadjuvant rectal setting. While the addition of oxaliplatin resulted in numerically fewer local recurrence events, the differences did not reach statistical significance even in the highest-risk patient population where one might expect the greatest absolute benefit to be realized. An unplanned retrospective exploratory analysis of only those patients with either clinical stage TIII with node positivity or those with T4 lesions showed that the addition of oxaliplatin did not result in statistically significant benefit in any of the examined endpoints. While we could not demonstrate enhanced therapeutic activity with the addition of oxaliplatin, we did find a substantial increase in toxicity associated with the use of this agent, in particular, an increase in grade 3 and 4 diarrhea. This result is consistent with three other large randomized studies that tested the benefit of adding oxaliplatin to fluoropyrimidines as radiation sensitizers in the neoadjuvant rectal setting (31–33). All three of these studies, along with the present study, failed to demonstrate a benefit with the addition of oxaliplatin in the neoadjuvant rectal setting. An unplanned retrospective analysis of a fifth study (34) did find a marginal benefit, as manifested by an increase in pathologic CR rate from 13% to 17% with the addition of oxaliplatin to radiotherapy and 5-fluorouracil in the rectal neoadjuvant setting. Given the lack of a statistically significant beneficial effect in efficacy outcomes coupled with the substantial increase in toxicity, the use of oxaliplatin in the rectal neoadjuvant setting is not recommended.
This study also had some limitations. As is the case with clinical trials in general, this study shares the limitation that the outcomes data may only apply to a patient who shares the eligibility criteria used for enrollment into the present trial along with possible selection bias of patients willing to participate in a randomized clinical trial. The other limitation that should be considered is the fact that we do not have complete information concerning the type and use of adjuvant therapy in the study patients.
In summary, the mature data from NSABP R-04 demonstrate that the neoadjuvant use of capecitabine is comparable with continuous infusion 5-FU when combined with radiation therapy in patients with stage II or III rectal cancer. The addition of oxaliplatin does not confer additional benefit but does result in substantially more toxicity when added to either fluoropyrimidine along with radiation therapy in the neoadjuvant setting.
Funding
This work was supported by the US National Cancer Institute at the National Institutes of Health, US Department of Health and Human Services, Public Health Service grants (grant numbers U10-CA180868, U10-CA180822, UG1-CA189867, U10-CA180888, U10-CA180820, and U10-CA180821). The National Surgical Adjuvant Breast and Bowel Project is now a member of NRG Oncology.
The work described in this manuscript is original research and has not been previously published.
Clinical Trials registration: NCT00058474.
The study sponsor played no role in the design, collection of data, analysis, or interpretation of the study, the writing of the manuscript, nor the decision to submit the manuscript for publication. Human investigations were performed after approval by a local Human Investigations Committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services.
References
- 1. Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64 (5):9–29. [DOI] [PubMed] [Google Scholar]
- 2. O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331 (8):502–507. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19 (21):4097–4106. [DOI] [PubMed] [Google Scholar]
- 4. Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19 (8):2282–2292. [DOI] [PubMed] [Google Scholar]
- 5. Glen H, Cassidy J. Redefining adjuvant chemotherapy in patients with stage III colon cancer: X-ACT trial. Expert Rev Anticancer Ther. 2008;8 (4):547–551. [DOI] [PubMed] [Google Scholar]
- 6. Schilsky RL. Pharmacology and clinical status of capecitabine. Oncology. 2000;14 (9):1297–1306. [PubMed] [Google Scholar]
- 7. Schüller J, Cassidy J, Dumont E, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45 (4):291–297. [DOI] [PubMed] [Google Scholar]
- 8. Sawada N, Ishikawa T, Sekiguchi F, et al. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5 (10):2948–2953. [PubMed] [Google Scholar]
- 9. Bosset J, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355 (11):1114–1123. [DOI] [PubMed] [Google Scholar]
- 10. Gerard J, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24 (28):4620–4625. [DOI] [PubMed] [Google Scholar]
- 11. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30 (16):1926–1933. [DOI] [PubMed] [Google Scholar]
- 12. Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27 (31):5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18 (16):2938–2947. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22 (1):23–30. [DOI] [PubMed] [Google Scholar]
- 15. Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21 (11):2059–2069. [DOI] [PubMed] [Google Scholar]
- 16. deGramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997;33 (2):214–219. [DOI] [PubMed] [Google Scholar]
- 17. Hochster H, Chachoua A, Speyer J, et al. Oxaliplatin with weekly bolus fluorourcil and low-dose leucovorin as first-line therapy for patients with colorectal cancer. J Clin Oncol. 2003;21 (14):2703–2707. [DOI] [PubMed] [Google Scholar]
- 18. Maindrault-Goebel F, Louvet C, André T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). Eur J Cancer. 1999;35 (9):1338–1342. [DOI] [PubMed] [Google Scholar]
- 19. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29 (28):3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27 (10):3109–3116. [DOI] [PubMed] [Google Scholar]
- 21. NCT00058474 NSABP Protocol R-04. https://clinicaltrials.gov/ct2/show/NCT00058474?term=NCT00058474&rank=1. Accessed August 24, 2015.
- 22. White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer. 1978;37 (5):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50 (3):163–170. [PubMed] [Google Scholar]
- 24. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53 (282):457–481. [Google Scholar]
- 25. Cox DR. Regression models and life-tables. J Royal Stat Soc Series B. 1972;34 (2):187–220. [Google Scholar]
- 26. O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32 (18):1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Das P, Lin EH, Bhatia S, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: a matched pair analysis. Int J Radiat Oncol Biol Phys. 2006;66 (5):1378–1383. [DOI] [PubMed] [Google Scholar]
- 28. Ramani VS, Myint AS, Montazeri A, et al. Preoperative chemoradiotherapy for rectal cancer: a comparison between intravenous 5-fluorouracil and oral capecitabine. Colorectal Dis. 2010;12(Suppl 2):37–46. [DOI] [PubMed] [Google Scholar]
- 29. Yothers G, Ganz PA, Lopa SH, et al. Patient-reported outcomes (PROs) comparison of 5-FU and capecitabine (cape) with concurrent radiotherapy (RT) for neoadjuvant treatment of rectal cancer: results of NSABP R-04. 2012 Gastrointestinal Cancers Symposium. J Clin Oncol. 2012;30:4 (Suppl);Abstract 391. [Google Scholar]
- 30. Ganz P, Lopa SH, Yothers G, et al. Comparative effectiveness of sphincter-sparing surgery versus abdomino-peritoneal resection in rectal cancer: patient-reported outcomes from NSABP R-04. J Clin Oncol. 2012;30(Suppl);Abstract 3545. [Google Scholar]
- 31. Gérard J, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28 (10):1638–1644. [DOI] [PubMed] [Google Scholar]
- 32. Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29 (20):2773–2780. [DOI] [PubMed] [Google Scholar]
- 33. Schmoll H, Haustermans K, Jay Price T, et al. Preoperative chemotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: first results of the PETACC-6 randomized phase III trial. J Clin Oncol. 2013;31(Suppl):Abstract 3531. [Google Scholar]
- 34. Rödel C, Liersch T, Becker H, et al. Preoperative chemotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomized phase 3 trial. Lancet Oncol. 2012;13 (7):679–687. [DOI] [PubMed] [Google Scholar]