Abstract
Porous aromatic frameworks (PAFs) incorporating a high concentration of acid functional groups possess characteristics that are promising for use in separating lanthanide and actinide metal ions, as required in the treatment of radioactive waste. These materials have been shown to be indefinitely stable to concentrated acids and bases, potentially allowing for multiple adsorption/stripping cycles. Additionally, the PAFs combine exceptional features from MOFs and inorganic/activated carbons giving rise to tunable pore surfaces and maximum chemical stability. Herein, we present a study of the adsorption of selected metal ions, Sr2+, Fe3+, Nd3+, and Am3+, from aqueous solutions employing a carbon-based porous aromatic framework, BPP-7 (Berkeley Porous Polymer-7). This material displays high metal loading capacities together with excellent adsorption selectivity for neodymium over strontium based on Langmuir adsorption isotherms and ideal adsorbed solution theory (IAST) calculations. Based in part upon X-ray absorption spectroscopy studies, the stronger adsorption of neodymium is attributed to multiple metal ion and binding site interactions resulting from the densely functionalized and highly interpenetrated structure of BPP-7. Recyclability and combustibility experiments demonstrate that multiple adsorption/stripping cycles can be completed with minimal degradation of the polymer adsorption capacity.
Short abstract
A highly interpenetrated carboxylic acid functionalized porous aromatic framework (PAF) has been demonstrated to undergo selective uptake of neodymium ions over iron and strontium ions, a promising development toward Ln/An group separation in the treatment of fission products.
Introduction
The fission and neutron capture reactions occurring in nuclear reactors generate a waste stream of more than 40 elements, which includes the entirety of the periodic table from germanium to erbium, in addition to the transuranic elements from neptunium to curium.1 Additionally, the unavoidable corrosion of stainless steel structural elements delivers numerous first row transition metals.2 Effective conversion of such a complex and highly radioactive mixture into waste forms suitable for long-term storage, along with recovering and reprocessing fissile uranium and plutonium, demands the separation of this mixture into separate groups (Figure 1, FP, fission products; MA, minor actinides).3 Initial separation of uranium and plutonium for reprocessing is accomplished by the PUREX (plutonium uranium redox extraction) process,4 which can be modified to include coextraction of neptunium (Figure 1, separation A).5 Of the elements remaining in the raffinate, the minor actinides—especially americium—dominate the long-term radiotoxicity and heat load of spent fuels.6,7 Provided they can be isolated in sufficient purity, such species could be recycled and utilized for energy production or transmuted into alternative isotopes that would shorten waste storage timeframes.
Figure 1.
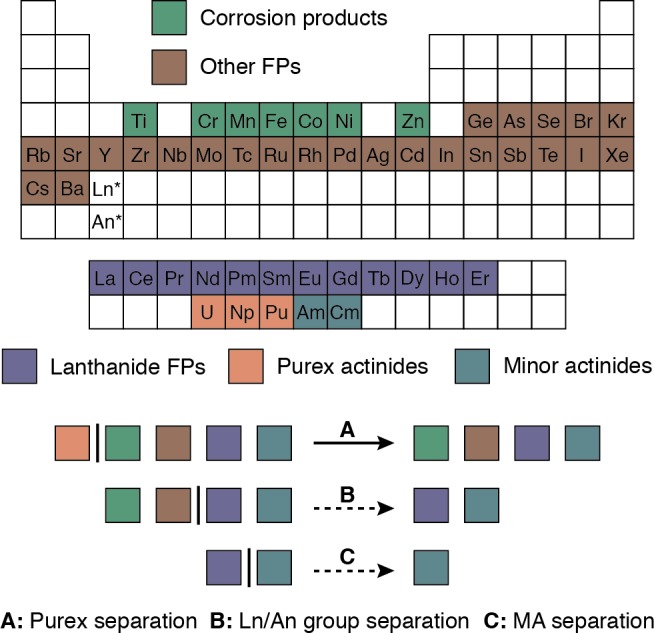
Separation of fission products (FPs) including highly radioactive PUREX actinides and minor actinides (MA).
Complete purification of the minor actinides requires the development of two selective separations.3 First, the lanthanide fission products must be partitioned together with the minor actinides in a process known as group separation (Figure 1, separation B). For this, biphasic solvent extraction using chelating diamides8,9 (i.e., the diamide extraction or DIAMEX process) or carbamoylphosphine oxides10,11 (i.e., the transuranic extraction or TRUEX process) represents the current state of the art. The group separation is required to provide solutions containing exclusively lanthanide and actinide ions, so that the increased strength of the actinide–ligand interaction can subsequently be exploited for selective extraction of the minor actinides (Figure 1, separation C). Soft ligand sets such as triazinylpyridines12 and alkylated thiophosphates13 have demonstrated promising efficiencies in separating actinides from lanthanides, but these ligands would be rendered inoperable by the presence of transition metal impurities.
Importantly, none of these processes have been successfully demonstrated or utilized beyond a laboratory scale. As is true for the PUREX process, solvent extractions will generate large volumes of organic waste via hydrolytic and radiolytic degradation of the solvents and extractants. In addition, the equipment required for multistage extraction and stripping, such as mixer-settlers and centrifugal contactors, greatly increases capital costs. An alternative approach—solid-phase extraction—has been pursued by impregnating the pores of macroporous polymer substrates with extractant solutions.14 Such methods have received considerable attention because they eliminate the agitated contactors demanded by solvent extraction, while maintaining the binding selectivity of conventional ligand sets. In addition, nanoparticles and mesoporous materials have been tested for the encapsulation of early actinides.15−17 However, the stability of these composite materials against radiation damage and acid hydrolysis is questionable due to the weak noncovalent interaction between the extractants and porous substrates. It has recently been shown that high acid concentration, heat, and γ-radiation all result in loss of extractants from substrates, with concomitant reduction in extraction capacity and separation efficiency.18,19 In addition, concerns about generating secondary solid wastes that are difficult to degrade—and so, must themselves be disposed of—may also hinder the industrial adoption of solid-phase extraction processes.
The development of porous adsorbents densely furnished with selective binding sites appended through covalent bonds could provide materials with both unprecedented separation performance and adequate stability in these extremely challenging conditions. However, the most widely investigated microporous materials face specific limitations for lanthanide group separation (Figure 2). Metal–organic frameworks (MOFs), which have displayed noteworthy performance in gas-phase separation, storage, sensing, and catalysis applications,20−27 generally do not possess the hydrolytic stability necessary for long-term application in highly acidic solutions (Figure 2). A zirconium-based MOF has recently displayed promising UO22+ adsorption from mildly acidic (pH = 2.5) solution, though further investigations on this material showed some sensitivity to more vigorous conditions involving dissolution in 0.1 M H3PO4/DMSO.28 Conversely, traditional adsorbents such as zeolites and activated carbons, while extremely robust, cannot be fine-tuned with the myriad reactions available from synthetic organic chemistry. Additionally, the inability of purely inorganic or MOF supports to be completely converted to volatile products by combustion would generate a large volume of secondary radiological waste.
Figure 2.
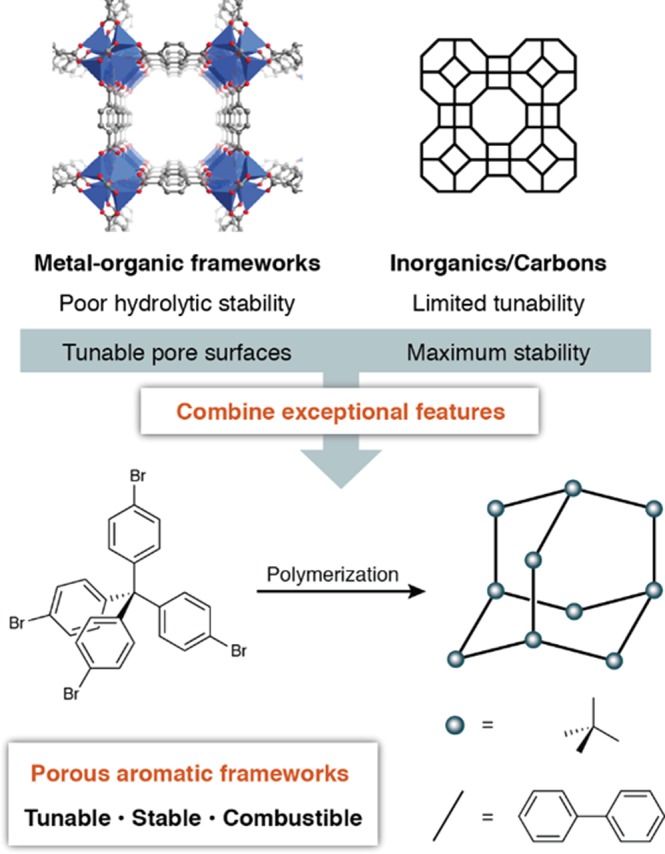
Potential advantages of porous aromatic frameworks (PAFs) for challenging adsorptions.
Preliminary studies performed on densely functionalized porous aromatic frameworks (PAFs)29 present clear advantages over both microporous materials and solid-phase extraction composites. First, these materials have been demonstrated to be indefinitely stable to concentrated acids (e.g., 6 M HCl, 99% ClSO3H) and bases (e.g., 6 M KOH),30−32 potentially allowing for multiple adsorption/stripping cycles. Second, like MOFs, the more chemically stable PAFs are amenable to targeted surface functionalization using synthetic organic chemistry, either before or after framework assembly.
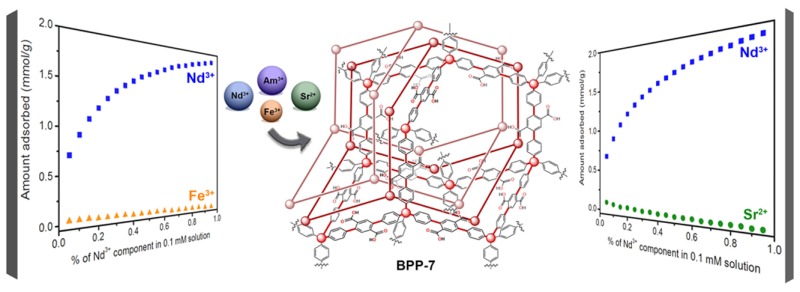
Recently, we reported catalytic routes to different porous aromatic polymers densely functionalized with carboxylic acids, together with a demonstration of their utility in ammonia capture.33 Among these, the framework BPP-7, which can be readily prepared through polymerization of 1-nonyl terephthalate ester followed by side chain cleavage, showed a remarkable NH3 uptake performance. This material features carboxylic acid lined pores ranging from ∼6.0 to 6.5 Å in diameter and a BET surface area of 705 m2/g, which are indicative of a multifold34 interpenetrated structure (Figure 3). To obtain a high adsorption capacity for the extraction of metal ions from a low-concentration solution, a large enthalpy of adsorption35 (ΔHads) is necessary, which can be achieved by promoting multiple chemical interactions between the metal ion and the binding site. BPP-7 was chosen as a densely functionalized PAF with a great potential for cooperative binding as a result of its highly interpenetrated structure. Its relatively larger pore size compared to other interpenetrated PAFs further allowed for improved uptake kinetics in ammonia adsorption.33 BPP-7 features binding sites with numerous carboxylic acid groups and is an excellent candidate for lanthanide and actinide group separation given that these ions are notoriously oxophilic.
Figure 3.
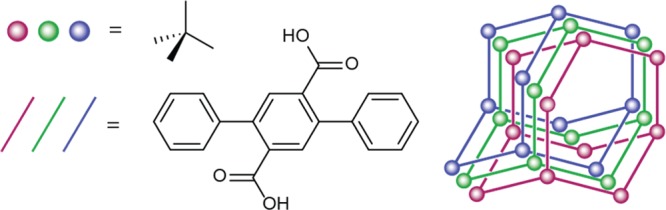
Multifold interpenetrated structure of BPP-7.
Herein, we report the utilization of the densely functionalized PAF BPP-7 for metal ion separation, an approach to simultaneously address the need for separation selectivity, adsorption capacity, hydrolytic stability, and combustibility demanded by a real-world lanthanide/actinide group separation.
Results and Discussion
Metal Ion Adsorption Isotherms and Langmuir Fitting
The uptake of metal ions by BPP-7 was measured at various concentration ranges for neodymium(III) (2.76 × 10–6 mM to 2.52 mM), iron(III) (0.113 mM to 4.33 mM), and strontium(II) (2.17 × 10–3 mM to 2.44 mM). As an initial investigation, the Nd3+, Fe3+, and Sr2+ ions were chosen as representative ions for group separation of the corrosion products, other fission products, and lanthanide fission products, respectively. The Fe3+ ion was chosen due to its high concentration in nuclear waste streams as a result of the corrosion of steel.2 Strontium-90 is environmentally toxic, and is an intermediate activity waste product.36 The Nd3+ ion was chosen as a midsize lanthanide ion.
The adsorption isotherms for Nd3+, Fe3+, and Sr2+ uptake were fitted with Langmuir models (Table 1), and the fits agree to within 4% of the measured values, which is within the experimental error (Figure 4). While a single-site Langmuir equation could be used to describe the Fe3+ adsorption isotherm, dual-site Langmuir equations were necessary for Nd3+ and Sr2+. Modeling the adsorption sites for Nd3+ and Sr2+ required including both a strong and a weak adsorption site, while the Fe3+ data could be modeled with only one weak site. Thus, the Fe3+ weak site interactions represent an average binding against which the Nd3+ and Sr2+ binding can be compared. There is evidence provided by Extended X-ray Absorption Fine Structure (EXAFS) data (see EXAFS section for more detail) that the strong adsorption site for Nd3+ and Sr2+ likely involves interactions with multiple carboxylate groups. Presumably, the larger ionic radii of Nd3+ and Sr2+ allow for such interactions, as opposed to a smaller Fe3+ ion.
Table 1. Single- and Dual-Site Langmuir Fit Parameters for Single-Component Adsorption Isothermsa.
| Nd3+ | Sr2+ | Fe3+ | |
|---|---|---|---|
| nsat,1 | 0.43 | 0.39 | 7.7 |
| b1 | 97350 | 278 | 0.26 |
| nsat,2 | 1.9 | 2.6 | |
| b2 | 34.8 | 0.59 |
Units for n are mmol/g, and units for b are mM–1.
Figure 4.
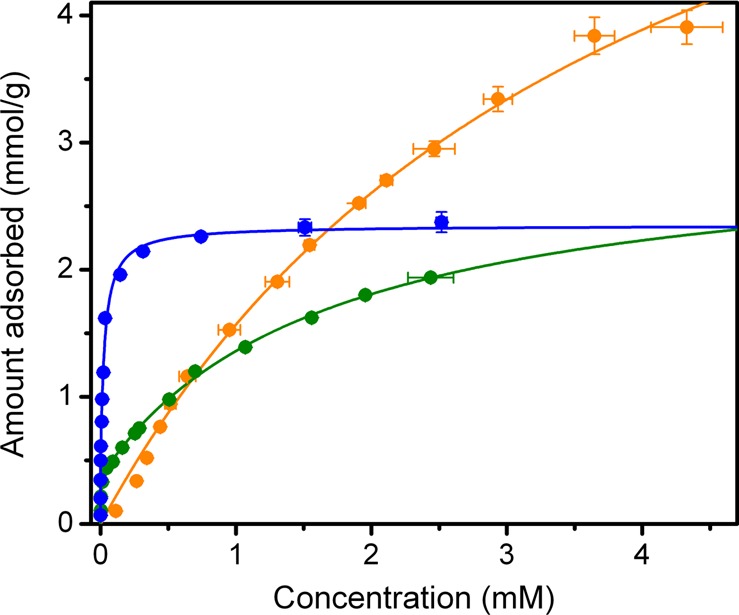
Room temperature Nd3+ (blue), Sr2+ (green), Fe3+ (orange) ion adsorption characteristics of BPP-7.
In Table 1, the b1 and b2 Langmuir parameters represent the affinities of the metal ion for the binding sites. Since Fe3+ is modeled with a single weak site interaction, only a b1 parameter was calculated. The Nd3+ and Sr2+ data were fitted with two b parameters, with b1 as the strong site interaction and b2 as the weak site interaction. Notably, the b1 parameter for Nd3+ (97350 mM–1) is orders of magnitude greater than those of Sr2+ (278 mM–1) and Fe3+ (0.26 mM–1) ions, which suggests that adsorption of Nd3+ is much stronger than Sr2+ and Fe3+ adsorption. Indeed, this can be observed in the relative steepness of the adsorption isotherms at low concentrations (Figure 4). The near vertical steepness of the Nd3+ adsorption isotherm indicates that Nd3+ binds the framework most strongly, followed by Sr2+ and Fe3+. While Nd3+ has the highest uptake at low concentrations, Fe3+ has the highest uptake at saturation owing to its smaller size. One would expect the uptake saturation to be inversely related to ionic radii, and since Nd3+ and Sr2+ have nearly equivalent ionic radii, their saturation capacities are similar and much lower than that of Fe3+. The nsat,1 and nsat,2 parameters measure the saturation capacities (mmol/g) of the two adsorption sites. For both Nd3+ and Sr2+, the saturation capacity of the stronger binding site is lower, which is expected. The relation of all Langmuir b1 parameters indicates that overall Nd3+ ions interact with one site more strongly than Sr2+ or Fe3+ ions. Presumably, this can be ascribed to the greater charge density of Nd3+ relative to Sr2+ and the larger ionic radius relative to Fe3+, which provides a better match for certain rigid binding pockets within BPP-7 involving multiple carboxylate/carboxylic acid groups.
Another important feature to note is that the adsorption capacity of Nd3+ within BPP-7 approaches 2.5 mmol/g, which is much higher than the uptake of lanthanides in similarly functionalized materials used for actinide uptake, such as the family of phosphoric acid functionalized silicas (exhibiting a maximum Eu uptake of 0.38 mmol/g).37 The large uptake capacity and multisite interactions result from a very high density of active sites and large overall surface area (705 m2/g), which in turn arises from the highly interpenetrated structure of BPP-7.33 Minor deviations from the proposed single- and dual-site Langmuir models may be attributed to electrostatic repulsion between adsorbed metal ions, which would reduce the binding affinity, resulting in occupation of fewer active sites than those available in the framework.38
IAST Calculations
Since binary adsorption isotherms are difficult to measure, it is often necessary to use an adsorption model such as ideal adsorbed solution theory (IAST) to predict mixture behavior from experimentally determined single-component isotherms.39 The IAST method has been well established for metal ion uptake40,41 and for a variety of adsorbents, including zeolites42,43 and metal–organic frameworks.44,45 The details of the IAST evaluation of selectivities, adsorbed quantities, and mixture purities for Nd3+, Fe3+, and Sr2+ are reported in the Experimental Section. IAST calculations were performed using the appropriate single- and dual-site Langmuir isotherm fits (Table 1).
Since the relative concentrations of isotopes of Nd3+, Fe3+, and Sr2+ ions can fluctuate in a radioactive waste stream,46 IAST selectivities were calculated over a wide range of compositions for total concentrations of 0.1 and 1 mM. At both concentrations, BPP-7 exhibits a high selectivity for Nd3+ over Sr2+ and Fe3+, with a more pronounced effect for the 0.1 mM mixtures (Figure 5). Importantly, selectivity for Nd3+ is most desired, since this ion is often used as an analogue for Am3+, and shares similar physical and chemical properties with other lanthanides and actinides.47 The relationship between Nd3+ and Am3+ uptake is explored further by experimentation below.
Figure 5.
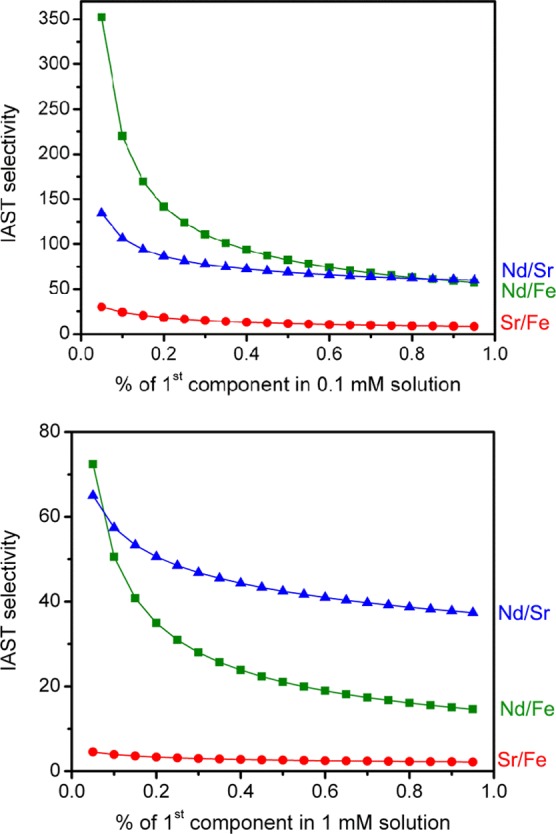
Ideal adsorbed solution theory (IAST) selectivities for 0.1 mM (top) and 1 mM (bottom) mixtures of Nd3+/Sr2+ (blue triangles), Nd3+/Fe3+ (green squares), and Sr2+/Fe3+ (red circles) ions at rt.
At all concentrations and compositions investigated, BPP-7 exhibits a high selectivity for Nd3+ over Fe3+ and Sr2+ and a moderate selectivity for Sr2+ over Fe3+. For instance, for a solution containing just 5% Nd and 95% Fe or Sr, BBP-7 will selectively adsorb Nd with an IAST selectivity of 350 and 130, respectively, assuming a total concentration of 0.1 mM. IAST selectivity is also higher for Sr2+ than Fe3+. Higher Nd3+ and Sr2+ selectivities at lower concentration can be attributed to the larger ionic radii for these ions, which presumably facilitates the simultaneous interaction with multiple carboxylic acid/carboxylate groups. The selectivity for Sr2+ over Fe3+ is also particularly important, in view of the environmentally hazardous nature of Sr-90.36 Many contaminated sites where Sr2+ is present also contain large quantities of iron and other transition metals,2 making this type of separation ideal for remediation purposes.
IAST purity predictions listed in Figure 6 further illustrate the effectiveness of metal ion selectivity in BPP-7, as they suggest that Nd3+ can be isolated from Sr2+ and Fe3+ solutions at very high purity, reaching 99.6% purity over Fe3+ at a composition of 0.95% Nd3+ in a 1 mM mixture. Even at very low percent composition, Nd3+ shows an adsorbed phase that is 75% pure. This effect is even more pronounced for the 0.1 mM mixture, where the purity of Nd3+ rapidly surpasses 90%. High purity separations are also predicted for Sr2+ in competition with Fe3+, for 0.6% composition in Sr and higher. This suggests that the coordination environment and strength of the metal ion–framework interactions are very uniform, and preferential binding is relatively consistent across all sites.
Figure 6.
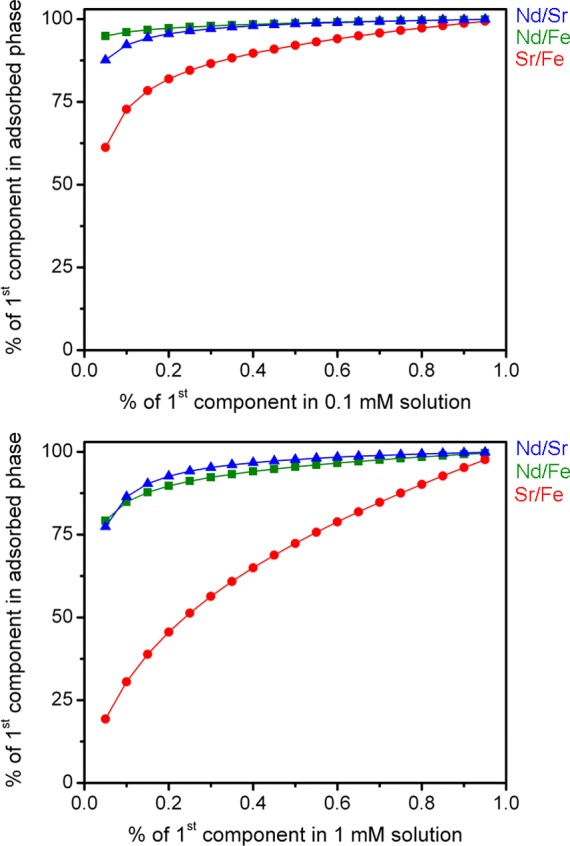
Ideal adsorbed solution theory (IAST) purities for 0.1 mM (top) and 1 mM (bottom) mixtures of Nd3+/Sr2+ (green squares), Nd3+/Fe3+ (blue triangles), and Sr2+/Fe3+ (red circles) ions.
Additional IAST calculations were performed to evaluate the exact amounts of metal ion adsorbed from two-component mixtures at 0.1 and 1.0 mM concentrations. Figure S1 shows the results of the analysis for a binary mixture containing Nd3+ and Fe3+. IAST adsorption for binary mixtures of Nd3+/Sr2+ and Sr2+/Fe3+ are presented in Figures S2 and S3. As expected, the adsorbed quantities were directly in line with IAST purities and selectivities, where the adsorption of Nd3+ is significantly higher in comparison to Sr2+ or Fe3+.
XAFS Measurements
The X-ray Absorption Near Edge Structure (XANES) spectra confirm the trivalent nature of Nd in the Nd-BPP-7 materials by comparison with Nd3+ in aqueous solution (see Figure S4). The edge-jump obtained from the near-edge spectra confirms the original Nd concentration in Nd-BBP-7 to be ∼20 wt %. No radiation damage was observed from the response at the Nd K-edge during the course of the X-ray Absorption Fine Structure (XAFS) experiments.
The EXAFS function, k3χ(k), and the Fourier transform magnitude, FT(k3χ(k)), of Nd-BPP-7 are shown in Figure 7. The fits to the spectra were performed based on the interpretation of two-shell models for the largest first (∼2.0 Å) and second (∼2.5 Å) peaks in the FT using metrical parameters obtained from previous studies of similar systems.48,49 According to proton replacement reactions (see below), two protons are lost per complexed Nd atom, which infers that two carboxylate groups are likely involved in the PAF coordination of Nd; therefore, on average two carboxylate groups provide coordination sites, in which a carboxylate oxygen atom contributes to form a part of the first coordination shell. Accordingly, Figure 7 shows the EXAFS data with the corresponding two-shell fits, which are superior to single-shell fits. The conceptual model for the fits considers the possibility of Nd interactions with oxygen atoms from carboxylate and carboxylic acid groups, as well as guest water molecules, for the first peak; Nd interactions with the secondary, noncoordinating carbon and oxygen atoms of the carboxylate and carboxylic acid groups, and potentially carbon atoms from the interpenetrating PAF network, form the second peak. The first shell fit incorporates the knowledge that there will be distances characteristic of Nd–O(carboxylate/carboxylic acid) and Nd–O(water) ligation, as mentioned previously. Interactions with hydroxide under the experimental conditions, like the possibility of Cl– coordination, are unlikely and would be difficult to resolve in the experiment (see Figure S4 for a schematic of possible interactions). There is no apparent multiple scattering peak in Figure 7, indicating that, if there is bidentate coordination by carboxylate groups, it is distorted and/or disordered. The initial distances obtained from EXAFS fitting (Table 2, Figure S5) suggest that carboxylate groups may coordinate to Nd in a monodentate fashion, and the resulting bond angle of Nd–O–C is less than 150°, since the focusing effect derived from a three-atom linear arrangement is also absent in this coordination mode. However, the large degree of disorder and broadening within the first shell supports that there may be more than a single interaction mode for coordination.
Figure 7.
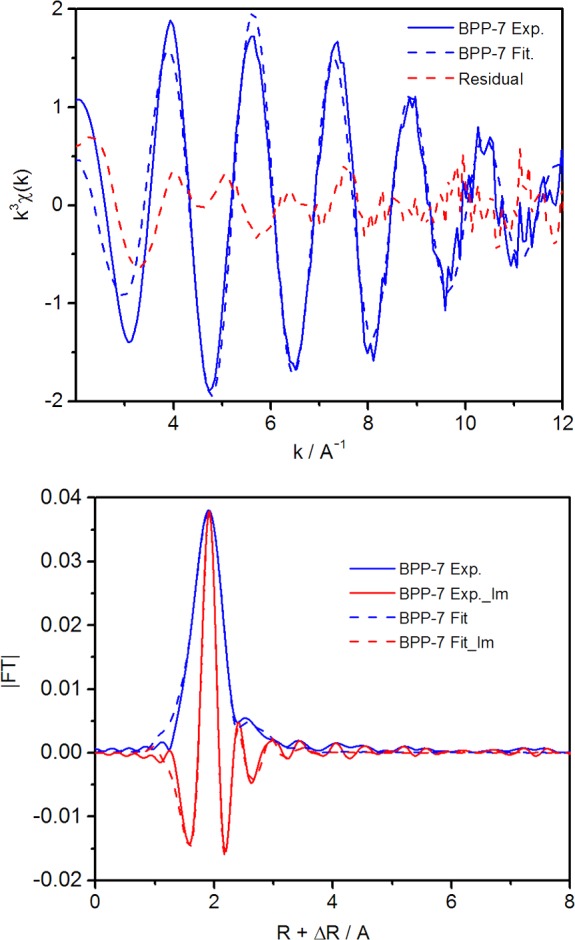
EXAFS function k3χ(k) and the two-shell fitting results for the Nd-BPP-7 (top) and Fourier transform magnitudes [FT(k3(k))] and two-shell fitting curves for the Nd-BPP-7 (bottom). The imaginary components are shown in red traces.
Table 2. Structural Parameters Obtained from the Two-Shell Nd K-Edge EXAFS Analysisa.
| S02 | N | R/Å | σ2/Å2 | E0/eV | Res./% | |
|---|---|---|---|---|---|---|
| Nd–O(1) (Lig., H2O) |
0.9 | 9 | 2.483 ± 0.003 | 0.009 | 4.8 | 3.6 |
| Nd···O(2) (Lig.) | 0.9 | 10 | 3.310 ± 0.009 | 0.019 | 4.8 | 3.6 |
| Nd···C (Lig.) |
The error in R was determined as 2σ by fitting. Lig. = BPP-7 ligand.
A summary of the metric parameters obtained from the curve fitting results for the two-shell fit are summarized in Table 2. In this model, the Nd–O(1) distances are ∼2.48 Å; the bond angle of Nd–O–C is less than 150°, while the second shell with surrounding carboxylate oxygens and multiple network carbons (Nd···O(2) and Nd···C) are detected at ∼3.31 Å, together with a relatively large Debye–Waller factor (DWF). The large DWF includes disorder resulting from the various conformations of BPP-7 coordinating to the Nd3+ ions. As shown in Figure S4 and corresponding to known distances, the Nd–O(1) distance corresponds to an average of all Nd–carboxyl interactions.50−52 The Nd–O(1) coordination number was determined to be ∼9 during the fitting process, and the best fit was found with this value. This value agrees with the general Nd coordination number of 9, derived from the interactions with oxygen, which corresponds well to the known coordination number of Nd3+ in solutions and solids. It is clear that the interpenetrating nature of the BPP-7 materials leads to Nd bonding with more than one carboxylate unit identified in the first shell (O(1)), based on the coordination number of the second shell. Further details of the exact, detailed coordination environment of Nd with respect to oxygen are not readily discernible, as a result of the degree of disorder exhibited by Nd-BPP-7, the average environments yielded by EXAFS, and the coordination number error of ±25%. It is, however, clear that several water molecules are included in the first coordination sphere of Nd. The possibility of a charge-compensating perchlorate in a higher coordination shell further provides for the proper charge balance for the reaction.
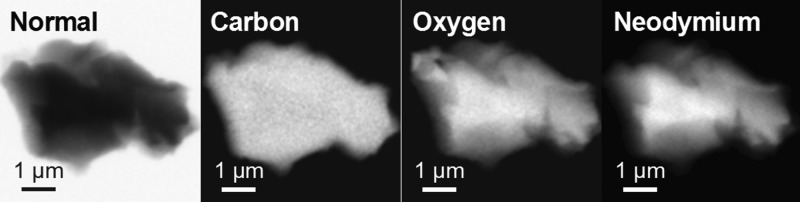
Scanning Transmission X-ray Microscope (STXM) Measurements
STXM is a powerful tool for characterizing materials of biological, environmental, extraterrestrial, or synthetic origins with high spatial resolution and chemical or electronic structure contrast.53−61 In this study, STXM was used to record images, elemental maps, and X-ray absorption near-edge structure (XANES) spectra to evaluate structure and morphology on the micrometer scale. Figure 8 shows a normal contrast image and C, O, and Nd elemental maps of a representative particle of Nd-BPP-7. XANES spectra obtained from this particle at the carbon and oxygen K-edges and Nd M5,4-edges are provided in Figures S6–S8. The images in Figure 8 indicate that the sample was uniform in composition of C, O, and Nd at the nanometer scale.
Figure 8.
Four images of a representative particle from Nd-BPP-7 from which X-ray absorption spectra were collected: (from left to right) normal contrast image obtained with a photon energy of 960 eV; elemental map of carbon obtained by subtraction using photon energies of 280 and 300 eV with the regions containing C shown as white using a standard grayscale; elemental maps of oxygen and neodymium obtained by the same method using photon energies of 525 and 540 eV (O) and 960 and 980 eV (Nd).
To determine the efficiency of Nd binding by BPP-7, the Nd concentration relative to the C and O content in the PAF particle was evaluated using singular value decomposition (SVD) analysis, which has been established previously for a variety of environmental and geological materials.62 The optical density of a target area in a given image is governed by OD = μ × ρ × t, where μ is the mass absorption coefficient,63 ρ is the density, and t is the sample thickness or path length. In this study, the intensity of the Nd M5-edge step (μ) was proportional to the Nd concentration, [Nd], in the target area of a given particle. Similarly, the intensity of the edge step at the C or O K-edges was proportional to [C] and [O] in the same target area, respectively. Two values [Nd]/[C] and [Nd]/[O] were defined as the molar ratios of Nd and C or O in Nd-BPP-7. To provide an accurate measurement, analyses were conducted on more than 10 different target areas from several particles with approximately 10,000 total pixels, and errors are reported as the standard deviation from the multiple measurements. In this manner, the [Nd]/[C] molar ratio obtained was 0.011(3), which corresponds to 1.2(4) mmol of Nd per gram of BPP-7. The slightly lower Nd concentration relative to results from ICP-MS may reflect small inaccuracies of the theoretical values for the mass absorption coefficients,63 or effects from surface contamination and saturation during the STXM measurement.
As described above, the EXAFS studies indicate that Nd is coordinated by two carboxylates in Nd-BPP-7. Hence, the uptake of Nd by BPP-7 can in this case be described by the following reaction, eq 1.
| 1 |
This stoichiometry affords a theoretical [Nd]/[O] molar ratio for the (C41O8H24)Nd(ClO4) product of 0.083. The significantly smaller experimental value of 0.027(5) reflects the presence of water molecules in the Nd coordination sphere. However, the exact number of water molecules could not be determined with confidence because of the possibility of incomplete HClO4 removal following Nd adsorption.
Am3+ Adsorption
The final and arguably most challenging step in the separation of fission products is the separation of minor actinides from lanthanides (step C, Figure 1). Accordingly, Am3+ adsorption studies were also performed with BPP-7. Two sets of adsorption experiments were carried out to establish americium(III) (243Am3+) uptake in the material, and to evaluate the relative behavior of Nd3+ and Am3+ when interacting with BPP-7. Initially, the distribution coefficients of adsorption of Nd3+ and Am3+ were measured at varying pH and fixed concentration to evaluate the proton replacement ratios and coordination behavior of these species. Plots of the log(Kd) versus the measured pH for both metal ions are displayed in Figure 9. Both plots have a linearly increasing region, which corresponds to the equilibrium between protonated and deprotonated carboxylic acid groups, as determined by the concentrations of protons in solution. At pH > 3, the decrease in concentration of protons is likely not great enough to cause a change in the distribution of adsorbed metal ions. Therefore, log(Kd) shows no dependence upon pH in this region. In the pH-dependent region of the plot, the slope of the best-fit line corresponds to the proton replacement ratio, as determined from the balanced equation, eq 2, where n is the number of protons replaced per adsorbed metal ion.
| 2 |
| 3 |
Figure 9.
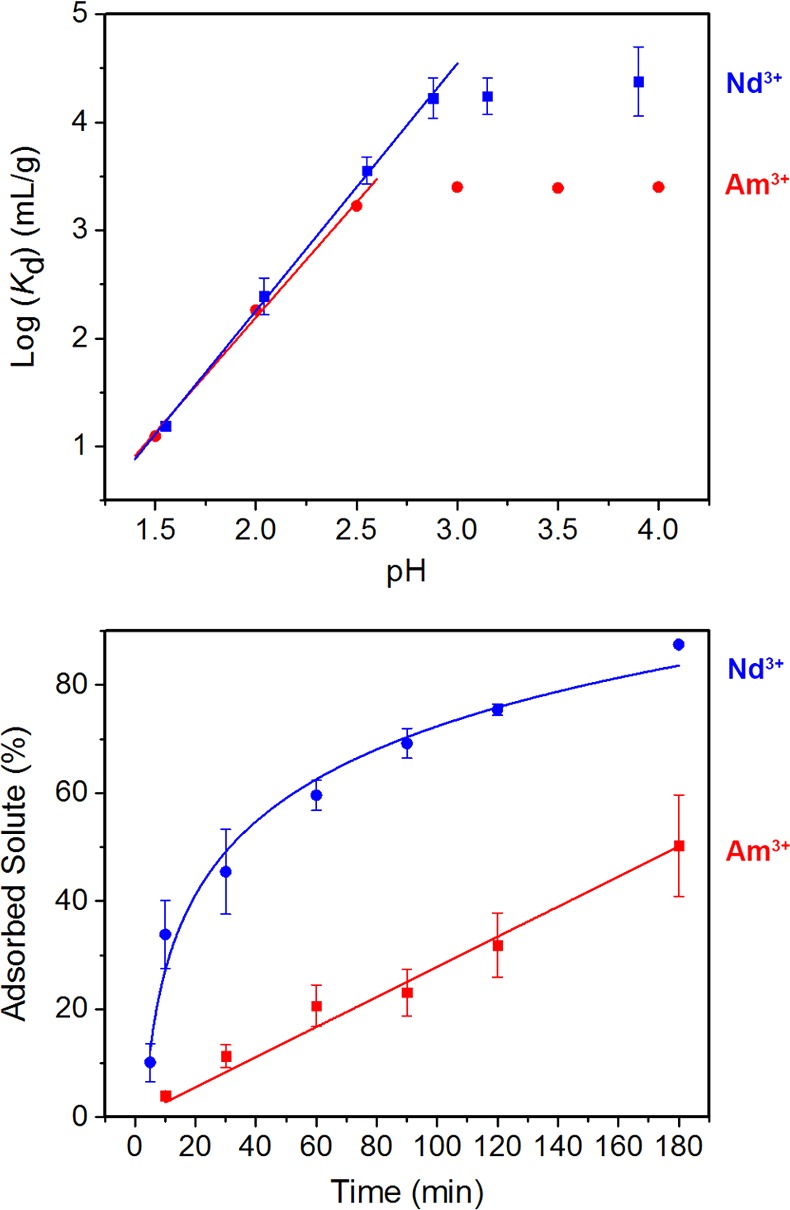
A plot of the log(Kd) versus measured pH for aqueous solutions containing Nd3+ and Am3+ ions (top), and kinetics for the uptake of Nd3+ and Am3+ (bottom).
The relationship between proton replacement ratio and log(Kd) is described with eq 3. The experimental proton replacement ratios for Nd3+ and Am3+ are n = 2.29 ± 0.06 and 2.13 ± 0.12, respectively. These values are obtained from the slopes of the linear fit lines of the pH-dependent region in the pH versus log(Kd) plots. The experimental proton replacement ratio for Nd3+ adsorption validates the dual-site binding approximation made for Langmuir fitting of the Nd3+ adsorption isotherm. The experimental proton replacement ratio for Am3+ was slightly lower than that of Nd3+, possibly due to its lower charge density, an effect previously established with Fe3+ and Sr2+ to a greater extent by IAST calculations. The lower charge density likely accounts for the lower maximum Kd value of Am3+ with respect to Nd3+. In the high pH plateau region, the average observed Kd value is lower for Am3+ than Nd3+, suggesting that the larger radius of Am3+ results in size exclusion and, hence, reduced adsorption by the framework. Thus, the maximum availability of sites for uptake is slightly lower for Am3+ adsorption than for Nd3+ adsorption. Despite these observations, the similarity between the derived proton replacement values for these species validates the choice of Nd3+ as an analogue for Am3+.
Nd3+ Recovery
Two distinct postadsorption processes were investigated to evaluate their propensity for isolation or removal of adsorbed metal ions from Nd-BPP-7. To assess the feasibility of recovering the adsorbed metal by thermal decomposition of the framework, thermogravimetric analysis experiments were performed on BPP-7 and Nd-BPP-7, and the resulting combustion product was analyzed. The resulting data indicate near-complete combustion of the unloaded framework (<2% remaining) into volatile products (Figure 10). The postcombustion residue from Nd-BPP-7 is an oxide of Nd3+, which is apparent from the presence of a slight digression in its combustion curve at ∼160 min. This suggests a phase change during the thermal decomposition of neodymium nitrate, corresponding to the formation of the intermediate product NdO(NO3), as previously proposed.64 The near-complete decomposition of BPP-7 and isolation of the adsorbed metal ions in a dense oxide form illustrates a distinct advantage of employing porous aromatic frameworks as media for solution-based adsorption and subsequent removal of cationic nuclides.
Figure 10.
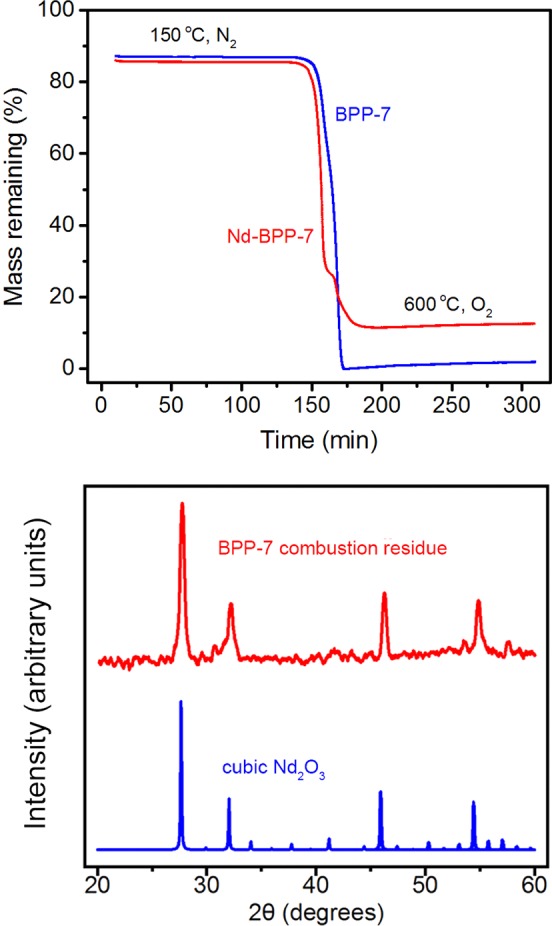
Top: Thermogravimetric analysis of Nd3+ adsorbed BBP-7 (red) and unloaded BBP-7 (blue). Both samples were treated identically, initially heated to 150 °C under an N2 atmosphere for 150 min followed by a second heating to 600 °C under an O2 atmosphere for another 150 min. Bottom: Powder X-ray diffraction patterns for the Nd-BPP-7 combustion product (red) and cubic Nd2O3 (blue).
The presence of neodymium oxide was investigated by powder X-ray diffraction on the residual combustion product of thermogravimetric analysis of Nd-BPP-7 (Figure 10) and was compared to the diffraction pattern of cubic Nd2O3 (blue).65 The residue shows four diffraction peaks characteristic of cubic Nd2O3, suggesting that the Nd3+ was retained in the structure of the framework, and that the loaded framework may be thermally decomposed to yield the oxide form of the metal in crystalline form. The presence of Nd2O3 is further supported by the infrared spectrum of the neodymium-loaded combustion product (see Figure S9). Separation of solvated nuclear species into distinct solid forms is ideal for nuclear waste remediation and recyclability, as it maximizes the density of isotopes in the sample. Thus, this material has potential not only to separate particular components of a nuclear fuel but also to preserve them in a stable, solid, insoluble form. This solidification process is particularly important for fuel storage, as the long-term stability of a stored fuel is heavily dependent upon the nature of the solid, preventing leaching of contaminants over time, as well as reducing the potential for highly penetrating radiation to escape the containment vessel into the environment.66 Industrial, large-scale processes are conducted as a covitrification with silica, which may cause separation of radionuclides and metallic phases, and poor solid phase stability.67 However, the process described in our study allows for separation of nuclides prior to vitrification, with the added benefit of minimal contamination by the adsorbent, which can readily be discarded as volatile combustion products.
Desorption and Recyclability
The desorption of metal ions and recyclability of BPP-7 were also investigated by replacement of the adsorbed metal ions through reacidification of the framework with HClO4. Following aqueous HClO4 treatment, the adsorption isotherm of Nd3+ exhibited only a minimal (0.7%) decrease in uptake capacity compared to the freshly synthesized PAF (see Figure 11, top). Thus, not only is desorption of the loaded metal ions possible but the material may be reused for further separations. The recyclability of the framework was tested over five desorption/adsorption cycles at a single starting concentration (2.1 mM) (Figure 11, bottom). A near-constant decrease in uptake was observed for each adsorption/desorption cycle, resulting in a total decrease in adsorption from ∼2 mmol/g to ∼1.75 mmol/g after 5 recycling processes. These values suggest that approximately 20 iterations of BPP-7 recycling could be achieved before the material degrades to 50% capacity. The minimal loss of sorption capability may be attributed to deactivation of carboxylic acid functional groups, perhaps through decomposition of the framework by reaction with the strong acid. It is very likely that the regeneration conditions could be further optimized to improve recyclability.
Figure 11.
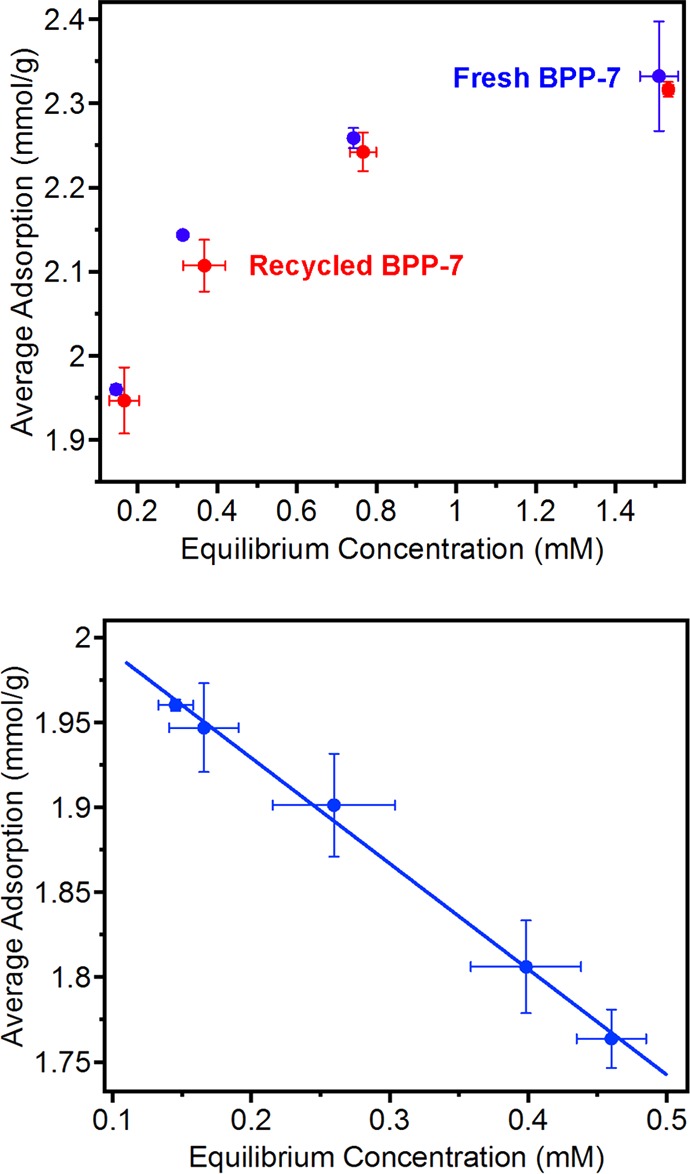
Recyclability of BPP-7 tested over 5 desorption/adsorption cycles at various equilibrium concentrations.
Experimental Section
General
The manipulations described below were performed primarily under aerobic conditions. BPP-7 was prepared according to a literature method33 and desolvated under vacuum at 150 °C for 24 h prior to use. The compounds Nd(NO3)3·6H2O (Sigma-Aldrich, 99.9%, Trace Metals Basis), Sr(NO3)2 (Alfa Aesar, 99.97%, Metals Basis), and Fe(NO3)3·9H2O (Alfa Aesar, >98%, Metals Basis) were purchased from commercial vendors, and used as received. Arsenazo III (1,8-dihydroxynaphthalene-3,6-disulfonic acid-2,7-bis[(azo-2)-phenylarsonic acid]) was purchased from Aldrich, and used as received. A Varian Cary 5 UV–visible spectrophotometer was used for all absorbance measurements.
Metal Ion Adsorption Measurements Employing BPP-7
Nd
Nd(NO3)3·6H2O (108.7 ± 0.25 mg) was dissolved in 250 mL of milli-Q water. From this stock solution, aliquots were directly added to the reaction mixtures to create solutions of concentrations of 20 μM to 1.0 mM.
Sr
Sr(NO3)2 (211.6 ± 0.3 mg) was dissolved in 20 mL of milli-Q water. From this stock solution, aliquots were directly added to the reaction mixtures to create solutions of concentrations 20 μm to 1.4 mM.
Fe
Fe(NO3)3·9H2O (5049.6 ± 0.3 mg) was dissolved in 200 mL of milli-Q water. From this stock solution, aliquots were directly added to the reaction mixtures to create solutions of concentrations 23 μM to 0.77 mM.
To 1 mL of each of these metal nitrate batches, 1.525 ± 0.025 mg of BPP-7 was added and allowed to react on an orbital shaker for 48 h. Subsequently, the reaction mixtures were centrifuged to separate the metalated BPP-7 from solution, and the supernatant solutions were then titrated to pH 5 ± 0.5 with 0.1 N KOH. From these samples, aliquots were taken for UV–vis analysis and ICP-MS analysis, respectively.
Adsorption Isotherms of Nd(III), Fe(III), and Sr(II)
Each data point of the isotherms corresponds to an independent adsorption experiment. The equilibrium concentrations of Nd(III) samples (high concentration, >2 mM), Sr(II), and Fe(III) were determined via UV–vis analysis. By contrast, the equilibrium concentrations of Nd(III) samples (<2 mM) were determined by ICP-MS. All data points from UV–vis were conducted in triplicate and the data points from ICP-MS analysis were performed in duplicate to estimate the standard error in the adsorption isotherm values.
To estimate selectivities, isotherm data were fitted with either a single- or dual-site Langmuir equation (eq 4), where n is the total amount adsorbed in mmol/g, C is the concentration in mM, nsat,i is the saturation capacity in mmol/g, and bi is the Langmuir parameter in bar–1 for up to two sites, 1 and 2. The fitted parameters can be found in Table 1. Ideal adsorbed solution theory (IAST)39 was then used to estimate the selectivity, Sads, and amount of each component adsorbed for binary mixtures of Nd3+/Fe3+, Nd3+/Sr2+, and Fe3+/Sr2+. Note that the selectivity factor, Sads, is defined according to eq 5, where ni is the amount adsorbed of each component, as determined from IAST, and xi is the mole fraction of each component in the solution phase at equilibrium.
| 4 |
| 5 |
Nd(III) Adsorption Kinetics and Proton Replacement
For the proton replacement reactions, 5 μM Nd(NO3)3 dissolved in water and combined with various concentrations (0.0001–0.032 M) of aqueous HNO3 were allowed to react with BPP-7 for 24 h. The equilibrium concentrations of the solutions after separation from BPP-7 were measured by ICP-MS. The distribution coefficient, Kd, was calculated as described in eq 6, where C0 and Cf are the initial and final metal ion concentrations in solution, respectively, V is the volume of the solution in mL, and M is the mass in grams of the adsorbent.
| 6 |
Kinetics studies were performed with a 2 mM aqueous solution of Nd(NO3)3. Percent adsorption was calculated based upon the final and initial concentrations of Nd(NO3)3 in solution. All Nd(III) kinetics and proton replacement reactions were performed in duplicate.
Am(III) Adsorption Kinetics and Proton Replacement
Kinetics and proton replacement experiments for Am(III) were performed under the same conditions as the analogous Nd(III) reactions. Both adsorption experiments used 6 μM aqueous solutions of 243Am(NO3)3. The equilibrium concentration of Am(III) in solution was measured with liquid scintillation counting (LSC). The distribution coefficient was determined using eq 5.
UV–Visible Spectroscopic Analysis
Reaction solutions containing Nd(III) and Sr(II) ions were prepared for visible spectroscopy using arsenazo III. Specifically, a 760 μL aliquot of each reaction mixture was added to 200 μL of buffer solution, and 40 μL of 0.1% arsenazo III in water. Following a literature procedure,68 the Nd(III)/arsenazo solution was fixed at pH 3 with a 0.1 M acetic acid buffer, while the Sr(II)/arsenazo solution was fixed at pH 8 with a 0.1 M HEPES buffer. The resulting metal–arsenazo complexes absorb at 653 and 647 nm, respectively, and extinction coefficients at these wavelengths were used to determine the concentration of Nd(III) and Sr(II) ions in solution via Beer’s law. Extinction coefficients were determined using an appropriate calibration curve. The concentration of Fe(III) ions was determined in a similar manner using Beer’s law and the direct absorbance of Fe(NO3)3 at 200 nm.
Recycling Experiment
BPP-7 was recycled by shaking for 12 h in 1 M HClO4 to displace adsorbed metal ions, and then washing four times with tetrahydrofuran at 60 °C. The material was then dried by heating under vacuum at 150 °C for 24 h.
Physical Measurements
A PerkinElmer SCIEX Elan DRC II inductively coupled mass spectrometer within the Geochemistry Division at Lawrence Berkeley National Laboratory was used to determine the concentrations of Nd(III) samples at low concentrations (<2 mM). A Wallac 1414 liquid scintillation counter was used for liquid scintillation spectroscopy of all Am(III) samples. A 50 μL aliquot of each reaction solution was added to a scintillation vial containing 5 mL of MP Biomedicals EcoLumeTM liquid scintillation cocktail, and counted for 1 min. Thermogravimetric analyses of BPP-7 samples were carried out with a TA Instruments Q5000 TGA. The samples were heated to 150 °C under an N2 atmosphere for 150 min, to remove any adsorbed solvents and gases, and were then heated to 600 °C under an O2 atmosphere for 150 min. The Nd-loaded BPP-7 combustion product was characterized using a Bruker Advance D8 powder X-ray diffractometer. The simulated diffraction pattern was calculated from the crystallographic dimensions of cubic Nd2O3. Infrared spectra were collected with a PerkinElmer Advance Spectrum 400 FTIR spectrometer equipped with a Pike attenuated total reflectance accessory.
X-ray Absorption Fine Structure (XAFS)
Near-edge and extended X-ray absorption fine structure (EXAFS) measurements were performed on BL11XU and BL14B1 at SPring-8. The operating energy and the ring current were 8 GeV and 99 mA in the topoff operation mode, respectively. The synchrotron radiation was monochromatized by liquid N2 cooled Si(311) double crystal monochromators for the XAFS measurements. The optics and EXAFS measurement systems of BL11XU have been previously described.69 Nd K-edge absorption spectra (43.569 keV) were collected in transmission using normal and quick monochromator scan mode (QXAFS mode) with Ar+N2-filled ionization chambers at ambient pressure and temperature. Nd-BPP-7 powder was mixed with boron nitride powder and pressed to make a tablet with 1 mm thickness. Fifty scans were performed for the Nd-BPP-7 sample, and the spectra were averaged for the data analysis. EXAFS data analysis was performed according to a standard procedure using the program WinXAS (version 3.1).70 Theoretical phases and amplitude required for the curve fitting were calculated by FEFF 8.2 code,71 using the model information from refs (51) and (72). Details of the fitting procedure can be found in the Supporting Information.
Scanning Transmission X-ray Microscope (STXM)–X-ray Absorption Near Edge Structure Measurements
Data were collected using the STXM at the Advanced Light Source-Molecular Environmental Sciences (ALS-MES) elliptically polarizing undulator beamline 11.0.2, which is operated in topoff mode at 500 mA, in a ∼0.5 atm He filled chamber.73 Samples for STXM measurements were encapsulated between two 100 nm Si3N4 membranes (Silson). Energy calibrations were performed at the Ne K-edge for Ne (867.3 eV). The energy resolution (fwhm) was estimated at 0.2 eV, and spectra were collected using linearly polarized radiation. Spectra at each image pixel or particular regions of interest in the sample image were extracted from the “stack”, which is a collection of images recorded at multiple, closely spaced photon energies across the absorption edge.74−76 Standard data analysis procedures were followed, as described previously.75,77−79
The XAFS and STXM measurements were performed on BPP-7 samples that were exposed to Nd perchlorate solutions with low concentrations analogous to the nitrate solutions used for the adsorption determinations. At the low concentrations employed, the characteristics of weak perchlorate coordination are similar to those of nitrate.80
Conclusions
The foregoing results demonstrate the enormous potential of the interpenetrated porous aromatic framework BPP-7, which is densely functionalized with carboxylic acid binding groups, for lanthanide and actinide group separation, as needed for the treatment of fission products. Significantly, BPP-7 exhibits a high selectivity for the uptake of neodymium in the presence of strontium and iron. Particularly important is the preferential binding of neodymium at low concentrations compared to iron, which represents a realistic scenario in the waste mixture, where the corrosion of stainless steel from tanks and iron-containing reagents would deliver metal ions such as iron in much higher concentrations. Adsorption of the ions was evaluated by the Langmuir model, which required including both a strong and a weak adsorption site for Nd3+ and Sr2+, while the Fe3+ data could be modeled with only one weak site. The stronger binding for Sr2+ and particularly for Nd3+ is ascribed to an appropriately sized binding pocket featuring multiple carboxylic acid groups. Further encouraging the use of BPP-7 for the purpose of lanthanide/actinide group separation is the excellent recyclability and combustibility of BPP-7. Tests have shown that desorption of BPP-7 to recover the loaded metal ions is possible, and that subsequent reuse of the material for further metal ion uptake shows only minor decrease in adsorption capacity.
Acknowledgments
Measurement of metal ion uptake in BPP-7 and characterization of the resulting materials were supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences Heavy Element Chemistry Program of the U.S. Department of Energy at Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231. The synthesis of BPP-7 and analysis of its metal ion adsorption behavior were supported through the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award DE-SC0001015. S.W. was supported by the Laboratory Directed Research and Development Program at LBNL. Work at the MES Beamline 11.0.2 was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences Condensed Phase and Interfacial Molecular Sciences Program of the U.S. Department of Energy at Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231. The Advanced Light Source and TT were supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The synchrotron radiation XAFS experiments were performed at the BL11XU and BL14B1 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2013B3504, 2013B3613, and 2012B3613).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00066.
IAST selectivities and purities for binary mixtures, XAFS fitting details, carbon/oxygen/Nd M5,4 edges, infrared spectra, and uptake of recycled BPP-7 (PDF)
Author Present Address
∥ J.F.V.H.: Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139.
Author Present Address
# S.W.: School of Radiation Medicine and Protection (SRMP) and School of Radiological and Interdisciplinary Sciences (RAD-X), Soochow University, Suzhou, Jiangsu 215123, China.
Author Contributions
§ S.D. and N.K.B. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Carbol P.; Wegen D. H.; Wiss T.; Fors P.. Spent Fuel as Waste Material. In Comprehensive Nuclear Materials; Rudy J. M. K., Ed.; Elsevier: Oxford, 2012; Vol. 5, pp 389–420. [Google Scholar]
- Ehrnstén U.Corrosion and Stress Corrosion Cracking of Austenitic Stainless Steels. In Comprehensive Nuclear Materials; Rudy J. M. K., Ed.; Elsevier: Oxford, 2012; Vol. 5, pp 93–104. [Google Scholar]
- Glatz J. P.Spent Fuel Dissolution and Reprocessing Processes. In Comprehensive Nuclear Materials; Rudy J. M. K., Ed.; Elsevier: Oxford, 2012; Vol. 5, pp 343–366. [Google Scholar]
- Anderson H. H.; Asprey L. B.. Solvent Extraction Process for Plutonium. US292406 A, May 8, 1947.
- Kumari N.; Pathak P. N.; Prabhu D. R.; Manchanda V. K. Comparison of Extraction Behavior of Neptunium from Nitric Acid Medium Employing Tri-n-Butyl Phosphate and N,N-dihexyl Octanamide as Extractants. Sep. Sci. Technol. 2012, 47, 1492–1497. 10.1080/01496395.2011.653034. [DOI] [Google Scholar]
- Salvatores M.; Palmiotti G. Radioactive Waste Partitioning and Transmutation within Advanced Fuel Cycles: Achievements and Challenges. Prog. Part. Nucl. Phys. 2011, 66, 144–166. 10.1016/j.ppnp.2010.10.001. [DOI] [Google Scholar]
- Magill J.; Berthou V.; Haas D.; Galy J.; Schenkel R.; Wiese H. W.; Heusener G.; Tommasi J.; Youinou G. Impact Limits of Partitioning and Transmutation Scenarios on the Radiotoxicity of Actinides in Radioactive Waste. Nucl. Energy 2003, 42, 263–277. 10.1680/nuen.42.5.263.37622. [DOI] [Google Scholar]
- Sasaki Y.; Tachimori S. Extraction of Actinides(III), (IV), (V), (VI), and Lanthanides(III) by Structurally Tailored Diamides. Solvent Extr. Ion Exch. 2002, 20, 21–34. 10.1081/SEI-100108822. [DOI] [Google Scholar]
- Sasaki Y.; Sugo Y.; Suzuki S.; Tachimori S. The Novel Extractants, Diglycoamides, for the Extraction of Lanthanides and Actinides in HNO3-n-Dodecane System. Solvent Extr. Ion Exch. 2001, 19, 91–103. 10.1081/SEI-100001376. [DOI] [Google Scholar]
- Schulz W. W.; Horwitz E. P. The Truex Process and the Management of Liquid Tru Uwaste. Sep. Sci. Technol. 1988, 23, 1191–1210. 10.1080/01496398808075625. [DOI] [Google Scholar]
- Philip Horwitz E.; Kalina D. C.; Diamond H.; Vandegrift G. F.; Schulz W. W. The TRUEX Process - A Process for the Extraction of the Transuranic Elements from Nitric AC in Wastes Utilizing Modified PUREX Solvent. Solvent Extr. Ion Exch. 1985, 3, 75–109. 10.1080/07366298508918504. [DOI] [Google Scholar]
- Panak P. J.; Geist A. Complexation and Extraction of Trivalent Actinides and Lanthanides by Triazinylpyridine N-Donor Ligands. Chem. Rev. 2013, 113, 1199–1236. 10.1021/cr3003399. [DOI] [PubMed] [Google Scholar]
- Peterman Dean R.; Law Jack D.; Todd Terry A.; Tillotson Richard D.. Use of Cyanex-301 for Separation of Am/Cm from Lanthanides in an Advanced Nuclear Fuel Cycle. In Separations for the Nuclear Fuel Cycle in the 21st Century; ACS Symposium Series; American Chemical Society: Washington, DC, 2006; Vol. 933, pp 251–259. [Google Scholar]
- Tranter T. J.Solid-phase Extraction Technology for Actinide and Lanthanide Separations in Nuclear Fuel Reprocessing; Woodhead Publishing: Cambridge, 2011. [Google Scholar]
- Ling L.; Zhang W.-x. Enrichment and Encapsulation of Uranium with Iron Nanoparticle. J. Am. Chem. Soc. 2015, 137, 2788–2791. 10.1021/ja510488r. [DOI] [PubMed] [Google Scholar]
- Makowski P.; Deschanels X.; Grandjean A.; Meyer D.; Toquer G.; Goettmann F. Mesoporous Materials in the Field of Nuclear Industry: Applications and Perspectives. New J. Chem. 2012, 36, 531–541. 10.1039/C1NJ20703B. [DOI] [Google Scholar]
- Batuk O. N.; Szabo V. D.; Denecke M. A.; Vitova T.; Kalmykov S. N. Synthesis and Characterization of Thorium, Uranium and Cerium Oxide Nanoparticles. Radiochim. Acta 2013, 101, 233. 10.1524/ract.2012.2014. [DOI] [Google Scholar]
- Zhang A.; Wei Y.; Hoshi H.; Kumagai M.; Kamiya M.; Koyama T. Resistance Properties of a Macroporous Silica-based N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide-impregnated Polymeric Adsorption Material against Nitric Acid, Temperature and γ-Irradiation. Radiat. Phys. Chem. 2005, 72, 669–678. 10.1016/j.radphyschem.2004.05.050. [DOI] [Google Scholar]
- Zhang A.; Wei Y.; Kumagai M.; Koma Y.; Koyama T. Resistant Behavior of a Novel Silica-based Octyl(phenyl)-N,N-diisobutyl Carbamoylmethylphoshine Oxide Neutral Extraction Resin against Nitric Acid, Temperature and γ-Radiation. Radiat. Phys. Chem. 2005, 72, 455–463. 10.1016/j.radphyschem.2004.01.004. [DOI] [Google Scholar]
- O’Keeffe M.; Yaghi O. M. Deconstructing the Crystal Structures of Metal-Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. 10.1021/cr200205j. [DOI] [PubMed] [Google Scholar]
- Getman R. B.; Bae Y.-S.; Wilmer C. E.; Snurr R. Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 703–723. 10.1021/cr200217c. [DOI] [PubMed] [Google Scholar]
- Sumida K.; Rogow D. L.; Mason J. A.; McDonald T. M.; Bloch E. D.; Herm Z. R.; Bae T.-H.; Long J. R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- Suh M. P.; Park H. J.; Prasad T. K.; Lim D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. 10.1021/cr200274s. [DOI] [PubMed] [Google Scholar]
- Wu H.; Gong Q.; Olson D. H.; Li J. Commensurate Adsorption of Hydrocarbons and Alcohols in Microporous Metal-Organic Frameworks. Chem. Rev. 2012, 112, 836–868. 10.1021/cr200216x. [DOI] [PubMed] [Google Scholar]
- Yanai N.; Kitayama K.; Hijikata Y.; Sato H.; Matsuda R.; Kubota Y.; Takata M.; Mizuno M.; Uemura T.; Kitagawa S. Gas Detection by Structural Variations of Fluorescent Guest Molecules in a Flexible Porous Coordination Polymer. Nat. Mater. 2011, 10, 787–793. 10.1038/nmat3104. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xie Z.; deKrafft K. E.; Lin W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. 10.1021/ja203564w. [DOI] [PubMed] [Google Scholar]
- Li J.-R.; Sculley J.; Zhou H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Carboni M.; Abney C. W.; Liu S.; Lin W. Highly Porous and Stable Metal-Organic Frameworks for Uranium Extraction. Chem. Sci. 2013, 4, 2396–2402. 10.1039/c3sc50230a. [DOI] [Google Scholar]
- Ben T.; Ren H.; Ma S.; Cao D.; Lan J.; Jing X.; Wang W.; Xu J.; Deng F.; Simmons J. M.; Qiu S.; Zhu G. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem., Int. Ed. 2009, 48, 9457–9460. 10.1002/anie.200904637. [DOI] [PubMed] [Google Scholar]
- Lu W.; Yuan D.; Sculley J.; Zhao D.; Krishna R.; Zhou H.-C. Sulfonate-Grafted Porous Polymer Networks for Preferential CO2 Adsorption at Low Pressure. J. Am. Chem. Soc. 2011, 133, 18126. 10.1021/ja2087773. [DOI] [PubMed] [Google Scholar]
- Lu W.; Sculley J. P.; Yuan D.; Krishna R.; Wei Z.; Zhou H.-C. Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas. Angew. Chem., Int. Ed. 2012, 51, 7480–7484. 10.1002/anie.201202176. [DOI] [PubMed] [Google Scholar]
- Additional stability observations, especially in the presence of strong base, have also been made in our laboratory.
- Van Humbeck J. F.; McDonald T. M.; Jing X.; Wiers B. M.; Zhu G.; Long J. R. Ammonia Capture in Porous Organic Polymers Densely Functionalized with Brønsted Acid Groups. J. Am. Chem. Soc. 2014, 136, 2432–2440. 10.1021/ja4105478. [DOI] [PubMed] [Google Scholar]
- The illustration in Figure 3 is meant to illustrate multifold interpenetration (>2-fold) rather than precise and uniform 3-fold interpenetration.
- For simplicity, we will designate metal ion binding to the surfaces within the porous carboxylic acid functionalized materials discussed throughout this article as corresponding to “adsorption”. It is important to note, however, that the metal ion binding phenomenon in these materials will generally not just involve interactions with the carboxylic acid groups, but may also involve some degree of ion exchange to release protons and afford metal–carboxylate interactions.
- Centi G.; Joester D. Nuclear Energy: A Perspective on Recent Results on the Removal of Strontium from Waste. ChemSusChem 2011, 4, 419–420. 10.1002/cssc.201100160. [DOI] [PubMed] [Google Scholar]
- Zhang W.; He X.; Ye G.; Yi R.; Chen J. Americium(III) Capture Using Phosphonic Acid-Functionalized Silicas with Different Mesoporous Morphologies: Adsorption Behavior Study and Mechanism Investigation by EXAFS/XPS. Environ. Sci. Technol. 2014, 48, 6874–6881. 10.1021/es500563q. [DOI] [PubMed] [Google Scholar]
- Yabutani T.; Sumi H.; Nakamura T.; Akatsuki S.; Thuy L. T. X. Multielemental Elution Behavior of Metal Ions Adsorbed on Iminodiacetic Acid Chelating Resin by Using Hydrogen Peroxide as an Eluent. Anal. Sci. 2012, 28, 463–468. 10.2116/analsci.28.463. [DOI] [PubMed] [Google Scholar]
- Myers A. L.; Prausnitz J. M. Thermodynamics of Mixed-gas Adsorption. AIChE J. 1965, 11, 121–127. 10.1002/aic.690110125. [DOI] [Google Scholar]
- Al-Asheh S.; Banat F.; Al-Omari R.; Duvnjak Z. Predictions of Binary Sorption Isotherms for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data. Chemosphere 2000, 41, 659–665. 10.1016/S0045-6535(99)00497-X. [DOI] [PubMed] [Google Scholar]
- Papageorgiou S. K.; Katsaros F. K.; Kouvelos E. P.; Kanellopoulos N. K. Prediction of Binary Adsorption Isotherms of Cu2+, Cd2+ and Pb2+ on Calcium Alginate Beads from Single Adsorption Data. J. Hazard. Mater. 2009, 162, 1347–1354. 10.1016/j.jhazmat.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Krishna R.; van Baten J. M. Using Molecular Simulations for Screening of Zeolites for Separation of CO2/CH4 Mixtures. Chem. Eng. J. 2007, 133, 121–131. 10.1016/j.cej.2007.02.011. [DOI] [Google Scholar]
- Krishna R.; Smit B.; Calero S. Entropy Effects During Sorption of Alkanes in Zeolites. Chem. Soc. Rev. 2002, 31, 185–194. 10.1039/b101267n. [DOI] [PubMed] [Google Scholar]
- Peng X.; Cheng X.; Cao D. Computer Simulations for the Adsorption and Separation of CO2/CH4/H2/N2 Gases by UMCM-1 and UMCM-2 Metal-Organic Frameworks. J. Mater. Chem. 2011, 21, 11259–11270. 10.1039/c1jm10264h. [DOI] [Google Scholar]
- Mason J. A.; Sumida K.; Herm Z. R.; Krishna R.; Long J. R. Evaluating Metal-organic Frameworks for Post-combustion Carbon Dioxide Capture via Temperature Swing Adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. 10.1039/c1ee01720a. [DOI] [Google Scholar]
- Burcl R.Strategy and Methodology for Radioactive Waste Characterization. International Atomic Energy Agency. In Strategy and Methodology for Radioactive Waste Characterization. International Atomic Energy Agency; International Atomic Energy Agency: Austria, 2007; pp 1–169. [Google Scholar]
- Edelstein N. M.; Fuger J.; Katz J. J.; Morss L. R.. Summary and Comparison of Properties of the Actinide and Transactinide Elements. In The Chemistry of the Actinide and Transactinide Elements; Morss L. R., Edelstein N. M., Fuger J., Eds.; Springer: Dordrecht, The Netherlands, 2010; Vol. 3. [Google Scholar]
- David F. H.; Fourest B. Structure of Trivalent Lanthanide and Actinide Aquo Ions. New J. Chem. 1997, 21, 167. [Google Scholar]
- Yaita T.; Narita H.; Suzuki S.; Tachimori S.; Motohashi H.; Shiwaku H. Structural Study of Lanthanides(III) in Aqueous Nitrate and Chloride Solutions by EXAFS. J. Radioanal. Nucl. Chem. 1999, 239, 371–375. 10.1007/BF02349514. [DOI] [Google Scholar]
- Mondry A.; Starynowicz P. Crystal Structure and Absorption Spectroscopy of a Neodymium(III) Complex with Triethylenetetraaminehexaacetic Acid, Na3[Nd(TTHA)]·2.5NaClO4·7.617H2O. Inorg. Chem. 1997, 36, 1176–1180. 10.1021/ic9604728. [DOI] [PubMed] [Google Scholar]
- Tang X.; Yue S.; Li P.; Wang N.; Liu Y. Hydrothermal Synthesis and Crystal Structure Study of Two Novel 3-D Mellitates {Nd2[C6(COO)6](H2O)6} and {Ho2[C6(COO)6](H2O)6}. J. Rare Earths 2008, 26, 800–803. 10.1016/S1002-0721(09)60009-0. [DOI] [Google Scholar]
- Huskowska E.; Legendziewicz J.; Schleid T.; Meyer G. A Special Double Issue Devoted to the Workshop on the Basic and Applied Aspects of Rare Earths do the Lanthanides Form Inner Sphere Complexes with ClO4– Ions in Competition with H2O Molecules?. Mater. Chem. Phys. 1992, 31, 117–122. 10.1016/0254-0584(92)90163-3. [DOI] [Google Scholar]
- Cosmidis J.; Benzerara K.; Nassif N.; Tyliszczak T.; Bourdelle F. Characterization of Ca-phosphate Biological Materials by Scanning Transmission X-ray Microscopy (STXM) at the Ca L 2,3 -, P L 2,3 -and C K- Edges. Acta Biomater. 2015, 12, 260–269. 10.1016/j.actbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Shapiro D. A.; Yu Y.-S.; Tyliszczak T.; Cabana J.; Celestre R.; Chao W.; Kaznatcheev K.; Kilcoyne A. L. D.; Maia F.; Marchesini S.; Meng Y. S.; Warwick T.; Yang L. L.; Padmore H. A. Chemical Composition Mapping with Nanometre Resolution by Soft X-ray Microscopy. Nat. Photonics 2014, 8, 765–769. 10.1038/nphoton.2014.207. [DOI] [Google Scholar]
- Kaulich B.; Thibault P.; Gianoncelli A.; Kiskinova M. Transmission and Emission X-ray Microscopy: Operation Modes, Contrast Mechanisms and Applications. J. Phys.: Condens. Matter 2011, 23, 083002–083025. 10.1088/0953-8984/23/8/083002. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Saiers J. E.; Xu N.; Minasian S. G.; Tyliszczak T.; Kozimor S. A.; Shuh D. K.; Barnett M. O. Impact of Natural Organic Matter on Uranium Transport through Saturated Geologic Materials: From Molecular to Column Scale. Environ. Sci. Technol. 2012, 46, 5931–5938. 10.1021/es300155j. [DOI] [PubMed] [Google Scholar]
- Brownlee D.; Tsou P.; Aleon J.; Alexander C. M. O. D.; Araki T.; Bajt S.; Baratta G. A.; Bastien R.; Bland P.; Bleuet P.; Borg J.; Bradley J. P.; Brearley A.; Brenker F.; Brennan S.; Bridges J. C.; Browning N. D.; Brucato J. R.; Bullock E.; Burchell M. J.; Busemann H.; Butterworth A.; Chaussidon M.; Cheuvront A.; Chi M.; Cintala M. J.; Clark B. C.; Clemett S. J.; Cody G.; Colangeli L.; Cooper G.; Cordier P.; Daghlian C.; Dai Z.; D’Hendecourt L.; Djouadi Z.; Dominguez G.; Duxbury T.; Dworkin J. P.; Ebel D. S.; Economou T. E.; Fakra S.; Fairey S. A. J.; Fallon S.; Ferrini G.; Ferroir T.; Fleckenstein H.; Floss C.; Flynn G.; Franchi I. A.; Fries M.; Gainsforth Z.; Gallien J. P.; Genge M.; Gilles M. K.; Gillet P.; Gilmour J.; Glavin D. P.; Gounelle M.; Grady M. M.; Graham G. A.; Grant P. G.; Green S. F.; Grossemy F.; Grossman L.; Grossman J. N.; Guan Y.; Hagiya K.; Harvey R.; Heck P.; Herzog G. F.; Hoppe P.; Hoerz F.; Huth J.; Hutcheon I. D.; Ignatyev K.; Ishii H.; Ito M.; Jacob D.; Jacobsen C.; Jacobsen S.; Jones S.; Joswiak D.; Jurewicz A.; Kearsley A. T.; Keller L. P.; Khodja H.; Kilcoyne A. L. D.; Kissel J.; Krot A.; Langenhorst F.; Lanzirotti A.; Le L.; Leshin L. A.; Leitner J.; Lemelle L.; Leroux H.; Liu M.-C.; Luening K.; Lyon I.; MacPherson G.; Marcus M. A.; Marhas K.; Marty B.; Matrajt G.; McKeegan K.; Meibom A.; Mennella V.; Messenger K.; Messenger S.; Mikouchi T.; Mostefaoui S.; Nakamura T.; Nakano T.; Newville M.; Nittler L. R.; Ohnishi I.; Ohsumi K.; Okudaira K.; Papanastassiou D. A.; Palma R.; Palumbo M. E.; Pepin R. O.; Perkins D.; Perronnet M.; Pianetta P.; Rao W.; Rietmeijer F. J. M.; Robert F.; Rost D.; Rotundi A.; Ryan R.; Sandford S. A.; Schwandt C. S.; See T. H.; Schlutter D.; Sheffield-Parker J.; Simionovici A.; Simon S.; Sitnitsky I.; Snead C. J.; Spencer M. K.; Stadermann F. J.; Steele A.; Stephan T.; Stroud R.; Susini J.; Sutton S. R.; Suzuki Y.; Taheri M.; Taylor S.; Teslich N.; Tomeoka K.; Tomioka N.; Toppani A.; Trigo-Rodriguez J. M.; Troadec D.; Tsuchiyama A.; Tuzzolino A. J.; Tyliszczak T.; Uesugi K.; Velbel M.; Vellenga J.; Vicenzi E.; Vincze L.; Warren J.; Weber I.; Weisberg M.; Westphal A. J.; Wirick S.; Wooden D.; Wopenka B.; Wozniakiewicz P.; Wright I.; Yabuta H.; Yano H.; Young E. D.; Zare R. N.; Zega T.; Ziegler K.; Zimmerman L.; Zinner E.; Zolensky M. Research Aticle - Comet 81P/Wild 2 Under a Microscope. Science 2006, 314, 1711–1716. 10.1126/science.1135840. [DOI] [PubMed] [Google Scholar]
- Cody G. D.; Ade H.; Alexander C. M. O. D.; Araki T.; Butterworth A.; Fleckenstein H.; Flynn G.; Gilles M. K.; Jacobsen C.; Kilcoyne A. L. D.; Messenger K.; Sandford S. A.; Tyliszczak T.; Westphal A. J.; Wirick S.; Yabuta H. Quantitative Organic and Light-element Analysis of Comet 81P/Wild 2 Particles Using C-, N-, and O-mu-XANES. Meteorit. Planet. Sci. 2008, 43, 353–365. 10.1111/j.1945-5100.2008.tb00627.x. [DOI] [Google Scholar]
- Dynes J. J.; Tyliszczak T.; Araki T.; Lawrence J. R.; Swerhone G. D. W.; Leppard G. G.; Hitchcock A. P. Speciation and Quantitative Mapping of Metal Species in Microbial Biofilms using Scanning Transmission X-ray Microscopy. Environ. Sci. Technol. 2006, 40, 1556–1565. 10.1021/es0513638. [DOI] [PubMed] [Google Scholar]
- Lawrence J. R.; Swerhone G. D. W.; Leppard G. G.; Araki T.; Zhang X.; West M. M.; Hitchcock A. P. Scanning Transmission X-ray, Laser Scanning, and Transmission Electron Microscopy Mapping of the Exopolymeric Matrix of Microbial Biofilms. Appl. Environ. Microb. 2003, 69, 5543–5554. 10.1128/AEM.69.9.5543-5554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart S. G.; Hitchcock A. P.; Smith A. P.; Ade H. W.; Lidy W.; Rightor E. G.; Mitchell G. E. NEXAFS Spectromicroscopy of Polymers: Overview and Quantitative Analysis of Polyurethane Polymers. J. Electron Spectrosc. Relat. Phenom. 1999, 100, 119–135. 10.1016/S0368-2048(99)00043-2. [DOI] [Google Scholar]
- Koprinarov I. N.; Hitchcock A. P.; McCrory C. T.; Childs R. F. Quantitative Mapping of Structured Polymeric Systems Using Singular Value Decomposition Analysis of Soft X-ray Images. J. Phys. Chem. B 2002, 106, 5358–5364. 10.1021/jp013281l. [DOI] [Google Scholar]
- Chantler C. T. Detailed Tabulation of Atomic Form Factors, Photoelectric Absorption and Scattering Cross Section, and Mass Attenuation Coefficients in the Vicinity of Absorption Edges in the Soft X-Ray (Z = 30–36, Z = 60–89, E = 0.1 keV–10 keV), Addressing Convergence Issues of Earlier Work. J. Phys. Chem. Ref. Data 2000, 29, 597–1056. 10.1063/1.1321055. [DOI] [PubMed] [Google Scholar]
- Van Vuuren C. P. J.; Strydom C. A. The Thermal Decomposition of Neodymium Nitrate. Thermochim. Acta 1986, 104, 293–298. 10.1016/0040-6031(86)85204-2. [DOI] [Google Scholar]
- Hirosaki N.; Ogata S.; Kocer C. Ab Initio Calculation of the Crystal Structure of the Lanthanide Ln2O3 Sesquioxides. J. Alloys Compd. 2003, 351, 31–34. 10.1016/S0925-8388(02)01043-5. [DOI] [Google Scholar]
- Wu L.; Liu N.; Qin Z.; Shoesmith D. W. Modeling the Radiolytic Corrosion of Fractured Nuclear Fuel under Permanent Disposal Conditions. J. Electrochem. Soc. 2014, 161, E3259–E3266. 10.1149/2.032408jes. [DOI] [Google Scholar]
- West Valley Demonstration Project: Draft Waste-Incidental-to-Reprocessing Evaluation for the Concentrator Feed Makeup Tank and the Meter Feed Hold Tank. In West Valley Demonstration Project: Draft Waste-Incidental-to-Reprocessing Evaluation for the Concentrator Feed Makeup Tank and the Meter Feed Hold Tank; U.S. Department of Energy: West Valley, NY, 2012; p 1. [Google Scholar]
- Hosten E.; Rohwer H. Interaction of Anions with Arsenazo III-lanthanide (III) Complexes. Anal. Chim. Acta 1997, 345, 227–233. 10.1016/S0003-2670(97)00100-1. [DOI] [Google Scholar]
- Shiwaku H.; Mitsui T.; Tozawa K.; Kiriyama K.; Harami T.; Mochozuki T. Cryogenically Cooled Monochromator with Multi-crystal Switching System on BL11XU at SPring-8. AIP Conf. Proc. 2004, 705, 659. 10.1063/1.1757882. [DOI] [Google Scholar]
- Ressler T. WinXAS: a Program for X-ray Absorption Spectroscopy Data Analysis under MS-Windows. J. Synchrotron Radiat. 1998, 5, 118–122. 10.1107/S0909049597019298. [DOI] [PubMed] [Google Scholar]
- Ankudinov A. L.; Rehr J. J. Theory of Solid-state Contributions to the X-ray Elastic Scattering Amplitude. Phys. Rev. B: Condens. Matter Mater. Phys. 2000, 62, 2437–2445. 10.1103/PhysRevB.62.2437. [DOI] [Google Scholar]
- Schauer C. K.; Anderson O. P. Highly polydentate ligands. Part 4. Crystal Structures of Neodymium(III) and Erbium(III) Complexes of 3,12-bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioate(4-). J. Chem. Soc., Dalton Trans. 1989, 185–191. 10.1039/DT9890000185. [DOI] [Google Scholar]
- Bluhm H.; Andersson K.; Araki T.; Benzerara K.; Brown G. E.; Dynes J. J.; Ghosal S.; Gilles M. K.; Hansen H. C.; Hemminger J. C.; Hitchcock A. P.; Ketteler G.; Kilcoyne A. L. D.; Kneedler E.; Lawrence J. R.; Leppard G. G.; Majzlam J.; Mun B. S.; Myneni S. C. B.; Nilsson A.; Ogasawara H.; Ogletree D. F.; Pecher K.; Salmeron M.; Shuh D. K.; Tonner B.; Tyliszczak T.; Warwick T.; Yoon T. H. Soft X-ray Microscopy and Spectroscopy at the Molecular Environmental Science Beamline at the Advanced Light Source. J. Electron Spectrosc. Relat. Phenom. 2006, 150, 86–104. 10.1016/j.elspec.2005.07.005. [DOI] [Google Scholar]
- Gianetti T. L.; Nocton G.; Minasian S. G.; Tomson N. C.; Kilcoyne A. L. D.; Kozimor S. A.; Shuh D. K.; Tyliszczak T.; Bergman R. G.; Arnold J. Diniobium Inverted Sandwich Complexes with μ-η6:η6-Arene Ligands: Synthesis, Kinetics of Formation, and Electronic Structure. J. Am. Chem. Soc. 2013, 135, 3224–3236. 10.1021/ja311966h. [DOI] [PubMed] [Google Scholar]
- Minasian S. G.; Keith J. M.; Batista E. R.; Boland K. S.; Bradley J. A.; Daly S. R.; Sokaras D.; Kozimor S. A.; Lukens W. W.; Martin R. L.; Nordlund D.; Seidler G. T.; Shuh D. K.; Tyliszczak T.; Wagner G. L.; Weng T. C.; Yang P. Covalency in Metal-Oxygen Multiple Bonds Evaluated Using Oxygen K-edge Spectroscopy and Electronic Structure Theory. J. Am. Chem. Soc. 2013, 135, 1864–1871. 10.1021/ja310223b. [DOI] [PubMed] [Google Scholar]
- Minasian S. G.; Keith J. M.; Batista E. R.; Boland K. S.; Kozimor S. A.; Martin R. L.; Shuh D. K.; Tyliszczak T.; Vernon L. J. Carbon K-Edge X-ray Absorption Spectroscopy and Time-Dependent Density Functional Theory Examination of Metal–Carbon Bonding in Metallocene Dichlorides. J. Am. Chem. Soc. 2013, 135, 14731–14740. 10.1021/ja405844j. [DOI] [PubMed] [Google Scholar]
- Minasian S. G.; Krinsky J. L.; Rinehart J. D.; Copping R.; Tyliszczak T.; Janousch M.; Shuh D. K.; Arnold J. A Comparison of 4f vs 5f Metal-Metal Bonds in (CpSiMe3)3M-ECp* (M = Nd, U; E = Al, Ga; Cp* = C5Me5): Synthesis, Thermodynamics, Magnetism, and Electronic Structure. J. Am. Chem. Soc. 2009, 131, 13767–13783. 10.1021/ja904565j. [DOI] [PubMed] [Google Scholar]
- Bugaris D. E.; Copping R.; Tyliszczak T.; Shuh D. K.; Ibers J. A. La2U2Se9: An Ordered Lanthanide/Actinide Chalcogenide with a Novel Structure Type. Inorg. Chem. 2010, 49, 2568–2575. 10.1021/ic902503n. [DOI] [PubMed] [Google Scholar]
- Bugaris D. E.; Choi E. S.; Copping R.; Glans P.-A.; Minasian S. G.; Tyliszczak T.; Kozimor S. A.; Shuh D. K.; Ibers J. A. Pentavalent and Tetravalent Uranium Selenides, Tl3Cu4USe6 and Tl2Ag2USe4: Syntheses, Characterization, and Structural Comparison to Other Layered Actinide Chalcogenide Compounds. Inorg. Chem. 2011, 50, 6656–6666. 10.1021/ic200565n. [DOI] [PubMed] [Google Scholar]
- Csoregh I.; Huskowska E.; Ertan A.; Legendziewicz J.; Kierkegaard P. Crystal Structure of a Novel Neodymium Hydroxide Perchlorate Hydrate, Nd2(OH)3(ClO4)3.5H2O. Acta Chem. Scand. 1989, 43, 829–833. 10.3891/acta.chem.scand.43-0829. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.