Abstract
Background & Aims
The immunosuppressant rapamycin frequently causes non-infectious diarrhea in recipients of organ transplants. We investigated the mechanisms of this process.
Methods
We performed a retrospective analysis of renal transplant recipients treated with rapamycin from 2003 through 2010 at Albany Medical College, collecting data on serum levels of rapamycin. Levels of the Na+/H+ exchanger 3 (NHE3) were measured in human ileal biopsies from patients who did and did not receive rapamycin (controls), in ileum tissues from rats or mice given rapamycin, and in mice with intestine-specific disruption of Mtor (mTORf/f:Villin-cre mice) or Atg7 (Atg7flox/flox; Villin-Cre). Exchange activity and intestinal water absorption were measured using a pH-sensitive dye and small intestine perfusion, respectively.
Results
Episodes of non-infectious diarrhea occurred in organ recipients following increases in serum levels of rapamycin. Expression of NHE3 was reduced in the ileal brush border of patients with diarrhea. In rats and mice, continuous administration of low doses of rapamycin reduced levels of NHE3 in intestinal tissues; this effect was not observed in mice with intestinal deletion of ATG7, indicating that autophagy is required for the reduction. Administration of single high doses of rapamycin to mice, to model the spikes in rapamycin levels that occur in patients with severe diarrheal episodes, resulted in reduced phosphorylation of S6 and AKT in ileal tissues, indicating inhibition of the mTOR complex (mTORC1 and mTORC2). Intestines of mice with intestine-specific deletion of mTOR were dilated and contained large amount of liquid stools; they also had reduced levels of total NHE3 and NHERF1, compared with control mice. We observed a significant reduction in Na+/H+ exchange activity in ileum tissues from these mice.
Conclusions
Rapamycin inhibition of mTOR reduces levels of NHE3 and Na+/H+ exchange activity in intestinal tissues of patients and rodents. This process appears to require the autophagic activity mediated by ATG7. Loss of mTOR regulation of NHE3 could mediate the development of diarrhea in patients undergoing rapamycin therapy.
Keywords: transplantation, sodium transport, mTOR, immunosuppression
INTRODUCTION
Non-infectious diarrhea is a gastrointestinal symptom frequently associated with the use of immunosuppressants, such as rapamycin, in organ transplant recipients and can lead to adverse post-transplantation outcomes, including graft loss and death1. The incidence of rapamycin-associated diarrhea is from 2% to 38%, and appears to be dose-dependent2, 3. Although rapamycin is known to cause malabsorption and weight loss4, the pathological mechanisms causing the diarrhea are unknown.
The mammalian target of rapamycin (mTOR) has been implicated in various cellular processes5. mTOR resides in two distinct signaling complexes (mTORC1 and mTORC2), in which mTORC1 regulates protein translation and autophagy activity6, whereas mTORC2 acts as a phosphoinositide-dependent kinase (PDK)-2 to directly regulate Akt activity6. Rapamycin is a potent mTOR inhibitor and inhibits mTORC1 at low concentrations, but also inhibits mTORC2 at higher concentrations7. The mTOR pathway, particularly mTORC1, helps to maintain the structure of the mammalian intestinal mucosa8 and is important in stem cell self-renewal9. Inhibition of mTOR by rapamycin has been shown to disrupt intestinal growth and regeneration in healthy animals4, 10.
mTOR is a regulator of autophagy, the process which mediates the turnover of intracellular proteins and organelles via lysosomal degradation and plays a role in cell survival during nutrient deprivation11. Rapamycin has been shown to activate autophagy via inhibition of mTORC112. Defects in autophagy, such as caused by deletion of either the Atg5 or Atg7 gene, result in the formation of aberrant Paneth cell granules13.
NHE3 is an abundant intestinal epithelial brush-border Na+/H+ exchanger protein located on the apical membrane of enterocytes where it facilitates neutral NaCl absorption14–16. NHE3 activity is highly regulated under both physiological and pathophysiological conditions17. Mice with NHE3 deletion develop diarrhea with increased fluid in the small intestine and colon, suggesting that NHE3 is essential for intestinal sodium absorption and water homeostasis15, 18, 19. One of the mechanisms of NHE3 stimulation is through Akt2-dependent ezrin phosphorylation, which leads to NHE3 activation20, 21. NHERF1and NHERF2 are also necessary for NHE3 regulation22, 23.
In this study, we demonstrate a novel role for the mTOR/autophagy pathways in NHE3 regulation. These findings may provide insights into the underlying mechanisms of rapamycin-associated diarrhea commonly observed in post-transplant patients.
MATERIALS AND METHODS
Chart Review
Clinical charts of renal transplant recipients treated with rapamycin from 2003 to 2010 at Albany Medical College (AMC) were retrospectively reviewed via a study approved by the AMC Institutional Review Board (Fig. 1 and S1).
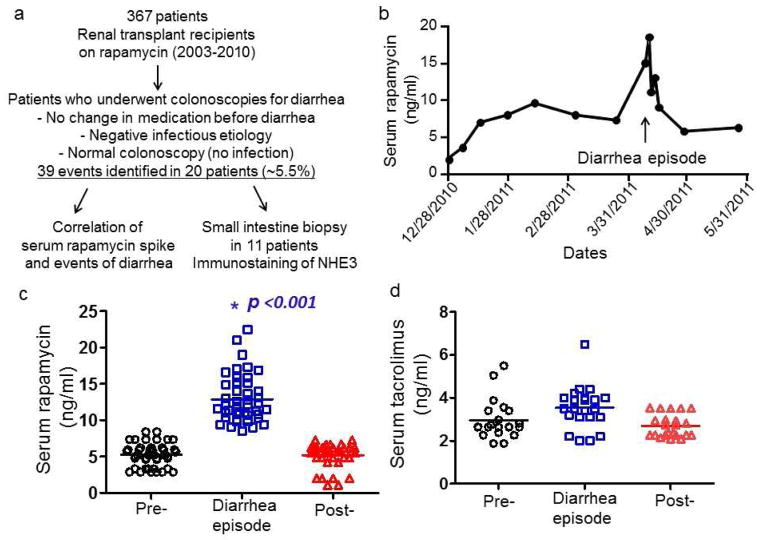
Figure 1.
(a) A schematic for patient selection. (b) An example of serum rapamycin level before, during and after a diarrheal episode in a single patient. Plots showing each serum rapamycin level in 20 patients with 39 episodes of profound diarrhea (c) and each serum tacrolimus level in 12 patients (d) at baseline, during and after the diarrheal episodes. * indicates p<0.001 (comparison between during and pre- or post-diarrheal episode) (one-way ANOVA with a Bonferroni test).
Human Tissue Selection and Immunostaining
Paraffin blocks containing tissue biopsies of the terminal ileum were obtained from 11 renal transplant patients on rapamycin referred for colonoscopy to evaluate diarrhea and from 10 control patients. All tissues were identically processed (S3).
Animals
Three-week-old male Sprague-Dawley rats (Taconic), mTORf/f(B6.129S4-Mtortm1.2Koz/mTOR) and villin-cre (B6.SJL-Tg(Vil-cre)997Gum/J) (Jackson laboratory), and Atg7flox/flox mice24 were housed in the AMC Animal Resource Facility (S4).
Rapamycin Treatment
Four- to 8-week-old rats or mice were treated intraperitoneally with rapamycin (Tecoland Corp., Irvine, CA) at either 5 mg/kg/day or vehicle (5% Tween-20 and 4% ethanol) for 2 weeks to model chronic treatment or a single dose of 20 mg/kg for 2 h to evaluate acute high-dose effects.
Isolation of Intestinal Epithelial Cells
Intestinal epithelial cells were prepared as described with a slight modification25 (S5).
Tissue Biotinylation and Western blot
Ileal tissues were collected for biotinylation, followed by lysis. Biotinylated plasma membrane proteins were pulled down by neutravidin-agarose beads (Thermo Scientific). Protein lysates were used for western blot (S2, S6 and S7).
Immunohistochemistry
Rat and mouse ileal tissues were harvested, followed by fixation and embedding. Twenty-micron sections were collected, followed by immunofluorescence staining as described (S8).
Measurement of Na+/H+ Exchange Activity
NHE3 exchange activity was measured and analyzed as described previously (S9 & S11)20, 26, 27.
Statistical Analysis
Data will be analyzed by GraphPad Prism version 5 using either student t-test or one-way ANOVA as appropriate.
RESULTS
Serum Rapamycin Levels and Diarrhea
We performed a chart review of all patients who received renal transplantation at AMC during 2003–2010 and were given rapamycin as a post-transplant immunosuppressant. Of 367 records screened, 20 patients had 39 events of acute, severe diarrhea requiring hospitalization (some admitted more than once) (Figure 1a). All 20 patients had no evidence of infection, recent medication changes, and graft-versus-host disease, with ages ranging from 26 to 68 years old. Twelve patients were female (S1). Eleven patients had colonoscopic biopsies that revealed normal histology of the terminal ileum. Figure 1b presents serum rapamycin data from a single renal transplant patient admitted for severe watery diarrhea and illustrates our general observations concerning serum rapamycin levels and the development of diarrhea. This patient’s serum rapamycin was 18.5 ng/ml at the time of diarrhea compared to his average baseline of 7 ng/ml. The diarrhea resolved shortly after the patient’s rapamycin levels returned to baseline.
In all 39 diarrheal episodes, rapamycin levels at the time of diarrhea were significantly elevated, ranging from 2–7 times the baseline levels (Figure 1c). In all cases, diarrhea resolved within 3–5 days after the serum rapamycin levels returned to baseline. Of the 20 patients with acute severe diarrhea, 12 were also co-administered tacrolimus, but the serum levels of tacrolimus did not significantly differ from their average baseline levels (Figure 1d).
NHE3 Expression in Rapamycin-treated Transplant Patients
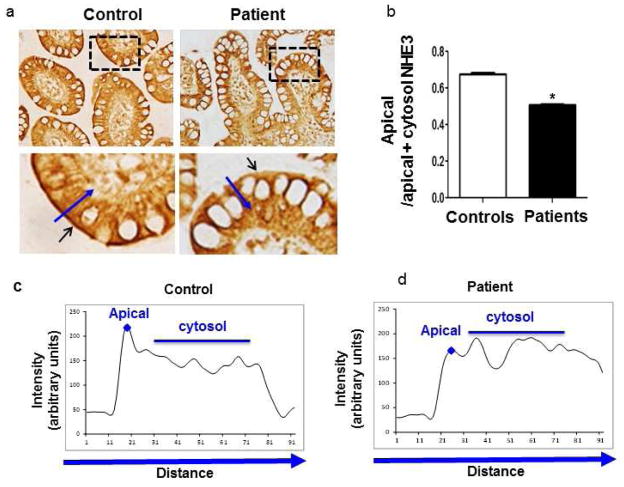
Mice with NHE3 knockout developed diarrhea, suggesting a role for NHE3 in intestinal sodium absorption and water homeostasis15. We hypothesized that rapamycin might interfere with NHE3 expression in the intestine of patients with acute diarrhea. We acquired fixed biopsy tissues from the Pathology Department, including terminal ileal biopsies from 11 renal transplant patients who developed diarrhea with spikes of serum rapamycin level, as well as from 10 adult control subjects. Biopsy tissues were acquired via colonoscopy at the time of the diarrheal episode from 11 transplant patients, and DAB (diaminobenzidine) staining revealed a marked reduction of NHE3 at the apical membranes in ileal biopsies. Quantification of apical/apical+cytosol NHE3 revealed an approximately 25% reduction of that ratio in rapamycin-treated patients compared to control subjects (Figure 2, S3), suggesting that apical NHE3 expression appears to be down-regulated by rapamycin.
Figure 2. Decreased NHE3 expression in ileal apical membranes from rapamycin-treated patients with acute diarrhea.
(a) Representative images of immunostaining of NHE3 in the terminal ileum from a control and one rapamycin-treated patient. The dashed areas were magnified as shown in the bottom panels. The small arrows point to the apical membrane with staining of NHE3. A long line with an arrowhead was drawn over the apical membrane and cytoplasmic area for quantification. (b & c) The intensity of NHE3 staining along the line with an arrowhead (a). The peak labeled with a diamond symbol represents NHE3 staining at the apical membrane. The average intensity of all data points underneath the line serves as the cytosolic NHE3 staining. (d) The bar graph presents the average of apical/apical+cytosol NHE3 in ileum samples from renal transplant patients compared to controls (*p<0.05 t-test, n=5 areas/slide, N=11 patients and 10 controls).
Effect of Rapamycin on NHE3 Expression in Rats
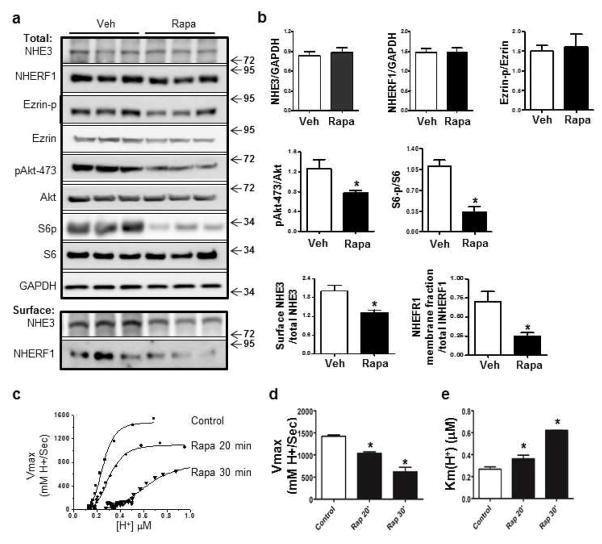
We employed a rat model to confirm that expression of NHE3 was down-regulated by chronic use of rapamycin. Sprague-Dawley rats were treated with rapamycin at 5mg/kg for 14 days, a dose that is sufficient to inhibit mTOR activity and to generate an anti-rejection effect28, 29. Ileal tissues were harvested from control and rapamycin-treated rats. There was a significant reduction in total NHE3 in the ileum of rapamycin-treated rats (Figure 3a–b). This effect could reflect a general inhibition of protein translation or an increase in turnover of NHE3. However, we found no significant reduction of the amount of other proteins, including Ezrin, NHERF1, and CFTR (Figure 3a–b). Notably, NHERF1 and Ezrin are NHE3-associated proteins that regulate NHE3 membrane-targeting23, 30. NHE3 reduction was accompanied by inhibition of phosphorylation of ribosomal protein S6 (p-S6), an indicator of mTOR activity (Figure 3a–b). The levels of phospho-Ezrin (p-Ezrin) and phospho-Akt (ser473) (p-Akts473), proteins that have previously been reported to regulate NHE3 activity20, 21, were not significantly altered by chronic exposure to rapamycin (Figure 3a–b).
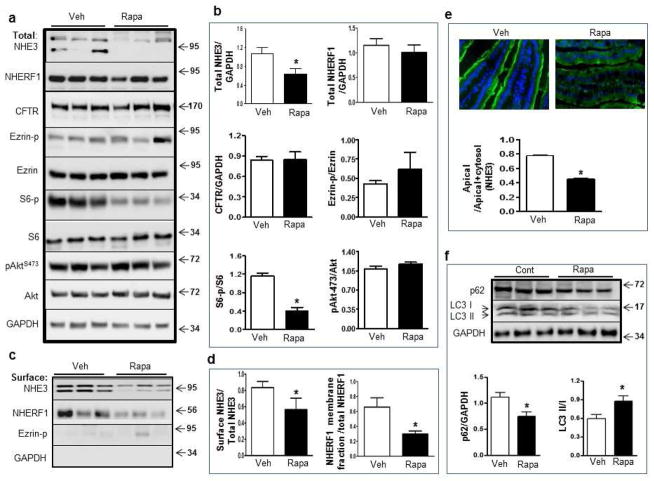
Figure 3. Rapamycin reduces the expression of NHE3 in rat ileum.
(a–b) Western blot analysis (a) and quantification (b) of NHE3, NHERF1, CFTR, Ezrin, p-Ezrin, S6, p-S6, Akt, p-Aktser473 in the small intestine from vehicle (Veh)- and rapamycin (Rapa)-treated rats. (c–d) Surface biotinylation assay (c) and quantification (d) for surface expression of NHE3 and NHERF1 in the membrane fraction. (e) Representative images and quantification of NHE3 at the apical membrane by immunostaining of NHE3 in mid-villus of the ileum from vehicle- and rapamycin-treated rats. (f) Western blot and quantification of p62, LC3I and LC3II in control and rapamycin-treated rat intestine (*p<0.05, t-test, N=3).
To further evaluate the effects of rapamycin on NHE3, a tissue surface biotinylation assay was developed to quantify the apical expression of NHE3. Plasma membrane proteins were biotinylated and the surface levels of NHE3 were analyzed by western blot. We observed that surface NHE3 was decreased in the rapamycin-treated group compared to controls (Figure 3c–d). Immunohistochemistry revealed that, while NHE3 was concentrated at the apical membrane of the ileal villus cells in control rats, a significant reduction of apical NHE3 was noted in rats treated with rapamycin (Figure 3e). Notably, rapamycin did not appear to significantly alter total NHERF1 levels (Figure 3a–b). However, there was a reduction in the amount of NHERF1 in the membrane fraction, as evidenced by the reduced recovery of NHERF1 among the avidin-biotinylated proteins (Figure 3c–d).
Autophagy and NHE3 Expression
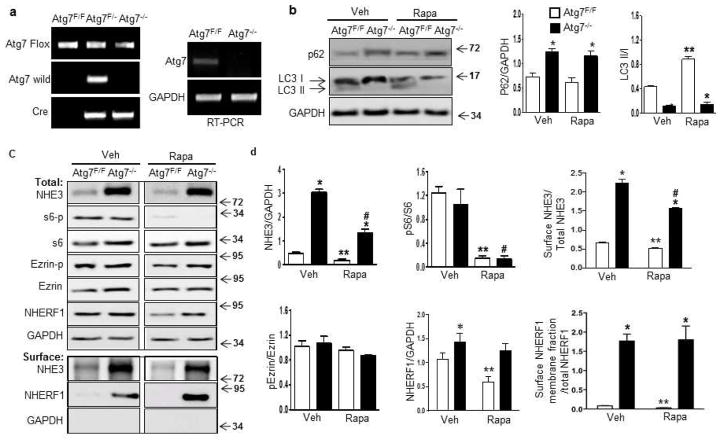
Rapamycin is a potent activator of autophagy, a process that turns over intracellular organelles and membrane receptors31. We evaluated the effect of rapamycin on autophagy activity in rat ileum by using the ubiquitin-binding protein p62/SQSTM1, which is degraded by autophagy and frequently used as a marker of autophagic activity32. Western blotting revealed a significant reduction of p62 in rat ileum treated with rapamycin (Figure 3f). Also the conversion of LC3I to LC3II, another marker of autophagic activity33, was significantly higher in rapamycin-treated animals than in controls (Figure 3f). Thus, long-term rapamycin exposure stimulates autophagy in the intestine. To verify an association between autophagy and the expression of NHE3, a mouse line was created with deletion restricted to intestinal epithelial cells of the autophagy gene Atg7 (Atg7flox/flox; Villin-Cre) (Atg7−/−). The Atg7−/− mice were viable, and Atg7 was verified to be deleted in intestinal epithelial cells as Atg7 RNA was almost undetectable in isolated intestinal epithelial cells of these mice (Figure 4a). Western blot revealed that p62 was detectable in adult control mice, but showed moderate accumulation in Atg7−/− mice, and rapamycin appeared to have little effect on p62 levels in control and Atg7−/− mice (Figure 4b), suggesting p62 is less sensitive to rapamycin in the mice. Although the level of LC3II was low in control mice, it was nearly absent in intestinal epithelial cells of Atg7−/− mice (Figure 4b), supporting the observation that ablation of Atg7 resulted in inhibition of autophagy. Rapamycin treatment promotes conversion of LC3I to LC3II, as indicated by the ratio of LC3II/LC3I in control mice. However, LC3II remains absent in Atg7−/− mice treated with rapamycin, confirming that deletion of the Atg7 autophagy gene nullifies the stimulatory effect of rapamycin on autophagy.
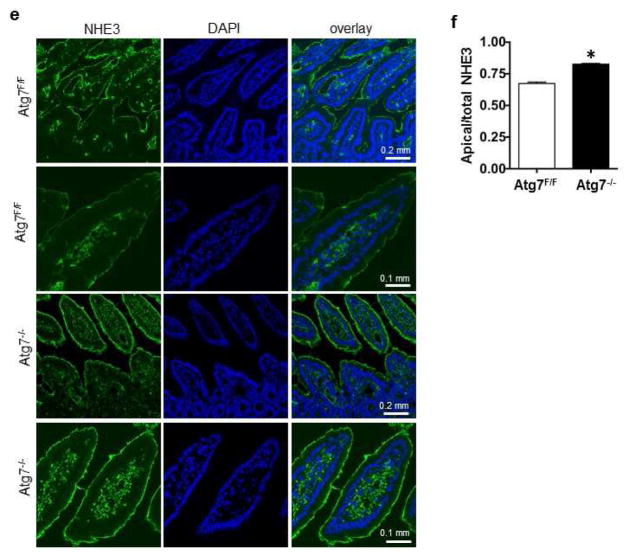
Figure 4. Deletion of Atg7 increases NHE3 expression in mouse intestine.
(a) Genotyping and RT-PCR of Atg7 in mouse intestinal epithelial cells (Atg7−/−). (b) Western blot and quantification of p62, LC3I, and LC3III in Atg7−/− mouse intestine treated with vehicle or rapamycin. (c) Western blot analysis of NHE3 and other proteins as indicated in control and Atg7−/− mice with and without rapamycin treatment, and surface biotinylation assay to evaluate the surface levels of NHE3 and NHERF1 in the membrane fraction. (d) Quantification of NHE3 and other proteins in control (open bar) and Atg7−/− mice (filled bar) are shown, as is quantification of NHE3 and NHERF1 in the membrane fraction. The comparisons that show statistically significant differences among the groups, with p < 0.05, are indicated as follows: *:Atg7−/−/Veh versus Atg7F/F/veh or Atg7−/−/Rapa versus Atg7F/F/Rapa; **: Atg7F/F/veh versus Atg7F/F/Rapa; and #: Atg7−/−/Rapa versus Atg7−/−/veh (N=3, one-way ANOVA with Bonferroni’s multiple comparison test). (e–f) Representative images (e) and quantification (f) of immunostaining of NHE3 in the ileum from Atg7F/F and Atg7−/− mice (*p<0.05, t-test, N= 3).
We next evaluated the effect of inactivation of autophagy on NHE3 expression. We observed a robust (6-fold) elevation of total NHE3 in the intestinal epithelial cells of Atg7−/− mice (Figure 4c–d), suggesting that basal autophagy is strongly involved in regulating NHE3 under basal conditions. As observed, rapamycin treatment reduces NHE3 levels in intestinal epithelial cells from control mice. In contrast, the amount of NHE3 in epithelial cells of rapamycin-treated Atg7−/− mice remained 3-fold higher compared to that of control mice. Thus, deletion of Atg7 appears to partially block the ability of rapamycin to reduce NHE3 levels, suggesting linkage between rapamycin-mediated stimulation of autophagy and down-regulation of NHE3. We also observed a moderate elevation of total NHERF1 in Atg7−/− mice. While rapamycin reduced NHERF1 expression in control mice, it had no significant effect in Atg7−/− mice. Likewise, there was no statistically significant impact on the levels of Ezrin and p-Ezrin. The levels of surface NHE3 and NHERF1 in the membrane fraction were further evaluated. There was an approximately 4-fold increase in surface expression of NHE3 in Atg7−/− mice. Rapamycin treatment reduced surface NHE3 expression in both control and Atg7−/− mice. However, the levels of surface/total NHE3 remained higher in rapamycin-treated Atg7−/− mice (Figure 4c–d). Immunohistochemistry revealed a significant increase of apical NHE3 in Atg 7−/− mice (Figure 4e–f). NHERF1 in the membrane fraction was also increased in Atg7−/− mice, which appears to be unaffected by rapamycin treatment (Figure 4d).
Effect of High Doses of Rapamycin on NHE3 Expression in Mice
Although our cohort of renal transplant recipients was continuously treated with rapamycin, diarrhea occurred only when the serum rapamycin levels were acutely increased (Figure 1). We therefore examined if high levels of rapamycin have an adverse impact on NHE3 expression. Previous studies have reported that low doses of rapamycin (0.5–100 nM) inhibit mTORC1 and stimulate autophagic activity5. However, at high doses (0.2–20 μM) rapamycin also inhibits mTORC2, which leads to decreased phosphorylation of Akt at Ser473, resulting in Akt inactivation7, 34. To simulate the serum spikes (2–7 times basal levels) observed in patients on rapamycin therapy, mice were treated with a single high dose of rapamycin (20 mg/kg). Ileal tissues were harvested 2 h after dosing and analyzed by western blot. Significant down-regulation of phosphorylation of both S6 and Akt (at Ser473) was observed (Figure 5a–b), suggesting that both mTORC1 and mTORC2 are inhibited at higher doses of rapamycin. Interestingly, acute exposure to high doses of rapamycin had little effect on the total levels of NHE3 and NHERF1 (Figure 5a–b). However, we observed markedly decreased levels of surface NHE3 and NHERF1 in the membrane fractions (Figure 5a–b).
Figure 5. An acute effect of high-dose rapamycin on NHE3 expression.
(a–b) Western blot analysis (a) and quantification (b) of NHE3, NHERF1, total and p-Ezrin, total and p-S6, total Akt and p-Akt at Ser473. (a) Surface biotinylation assay and quantification (b) of NHE3 and NHERF1 in the membrane fraction of intestinal tissue from vehicle- and rapamycin-treated mice (*p<0.05, t-test, N=3). (c) Representative traces showing the effect of rapamycin on Na+/H+ activity in PS120 cells stably expressing NHE3 and NHERF2. Bar graph showing Vmax (d) and K(H+)i (μM) (e) in vehicle- and rapamycin-treated groups (*P<0.05, One way ANOVA with Dunnett’s post hoc test, N=3).
Effect of Rapamycin on NHE3 Activity in PS120 Cells
To determine if rapamycin has a direct inhibitory effect on NHE3 exchange activity, we used PS120 cells that were stably transfected with HA-human NHE3 and NHERF2, as NHERF2 is required for many aspects of regulation of NHE3 activity22, 23, 30. Acute treatment with rapamycin at 200 nM resulted in a 36% and a 64% decrease in Vmax in NHE3 transporter activity as well as a 1.5- and 2.6-fold increase in Km at 20 min and 30 min, respectively (Figure 5c–e).
NHE3 Expression in mTOR-Deficient Mice
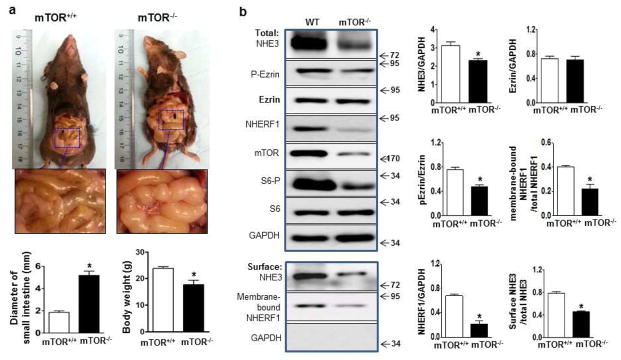
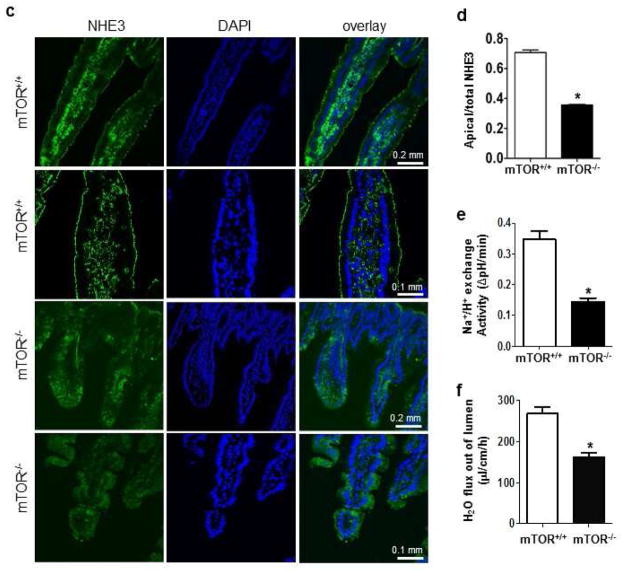
Having demonstrated that chronic inhibition of mTOR by rapamycin reduces the total levels of NHE3, and acute inhibition of mTOR at higher doses of rapamycin reduces surface levels of NHE3, we next investigated if reduced NHE3 expression is through direct inhibition of mTOR in intestinal epithelial cells. We generated mTORf/f:Villin-cre (mTOR−/−) mice to delete mTOR conditionally in intestinal epithelial cells (Suppl. Fig 1). Animals were viable. However, about 30% of them displayed low body weight compared with control mice from the same dam, suggesting growth stunting (Figure 6a). Interestingly, these mice also exhibited dilated intestines (approximately twice normal size), containing large amount of liquid stools (Figure 6a). Intestinal epithelial cells were isolated from the ileum of mTOR−/− and control mice. mTOR protein levels were markedly reduced in mTOR−/− mice. A small amount of mTOR protein was present in the samples prepared from mTOR−/− mice, which perhaps originated from non-epithelial cells. Analysis of total NHE3 and NHERF1 revealed the amounts to be significantly reduced in mTOR−/− mice (Figure 6b). Tissue surface biotinylation assay revealed the levels of surface NHE3 and NHERF1 in the membrane fractions also to be reduced in mTOR−/− mice (Figure 6b). Immunohistochemistry detected a significant reduction of apical NHE3 in mTOR−/− mice (Figure 6c–d). By using two-photon confocal microscopy with a ratiometric pH-sensitive dye (SNARF-4FAM) 27, 30, we observed a significant reduction in Na+/H+ exchange activity in the ileum of mTOR−/− mice (Figure 6e). By applying a perfusion assay to the small intestine 35, we confirmed a marked reduction of water absorption in the small intestine of mTOR−/− mice (Figure 6f).
Figure 6.
The effect of mTOR deletion on NHE3 expression. (a) Representative images of exposed intestines and quantification of the diameter of intestines and body weight. (b) Western blot and surface biotinylation assay analyses of total and surface NHE3 and related proteins in intestinal epithelial cells. (b) Quantification of total and surface NHE3, total and membrane-bound NHEFR1. (c) Representative images and quantification (d) of immunostaining of NHE3 in mouse ileum. Experiments from mTORF/F and mTOR−/− mice were repeated at least 3 times (*p<0.05, t-test). (e) Na+/H+ exchange activity in ileal villus Na+ absorptive cells in mTORF/F and mTOR−/− mice. n=4, (*p<0.05, t-test). (f) Water absorption of small intestine in mTORF/F and mTOR−/− mice. n=5, (*p<0.05, t-test).
DISCUSSION
In the present study, we report that severe acute diarrhea develops following a sharp rise in serum rapamycin levels. Acute diarrhea tends to resolve when serum rapamycin levels return to their therapeutic baselines. We also provide evidence that the NHE3 level of the apical domains of intestinal cells is reduced by rapamycin. Using animal models, we demonstrated that chronic low doses of rapamycin reduce the total levels of NHE3, in part through activation of the autophagy pathway. In contrast, exposure of animals to a single high dose of rapamycin impairs NHE3 transporter activity and reduces apical levels of NHE3 without causing changes in the total levels of NHE3. Thus, there appears to be two distinct mechanisms that explain the dose-dependent inhibition of NHE3 by rapamycin. Finally, we confirmed that deletion of mTOR (to inactivate both mTORC1 and mTORC2) in intestinal epithelial cells results in reductions of both total and apical NHE3 in the small intestine, and reduces both Na+/H+ exchange activity and water absorption. Significantly, a portion of these mice developed fluid-filled dilated intestine, recapitulating the diarrhea in patients. These findings demonstrate a critical role of the mTOR/autophagy pathway in the expression of NHE3 and its homeostasis.
Linkages between Serum Rapamycin Spikes and Diarrhea
We observed that diarrheal episodes were associated with a sharp rise in serum levels of rapamycin in patients receiving prolonged rapamycin treatment. Because diarrhea causes excessive fluid loss, it is conceivable that the observed serum rapamycin spikes could be related to dehydration. However, dehydration alone is unlikely to be the primary cause, as serum levels of tacrolimus, a co-administered immunosuppressant, were not found to be significantly elevated during diarrheal episodes. Previous studies have suggested that fluctuations (5–6 fold) of serum rapamycin levels might be due to variations in medication or to diet, changes in dietary fats in particular36. In the present study, however, we were unable to address the cause of the serum rapamycin spikes. In our animal models, we treated animals with 5 mg/kg/day, a relatively low dose that has been used in previous studies28, 37. This dosage is sufficient to reduce phosphorylation of S6, a downstream substrate of mTORC1, but had little effect on phosphorylation of Aktser473 (Figure 3a–b), the target of mTORC2. However, rapamycin at 20 mg/kg inhibited phosphorylation of Aktser473 (Figure 5a–b), suggesting that this fourfold increase in rapamycin resulted in inhibition of both mTORC1 and mTORC2. However, the pharmacokinetics of rapamycin may vary between rodents and humans28, thereby limiting the utility of rapamycin dosing in rodents when modeling human conditions. In the present study, we evaluated the levels of surface NHE3 in the terminal ileum of two groups: controls and diarrheic transplant recipients treated with rapamycin. However, a lack of biopsies precluded us from examining this aspect in transplant recipients who received chronic treatment with rapamycin but did not have diarrhea. Our rodent models were used to model chronic exposure to rapamycin in humans. Given that chronic treatment with a low dose of rapamycin reduced the total levels of NHE3 expression, it may be that expression of NHE3 is reduced in the intestine of renal recipients under a rapamycin regimen.
Two Distinct Routes Regulate NHE3
NHE3 appears to be constantly turned over by autophagy under normal conditions, as we observed a marked accumulation of NHE3 in intestinal epithelial cells in Atg7−/− mice. Deletion of mTOR from intestinal epithelial cells reduced NHE3 expression, further suggesting a homeostatic role of both the mTOR and autophagy pathways on NHE3 regulation. The inhibitory effect of rapmaycin on NHE3 expression is significantly reduced when autophagy is inactivated, suggesting that rapamycin-induced down-regulation of NHE3 is mediated at least partially through the autophagy pathway. This highlights a significant role for autophagy in regulating homeostasis of NHE3 in intestinal epithelial cells when mTOR or autophagy activity is altered, such as by stress or starvation. However, we observed that, even when autophagy was inactivated, NHE3 expression was partially inhibited by rapamycin. This could reflect contamination from other villin-cre negative cells in our sample preparation. We also cannot rule out unrecognized mechanisms such as protein translation or systemic effects by rapamycin that regulate expression of NHE3.
Rapamycin is well known to have a dose-dependent effect on the activity of mTOR complexes. It inhibits mTORC1 at low doses whereas mTORC2 at higher doses6. In the present study, we found that long-term treatment with rapamycin at a low dose (5mg/kg/day) appears to inhibit mTORC1 (indicated by reduced phosphorylation of S6), without affecting mTORC2 (indicated by unchanged phosphorylation of Aktser473). These data suggest that rapamycin-mediated stimulation of autophagy via inhibition of mTORC1 is an important mechanism of down-regulation of intestinal NHE3. We demonstrated that acute administration of rapamycin at a high dose (20 mg/kg) was associated with: 1) reduced phosphorylation of AktSer473, a downstream target of mTORC26; 2) reduced surface expression of NHE3 and NHERF1 in the membrane fraction without significant influence on total expression of NHE3 in intestinal epithelial cells; and 3) reduction of NHE3 transporter activity in PS120 acutely treated with 0.2μM rapamycin. Therefore, acute exposure to high serum levels of rapamycin appears to impact NHE3 activity via a different route from that elicited by long-term exposure to a low dose of rapamycin.
Previous studies have shown that activation of Akt stimulates NHE3 activity in PS120 fibroblasts38 and promotes NHE3 apical movement with activation20, 21. It is conceivable that inactivation of Akt due to mTORC2 inhibition may contribute to reduced NHE3 surface expression, although the precise mechanism remains to be elucidated. Given that NHERF1 plays a critical role in regulating NHE3 membrane targeting and the fact that its levels in the membrane fraction are reduced when exposed to rapamycin, it is possible that down-regulation of NHE3 in the apical membrane involves NHERF1. We observed that rapamycin decreased the Vmax and increased the Km of NHE3 transporter in PS120 cells. This is consistent with the observed reduction in surface levels of NHE3 in intestinal epithelial cells, plus an effect on NHE3 Na+/H+ turnover number, possibly by a mechanism yet to be described.
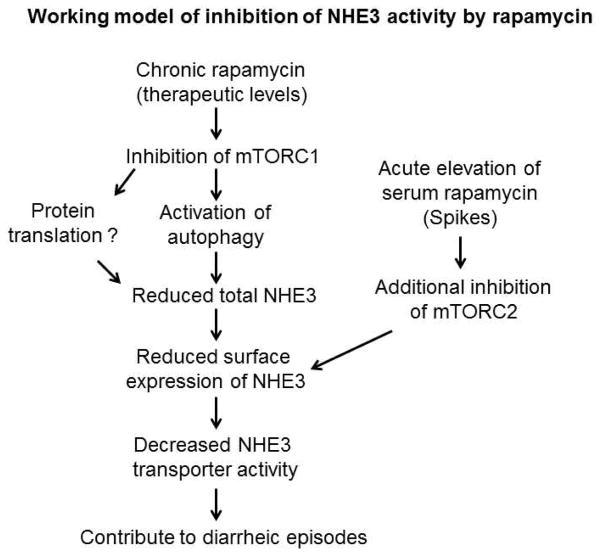
A “Double Hit” Model that Causes Acute Diarrhea
Although renal recipients in this study were constantly exposed to rapamycin at therapeutic levels that are sufficient to inhibit mTOR, acute diarrhea in this cohort occurred only when serum rapamycin levels were significantly elevated above the baseline. Data from our animal studies indicate that low doses of rapamycin which inhibit mTORC1, can be thought of as the ‘primary hit’ in the reduction of total NHE3 expression. However, when a single high dose of rapamycin was given, mTORC2 appears to also be inhibited. This ‘second hit’ caused by increased serum rapamycin could further alter NHE3 membrane-targeting, contributing to the production of acute, non-infectious diarrhea (Figure 7). Consistent with this notion, a portion of rats (3/16) treated with a high dose rapamycin for two weeks developed fluid-filled dilated intestine (Suppl. Figure 2). Moreover, animals with deletion of mTOR, thereby inactivating both mTORC1 and mTORC2, had altered NHE3 homeostasis and also developed diarrhea (Figure 6a). Thus, these animal models largely recapitulate the clinical observations of patients who developed serum spikes of rapamycin.
Figure 7. A proposed model of inhibition of NHE3 by rapamycin.
Rapamycin inhibits NHE3 activity through two distinct routes: 1) chronic exposure to rapamycin at a therapeutic dose stimulates autophagic activity, contributing in part to the down-regulation of NHE3; and 2) further elevation of serum rapamycin additionally impairs NHE3 activity through acute inhibition of membrane trafficking of NHE3. This described a mechanism that potentially contributes to the development of diarrhea.
Mechanism of Autophagy-mediated Down-Regulation of NHE3 and NHERF1
We observed that the total levels of NHERF1 parallel the change of total levels of NHE3 observed upon rapamycin treatment or from deletion of mTOR or Atg7 in mice. Although autophagy could independently mediate the turnover of NHE3 and/or NHERF1, given that NHERF1 interacts with NHE3, it is also conceivable that NHE3/NHERF1 together as a complex could be targeted by autophagy. Of note, the effect of rapamycin on total NHERF1 in rats is not significant, whereas its effect in mice is marked. This may reflect species-related differences in the effect of rapamycin, similar to that observed with p62, whose levels were significantly reduced by rapamycin in rats but not in mice. The reduction of surface expression of NHE3 and NHERF1 in the membrane fraction could be attributed to: 1) reduction of total levels of NHE3 and NHERF1 (except NHERF1 in rats); 2) impairment of membrane targeting; and 3) both mechanisms. NHERF1 and its family of related proteins have been implicated in regulating membrane-targeting of NHE323, 39. It is conceivable that mTOR inhibition and autophagy activation could impair NHERF1 function, which could in turn reduce the levels of NHE3 and NHERF1 in the membrane fraction. Nevertheless, the mechanisms underlying how mTOR inhibition and/or autophagy activation reduce NHE3 expression needs to be elucidated.
Summary
We conclude that rapamycin suppresses NHE3 activity through two distinct mechanisms: 1) by autophagic degradation of NHE3 as a consequence of long-term exposure to therapeutic levels of rapamycin; and 2) by acute inhibition of surface expression of NHE3 by high levels of serum rapamycin. These two distinct routes (Figure 7) could act together to severely inhibit NHE3 activity, contributing to the development of diarrhea in renal transplant recipients. Given that rapamycin and its analogs have been increasingly used in many medical conditions, including cancer, autoimmune dysfunction, and organ transplantation, understanding the mechanisms of rapamycin-associated diarrhea has at least three significant clinical and economic implications. First, this non-infectious etiology should be considered when the serum rapamycin level is higher than baseline. Secondly, understanding and controlling the factors that lead to serum spikes of rapamycin could significantly reduce the incidence of rapamycin-associated diarrhea. Finally, an understanding of the mechanisms of rapamycin-associated diarrhea may lead to the identification of compensating therapeutic practices that can mitigate the negative effects of this valuable family of immunosuppressive drugs.
Supplementary Material
Acknowledgments
This work was supported by NIDDK: K08DK088950 & R03 DK099566 (XJZ) and NINDS: NS062068 (YFH). We thank Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) for providing Atg7flox/flox mice. We thank Drs. Mark Donowitz and Bradford A Kay for their critique and comments on the manuscript.
Abbreviations
- mTOR
Mammalian Target of rapamycin
- NHE3
Na+/H+ exchanger 3
Footnotes
Disclosures: no disclosure
Contributions: YJ and XZ: data acquisition and analysis. PA, AGS, P R and DM: chart review. CD, JM, and JD: material support. HYF and ZXJ: study concept and design, data interpretation and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bunnapradist S, Neri L, Wong W, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51:478–86. doi: 10.1053/j.ajkd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg PM, Thuluvath PJ. Diarrhea in liver transplant recipients: etiology and management. Liver Transpl. 2005;11:881–90. doi: 10.1002/lt.20500. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib AA. Sirolimus-induced intractable chronic diarrhea: a case report. Transplant Proc. 2006;38:1298–300. doi: 10.1016/j.transproceed.2006.02.123. [DOI] [PubMed] [Google Scholar]
- 4.Dias VC, Madsen KL, Mulder KE, et al. Oral administration of rapamycin and cyclosporine differentially alter intestinal function in rabbits. Dig Dis Sci. 1998;43:2227–36. doi: 10.1023/a:1026610404647. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura A, Hara K, Yamamoto K, et al. Role of the mTOR complex 1 pathway in the in vivo maintenance of the intestinal mucosa by oral intake of amino acids. Geriatr Gerontol Int. 2012;12:131–9. doi: 10.1111/j.1447-0594.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz OH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–5. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francavilla A, Starzl TE, Scotti C, et al. Inhibition of liver, kidney, and intestine regeneration by rapamycin. Transplantation. 1992;53:496–8. doi: 10.1097/00007890-199202010-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadwell K, Patel KK, Komatsu M, et al. A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy. 2009;5:250–2. doi: 10.4161/auto.5.2.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun CH, Tse CM, Nath SK, et al. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol. 1995;269:G1–11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 15.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–5. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol. 2003;285:C1246–54. doi: 10.1152/ajpcell.00598.2002. [DOI] [PubMed] [Google Scholar]
- 17.Donowitz M, Mohan S, Zhu CX, et al. NHE3 regulatory complexes. J Exp Biol. 2009;212:1638–46. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gawenis LR, Stien X, Shull GE, et al. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G776–84. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 19.Ledoussal C, Woo AL, Miller ML, et al. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1385–96. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Leu S, Cheong A, et al. Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation. Gastroenterology. 2004;126:122–35. doi: 10.1053/j.gastro.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 21.Shiue H, Musch MW, Wang Y, et al. Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J Biol Chem. 2005;280:1688–95. doi: 10.1074/jbc.M409471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Cha B, Zachos NC, et al. Elevated calcium acutely regulates dynamic interactions of NHERF2 and NHE3 proteins in opossum kidney (OK) cell microvilli. J Biol Chem. 2011;286:34486–96. doi: 10.1074/jbc.M111.230219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Singh V, Cha B, et al. NHERF2 protein mobility rate is determined by a unique C-terminal domain that is also necessary for its regulation of NHE3 protein in OK cells. J Biol Chem. 2013;288:16960–74. doi: 10.1074/jbc.M113.470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon J, Huang X, Yang J, et al. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32:15704–14. doi: 10.1523/JNEUROSCI.2392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans GS, Flint N, Somers AS, et al. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–31. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 26.Levine SA, Montrose MH, Tse CM, et al. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem. 1993;268:25527–35. [PubMed] [Google Scholar]
- 27.Murtazina R, Kovbasnjuk O, Zachos NC, et al. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem. 2007;282:25141–51. doi: 10.1074/jbc.M701910200. [DOI] [PubMed] [Google Scholar]
- 28.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmid C, Heemann U, Tilney NL. Factors contributing to the development of chronic rejection in heterotopic rat heart transplantation. Transplantation. 1997;64:222–8. doi: 10.1097/00007890-199707270-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lin R, Murtazina R, Cha B, et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–71. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He C, Wei Y, Sun K, et al. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154:1085–99. doi: 10.1016/j.cell.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–6. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 33.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods in molecular biology. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 34.Moore SF, Hunter RW, Hers I. mTORC2 protein complex-mediated Akt (Protein Kinase B) Serine 473 Phosphorylation is not required for Akt1 activity in human platelets [corrected] J Biol Chem. 2011;286:24553–60. doi: 10.1074/jbc.M110.202341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayburgh DR, Musch MW, Leitges M, et al. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–94. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerman JJ, Ferron GM, Lim HK, et al. The effect of a high-fat meal on the oral bioavailability of the immunosuppressant sirolimus (rapamycin) J Clin Pharmacol. 1999;39:1155–61. [PubMed] [Google Scholar]
- 37.Bishu K, Ogut O, Kushwaha S, et al. Anti-remodeling effects of rapamycin in experimental heart failure: dose response and interaction with Angiotensin receptor blockade. PLoS One. 2013;8:e81325. doi: 10.1371/journal.pone.0081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee-Kwon W, Johns DC, Cha B, et al. Constitutively active phosphatidylinositol 3-kinase and AKT are sufficient to stimulate the epithelial Na+/H+ exchanger 3. J Biol Chem. 2001;276:31296–304. doi: 10.1074/jbc.M103900200. [DOI] [PubMed] [Google Scholar]
- 39.Sarker R, Valkhoff VE, Zachos NC, et al. NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity. Am J Physiol Cell Physiol. 2011;300:C771–82. doi: 10.1152/ajpcell.00119.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.