Abstract
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors found in the gastrointestinal (GI) tract, with the stomach being the most common site. They represent a distinct group of GI tumors originating from the interstitial cells of Cajal and are characterized by gain-of-function mutations of KIT. KIT oncoprotein serves as both diagnostic and therapeutic targets. Prognosis is related to size, mitotic activity, and site of the tumor. Asymptomatic, small endoscopic ultrasonography (EUS)-suspected GISTs are increasingly encountered with the wide availability of endoscopic/endosonographic examination. The majority of small GISTs are biologically indolent, albeit possibly harboring c-KIT gene mutations. An ongoing controversy exists regarding the management and surveillance policy for small gastric GISTs. A number of reports on the management of GISTs have been published, not confidently addressing the issue of gastric GISTs of small size. This work provides an overview on the current state of management considerations, specifically focusing on small EUS-suspected gastric GISTs, which are increasingly encountered by clinicians.
Keywords: Gastrointestinal stromal tumor (GIST), small hypoechoic muscularis propria (MP) lesion, surgery, surveillance
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract.[1,2] Although they are relatively rare, comprising only 0.1%-3% of all GI tumors,[3,4] their diagnosis has increased dramatically as the result of greater awareness and improved histopathological detection rates.[5]
GISTs, once classified among smooth muscle tumors, are now believed to arise from a common precursor cell that gives rise to the interstitial cells of Cajal, the GI pacemaker cells in the myenteric plexus.[6,7] The majority arise in the wall of the stomach (60%-70%) and small intestine (30%); the colon, rectum, esophagus, and appendix are other less common sites.[5,8] Rarely, GISTs develop in extra-GI sites such as the mesentery, omentum, or retroperitoneum.[9,10,11] The symptoms are related to the size and location of the tumor and are usually nonspecific (i.e., early satiety, bloating), unless they ulcerate, bleed, or enlarge causing pain or obstruction.[12]
In 1998, Hirota et al. described the presence of oncogenic c-KIT mutations resulting in gain-of-function of the enzymatic activity of the KIT, a transmembrane tyrosine kinase receptor for stem cell factor.[13] In a small subset of GISTs, mutations were identified in the platelet-derived growth factor receptor alpha (PDGFR-α) gene rather than the KIT.[14,15,16] GISTs can be pathologically distinguished from other mesenchymal tumors by CD117 antibody immunohistochemical (IHC) staining for the expression of KIT receptor protein.[7]
Asymptomatic, small, and incidentally detected GISTs are attracting attention in recent publications.[17,18,19] In fact GISTs vary greatly in size, ranging from millimetric incidental lesions to large masses of 35 cm or more.[5] They are known to be rare when only clinically relevant ones are covered; nevertheless, the frequency is estimated to be as high as 35% of the population when all small or microscopic tumors are included,[20] as reported by the data from the autopsy series and gastric surgical specimens.[21,22] Although these lesions are presumed to have no malignant potential,[9,23] their natural course is not well defined. A small proportion is thought to evolve into clinically significant GISTs,[22] and rarely these small lesions with low mitotic activity may ultimately metastasize.[24,25] The appropriate strategy for the management of asymptomatic small subepithelial lesions (SELs) originating from the muscularis propria (MP) of the gastrointestinal wall (i.e., MP-SELs) particularly those in a gastric location detected by endoscopic ultrasonography (EUS), remains controversial.
This work aims to provide an overview of management considerations, specifically focusing on small gastric SELs originating from MP with a presumptive diagnosis of GIST based on EUS examination.
DIAGNOSIS OF “SMALL GISTS”
As upper endoscopy has become widely available, clinicians more often encounter bulges arising beneath the epithelium with variable clinical significance ranging from insignificant to malignant lesions. Endoscopic biopsies are unlikely to determine the diagnosis because these lesions lie deep in the GI wall. For SELs larger than 10 mm, evaluation with EUS is recommended to ascertain the size, layer of origin, features of echogenicity, and high-risk features for malignancy suspicion.[26] In most cases, EUS findings only allow a presumptive diagnosis and determine the need for further explorations such as tissue sampling, surgery, or follow-up. MP (fourth layer) hypoechoic lesions are mostly GISTs if they are localized in the stomach, but EUS alone is not sufficient to differentiate GIST from other causes of hypoechoic MP masses such as schwannoma, leiomyoma, and lymphoma. Therefore, a tissue diagnosis with EUS-guided fine-needle aspiration (EUS-FNA) is generally performed. Figure 1 depicts the EUS view of features consistent with a gastric MP-SEL histologically proven to be GIST.
Figure 1.
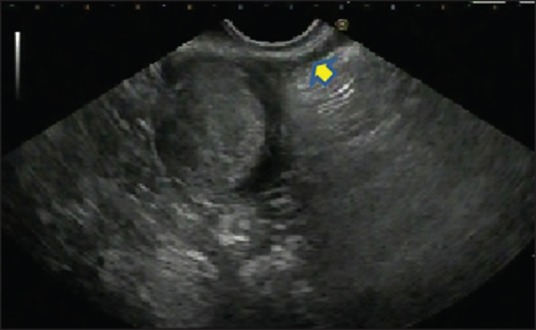
The EUS shows an incidental small (18.3 × 18 mm2) gastric hypoechoic SELs arising from the fourth layer (arrow, MP) suggesting GIST. EUS-assisted fine-needle aspiration (FNA) was performed and the IHC analysis revealed GIST
At present, although EUS-FNA is considered the procedure of choice for preoperative diagnosis of GIST by IHC analysis of the sample for c-KIT,[24,27] it provides inadequate material in up to 33.3% of the cases.[28,29] In particular, smaller tumors are technically more difficult to obtain adequate histological samples compared to larger ones.[27,30] In the study by Akahoshi et al., the reported diagnostic yields for tumors less than 20 mm, 20 mm-40 mm, and 40 mm or more were 71%, 86%, and 100%, respectively, when only the adequate specimens were considered.[27] The literature shows discrepant rates of sample adequacy, ranging 58%-81.3 % with EUS-FNA in upper GI SELs, which influence the final yield for GIST diagnosis.[27,31,32,33] One of the reasons for this discrepancy may be the presence of a cytologist for a rapid onsite evaluation (ROSE) of the sample in some studies. A recent study reported a sampling adequacy reaching 100% in both the <20 mm and the ≥20 mm groups of upper GI SELs; with a sensitivity of 81.3% for the diagnosis of small GIST<20 mm using 22- or 25-gauge (G) needles and a forward-viewing type ultrasound endoscope in difficult circumstances, in the presence of on-site FNA cytology review.[33] Thus, the diagnosis of small GISTs may be only presumptive based on EUS appearance, but performing EUS-FNA and management decisions may also be challenging in most circumstances where optimized conditions are not available. Nevertheless, GIST appeared to be the most common diagnosis among the MP-SELs in many studies evaluating the postoperative results. In one study, postoperative histological examination revealed GIST in 16 of 19 (84.2%) resected gastric MP-SELs ≤3 cm in size (the others were 2 schwannomas and 1 leiomyoma).[19] In another study, 21 of 23 (91.3%) gastric submucosal tumors having a median diameter of 1.8 (0.5-4.0) cm (the other 2 were schwannomas) were GISTs.[34] Even in the case of a GIST diagnosis accomplished by EUS-FNA, evaluation of the malignant potential of the tumor based on the mitotic index may not be possible due to the lack of sufficient material as required for prompt investigation.[5] Thus, EUS-FNA may not change the management strategy, at least in a significant subset of patients with asymptomatic small MP-SELs.
Choice of needle size does not seem to offer a significant advantage in the tissue acquisition of GI SELs. In fact, that choice largely remains at the discretion of the endosonographer. Only one randomized prospective study could demonstrate a nonsignificant advantage of 22G needles over 25G needles in a small population of subepithelial GI tumors having a mean diameter of 32 mm.[35] The assumption that the 19G needle may deliver superior results over smaller-caliber (22G and 25G) needles in EUS-FNA of suspected GISTs has not been proved.[31,36,37] In a prospective multicenter study, the definite diagnostic yield with IHC of 19G FNA was only 52% from 46 gastric SELs having a median size of 15 mm × 24 mm.[37] Targeting a small SEL using a 19G fine needle might be more difficult and the needle's movement might be restricted within such a small SEL. Forward exit of the needle using the forward-viewing therapeutic linear echoendoscope may provide easier application and higher diagnostic yield. A diagnostic yield of 93.4% was reported with a technique named EUS-guided fine-needle tissue acquisition (EUS -FNTA) using the forward-viewing therapeutic linear echoendoscope among 121 patients with SELs (13 in the esophagus, 96 in the stomach, 10 in the duodenum, 2 in the rectum); high performance was also observed for small lesions (< 20 mm).[38] Nevertheless, no correlation between the mitotic index from EUS - FNTA samples and surgical specimen was found, despite the very high diagnostic yield for GISTs allowing adequate samples for histology and IHC in 100% of the cases with EUS - FNTA.[38]
One option to overcome the limitations of EUS-FNA would be tissue acquisition via EUS-guided Tru-Cut biopsy (EUS-TCB). However, it is difficult to apply EUS-TCB for GISTs smaller than the length of the needle. Moreover, the mitotic index on the final surgical pathology specimen was poorly correlated with the mitotic index determined on EUS-TCB in one study,[39] and also two severe septic complications were reported in this study. EUS-TCB could not yield superior overall diagnostic accuracy than EUS-FNA in GISTs owing to the high technical failure rate of EUS-TCB in another study.[40] The yield with EUS-TCB may be better in the case of larger MP-SELs. EUS-TCB provided diagnostic histology and IHC for 79% and 97%, respectively, of the 38 patients mainly with upper GI MP-SELs all with a diameter ≥20 mm, while a EUS-FNA diagnostic final cytology was achieved in 76% of patients.[41]
PROGNOSTIC FACTORS AND NATURAL HISTORY OF “SMALL GISTS”
The clinical behavior of GISTs is quite variable, and criteria to predict the lesions with progressive disease potential have been investigated for many years. Tumor size, mitotic count, and anatomic site gained acceptance as prognostic parameters.[42] Macroscopically positive surgical margins and tumor rupture are additional factors affecting the prognosis for resected lesions.[20,43,44,45] The clinical behavior of these tumors remains difficult to predict except for the presence of obvious metastasis, thus the question of which parameters can reliably predict disease behavior in GISTs has long been discussed.
Schemes were proposed to stratify the malignant risk rather than strictly labeling a given GIST as benign or malignant. Accordingly, the National Institutes of Health (NIH) consensus[42] classified GISTs into very low-, low-, intermediate-, and high-risk categories by using the two parameters of tumor size and mitotic count. Miettinen and Lasota incorporated tumor location (stomach, duodenum, jejunum/ileum, rectum) as a third parameter, based on their long-term follow-up data from more than 1500 patients.[8,9] They indicated that small GISTs of <20 mm with a mitotic index of <5/50 high-power field (HPF) have no metastasis risk irrespective of the anatomic site, thus defining these lesions as benign. However, the NIH consensus classified these lesions as “very low-risk,” avoiding the term “benign,” indicating that no GIST can be defined as definitely benign on the basis of currently available evidence.[42] Regular endosonographic or endoscopic surveillance is the commonly employed conservative strategy for these small lesions to monitor tumor progression.
There are few studies revealing the natural history and outcome of small MP-SELs, most of which rely on retrospective data. These studies suggest that most patients with small MP-SELs are not candidates for surgical resection. In the retrospective analysis conducted by our group, among 28 patients with small gastric MP-SELs who had been managed conservatively (75% of cases <20 mm at the index EUS), there was no tumor-related death or any new symptom related to disease progression after a mean follow-up of 48.5 (12-99) months.[30] Additionally, no significant-sized change or development of high-risk sonographic feature was detected in the patient group compliant with EUS surveillance.[30] Lachter et al.[46] consistently reported that the majority of small (<17 mm) GISTs did not change in echogenicity or size during a median period of 5-year surveillance, and enlargement in size was detected significantly more in GISTs over 17 mm diameter (P < 0.018). In one retrospective study by Lok et al., an increase in tumor size was revealed in only 3 of 23 patients (13.0%) over a mean period of 17.3 ± 10.2 months follow-up with EUS for small MP-SELs (mostly gastric with a median size of 12.9 ± 6.9 mm) without high-risk EUS features.[17] In another study by Kim et al., 8.5% of small gastric SELs (≤30 mm) showed changes in size or echo pattern over a median period of 24 months when followed up endoscopically or by EUS.[19] Imaoka et al.[47] followed 132 gastric SELs with upper GI endoscopy for 5 years and found that only two lesions increased in size; surgical resection was performed for these tumors, which were eventually diagnosed as GISTs, and 1 patient had liver metastasis after resection. Endoscopic surveillance studies may underestimate the size changes owing to ill-defined inspection by endoscopy.[48]
EUS surveillance has the advantage of measuring the exact dimensions and provides information on any change to high-risk sonographic features related to the increased likelihood of malignant behavior. These high-risk EUS features suspicious of malignancy for GISTs were defined as larger size, irregular borders, heterogeneous echo patterns, presence of anechoic spaces, and echogenic foci[49,50] [Table 1]. In addition, efforts have been made to identify the EUS features that could differentiate GISTs from the benign SEL leiomyomas. Heterogeneity, hyperechogenic reflexes, a marginal halo, and higher echogenicity in comparison with the MP layer were the EUS features that were significantly more frequent (P < 0.05) in the GISTs than in the leiomyomas, and EUS could distinguish these lesions with a sensitivity of 89.1% and specificity of 85.7% if two of these four features were present.[51] Another study attempted to use digital image analysis to overcome the subjective judgment of these EUS features, and reported a sensitivity of 94%, specificity of 80%, and accuracy of 90.8% in differentiating GIST from leiomyoma or schwannoma.[52] Some of EUS features mentioned above may be more difficult to characterize for very small lesions, and their predictive value is unsettled for these cases. In one retrospective study, EUS features such as heterogeneity, hyperechoic foci, cystic change, hypoechoic foci, lobulation, and ulceration could not distinguish very low-risk lesions from moderate-risk ones preoperatively among 55 medium-sized gastric (20-50 mm) GISTs.[53] It is possible that risk stratification can be even less accurate in smaller lesions.
Table 1.
Features predictive of the malignant potential of GISTs by endoscopy, endoscopic ultrasound and contrast-enhanced endoscopic ultrasound
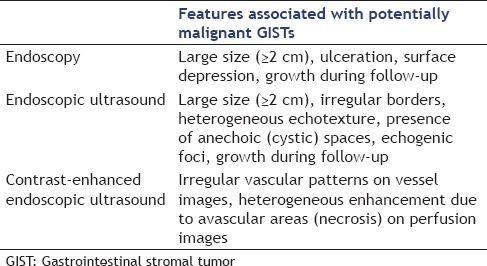
Although EUS features give important clues about the etiology and malignant potential of a SEL, EUS-FNA tissue acquisition is recommended for more accurate diagnosis, but a significant number of endosonographers will not perform FNA for EUS-suspected GISTs in clinical practice.[54] As EUS-FNA of SELs is technically demanding with a moderate diagnostic yield and is frequently unable to determine the mitotic index, newer technologies such as contrast-enhanced EUS (CE-EUS) and EUS-elastography have emerged and have been utilized to improve the diagnostic yield of EUS. EUS-elastography evaluates the strain generated by the slight compression of the tissue by the probe, and the elasticity is displayed as a real-time quantification on a color-coded image superimposed on the grayscale B-mode image. EUS-elastography is a promising technique that might enhance characterization of benign and malignant SELs during the usual EUS procedure. Its clinical impact on discrimination between benign and malignant pancreatic masses and lymph nodes has been evaluated,[55,56] but further studies are required to establish the complementary role of sonoelastography images in defining the tissue characteristics of benign and malignant GI SELs. The vascular characteristics of the SELs can be visualized by CE-EUS with the aim of improved differential diagnosis and malignant potential prediction. In a study of 17 patients with suspicious gastric or esophageal submucosal lesions, the enhancement pattern of contrast-enhanced harmonic EUS (CEH-EUS) could distinguish GISTs from benign SELs.[57] All eight histologically proven GISTs showed hyperenhancement, and all the nine hypoenhanced lesions were benign SELs (leimyoma or lipoma).[57] Additionally, CE-EUS has been used to predict the malignant potential of GISTs in one study according to the image patterns; by identifying irregular vessels on the vessel image and heterogeneous enhancement on the perfusion image, it was found that high-grade malignancy GISTs could be predicted with a sensitivity, specificity, and accuracy of 100%, 63%, and 83%, respectively.[58] These promising techniques are not studied yet specifically for small gastric MP-SELs, but their application has the potential to improve the accuracy of noninvasive characterization with EUS, and may lead to more selective use of tissue acquisition and more individualized management strategies. Features found to be predictive of the malignant potential of GISTs by endoscopy, EUS, and CE-EUS are summarized in Table 1.
Tumor growth has been considered as the main indicator to distinguish the potentially malignant SELs from their benign counterparts during surveillance, but what extent of growth can be considered significant has not been established. It is plausible that only a limited fraction of small lesions presenting with increase in size will be of clinical significance, as metastasis rates do not correlate with the rates of size change. Bearzi et al. described GIST cases with low mitotic counts that grew to larger sizes in the absence of any aggressive behavior.[59] By contrast, a small GIST may acquire the genetic mutations that can transform it into a metastasizing lesion without any detectable increase in its size during the surveillance period. Agaimy et al. grossly identified small GISTs (1-10 mm) as frequent as 22.5% in a population older than 50 years in their autopsy series, and many of them carried an oncogenic activation in the c-KIT (46%) or PDGFR-α (4%) gene.[22] Most such lesions remain small or may regress, demonstrating an indolent course, and only a small fraction is the precursor of clinically overt GISTs. The neoplastic process after acquisition of c-KIT mutations that results in malignant behavior is thought to be related to cytogenetic aberrations such as chromosomal depletion and molecular mutations in tumor suppressor genes.[60,61,62,63,64] Eventually, further research is needed to clarify the genetic determinants for neoplastic transformation and to identify the molecular surrogate markers for acquiring aggressive mutations, rather than applying indefinite surveillance strategies for all patients.
CURRENT MANAGEMENT RECOMMENDATIONS FOR “SMALL GISTS”
Currently, the initial management strategy for small GISTs is tailored by tumor site and size. Surgical resection with preceding metastasis work-up is the mainstay of treatment for lesions larger than 20-30 mm that are highly suspicious of GIST due to their considerable risk of malignancy.[20,49,50] GISTs in a nongastric primary location such as the small intestine or rectum are regarded as having more aggressive malignant potential than gastric GISTs.[9,43,65,66] A series of 127 patients with localized GISTs who underwent complete resection reported small-intestinal tumor location as an independent factor associated with increased risk of recurrence.[66] Because of the varying potentials for neoplastic transformation risk, the surgical approach is generally chosen for duodenal or rectal GISTs, even for small ones.[43,67]
Practice guidelines do not provide clear and consistent recommendations toward management or follow-up of small-size GISTs. The American Gastroenterological Association (AGA) recommends surgical resection for gastric GISTs >30 mm in size and to consider surveillance for smaller lesions with no concerning endosonographic features.[68] The US National Comprehensive Cancer Network (NCCN) recommends resection for all GISTs ≥20 mm; no clear statement is made regarding incidentally encountered small gastric GISTs (<20 mm) due to the insufficient data, but complete surgical resection is suggested for those having high-risk EUS features, and endoscopic surveillance at 6-12-month intervals for the lesions without high-risk features.[5] European Society for Medical Oncology (ESMO) guidelines suggest control at short-term (e.g., at 3 months) and then to prolong the follow-up intervals in case of no growth for small lesions, if follow-up strategy is the preferred choice.[43] Japanese guidelines state that lesions <20 mm in size and without ulceration or surface depression can be followed up endoscopically once or twice a year.[69]
Institutions avoid suggesting strict recommendations because of the lack of evidence demonstrating the usefulness of regular surveillance policy for small lesions. Therefore, data from properly designed large-scale studies are needed. Generally, the suggested cutoff diameter is <20 mm for a conservative approach,[5,43,69] although the AGA recommends <30 mm cutoff.[68] Thus, the issue of whether asymptomatic gastric GISTs smaller than 20 mm or 30 mm may safely be followed up is another unresolved question as the current cutoffs seem somewhat arbitrarily chosen in the limited published data in hand.
With the widespread availability of endoscopy, it is likely that these small gastric MP-SELs will be more commonly encountered. An indefinite surveillance strategy for such lesions increases the hospital workload, and considering the very small percentage turning out to be clinically significant, this approach may lack cost-effectiveness. Additionally, the unrevealing surveillance procedures with their discomfort and potential morbidity may induce emotional distress and frustration in many patients. In fact, this concern is reflected by the high rates of poor compliance (up to 61%) in many studies confined to the follow-up guidelines.[17,30,70] Thus, the efficiency of the short-interval surveillance approach should be well balanced against the poor compliance by patients. Obviously, if the surveillance is the chosen management option, less invasive, cheaper, and more comfortable tools are needed. In line with this objective, Polkowski et al. reported that 69% of EUS-diagnosed gastric SELs could be visualized and measured using transabdominal ultrasound in water-filled stomach,[71] thus they suggested transabdominal ultrasound may serve as an alternative to EUS for surveillance. Many experts do not consider contrast-enhanced computed tomography (CT) scan imaging useful for these lesions because of its low sensitivity in detecting small gastric GISTs.
Surgery is the current standard of treatment for GISTs larger than 20-30 mm, but postoperative recurrence can be expected depending on the presence of high-risk parameters. However, it is debatable whether surgery for small gastric GISTs would be an overtreatment or not for a lesion with very low malignant potential. Miettinen reported that no metastasis occurred after the resection of small GISTs in their follow-up series,[8] which means that surgical resection achieves nearly 100% cure for small GISTs. Some authors suggest surgery for all small tumors because of their occasional metastatic behavior,[27,72,73] while others argue against it because of the high cost and the surgical risks for a lesion with very low malignant potential.[17] Given the lack of sufficient evidence for optimal management, it may be logical to tailor a decision individually after discussing the risks and benefits of all potential management strategies with the patient considering his/her age, life expectancy, and comorbidities. At present, an evidence-based optimal algorithm has not been established for diagnostic or therapeutic work-up of small (< 2 cm) MP-SELs discovered on endoscopy. For this purpose, we suggest an algorithm [Figure 2] for the management of these lesions after reviewing the currently available data and opinions on the subject.
Figure 2.
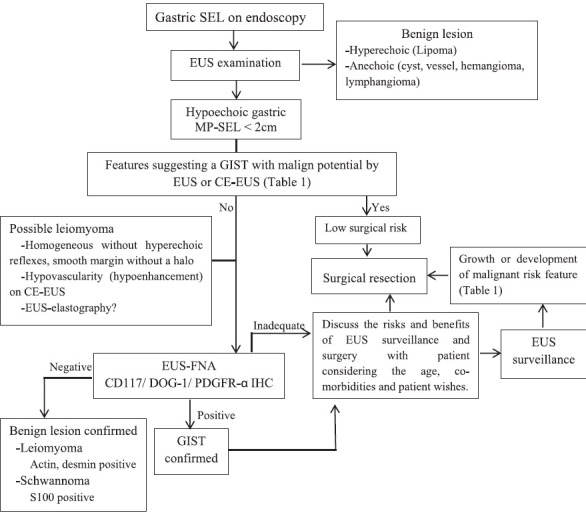
Approach for the management of small gastric MP-SELs
CE-EUS = Contrast-enhanced EUS, DOG-1 = Discovered on GIST-1, EUS = Endoscopic ultrasonography, GIST = Gastrointestinal stromal tumor, IHC = Immunohistochemistry, MP = Muscularis propria, PDGFR-α = Platelet derived growth factor receptor alpha, SEL = Subepithelial lesion
Several endoscopic treatment modalities have been reported for small MP-SELs, such as band ligation of the tumor, resection using polypectomy snare, and endoscopic submucosal dissection techniques.[20,74,75,76,77,78,79,80,81,82,83,84,85,86,87] They remain controversial due to concerns about the risks of perforation, incomplete resection with macroscopic residue, removal of tumor tissue without a sufficiently safe margin, tumor spillage, and the continuing need for long-term follow-up to ensure complete removal.[20,68,74,80,84] Currently, such endoscopic approaches are mainly considered in the context of clinical trials.
CONCLUSIONS
Drawing definite conclusions for small gastric GISTs may be limited by the insufficient sample size of studies, mostly due to the fact that GISTs are rare tumors. Available data support the notion of a conservative management strategy, rather than a surgical approach, for small gastric MP-SELs, but in light of the uncertainties expressed herein, the final decision for these small lesions must be individualized after options are thoroughly discussed with the patient. More accurate and less invasive characterization with the newer imaging techniques and the improvements in needle design will allow a more precisely targeted puncture, and larger and safer tissue acquisition. Hopefully, identification of the molecular and genetic aspects of malignant transformation profiles at the early stage of small gastric MP-SELs will provide superior risk estimation and refinement of management strategies in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–20. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): A review. Eur J Cancer. 2002;38(Suppl 5):S39–51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim KM, Kang DW, Moon WS, et al. Gastrointestinal Stromal Tumor Committee; Korean Gastrointestinal Pathology Study Group. Gastrointestinal stromal tumors in Koreans: İt›s incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005;20:977–84. doi: 10.3346/jkms.2005.20.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: Results of a nation-wide study. Eur J Cancer. 2005;41:2868–72. doi: 10.1016/j.ejca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, Mehren MV, Antonescu CR, et al. NCCN Task Force Report: Update on the Management of Patients with Gastrointestinal Stromal Tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–44. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 7.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–69. [PMC free article] [PubMed] [Google Scholar]
- 8.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 11.Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23:49–68. doi: 10.1016/j.hoc.2008.12.002. vii-viii. [DOI] [PubMed] [Google Scholar]
- 12.Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013;5:102–12. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 15.Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–7. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 16.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–64. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 17.Lok KH, Lai L, Yiu HL, et al. Endosonographic surveillance of small gastrointestinal tumors originating from muscularis propria. J Gastrointestin Liver Dis. 2009;18:177–80. [PubMed] [Google Scholar]
- 18.Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–7. doi: 10.1016/j.gie.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, Jung HY, Choi KD, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol. 2011;45:330–6. doi: 10.1097/MCG.0b013e318206474e. [DOI] [PubMed] [Google Scholar]
- 20.Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 2013;13:3–18. doi: 10.5230/jgc.2013.13.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–35. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Agaimy A, Wünsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–20. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 23.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Akahoshi K, Oya M. Gastrointestinal stromal tumor of the stomach: How to manage? World J Gastrointest Endosc. 2010;2:271–7. doi: 10.4253/wjge.v2.i8.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: More questions than answers. A review emphasizing the need for a standardized GIST reporting? Int J Clin Exp Pathol. 2010;3:461–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–7. doi: 10.1053/j.gastro.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez SA, Faigel DO. Endoscopic diagnosis of gastrointestinal stromal cell tumors. Curr Opin Gastroenterol. 2007;23:539–43. doi: 10.1097/MOG.0b013e32829fb39f. [DOI] [PubMed] [Google Scholar]
- 29.Wiersema MJ, Wiersema LM, Khusro Q, et al. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc. 1994;40:199–206. doi: 10.1016/s0016-5107(94)70167-9. [DOI] [PubMed] [Google Scholar]
- 30.Yegin EG, Kani T, Banzragch M, et al. Survival in patients with hypoechoic muscularis propria lesions suggestive of gastrointestinal stromal tumors in gastric wall. Acta Gastroenterol Belg. 2015;78:12–7. [PubMed] [Google Scholar]
- 31.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–5. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 32.Akahoshi K, Oya M, Koga T, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23:405–12. doi: 10.15403/jgld.2014.1121.234.eug. [DOI] [PubMed] [Google Scholar]
- 33.Sekine M, Imaoka H, Mizuno N, et al. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2015;27:44–52. doi: 10.1111/den.12333. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki Y, Nakajima K, Kurokawa Y, et al. Clinical significance of surgery for gastric submucosal tumours with size enlargement during watchful waiting period. Eur J Cancer. 2013;49:2681–8. doi: 10.1016/j.ejca.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy. 2011;43:709–15. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 36.Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: Sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–61. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Eckardt AJ, Adler A, Gomes EM, et al. Endosonographic large-bore biopsy of gastric subepithelial tumors: A prospective multicenter study. Eur J Gastroenterol Hepatol. 2012;24:1135–44. doi: 10.1097/MEG.0b013e328356eae2. [DOI] [PubMed] [Google Scholar]
- 38.Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39–45. doi: 10.1055/s-0033-1344895. [DOI] [PubMed] [Google Scholar]
- 39.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 41.DeWitt J, Emerson RE, Sherman S, et al. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc. 2011;25:2192–202. doi: 10.1007/s00464-010-1522-z. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 43.The ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2012;23(Suppl 7):vii49–vii55. doi: 10.1093/annonc/mds252. 2012. [DOI] [PubMed] [Google Scholar]
- 44.Gouveia AM, Pimenta AP, Capelinha AF, et al. Surgical margin status and prognosis of gastrointestinal stromal tumor. World J Surg. 2008;32:2375–82. doi: 10.1007/s00268-008-9704-8. [DOI] [PubMed] [Google Scholar]
- 45.Unalp HR, Derici H, Kamer E, et al. Gastrointestinal stromal tumours: Outcomes of surgical management and analysis of prognostic variables. Can J Surg. 2009;52:31–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Lachter J, Bishara N, Rahimi E, et al. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653–6. [PubMed] [Google Scholar]
- 47.Imaoka H, Sawaki A, Mizuno N, et al. Incidence and clinical course of submucosal lesions of the stomach. Gastrointesc Endosc. 2005;61:AB16. [Google Scholar]
- 48.Margulies C, Krevsky B, Catalano MF. How accurate are endoscopic estimates of size? Gastrointest Endosc. 1994;40:174–7. doi: 10.1016/s0016-5107(94)70162-8. [DOI] [PubMed] [Google Scholar]
- 49.Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468–73. doi: 10.1016/s0016-5107(97)70175-5. [DOI] [PubMed] [Google Scholar]
- 51.Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376–81. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim GH, Kim KB, Lee SH, et al. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. BMC Gastroenterol. 2014;14:7. doi: 10.1186/1471-230X-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim MN, Kang SJ, Kim SG, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013;7:642–7. doi: 10.5009/gnl.2013.7.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha CY, Shah R, Chen J, et al. Diagnosis and management of GI stromal tumors by EUS-FNA: A survey of opinions and practices of endosonographers. Gastrointest Endosc. 2009;69:1039–44. doi: 10.1016/j.gie.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 55.Săftoiu A, Vilmann P, Gorunescu F, et al. European EUS Elastography Multicentric Study Group. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 56.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: The first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344–8. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 57.Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–20. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Bearzi I, Mandolesi A, Arduini F, et al. Gastrointestinal stromal tumor. A study of 158 cases: Clinicopathological features and prognostic factors. Anal Quant Cytol Histol. 2006;28:137–47. [PubMed] [Google Scholar]
- 60.Heinrich M, Rubin B, Longley B, et al. Biology and genetic aspects of gastrointestinal stromal tumours: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–95. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 61.El-Rifai W, Sarlomo-Rikala M, Andersson LC, et al. High-resolution deletion mapping of chromosome 14 in stromal tumors of thegastrointestinal tract suggests two distinct tumor suppressor loci. Genes Chromosomes Cancer. 2000;27:387–91. [PubMed] [Google Scholar]
- 62.Chen Y, Liou CP, Tseng HH, et al. Deletions of chromosome 1p and 15q are associated with aggressiveness ofgastrointestinal stromal tumors. J Formos Med Assoc. 2009;108:28–37. doi: 10.1016/S0929-6646(09)60029-2. [DOI] [PubMed] [Google Scholar]
- 63.Kim NG, Kim JJ, Ahn JY, et al. Putative chromosomal deletions on 9P, 9Q and 22Q occur preferentially in malignantgastrointestinal stromal tumors. Int J Cancer. 2000;85:633–8. doi: 10.1002/(sici)1097-0215(20000301)85:5<633::aid-ijc6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 64.Wozniak A, Sciot R, Guillou L, et al. Array CGH analysis in primary gastrointestinal stromal tumors: Cytogenetic profilecorrelates with anatomic site and tumor aggressiveness, irrespective of mutationalstatus. Genes Chromosomes Cancer. 2007;46:261–76. doi: 10.1002/gcc.20408. [DOI] [PubMed] [Google Scholar]
- 65.Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth muscle (stromal) tumors: Dependence onanatomic site. Am J Surg Pathol. 1999;23:82–7. doi: 10.1097/00000478-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence afterresection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–15. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 67.Pisters PW, Patel SR. Gastrointestinal stromal tumors: Current management. J Surg Oncol. 2010;102:530–8. doi: 10.1002/jso.21460. [DOI] [PubMed] [Google Scholar]
- 68.Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217–28. doi: 10.1053/j.gastro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 69.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–30. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 70.Nickl N. Endoscopic approach to gastrointestinal stromal tumors. Gastrointest Endosc Clin N Am. 2005;15:455–66. doi: 10.1016/j.giec.2005.04.001. viii. [DOI] [PubMed] [Google Scholar]
- 71.Polkowski M, Palucki J, Butruk E. Transabdominal ultrasound for visualizing gastric submucosal tumors diagnosed by endosonography: Can surveillance be simplified? Endoscopy. 2002;34:979–83. doi: 10.1055/s-2002-35839. [DOI] [PubMed] [Google Scholar]
- 72.Bandoh T, Isoyama T, Toyoshima H. Submucosal tumors of the stomach: A study of 100 operative cases. Surgery. 1993;113:498–506. [PubMed] [Google Scholar]
- 73.Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 74.Guo J, Liu Z, Sun S, et al. Ligation-assisted endoscopic enucleation for the diagnosis and resection of small gastrointestinal tumors originating from the muscularis propria: A preliminary study. BMC Gastroenterol. 2013;13:88. doi: 10.1186/1471-230X-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024–8. doi: 10.1055/s-2006-944814. [DOI] [PubMed] [Google Scholar]
- 76.Liu BR, Song JT, Qu B, et al. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26:3141–8. doi: 10.1007/s00464-012-2305-5. [DOI] [PubMed] [Google Scholar]
- 77.Hwang JC, Kim JH, Kim JH, et al. Endoscopic resection for the treatment of gastric subepithelial tumors originatedfrom the muscularis propria layer. Hepatogastroenterology. 2009;56:1281–6. [PubMed] [Google Scholar]
- 78.Białek A, Wiechowska-Kozłowska A, Huk J. Endoscopic submucosal dissection of large gastric stromal tumor arising frommuscularis propria. Clin Gastroenterol Hepatol. 2010;8:e119–20. doi: 10.1016/j.cgh.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Jeong ID, Jung SW, Bang SJ, et al. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. 2011;25:468–74. doi: 10.1007/s00464-010-1195-7. [DOI] [PubMed] [Google Scholar]
- 80.Park YS, Park SW, Kim TI, et al. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tipelectrosurgical knife. Gastrointest Endosc. 2004;59:409–15. doi: 10.1016/s0016-5107(03)02717-2. [DOI] [PubMed] [Google Scholar]
- 81.Sun S, Jin Y, Chang G, et al. Endoscopic band ligation without electrosurgery: A new technique for excision ofsmall upper-GI leiomyoma. Gastrointest Endosc. 2004;60:218–22. doi: 10.1016/s0016-5107(04)01565-2. [DOI] [PubMed] [Google Scholar]
- 82.Sun S, Ge N, Wang C, et al. Endoscopic band ligation of small gastric stromal tumors and follow-up byendoscopic ultrasonography. Surg Endosc. 2007;21:574–8. doi: 10.1007/s00464-006-9028-4. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Hong YS, Lee JM, et al. Duodenal gastrinoma treated with endoscopic band ligation. Gastrointest Endosc. 2009;69:964–7. doi: 10.1016/j.gie.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 84.Huang WH, Feng CL, Lai HC, et al. Endoscopic ligation and resection for the treatment of small EUS-suspected gastric GI stromal tumors. Gastrointest Endosc. 2010;71:1076–81. doi: 10.1016/j.gie.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Ren W, Fan CQ, et al. Full thickness endoscopic resection of nonintracavitary gastric stromal tumors: A novel approach. Surg Endosc. 2011;25:641–7. doi: 10.1007/s00464-010-1189-5. [DOI] [PubMed] [Google Scholar]
- 86.Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: A new technique for treating upper GIsubmucosal tumors originating from the muscularis propria layer (with videos) Gastrointest Endosc. 2012;75:195–9. doi: 10.1016/j.gie.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Ge N, Sun S, Wang S, et al. Endoscopic ultrasound-assisted tunnel-type endoscopic submucosal dissectionfor the treatment of esophageal tumors arising in the muscularis propria (withvideo) Endosc Ultrasound. 2013;2:11–5. doi: 10.7178/eus.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


