Abstract
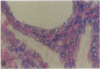
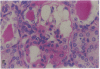
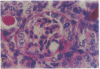
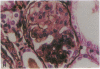
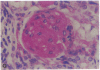
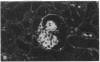
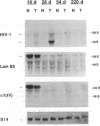
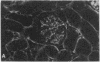
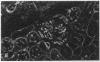
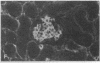
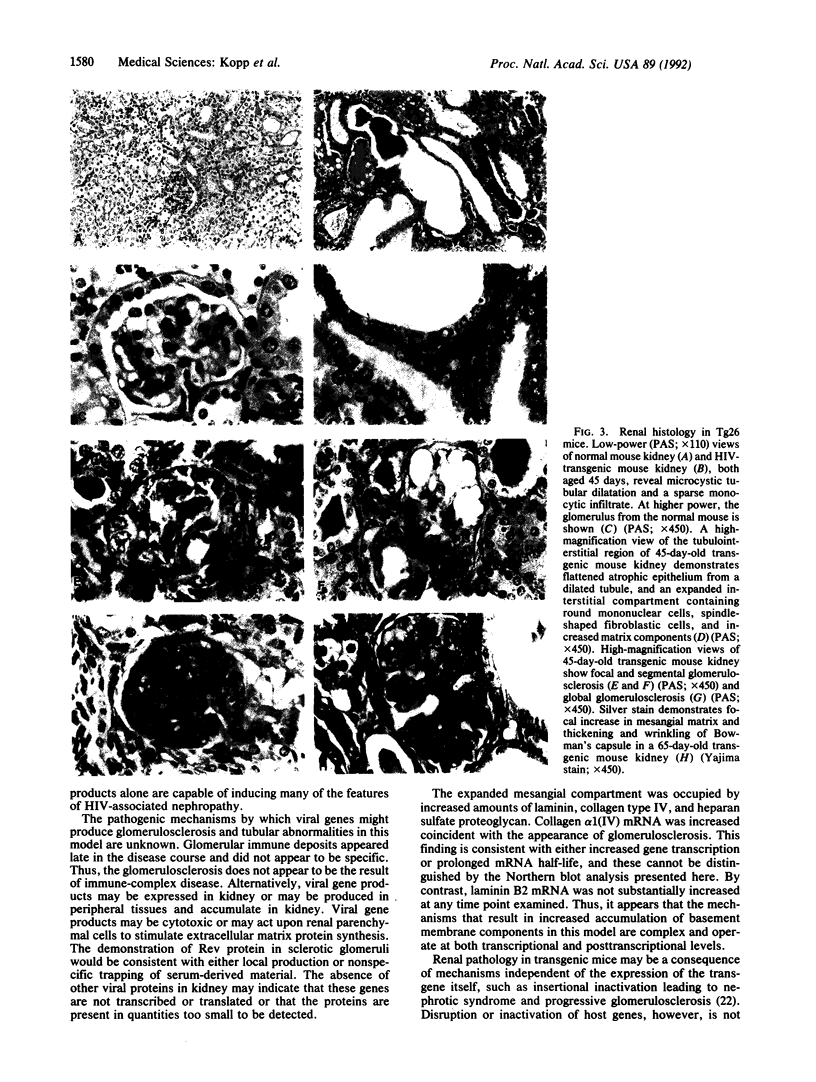
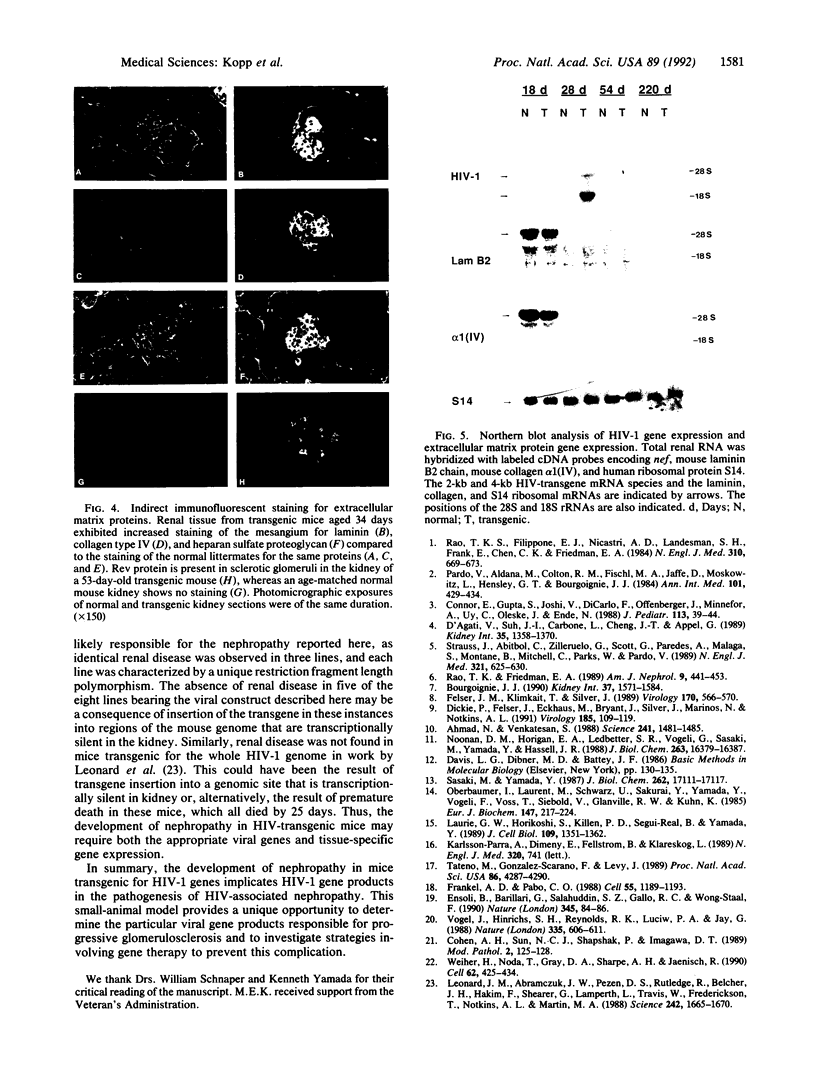
Patients infected with human immunodeficiency virus type 1 (HIV-1) develop a renal syndrome characterized by proteinuria, renal failure, and focal segmental glomerulosclerosis. By using a noninfectious HIV-1 DNA construct lacking the gag and pol genes, three transgenic mouse lines have been generated that develop a syndrome remarkably similar to the human disease. In the present study, we have characterized in detail one of these lines, Tg26. In Tg26 mice, proteinuria was detectable at approximately 24 days of age, followed by severe nephrotic syndrome and rapid progression to end-stage renal failure. Renal histology showed focal segmental glomerulosclerosis and microcystic tubular dilatation. Indirect immunofluorescence studies demonstrated increased accumulation of the basement membrane components laminin, collagen type IV, and heparan sulfate proteoglycan. The viral protein Rev was present in sclerotic glomeruli. Northern blot analysis of total renal RNA showed expression of viral genes prior to the appearance of histologic renal disease, with greatly diminished viral gene expression late in the disease course. Kidneys from transgenic mice expressed increased steady-state levels of collagen alpha 1(IV) mRNA when glomerulosclerosis was present. We conclude that the presence of HIV-1 genes is associated with progressive renal dysfunction and glomerulosclerosis in transgenic mice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N., Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988 Sep 16;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- Bourgoignie J. J. Renal complications of human immunodeficiency virus type 1. Kidney Int. 1990 Jun;37(6):1571–1584. doi: 10.1038/ki.1990.151. [DOI] [PubMed] [Google Scholar]
- Cohen A. H., Sun N. C., Shapshak P., Imagawa D. T. Demonstration of human immunodeficiency virus in renal epithelium in HIV-associated nephropathy. Mod Pathol. 1989 Mar;2(2):125–128. [PubMed] [Google Scholar]
- Connor E., Gupta S., Joshi V., DiCarlo F., Offenberger J., Minnefor A., Uy C., Oleske J., Ende N. Acquired immunodeficiency syndrome-associated renal disease in children. J Pediatr. 1988 Jul;113(1 Pt 1):39–44. doi: 10.1016/s0022-3476(88)80525-0. [DOI] [PubMed] [Google Scholar]
- D'Agati V., Suh J. I., Carbone L., Cheng J. T., Appel G. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int. 1989 Jun;35(6):1358–1370. doi: 10.1038/ki.1989.135. [DOI] [PubMed] [Google Scholar]
- Dickie P., Felser J., Eckhaus M., Bryant J., Silver J., Marinos N., Notkins A. L. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991 Nov;185(1):109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Felser J. M., Klimkait T., Silver J. A syncytia assay for human immunodeficiency virus type I (HIV-I) envelope protein and its use in studying HIV-I mutations. Virology. 1989 Jun;170(2):566–570. doi: 10.1016/0042-6822(89)90448-0. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Karlsson-Parra A., Dimény E., Fellström B., Klareskog L. HIV receptors (CD4 antigen) in normal human glomerular cells. N Engl J Med. 1989 Mar 16;320(11):741–741. doi: 10.1056/NEJM198903163201119. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Horikoshi S., Killen P. D., Segui-Real B., Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989 Sep;109(3):1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. M., Abramczuk J. W., Pezen D. S., Rutledge R., Belcher J. H., Hakim F., Shearer G., Lamperth L., Travis W., Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988 Dec 23;242(4886):1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Noonan D. M., Horigan E. A., Ledbetter S. R., Vogeli G., Sasaki M., Yamada Y., Hassell J. R. Identification of cDNA clones encoding different domains of the basement membrane heparan sulfate proteoglycan. J Biol Chem. 1988 Nov 5;263(31):16379–16387. [PubMed] [Google Scholar]
- Oberbäumer I., Laurent M., Schwarz U., Sakurai Y., Yamada Y., Vogeli G., Voss T., Siebold B., Glanville R. W., Kühn K. Amino acid sequence of the non-collagenous globular domain (NC1) of the alpha 1(IV) chain of basement membrane collagen as derived from complementary DNA. Eur J Biochem. 1985 Mar 1;147(2):217–224. doi: 10.1111/j.1432-1033.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]
- Pardo V., Aldana M., Colton R. M., Fischl M. A., Jaffe D., Moskowitz L., Hensley G. T., Bourgoignie J. J. Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Oct;101(4):429–434. doi: 10.7326/0003-4819-101-4-429. [DOI] [PubMed] [Google Scholar]
- Rao T. K., Filippone E. J., Nicastri A. D., Landesman S. H., Frank E., Chen C. K., Friedman E. A. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Mar 15;310(11):669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- Rao T. K., Friedman E. A. AIDS (HIV)-associated nephropathy; does it exist? An in-depth review. Am J Nephrol. 1989;9(6):441–453. doi: 10.1159/000168011. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Yamada Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J Biol Chem. 1987 Dec 15;262(35):17111–17117. [PubMed] [Google Scholar]
- Strauss J., Abitbol C., Zilleruelo G., Scott G., Paredes A., Malaga S., Montané B., Mitchell C., Parks W., Pardo V. Renal disease in children with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Sep 7;321(10):625–630. doi: 10.1056/NEJM198909073211001. [DOI] [PubMed] [Google Scholar]
- Tateno M., Gonzalez-Scarano F., Levy J. A. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4287–4290. doi: 10.1073/pnas.86.11.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Weiher H., Noda T., Gray D. A., Sharpe A. H., Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990 Aug 10;62(3):425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]