Abstract
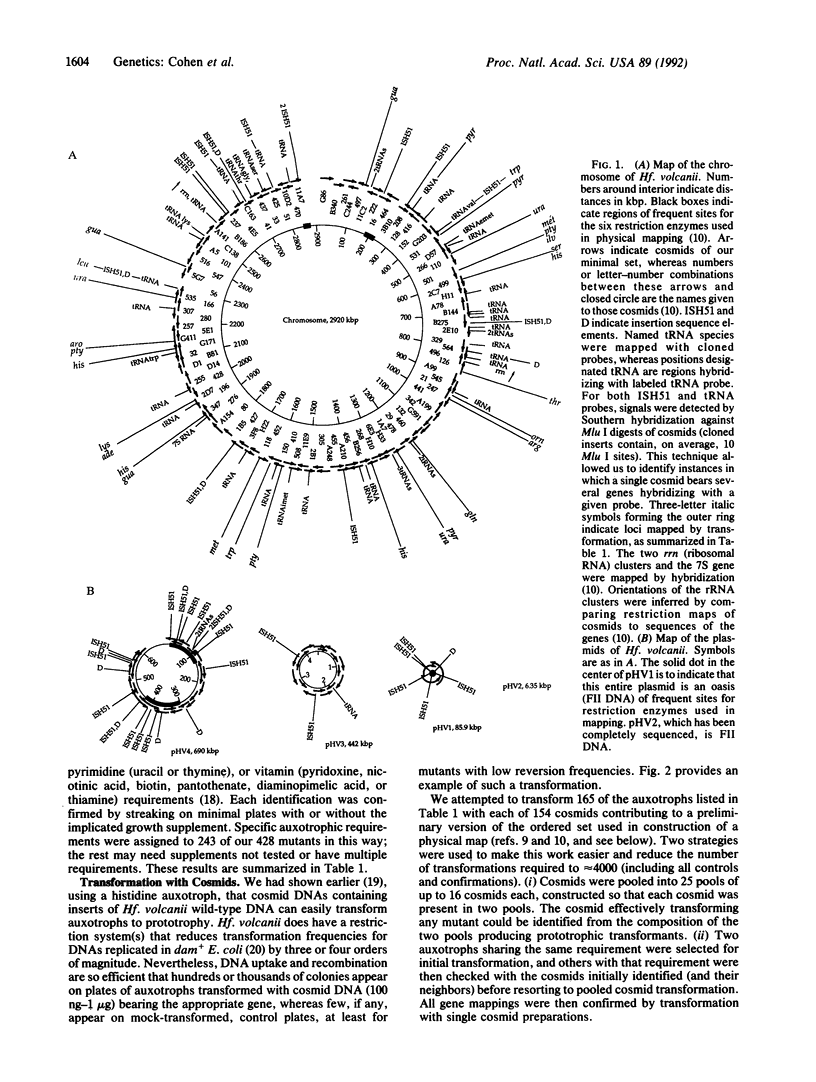
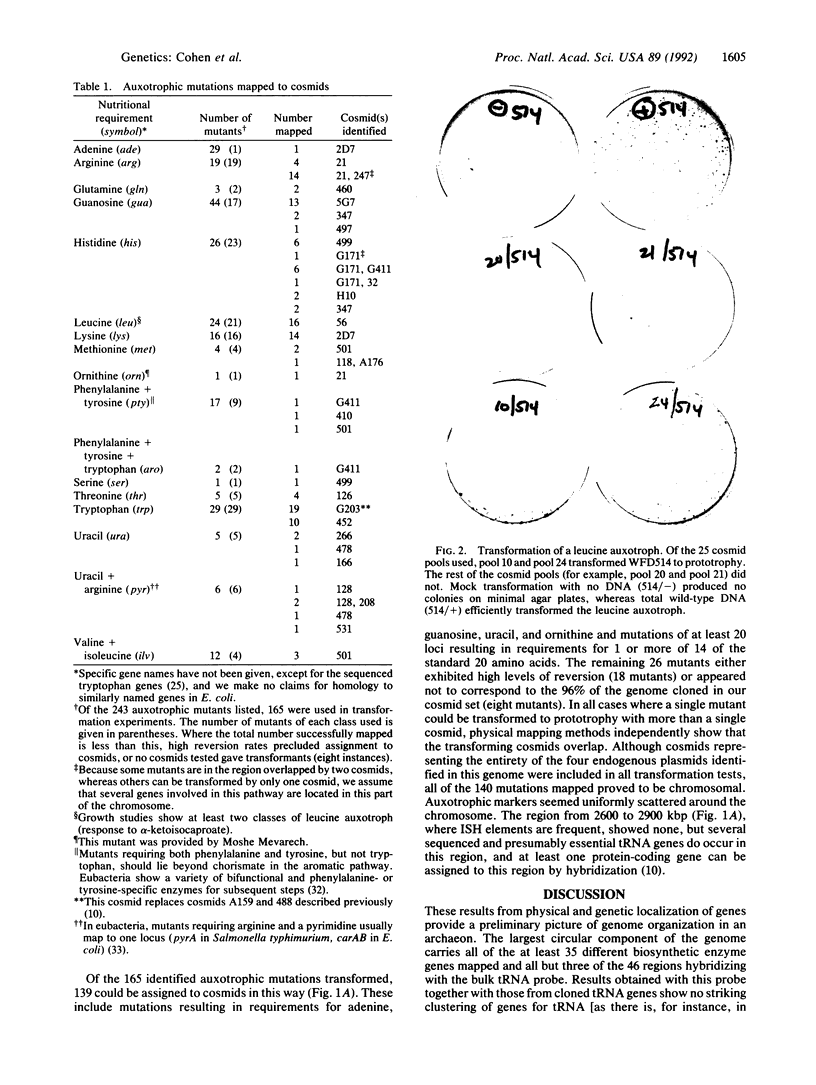
We have assigned genetic markers to locations on the physical map of the genome of the archaeon Haloferax volcanii, using both a physical method (hybridization) and a more specific genetic technique (transformation with cosmids). Hybridizations were against restriction digests of each of 151 cosmids making up a minimally overlapping set and covering 96% of the genome. Results with a cloned insertion sequence and a tRNA probe indicated that transposable elements are concentrated on two of the four plasmids of this species, whereas regions complementary to tRNA are largely chromosomal. For a genetic analysis of genes involved in the biosynthesis of amino acids, purines, and pyrimidines, we used cosmid transformation to assign 139 of 243 ethyl methanesulfonate-induced auxotrophic mutations, generated and characterized for this study, to single cosmids or pairs of cosmids from the minimal set. Mutations affecting the biosynthesis of uracil, adenine, guanine, and 14 amino acids have been mapped in this way. All mutations mapped to the 2920-kilobase-pair chromosome of Hf. volcanii and seemed uniformly distributed around this circular replicon. In some cases, many mutations affecting a single pathway map to the same or overlapping cosmids, as would be expected were genes for the pathway linked. For other biosynthetic pathways, several unlinked genetic loci can be identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer J., Lechner K., Böck A. Gene organization and structure of two transcriptional units from Methanococcus coding for ribosomal proteins and elongation factors. Can J Microbiol. 1989 Jan;35(1):200–204. doi: 10.1139/m89-031. [DOI] [PubMed] [Google Scholar]
- Charlebois R. L., Hofman J. D., Schalkwyk L. C., Lam W. L., Doolittle W. F. Genome mapping in halobacteria. Can J Microbiol. 1989 Jan;35(1):21–29. doi: 10.1139/m89-004. [DOI] [PubMed] [Google Scholar]
- Charlebois R. L., Lam W. L., Cline S. W., Doolittle W. F. Characterization of pHV2 from Halobacterium volcanii and its use in demonstrating transformation of an archaebacterium. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8530–8534. doi: 10.1073/pnas.84.23.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlebois R. L., Schalkwyk L. C., Hofman J. D., Doolittle W. F. Detailed physical map and set of overlapping clones covering the genome of the archaebacterium Haloferax volcanii DS2. J Mol Biol. 1991 Dec 5;222(3):509–524. doi: 10.1016/0022-2836(91)90493-p. [DOI] [PubMed] [Google Scholar]
- Cline S. W., Doolittle W. F. Efficient transfection of the archaebacterium Halobacterium halobium. J Bacteriol. 1987 Mar;169(3):1341–1344. doi: 10.1128/jb.169.3.1341-1344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Lam W. L., Charlebois R. L., Schalkwyk L. C., Doolittle W. F. Transformation methods for halophilic archaebacteria. Can J Microbiol. 1989 Jan;35(1):148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- Conover R. K., Doolittle W. F. Characterization of a gene involved in histidine biosynthesis in Halobacterium (Haloferax) volcanii: isolation and rapid mapping by transformation of an auxotroph with cosmid DNA. J Bacteriol. 1990 Jun;172(6):3244–3249. doi: 10.1128/jb.172.6.3244-3249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. K., Hawkins L. K., Gupta R. Presence of an intron in elongator methionine-tRNA of Halobacterium volcanii. Can J Microbiol. 1989 Jan;35(1):189–194. doi: 10.1139/m89-029. [DOI] [PubMed] [Google Scholar]
- Fan J. B., Korman S. H., Cantor C. R., Smith C. L. Giardia lamblia: haploid genome size determined by pulsed field gel electrophoresis is less than 12 Mb. Nucleic Acids Res. 1991 Apr 25;19(8):1905–1908. doi: 10.1093/nar/19.8.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Hofman J. D., Schalkwyk L. C., Doolittle W. F. ISH51: a large, degenerate family of insertion sequence-like elements in the genome of the archaebacterium, Halobacterium volcanii. Nucleic Acids Res. 1986 Sep 11;14(17):6983–7000. doi: 10.1093/nar/14.17.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Nuttall S. D., Dyall-Smith M. L. Construction and use of halobacterial shuttle vectors and further studies on Haloferax DNA gyrase. J Bacteriol. 1991 Jun;173(12):3807–3813. doi: 10.1128/jb.173.12.3807-3813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Cohen A., Tsouluhas D., Doolittle W. F. Genes for tryptophan biosynthesis in the archaebacterium Haloferax volcanii. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6614–6618. doi: 10.1073/pnas.87.17.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Doolittle W. F. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5478–5482. doi: 10.1073/pnas.86.14.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985 Apr;162(1):461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh U. G., Kurtti T. J., Ross S. E. Electrophoretic characterization of chromosomal DNA from two microsporidia. J Invertebr Pathol. 1990 Sep;56(2):243–248. doi: 10.1016/0022-2011(90)90107-h. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Betlach M. Genome organization in Halobacterium halobium: a 70 kb island of more (AT) rich DNA in the chromosome. Mol Gen Genet. 1985;198(3):449–455. doi: 10.1007/BF00332938. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Blaseio U. Transposition burst of the ISH27 insertion element family in Halobacterium halobium. Nucleic Acids Res. 1990 Dec 11;18(23):6921–6925. doi: 10.1093/nar/18.23.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzmann J., Klein A. Physical and genetic map of the Methanococcus voltae chromosome. Mol Microbiol. 1991 Feb;5(2):505–513. doi: 10.1111/j.1365-2958.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Palm P., Reiter W. D., Gropp F., Pühler G., Klenk H. P. Comparative evaluation of gene expression in archaebacteria. Eur J Biochem. 1988 May 2;173(3):473–482. doi: 10.1111/j.1432-1033.1988.tb14023.x. [DOI] [PubMed] [Google Scholar]



