Abstract
Objective
Increased plasma concentrations of angiotensin II (Ang II) have been implicated in many cardiovascular diseases such as atherosclerosis, aortic aneurysms and myocardial infarction in humans. However, it is not known whether high levels of plasma Ang II affect coronary plaque stability and subsequent myocardial infarction. The current study was designed to examine whether elevated plasma Ang II can directly induce coronary events such as acute coronary syndrome.
Approach and Results
To examine the above hypothesis, we infused Ang II [100 ng/min/kg (low group) and 200 ng/min/kg (high group)] or saline vehicle via osmotic minipumps into WHHL rabbits, a model of human familial hypercholesterolemia and atherosclerosis. Infusion of Ang II resulted in mortality rates of 50% and 92% in the low- and high-Ang II groups, respectively, while there were no deaths in the vehicle group. Pathological analysis revealed that Ang II-infused WHHL rabbits that died showed myocardial infarction. Furthermore, Ang II-infused WHHL rabbits exhibited coronary plaque erosion and rupture that were associated with thrombosis.
Conclusions
These findings suggest that increased blood levels of Ang II can destabilize coronary plaques and trigger the thrombosis, which possibly induces myocardial infarction. The model described in this study provides a novel means for the study of human acute coronary syndrome.
Keywords: Angiotensin II, acute coronary syndrome, myocardial infarction, WHHL, hypercholesterolemia
Introduction
Angiotensin II (Ang II) is an octapeptide hormone that plays an important role in cardiovascular homeostasis. Ang II mediates blood pressure through vascular constrictive functions by binding to two types of Ang receptor (designated as AT1R and AT2R) on the target cells1, 2. Moreover, elevation of plasma Ang II has been shown to play pathophysiological roles in many cardiovascular diseases. Several lines of evidence suggest that Ang II is associated with coronary heart disease including acute coronary syndrome and myocardial infarction (MI)3-5. This notion was first suggested by the pathological observations that Ang II and ATR immunoreactive proteins were present in the lesions of coronary plaques of patients with unstable angina and acute MI6, 7. Second, patients with high plasma renin, a protein that is a rate-limiting factor for the production of Ang II, have a five-fold increase in the rate of MI compared with subjects who have a low plasma renin profile8. Furthermore, an angiotensin-converting-enzyme (ACE) inhibitor, which lowers the level of circulating Ang II, has been shown to reduce aortic atherosclerosis in hyperlipidemic rabbits9-11 and ATR blocker, losartan inhibits early atherosclerosis in monkeys12. In human studies; however, it is generally accepted that ACE inhibitors can reduce the risk of MI whereas it is controversial whether Ang II receptor blockers are beneficial or detrimental to MI13-15. In spite of this, genetic polymorphisms of the AT1R gene as well as the ACE gene are associated with a high risk for coronary events16-19. It is well known that Ang II infusion leads to the enhancement of atherosclerosis and the formation of aortic aneurysms in hyperlipidemic mice20, 21. In addition, Tsukuba hypertensive mice, which transgenically express both human angiotensinogen and renin genes, exhibited extensive atherosclerosis in the aortic root when challenged with a high-fat diet22.
Although there is a clear link between Ang II and the increased risk of MI23 and Ang II has been implicated in the development of atherosclerosis7, 24, it is not known whether elevated plasma Ang II per se can directly induce coronary events such as acute coronary syndrome.
To examine this hypothesis, we infused Ang II into Watanabe heritable hyperlipidemic (WHHL) rabbits, a model of human familial hypercholesterolemia, which spontaneously develop coronary atherosclerosis25. Our results demonstrated that the infusion of Ang II resulted in a high prevalence of death in these rabbits. Furthermore, coronary plaque erosion and thrombosis are frequently observed in the lesions of Ang II-infused WHHL rabbits, whereas such lesions were rarely found in the vehicle group. These results indicate that increased plasma levels of Ang II can lead to coronary complications.
Materials and Methods
Materials and methods are available in the online-only Data Supplement.
Results
Ang II infusion induces a high prevalence of WHHL rabbit death
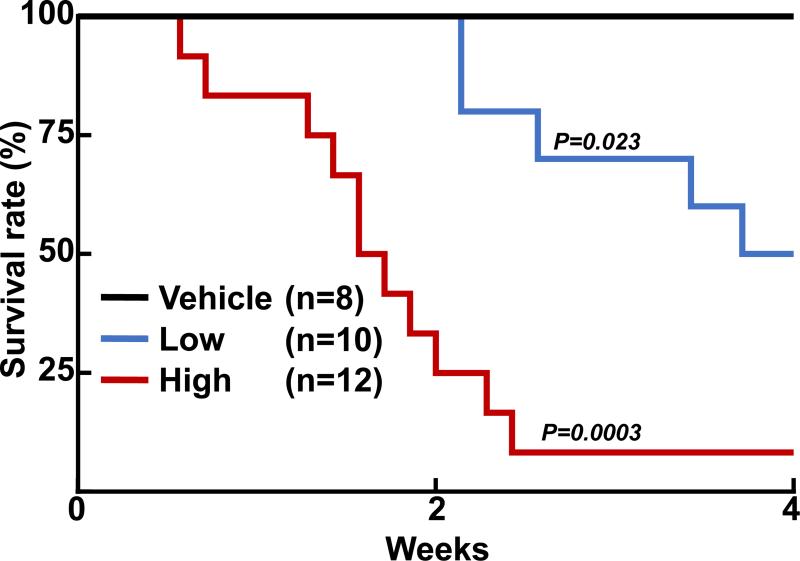
The first striking finding after Ang II infusion was gradual death of WHHL rabbits: the mortality rates reached 50% (5/10) in the low-Ang II group at 4 wks and 92% (11/12) in the high-Ang II group by 16 days (Fig. 1). Many rabbits looked inactive, showed dyspnea, and ate less before they died but two healthy rabbits died suddenly. These rabbits died at various times, while none of the vehicle rabbits died. Surviving rabbits of the Ang II groups at 4 wks showed high blood pressure along with an increased number of blood neutrophils and monocytes compared with the vehicle group (S-Fig. III), but plasma lipid levels were unchanged (S-Table III). Autopsy examinations revealed that all dead rabbits in both low-Ang II and high-Ang II groups showed severe pulmonary edema, congestion and/or hemorrhage (S-Fig. IV), which was not present in all surviving rabbits sacrificed at 4 wks. There were no abnormalities in other organs such as liver, kidneys, brain, adrenals, stomach and intestines. The pulmonary pathological features suggested that the death of the WHHL rabbits was possibly caused by acute left heart failure.
Figure 1.
Ang II infusion induces a high WHHL mortality rate. Kaplan-Meier analysis of cumulative rates of survival in WHHL rabbits after Ang II infusion.
Ang II infusion increases myocardial infarction
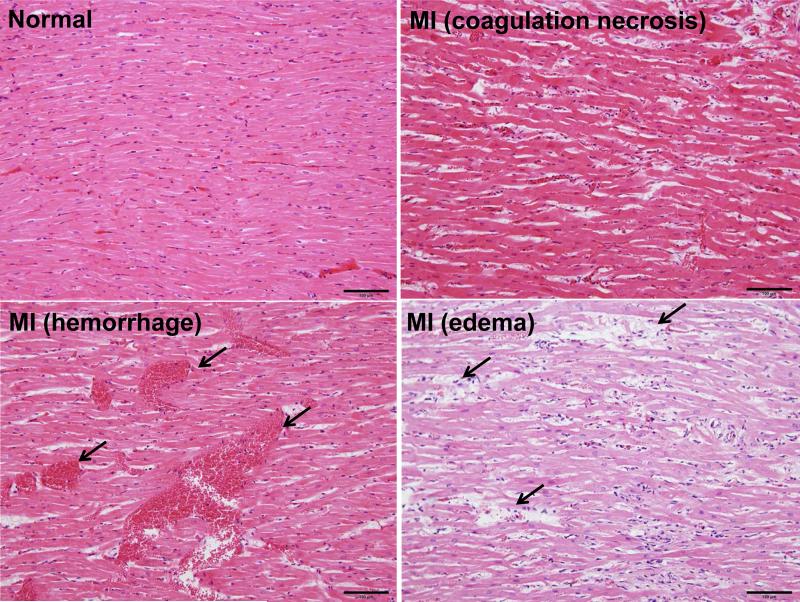
Histological examinations revealed that 80% (8/10) of low-Ang II and 100% (12/12) of high-Ang II groups exhibited histological features of fresh MI (MI), including myocardial eosinophilic degeneration, disappearance of striation, coagulation necrosis, edema, neutrophil infiltration and hemorrhage (Fig. 2). In addition, focal or diffuse fibrosis and calcification accompanied by mononuclear cell infiltration were observed (S-Fig. VA). MI changes were found in the right, left ventricle wall, interventricular septum and papillary muscles. In some areas, subendomyocardial infarction, transmural infarction, subepicardial infarction and pericarditis were observed (S-Fig. VB-C). MI changes were also observed in one rabbit in the vehicle group.
Figure 2.
Representative micrographs of fresh MI in WHHL rabbits induced by Ang II infusion. Compared with the normal heart structure (top left), diffuse myocardial eosinophilic degeneration and coagulation necrosis, edema and hemorrhage (indicated by arrowheads) were seen in the hearts of Ang II-infused WHHL rabbits. These features represent those of fresh MI. All sections are stained with HE staining.
Coronary atherosclerotic lesions
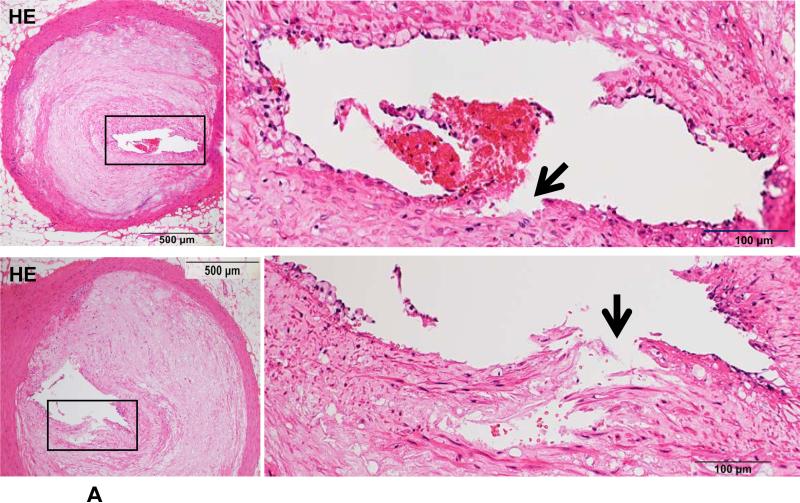
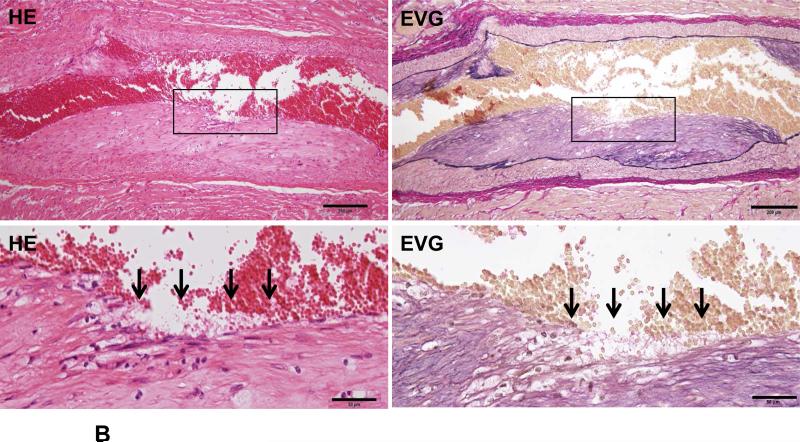
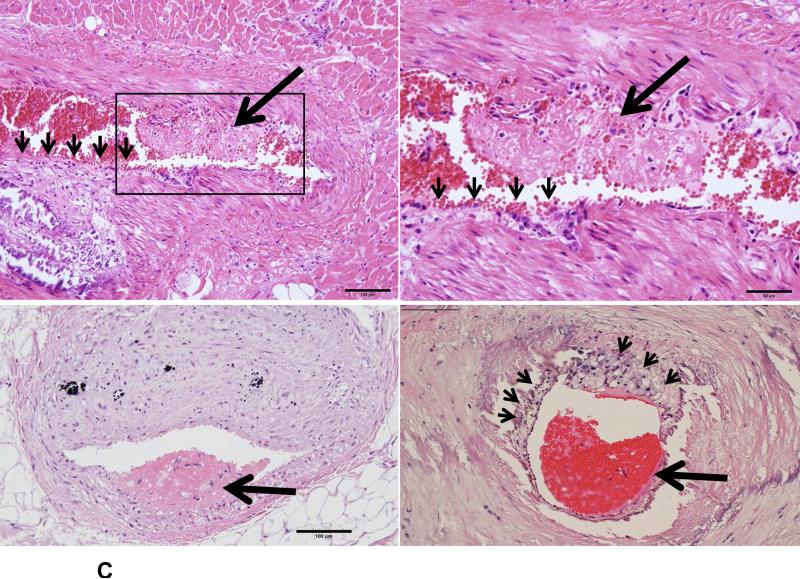
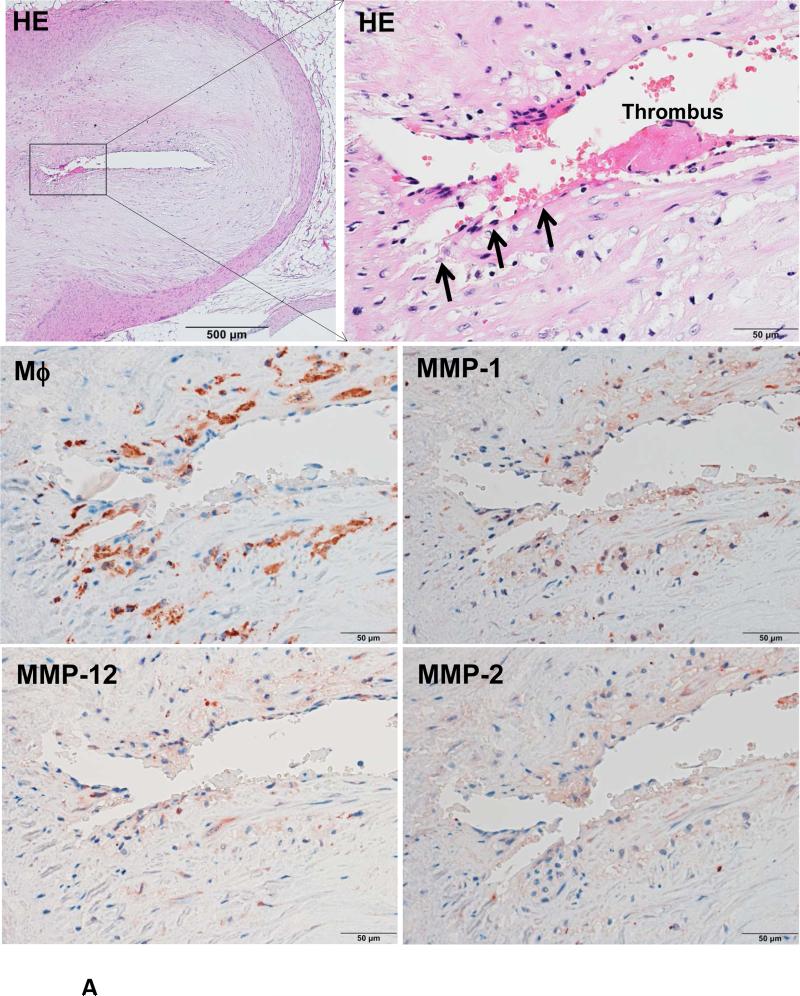
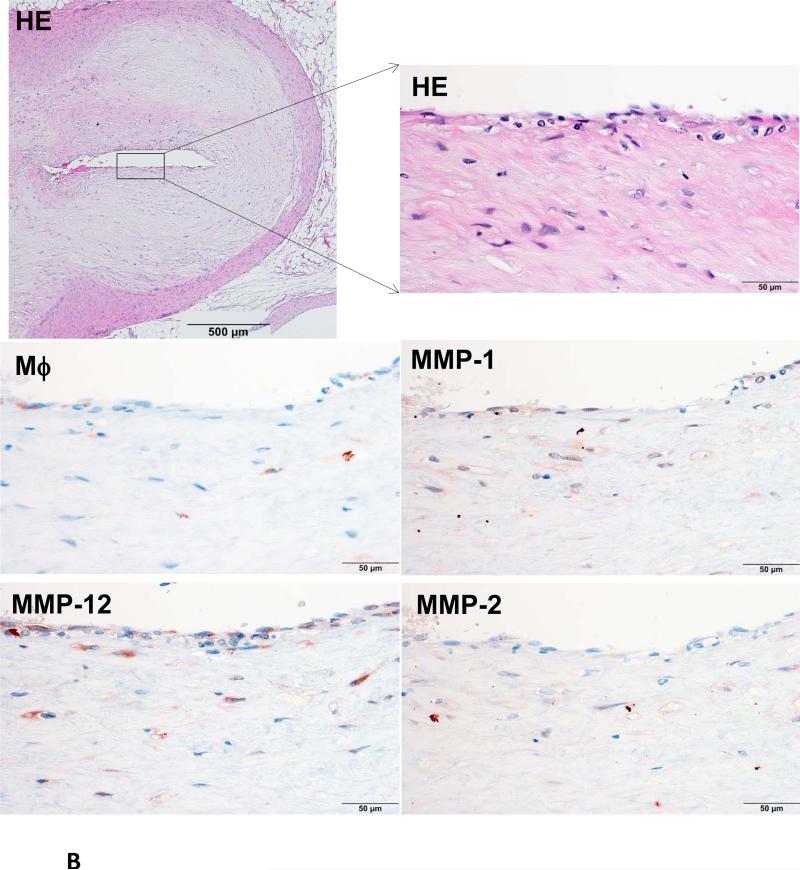
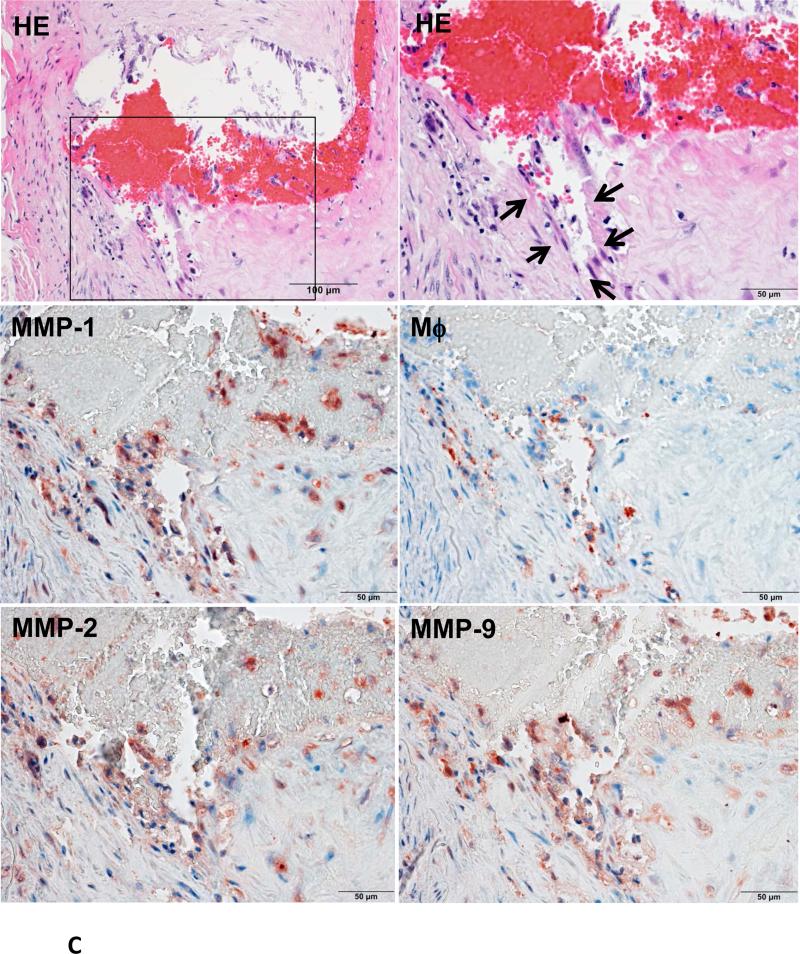
Because Ang II infusion led to a high prevalence of MI and a high mortality rate, we examined whether coronary plaque erosion/rupture was present, which may be responsible for MI observed in Ang II-infused WHHL rabbits. The whole hearts were dissected into 5 blocks and the coronary lesions were investigated extensively as described in the Materials and Methods and shown in S-Fig. VI. Although coronary atherosclerosis was observed in different sized-arteries varying from large epicardial arteries (blocks I and II) to small arteries and arterioles (blocks III-V), the lesions of epicardial arteries in blocks I (left coronary artery) and II (right coronary artery) were consistently present in all rabbits. These lesions were either characterized by fibrosis with more smooth muscle cells and few macrophages (S-Fig. VIIA) or by the accumulation of foam cells on the lumen surface or a typical necrotic core covered by a thin fibrous cap (S-Fig. VIIB-C). To examine whether Ang II can affect coronary complications, we observed all sections under a light microscope and found that erosion along with superimposed lumen thrombi was indeed present in Ang II-infused WHHL rabbits (Fig. 3A-C). The close association of coronary plaque erosion/rupture with thrombosis could be further illustrated using step sections (S-Fig. VIII). To show the lesion distribution, we quantified the number of coronary plaques with erosion, rupture and thrombosis in each block. As shown in Table 1, coronary erosion/rupture along with thrombosis were found in both low and high-Ang II groups but not in the vehicle group. These coronary erosions/rupture were mainly in the main trucks (epicardial arteries) of the left (blocks I, III) and the right (block II) but not seen in the small arteries and arterioles (blocks IV and V). Because matrix metalloproteinases (MMP) may be involved in the plaque rupture26, we examined MMP expression in the erosion/rupture area by immunohistochemical staining. MMP-1, -2, -9, and -12 immunoreactive proteins were stained in these areas especially where macrophages were present but not in the normal-appearance area of the same coronary artery (Fig. 4A-B). This was also seen in the lesions which were associated with thrombosis (Fig. 4C). Regardless of these morphologic features, all coronary lesions resulted in various degrees of coronary lumen stenosis although coronary stenosis of the Ang II groups was not significantly different from that of the vehicle group (S-Fig. IX).
Figure 3. Coronary plaque rupture (A), erosion (B) and thrombosis (C) in Ang II-infused WHHL rabbits.
A. Two representative micrographs of coronary plaque rupture are shown. The lesions show marked lumen stenosis (>80%). At high magnification, the top lesion shows distinct disruption (arrowhead) associated with a thrombus. The bottom lesion shows an intimal split (arrowhead) in which some red blood cells are contained.
B. The surface of a fibrotic plaque was partially eroded or defective (arrows), covered by blood cells. The left panel shows HE staining and the right panel shows EVG staining.
C. Typical mural thrombi are shown in three coronary lesions. The coronary lumen was partially filled with a thrombus (arrows) which contains fibrin and blood cells (top and bottom panels). Please note that opposite to the thrombus, the lesion surface is eroded (arrowheads) and calcification is seen at the bottom of the lesions (top panels). Foam cells are accumulated beneath the thin cap of a vulnerable plaque (arrowheads) (bottom right).
Table 1.
The number of coronary erosion, rupture and thrombosis in each block
| Erosions | |||||
|---|---|---|---|---|---|
| Block I | Block II | Block III | Block IV | Block V | |
| Vehicle (n=8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) |
| Ang II-L (n=10) | 1 ± 1.25 (5/10) | 0.7 ± 0.68* (6/10) | 0.4 ± 0.966 (2/10) | 0 ± 0 (0/10) | 0 ± 0 (0/10) |
| Ang II-H (n=12) | 1.08 ± 0.9** (9/12) | 1.0 ± 1.04* (7/12) | 0.73 ± 0.65** (7/12) | 0 ± 0 (0/12) | 0 ± 0 (0/12) |
|
Ruptures | |||||
| Block I | Block II | Block III | Block IV | Block V | |
| Vehicle (n=8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) |
| Ang II-L (n=10) | 0 ± 0 (0/10) | 0 ± 0 (0/10) | 0.1±0.316 (1/10) | 0 ± 0 (0/10) | 0 ± 0 (0/10) |
| Ang II-H (n=12) | 0.17 ± 0.58 (1/12) | 0 ± 0 (0/12) | 0 ± 0 (0/12) | 0 ± 0 (0/12) | 0 ± 0 (0/12) |
|
Erosions/rupture-associated thrombosis | |||||
| Block I | Block II | Block III | Block IV | Block V | |
| Vehicle (n=8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) | 0 ± 0 (0/8) |
| Ang II-L (n=10) | 1.0 ± 1.25 (5/10) | 0.6 ± 0.52* (6/10) | 0.5 ± 0.972 (3/10) | 0 ± 0 (0/10) | 0 ± 0 (0/10) |
| Ang II-H (n=12) | 1.33 ± 1.07** (9/12) | 1.0 ± 1.04* (7/12) | 0.73 ± 0.65* (7/12) | 0 ± 0 (0/12) | 0 ± 0 (0/12) |
The number of each type of lesions was calculated from all sections of each block. The largest number on the section was used to represent each block. The data are expressed as the mean ± SD.
P<0.05
P<0.01 vs vehicle group using the Mann-Whitney U test.
Parentheses indicate the ratio of animals with the lesions to total animal number. Thrombosis includes both partial and occluded thrombi.
Figure 4. Immunohistochemical staining of macrophages and MMPs.
A representative eroded lesion (arrowheads) with a thrombus on the surface shows accumulation of a few macrophages and stained with MMP-1, -2 and -12 Abs (A). In the normal area adjacent to the erosion of the same artery, there are only a few macrophages that are stained with MMP Abs (B). Another representative ruptured lesion (arrowheads) associated with an occlusive thrombus shows accumulated macrophages with MMPs staining at the rupture area (C).
Escalation increase of plasma Ang II also induces coronary complications and myocardial infarction
Experiment 1 described above showed that the abrupt increase of plasma Ang II resulted in high prevalence of MI and death, which may possibly be caused by or related with coronary atherosclerosis. To investigate whether escalation increase of plasma Ang II also exhibited such effects, we performed a second experiment in which WHHL rabbits were first infused with relatively low doses of Ang II for 4 wks and then further infused with high doses of Ang II for another 4 wks (S-Fig. I). Except for one rabbit in the low-Ang II group that died at 2 wks, all rabbits survived until 4 wks and further received another infusion of Ang II for another 4 wks (S-Fig. X). Similar to experiment 1, the levels of blood pressure (both groups) and blood leukocytes (high-Ang II group) were significantly higher in the Ang II groups than in the vehicle group (S-Fig. XI), but plasma lipids were unchanged (S-Table III). However, after changing to high doses of Ang II, 71% (5/7) of rabbits died in the high-Ang II group and one rabbit in the low-Ang II group died, although none of the rabbits died in the vehicle group (S-Fig. X). Pathological examinations disclosed that pulmonary edema/hemorrhage and MI were present in the Ang II groups, which was similar to experiment 1. MI was found in all dead rabbits (5/7 in the high-Ang II group and 2/7 in the low group), but not in the alive rabbits of both Ang II and control groups. Similar to the experiment 1, coronary plaque rupture/erosion or with thrombosis were found in Ang II groups (S-Table IV) but coronary stenosis of the Ang II groups was not significantly different from the vehicle group (S-Fig. XII).
Aortic atherosclerosis
Grossly, we did not observe aortic aneurysms (such as bulbous dilation of the aorta) in Ang II groups, as reported in Ang II-infused apoE KO mice20. Extensive aortic atherosclerotic lesions were observed in both Ang II and vehicle groups and occupied for more 80% of the aortic surface, but there was no significant difference between Ang II-infused groups and the vehicle group in both experiments (data not shown). Histological examinations showed that luminal surface macrophages of the aortic arch were increased in Ang II-infused groups (data not shown). Moreover, RT-PCR showed that aortic lesions of Ang II groups had significantly higher mRNA expression of interleukin-6 (IL-6) and plasminogen activator inhibitor-1 (PAI-1). Expression of monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), MMP-9 were also increased while expression of collagen I and III were reduced compared with those in the vehicle group, although the differences were not statistically significant (S-Fig. XIII).
Ang II infusion does not cause coronary plaque erosion and MI in wild-type JW rabbits
To rule out the possibility that Ang II infusion had any effects on the wild-type JW rabbits, we performed experiments using the same protocol. Although Ang II infusion led to the elevation of blood pressure in JW rabbits, as may be expected, there was no discernible atherosclerosis in both aortas and coronary arteries or MI (data not shown).
Discussion
In the current study, we demonstrated for the first time that increased plasma levels of Ang II led to a high prevalence of death in WHHL rabbits. All dead rabbits showed pathological features of MI and many of them also showed culprit coronary lesions, suggesting that excessive levels of Ang II can trigger coronary plaque erosion/rupture and possibly cause subsequent MI because plaque erosion/rupture was predominantly present in epicardial arteries. Although both abrupt (expt 1) and escalation (expt 2) increases of Ang II in the circulation increased the coronary complications, the death rate in the experiment 2 was lower than in the experiment 1, suggesting that both doses and durations of Ang II infusion affect the severity of coronary complications and MI. Nevertheless, the current study indicates that WHHL rabbits treated with Ang II may become a new model for investigation of human coronary atherosclerosis and its complications such as acute coronary syndromes of unstable angina, MI and sudden cardiac death27. Vulnerable plaques have been reported in the innominate (brachiocephalic) arteries of apoE KO mice28, 29. Aortic plaque rupture has been also attempted in cholesterol-fed rabbits injected with catecholamines and Russel's viper venom30. Although these models are valuable for the study of lesion development, these models bear little resemblance to human coronary plaque rupture or thrombosis. Metal stress or hypoxia can induce MI in hypercholesterolemic mice but plaque rupture and thrombosis have not been shown to be the underlying mechanism31. Furthermore, SR-B1 and apoE double KO mice developed high prevalence of myocardial death or MI due to the coronary occlusions rather than plaque rupture and subsequent thrombosis32. Therefore, Ang II-treated WHHL rabbits may be useful not only for disclosing many aspects of human ACS mechanisms but also for the development of new therapeutics. In the future studies, it will be interesting to investigate whether different therapeutics (such as lipid-lowering drugs, anti-hypertensive drugs, and anti-coagulant drugs) will prevent coronary complications or MI or death in this model.
In spite of this, the molecular mechanisms for Ang II-induced coronary complications in WHHL rabbits are not fully understood. Several possible mechanisms may operate in concert for Ang II-induced coronary events in WHHL rabbits. Essential functions of Ang II are to elevate the blood pressure; therefore, it is reasonable to consider that the hemodynamic influence on the plaques is the first cause of the coronary events in WHHL rabbits. To verify this possibility, we generated hypertensive WHHL rabbits by unilateral removal of a kidney. Although these WHHL rabbits indeed had high blood pressure, all of them could survive for more than 30 weeks (Waqar et al., unpublished data), suggesting that hypertension alone is insufficient to trigger the plaque erosion/rupture in WHHL rabbits. The second possible mechanism may be associated with the multiple local effects of Ang II on the arterial wall. Ang II infusion elevated blood leukocytes in WHHL rabbits, indicating the presence of systemic inflammation. Ample evidence showed that Ang II affects a number of functions of the arterial wall cells, such as endothelial cells, smooth muscle cells and macrophages3, 4, thereby provoking vascular inflammation. In support of this notion, aortic lesions of Ang II-treated WHHL rabbits showed significantly higher expression of PAI-1 and IL-6 along with increased trend of other proinflammatory cytokines and MMP-9 (presumably derived from accumulated macrophages). Furthermore, our preliminary studies revealed that Ang II treatment increases the mRNA of tissue factor and MMP-9 in THP-1 macrophages and reduces the expression of eNOs of human umbilical vein endothelial cells (data not shown). Taken together, these observations prompted us to envision that an Ang II-induced pro-inflammatory state facilitates the transformation of a stable plaque into a vulnerable plaque prone to rupture. Previous studies have shown that MMPs produced by macrophages may cause the rupture of vulnerable plaques26, 33, 34. Increased tissue factor along with PAI-1 activity in the lesions renders lesions rather thrombogenic35, which further promotes the formation of thrombosis in case plaques rupture. The third possibility for Ang II-induced plaque rupture is associated with the fact that Ang II is notorious for inducing vascular oxidative stress due to the generation of reactive oxygen species, such as superoxide and hydrogen peroxide, by stimulating the activity of NADP(H) and xanthine oxidase from a variety of vascular cells36,37, which has been considered to play an important role in the pathogenesis of atherosclerosis38.
It should be pointed out that it is currently unknown whether MI was totally caused by coronary complications induced by Ang II infusion or whether MI is responsible for rabbit death because Ang II can directly exert detrimental effects on the heart or other organs. Therefore, it remains to be investigated in future whether Ang II can also cause myocardial injury or myocardial death. In addition, we observed that many eroded areas were not associated with occlusive thrombosis in Ang II-infused rabbits. We speculate that this may be due to: (1) plaques with occlusive thrombi were overlooked, (2) luminal thrombosis superimposed on the eroded area was quickly dissolved by the fibrinolytic system, or (3) MI could be caused by coronary spasm induced by Ang II39-41, which cannot be evaluated by pathological methods. Therefore, it is necessary to evaluate cardiac functions induced by Ang II infusion by other methods such as ECG, plasma cardiac markers, ultrasonic cardiogram and angiography of this model. Regardless of this, the current animal model may become a novel means to investigate many facets of human coronary syndrome and to develop new therapeutics for MI in the future.
In conclusion, we showed herein that Ang II infusion induces coronary complications and increases the prevalence of MI and mortality in WHHL rabbits. Although the molecular mechanisms are not yet completely clear, vigorous treatment of Ang II-induced deleterious effects may be beneficial for the prevention of cardiovascular events.
Supplementary Material
Significance.
Although increased plasma concentrations of angiotensin II have been implicated in many cardiovascular diseases, it is not known whether high levels of plasma angiotensin II affect coronary plaque rupture and subsequent myocardial infarction. In the current study, we infused angiotensin II into WHHL rabbits, a model of human familial hypercholesterolemia and demonstrated that increased blood levels of angiotensin II can induce coronary complications and myocardial infarction. The model described in this study provides a novel means for the study of human acute coronary syndrome.
Acknowledgements
We thank Suiimi A and Nakagawa Y for their technical assistance in making pathological specimens.
Sources of funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports and Technology, Japan (22390068, 25670190 and 15H04718 to J.F.) and NIH grant (R01HL117491 to EYC).
Non-standard Abbreviations and Acronyms
- Ang II
angiotensin II
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- PAI-1
plasminogen activator inhibitor-1
- TNF-α
tumor necrosis factor-α
- WHHL
Watanabe heritable hyperlipidemic
Footnotes
Disclosure
None
The online-only Data Supplement is available with this article.
Contributor Information
Shen Li, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Yan-ning Wang, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Manabu Niimi, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Bo Ning, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Yajie Chen, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Dedong Kang, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Ziyun Wang, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Qi Yu, Department of Pathology, Xi'an Medical University, Xi'an, China.
Ahmed Bilal Waqar, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan.
Enqi Liu, Research Institute of Atherosclerotic Disease and Laboratory Animal Center, Xi'an Jiaotong University School of Medicine, Xi'an, China.
Jifeng Zhang, Center for Advanced Models for Translational Sciences and Therapeutics, University of Michigan Medical Center, Ann Arbor, MI, USA.
Masashi Shiomi, Institute for Experimental Animals, Kobe University School of Medicine, Kobe, Japan.
Y. Eugene Chen, Center for Advanced Models for Translational Sciences and Therapeutics, University of Michigan Medical Center, Ann Arbor, MI, USA.
Jianglin Fan, Department of Molecular Pathology, Faculty of Medicine, Graduate School of Medical Sciences, University of Yamanashi, Yamanashi 409-3898, Japan; Department of Pathology, Xi'an Medical University, Xi'an, China.
References
- 1.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin ii-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 2.Benigni A, Cassis P, Remuzzi G. Angiotensin ii revisited: New roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavras I, Gavras H. Angiotensin ii as a cardiovascular risk factor. J Hum Hypertens. 2002;16(Suppl 2):S2–6. doi: 10.1038/sj.jhh.1001392. [DOI] [PubMed] [Google Scholar]
- 4.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BMW. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 5.da Silva AR, Fraga-Silva RA, Stergiopulos N, Montecucco F, Mach F. Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest. 2015;45:274–287. doi: 10.1111/eci.12401. [DOI] [PubMed] [Google Scholar]
- 6.Potter DD, Sobey CG, Tompkins PK, Rossen JD, Heistad DD. Evidence that macrophages in atherosclerotic lesions contain angiotensin ii. Circulation. 1998;98:800–807. doi: 10.1161/01.cir.98.8.800. [DOI] [PubMed] [Google Scholar]
- 7.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin ii and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 8.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. The New England journal of medicine. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Haudenschild CC, Nickerson C, Drago R. Antiatherogenic effect of captopril in the watanabe heritable hyperlipidemic rabbit. Hypertension. 1990;15:327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- 10.Campbell JH, Fennessy P, Campbell GR. Effect of perindopril on the development of atherosclerosis in the cholesterol-fed rabbit. Clin Exp Pharmacol Physiol Suppl. 1992;19:13–17. doi: 10.1111/j.1440-1681.1992.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 11.Schuh JR, Blehm DJ, Frierdich GE, McMahon EG, Blaine EH. Differential effects of renin-angiotensin system blockade on atherogenesis in cholesterol-fed rabbits. J Clin Invest. 1993;91:1453–1458. doi: 10.1172/JCI116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000;101:1586–1593. doi: 10.1161/01.cir.101.13.1586. [DOI] [PubMed] [Google Scholar]
- 13.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: Unraveling the arb-mi paradox. Circulation. 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 14.Tsuyuki RT, McDonald MA. Angiotensin receptor blockers do not increase risk of myocardial infarction. Circulation. 2006;114:855–860. doi: 10.1161/CIRCULATIONAHA.105.594978. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. The New England journal of medicine. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 16.Kruzliak P, Kovacova G, Pechanova O, Balogh S. Association between angiotensin ii type 1 receptor polymorphism and sudden cardiac death in myocardial infarction. Dis Markers. 2013;35:287–293. doi: 10.1155/2013/731609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice GI, Foy CA, Grant PJ. Angiotensin converting enzyme and angiotensin ii type 1-receptor gene polymorphisms and risk of ischaemic heart disease. Cardiovascular research. 1999;41:746–753. doi: 10.1016/s0008-6363(98)00246-6. [DOI] [PubMed] [Google Scholar]
- 18.Xu M, Sham P, Ye Z, Lindpaintner K, He L. A1166c genetic variation of the angiotensin ii type i receptor gene and susceptibility to coronary heart disease: Collaborative of 53 studies with 20,435 cases and 23,674 controls. Atherosclerosis. 2010;213:191–199. doi: 10.1016/j.atherosclerosis.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Cambien F, Costerousse O, Tiret L, Poirier O, Lecerf L, Gonzales MF, Evans A, Arveiler D, Cambou JP, Luc G, et al. Plasma level and gene polymorphism of angiotensin-converting enzyme in relation to myocardial infarction. Circulation. 1994;90:669–676. doi: 10.1161/01.cir.90.2.669. [DOI] [PubMed] [Google Scholar]
- 20.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss D, Kools JJ, Taylor WR. Angiotensin ii-induced hypertension accelerates the development of atherosclerosis in apoe-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama F, Haraoka S, Watanabe T, Shiota N, Taniguchi K, Ueno Y, Tanimoto K, Murakami K, Fukamizu A, Yagami K. Acceleration of atherosclerotic lesions in transgenic mice with hypertension by the activated renin-angiotensin system. Laboratory investigation; a journal of technical methods and pathology. 1997;76:835–842. [PubMed] [Google Scholar]
- 23.Alderman MH. Is there a link between the circulating renin-angiotensin system and coronary disease? A buoyant view. Heart. 1996;76:18–22. doi: 10.1136/hrt.76.3_suppl_3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss D, Sorescu D, Taylor WR. Angiotensin ii and atherosclerosis. Am J Cardiol. 2001;87:25C–32C. doi: 10.1016/s0002-9149(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Liang J, Niimi M, Bilal Waqar A, Kang D, Koike T, Wang Y, Shiomi M, Fan J. Probucol suppresses macrophage infiltration and mmp expression in atherosclerotic plaques of whhl rabbits. J Atheroscler Thromb. 2014;21:648–658. doi: 10.5551/jat.21600. [DOI] [PubMed] [Google Scholar]
- 26.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 27.Shah PK. Pathophysiology of coronary thrombosis: Role of plaque rupture and plaque erosion. Progress in cardiovascular diseases. 2002;44:357–368. doi: 10.1053/pcad.2002.123473. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the apoe knockout mouse. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 29.Bond AR, Jackson CL. The fat-fed apolipoprotein e knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. Journal of biomedicine & biotechnology. 2011;2011:379069. doi: 10.1155/2011/379069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abela GS, Picon PD, Friedl SE, Gebara OC, Miyamoto A, Federman M, Tofler GH, Muller JE. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation. 1995;91:776–784. doi: 10.1161/01.cir.91.3.776. [DOI] [PubMed] [Google Scholar]
- 31.Caligiuri G, Levy B, Pernow J, Thoren P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of sr-bi expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein e-deficient mice. Circulation research. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 33.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 34.Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A. 1995;92:402–406. doi: 10.1073/pnas.92.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Gaboury CL, Conlin PR, Seely EW, Williams GH, Vaughan DE. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin ii. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation. 1993;87:1969–1973. doi: 10.1161/01.cir.87.6.1969. [DOI] [PubMed] [Google Scholar]
- 36.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin ii stimulates nadh and nadph oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin ii in atherosclerosis. Regul Pept. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 38.Mugge A. The role of reactive oxygen species in atherosclerosis. Z Kardiol. 1998;87:851–864. doi: 10.1007/s003920050241. [DOI] [PubMed] [Google Scholar]
- 39.Gavras H, Kremer D, Brown JJ, Gray B, Lever AF, MacAdam RF, medina A, Morton JJ, Robertson JI. Angiotensin- and norepinephrine-induced myocardial lesions: Experimental and clinical studies in rabbits and man. Am Heart J. 1975;89:321–332. doi: 10.1016/0002-8703(75)90082-4. [DOI] [PubMed] [Google Scholar]
- 40.Gavras H, Lever AF, Brown JJ, Macadam RF, Robertson JI. Acute renal failure, tubular necrosis, and myocardial infarction induced in the rabbit by intravenous angiotensin ii. Lancet. 1971;2:19–22. doi: 10.1016/s0140-6736(71)90008-0. [DOI] [PubMed] [Google Scholar]
- 41.Shiomi M, Ishida T, Kobayashi T, Nitta N, Sonoda A, Yamada S, Koike T, Kuniyoshi N, Murata K, Hirata K, Ito T, Libby P. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction-prone strain of the watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2013;33:2518–2523. doi: 10.1161/ATVBAHA.113.301303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.