Abstract
We describe here the synthesis and evaluation of a series of tetrahydroisoquinolines that show subunit-selective potentiation of NMDA receptors containing the GluN2C or GluN2D subunits. Bischler-Napieralski conditions were employed in the key step for the conversion of acyclic amides to the corresponding tetrahydroisoquinoline containing analogs. Compounds were evaluated using both two electrode voltage clamp recordings from Xenopus laevis oocytes and imaging of mammalian BHK cells loaded with Ca2+-sensitive dyes. The most potent analogues had EC50 values of 300 nM and showed over 2-fold potentiation of the response to maximally effective concentrations of glutamate and glycine, but had no effect on responses from NMDA receptors containing the GluN2A or GluN2B subunits, AMPA, kainate, GABA, or glycine receptors or a variety of other potential targets. These compounds represent a potent class of small molecule subunit-selective potentiators of NMDA receptors.
Introduction
The ionotropic family of glutamate receptors comprises N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptor subtypes, subdivided on the basis of amino acid sequence homology, structure homology, and pharmacology. The NMDA receptor mediates a slow, Ca2+-permeable component of excitatory synaptic transmission in the central nervous system, and plays a prominent role in normal brain processes such as learning, memory, synaptic plasticity, and neuronal development.1–8 In addition, dysfunction of NMDA receptors, either overactivation or hypofunction, has been implicated as a contributing factor to a wide range of neurological disorders including schizophrenia9–11, Alzheimer’s disease12, Parkinson’s disease13, Huntington’s chorea14, depression15, 16, epilepsy6, neuropathic pain17, and acute brain injury following ischemia18–20, hypoxia or trauma.8, 21
NMDA receptors are ligand-gated cation channels that are tetramers of two glycine-binding GluN1 subunits and two glutamate-binding GluN2 subunits. There are four different GluN2 subunits (GluN2A-D), each of which endows the receptor with unique open probability, single channel conductance, and deactivation time course.22, 23 For example, GluN2C- and GluN2D-containing NMDA receptors have a lower open probability,24–26 decreased sensitivity to block by Mg2+,27 and can be activated by lower concentrations of glycine and glutamate than GluN2A- and GluN2B-containing receptors.28, 29 The four different subunits are differentially expressed throughout the brain, with particularly prominent GluN2C and GluN2D expression in the cerebellum, basal ganglia, and cortical and hippocampal interneurons.30–32 The distinct localized expression and the unique functional properties of each of the four subunits, along with the potential involvement of NMDA receptors in disease states and injuries, creates a compelling rationale for development of subunit-selective modulators with potential use in a variety of neuropathological conditions. Compounds that increase the strength of glutamatergic synapses have been hypothesized to be therapeutically useful in treating schizophrenia.33–35 For examples, agonists at the GluN1 subunit, e.g. glycine and D-serine, have received attention as potential therapies for schizophrenia36; however, these molecules will increase the activity of all NMDA receptor subtypes to a similar degree and have activity at other cell-surface receptors expressed in the CNS.37 By contrast, small molecules directly potentiating the NMDA receptor at regions other than the agonist binding site might exhibit advantageous subunit-selectivity and be more selective for the NMDA receptor over other receptors.38 Moreover the GluN2C and GluN2D subunits are particularly interesting targets in this context because they are expressed on hippocamapal and cortical interneurons,27 whose hypofunction is thought to cause disinhibition of pyramidal cells leading to excessive drive of the dopaminergic system.35, 39, 40
Each NMDA receptor subunit contains four semi-autonomous domains: an extracellular amino-terminal domain (ATD), an extracellular ligand binding domain (LBD), a transmembrane domain that contributes to the ion conduction pore, and an intracellular carboxy-terminus. The binding sites for at least six classes of antagonists of the NMDA receptor are known. Voltage-dependent channel blockers, typically rigid organic cations such as phencyclidine (PCP), bind deep within the ion conduction pore in a voltage-dependent fashion.41–43 Two additional classes of NMDA receptor antagonists include competitive antagonists that bind with high affinity to either the glycine site on the GluN1 subunit or the glutamate site on the GluN2 subunit.44–47 A fourth class of non-competitive antagonists, which includes quinazoline-4-ones and dihydroquinoline-pyrazolines, act at the membrane-proximal portion of the ligand binding domain and are more potent after glutamate but not glycine binding.48–50 A fifth class of NMDA receptor antagonists binds to the weakly conserved ATD, and is highly selective for the GluN2B subunit.51 Antagonists in this class, which include ifenprodil and a wide range of related structures, show well over 200-fold selectivity for GluN2B over GluN2A, GluN2C and GluN2D. Finally, a sixth class of antagonists selectively inhibits GluN2A-containing receptors through actions at the dimer interface between the GluN1 and GluN2 ligand binding domains. These compounds, typified by TCN201, are allosteric regulators of glycine binding.52, 53 In addition to these six classes, several phenanthroic acid and napthoic acid analogues are noncompetitive antagonists that act outside the ATD.54, 55
In contrast to NMDA receptor antagonists, relatively few compounds have been shown to potentiate NMDA receptor function. Polyamines (e.g. spermine)56–58, aminoglycosides59, and sulfated neurosteroids60 can enhance the function of GluN2B-containing NMDA receptors with EC50 values in range of 40–130 µM. Phenanthroic acid and napthoic acid derivatives increase the current response of GluN2A- and GluN2B-containing receptors at concentrations around 100 µM.61, 62 In addition, D-cycloserine binds the GluN1 subunit and is a partial agonist at GluN2A, GluN2B, or GluN2D receptors, but can activate GluN2C-containing receptors to a greater extent than glycine.63, 64 Thus, the few potentiators that are known show strong GluN2 subunit dependence, suggesting potentiation of NMDA receptors is mediated by less conserved portions of the receptor.
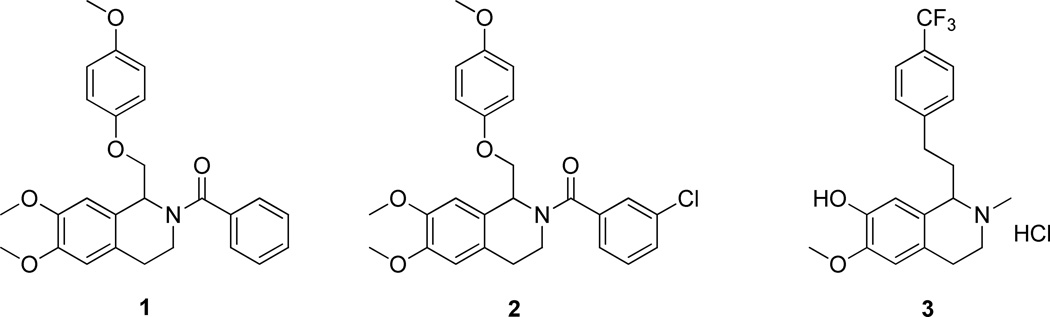
To identify small drug-like subunit-selective modulators of NMDA receptors, we previously employed a reverse chemical genetics approach utilizing a fluorescence Ca2+ imaging-based screen of a diversity library containing 100,000 compounds on BHK cell lines expressing GluN1/GluN2C or GluN1/GluN2D receptors.65, 66 We identified an NMDA receptor potentiator with micromolar potency that selectively enhanced the function of both GluN2C- and GluN2D-containing NMDA receptors, but was without action on GluN2A- and GluN2B-containing receptors. This compound, (6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone), had a tetrahydroisoquinoline backbone (1). Compound 1 enhanced the response of GluN2C/D-containing NMDA receptors by 1.5-fold in the presence of maximally effective concentrations of co-agonists glutamate and glycine with an EC50 value estimated to be 12 µM, but the solubility limit prevented accurate determination of the maximal effect. Compound 1 and related analogues had no agonist activity on their own. We recently described the mechanism and site of action of a related compound, 3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (2, also referred to as CIQ), which had a reported EC50 value of 3 µM and enhanced receptor responses less than 2-fold.66
In this study, we report synthetic efforts towards the optimization of substituents on the core structure of 2 that have led to the development of a structure-activity relationship (SAR) for this class of compounds.67 The active compounds in this class selectively potentiated current response from GluN2C- and GluN2D-containing receptors. While the core backbone of these compounds is similar to that of the previously identified GluN2B-selective antagonist, HON0001 (3, Figure 1)68, none of the compounds studied here inhibit GluN2B at relevant concentrations. Moreover, this class of compounds did not affect responses from GluN2A-containing receptors nor from GluA1 AMPA receptors and GluK2 kainate receptors, suggesting this class of compounds shows strong subunit selectivity.
Figure 1.
Structures for (6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (1), CIQ (2) and HON0001 (3).
Results
Chemistry
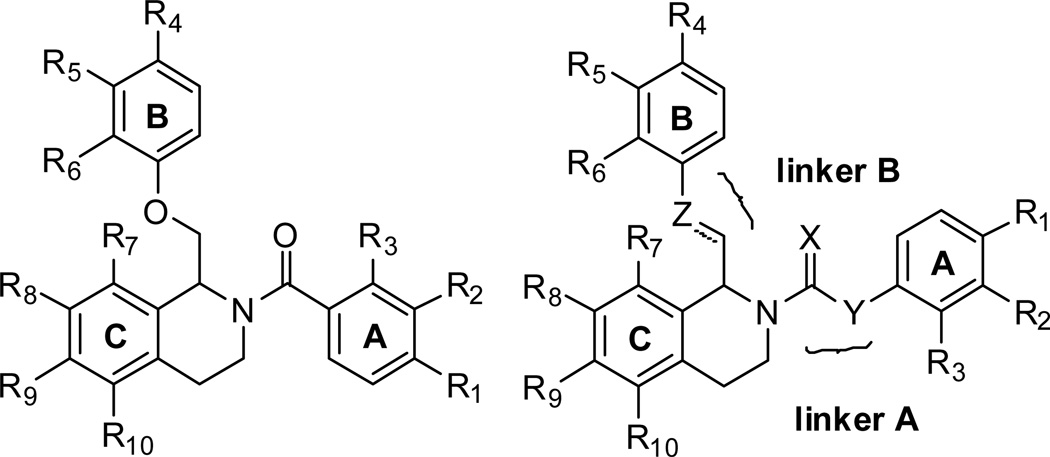
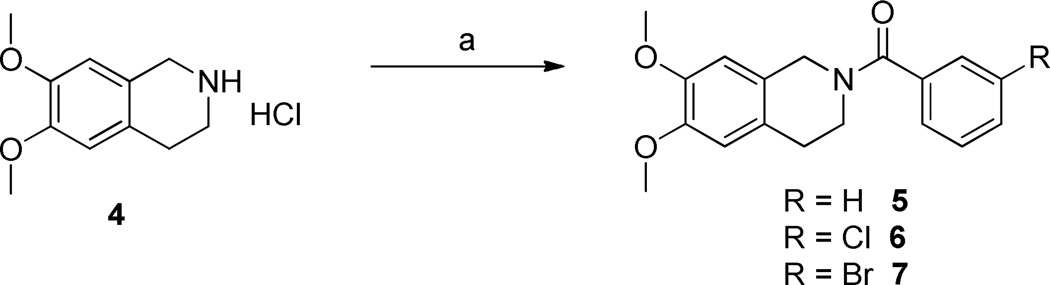
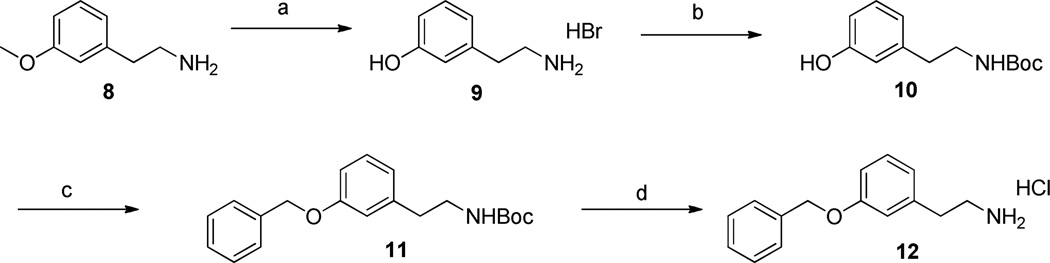
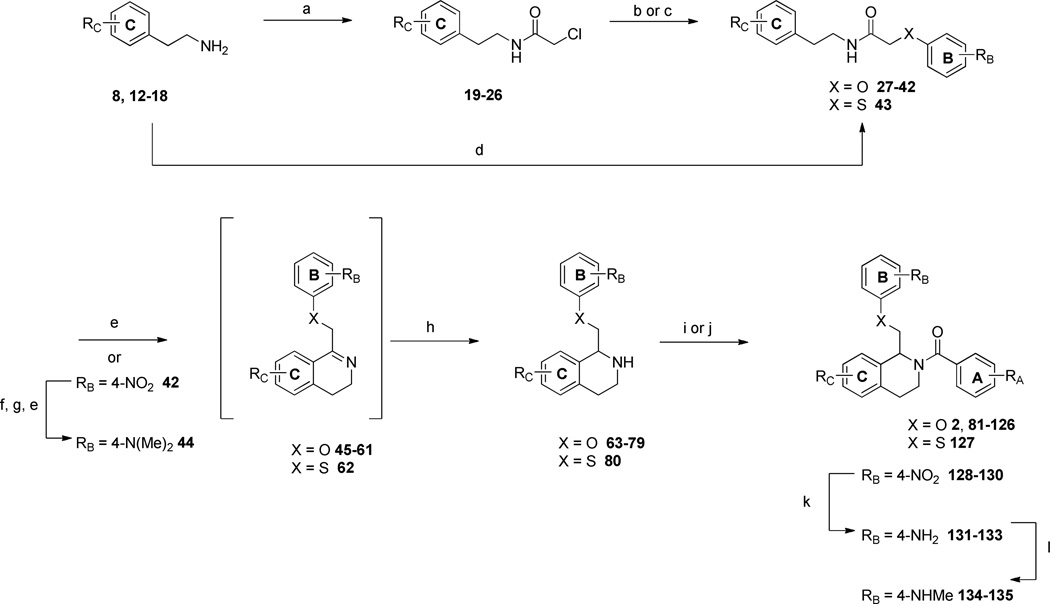
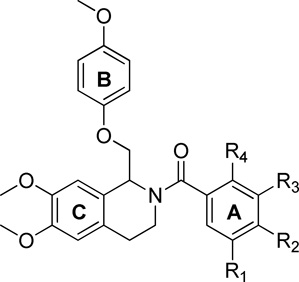
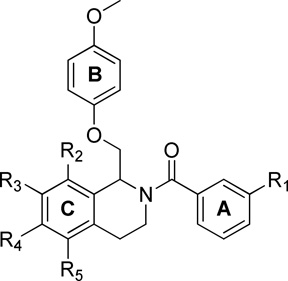
To explore the SAR of the tetrahydroisoquinoline compound class as posititve allosteric modulators of GluN2C/2D-containing NMDA receptors, a series of derviatives were synthesized. For SAR development, the core tetrahydroisoquinoline was divided into 3 modifiable rings (A, B, and C) and 2 modifiable linker regions (Linker-A and Linker-B) (Figure 2). Compounds 5, 6, and 7 were synthesized from 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (4) and the corresponding benzoyl chloride (Scheme 1). All other synthetically derived analogues were generated via one of three pathways, each starting with substituted phenethylamines. 2-(3-(benzyloxy)phenyl)ethanamine (12) was prepared from 3-methoxyphenethylamine (8) in the sequence shown in Scheme 2. The starting material (8) was demethylated in a refluxing mixture of acetic acid and hydrobromic acid to form the phenol 9. The primary amine was then Boc-protected to form 10 followed by an alkylation of the phenol with benzyl bromide to give 11. The Boc group was removed under acidic conditions to give the phenethylamine 12.
Figure 2.
Generic structures showing A-,B-, and C-rings and A- and B-linkers for SAR development on the screening hit, 1180.
Scheme 1.
a) substituted benzoyl chloride, NaHCO3, DCM/H2O, 67–85% (Procedure I).
Scheme 2.
a) HBr, AcOH, reflux, 4h, quant.; b) Et3N, Boc2O, DMF/dioxane, 18 h, 84%; c) Cs2CO3, BnBr, MeCN, 24 h, 75%; d) HCl, Et2O.
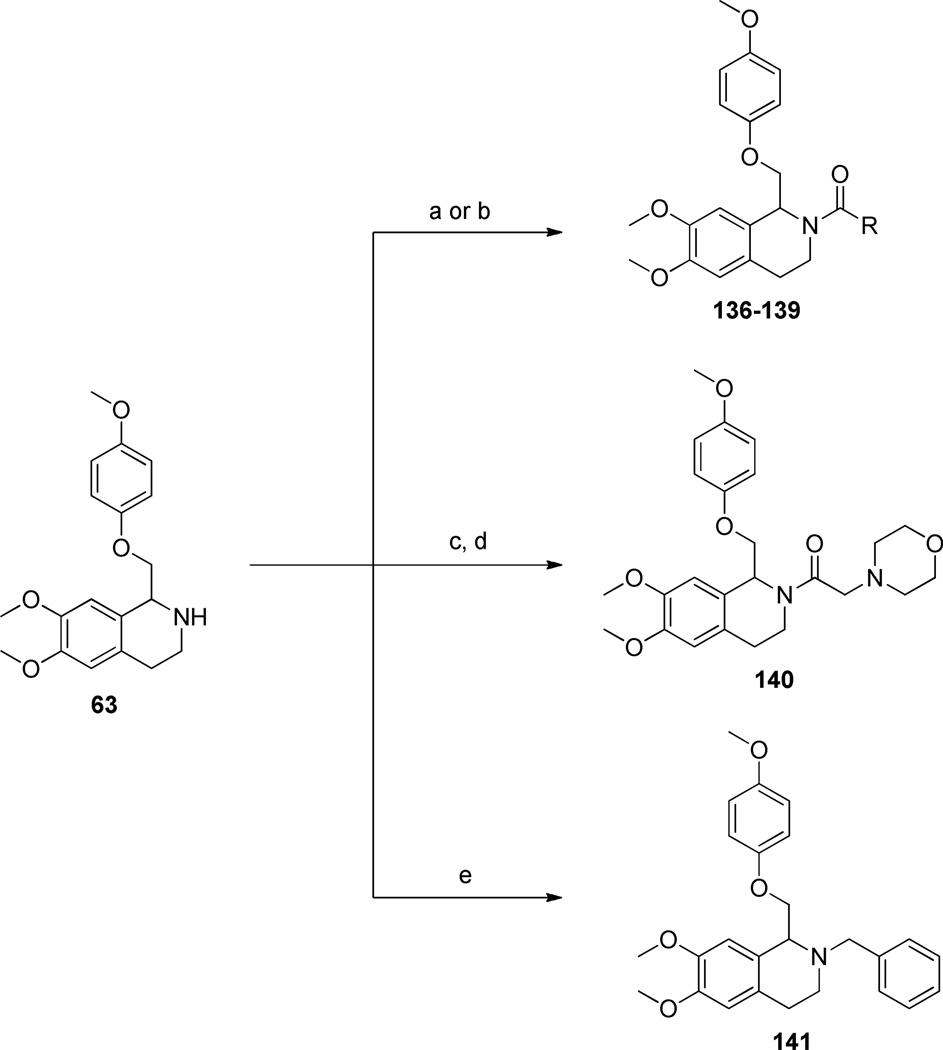
The synthetic scheme employed for the preparation of each analogue was dependent upon the identity of the linker between the tetrahydroisoquinoline core and the B ring (Figure 2). For compounds containing an ether or thioether linker, an appropriately substituted phenethylamine (8, 12–18) was acylated with chloroacetyl chloride to afford the α-chloroamide-containing compounds 19–26. Displacement of the chlorine with a phenol or thiophenol in the presence of base afforded 27–42 and 43, respectively. The amides 27–43 were then subjected to Bischler-Napieralski conditions to form the dihydroisoquinolines (45–62). The nitro-containing amide compound 42 was subjected to hydrogenolysis, followed by a reductive amination to afford the dimethylamino-containing intermediate which was then cyclized using Bischler-Napieralski conditions to give the dihydroisoquinoline 44. The dihydroisoqunolines were subsequently reduced with sodium borohydride (NaBH4) to afford the racemic tetrahydroisoquinolines 63–80. Acylation of 63–80 with a substituted benzoyl chloride afforded analogues 2 and 81–127 (Scheme 3). Nitro-containing analogues 128–130 were reduced to the corresponding anilines (131–133) with tin (II) chloride dehydrate.69 The anilines (131–133) were then subjected to reductive amination conditions to give the methylamino-containing analogues 134 and 135.69, 70 Although the A-ring in each of the compounds described above is a substituted phenyl ring, a number of compounds were synthesized with various aromatic or heterocyclic moieties in place of the phenyl. These compounds were synthesized in one step from tetrahydroisoquinoline 63 in an acylation reaction with an aryl chloride or 4-morpholinyl chloride (Scheme 4). In addition, tetrahydroisoquinoline 63 was acylated with chloroacetyl chloride followed by chlorine displacement with morpholine to afford 23, with a modified A-ring and linker A. Tetrahydroisoquinoline 63 was also subjected to alkylation conditions to form the tertiary amine 141 (Scheme 4).
Scheme 3.
a) chloroacetyl chloride, Et3N, DCM, 0°C, 1.5–2h, 68–83% (Procedure II); b) substituted phenol, Cs2CO3, MeCN, 18h, 66–90% (Procedure III); c) 4-nitrophenol, CsF, DMF, 76%; d) phenoxyacetyl chloride, Et3N, DCM, 0°C, 1.5–2h, 69%; e) POCl3, toluene, reflux, 1.5–12 hrs (Procedure IV); f) Pd/C, H2, MeOH, 97%; g) paraformaldehyde, NaCNBH3, MeOH, 89%; h) NaBH4, MeOH, 18h, 18–70% over two steps (Procedure VI); i) substituted benzoyl chloride, Et3N, DCM, 0°C, 2h, 21–99% (Procedure VII); j) substituted benzoic acid, EDCI, DMAP, 18h, 22–68% (Procdure VIII); k) tin(II)chloride dihydrate, EtOH 33–66%; l) NaOMe, paraformaldehyde, sodium borohydride, MeOH, 68–72%.
Scheme 4.
a) aryl chloride, Et3N, DCM, 0°C, 21–99% (Procedure VII); b) 4-morpholinecarbonyl chloride, Et3N, DCM, 0°C, 31%; c) chloroacetyl chloride, Et3N, DCM, 0°C, 78%; d) morpholine, EtOH, 53%; e) BnBr, K2CO3, DMF, 39%.
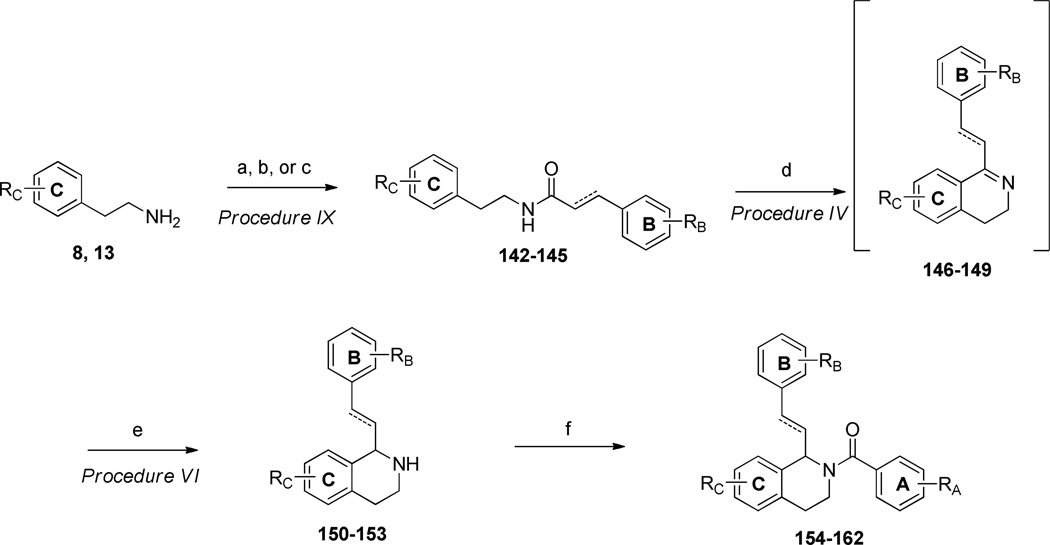
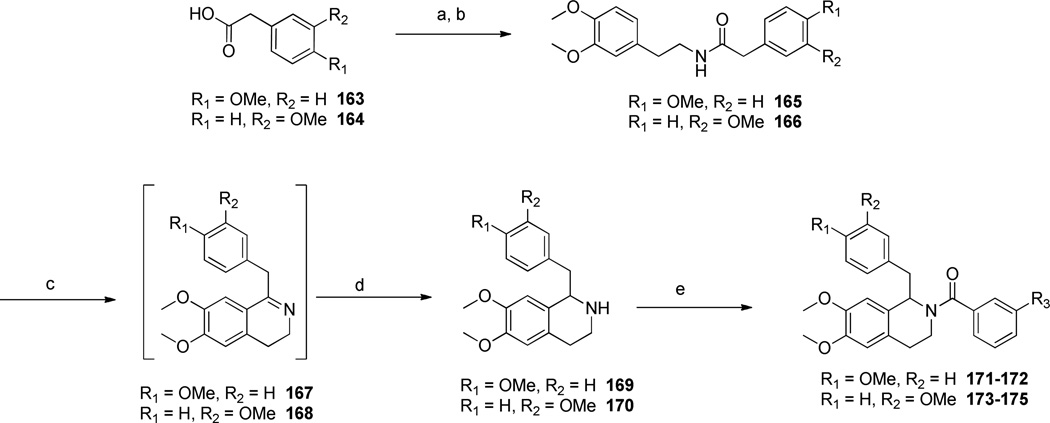
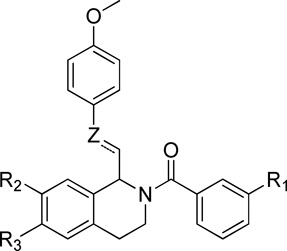
Additionally, compounds with either a saturated or an unsaturated carbon linker, a substituted cinnamic acid or phenylpropionic acid underwent standard EDC-coupling conditions with a substituted phenethylamine (8 or 13) to afford amides 142–145. Amides 142–145 were then subjected to Bischler-Napieralski conditions in refluxing POCl3 to afford the corresponding dihydroisoquinolines (146–149), which were subsequently reduced with NaBH4 to afford the tetrahydroisoquinolines 150–153. Acylation of 150–153 with a series of substituted benzoyl chlorides afforded the final analogues 154–162 (Scheme 5). Compounds with a shorter linker than those described above were synthesized according to the procedure shown in Scheme 6. The commercially available phenylacetic acids (163 and 164) were each treated with thionyl chloride to form the corresponding acid chlorides, which were subsequently reacted with 3,4-dimethoxyphenethylamine (13) to give amides 165 and 166. The amides were then subjected to Bischler-Napieralski reaction conditions to form the dihydroisoquinoline intermediates (167 and 168), which were further reduced to the tetrahydroisoquinolines (169 and 170) with sodium borohydride. The core was then acylated with an appropriately substituted benzoyl chloride to afford final compounds 171–175.
Scheme 5.
a) 4-methoxycinnamic acid, EDC, DMAP, DMF/DCM, 18h, 42–66% (Procedure IX); b) 4-methoxyphenylpropionic acid, EDC, DMAP, DCM/DMF, 18h, 66% (Procedure IX); c) phenylpropionyl chloride, Et3N, DCM, 99%; d) POCl3, toluene, reflux, 1.5h (Procedure VI); e) NaBH4, MeOH, 18h, 58–73% over two steps (Procedure VI); f) substituted benzoyl chloride, Et3N, DCM, 0°C, 38–84% (Procedure VII).
Scheme 6.
a) SOCl2, DCM; b) 3,4-dimethoxyphenethylamine, Et3N, DCM, 26–84% over two steps; c) POCl3, toluene, reflux (Procedure IV); d) NaBH4, MeOH, 18h, 26% over two steps (Procedure VI); e) substituted benzoyl chloride, Et3N, DCM, 31–64% (Procedure VII).
Stereoselective synthesis of compound 2
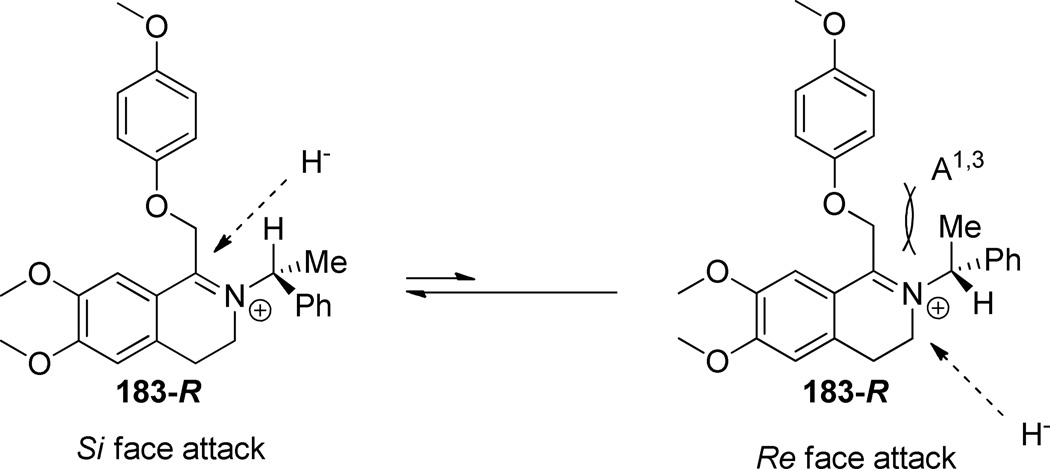
The stereoisomers for 2 were synthesized according to the procedure shown in Scheme 7. Either enantiomer of the commercially available chiral amine (177) was subjected to acylation conditions with 3,4-dimethoxyphenylacetyl chloride (176), to give the secondary amide 178. Reduction of the amide with borane dimethylsulfide complex and boron trifluoride etherate gave the corresponding amine (179) in excellent yields.71 Acylation with chloroacetyl chloride followed by halogen displacement with 4-methoxyphenol afforded the tertiary amide (181). Treatment of amide 181 with phosphorus oxychloride afforded the chiral iminium intermediate, which was subsequently reduced to the tetrahydroisoquinoline (182) with sodium borohydride. Although crystallographic data will be needed to confirm the assignment, the stereochemistry of the reduction has been assigned based on the model shown in Figure 3 and on literature precedent for the use of this chiral directing group in similar reductions72–74. Tetrahydroisoquinoline 182 was subjected to hydrogenolysis to remove the chiral directing group, followed by acylation with 3-chlorobenzoyl chloride, to give the assigned R-(+) and S-(−) enantiomers of 2.
Scheme 7.
a) NaOH, DCM/H2O, R = 72%, S = 82%; b) BF3·OEt2O, then BH3·THF, THF, R = 90%, S = 95% c) 2-chloroacetyl chloride, Et3N, DCM, R = 80%, S = 73%; d) 4-methoxyphenol, Cs2CO3, MeCN, R = 74%, S = 73%; e) POCl3, toluene, reflux; f) NaBH4, MeOH, −78 °C, R, R = 34%, S, S = 34%; g) H2, Pd/C, EtOH; h) 3-chlorobenzoyl chloride, Et3N, DCM, R = 51%, S = 34%.
Figure 3.
Proposed model to predict stereochemical outcome of the reduction of iminium 185-R
Potentiation of NMDA Receptors
All of the compounds synthesized in addition to 33 commercially available analogues (184–217) were evaluated using two electrode voltage clamp recordings of currents from four recombinant NMDA receptor subtypes: GluN1/GluN2A, GluN1/GluN2B, GluN1/GluN2C, and GluN1/GluN2D. We initially evaluated the conditions under which to best assess compound activity. At low concentrations of agonist (0.3 µM glutamate, 0.1 µM glycine), compound 2 produced strong potentiation (353±36% of control at 10 µM for GluN2D), but full concentration-effect curves could not be fitted with the Hill equation, limiting our ability to obtain EC50 values with which to compare analogues. By contrast, concentration-effect curves for compound 2 recorded in response to maximally-effective concentrations of glutamate (100 µM) and glycine (30 µM) were well-fitted by the Hill equation (maximum 228±8.8% of control for GluN2D). For all subsequent experiments, concentration-response curves for each compound were generated by co-applying increasing concentrations of compound with maximally effective concentrations of glutamate and glycine. Each compound was tested in at least 5 oocytes from two different frogs. The mean degree of potentiation measured at 10 µM for each compound at GluN1/GluN2C and GluN1/GluN2D is presented in Tables 1–9. For compounds exhibiting more than 115% potentiation at 10 µM, the EC50 values were determined by fitting composite concentration-response curves to equation 1 (Table 1–9). None of the compounds tested potentiated responses from receptors comprised of GluN1/GluN2A or GluN1/GluN2B, and thus these data are not shown. A small subset of compounds produced weak inhibition (70% of the control response at 100 µM) of GluN1/GluN2A (compounds 7, 84, 110, 186) or GluN1/GluN2B (compounds 1, 96, 100, 101, 109, 139, 192, 206); these inhibitory effects were not studied further.
Table 1.
Optimization of substituent placement on Ring A.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||||
| R1 | R2 | R3 | R4 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | H | H | H | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 184 | H | Me | H | H | 106 ± 5.1 | 107 ± 2.4 | -- | -- |
| 185 | H | H | Me | H | 171 ± 11 | 132 ± 10 | 6.2 (211%) | 5.5 (174%) |
| 186 | H | H | H | Me | 108 ± 2.0 | 96 ± 1.7 | -- | -- |
| 187 | H | Cl | H | H | 128 ± 3.2 | 111 ± 4.1 | 4.1 (135%) | -- |
| 2 | H | H | Cl | H | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 188 | H | H | H | Cl | 102 ± 7.1 | 101 ± 8.9 | -- | -- |
| 189 | H | Br | H | H | 107 ± 3.2 | 108 ± 3.1 | -- | -- |
| 190 | H | H | Br | H | 183 ± 11 | 178 ± 6.6 | 0.9 (195%) | 2.2 (188%) |
| 191 | H | OMe | H | H | 101 ± 1.1 | 96 ± 1.9 | -- | -- |
| 192 | H | H | OMe | H | 147 ± 12 | 132 ± 10.5 | 5.8 (181%) | 12 (179%) |
| 193 | H | NO2 | H | H | 96 ± 1.4 | 102 ± 1.8 | -- | -- |
| 84 | H | H | NO2 | H | 160 ± 7.4 | 151 ± 6.3 | 11 (250) | 13 (261) |
| 85 | H | Cl | Cl | H | 126 ± 5.2 | 136 ± 5.0 | 21 (178%) | 24 (220%) |
| 86 | H | H | Cl | Cl | 120 ± 3.1 | 109 ± 8.4 | 1.4 (127%) | -- |
| 194 | H | Cl | H | Cl | 112 ± 5.0 | 99 ± 3.1 | -- | -- |
| 87 | Cl | H | Cl | H | 149 ± 8.0 | 138 ± 8.2 | 4.8 (201%) | 1.9 (159%) |
| 88 | H | F | Cl | H | 183 ± 13 | 168 ± 10 | 5.3 (232%) | 5.3 (206%) |
| 195 | H | O-CH2-O | H | 106 ± 3.6 | 99 ± 1.5 | -- | -- | |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 6–25 oocytes from 2–5 frogs for each compound and receptor tested.
Table 9.
Optimization of Linker B
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
||||||||
| R1 | R2 | R3 | Z | C=C | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | OMe | OMe | O | No | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 2 | Cl | OMe | OMe | O | No | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 190 | Br | OMe | OMe | O | No | 183 ± 11 | 178 ± 6.6 | 0.9 (195%) | 2.2 (188%) |
| 154 | F | OMe | OMe | C | No | 92 ± 1.7 | 95 ± 2.3 | -- | -- |
| 155 | H | OMe | OMe | C | Yes | 122 ± 4.4 | 127± 5.1 | 35 (192%) | 35 (199%) |
| 156 | Cl | OMe | OMe | C | Yes | 169 ± 6.9 | 155 ± 6.4 | 1.1 (172%) | 1.3 (157%) |
| 157 | Br | OMe | OMe | C | Yes | 181 ± 17 | 169 ± 7.3 | 2.8 (188%) | 2.8 (174%) |
| 158 | Cl | H | OMe | C | Yes | 184 ± 13 | 187 ± 15 | 1.0 (190%) | 1.0 (192%) |
| 159 | Br | H | OMe | C | Yes | 172 ± 21 | 164 ± 11 | 1.5 (176%) | 1.7 (185%) |
| 160 | H | OMe | OMe | C | No | 102 ± 3.4 | 100 ± 2.9 | -- | -- |
| 161 | Cl | OMe | OMe | C | No | 114 ± 2.6 | 108 ± 2.0 | -- | -- |
| 162 | Br | OMe | OMe | C | No | 128 ± 5.2 | 134 ± 7.4 | 11 (129%) | 15 (128%) |
| 127 | H | OMe | OMe | S | No | 103 ± 4.3 | 94 ± 1.0 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeds 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) responses. Data are from between 6–25 oocytes from between 2–5 frogs for each compound and receptor tested. Compounds 1, 2, 190 are also shown in preceding Tables, and are included here for comparison.
In addition, several active compounds were tested against a representative member of the AMPA-selective class of glutamate receptors (GluA1) and of the kainate-selective glutamate receptor class (GluK2). Compounds 1, 2, 5, 87, 96, 98, 111, 117, 118, 138, 140, 155, 157, 158, 159, 161, 162, 190, 197 co-applied with 100 µM glutamate had no effects on homomeric GluA1 responses recorded under two electrode voltage clamp. Compounds 1, 2, 190, and 197 were co-applied with 100 µM glutamate to GluK2 expressing oocytes and had no effect on GluK2-mediated currents. Compound 83 was tested on an additional 19 receptors (e.g., AMPA, GABA, glycine, nicotinic acetylcholine, purinergic) using the oocyte expression system (see below). Together, these results suggest strong GluN2C/D-selectivity for this series of compounds.
Effect of removing Linker-B and the B-ring
In order to determine the importance of each portion of the compound for potency, fragments of the lead compound 1 and two lead compounds 2, 192 were synthesized and tested. Compounds 5, 6, and 7 correspond to compounds 1, 2, and 190 without the Linker-B and the B-ring (Figure 4). All three of these compounds were inactive against all recombinant NMDA receptors (n=6–10 oocytes for GluN1/GluN2A, GluN1/GluN2B, GluN1/GluN2C, GluN1/GluN2D, data not shown). Reducing the length of the linker to a single methylene also removed all activity (171–175 in Figure 4; data not shown). Thus, the B-ring and the two atom linker are essential for activity in this series.
Figure 4.
Structure of analogues with B-ring and linker B removed (5–7) and analogues with a one atom linker B (173–177)
Optimization of A- and B-Ring Substituent Position
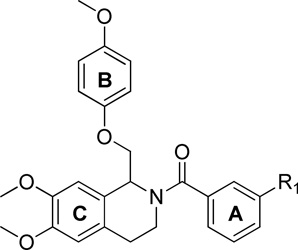
We evaluated the optimal placement of substituents on the A-ring (Table 1). Substituents in the 3-position, (R3 in Table 1) showed the strongest potentiation compared to substitution in the 2- (R4) or 4- (R2) position. This is evident when comparing potentiation associated with the methyl-containing analogues 184, 185, and 186. For this subset of compounds, 185, with a methyl in the 3-position (R3, Table 1), is the most effective and potentiates GluN2C- and GluN2D-containing receptors to a greater extent. When a chlorine substituent was placed on the A-ring, as in compounds 187, 2, and 188, the 3-chloro analogue (2) showed greater potentiation compared to the 2- and 4-chloro derivatives (187, 188; Table 1). Analogues with dichloro (86, 85, 194, 87) or 3-chloro-4-fluoro (88) substitutions on the A-ring showed EC50 values (when active) ranging from 1.4–21 µM on GluN2C and 1.9–24 µM on GluN2D (Table 1).
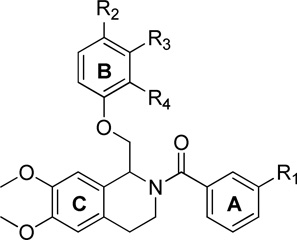
We also evaluated the positional effects of B-ring substitutions. Data in Table 2 show that the para-position (R2, Table 2) on the B-ring is the only position that affords any detectable activity for methoxy substituents. When a methoxy substituent is placed in either the ortho- or meta-position, all activity is lost (Table 2).
Table 2.
Optimization of substituent placement on Ring B.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||||
| R1 | R2 | R3 | R4 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 89 | H | H | H | H | 95 ± 2.6 | 99 ± 1.9 | -- | -- |
| 1 | H | OMe | H | H | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 90 | H | H | OMe | H | 95 ± 2.2 | 92 ± 1.5 | -- | -- |
| 196 | H | H | H | OMe | 94 ± 3.7 | 95 ± 2.5 | -- | -- |
| 2 | Cl | OMe | H | H | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 91 | Cl | H | OMe | H | 100 ± 2.0 | 96 ± 1,4 | -- | -- |
| 92 | Cl | H | H | OMe | 91 ± 2.2 | 97 ± 1.1 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Compounds 1 and 2 were also shown in Table 1, and are included here for comparison. Data are from between 6–25 oocytes from between 2–4 frogs for each compound and receptor tested.
Optimization of Substituent Identity on A- and B-Rings
Since substitution in the meta-position on the A-ring yielded the most potent and effective potentiators, a number of modifications were made to this position (R1 in Table 3). Substitution on the A-ring with 3-bromo (190), 3-iodo (93), and 3-trifluoromethyl (198) gave strong potentiation with potencies similar to compound 266. All other substitutions on the 3-position on the A-ring showed either decreased potency or decreased maximum degree of potentiation. These data suggest that the binding pocket has both size and electronic requirements. Since 2 and 190 show similar potencies and maximal effects, and they are more active than the 3-fluoro (197) analogue, it was expected that the 3-iodo (93) analogue would be active. While not as potent as the 3-bromo (190) derivative, the 3-iodo (93) analogue showed enhanced maximal potentiation. Interestingly, addition of a similarly large phenyl (94) ring shows reasonable potency but a diminished degree of potentiation. In addition, when R3 is an electron-withdrawing group, such as nitro (84), the compounds are less potent but more efficacious (Table 3). Figure 5A summarizes optimal substitutions on the A ring. We subsequently evaluated a range of different ring systems in place of Ring-A (Table 4). Only 2-thiophene (203) showed weak activity at GluN2C-containing receptors with no or minimal potentiating effects at GluN2D-containing receptors. The weak selectivity of this ring system for GluN2C suggests a possible starting point from which to develop future compounds with selectivity for GluN2C over GluN2D.
Table 3.
Optimization of A Ring substituents
 | |||||
|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
||||
| R1 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 197 | F | 154 ± 7.0 | 142 ± 4.5 | 7.2 (184%) | 7.0 (169%) |
| 2 | Cl | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 190 | Br | 183 ± 11 | 178 ± 6.6 | 0.9 (195%) | 2.2 (188%) |
| 93 | I | 209 ± 9.8 | 163 ± 13 | 4.0 (251%) | 4.9 (239%) |
| 185 | Me | 171 ± 11 | 151 ± 7.5 | 6.2 (211%) | 5.5 (174%) |
| 94 | Ph | 140 ± 9.4 | 132 ± 5.4 | 2.9 (142%) | 3.6 (136%) |
| 198 | CF3 | 187 ± 12 | 202 ± 7.0 | 2.4 (201%) | 2.4 (218%) |
| 192 | OMe | 147 ± 12 | 132 ± 10 | 5.8 (181%) | 12 (179%) |
| 95 | OH | 96 ± 0.8 | 96 ± 2.3 | -- | -- |
| 84 | NO2 | 160 ± 7.4 | 151 ± 6.3 | 11 (250%) | 13 (261%) |
| 96 | CH2Cl | 138 ± 2.7 | 115 ± 2.2 | 17 (205%) | -- |
| 97 | CHCl2 | 149 ± 7.3 | 121± 2.9 | 3.7 (161%) | 5.4 (127%) |
| 98 | CN | 119 ± 4.2 | 139 ± 5.8 | 29 (164%) | 7.7 (166%) |
| 99 | C(O)Me | 105 ± 2.4 | 104 ± 1.2 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Compounds 1, 2, 84, 185, 190, 192 were also shown in preceding Tables, and are included here for comparison. Data are from between 6–25 oocytes from between 2–5 frogs for each compound and receptor tested.
Figure 5.
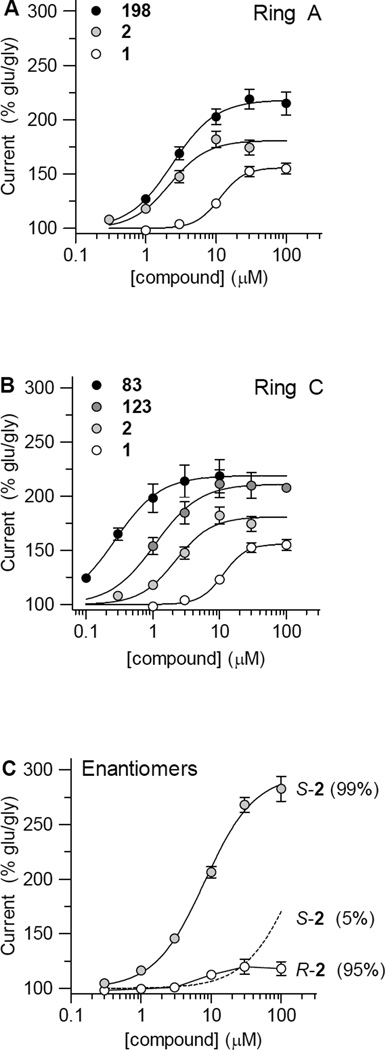
A, Composite concentration-effect data and fitted curves are shown for the initial screening hit (1), as well as for two modifications to Ring A that improve activity (2, or CIQ, and 198). B. Composite concentration-effect data and fitted curves are shown for the initial screening hit (1), 2 or CIQ, and two analogues with Ring C modifications that yield more potent and efficacious potentiation. C. Composite concentration-effect data and fitted curves are show for R-(−)-2 (90% ee), and S-(+)-2 (>98% ee). The broken line indicates predicted activity of 5% contaminant S-(+)-2. Data represent mean ± SEM.
Table 4.
Replacement of Ring A
 | |||||
|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
||||
| R | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | phenyl | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 199 | t-butyl | 90 ± 3.6 | 95 ± 1.4 | -- | -- |
| 200 | 2-pyridine | 87 ± 4.3 | 99 ± 2.4 | -- | -- |
| 201 | 3-pyridine | 98 ± 1.5 | 94 ± 1.7 | -- | -- |
| 202 | 4-pyridine | 100 ± 1.3 | 97 ± 1.8 | -- | -- |
| 203 | 2-thiophene | 117 ± 2.1 | 105 ± 1.6 | 9.0 (134%) | -- |
| 136 | 2-furan | 102 ± 1.3 | 100 ± 1.4 | -- | -- |
| 137 | 5-isoxazole | 93 ± 2.3 | 97 ± 2.4 | -- | -- |
| 204 | 1-napthyl | 97 ± 2.8 | 96 ± 0.6 | -- | -- |
| 138 | 2-napthylb | 113 ± 2.8 | 106 ± 1.5 | -- | -- |
| 139 | N-morpholine | 95 ± 1.9 | 96 ± 2.0 | -- | -- |
| 140 | -CH2-N-morpholine | 92 ± 2.7 | 94 ± 0.5 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 5–20 oocytes from between 2–4 frogs for each compound and receptor tested. Compound 1 was included here for comparison.
In contrast to 1-naphthyl, higher concentrations of 2-naphthyl did show activity (136±5.5% potentiation of GluN2C at 100 µM).
As shown in Table 2, only compounds with a para-substitution pattern around the B-ring have detectable potentiation on recombinant GluN2C/GluN2D receptors. Several modifications were made to the para-position substituent on the B-ring to test for the optimal functionality in this position (R2 in Table 5). When a thiomethyl is placed in the 4-position, there is potentiation if the analogue also contains a 3-chloro on the A-ring (104), but not a hydrogen (103). The potency of 104 is similar to the 4-methoxy containing analogue (2), but the degree of potentiation is reduced. The 4-ethyl analogue is weakly active at GluN2C (207; Table 5). A 4-dimethylamino substituent shows potentiation preferentially of GluN2C if the analogue also contains a 3-chloro or 3-bromo on Ring-A (107 and 108, respectively), but not a hydrogen (106). Substitution with ethoxy (100, 101), benzyloxy (109), amino (133, 132, 131), or nitro (129, 130, 128) led to compounds with no detectable activity on recombinant GluN2C- or GluN2D-containing receptors. These results suggest that the methyl ether is important for binding. Moreover, steric effects may explain the lack of efficacy for the larger substituents such as benzyloxy (109; Table 5).
Table 5.
Optimization of B Ring substituents
 | ||||||
|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||
| R1 | R2 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | OMe | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 2 | Cl | OMe | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 100 | Cl | OEt | 93 ± 5.5 | 92 ± 1.4 | -- | -- |
| 101 | Br | OEt | 99 ± 2.0 | 81 ± 1.7 | -- | -- |
| 102 | Cl | OH | 105 ± 1.9 | 98 ± 0.8 | -- | -- |
| 103 | H | SMe | 102 ± 5.3 | 100 ± 1.9 | -- | -- |
| 104 | Cl | SMe | 164 ± 6.7 | 143 ± 3.9 | 5.1 (191%) | 5.3 (159%) |
| 205 | H | Et | 120 ± 2.2 | 108 ± 2.6 | 23 (160%) | -- |
| 105 | Cl | OCF3 | 102 ± 1.1 | 97 ± 1.5 | -- | -- |
| 131 | H | NH2 | 102 ± 4.4 | 93 ± 0.9 | -- | -- |
| 132 | Cl | NH2 | 97 ± 1.8 | 100 ± 1.1 | -- | -- |
| 133 | Br | NH2 | 93 ± 0.8 | 95 ± 0.7 | -- | -- |
| 134 | Cl | NHMe | 97 ± 0.9 | 97 ± 1.7 | -- | -- |
| 135 | Br | NHMe | 108 ± 2.3 | 100 ± 1.1 | -- | -- |
| 106 | H | NMe2 | 99 ± 2.0 | 97 ± 2.0 | -- | -- |
| 107 | Cl | NMe2 | 120 ± 3.4 | 110 ± 1.3 | 35 (198%) | -- |
| 108 | Br | NMe2 | 127 ± 4.1 | 108 ± 1.5 | 12 (167%) | -- |
| 206 | Cl | CO2Me | 103 ± 2.3 | 107 ± 1.7 | -- | -- |
| 207 | Br | CO2Me | 107 ± 4.0 | 102 ± 0.8 | -- | -- |
| 109 | Cl | OBn | 93 ± 1.6 | 93 ± 2.5 | -- | -- |
| 128 | H | NO2 | 94 ± 2.0 | 91 ± 1.7 | -- | -- |
| 129 | Cl | NO2 | 90 ± 0.7 | 93 ± 0.8 | -- | -- |
| 130 | Br | NO2 | 95 ± 3.4 | 92 ± 2.6 | -- | -- |
| 208 | F | F | 85 ± 1.3 | 86 ± 1.2 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 5–25 oocytes from 2–5 frogs for each compound. Compounds 1 and 2 were also shown in Tables 1–4, and are included here for comparison.
Optimization of C-Ring Substitutions
All of the compounds described above contain a dimethoxy substitution pattern on the C-ring (R3 and R4, Table 6). Substitution of methyl groups in place of both methoxy substituents results in modestly enhanced potency (~ 1 µM EC50) and increased maximal potentiation (compare 122 and 123 to 2 and 190). When a dioxolane is fused at these two positions (110, 111, 112), the potency is decreased when compared to the dimethoxy analogues (1, 2, 190). When the R3-methoxy is removed and a R2-methoxy is installed (Table 6), the resulting compounds show modest changes in either potency or maximal effect. Compounds with dimethoxy substitutions at R4 and R5 (120 and 121; Table 6) are inactive. This suggests that substitution at the R5 position introduces unfavorable steric interactions within the binding pocket. In addition, the decrease in potency associated with a non-hydrogen substituent at R2 may be explained by steric interactions between linker-B and the substituent at R2 on the C-ring.
Table 6.
Optimization of C ring substituents
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
||||||||
| R1 | R2 | R3 | R4 | R5 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | H | OMe | OMe | H | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 2 | Cl | H | OMe | OMe | H | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 190 | Br | H | OMe | OMe | H | 183 ± 11 | 178 ± 6.6 | 0.9 (195%) | 2.2 (188%) |
| 110 | H | H | O-CH2-O | H | 99 ± 2.7 | 99 ± 1.9 | -- | -- | |
| 111 | Cl | H | O-CH2-O | H | 126 ± 4.3 | 134 ± 6.6 | 16 (200%) | 7.4 (186%) | |
| 112 | Br | H | O-CH2-O | H | 170 ± 9.9 | 136 ± 6.8 | 3.2 (177%) | 3.4 (138%) | |
| 113 | H | OMe | H | OMe | H | 143 ± 5.0 | 141 ± 9.0 | 10 (182%) | 14 (190%) |
| 114 | Cl | OMe | H | OMe | H | 178 ± 21 | 166 ± 7.0 | 6.1 (234%) | 9.2 (233%) |
| 115 | Br | OMe | H | OMe | H | 178 ± 8.6 | 166 ± 4.3 | 9.0 (261%) | 7.8 (228%) |
| 116 | H | H | H | OMe | H | 136 ± 13 | 144 ± 6.5 | 7.5 (174%) | 5.7 (167%) |
| 117 | Cl | H | H | OMe | H | 210 ± 12 | 216 ± 18 | 1.3 (240%) | 1.0 (219%) |
| 118 | Br | H | H | OMe | H | 263 ± 24 | 281 ± 24 | 2.0 (294%) | 2.9 (373%) |
| 119 | C(O)Me | H | H | OMe | H | 140 ± 4.1 | 124 ± 2.9 | 21 (241%) | 32 (242%) |
| 120 | Cl | H | H | OMe | OMe | 96 ± 3.4 | 93 ± 0.6 | -- | -- |
| 121 | Br | H | H | OMe | OMe | 98 ± 2.1 | 95 ± 0.9 | -- | -- |
| 122 | Cl | H | Me | Me | H | 238 ± 11 | 179 ± 8.2 | 1.4 (244%) | 1.5 (180%) |
| 123 | Br | H | Me | Me | H | 195 ± 14 | 211 ± 0.9 | 0.9 (197%) | 1.1 (211%) |
| 124 | H | H | H | Me | H | 116 ± 3.9 | 109 ± 1.8 | 24 (182%) | -- |
| 125 | Cl | H | H | Me | H | 207 ± 10 | 201 ± 11 | 5.0 (252%) | 4.7 (237%) |
| 126 | Br | H | H | Me | H | 205 ± 12 | 230 ± 9.2 | 3.2 (227%) | 3.6 (257%) |
| 81 | H | H | H | OBn | H | 212 ± 11 | 182 ± 9.1 | 0.7 (222%) | 2.3 (177%) |
| 82 | Cl | H | H | OBn | H | 252 ± 13 | 195 ± 17 | 0.4 (254%) | 0.4 (198%) |
| 83 | Br | H | H | OBn | H | 253 ± 23 | 219 ± 15 | 0.3 (257%) | 0.3 (219%) |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 5–25 oocytes from between 2–5 frogs for each compound and receptor tested. Compounds 1, 2, 190, are also shown in preceding tables, and are included here for comparison.
Interestingly, when a single methoxy is placed at R4 on the C-ring, as in compounds 116 and 117, potency is increased when compared to the dimethoxy counterparts (Table 6). While compound 118 does not show increased potency compared to its dimethoxy analog, it does exhibit a greater maximum potentiation, further suggesting that a single substitution at R4 on the C-ring is optimal for activity. This trend of increased effectiveness also appears to hold for the methyl-substituted C ring. Compounds 122 and 123 show increased potency compared to 2 and 190, and when a single methyl is placed at R4 on the C-ring as in compounds 124, 125, and 126 the maximal effect is slightly increased. Interestingly, introduction of O-benzyl substitutions at the R4 position results in the most potent compounds tested. Compounds 82 and 83 show submicromolar EC50 values (0.3–0.4 µM) with strong potentiation of 254–257% at GluN2C and 198–219% at GluN2D, suggesting that space exists within the binding pocket that can be exploited to enhance potency. Figure 5B summarizes optimal substitutions on the C ring.
Optimization of Linker-A and Linker-B
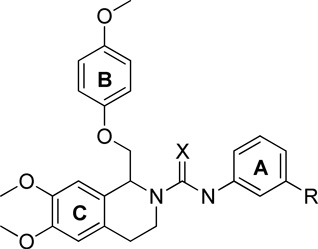
There are two regions on the backbone that link the 3 rings together: linker-A and linker-B (Figure 2). In both the original screening hit (1) and 2, linker-A is an amide linkage. As shown in Table 7, extension of this linker into a urea (211, 212) or thiourea (211, 212, 213, 214, and 215) leads to a loss of activity for all but one analogue (215), which retained only weak potentiating activity (Table 7). Substitution of the amide with a sulfonamide (216, 217) eliminated all activity on GluN1/GluN2C and GluN1/GluN2D receptors (Table 8), as did reduction of the amide linkage to a tertiary amine (141; Table 8). These data suggest that both the potency and maximal degree of potentiation of this series is dependent on the amide linkage.
Table 7.
Optimization of linker A
 | ||||||
|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||
| R | X | GluN2C | GluN2D | GluN2C | GluN2D | |
| 209 | H | O | 99 ± 3.7 | 99 ± 1.5 | -- | -- |
| 210 | Cl | O | 93 ± 1.9 | 90 ± 2.0 | -- | -- |
| 211 | H | S | 99 ± 2.6 | 89 ± 1.2 | -- | -- |
| 212 | Cl | S | 99 ± 1.1 | 98 ± 1.5 | -- | -- |
| 213 | F | S | 98 ± 1.9 | 103 ± 2.0 | -- | -- |
| 214 | Br | S | 99 ± 1.0 | 110 ± 6.0 | ||
| 215 | Me | S | 116 ± 2.0 | 126 ± 6.6 | 5.2 (126%) | 4.9 (130%) |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeds 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 8–14 oocytes from between 2–3 frogs for each compound and receptor tested.
Table 8.
Optimization of linker A
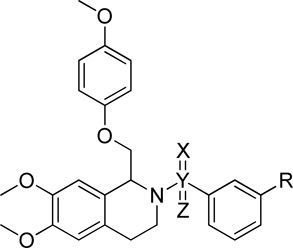 | ||||||||
|---|---|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||||
| R | X | Y | Z | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | O | C | - | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 216 | H | O | S | O | 92 ± 1.5 | 98 ± 0.6 | -- | -- |
| 141 | H | - | CH2 | - | 102 ± 3.4 | 92 ± 2.4 | -- | -- |
| 2 | Cl | O | C | - | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 217 | Cl | O | S | O | 97 ± 1.8 | 96 ± 0.5 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeds 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) responses. Compounds 1 and 2 from preceding Tables are included here for comparison. Data are from between 6–25 oocytes from between 2–5 frogs for each compound and receptor tested.
All of the compounds described thus far contained an ether linkage in linker-B. Table 9 shows modifications to this linker region. When the linker is aliphatic, as in 154, 160, 161, 162, the compounds either show a significant reduction in potency or are inactive. Similarly, replacing the linkage with a thioether (127) eliminated activity of the compounds. When the compounds contain unsaturation within linker-B, the compounds appear less soluble and show varying effects. For example, 156, 158, and 159 all showed increased potency compared to the related compounds containing an ether linkage (2, 117, and 118, respectively). By contrast, 155 shows decreased potency compared to 1. Analogues with a single methoxy on the C-ring (158 and 159) were similarly potent as analogs with a dimethoxy (156 and 157), indicating two different changes (single methoxy, removal of ether) did not have additive effects. These data suggest modest enhancements in potency can be gained through alterations in the composition of this linker.
Off-Target Testing
We subsequently tested the most potent analogue (83) against a variety of other targets. We used two electrode voltage clamp recordings to assess the ability of 3 µM 83 (10 times the EC50) to inhibit GABA, glycine, serotonin, nicotinic acetylcholine, AMPA, kainate, and purinergic receptors. Although compound 83 had a statistically significant effect on seven of the receptors tested (Table 10), it showed less than 10% inhibition or 5% potentiation, suggesting compound 83 shows approximately 100-fold selectivity for GluN2C or GluN2D compared to these receptors. Compound 83 was also submitted to the National Institute of Mental Health Psychoactive Drug Screening Program (PDSP, http://pdsp.med.unc.edu/) for further off-target profiling (Supplementary Table S1). In the PDSP assays less than 50% inhibition of ligand binding was observed with 5 µM compound 83 at serotonin receptors (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2C, 5-HT3, 5-HT5A, 5-HT6, 5-HT7), adrenergic receptors (α1A, α1B, α1D, α2A, α2B, α2C, β2, β3), brain and peripheral benzodiazepine sites, dopamine receptors (D1, D2, D3, D4, D5), opioid receptors (δ and μ), histamine receptors (H1, H2, H3), acetylcholine muscarinic receptors (M1, M2, M3, M4, M5), sigma receptors (σ1, σ2), as well as serotonin, dopamine, and norepinephrine transporters (n=4 for each). The initial screen determined significant inhibition at the κ opioid receptors, which was studied further. Compound 83 inhibited antagonist binding to kappa opioid with a Ki greater than 5 µM, 15-fold higher than the EC50 for NMDA receptor potentiation. The combined data from these off-target screens highlights the remarkable selectivity of this series for GluN2C- and GluN2D-containing receptors.
Table 10.
Off-target actions of compound 83
| Receptor | Agonist (µM) | ITEST / ICONTROL (mean ± SEM, %) |
n |
|---|---|---|---|
| GluN1/GluN2A | 100 glutamate, 30 glycine | 105 ± 2.3 | 7 |
| GluN1/GluN2B | 100 glutamate, 30 glycine | 102 ± 4.0 | 4 |
| GluN1/GluN2C | 100 glutamate, 30 glycine | 247 ± 20.1* | 10 |
| GluN1/GluN2D | 100 glutamate, 30 glycine | 213 ± 14.9* | 8 |
| GluA1 | 100 glutamate | 100 ± 3.1 | 4 |
| GluA2 | 100 glutamate | 97 ± 1.5 | 4 |
| GluA3 | 100 glutamate | 99 ± 0.5 | 4 |
| GluA4 | 100 glutamate | 100 ± 1.0 | 4 |
| GluK1 | 100 glutamate | 101 ± 1.8 | 4 |
| GluK2 | 100 glutamate | 98 ± 1.3 | 4 |
| GluK2/GluK5 | 100 glutamate | 103 ± 1.1 | 4 |
| Serotonin 5-HT3A | 100 serotonin | 93 ± 0.8 * | 4 |
| GABAC (ρ1)(human) | 2 GABA | 99 ± 2.1 | 3 |
| Glycine α1 | 50 glycine | 91 ± 9.0 | 4 |
| Nicotinic α1β1γδ(mouse) | 1 acetylcholine | 105 ± 1.6* | 9 |
| Nicotinic α4β2(human) | 10 acetylcholine | 91 ± 1.5* | 7 |
| Nicotinic α3β4(human) | 10 acetylcholine | 95 ± 0.9* | 5 |
| Nicotinic α7(human) | 300 acetylcholine | 90 ± 3.3 | 3 |
| Nicotinic α9α10 | 1 acetylcholine | 92 ± 1.4* | 4 |
| Purinergic P2x2(human) | 9 ATP | 94 ± 0.9* | 4 |
| Purinergic P2x2 | 9 ATP | 94 ± 2.5 | 4 |
Recombinant receptors were expressed in Xenopus oocytes and responses the agonists indicated were recorded under two electrode voltage clamp. All cDNAs encoded rat receptors unless otherwise indicated. Compound 83 tested at 3 µM
p < 0.05, paired t-test.
Potency of Stereoisomers
All compounds in this series thus far have been synthesized and tested as racemic mixtures. We studied the stereoisomers of compound 2 by first separating the two enantiomers using chiral supercritical fluid chromatography (SFC; see Methods). We assessed the activity of each enantiomer in BHK cells that stably express GluN1/GluN2C or GluN1/GluN2D using the Ca2+ sensitive dye Fluo4 and in oocytes expressing GluN1/GluN2C or GluN1/GluN2D using two electrode voltage clamp recordings. The activity determined in these two assays suggested that one enantiomer potentiates the response of GluN1/GluN2C and GluN1/GluN2D receptors to a far greater extent than the other. In Ca2+-imaging experiments with BHK cells, the active SFC peak potentiated GluN2C and GluN2D responses with EC50 values of 7.1 and 6.6 µM, respectively (N=4). We therefore carried out stereoselective-synthesis of the two enantiomers of compound 2 and directly compared the activity of these two enantiomers using two electrode voltage clamp recordings. The results suggested that the S-(−)-enantiomer (>98% ee) of compound 2 (with stereochemistry assigned based on literature precedent72–74) potentiates the response of GluN2C and GluN2D by 304% and 294% with estimated EC50 values of 9.0 and 8.0 µM, respectively. Like the racemic form, 2-S-(−) had no effect on GluN2A- or GluN2B-containing receptors. By contrast, the R-(+)-enantiomer (90% ee) possessed minimal activity, which was indistinguishable from that predicted for the 5% residual S-(−)-enantiomer (Fig 5C). These data suggest that the binding pocket preferentially accommodates one enantiomer, and this stereoselectivity may provide a means to further enhance the selectivity of this compound class over other central nervous system targets. Given the limited solubility of compound 2, we attribute the slightly reduced potency of the purified enantiomer (8–9 µM) compared to the racemic mixture (4.6–5.0 µM) to the greater amount of active species in solution for the purified enantiomer compared to racemic mixture at concentrations near the limit of solubility. The larger amount of the active enantiomer in solution before the solubility limit allows a better estimate of the maximum potentiation, which was higher for the S-(−)-isomer (304% and 294% for GluN2C and GluN2D) than for racemic 2 (233% and 215% for GluN2C and GluN2D). The higher potentiation led to increases in EC50 values compared to racemic mixture.
Conclusion
Introduction of a single of O-benzyl substitution at the R4 position of the tetrahydroisoquinoline of compound 2 provided compound 83, a GluN2C- and GluN2D-selective potentiator with 300 nM potency. Moreover, the majority of the activity attributed to the compounds in this class of tetrahydroisoquinoline potentiators appears to arise from a single enantiomer, tentatively assigned with S-stereochemistry. This class of potentiators also demonstrates strong selectivity for GluN2C and GluN2D receptors with the most potent analogue showing no activity at over 65 receptors, channels, and transporters expressed in the central nervous system. Additionally, compounds 107, 108, 205 and 203 may represent potential starting point for developing GluN2C-selective modulators.
Biology Experimentals
Two-electrode voltage-clamp recordings were performed on Xenopus laevis oocytes expressing recombinant rat GluN1/GluN2A, GluN1/GluN2B, GluN1/GluN2C, GluN1/GluN2D, GluA1, or GluK2 receptors. cDNAs for rat GluN1-1a (GenBank accession numbers U11418 and U08261; hereafter GluN1), GluN2A (D13211), GluN2B (U11419), GluN2C (M91563), GluN2D (L31611), GluA1 (X17184), GluK2 (Z11548) were provided by Drs. S. Heinemann (Salk Institute), S. Nakanishi (Kyoto University), and P. Seeburg (University of Heidelberg). Oocyte isolation, RNA synthesis, and RNA injection were completed as described in detail elsewhere75; all protocols involving Xenopus laevis were approved by the Emory University Institutional Animal Care and Use Committee. During two-electrode voltage-clamp recordings, oocytes were placed into a perfusion chamber and continually washed with recording solution containing (in mM) 90 NaCl, 1.0 KCl, 0.5 BaCl2, 0.005 EDTA, and 10 HEPES at pH 7.4 (23°C). Glass electrodes with a tip resistance of 0.3–1.0 MΩ were pulled from thin-walled glass capillary tubes and filled with 3.0 M KCl. An OC-725C amplifier (Warner Instruments) was used to hold the membrane potential of the oocytes at −40 mV during current recording. All compounds were made as 20 mM stock solutions in DMSO, and dissolved to reach the desired final concentration in recording solution containing 100 µM glutamate and 30 µM glycine for use on oocytes expressing NMDA receptors. Final DMSO content was 0.05–0.5% (vol/vol). Oocytes expressing GluK2 receptors were pre-treated with 10 µM concanavalin A for 10 minutes. Recombinant GluA1 and GluK2 receptors were activated by 100 µM glutamate. In order to prevent a gradual increase in current response over the course of the experiment, which is a common feature of GluN1/GluN2A receptor responses in oocytes, some oocytes expressing GluN1/GluN2A were either pretreated for 10 minutes with 50 µM BAPTA-AM (1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester)) or injected with 50 nl of 2 mM K-BAPTA (potassium 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid). For every test compound, we recorded 5–7 concentrations in at least 5 oocytes obtained from two different frogs. We subsequently determined mean potentiation at 10 µM (±SEM), and when potentiation exceeded 115% of control we determined the EC50 (half-maximally effective concentration of potentiator) by fitting the equation
| (1) |
to the average composite concentration-response data normalized to the current in the absence of potentiator (100%). N is the Hill slope, which ranged between 1 and 2 and is not reported; maximum is the maximal response predicted for saturating concentration of potentiator. A few compounds produced modest inhibition (70% of controls at 100 µM); these inhibitory actions were not studied further.
For off-target data, GABAC (ρ1), glycine (α1), serotonin (5-HT3A), nicotinic acetylcholine (nAChR, α1β1δγ, α4β2, α3β4, α9α10, α7), and purinergic (P2X2 rat, P2X2 human) receptors were expressed in Xenopus oocytes. cRNA encoding the receptor subunits was synthesized from linear template cDNA using the mMessage mMachine kit (Ambion). Oocytes were injected with 1–5 ng cRNA in a 50 nL volume and incubated in Barth's solution at 15°C for 2–5 days prior to recording. α1β1δγ nAChR subunits were injected at a 1:1:1:1 ratio while α4β2, α3β4, and α9α10 nAChR subunits were injected at a 1:1 ratio. The cDNAs encoding GABAC, glycine, and serotonin subunits were provided by Dr. D. Weiss (University of Texas Health Science Center at San Antonio). cDNAs encoding nicotinic acetylcholine receptor subunits were provided by Drs. R. Papke (University of Florida) and S. Heinemann (Salk Institute). cDNAs encoding the purinergic receptors were provided by Dr. R. Hume (University of Michigan). GABAC receptors were activated by 2 µM GABA. Acetylcholine was used at the indicated concentrations (in µM) to activate the nicotinic acetylcholine receptors: α1β1δγ (1), α4β2 (10), α3β4 (10), α9α10 (1), α7(300). 50 µM glycine was used to activate the glycine receptor, 100 µM serotonin was used to activate 5-HT3A receptor, and 9 µM ATP was used to activate the purinergic receptors. Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. For experimental details please refer to the PDSP web site http://pdsp.med.unc.edu/
Ca2+ imaging experiments were performed as described previously76 with the following modifications. One day prior to the experiment, the cells were seeded in 20 µl media at 600,000 cells/ml in black clear bottom 384 well plates (Corning CellBind). On the day of the experiment, the media was aspirated and replaced with Flou-4 NW (Invitrogen) dissolved in HEPES-buffered saline (HBSS, Gibco #14175-053) comprised of (in mM) 5.33 KCl, 0.441 KH2PO4, 4.17 NaHCO3, 137.9 NaCl, 0.338 Na2HPO4, 5.56 D-glucose, 2 CaCl2, and 20 HEPES (pH 7.4), with 2.5 mM (1%) Probenecid, and 30 µM 7-chlorokynurenic acid for 60 minutes at 37° C in the dark. Cells were then gently washed with 30 µl/well using the same buffer without the Flou-4 dye, and placed in 20 µl/well buffer. Using a FDSS7000 instrument (Hamamatsu) real time recordings of changes in Fluo-4 emission was performed (excitation 480 nm and emission 540 nm) at room temperature (20–22° C). After 10 seconds of baseline recordings, 10 µl/well of 3× concentrated test compound, controls, or assay buffer in HBSS (pH 7.4) and 1 mM glycine (final concentration) were added. After 2 minutes, an additional 10 µl/well were added containing a 4× concentrated solutions of NMDA (GluN2C: 1000 µM and GluN2D: 300 µM). Changes in fluorescence were recorded for subsequent 2 minutes. For determination of the concentration-response relationships, test compounds were serial diluted 3-fold over 10 concentration steps. Responses (fluorescence units, FU) were normalized to the first recording and expressed as percent of maximally effective concentration of NMDA (see above). The EC50 value was determined by non-linear least squares fitting of equation 1 to the data.
The maximum solubility (20 µM) was determined for compound 2 using a BMG Labtech Nephelostar nephelometer (Offenburg, Germany), according to manufacturer’s instructions. Only responses for concentrations below the experimentally determined limit of solubility were measured; whenever visual evidence of precipitation at higher concentrations was observed, experiments were repeated with 1–10 mM 2-hydroxypropyl-β-cyclodextrin added to the recording solution to ensure that the compounds remained in solution. 2-hydroxypropyl-β-cyclodextrin had no detectable effect on NMDA receptor response amplitude (data not shown). We also evaluated the stability of potentiator activity by making a solution of 10 µM 2 and continuously agitating it. After increasing periods of time, some of the solution was filtered (0.2 µm nylon filter) and used to measure potentiation of GluN1/GluN2D receptors expressed in X. laevis oocytes. Similarly, we tested the concentration-response effects of 2 on maximal responses induced by NMDA on GluN1/GluN2C in BHK cells by measuring changes in fluorescence of Fluo-4 at different time points after making the compound solutions. Activity (potency and efficacy) was retained without loss until 6 hours, whereas no activity was seen after 24 hours. Results from these two experiments suggested that compound 2 remains active in solution up to 2–3 hours, with activity decreasing thereafter until by 24 hours there is no detectable activity.
The enantiomers of 2 were obtained by supercritical fluid chromatography on a Berger Multigram II operating at 50 ml/min at 35 °C and 100 bar backpressure. The column was a Phenomenex Lux 5u Cellulose-1 (250 × 21.2 mm). The eluent was CO2 (60 %) and methanol + 0.1 % diethylamine (40%). 15 mg were dissolved in methanol and 200 stacked injections of 0.4 ml were performed. Enantiomeric excess was determined on an Aurora Fusion A5 SFC system operating at 3 ml/min at 40 °C and 100 bar backpressure. The column was a Phenomenex Lux 3u Cellulose-1 (150 × 4.6 mm). The eluent was CO2 (70 %) and ethanol + 0.1 % diethylamine (30%). EE of peak 1 (rt 1.584 min) determined at 220 nM 98.3 %. Peak 2 (rt 2.708 min) 98.2 %.
Chemistry Experimentals
Compounds not described were purchased from either Life Chemicals (Compounds 184, 187, 189–194, 196–197, 200–201, 203–206, 208, 210–217) or Chem Div (Compounds 2, 185–186, 188, 195, 198, 127, 207, 209)). All purchased compounds were greater than 90% pure, as determined by the suppliers, by HPLC or NMR.
All reagents for synthesis were obtained from commercial suppliers and used without further purification. Reaction progress was monitored by thin layer chromatography (TLC) on precoated aluminum plates (silica gel 60 F254, 0.25 mm). Proton and carbon NMR spectra were recorded on an INOVA-400 (400 MHz) or VNMRS 400 (400 MHz). The spectra obtained were referenced to the residual solvent peak. Mass spectra were performed by the Emory University Mass Spectroscopy Center on either a VG 70-S Nier Johnson or JEOL instrument. Elemental analyses were performed by Atlantic Microlab Inc. Purity was established using HPLC, unless indicated by combustion analysis, and were found to be ≥ 95%. C, H, N agreed with proposed structures ±0.4% of theoretical values unless indicated. Optical activity was measured at 20°C with a Perkin-Elmer model 341 polarimeter. Flash chromatography was performed on a Teldyne ISCO CombiFlash Companion System with prepackaged Teledyne RediSep or Silicycle normal phase columns with silica gel.
General preparation for 1,2,3,4-tetrahydroisoquinoline compounds (Procedure I)
6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (1.0 g, 4.4 mmol) was dissolved in DCM (20 mL) and saturated aqueous sodium bicarbonate solution (20 mL). The biphasic reaction mixture was cooled to 0 °C in an ice bath and benzoyl chloride (3 equiv) was added dropwise. After complete addition, the reaction was warmed to room temperature and stirred for an additional 2 hours, when TLC indicated complete conversion. The organics were separated and the aqueous phase was extracted with DCM (2×). The organics were combined, washed with brine and water, dried over MgSO4, filtered and concentrated in vacuo. The resulting residue was subjected to flash column chromatography.
General preparation for 2-chloro-N-phenethylacetamide compounds (Procedure II)
A solution of a phenethylamine (1.0 equiv) in DCM was cooled to 0 °C in an ice bath. To this solution, Et3N (3.0 equiv) was added followed by dropwise addition of an acid chloride (1.2 equiv). Upon complete addition, the reaction was warmed to room temperature and stirred for 2 hours. When TLC indicated complete conversion, the reaction mixture was concentrated in vacuo. The resulting residue was taken up into DCM and washed with a saturated solution of NH4Cl and brine. The aqueous layer was then extracted with DCM (2×). The organic layers were combined, washed with water, dried over MgSO4 and filtered. The solvent was removed in vacuo. The resulting solid was subjected to flash column chromatography.
General preparation for N-(phenethyl)-2-(phenoxy)acetamide and N-(phenethyl)-2-(phenyl)thio)acetamide compounds (Procedure III)
To a solution of phenol or thiophenol (7.3 mmol) in MeCN (15 mL) was added Cs2CO3 (3.0 equiv.) at room temperature and the reaction mixture was allowed to stir for 2 hours. A solution of the α-chloroamide (1.2 equiv.) dissolved in dry MeCN (15 mL) was added and the resulting reaction mixture was stirred for 18 h under an argon atmosphere. After TLC indicated complete conversion, the volatiles were removed in vacuo and the resulting residue was treated with NH4Cl and extracted into EtOAc (2×). The resulting organic layer was washed with brine and water, dried over MgSO4, filtered, and concentrated in vacuo. The resulting solid was subjected to flash column chromatography.
General preparation for 3,4-dihydroisoquinoline compounds (Procedure IV)
To a solution of 3 (3.5 mmol) suspended in toluene (40 mL) was added POCl3 (3.0 equiv.), dropwise. The reaction mixture was brought to reflux and allowed to stir for 90 minutes before cooling to room temperature. The resulting precipitate was filtered and carried on without further purification.
General preparation for 3,4-dihydroisoquinoline compounds (Procedure V)
The amide (1.0 equiv) was dissolved in dry toluene (30 mL) and brought to reflux. Phosphorus pentoxide (7.0 equiv) was added to the refluxing solution over about 15 minutes. The reaction was refluxed for an additional 30 minutes. The toluene was decanted and the remaining residue was dissolved in water and washed with ether (2×). The aqueous solution was treated with NH4OH and extracted into DCM (3×). The organic layer was separated, dried over MgSO4, filtered and concentrated in vacuo. The crude product was taken on without further purification.
General preparation for 1,2,3,4-tetrahydroisoquinoline compounds (Procedure VI)
The dihydroisoquinoline 4 (4.0 mmol) was suspended in anhydrous MeOH (40 mL). The reaction mixture was cooled to 0 °C in an ice bath. NaBH4 (3.0–4.0 equiv.) was added slowly to the reaction mixture under an argon atmosphere. It was then allowed to warm to room temperature and stirred for 6 hours to overnight, until TLC indicated complete conversion. Volatiles were removed in vacuo. The resulting residue was dissolved in DCM and washed with 1 N HCl, water, and brine. The aqueous layer was extracted with DCM (2×). The organic layers were combined, dried over MgSO4 and filtered. Volatiles were removed in vacuo. The crude residue was subjected to flash column chromatography.
General preparation for 3,4-dihydroisoquinolin-2(1H)-yl)methanone compounds (Procedure VII)
The tetrahydroisoquinoline 5 (1 mmol) was dissolved in anhydrous DCM (10 mL). The solution was cooled to 0 °C in an ice bath. Triethylamine (3.0 equiv.) was added to the cooled solution followed by dropwise addition of a benzoyl chloride (1.2 equiv.). The reaction mixture was allowed to warm to room temperature and stirred for an additional 90 minutes. Volatiles were removed in vacuo. The resulting residue was treated with NH4Cl saturated solution followed by extraction with DCM (2×). The organic phase was washed with brine and water, dried over MgSO4, filtered, and concentrated to yield a residue. The resulting residue was subjected to column chromatography to afford the final products as a mixture of two rotamers.
General preparation for 3,4-dihydroisoquinolin-2(1H)-yl)methanone compounds (Procedure VIII)
Benzoic acid (1.0–1.3 equiv) was dissolved in dry DCM (15 mL) and cooled to 0 °C in an ice bath. DMAP (1.2 equiv) and EDC (1.2 equiv) were added and the reaction was stirred at 0 °C for 2 hours. Tetrahydroisoquinoline (1.0 equiv) dissolved in DCM (10 mL) was added to the cooled reaction and it was warmed to room temperature and stirred for an additional 18 hours. When TLC indicated complete conversion, 1M HCl was added and the aqueous phase was extracted with DCM (2×). The organics were combined and washed with brine and water, dried over MgSO4, filtered and concentrated in vacuo. The resulting residue was subjected to column chromatography to afford the final products as a mixture of two rotamers.
General preparation of N-(phenethyl)-3-phenylpropanamide and (E)-N-(phenethyl)-3-(phenyl)acrylamide compounds (Procedure IX)
Phenylpropionic acid (6.7 mmol, 1.0 equiv) or cinnamic acid (5.6 mmol, 1.0 equiv) was dissolved in dry DCM (20 mL) and DMF (10 mL) and the reaction mixture was brought to 0 °C in an ice bath. EDC (1.3 equiv) and DMAP (1.1 equiv) were added and the reaction mixture was stirred at 0 °C for 2 hours. 3,4-dimethoxyphenethylamine (6.7 mmol, 1.0 equiv) was added dropwise. The solution was warmed to room temperature and stirred overnight. After TLC indicated complete conversion, 1M HCl was added and the reaction mixture was extracted into DCM (3×). The organics were combined, washed with brine and water, dried over MgSO4, filtered and concentrated in vacuo. The resulting solid was subjected to flash column chromatography.
(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (5)
Compound 5 was prepared according to Procedure I using benzoyl chloride (1.5 mL, 13 mmol, 3.0 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white solid (mixture of rotamers, 0.9 g, 67%). 1H NMR (CDCl3, 400 MHz) δ: 7.45-7.39 (m, 7H), 6.62-6.38 (m, 1H), 4.81 (m, 1H), 3.97 (m, 1H), 3.86 (s, 6H), 3.76 (m, 1H), 3.60 (m, 1H), 2.81 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 171.1, 170.6, 147.9, 136.4, 129.9, 128.7, 127.4, 127.0, 125.8, 125.0, 111.5, 109.6, 108.8, 56.2, 56.1, 49.8, 45.6, 44.7, 29.3, 28.1. HRMS calcd. for C18H20NO3, 298.14377 [M + H]+; found, 298.14362 [M + H]+.
(3-chlorophenyl)(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone (6)
Compound 6 was prepared according to Procedure I using 3-chlorobenzoyl chloride (1.7 mL, 13 mmol, 3.0 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 24 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white solid (mixture of rotamers, 1.1 g, 76%). 1H NMR (CDCl3, 400 MHz) δ: 7.51-7.31 (m, 4H), 6.65-6.39 (m, 2H), 4.80 (s, 1H), 3.96 (m, 1H), 3.84 (s, 6H), 3.77 (m, 1H), 3.60 (m, 1H), 2.88 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 169.6, 169.2, 148.2, 137.9, 134.8, 133.2, 130.5, 129.9, 127.3, 125.6, 125.2, 124.7, 111.6, 109.5, 108.8, 56.2, 56.1, 49.7, 45.6, 44.7, 29.3, 27.9. HRMS calcd. for C18H19NO3Cl, 332.10480 [M + H]+; found, 332.10483 [M + H]+.
(3-bromophenyl)(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone (7)
Compound 7 was prepared according to Procedure I using 3-bromobenzoyl chloride (1.7 mL, 13 mmol, 3.0 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 24 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white solid (1.4 g, 85%). 1H NMR (CDCl3, 400 MHz) δ: 7.56-7.52 (m, 2H), 7.30 (d, J= 7.6 Hz, 1H), 7.29-7.24 (m, 1H), 6.63-6.68 (m, 2H), 4.76 (m, 1H), 4.45 (m, 1H), 3.93 (m, 1H), 3.82 (s, 6H), 3.75 (m, 1H), 3.57 (m, 1H), 2.81 (m, 1H).13C NMR (CDCl3, 100 MHz) δ:169.4, 148.2, 138.2, 133.1, 130.4, 130.2, 126.6, 125.9, 125.6, 124.7, 124.4, 122.9, 111.7, 109.5, 56.2, 56.1, 54.1, 49.7, 45.6, 44.7, 29.2, 28.1. HRMS calcd. for C18H19NO3Br, 376.05428 [M + H]+; found, 376.05471 [M + H]+.
(6-(benzyloxy)-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (81)
Compound 81 was prepared according to Procedure VII using tetrahydroisoquinoline 63 (0.2 g, 0.53 mmol) and benzoyl chloride (0.09 g, 0.07 mL, 0.64 mmol, 1.2 equiv). The crude residue was purifed by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to give the title compound as an off-white amorphous solid (mixture of rotamers, 0.18 g, 71%). 1H NMR (CDCl3, 400 MHz) δ: 7.52-7.22 (m, 10H), 6.93-6.71 (m, 7H), 1H [6.01, 5.19 (t, J = 4.4 Hz; dd, J1 = 3.8 Hz, J2 = 8.6 Hz)], 5.05 (s, 2H), 1H [4.88, 4.10 (dd, J1 = 5.4 Hz, J2 = 13 Hz; t, J = 9.6 Hz), 4.36 (d, J = 4.4 Hz, 1H), 3.93-3.66 (m, 4H), 3.27-3.15 (m, 1H), 3.93-2.70 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 172.0, 171.3, 158.4, 157.9, 154.2, 153.1, 152.7, 137.0, 136.8, 136.7, 136.6, 135.9, 129.8, 129.7, 128.9, 128.6, 128.3, 127.7, 126.8, 126.5, 126.1, 125.1, 115.9, 115.6, 115.4, 115.2, 114.9, 113.8, 113.6, 96.1, 71.4, 70.4, 70.2, 57.2, 56.0, 51.8, 42.9, 35.4. 30.1, 28.7. HRMS calcd. for C31H30NO4, 480.21694 [M + H]+; found,480.21792 [M + H]+.
(6-(benzyloxy)-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(3-chlorophenyl)methanone (82)
Compound 82 was prepared according to Procedure VII using tetrahydroisoquinoline 63 (0.2 g, 0.53 mmol) and 3-chlorobenzoyl chloride (0.1 g, 0.08 mL, 0.64 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to give the title compound as an off white amorphous solid (mixture of rotamers, 0.20 g, 70%). 1H NMR (CDCl3, 400 MHz) δ: 7.59-7.21 (m, 9H), 6.93-6.76 (m, 7H), 1H [5.98, 5.14 (t, J = 4.8 Hz; dd, J1 = 4.4 Hz, J2 = 9.2 Hz)], 5.04 (s, 2H), 1H [4.85, 4.13 (dd, J1= 5.2 Hz, J2 = 13 Hz; m)], 4.35 (m, 1H), 3.94-3.67 (m, 4H), 3.26-2.73 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.5, 169.7, 168.5, 158.5, 158.0, 154.4, 153.0, 152.6, 138.3, 137.1, 137.0, 136.7, 135.7, 134.9, 134.6, 133.4, 130.3, 129.9, 128.9, 128.4, 127.7, 127.1, 125.8, 125.7, 124.9, 124.7, 115.9, 115.5, 115.3, 114.9, 113.9, 113.7, 71.3, 70.2, 57.3, 56.0, 52.0, 42.9, 35.5, 30.0, 28.6. HRMS calcd. for C31H29NO4Cl, 514.17796 [M + H]+; found, 514.17923 [M + H]+.
(6-(benzyloxy)-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(3-bromophenyl)methanone (83)
Compound 83 was prepared according to Procedure VII using tetrahydroisoquinoline 63 (0.2 g, 0.53 mmol) and 3-bromobenzoyl chloride (0.1 g, 0.08 mL, 0.64 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to give the title compound as an off-white amorphous solid (mixture of rotamers, 0.21 g, 71%). 1H NMR (CDCl3, 400 MHz) δ: 7.56-7.21 (m, 9H), 6.94-6.76 (m, 7H), 1H [5.98, 5.13 (t, J = 4.7 Hz; dd, J1 = 2.6 Hz, J2 = 9.4 Hz)], 5.05 (s, 2H), 1H [4.85, 4.13 (m, m)], 4.34 (m, 1H), 3.93-3.67 (m, 4H), 3.26-2.72 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 158.5, 158.0, 154.4, 153.1, 152.6, 138.6, 138.4, 136.9, 136.7, 135.7, 132.9, 132.8, 131.1, 130.5, 130.2, 129.9, 129.0, 128.9, 128.3, 127.7, 126.3, 125.7, 125.3, 124.7, 123.0, 122.7, 115.9, 115.5, 115.3, 115.0, 114.9, 114.0, 113.7, 71.3, 70.2, 57.3, 56.0, 52.0, 42.9, 35.5, 30.0, 29.0. HRMS calcd. for C31H29NO4Br, 558.12745 [M + H]+; found, 558.12878 [M + H]+.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(3-nitrophenyl)methanone (84)
Compound 84 was prepared according to Procedure VII using 63 (0.2 g, 0.6 mmol, 1.0 equiv) and 3-nitrobenzoyl chloride (0.1 g, 0.6 mmol, 1.0 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes) to afford the title compound as a white amorphous solid (0.1, 33%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 1H [8.57, 8.30 (s, s)], 8.28 (d, J = 7.6 Hz, 1H), 1H [7.86, 7.74 (d, J = 8.0 Hz; d, J = 7.2 Hz), 7.64-7.57 (m, 1H), 6.89 (d, J = 8.8 Hz, 1H), 6.86-6.81 (m, 3H), 1H [6.70, 6.65 (s, s)], 1H [6.46, 5.98 (s, m)], 1H [5.04, 4.90 (dd, J1 = 3.4 Hz, J2 = 9.8 Hz; dd, J1 = 5.6 Hz, J2 = 13.2 Hz)], 4.39 (d, J = 5.2 Hz, 1H), 4.23-3.97 (m, 1H), 3.88-3.74 (m, 9H), 3.74-3.72 (m, 1H), 3.30-3.12 (m, 1H), 2.91-2.71 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.3,168.5, 154.5, 152.9, 152.3, 149.0, 148.6, 148.2, 147.9, 138.1, 133.9, 133.0, 130.2, 129.8, 127.2, 126.0, 124.9, 124.5, 123.5, 122.1, 115.9, 115.4, 115.0, 112.1, 111.5, 110.3, 109.8, 71.0, 69.9, 57.7, 56.3, 56.2, 55.9, 52.2, 43.1, 35.7, 29.2, 27.7. HRMS calcd. for C26H27N2O7, 479.18128 [M + H]+; found, 479.18062 [M + H]+.
(3,4-dichlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (85)
Compound 85 was prepared according to Procedure VII using 64 (0.2 g, 0.6 mmol) and 3,4-dichlorobenzoyl chloride (0.2 g, 0.7 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.2 g, 67%). 1H NMR (CDCl3, 400 MHz) δ: 7.74-7.44 (m, 2H), 7.37-7.20 (m, 1H), 6.86-6.77 (m, 4H), 1H [6.66, 6.60 (s, s)], 1H [6.47, 5.93 (s, m)], 1H [5.07, 5.05 (dd, J1 = 3.6 Hz, J2 = 9.2 Hz; dd, J1 = 6.0 Hz, J2 = 13 Hz)], 4.34 (d, J = 4.0 Hz, 1H), 1H [4.16, 3.96 (t, J = 10.0 Hz; dd, J1 = 4.0 Hz, J2 = 10.0 Hz)], 3.86-3.74 (m, 10H), 1H [td, J1 = 4.0 Hz, J2 = 13 Hz; td, J1 = 5.2 Hz, J2 = 10.0 Hz), 2.92-2.66 (m, 2H). 13C NMR (CDCl3, 400 MHz) δ: 169.6, 154.5, 154.3, 152.9, 152.4, 149.0, 148.5, 148.1, 136.3, 134.0, 132.9, 131.0, 130.7, 130.2, 127.2, 126.1, 125.0, 123.8, 115.9, 115.5, 114.9, 112.0, 111.5, 109.8, 96.8, 57.6, 56.3, 56.2, 56.0, 43.0, 35.8, 29.2, 27.7. HRMS calcd. for C26H26NO5Cl2, 502.11826 [M + H]+; found, 502.11841 [M + H]+. Anal. (C26H25NO5Cl2): C, H, N.
(2,3-dichlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (86)
Compound 86 was prepared according to Procedure VIII using 64 (0.2 g, 0.6 mmol, 1.0 equiv) and 2,3-dichlorobenzoic acid (0.1 g, 0.6 mmol, 1.0 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.1 g, 33%). 1H NMR (CDCl3, 400 MHz) δ: 7.50-7.45 (m, 1H), 7.30-7.22 (m, 1H), 1H [7.19, 7.12 (dd, J1 = 1.6 Hz, J2 = 5.2 Hz; dd, J1 = 1.6 Hz, J2 = 5.2 Hz)], 6.87-6.83 (m, 1H), 6.83-6.70 (m, 3H), 6.67-6.60 (m, 1H), 6.50-5.92 (m, 1H), 1H [4.93, 4.76 (m, m)], 4.41-4.32 (m, 1H), 1H [4.08, 3.93 (t, J1 =10.0 Hz; dd, J1 = 4.2 Hz, J2 = 10.0 Hz), 3.86-3.72 (m,9H), 3.60-3.44 (m, 1H), 3.27-2.96 (m, 1H), 2.84-2.64 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 174.1, 167.2, 166.8, 154.2, 152.8, 152.6, 148.4, 148.0, 147.8, 138.2, 133.8, 133.2, 130.9, 128.2, 127.2, 126.4, 126.2, 125.7, 125.1, 124.8, 124.0, 115.8, 115.4, 114.8, 111.9, 111.5, 109.9, 81.3, 70.9, 70.5, 56.3, 56.1, 55.9, 51.8, 42.4, 29.0, 28.0. HRMS calcd. for C26H26NO5Cl2, 502.11826 [M + H]+; found, 502.11832 [M + H]+. Anal. (C26H25NO5Cl2): C, H, N.
(3,5-dichlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (87)
Compound 87 was prepared according to Procedure VII using 64 (0.11 g, 0.33 mmol) and 3,5-dichlorobenzoyl chloride (0.08 g, 0.37 mmol. 1.1 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.06 g, 36%). 1H NMR (CDCl3, 400 MHz) δ: 7.51 (s, 1H), 7.42 (s, 1H), 7.25 (m, 1H), 6.9-6.79 (m, 4H), 1H [6.68, 6.64 (s, s)], 1H [6.51, 5.94 (s, m)], 1H [5.06, 4.85 (dd, J1 = 2.8 Hz, J2 = 9.6 Hz; dd, J1 = 5.6 Hz, J2 = 13.2 Hz)], 4.36 (d, J = 4.4 Hz, 1H), 1H [4.21, 3.99 (t, J = 10.0 Hz; dd, J1 = 3.6 Hz, J2 = 10.0 Hz), 3.89-3.73 (m, 9H), 3.72-3.68 (m, 1H), 3.27-3.08 (m, 1H), 2.94-2.70 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 168.2, 154.5, 154.4, 149.0, 148.5, 148.1, 147.9, 139.2, 135.7, 135.3, 129.9, 129.8, 127.2, 126.5, 126.1, 125.3, 124.9, 123.7, 115.9, 115.4, 115.0, 114.9, 112.0, 111.5, 110.3, 109.8, 71.0, 70.1, 57.6, 56.3, 56.2, 55.9, 52.1, 42.9, 35.8, 29.2, 27.7. HRMS calcd. for C26H26NO5Cl2, 502.11826 [M + H]+; found, 502.11748 [M + H]+.
(3-chloro-4-fluorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (88)
Compound 88 was prepared according to Procedure VIII using 64 (0.1 g, 0.3 mmol) and 3-chloro-4-fluorobenzoic acid (0.06 g, 0.4 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.10 g, 68%). 1H NMR (CDCl3, 400 MHz) δ: 8.08-7.74 (m, 1H), 7.46-7.13 (m, 2H), 6.88-6.80 (m, 4H), 6.72-6.63 (m, 1H) 1H [6.50, 5.95 (s, m)], 1H [5.10, 4.84 (m, dd, J1 = 5.2 Hz, J2 = 12 Hz)], 4.36 (m, 1H), 4.22-3.97 (m, 1H), 3.87-3.76 (m, 9H), 3.73-3.66 (m, 1H), 3.30-3.07 (m, 1H), 2.88-2.70 (m, 2H). 13C NMR (CDCl3, 400 MHz) δ: 169.7, 154.5, 152.9, 152.5, 148.9, 148.5, 148.1, 147.8, 133.5, 130.8, 127.3, 126.1, 125.1, 123.8, 115.9, 115.5, 114.9, 112.0, 109.8, 70.3, 57.7, 56.2, 56.1,55.9, 43.0, 35.8, 29.9, 29.2, 27.7, 15.5. HRMS (m/z): [M]+ calcd. for C26H26NO5ClF, 486.14781; found, 486.14826. Anal. (C26H25NO5ClF) C, H, N.
6,7-dimethoxy-1-(phenoxymethyl)-3,4-dihydroisoquinolin-2(1H)-yl(phenyl)methanone (89)
Compound 89 was prepared according to Procedure VII using 79 (0.6 g, 2 mmol) and benzoyl chloride (0.4 g, 0.3 mL, 3 mmol, 1.3 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, gradient 0–60% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.8 g, 99% yield). 1H NMR (CDCl3, 400 MHz) δ: 7.50 (m, 1H), 7.38 (m, 4H), 7.23 (m, 2H), 6.94-6.89 (m, 2H), 1H [6.81, 6.60 (s, s)], 1H [6.78, 6.66 (s, s)], 1H [6.47, 5.93 (s, m)], 1H [5.16, 4.84 (dd, J1 = 4.0 Hz, J2 = 9.0 Hz; dd, J1 = 6.0 Hz, J2 = 9.4 Hz), 4.39 (m, 1H), 1H [4.18, 3.99 (m, m)], 3.84-3.75 (m, 6H), 1H [3.78, 3.63 (m, m)], 3.28-3.02 (m, 1H), 2.91-2.61 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 171.9, 171.2, 158.9, 158.5, 148.8, 148.4, 148.0, 147.7, 136.6, 129.8, 128.8, 128.6, 127.7, 127.5, 126.8, 126.5, 125.4, 124.3, 121.5, 121.3, 114.9, 114.6, 112.1, 111.6, 110.5, 110.0, 70.3, 69.6, 57.2, 56.2, 56.1, 51.7, 43.0, 35.7, 29.3, 27.9. HRMS calcd. for C25H25NO4, 404.18564 [M + H]+; found, 404.18538 [M + H]+. Anal. (C25H24NO4): C, H, N.
(6,7-dimethoxy-1-((3-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (90)
Compound 90 was prepared according to Procedure VII using 65 (0.2 g, 0.6 mmol, 1.0 equiv) and benzoyl chloride (0.1 g, 0.7 mmol, 1.2 equiv, 0.08 mL). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.18 g, 60%). 1H NMR (CDCl3, 400 MHz) δ: 8.06-7.12 (m, 6H), 6.80-5.98 (m, 4H), 1H [5.16, 4.89 (dd, J1 = 4.0 Hz, J2 = 8.8 Hz; dd, J1 = 6.0 Hz, J2 = 12 Hz)], 4.40-3.96 (m, 2H), 3.85-3.75 (m, 10H), 3.66-2.64 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 171.9, 171.3, 161.1, 160.1, 159.7, 148.8, 148.4, 147.9, 147.7, 136.5, 133.5, 130.3, 129.9, 128.8, 128.6, 127.7, 127.5, 126.9, 126.4, 125.3, 124.2, 112.0, 111.5, 110.4, 109.9, 107.0, 106.8, 101.3, 70.4, 69.7, 57.2, 56.2, 56.1, 55.6, 51.6, 43.0, 35.7, 29.3, 27.9. HRMS calcd. for C26H28NO5, 434.19620 [M + H]+; found, 434.19602 [M + H]+. Anal. (C26H27NO5): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-((3-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (91)
Compound 91 was prepared according to Procedure VII using 65 (0.2 g, 0.6 mmol, 1.0 equiv) and 3-chlorobenzoyl chloride (0.1 g, 0.7 mmol, 1.2 equiv, 0.07 mL). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.20 g, 67%). 1H NMR (CDCl3, 400 MHz) δ: 8.03-7.46 (m, 1H), 7.39-7.29 (m, 3H), 7.16 (t, J = 8.4 Hz, 1H), 1H [6.78,6.66 (s, s)], 1H [6.61, 6.47 (s,s)], 6.52-5.95 (m, 3H), 1H [5.10, 4.86 (dd, J1 = 3.0 Hz J2 = 9.0 Hz; dd, J1 = 5.6 Hz, J2 = 13 Hz)], 4.38 (m, 1H), 1H [4.20, 3.98 (m, m)], 3.85-3.75 (m, 9H), 3.75-3.45 (m, 1H), 3.28-3.04 (m, 1H), 2.92-2.66 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.4, 169.6, 161.1, 160.0, 148.0, 147.8, 138.2, 134.6, 132.3, 130.3,130.0, 128.2, 128.0, 127.3, 127.1, 125.7, 125.0, 123.8, 111.5, 110.3, 109.9, 107.3, 107.0, 106.5, 101.3, 101.0, 70.3, 57.4, 56.2, 56.1, 55.6, 42.9, 35.7, 29.2, 27.8. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15689 [M + H]+. Anal. (C26H26NO5Cl): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-((2-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (92)
Compound 92 was prepared according to Procedure VII using 66 (0.5 g, 2 mmol, 1.0 equiv) and 3-chlorobenzoyl chloride (0.3 g, 2 mmol, 1.2 equiv, 0.22 mL). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.5 g, 70%). 1H NMR (CDCl3, 400 MHz) δ: 7.81-7.23 (m, 4H), 6.98-6.72 (m, 5H), 1H [6.64, 6.59 (s, s)], 1H [6.48, 5.98 (s, m)], 1H [5.12, 4.84 (dd, J1 = 4.0 Hz, J2 = 9.6 Hz; dd, J1 = 5.8 Hz, J2 =13 Hz)], 4.42-4.38 (m, 1H), 4.05-4.01 (m, 1H), 3.89-3.66 (m, 10H), 3.30-3.04 (m, 1H), 2.93-2.60 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.5, 169.6, 149.9, 149.7, 147.9, 147.7, 138.3, 138.2, 134.8, 134.6, 132.0, 130.2, 129.9, 128.3, 127.3, 127.1, 126.2, 126.1, 125.8, 125.2, 124.9, 124.1, 122.1, 121.1, 120.8, 114.2, 113.4, 112.3, 112.0, 111.8, 111.5, 110.6, 110.0, 71.1, 70.1, 57.5, 56.1, 56.0, 55.8, 51.7, 42.8, 35.6, 29.2, 27.7. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15685 [M + H]+. Anal. (C26H26NO5Cl): C, H, N.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(3-iodophenyl)methanone (93)
Compound 93 was prepared according to Procedure VIII using 64 (0.2 g, 0.6 mmol, 1.0 equiv) and 3-iodobenzoic acid (0.2 g, 0.6 mmol, 1.0 equiv). The crude material was subjected to silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.2 g, 59%). 1H NMR (CDCl3, 400 MHz) δ: 1H [7.94, 7.70 (s, s)], 7.74 (d, J = 7.6 Hz, 1H), 1H [7.46, 7.33 (d, J = 8.0 Hz; d, J = 7.6 Hz)], 7.13 (q, J = 8.0 Hz, 1H), 6.86 (d, J = 9.6 Hz, 1H), 6.80 (m, 3H), 1H [6.66, 6.61 (s, s)], 1H [6.47, 5.92 (s, m)], 1H [5.07, 4.83 (dd, J1 = 3.8 Hz, J2 = 9.8 Hz; dd, J1 = 6.8 Hz, J2 = 13 Hz)], 4.33 (m, 1H), 4.17-4.01 (m, 1H), 3.97-3.60 (m, 1H), 3.86-3.74 (m, 9H), 3.27-3.04 (m, 1H), 2.90-2.64 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.0, 169.3, 154.4, 154.3, 153.0, 152.5, 148.9, 148.4, 148.0, 147.8, 138.8, 138.6, 138.5, 136.7, 135.6, 130.3, 127.3, 126.8, 126.2, 125.2, 124.0, 115.9, 115.5, 114.9, 112.0, 111.5, 109.9, 94.5, 94.4, 71.1, 70.2, 57.5, 56.3, 56.1, 55.9, 43.0, 35.7, 29.2. HRMS calcd. for C26H27NO5I, 560.09285 [M + H]+; found, 560.09224 [M + H]+. Anal. (C26H26NO5I): C, H, N.
[1,1'-biphenyl]-3-yl(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (94)
Compound 94 was prepared according to Procedure VIII using 64 (0.5 g, 1.5 mmol) and 3-phenylbenzoic acid (0.3 g, 1.5 mmol, 1.0 equiv) in DCM (10 mL) and DMF (2.0 mL). The crude yellow residue was purified by silica gel chromatography (ISCO, Silicycle 12 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.26 g, 34%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.83-7.30 (m, 8H), 6.92-6.74 (m, 5H), 1H [6.68, 6.62 (s, s)], 1H [6.49, 6.01 (s, m)], 1H [5.21, 4.91 (dd, J1 = 3.8 Hz, J2 = 9.0 Hz; dd, J1 = 5.6 Hz, J2 = 12.8 Hz), 4.43-4.35 (m, 1H), 1H [4.22, 4.00 (m, m)], 3.90-3.72 (m, 9H), 1H [3.67, 3.26 (m, m)], 1H [3.15, 2.89 (m, m)], 2.78-2.65 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 171.8, 171.1, 154.4, 154.3, 153.1, 152.6, 148.8, 148.4, 148.0, 147.7, 141.8, 141.5, 140.6, 140.4, 137.2, 129.3, 129.1, 128.5, 128.4, 128.0, 127.9, 127.4, 126.6, 126.5 (2), 125.6, 125.5, 124.5, 115.9, 115.6, 114.9, 112.1, 111.6, 110.5, 110.0, 71.2, 70.5, 57.5, 56.3, 56.1, 55.9, 51.8, 43.0, 35.8, 29.3, 27.8. HRMS calcd. for C32H32NO5, 510.22750 [M + H]+; found, 510.22802 [M + H]+. Anal. (C32H31NO5): C, H, N.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(3-hydroxyphenyl)methanone (95)
Compound 95 was prepared according to Procedure VIII using 64 (0.16 g, 0.5 mmol) and 3-hydroxybenzoic acid (0.07 g, 0.5 mmol). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.09 g, 41%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 8.14-8.02 (m, 1H), 7.72-7.40 (m, 3H), 7.34-7.04 (m, 2H), 6.84-6.74 (m, 2H), 6.65-5.98 (m, 2H), 1H [5.20, 4.83 (m, m)], 4.32 (m, 1H), 4.18-3.90 (m, 1H), 3.84-3.70 (m, 9H), 3.70-3.68 (m, 1H), 3.30-3.02 (m, 1H), 2.84-2.59 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 171.7, 157.1, 154.3, 153.0, 152.6, 148.4, 147.9, 137.0, 130.0, 126.4, 125.1, 117.9, 117.5, 117.1, 115.9, 115.6, 115.4, 114.9, 114.3, 112.0, 111.5, 110.4, 71.1, 67.7, 63.2, 56.2, 56.1, 55.9, 52.0, 42.9, 29.2, 27.9. HRMS calcd. for C26H28NO6, 450.19111 [M + H]+; found, 459.19206 [M + H]+. Anal. (C26H27NO6) C, H, N.
(3-(chloromethyl)phenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (96)
Compound 96 was prepared according to Procedure VII using 64 (0.8 g, 2.4 mmol) and 3-(chloromethyl)benzoyl chloride (0.5 g, 2.9 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 12 g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.30 g, 26%). 1H NMR (CDCl3, 400 MHz) δ: 7.57-7.34 (m, 3H), 6.92-6.77 (m, 4H), 6.69-6.62 (m, 2H), 1H [6.50, 5.98 (s, m)], 1H [5.15, 4.90 (m, dd, J1 = 5.6 Hz, J2 = 13.2 Hz)], 4.60 (m, 2H), 4.43-4.31 (m, 1H), 4.19-4.11 (m, 1H), 3.88-3.67 (m, 10H), 3.32-3.08 (m, 1H), 2.96-2.66 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.7, 169.6, 154.4, 154.3, 153.0, 152.6, 148.8, 148.4, 148.0, 138.3, 138.0, 137.0, 133.5, 130.8, 130.3, 130.2, 130.0, 129.3, 129.1, 128.1, 127.7, 127.3, 127.1, 126.8, 126.3, 125.3, 124.2, 115.9, 115.6, 114.9, 112.0, 111.5, 110.4, 109.9, 71.1, 70.3, 57.5, 56.2, 56.1, 55.9, 51.9, 46.0, 45.7, 43.0, 35.7, 29.2, 27.9. HRMS calcd. for C27H29NO5Cl, 482.17288 [M + H]+; found, 482.17250 [M + H]+. Anal. (C27H28NO5Cl): C, H, N.
(3-(dichloromethyl)phenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (97)
Compound 97 was prepared according to Procedure VII using 64 (0.2 g, 0.6 mmol) and 3-(dichloromethyl)benzoyl chloride (0.16 g, 0.72 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 4 g column, 0–60% EtOAc/hexanes) to afford the title compound as a white amorphous solid (0.07 g, 21%). 1H NMR (CDCl3, 400 MHz) δ: 7.76-7.44 (m, 4H), 6.91-6.64 (m, 6H), 1H [6.50, 5.99 (s, m)], 1H [5.13, 4.90 (m, m)], 4.39 (m, 1H), 4.20-3.94 (m, 1H), 3.88-3.69 (m, 10H), 3.29-3.13 (m, 1H), 2.98-2.70 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.8, 170.1, 154.3, 153.0, 152.5, 148.9, 147.8, 141.1, 137.1, 129.4, 129.3, 128.3, 127.5, 125.8, 125.2, 124.8, 124.0, 115.5, 114.9, 71.2, 64.3, 57.5, 56.3, 56.2, 56.1, 55.9, 52.0, 43.0, 27.2. HRMS calcd. for C27H28NO5Cl2, 516.13391 [M + H]+; found, 516.13457 [M + H]+. Anal. (C27H27NO5Cl2): C, H, N.
3-(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)benzonitrile (98)
Compound 98 was prepared according to Procedure VII using 64 (0.21 g, 0.63 mmol) and 3-cyanobenzoyl chloride (0.13 g, 0.76 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.28 g, 99%). 1H NMR (CDCl3, 400 MHz) δ: 8.00-7.44 (m, 4H), 6.98-6.75 (m, 4H), 1H [6.70, 6.62 (s, s)], 1H [6.48, 6.00 (s, m)], 1H [5.02, 4.88 (m, m)], 4.38 (m, 1H), 4.06-3.71 (m, 10H), 3.28 (m, 1H), 3.14 (m, 1H), 2.96-2.69 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.4, 168.6, 154.4, 154.2, 152.7, 152.2, 148.9, 148.4, 147.9, 147.7, 137.6, 134.5, 134.0, 133.1, 133.0, 132.0, 131.5, 131.0, 130.3, 129.7, 129.3, 127.1, 125.9, 124.7, 123.4, 118.2, 118.0, 115.7, 115.3, 114.9, 114.7, 113.0, 112.6, 111.4, 110.2, 109.7, 70.8, 70.0, 57.5, 56.1, 55.9, 55.7, 51.9, 43.0, 35.6, 29.0, 27.6. HRMS calcd. for C27H27N2O5, 459.19145 [M + H]+; found, 459.19087 [M + H]+. Anal. (C27H27N2O5): C, H, N.
1-(3-(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)phenyl)ethanone (99)
Compound 99 was prepared according to Procedure VIII using 64 (1.0 g, 3.0 mmol) and 3-acetylbenzoic acid (0.5 g, 3.04 mmol, 1.0 equiv) in DCM (10 mL) and DMF (2.0 mL). The crude residue was purified by silica gel chromatography (ISCO, Silicycle 12 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.32 g, 22%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 8.15-7.97 (m, 2H), 7.72-7.46 (m, 2H), 6.87-6.78 (m, 4H), 1H [6.66, 6.61 (s, s)], 1H [6.43, 5.95 (s, m)], 1H [5.06, 4.87 (dd, J1 = 3.6 Hz, J2 = 9.2 Hz; dd, J1 = 5.8 Hz, J2 = 13 Hz)], 4.39-4.31 (m, 1H), 1H [4.14, 3.96 (m, m)], 3.84-3.72 (m, 9H), 1H [3.66, 3.25 (m, m)], 1H [3.12, 2.86 (m, m)], 2.77-2.61 (2H), 3H [2.58, 2.56 (s, s)]. 13C NMR (CDCl3, 100 MHz) δ: 197.8, 197.6, 170.9, 170.2, 154.5, 154.3, 153.0, 152.5, 148.9, 148.4, 148.0, 137.6, 137.4, 137.1, 132.3, 131.3, 129.6, 129.4, 129.2, 128.9, 128.0, 127.3, 126.8, 126.2, 125.2, 124.0, 115.9, 115.6, 114.9 (2), 112.1, 111.5, 110.4, 109.8, 71.1, 70.3, 57.5, 56.2, 56.1, 55.9, 51.9, 43.1, 35.7, 29.2, 27.8, 27.0. HRMS calcd. for C28H30NO6, 476.20676 [M + H]+; found, 476.20717 [M + H]+. Anal. (C28H29NO6): C, H, N.
(3-chlorophenyl)(1-((4-ethoxyphenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone (100)
Compound 100 was prepared according to Procedure VII using 75 (0.14 g, 0.4 mmol) and 3-chlorobenzoyl chloride (0.08 g, 0.5 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.1 g, 47%). 1H NMR (CDCl3, 400 MHz) δ: 7.60-7.24 (m, 4H), 6.86-6.76 (m, 4H), 1H [6.66, 6.61 (s, s)], 1H [6.47, 5.93 (s, m)], 1H [5.07, 4.84 (, J1 = 3.8 Hz, J2 = 9.4 Hz; dd, J1 = 5.4 Hz, J2 = 13 Hz)], 4.33 (m, 1H), 4.18-3.93 (m, 3H), 3.86-3.73 (m, 7H), 1H [3.65, 3.23 (m, m)], 1H [3.09, 2.85 (m, m)], 2.77-2.65 (m, 1H), 1.37 (t, J = 7.2 Hz, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 153.6, 152.9, 152.5, 148.9, 148.0, 147.8, 138.3, 124.9, 134.5, 130.2, 130.0, 129.8, 128.2, 127.3, 127.1, 126.2, 125.9, 125.2, 124.9, 124.0, 115.9, 115.7, 115.6, 115.4, 112.0, 111.5, 110.4, 109.9, 71.1, 70.2, 64.2, 57.5, 56.2, 56.1, 51.9, 42.9, 35.7, 29.2, 27.8, 15.1. HRMS calcd. for C27H29NO5Cl, 482.17288 [M + H]+; found, 482.17324 [M + H]+. Anal. (C27H28NO5Cl): C, H, N.
(3-bromophenyl)(1-((4-ethoxyphenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone (101)
Compound 101 was prepared according to Procedure VII using 75 (0.14 g, 0.4 mmol) and 3-bromobenzoyl chloride (0.10 g, 0.5 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.1 g, 51%). 1H NMR (CDCl3, 400 MHz) δ: 7.76-7.50 (m, 2H), 7.44-7.23 (m, 2H), 6.86-6.79 (m, 4H), 1H [6.66, 6.61 (s, s)], 1H [6.47, 5.93 (s, m)], 1H [5.07, 4.84 (dd, J1 = 3.2 Hz, J2 = 9.2 Hz; dd, J1 = 5.8 Hz, J2 = 13 Hz)], 4.33 (m, 1H), 4.18-3.92 (m, 3H), 3.85-3.73 (m, 7H), 1H [3.65, 3.23 (m, m)], 1H [3.09, 2.85 (m, m)], 2.76-2.65 (m, 1H), 1.36 (t, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.2, 169.5, 153.8, 153.6, 152.9, 152.5, 148.9, 148.0, 147.8, 138.5, 132.9, 132.7, 131.0, 130.5, 130.2, 129.9, 127.3, 126.3, 125.4, 125.2, 124.0, 122.9, 122.7, 115.9, 115.7, 115.6, 115.4, 112.0,111.5, 110.4, 109.8, 71.1, 70.2, 64.2, 57.5, 56.3, 56.2,51.9, 42.9, 35.7, 29.2, 27.8, 15.2. HRMS calcd. for C27H29NO5Br, 526.12236 [M + H]+; found, 526.12256 [M + H]+. Anal. (C27H28NO5Br): C, H, N.
(6,7-dimethoxy-1-((4-(methylthio)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (103)
Compound 103 was prepared according to Procedure VII using 73 (0.4 g, 1.2 mmol) and benzoyl chloride (0.8 g, 1.3 mmol, 1.1 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of rotamers, 0.12 g, 23%). 1H NMR (CDCl3, 400 MHz) δ: 1H [7.93, 7.79 (m, m)], 7.67 (dd, J1 = 1.4 Hz, J2 = 8.2 Hz, 1H), 7.60-7.47 (m, 1H), 7.44-7.36 (m, 1H), 7.28 (m, 2H), 7.16-7.07 (m, 2H), 6.77 (d, J = 8.8 Hz, 1H), 6.67-6.59 (m, 2H), 5.08-4.92 (m, 1H), 4.31-4.23 (m, 1H), 4.12-4.02 (m, 1H), 3.97-3.79 (m, 8H), 3.49-3.35 (m, 1H), 2.62-2.67 (m, 1H), 2.39 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ: 169.8, 161.4, 161.3, 157.1, 148.6, 148.4, 147.6, 134.4, 133.6, 132.1, 130.7, 130.6, 130.1, 129.5, 128.7, 127.7, 126.9, 126.8, 125.0, 115.4, 112.0, 110.4, 70.2, 66.1, 56.2, 56.1, 54.4, 54.2, 38.4, 27.9, 18.0, 15.5. HRMS calcd. for C26H28NO4S, 450.17336 [M + H]+; found, 450.17413 [M + H]+. Anal. (C26H27NO4S): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-((4-(methylthio)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (104)
Compound 104 was prepared according to Procedure VII using 73 (0.17 g, 0.5 mmol) and 3-chlorobenzoyl chloride (0.10 g, 0.6 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of two rotamers,0.16 g, 68%). 1H NMR (CDCl3, 400 MHz) δ: 7.86-7.65 (m, 1H), 7.59-7.44 (m, 2H), 7.37-7.30 (m, 1H), 7.24-7.20 (m, 1H), 7.16-7.07 (m, 2H), 6.77-6.74 (m, 1H), 6.65-6.59 (m, 2H), 5.05 -4.92 (m, 1H), 4.28-4.23 (m, 1H), 4.09-4.00 (m, 1H), 3.98-3.80 (m, 8H), 3.47-3.36 (m, 1H), 3.08-2.86 (m, 1H), 2.39 (s, 3H). 13C NMR (CDCl3,100 MHz) δ: 170.3, 169.7, 157.3, 156.8, 148.9, 148.5, 148.0, 147.8, 138.2, 134.9, 134.6, 130.3, 130.1, 129.9, 129.7, 128.2, 127.4, 127.0, 126.3, 125.8, 124.9, 123.8, 115.6, 115.2, 112.1, 111.6, 110.4, 109.9, 70.5, 69.7, 57.3, 56.3, 56.1, 51.8, 42.9, 35.7, 29.2, 27.8, 18.0, 17.9. HRMS (m/z): [M]+ calcd. for C26H27NO4SCl, 484.13438 [M + H]+; found, 484.13530 [M + H]+. Anal. (C26H26NO4SCl): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-((4-(trifluoromethoxy)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (105)
Compound 105 was prepared according to Procedure VII using 74 (0.33 g, 0.9 mmol) and 3-chlorobenzoyl chloride (0.2 g, 1.0 mmol, 1.3 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.3 g, 67%). 1H NMR (CDCl3, 400 MHz) δ: 7.42-7.34 (m, 3H), 7.27-7.25 (m, 1H), 7.16-7.13 (m, 2H), 6.95-6.79 (m, 2H), 6.69-6.64 (m, 1H), 1H [6.49, 5.98 (s, m)], 1H [5.14, 4.86 (dd, J1 = 3.2 Hz, J2 = 9.6 Hz; dd, J1 = 5.4 Hz, J2 = 12.6 Hz)], 4.41-4.38 (m, 1H), 4.02-3.98 (m, 1H), 3.91-3.65 (m, 7H), 3.29-3.00 (m, 1H), 2.95-2.74 (2H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.7, 157.3, 156.8, 149.0, 148.6, 148.1, 147.9, 143.3, 138.1, 134.9, 134.6,130.3, 130.1, 129.9, 128.1, 127.4, 127.0, 126.3, 125.8, 124.9, 124.8, 123.5, 122.8, 122.7, 122.0, 115.7, 115.3, 112.1, 11.6, 110.3, 109.8, 70.7, 69.9, 57.3, 56.2, 56.1, 51.8, 43.0, 35.7, 29.2, 27.7. HRMS calcd. for C26H23NO5ClF3, 522.12896 [M + H]+; found, 522.12847 [M + H]+.
(1-((4-(dimethylamino)phenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (106)
Compound 106 was prepared according to Procedure VII using tetrahydroisoquinoline 78 (0.25 g, 0.73 mmol, 1.0 equiv) and benzoyl chloride (0.10 mL, 0.88 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.17 g, 54%, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.14; 1H NMR (CDCl3, 400 MHz) δ: 7.53-7.51 (m, 1H), 7.39 (s, 4H), 6.88-6.81 (m, 1H), 6.76-6.60 (m, 4H), 1H [6.47, 5.96 (s, m) 1H [5.12, 4.87 (dd, J1 = 4.4 Hz, J2 = 9.2 Hz; dd, J1 = 5.6 Hz, J2 = 13 Hz)], 4.39-4.31 (m, 1H), 4.15-3.94 (m, 1H), 3.85-3.77 (m, 7H), 3.68-3.61 (m, 1H), 3.33-3.08 (m, 1H), 2.84 (s, 6H), 2.83-2.64 (m, 2H). 13C NMR (100 MHz) δ: 171.9, 171.2, 151.2, 150.8, 148.7, 148.3, 147.9, 147.6, 146.2, 136.7, 129.8, 128.6, 127.7, 127.4, 126.9, 126.4, 125.6, 124.6, 115.9, 115.6, 115.0, 114.9, 111.9, 111.5, 110.4, 110.0, 71.3, 70.6, 57.4, 56.2, 56.1, 51.7, 42.9, 41.9, 41.8, 35.7, 29.3, 27.9. HRMS calcd. for C27H31N2O4, 447.22783 [M + H]+; found, 447.22759 [M + H]+.
((3-chlorophenyl)(1-((4-(dimethylamino)phenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (107)
Compound 107 was prepared according to Procedure VII using tetrahydroisoquinoline 78 (0.25 g, 0.73 mmol, 1.0 equiv) and 3-chlorobenzoyl chloride (0.11 mL, 0.88 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.33 g, 94%, mixture of rotamers). TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.25; 1H NMR (CDCl3, 400 MHz) δ: 7.61-7.25 (m, 4H), 6.87-6.84 (m, 1H), 6.80-6.78 (m, 1H), 6.72-6.68 (m, 2H), 1H [6.63, 6.60 (s,s)], 1H [6.47, 5.94 (s,m)], 1H [5.06, 4.85 (dd, J1 = 4.0 Hz, J2 = 9.2 Hz; dd J1 = 5.6 Hz, J2 = 13 Hz)], 4.37-4.33 (m, 1H), 4.17-3.92 (m, 1H), 3.86-3.62 (m, 7H), 3.74-3.62 (m, 1H), 3.27-3.02 (m, 1H), 2.85 (s, 6H), 2.83-2.64 (m, 1H). 13C NMR (100 MHz) δ: 179.3, 169.6, 151.1, 148.8, 148.4, 147.9, 147.7, 146.4, 146.3, 138.3, 134.8, 134.5, 130.2, 129.9, 129.8, 128.2, 127.3, 127.1, 126.3, 125.9, 125.3, 124.9, 124.2, 115.9, 115.5, 114.8, 111.9, 111.5, 110.4, 109.9, 71.2, 70.4, 57.6, 56.2, 56.2, 51.9, 42.8, 41.9, 41.8, 35.6, 29.3, 27.8. HRMS calcd. for C27H30N2O4Cl, 481.18886 [M + H]+; found, 481.18843 [M + H]+; Anal. (C27H29N2O4Cl): C, H, N.
((3-bromophenyl)(1-((4-(dimethylamino)phenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (108
Compound 108 was prepared according to Procedure VII using tetrahydroisoquinoline 78 (0.25 g, 0.73 mmol, 1.0 equiv) and 3-bromobenzoyl chloride (0.12 mL, 0.88 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.25 g, 64%, mixture of rotamers). TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.25; 1H NMR (CDCl3, 400 MHz) δ 7.78-7.43 (m, 2H), 7.31-7.23 (m, 2H), 6.87-6.79 (m, 2H), 6.71-6.69 (m, 2H), 1H [6.65-6.61 (s,s)], 1H [6.48, 5.93 (s, m)], 1H [5.06, 4.84 (dd, J1 = 3.6 Hz, J2 = 9.2 Hz; dd, J1 = 5.6 Hz, J2 = 13 Hz)], 4.37-4.30 (m, 1H), 4.18-3.92 (m, 1H), 3.86-3.78 (m, 7H), 3.74-3.62 (m, 1H), 3.27-3.01 (m, 1H), 2.85 (s, 6H), 2.83-2.64 (m, 2H). 13C NMR (100 MHz) δ: 170.2, 169.4, 151.1, 150.7, 148.9, 148.4, 147.9, 147.8, 146.4, 146.2, 138.6, 132.8, 132.7, 131.1, 1330.4, 130.2, 129.9, 127.3, 126.3, 126.2, 125.4, 125.3, 124.2, 122.9, 122.6, 115.9, 115.5, 114.9, 111.9,111.5, 110.4, 109.9, 71.2, 70.4, 57.6, 56.3, 56.2, 51.9, 42.9, 41.9, 41.8, 35.7, 29.2, 27.8. HRMS calcd. for C27H30N2O4Br, 252.13835 [M + H]+; 525.13783 found, [M + H]+; Anal. (C27H29N2O4Br): C, H, N.
(1-((4-(benzyloxy)phenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(3-chlorophenyl)methanone (109)
Compound 109 was prepared according to Procedure VII using 72 (0.18 g, 0.44 mmol) and 3-chlorobenzoyl chloride (0.09 g, 0.5 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.13 g, 52%). 1H NMR (CDCl3, 400 MHz) δ: 7.61-7.24 (m, 9H), 6.90-6.78 (m, 4H), 1H [6.66, 6.61 (s, s)], 1H [6.47, 5.94 (s, m)], 1H [5.08, 4.85 (dd, J1 = 3.2 Hz, J2 = 6.0 Hz; dd, J1 = 5.4 Hz, J2 = 13.0 Hz)], 5.00 (s, 2H), 4.34 (m, 1H), 1H [4.16, 3.95 (m, m)], 3.85-3.78 (m, 6H), 3.75-3.64 (m, 1H), 3.28-3.05 (m, 1H), 2.92-2.64 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.4, 169.6, 153.5, 152.7, 148.9, 148.0, 147.8, 138.3, 137.4, 134.9, 134.6, 130.0, 128.8, 128.2, 127.7, 127.3, 127.1, 126.3, 126.0, 125.2, 124.0. 116.1, 115.5, 111.6, 109.9, 70.8, 57.5, 56.3, 56.2, 42.9, 29.2, 14.4. HRMS calcd. for C32H31NO5Cl, 544.18853 [M + H]+; found, 544.18945 [M + H]+. Anal. (C32H30NO5Cl): C, H, N.
(5-((4-methoxyphenoxy)methyl)-7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinolin-6(5H)-yl)(phenyl)methanone (110)
Compound 110 was prepared according to Procedure VII using 67 (0.14 g, 0.45 mmol) and benzoyl chloride (0.09 g, 0.68 mmol, 1.5 equiv). The crude material was purified by silica gel chromatography (ISCO, Silicycle 4g column, 0–60% EtOAc/hexanes gradient) afforded the title compound as a white amorphous solid (mixture of rotamers, 0.14 g, 73%). 1H NMR (CDCl3, 400 MHz) δ: 7.50-7.41 (m, 5H), 6.90-6.77 (m, 4H), 1H [6.66, 6.47 (s, s)], 6.61 (s, 1H), 5.91 (s, 2H), 1H [5.13, 5.85 (m, m)], 4.36 (m, 1H), 1H [4.12, 3.94 (m, m)], 3.81-3.71 (m, 4H), 1H [3.66, 3.26 (m, m)], 1H [3.09, 2.85 (m, m)], 2.78-2.64 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 172.1, 171.4, 154.5, 153.3, 152.9, 147.2, 146.9, 136.9, 130.1, 129.0, 128.8, 128.0, 127.9, 127.1, 126.8, 125.7, 116.1, 115.9, 115.1, 109.6, 108.9, 107.8, 107.3, 101.5, 71.6, 70.6, 57.8, 56.2, 52.4, 43.2, 35.8, 30.0, 28.6. HRMS calcd. for C25H24NO5, 418.16490 [M + H]+; found, 418.16432 [M + H]+. Anal. (C25H23NO5): C, H, N.
(3-chlorophenyl)(5-((4-methoxyphenoxy)methyl)-7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinolin-6(5H)-yl)methanone (111)
Compound 111 was prepared according to Procedure VII using 67 (0.1 g, 0.3 mmol) and 3-chlorobenzoyl chloride (0.07 g, 0.05 mL, 0.4 mmol, 1.2 equiv.). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.08 g, 56%). 1H NMR (CDCl3, 400 MHz) δ: 8.05-7.5 (m, 1H), 7.41-7.28 (m, 3H), 6.89-6.48 (m, 6H), 5.95-5.90 (m, 2H), 1H [5.07, 4.84 (dd, J1 = 3.4 Hz, J2 = 9.4 Hz; dd, J1 = 5.6 Hz, J2 = 13 Hz)], 4.36-4.34 (m, 1H), 4.17-3.92 (m, 1H), 3.77 (s, 3H), 3.75-3.66 (m, 1H), 3.30-3.03 (m, 1H), 2.90-2.66 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 157.6, 154.3, 152.9, 152.5, 147.6, 147.1, 146.5, 138.1, 134.9, 134.6, 130.2, 129.9, 128.6, 128.1, 127.6, 127.0, 126.2, 125.8, 124.9, 115.9, 115.5, 114.9, 108.8, 107.4, 101.3, 71.2, 57.7, 56.0, 55.9, 52.3, 43.0, 35.6, 29.7, 28.2. HRMS calcd. for C25H23NO5Cl, 452.12593 [M + H]+; found, 452.12608 [M + H]+. Anal. (C25H22NO5Cl): C, H, N.
(3-bromophenyl)(5-((4-methoxyphenoxy)methyl)-7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinolin-6(5H)-yl)methanone (112)
Compound 112 was prepared according to Procedure VII using 67 (0.14 g, 0.5 mmol) and 3-bromobenzoyl chloride (0.15 g, 0.68 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.08 g, 56%). 1H NMR (CDCl3, 400 MHz) δ: 7.73-7.52 (m, 2H), 7.42-7.28 (m, 2H), 6.89-6.81 (m, 4H), 1H [6.78, 6.61 (s, s)], 1H [6.67, 6.49 (s, s)], 5.96-5.91 (m, 2H), 1H [5.06, 4.83 (m, m)], 4.35 (d, J = 4.4 Hz, 1H), 4.16-3.93 (m, 1H), 3.77 (s, 3H), 3.75-3.63 (m, 1H), 3.28-3.04 (m, 1H), 2.90-2.66 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.4, 169.6, 154.6, 153.2, 147.3, 147.0, 138.7, 133.1, 133.0, 131.2, 130.7, 130.4, 130.1, 128.8, 127.8, 126.4, 125.6, 125.3, 123.2, 122.9, 116.1, 115.7, 115.2, 115.1, 109.6, 109.0, 107.7, 107.3, 101.6, 71.4, 70.4, 58.0, 56.2, 52.6, 43.2, 35.8, 29.9, 28.4. HRMS calcd. for C25H23NO5Br, 496.07541 [M + H]+; found, 496.07478 [M + H]+. Anal. (C25H22NO5Br): C, H, N.
(6,8-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (113)
Compound 113 was prepared according to Procedure VII using 68 (0.11 g, 0.3 mmol) and benzoyl chloride (0.06 g, 0.4 mmol, 1.2 equiv). The crude material was as a white solid (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.09 g, 62%). 1H NMR (CDCl3, 400 MHz) δ: 7.48-7.29 (m, 5H), 6.88-6.78 (m, 4H), 6.36-6.24 (m, 2H), 1H [5.43, 4.88 (dd, J1 = 3.4 Hz, J2 = 9.4 Hz; dd, J1 = 6.0 Hz, J2 = 13.2 Hz)], 1H [4.47, 4.32 (dd, J1 = 3.2 Hz, J2 = 10.4 Hz; dd, J = 7.4 Hz, J = 9.8 Hz)], 4.09-3.99 (m, 1H), 3.84-3.71 (m, 10H), 3.35-3.10 (m, 1H), 2.89-2.69 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 172.1, 160.2, 157.0, 154.1, 153.5, 153.0, 137.7, 137.0, 136.5, 130.3, 129.6, 128.8, 128.3, 127.8, 126.8, 115.8, 115.5, 114.8, 114.0, 105.0, 96.9, 67.4, 56.0, 55.6, 53.4, 48.2, 42.0, 35.1, 29.8. HRMS calcd. for C26H28NO5, 434.19620 [M + H]+; found, 434.19690 [M + H]+. Anal. (C26H27NO5): C, H, N.
(3-chlorophenyl)(6,8-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (114)
Compound 114 was prepared according to Procedure VII using 68 (0.11 g, 0.3 mmol) and 3-chlorobenzoyl chloride (0.07 g, 0.4 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.08 g, 51%). 1H NMR (CDCl3, 400 MHz) δ: 7.52-7.22 (m, 4H), 6.88-6.79 (m, 4H), 6.37-6.22 (m, 2H), 1H [5.36, 4.85 (dd, J1 = 3.2 Hz, J2 = 9.2 Hz; dd, J1 = 6.2 Hz, J2 = 13.4 Hz)], 1H [4.46, 4.30 (dd, J1 = 2.8 Hz, J2 = 10.0 Hz; dd, J1 = 7.2 Hz, J2 = 10.0 Hz)], 4.10-3.95 (m, 1H), 3.84-3.74 (m, 9H), 3.75-3.70 (m, 1H), 3.36-3.08 (m, 1H), 2.72-2.87 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.4, 169.5, 160.3, 159.9, 157.0, 154.1, 153.3, 152.8, 138.9, 138.1, 137.5, 136.5, 134.4, 130.2, 129.6, 128.3, 125.8, 115.8, 115.3, 114.9, 114.3, 113.6, 105.0, 104.5, 96.9, 67.3, 56.0, 55.6, 53.5, 42.0, 35.1, 30.0. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15753 [M + H]+. Anal. (C26H27NO5Cl): C, H, N.
(3-bromophenyl)(6,8-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (115)
Compound 115 was prepared according to Procedure VII using 68 (0.11 g, 0.3 mmol) and 3-bromobenzoyl chloride (0.09 g, 0.4 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.10 g, 58%). 1H NMR (CDCl3, 400 MHz) δ: 7.70-7.13 (m, 4H), 6.86-6.76 (m, 4H), 6.34-6.18 (m, 2H), 1H [5.34, 4.82 (dd, J1 = 3.4 Hz, J2 = 10.2 Hz; dd, J1 = 6.4 Hz, J2 = 13.2 Hz)], 1H [4.43, 4.27 (dd, J1 = 3.4 Hz, J2 = 10.2 Hz; dd, J1 = 7.2 Hz, J2 = 10.0 Hz), 4.10-3.92 (m, 2H), 3.81-3.69 (m, 9H), 3.33-3.06 (m, 1H), 2.84-2.69 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.4, 160.3, 159.9, 157.0, 154.1, 152.8, 138.3, 137.5, 136.5, 132.6, 131.1, 129.9, 126.3, 122.5, 115.8, 115.3, 114.9, 113.6, 105.0, 104.5, 96.9, 67.2, 56.0, 55.6, 53.5, 42.0, 35.1, 28.5. HRMS calcd. for C26H27NO5Br, 512.10671 [M + H]+; found, 512.10713 [M + H]+. Anal. (C26H26NO5Br): C, H, N.
(6-methoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (116)
Compound 116 was prepared according to Procedure VII using 69 (0.6 g, 2.0 mmol) and benzoyl chloride (0.34 g, 2.4 mmol, 1.2 equiv). The crude material was subjected to flash column chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.6 g, 74%). 1H NMR (CDCl3, 400 MHz) δ: 7.52-7.22 (m, 5H), 6.92-6.00 (m, 7H), 1H [5.20, 4.87 (dd, J1 = 3.2 Hz, J2 = 8.0 Hz; dd, J1 = 5.2 Hz, J2 = 12.0 Hz)], 4.35 (m, 1H), 4.13-3.90 (m, 1H), 3.80-3.63 (m, 1H), 3.77 (s, 3H), 3.73 (s, 3H), 3.30-3.14 (m, 1H), 2.94-2.70 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 171.3, 159.2, 158.7, 154.4, 154.2, 153.2, 152.7, 136.8, 135.8, 129.8, 128.8. 128.6, 127.7, 126.8, 125.7, 124.8, 116.0, 115.6, 114.9, 113.7, 113.1, 71.4, 56.0, 55.9, 55.6, 51.8, 42.8,35.4, 30.1, 28.7. HRMS calcd. for C25H26NO5, 404.18564 [M + H]+; found, 404.18527 [M + H]+. Anal. (C25H25NO5): C, H, N.
(3-chlorophenyl)(6-methoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (117)
Compound 117 was prepared according to Procedure VII using 69 (0.25 g, 0.8 mmol) and 3-chlorobenzoyl chloride (0.18 g, 1 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.1 g, 27%). 1H NMR (CDCl3, 400 MHz) δ: 7.61-6.94 (m, 5H), 6.89-5.98 (m, 6H), 1H [5.15, 4.87 (dd, J1 = 3.6 Hz, J2 = 9.6 Hz; dd, J1 = 5.4 Hz, J2 = 12.6 Hz)], 4.36 (m, 1H), 1H [4.15, 3.94 (m, m)], 3.81 (s, 3H), 3.77 (s, 3H), 3.76-3.69 (m, 1H), 3.31-3.14 (m, 1H), 2.98-2.74 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.4, 169.7, 159.2, 158.8, 154.4, 153.0, 152.6, 138.4, 138.2, 136.6, 135.6, 134.9, 134.5, 130.2, 129.9, 128.7, 128.3, 128.2, 127.1, 125.8, 125.4, 124.9, 124.4, 115.9, 115.5, 115.0, 114.9, 114.2, 113.8, 113.2, 113.0, 71.3, 70.2, 57.3, 55.9, 55.5, 52.0, 42.8, 35.4, 35.4, 30.0, 28.5. HRMS calcd. for C25H24NO4Cl, 438.14636 [M + H]+; found, 438.14666 [M + H]+. Anal. (C25H23NO4Cl): C, H, N.
(3-bromophenyl)(6-methoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (118)
Compound 118 was prepared according to Procedure VII using 69 (0.25 g, 0.8 mmol) and 3-bromobenzoyl chloride (0.22 g, 1.0 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.22 g, 55%). 1H NMR (CDCl3, 400 MHz) δ: 8.19-7.50 (m, 2H), 7.42-7.20 (m, 3H), 6.93-5.96 (6H), 1H [5.14, 5.12 (dd, J = 3.4 Hz, J = 9.4 Hz; dd, J = 5.2 Hz, 12.8 Hz)], 4.35-4.32 (m, 1H), 1H [4.12, 3.68 (m, m)], 3.98-3.84 (m, 1H), 3.30-3.08 (m, 1H), 2.94-2.72 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 158.8, 154.4, 153.1, 152.6, 138.5, 136.6, 136.3, 135.6, 133.2, 132.9, 131.0, 130.5, 130.1, 129.9, 128.8, 128.7, 128.3, 126.3, 125.3, 124.4, 122.7, 115.9, 115.5, 115.0, 114.9, 114.3, 113.8, 113.2, 113.0, 71.3, 70.2, 57.3, 55.9, 55.5, 52.0, 42.9, 35.5, 29.9, 28.5. HRMS calcd. for C25H25NO4Br, 482.09615 [M + H]+; found, 482.09600 [M + H]+. Anal. (C25H24NO4Br): C, H, N.
1-(3-(6-methoxy-1-((4-methoxyphenoxy)methyl)-1,2,3,4-tetrahydroisoquinoline-2-carbonyl)phenyl)ethanone (119)
Compound 119 was prepared according to Procedure VIII using tetrahydroisoquinoline 69 (0.4 g, 1.3 mmol, 1.0 equiv) and 3-acetylbenzoic acid (0.22 g, 1.3 mmol, 1.0 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (mixture of two rotamers, 0.3 g, 50%). 1H NMR (CDCl3, 400 MHz) δ: 8.13-7.97 (m, 2H), 7.71-7.45 (m, 2H), 6.91-5.97 (m, 7H), 1H [5.10, 4.87 (dd, J1 = 3.4 Hz, J2 = 9.4 Hz; dd, J1 = 5.8 Hz, J2 = 12.6 Hz)], 4.36 (m, 1H), 4.13-3.91 (m, 1H), 3.77-3.69 (m, 8H), 3.31-2.73 (m, 2H), 2.62-2.51 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 197.7, 171.0, 170.2, 159.3, 158.8, 154.5, 152.6, 137.6, 137.3, 137.1, 136.6, 132.2, 131.3, 129.4, 129.2, 128.9, 128.3, 128.0, 126.9, 115.9, 115.6, 114.9, 114.9, 114.3, 113.8, 113.2, 113.0, 71.4, 70.3, 57.4, 55.9, 55.5, 52.0, 43.0, 35.4, 30.0, 28.5, 27.0. HRMS calcd. for C27H28NO5, 446.19620 [M + H]+; found, 446.19649 [M + H]+. Anal. (C27H27NO5): C, H, N.
(3-chlorophenyl)(5,6-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (120)
Compound 120 was prepared according to Procedure VII using 76 (0.20 g, 0.61 mmol) and 3-chlorobenzoyl chloride (0.12 g, 0.67 mmol, 1.1 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.14 g, 49%). 1H NMR (CDCl3, 400 MHz) δ: 7.58-7.24 (m, 4H), 7.03-5.97 (m, 6H), 1H [5.12, 4.87 (dd, J1 = 3.2 Hz, J2 = 9.4 Hz; dd, J1 = 5.8 Hz, J2 = 13 Hz)], 4.33 (d, J = 4.8 Hz, 1H), 4.15-3.88 (m, 1H), 3.84-3.73 (m, 10H), 1H [3.60, 3.17 (m, m)], 3.08-2.68 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 154.4, 154.3, 153.0, 152.5, 152.1, 151.5, 147.0, 146.4, 138.2, 134.9, 134.5, 130.2, 129.8, 129.7, 128.8, 128.2, 127.1, 126.2, 125.9, 125.3, 124.9, 122.6, 115.9, 115.4, 114.9, 110.7, 71.2, 60.4, 57.2, 57.1, 56.1, 56.0, 55.9, 51.7, 42.4, 24.2, 22.9. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15639 [M + H]+. Anal. (C26H26NO5Cl): C, H, N.
(3-bromophenyl)(5,6-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (121)
Compound 121 was prepared according to Procedure VII using 76 (0.20 g, 0.61 mmol) and 3-bromobenzoyl chloride (0.15 g, 0.67 mmol, 1.1 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes) to afford the title compound as a white amorphous solid (0.16 g, 51%). 1H NMR (CDCl3, 400 MHz) δ: 7.73-7.50 (m, 2H), 7.43-7.21 (m, 2H), 6.89-5.96 (m, 6H), 1H [5.12, 4.87 (dd, J1 = 3.4 Hz, J2 = 9.4 Hz; dd, J1 = 5.8 Hz, J2 = 13 Hz), 4.32 (d, J = 4.4 Hz, 1H), 4.15-3.88 (m, 1H), 3.84-3.73 (m, 10H), 1H [3.60, 3.17 (m, m)], 3.08-2.66 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 170.2, 169.5, 154.3, 153.0, 152.5, 152.0, 151.5, 146.4, 138.4, 132.9, 132.7, 131.0, 130.4, 129.9, 129.7, 128.8, 126.2, 125.3, 123.0, 122.7, 115.5, 114.8, 110.6, 71.2, 60.3, 57.2, 56.1, 56.0, 55.9, 51.7, 42.5, 35.1, 24.2, 22.9. HRMS calcd. for C26H27NO5Br, 512.10671 [M + H]+; found, 512.10571 [M + H]+. Anal. (C26H26NO5Br): C, H, N.
(3-chlorophenyl)(1-((4-methoxyphenoxy)methyl)-6,7-dimethyl-3,4-dihydroisoquinolin-2(1H)-yl)methanone (122)
Compound 122 was prepared according to Procedure VII using 70 (0.25 g, 0.8 mmol) and 3-chlorobenzoyl chloride (0.18 g, 1.0 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.3 g, 82%). 1H NMR (CDCl3, 400 MHz) δ: 7.61-7.25 (m, 4H), 7.07-5.97 (m, 6H), 1H [5.14, 4.87 (dd, J1 = 3.2 Hz, J2 = 9.4 Hz; dd, J1 = 5.8 Hz, J2 = 13.0 Hz)], 4.38 (m, 1H), 1H [4.16, 3.96 (m, m)], 3.76 (s, 3H), 3.72-3.67 (m, 1H), 3.30-3.06 (m, 1H), 2.86-2.72 (m, 2H), 2.30-2.19 (m, 7H). 13C NMR (CDCl3, 100 MHz) δ: 170.5, 169.7, 154.4, 153.1, 152.6, 138.3, 136.7, 136.0, 135.2, 135.0, 134.9, 134.5, 132.5, 131.6, 130.8, 130.3, 129.9, 129.8, 129.6, 128.2, 127.1, 125.9, 124.9, 115.9,115.5, 114.9, 71.3, 57.5, 55.9, 55.9, 52.1, 43.1, 35.8, 27.8, 19.7. HRMS calcd. for C26H27NO3Cl, 436.16740 [M + H]+; found, 436.16736 [M + H]+. Anal. (C26H26NO3Cl): C, H, N.
(3-bromophenyl)(1-((4-methoxyphenoxy)methyl)-6,7-dimethyl-3,4-dihydroisoquinolin-2(1H)-yl)methanone (123)
Compound 123 was prepared according to Procedure VII using 70 (0.25 g, 0.8 mmol) and 3-bromobenzoyl chloride (0.22 g, 1.0 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes) to afford the title compound as a white amorphous solid (mixture of two rotamers, 0.3 g, 74%). 1H NMR (CDCl3, 400 MHz) δ: 7.77-7.22 (m, 4H), 7.07-5.98 (6H), 1H [dd, J1 = 3.2 Hz, J2 = 9.2 Hz; dd, J1 = 5.6 Hz, J2 = 12.8 Hz)], 4.38 (m, 1H), 1H [4.15, 3.96 (m, m)], 3.76 (s, 3H), 3.74-3.64 (m, 1H), 3.30-3.05 (m, 1H), 2.9-2.68 (m, 2H), 2.25-2.21 (m, 6H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.5, 154.4, 154.2, 153.1, 152.6, 138.7, 138.5, 136.7, 135.9, 135.2, 135.0, 132.8, 132.5, 131.6, 130.8, 130.5, 130.1, 129.9, 129.6, 128.5, 126.3, 125.4, 122.9, 122.7, 115.9, 115.5, 114.9, 76.8, 71.3, 70.2, 57.6, 56.0, 55.9, 52.1, 43.1, 35.8, 27.8, 19.8, 19.7. HRMS calcd. for C26H27NO3Br, 480.11688 [M + H]+; found, 480.11704 [M + H]+. Anal. (C26H26NO3Br): C, H, N.
(1-((4-methoxyphenoxy)methyl)-6-methyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (124)
Compound 124 was prepared according to Procedure VII using 71 (0.18 g, 0.64 mmol, 1.0 equiv) and benzoyl chloride (0.11 g, 0.76 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes) to afford the title compound as an off-white amorphous solid (mixture of two rotamers, 0.11 g, 45%). 1H NMR (CDCl3, 400 MHz) δ: 7.50-6.02 (m, 12H), 1H [5.21, 4.88 (m, m)], 1H [4.38, 4.27 (m, m)], 3.92-3.46 (m, 6H), 3.27-2.70 (m, 2H), 2.39-2.25 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 172.5, 171.3, 154.2, 138.4, 138.1, 137.0, 136.8, 134.4, 130.4, 129.8, 129.5, 128.8, 128.7, 128.6, 127.7, 127.6, 127.2, 126.8, 126.6, 126.0, 115.9, 115.6, 114.9, 71.5, 70.4, 67.1, 57.5, 56.0, 52.7, 52.0, 45.6, 43.0, 35.5, 34.0, 29.8, 28.3, 21.6, 21.3. HRMS calcd. for C25H27NO3, 390.20637 [M + H]+; found, 390.20671 [M + H]+.
(3-chlorophenyl)(1-((4-methoxyphenoxy)methyl)-6-methyl-3,4-dihydroisoquinolin-2(1H)-yl)methanone (125)
Compound 125 was prepared according to Procedure VII using 71 (0.18 g, 0.64 mmol, 1.0 equiv) and 3-chlorobenzoyl chloride (0.13 g, 0.76 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes) to afford the title compound as an off-white amorphous solid (mixture of two rotamers, 0.10 g, 37%). 1H NMR (CDCl3, 400 MHz) δ: 6.57-5.99 (m, 11H), 1H [5.14, 4.85 (m; dd, J1 = 5.6 Hz, J2 = 13.2 Hz)], 1H [4.36, 4.25 (m, m)], 4.20-3.56 (m, 6H), 3.48-2.71 (m, 3H), 2.33-2.26 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.9, 154.4, 138.2, 137.8, 134.6, 130.2, 129.8, 129.6, 128.8, 128.2, 127.7, 127.4, 127.0, 126.9, 126.1, 124.8, 115.9, 115.6, 115.5, 115.0, 70.2, 66.9, 57.6, 56.0, 52.6, 52.2, 45.5, 43.0, 35.6, 35.2, 29.7, 21.6, 21.3. HRMS calcd. for C25H25NO3Cl, 422.15175 [M + H]+; found, 422.15201 [M + H]+.
(3-bromophenyl)(1-((4-methoxyphenoxy)methyl)-6-methyl-3,4-dihydroisoquinolin-2(1H)-yl)methanone (126)
Compound 126 was prepared according to Procedure VII using 71 (0.18 g, 0.64 mmol, 1.0 equiv) and 3-bromobenzoyl chloride (0.17 g, 0.76 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes) to afford the title compound as an off-white amorphous solid (mixture of two rotamers, 0.11 g, 37%). 1H NMR (CDCl3, 400 MHz) δ: 7.74-5.99 (11H), 1H [5.15, 4.85 (m; dd, J1 = 5.6 Hz, J2 = 13.2 Hz)], 1H [4.36, 4.26 (m, m)], 4.17-3.57 (m, 6H), 3.45-3.08 (m, 1H), 2.98-2.72 (m, 2H), 2.34-2.27 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 170.7, 154.3, 152.6, 138.5, 137.8, 134.2, 132.8, 132.5, 130.3, 130.1,129.8, 129.7, 128.8, 127.8, 127.5, 127.2, 126.1, 125.2, 122.6, 115.9, 115.5, 114.9, 71.3, 57.6, 55.9, 52.7, 45.5, 28.2, 21.6, 21.3. HRMS calcd. for C25H25NO3Br, 466.10123 [M + H]+; found, 466.10158 [M + H]+.
(6,7-dimethoxy-1-(((4-methoxyphenyl)thio)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (127)
Compound 127 was prepared according to Procedure VII using 80 (0.2 g, 0.58 mmol) and benzoyl chloride (0.10 g, 0.70 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Silicycle 4 g column, 0–60% EtOAc/hexanes gradient) to afford the tile compound as an off-white amorphous solid (0.10 g, 36%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.48 (d, J = 8.8 Hz, 2H) 7.43-7.37 (m, 5H), 6.86 (d, J = 8.8 Hz, 2H), 6.62 (s, 1H), 6.57 (s, 1H), 5.86 (m, 1H), 1H [4.92, 4.75 (m, m)], 3.86-3.76 (m, 9H), 3.60-3.53 (m, 1H), 3.46-3.35 (m, 1H), 3.14-3.07 (m, 1H), 2.86-2.61 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ:171.2, 159.3, 148.3, 147.8, 136.7, 133.6, 129.8, 128.8, 127.8, 127.5, 126.9, 126.7, 125.7, 114.9, 114.8, 111.5, 110.6, 56.3, 56.2, 56.0, 55.6, 55.5, 51.3, 42.3, 29.1. HRMS calcd. for C26H28NO4S, 450.17336 [M + H]+; found, 450.17354 [M + H]+. Anal. (C26H27NO4S): C, H, N.
(6,7-dimethoxy-1-((4-nitrophenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (128)
Compound 128 was prepared according to Procedure VII using tetrahydroisoquinoline 77 (0.7 g, 2.1 mmol, 1.0 equiv) and benzoyl chloride (0.3 mL, 2.5 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a yellow amorphous solid (0.86 g, 68%, mixture of rotamers). TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.44; 1H NMR (CDCl3, 400 MHz) δ: 8.19-8.17 (m, 2H), 7.46-7.39 (m, 5H), 7.02-7.00 (m, 1H), 6.87-6.77 (m, 1H), 6.71-6.63 (m, 1H), 1H [6.46, 5.99 (s,m)], 1H [5.24, 4.89 (d, J = 5.2 Hz; d, J = 7.6)], 4.52-4.44 (m, 2H), 4.29-4.05 (m, 1H), 3.91-3.78 (m, 6H), 3.63-3.56 (m, 1H), 3.22-3.11 (m, 1H), 2.94-2.67 (m, 2H) 13C NMR (100 MHz) δ:171.8, 171.4, 163.8, 163.5, 149.1, 148.6, 148.1, 141.9, 136.2, 130.1, 128.9, 128.8, 127.6, 126.8, 126.6, 126.2, 124.6, 123.3, 114.9, 114.6, 112.1, 111.6, 110.4, 109.8, 106.5, 70.9, 70.2, 56.9, 56.3, 56.2, 53,7, 51.4, 43.1, 35.6, 29.2, 27.8. HRMS calcd. For C25H25N2O6, 449.17071 [M + H]+; found, 449.17080 [M + H]+; Anal. (C25H24N2O6): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-((4-nitrophenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (129)
Compound 129 was prepared according to Procedure VII using tetrahydroisoquinoline 77 (0.70 g, 2.0 mmol, 1.0 equiv) and 3-chlorobenzoyl chloride (0.3 mL, 2.5 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a yellow amorphous solid (0.86 g, 86 %, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.50; 1H NMR (CDCl3, 400 MHz) δ: 8.17-8.15 (m, 2H), 7.55-7.33 (m, 2H), 7.26-7.24 (m, 1H), 7.00-6.98 (m, 1H), 6.92-6.76 (m, 1H), 6.67-6.63 (m, 1H), 1H [6.47, 5.97 (s, m)], 1H [5.16, 4.86 (d, J = 6.8 Hz; d, J = 7.6 Hz)], 4.47-4.27 (m, 2H), 4.09-4.06 (m, 1H), 3.85-3.79 (m, 6H), 3.65-3.58 (m, 1H), 3.22-3.10 (m, 1H), 2.92-2.70 (m, 2H); 13C NMR (100 MHz) δ: 170.3, 169.8, 163.7, 163.1, 149.2, 148.7, 147.9, 142.0, 137.9, 134.9, 130.3, 130.2, 128.0, 127.5, 127.0, 126.4, 126.3, 125.6, 124.8, 124.3, 122.9, 114.9, 114.5, 112.1, 111.6, 110.3, 109.7, 70.8, 70.0, 60.6, 57.1, 56.3, 56.2, 53.7, 51.6, 49.6, 43.1, 35.7, 29.1, 27.7, 21.3. HRMS calcd. for C25H24N2O6Cl, 483.13174 [M + H]+; found, 483.13178 [M + H]+.
((3-bromophenyl)(6,7-dimethoxy-1-((4-nitrophenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (130)
Compound 130 was prepared according to Procedure VII using tetrahydroisoquinoline 77 (0.7 g, 2.1 mmol, 1.0 equiv) and 3-bromobenzoyl chloride (0.3 mL, 2.5 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a yellow amorphous solid (0.86 g, 68%, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.43; 1H NMR (CDCl3, 400 MHz) δ: 8.18-8.14 (m, 2H), 7.71-7.49 (m, 2H), 7.38-7.24 (m, 2H), 7.00-6.98 (m, 1H), 6.93-6.76 (m, 1H), 6.67-6.63 (m, 1H), 1H [6.48, 5.96 (s, m)], 1H [5.17, 4.85 (d, J = 6.8 Hz; dd, J1 = 4.8 Hz, J2 = 13 Hz)], 4.50-4.42 (m, 1H), 4.32-4.00 (m, 1H), 3.84-3.79 (m, 6H), 3.65-3.57 (m, 1H), 3.22-3.09 (m, 1H), 2.92-2.69 (m, 2H); 13C NMR (100 MHz) δ: 170.1, 169.7, 163.6, 163,1, 149.2, 148.7, 148.2, 147.9, 142.2, 142.1, 138.1, 133.1, 132.9, 130.9, 130.5, 130.3, 129.8, 127.5, 126.5, 125.3, 124.3, 123,0, 114.8, 114.6, 112.1, 111.6, 110.3, 109.7, 70.8, 69.9, 65.3, 64.2, 60.6, 57.1, 56.3, 56.2, 51.6, 43.1, 35.7, 29.1, 27.7, 21.3. HRMS calcd. For C25H24N2O6Br, 527.08122 [M + H]+; found, 527.08124 [M + H]+.
((1-((4-aminophenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone) (131)
Compound 128 (0.30 g, 0.7 mmol, 1.0 equiv) and tin (II) chloride dihydrate (0.7 g, 3.3 mmol, 5.0 equiv) were suspended in EtOH (2.7 ml) and heated to 70 °C. The reaction mixture was allowed to stir for three hours. Upon completion, the reaction was basified to pH 9 with saturated NaHCO3. DCM was added and the mixture was filtered through a silica plug eluting with 10% MeOH/DCM. The organics were separated and washed with water and brine, dried over MgSO4, and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to yield the title compound as a off-white amorphous solid (0.09 g, 33%, mixture of rotamers) TLC (MeOH/DCM, 1:10, v/v) Rf = 0.73; 1H NMR (CDCl3, 400 MHz) δ: 7.52-7.50 (m, 1H), 7.39 (s, 4H), 6.98-6.76 (m, 2H), 6.65-6.57 (m, 3H), 1H [6.46, 5.95 (s, m)], 1H [5.11, 4.87 (dd, J1 = 4.4 Hz, J2 = 8.8 Hz; dd, J1 = 5.6, J2 = 13)], 4.36-4.07 (m, 2H), 3.94-3.76 (m, 6H), 3.67-3.59 (m, 1H), 3.43 (s, 2H), 3.23-3.09 (m, 1H), 2.86-2.63 (m, 2H). 13C NMR (100 MHz) δ: 171.9, 171.2, 152.1, 151.7, 148.7, 148.3, 147.9, 147.7, 140.6, 136.7, 129.8, 129.7, 128.7, 128.6, 127.8, 127.4, 126.9, 126.5, 125.7, 124.6, 116.5, 116.2, 115.8, 114.2, 112.0, 111.6, 110.1, 71.3, 70.6, 57.4, 56.2, 56.1, 53.7, 51.7, 42.9, 35.7, 29.9, 29.3, 27.9, 22.9. HRMS calcd. for C25H27N2O4, 419.19653 [M + H]+; found, 419.19721 [M + H]+.
((1-((4-aminophenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(3-chlorophenyl)methanone) (132)
Compound 129 (0.56 g, 1.1 mmol, 1.0 equiv) and tin (II) chloride dihydrate (1.2 g, 5.7 mmol, 5.0 equiv) were suspended in EtOH (5.8 ml) and heated to 70 °C. The reaction mixture was allowed to stir for three hours. Upon completion, the reaction was basified to pH 9 with saturated NaHCO3. DCM was added and the mixture was filtered through a silica plug eluting with 10% MeOH/DCM. The organics were separated and washed with water and brine, dried over MgSO4, and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 40 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.21 g, 42%, mixture of rotamers) TLC (MeOH:DCM, 1:10, v/v) Rf = 0.64; 1H NMR (CDCl3, 400 MHz) δ: 7.60-7.31 (m, 3H), 7.29-7.24 (m, 1H), 6.74-6.75 (m, 1H), 6.70-6.60 (m, 4H), 1H [6.46, 5.92 (s,m)], 1H [5.04, 4.84 (dd, J1 = 3.6, J2 = 9.2; dd, J1 = 5.6, J2 = 13)], 4.32-4.29 (m, 1H), 4.14-3.91 (m, 2H), 3.85-3.77 (m, 6H), 3.74-3.64 (m, 1H), 3.44 (s, 2H), 3.23-3.07 (m, 1H), 2.85-2.64 (m, 2H). 13C NMR (100 MHz) δ: 170.3, 169.6, 151.9, 151.5, 148.8, 148.4, 147.9, 147.7, 140.8, 138.3, 138.1, 134.8, 134.5, 130.2, 129.9, 129.8, 128.2, 127.3, 127.1, 126.2, 125.9,125..3, 124.9, 124.1, 116.5, 116.1, 115.6, 111.9, 111.5, 110.8, 110.4, 109.9, 104.4, 87.2 81.2, 76.9, 71.2, 70.3, 65.6, 60.6, 57.5, 56.2, 56.1, 54.8, 51.9, 42.8, 35.7, 29.2, 27.8, 21.3. HRMS calcd. for C25H26N2O4Cl, 453.15756 [M + H]+; found, 453.15723 [M + H]+. Anal. (C25H26N2O4Cl): C, H, N.
((1-((4-aminophenoxy)methyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(3-bromophenyl)methanone) (133)
Compound 130 (0.20 g, 0.4 mmol, 1.0 equiv) and tin (II) chloride dihydrate (0.4 g, 1.9 mmol, 5.0 equiv) were suspended in EtOH (1.5 ml) and heated to 70 °C. The reaction mixture was allowed to stir for three hours. Upon completion, the reaction was basified to pH 9 with saturated NaHCO3. DCM was added and the mixture was filtered through a silica plug eluting with 10% MeOH/DCM. The organics were separated and washed with water and brine, dried over MgSO4, and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 25 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a brown amorphous solid (0.13 g, 66%, mixture of rotamers) TLC (MeOH:DCM, 1:10, v/v) Rf = 0.73; 1H NMR (CDCl3, 400 MHz) δ: 7.54-7.42 (m, 3H), 7.29-7.25 (m, 3H), 6.78-6.75 (m, 1H), 6.71 (m, 1H), 6.65-6.61 (m, 3H), 1H [6.46, 5.92 (s,m)] 1H [5.04, 4.83 (dd, J1 = 3.5, J2 = 9.1; dd, J1 = 6.4, J2 = 13)], 4.32-4.10 (m, 1H), 3.86-3.78 (m, 6H), 3.74-3.64 (m, 1H), 3.43 (s, 2H), 3.23-3.19 (m, 1H), 2.85-2.65 (m,2H). 13C NMR (100 MHz) δ: 170.2, 169.4, 151.9, 151.5, 148.9, 148.4, 148.0, 147.8, 140.9, 140.7, 138.6, 132.9, 132.7, 131.0, 130.5, 130.2, 129.9, 127.3, 126.3, 126.2, 125.4, 124.2, 122.9, 116.7, 116.1, 115.7, 112.0, 111.6, 109.9, 71.2, 70.3, 57.5, 56.3, 56.1, 53.7, 51.9, 42.8, 35.7, 29.2, 27.9. HRMS calcd. for C25H26N2O4Br, 497.10705 [M + H]+; found, 497.10746 [M + H]+.
(3-chlorophenyl)(6,7-dimethoxy-1-((4-(methylamino)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (134)
Compound 132 (0.1 g, 0.2 mmol, 1 equiv.) and paraformaldehyde (0.03 g, 1.10 mmol, 5 equiv.) were suspended in dry methanol (2.8 ml) and sodium methoxide (0.05 ml, 0.2 mmol, 1 equiv.) was added dropwise at 0°C. After the addition was complete, the reaction was heated to reflux. After stirring at reflux for one hour the reaction was allowed to cool to room temperature. Sodium borohydride (0.04 g, 1.10 mmol, 5 equiv) was added and the reaction was heated to reflux for one additional hour. The reaction was allowed to cool to room temperature and 1M NaOH was added. The reaction mixture was extracted with DCM, washed with water and brine, dried with MgSO4, filtered, and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 20 g column, 0–80 % EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.07 g, 72 %). TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.17; 1H NMR (CDCl3, 400 MHz) δ: 7.61 -7.27 (m, 4H), 6.82-7.78 (m, 1H), 6.76-6.73 (d, 1H, J = 8.4 Hz), 1H [6.65, 6.60 (s,s)]), 6.56-6.54 (m, 2H), 1H [6.47, 5.93 (s, m)], 1H [5.05, 4.85 (dd, J1 = 3.6 Hz, J2 = 9.2 Hz; dd, J1 = 5.6, J2 = 13 Hz)], 4.33-4.31 (m, 1H), 4.16-3.93 (m, 1H), 3.86-3.78 (m, 6H), 3.73-3.65 (m, 1H), 3.24-3.08 (m, 1H), 2.85-2.81 (m, 1H), 2.78 (s, 3H), 2.77-2.65 (m, 1H). 13C NMR (100 MHz) δ: 170.3, 169.7, 151.4, 150.9, 148.8, 148.4, 147.9, 147.7, 144.3, 114.1, 138.3, 134.9, 134.5, 130.2, 129.9, 129.8, 128.2, 127.3, 127.1, 126.2, 125.9, 125.3, 124.9, 1214.2, 116.2, 115.8, 113.9, 111.9, 111.5, 110.4, 109.9,71.3, 70.5, 66.1, 57.7, 56.3, 56.1, 52.5, 51.9, 42.8, 35.6, 31.9, 29.2, 27.8, 15.5. HRMS calcd. for C26H28N2O4Cl, 467.1732 [M + H]+; found, 467.17265 [M + H]+. Anal. (C26H27N2O4Cl): C, H, N.
(3-bromophenyl)(6,7-dimethoxy-1-((4-(methylamino)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (135)
Compound 133 (0.22 g, 0.44 mmol, 1 equiv.) and paraformaldehyde (0.066 g, 2.19 mmol, 5 equiv.) were suspended in dry methanol (5.5 ml) and sodium methoxide (0.10 ml, 0.44 mmol, 1 equiv.) was added dropwise at 0°C. After the addition was complete, the reaction was heated to reflux. After one hour at refluc, the reaction was allowed to cool to room temperature. Sodium borohydride (0.083 g, 2.19 mmol, 5 equiv.) was added and the reaction was heated to reflux for one additional hour. The reaction was allowed to cool to room temperature and 1 M NaOH was added. The reaction mixture was extracted with DCM, washed with water and brine, dried with MgSO4, filtered, and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 20 g column, 0–80 % EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.15 g, 68%). TLC (MeOH:DCM, 1:10, v/v) Rf = 0.85; 1H NMR (CDCl3, 400 MHz) δ: 7.77-7.51 (m, 2H), 7.45-7.25 (m, 2H), 6.83-6.65 (m, 3H), 6.60-6.58 (m, 2H), 1H [6.47, 5.93 (s, m)], 1H [5.05, 4.84 (d, J = 5.2 Hz; dd, J1 = 5.6 Hz, J2 = 12.8 Hz)], 4.33-4.32 (m, 1H), 4.16-3.92 (m, 2H),3.86-3.85 (m, 6H), 3.74-3.65 (m, 1H), 3.24-3.08 (m, 1H), 2.85-2.83 (m, 1H), 2.79 (s, 3H), 2.77-2.65 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 170.2, 169.4, 151.3, 150.8, 148.8, 148.4, 147.9, 147.7, 144.4, 138.5, 132.8, 131.7, 131.1, 130.4, 130.2, 129.9, 127.3, 126.3, 126.2, 125.4, 125.4, 124.2, 122.9, 233.6, 116.2, 115.8, 113.8, 111.9, 111.5, 110.4, 109.9, 71.3, 70.5, 57.6, 56.2, 56.2, 51.9, 42.9, 35.7, 32.1, 31.8, 30.8, 29.8, 29.6, 29.2, 27.8, 22.9, 14.4, 13.9. HRMS calcd. For C26H27N2O4Cl, 467.1732 [M + H]+; found, 467.17265 [M + H]+.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(furan-2-yl)methanone (136)
Compound 136 was synthesized according to Procedure VII using 64 (0.1 g, 0.3 mmol) and 2-furoyl chloride (0.05 g, 0.4 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.1 g, 99%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.50-6.60 (m, 7H), 6.48 (m, 1H), 5.79 (t, J = 5.8 Hz, 1H), 4.73-4.20 (m, 3H), 3.85-3.83 (m, 6H), 3.73 (s, 3H), 3.25 (m, 1H), 3.09-2.99 (m, 1H), 2.88-2.71 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 164.1, 163.8, 158.6, 158.1, 154.4, 154.3, 152.7, 152.4, 151.0, 150.5, 148.2, 144.1, 126.9, 126.4, 123.5, 116.6, 115.9, 114.8, 111.4, 111.0, 110.2, 109.3, 108.4, 71.0, 70.4, 56.2, 56.1, 55.9, 42.2, 37.5, 29.1, 27.4. HRMS calcd. for C24H26NO6, 424.17546 [M + H]+; found, 424.17636 [M + H]+. Anal. (C24H25NO6): C, H, N.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(isoxazol-5-yl)methanone (137)
Compound 137 was synthesized according to Procedure VII using 64 (0.1 g, 0.3 mmol) and isoxazole-5-carbonyl chloride (0.05 g, 0.4 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.1 g, 99%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 1H [8.37, 8.34 (d, J = 2Hz; dd, J1 = 3.6 Hz, J2 = 1.6 Hz)], 1H [6.99, 6.93 (d, J = 2 Hz; d, J = 2 Hz)], 6.96-6.67 (m, 6H), 1H [5.87, 5.65 (dd, J1 = 5.2 Hz, J2 = 5.6 Hz; dd, J1 = 4.4 Hz, J2 = 8.8 Hz)], 4.83-4.11 (m, 3H), 3.90 (s, 3H), 3.89 (s, 3H), 3.74 (s, 3H), 3.36-2.79 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 164.1, 163.8, 158.6, 158.3, 158.1, 154.4, 154.3, 152.7, 152.4, 151.0, 150.5, 148.9, 148.6, 148.0, 126.9, 126.4, 124.6, 123.5, 115.9, 115.6, 114.9, 111.8, 111.5, 110.3, 110.0, 109.6, 108.1, 70.9, 70.6, 56.6, 56.3, 56.1, 55.9, 53.2, 42.6, 37.1, 29.3, 27.9. HRMS calcd. for C23H25N2O6, 425.17071 [M + H]+; found, 425.17159 [M + H]+. Anal. (C23H25N2O6): C, H, N.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(naphthalen-2-yl)methanone (138)
Compound 138 was prepared according to Procedure VII using 64 (0.13 g, 0.4 mmol) and 2-napthoyl chloride (0.09 g, 0.5 mmol). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4g column, 0–60% EtOAc/hexanes) to afford the title compound as an off-white amorphous solid (mixture of rotamers, 0.04 g, 21%). 1H NMR (CDCl3, 400 MHz) δ: 8.07-7.83 (m, 4H), 7.66-7.49 (m, 3H), 6.95-6.84 (m, 2H), 6.80 (s, 2H), 1H [6.72, 6.65 (s, s)], 1H [6.45, 6.07 (s, m)], 1H [5.26, 4.97 (dd, J1 = 4.0 Hz, J2 = 8.8 Hz; dd, J1 = 5.4 Hz, J2 = 13 Hz)], 4.48-4.39 (m, 1H), 4.22-3.96 (m, 1H), 3.89-3.75 (m, 10H), 3.71-3.28 (m, 1H), 3.23-2.87 (m, 1H), 2.86-2.64 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 172.0, 171.2, 154.4, 153.1, 152.7, 148.8, 148.4, 147.9, 147.7, 133.9, 133.8, 133.0, 128.7, 128.0, 127.4, 127.3, 127.0, 126.5, 125.5, 125.4, 124.4, 124.2, 115.9, 115.4, 115.0, 110.5, 110.4, 110.0, 71.2, 56.2, 56.1, 51.8, 29.3. HRMS calcd. for C30H30NO5, 484.21185 [M + H]+; found, 484.21169 [M + H]+. Anal. (C30H29NO5): C, H, N.
(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(morpholino)methanone (139)
Compound 139 was synthesized according to Procedure VII using 64 (0.12 g, 0.36 mmol) and morpholine-4-carbonyl chloride (0.07 g, 0.44 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.05 g, 31%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 6.82 (s, 4H), 6.72 (s, 1H), 6.62 (s, 1H), 5.23-5.20 (m, 1H), 4.24-4.19 (m, 1H), 4.13-4.09 (m, 1H), 3.86 (m, 7H), 3.76 (s, 3H), 3.69 (m, 4H), 3.50-3.24 (m, 5H), 3.30-2.95 (m, 1H), 2.68-2.62 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 154.2, 153.1, 148.1, 147.4, 128.2, 126.5, 115.8, 114.8, 112.1, 109.7, 71.5, 56.2, 56.0, 55.9, 54.9, 39.9, 29.3. HRMS calcd. for C19 H24NO4, 330.16999 [M + H]+; found, 330.16968 [M + H]+. Anal. (C19H24NO4): C, H, N.
1-(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)-2-morpholinoethanone (140)
Compound 140 was prepared via a two step sequence. Tetrahydroisoquinoline 64 (0.7 g, 2.1 mmol) was dissolved in dry DCM (20 mL) and cooled to 0°C in an ice bath. Triethylamine (0.22 g, 2.1 mmol, 1.0 equiv) was added to the cooled mixture followed by dropwise addition of chloroacetyl chloride (0.24 g, 2.1 mmol, 1.0 equiv). The reaction was warmed to room temperature and stirred for an additional 2 hours. The reaction was quenched with 1M HCl and extracted into DCM (2×). The combined organics were washed with brine and water, dried over MgSO4, filtered and concentrated in vacuo. The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 0–60% EtOAc/hexanes gradient) to afford the α-chloro amide (0.67 g, 78%, mixture of rotamers) as a white amorphous solid. 1H NMR (CDCl3, 400 MHz) δ: 6.85-6.78 (m, 4H), 1H [6.74, 6.71 (s, s)], 1H [6.66, 6.65 (s, s)], 1H [5.72, 4.26 (t, J = 5.2 Hz; m)], 1H [5.25, 4.74 (dd, J1 = 3.8 Hz, J2 = 9.8 Hz; m)], 1H [4.58, 4.34 (d, J = 12.4 Hz; d, J = 12.4 Hz)], 4.22-4.07 (m, 3H), 3.89-3.74 (m, 10H), 3.13-2.68 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 166.7, 166.0, 154.6, 154.3, 152.3, 148.9, 148.4, 148.0, 127.5, 126.4, 125.3, 123.2, 115.9, 115.6, 114.9, 114.8, 111.9, 111.3, 110.4, 110.0, 70.9, 70.7, 56.3, 56.2, 56.1, 55.9, 42.3, 42.0, 41.8, 31.8, 27.8, 22.9. HRMS calcd. for C21H25NO5Cl, 406.14158 [M + H]+; found, 406.14197 [M + H]+. The α-chloro amide (0.20 g, 0.49 mmol, 1.0 equiv) was dissolved in absolute EtOH (10 mL). Morpholine (0.22 g, 0.22 mL, 2.5 mmol, 5.0 equiv) was added to this solution and the reaction was stirred at room temperature for 18 hours. After TLC indicated complete conversion, the volatiles were removed in vacuo and the crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 0–70% EtOAc/hexanes gradient, dry load) to afford the title compound as a white amorphous solid (0.12 g, 53%). 1H NMR (CDCl3, 400 MHz) δ: 6.84-6.77 (m, 4H), 1H [6.74, 6.71 (s, s)], 1H [6.64, 6.63 (s, s)], 1H [5.75, 5.63 (m, m)], 1H [4.74, 4.25 (dd, J1 = 4.4 Hz, J2 = 13 Hz; dd, J1 =5.4 Hz, J2 = 9.8 Hz)], 4.18-4.06 (m, 2H), 3.91-3.82 (m, 6H), 3.75-3.35 (m, 9H), 3.32-3.23 (m, 1H), 3.09-2.78 (m, 2H), 2.70-2.46 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ: 168.9, 168.6, 154.4, 154.2, 152.9, 152.6, 148.7, 148.3, 147.8, 127.5, 126.7, 125.7, 124.6, 115.7, 115.5, 114.8, 111.9, 111.4, 110.5, 110.1, 70.9, 67.1, 62.3, 61.6, 56.2, 53.8, 51.7, 29.3, 28.0. HRMS calcd. for C25H33N2O6, 457.23331 [M + H]+; found, 457.23263 [M + H]+. Anal. (C25H32N2O6): C, H, N.
2-benzyl-6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-1,2,3,4-tetrahydroisoquinoline (141)
The tetrahydroisoquinoline 64 (0.1 g, 0.30 mmol) was dissolved in dry THF (20 mL). Potassium carbonate (0.17 g, 1.2 mmol, 4.0 equiv) was added and the mixture was allowed to stir for 2 hours. Benzyl bromide (0.04 mL, 0.36 mmol, 1.2 mmol) was added and the reaction was allowed to stir at room temperature for 24 hours. Saturated aqueous NH4Cl was added and the resulting mixture was extracted into EtOAc (2×). The organics were combined and washed with brine and water, dried over MgSO4, filtered and concentrated in vacuo. The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–50% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.05 g, 39%). 1H NMR (CDCl3, 400 MHz) δ: 7.51-7.24 (m, 5H), 6.86-6.60 (m, 6H), 4.38-4.24 (m, 1H), 4.12-3.98 (m, 1H), 3.97-3.74 (m, 12H), 3.29-3.15 (m, 1H), 2.98-2.82 (m, 2H), 2.65-2.50 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 158.2, 152.3, 148.2, 147.6, 146.2, 134.1, 129.6, 129.2, 127.6, 126.8, 125.2, 114.7, 114.5, 110.6, 78.5, 63.1, 56.3, 56.0, 55.6, 51.2, 42.2, 29.3. HRMS calcd. for C26H30NO4, 420.21694 [M + H]+; found, 420.21721 [M + H]+. Anal. (C26H29NO4): C, H, N.
(6,7-dimethoxy-1-phenethyl-3,4-dihydroisoquinolin-2(1H)-yl)(3-fluorophenyl)methanone (154)
Compound 154 was prepared via Procedure VII using 153 (0.8 g, 3.0 mmol) and 3-fluorobenzoyl chloride (0.6 g, 3.0 mmol). The crude material was purified by silica gel chromatography (ISCO, Redisep 12 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.8 g, 71%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.42-7.03 (m, 8H), 1H [6.64, 6.61 (s, s)], 1H [6.56, 6.34 (s, s)], 5.81 (m, 1H), 1H [4.82, 4.73 (m, m)], 3.85-3.78 (m, 6H), 3.73 (m, 1H), 3.50 (m, 1H), 1H [3.32, 3.09 (m, m)], 2.97 (m, 2H), 2.69-2.40 (m, 1H), 2.31-1.90 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.6, 164.1, 161.6, 148.1, 148.0, 141.9, 141.0, 138.9, 138.8, 130.8, 130.7, 129.4, 128.7, 128.5, 128.3, 126.4, 126.2, 125.9, 124.8, 122.8, 122.3, 116.9, 116.6, 114.1,113.9, 112.0, 111.5, 110.3, 109.5, 58.0, 56.2, 56.1, 52.2, 41.2, 39.2, 38.5, 35.9, 33.1, 29.1, 27.8. HRMS calcd. for C26H27NO3F, 420.19695 [M + H]+; found, 420.19789 [M + H]+. Anal. (C26H26NO3F): C, H, N.
(E)-(6,7-dimethoxy-1-(4-methoxystyryl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (155)
Compound 155 was prepared according to Procedure VII using 151 (0.15 g, 0.46 mmol) and benzoyl chloride (0.07 g, 0.55 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white solid (0.10 g, 51%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.48-7.36 (m, 5H), 7.30-7.15 (m, 2H), 6.83 (d, J = 8.8 Hz, 2H), 6.69-6.54 (m, 1H), 6.42-6.16 (m, 3H), 1H [5.29, 4.76 (m, m)], 3.86-3.76 (m, 10H), 3.48-3.20 (m, 1H), 3.15-2.86 (m, 1H), 2.80-2.56 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 171.3, 170.5, 159.6, 148.3, 147.8, 136.5, 132.8, 131.5, 130.4, 129.9, 129.5, 128.7, 128.0, 126.9, 126.0, 114.2, 111.4, 52.3, 56.2, 56.1, 55.6, 55.5, 41.5, 29.3, 19.2, 14.4. HRMS calcd. for C27H28NO4, 430.20129 [M + H]+; found, 430.20090 [M + H]+. Anal. (C27H27NO4): C, H, N.
(E)-(3-chlorophenyl)(6,7-dimethoxy-1-(4-methoxystyryl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (156)
Compound 156 was prepared according to Procedure VII using 151 (0.15 g, 0.46 mmol) and 3-chlorobenzoyl chloride (0.10 g, 0.55 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white solid (0.12 g, 56%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.48-7.22 (m, 6H), 6.83 (d, J = 8.8 Hz, 2H), 6.67-6.54 (m, 1H), 6.43-6.33 (m, 1H), 6.30 (s, 1H), 6.19 (s, 1H), 1H [5.20, 4.73 (m, m)], 3.95-3.68 (m, 10H), 3.50-3.18 (m, 1H), 3.14-2.58 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 168.9, 159.7, 148.4, 147.9, 138.2, 134.8, 133.1, 131.8, 130.2, 129.4, 128.9, 128.1, 127.2, 126.9, 126.5, 125.8, 125.0, 114.2, 111.5, 56.3, 56.2, 56.1, 55.6, 55.5, 54.1, 41.5, 37.1, 29.2. HRMS calcd. for C27H27NO4Cl, 464.16231 [M + H]+; found, 464.16185 [M + H]+.
(E)-(3-bromophenyl)(6,7-dimethoxy-1-(4-methoxystyryl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (157)
Compound 157 was prepared according to Procedure VII using 151 (0.15 g, 0.46 mmol) and 3-bromobenzoyl chloride (0.12 g, 0.55 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white solid (0.15 g, 64%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.62-7.21 (m, 6H), 6.83 (d, J = 8.4 Hz, 2H), 6.66-6.54 (m, 1H), 6.43-6.19 (m, 3H), 1H [5.19, 4.72 (m, m)], 3.86-3.67 (m, 10H), 3.56-3.42 (m, 1H), 3.12-2.84 (m, 1H), 2.80-2.61 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ: 168.8, 159.7, 148.4, 147.9, 138.4, 133.1.31.8, 130.4, 129.4, 128.1, 126.5, 125.8, 125.4, 122.9, 114.2, 112.8, 111.2, 56.3, 56.2, 56.1, 55.6, 55.5, 54.2, 41.6, 29.1, 24.2, 19.9, 13.9. HRMS calcd. for C27H27NO4Br, 508.11096 [M + H]+; found, 508.11132 [M + H]+.
(E)-(3-chlorophenyl)(6-methoxy-1-(4-methoxystyryl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (158)
Compound 148 was prepared according to Procedure IV using 144 (4.1 g, 13 mmol, 1.0 equiv). The crude solid was filtered and carried on without further purification. Compound 152 was prepared via Procedure VI using dihydroisoquinoline 148 (3.0 g, 10.2 mmol). The crude residue was subjected to flash column chromatography (ISCO, Redisep 24 g column, 0–10% MeOH/DCM gradient) to afford 152 (0.6 g, 20%) in an impure form. The impurities were inseparable by chromatography. The product was visible by LCMS and was carried on without further purification. HRMS calcd. for C19H22NO2, 296.16451 [M + H]+; found, 269.16476 [M + H]+. Compound 158 was prepared according to Procedure VII using 152 (0.3 g, 1.0 mmol) and 3-chlorobenzoyl chloride (0.21 g, 1.2 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 20–70% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (0.37 g, 84%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.44-7.24 (m, 6H), 7.14 (m, 1H), 6.96-6.81 (m, 3H), 6.69 (m, 1H), 6.32-6.16 (m, 2H), 1H [5.25, 4.71 (m, m)], 3.80-3.75 (m, 6H), 1H [3.71, 3.48 (m, m)], 1H [3.45, 3.10 (m, m)], 2.96-2.69 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 168.9, 159.6, 158.7, 158.4, 158.1, 138.6, 138.2, 134.9, 133.9, 133.3, 132.7, 130.1, 129.8, 129.5, 128.8, 128.1, 127.2, 126.8, 125.0, 124.7, 114.2, 114.1, 113.1, 60.6, 55.5, 54.1, 52.2, 41.6, 41.2, 38.9, 32.1, 29.8, 28.7, 14.4. HRMS calcd. for C26H25NO3Cl, 434.15175 [M + H]+; found, 434.15147 [M + H]+.
(E)-(3-bromophenyl)(6-methoxy-1-(4-methoxystyryl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (159)
Compound 148 was prepared according to Procedure IV using 144 (4.1 g, 13 mmol, 1.0 equiv). The crude solid was filtered and carried on without further purification. Compound 152 was prepared via Procedure VI using dihydroisoquinoline 148 (3.0 g, 10.2 mmol). The crude residue was subjected to flash column chromatography (ISCO, Redisep 24 g column, 0–10% MeOH/DCM gradient) to afford 152 (0.6 g, 20%) in an impure form. The impurities were inseparable by chromatography. The product was visible by LCMS and was carried on without further purification. HRMS calcd. for C19H22NO2, 296.16451 [M + H]+; found, 269.16476 [M + H]+. Compound 159 was prepared according to Procedure VII with 152 (0.3 g, 1.0 mmol) and 3-bromobenzoyl chloride (0.27 g, 1.2 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 20–70% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (0.30 g, 62%, mixture of rotamers) as an off-white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.59-7.55 (m, 2H), 7.37-7.13 (m, 5H), 6.93-6.69 (m, 4H), 6.32-6.17 (m, 2H), 1H [5.24, 4.71 (m, m)], 3.78-3.68 (m, 7H), 3.48-2.69 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ: 168.4, 159.6, 158.6, 138.4, 133.5, 132.9, 130.4, 130.0, 129.4, 128.8, 128.1, 126.8, 125.4, 122.9, 115.8, 114.2, 113.6, 113.1, 104.0, 55.5, 54.1, 41.6, 29.9, 28.7, 15.2. HRMS calcd. for C26H25NO3Br, 478.10123 [M + H]+; found, 478.10162 [M + H]+.
(6,7-dimethoxy-1-(4-methoxyphenethyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (160)
Compound 160 was prepared according to Procedure VII using 150 (0.20 g, 0.6 mmol) and benzoyl chloride (0.10 g, 0.73 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (0.10 g, 38%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.43-7.32 (m, 5H), 2H [7.18, 6.94 (d, J = 5.5 Hz; d, J = 5.5 Hz)], 2H [6.84, 6.76 (d, J = 5.5 Hz; d, J = 5.5 Hz), 1H [6.65, 6.63 (s, s)], 1H [6.57, 6.32 (s, s)], 1H [5.83, 4.82 (dd, J = 2.8 Hz, J = 6.4 Hz; m), 3.86-3.71 (m, 10H), 3.53-3.28 (m, 1H), 3.12-2.73 (m, 3H), 2.62-2.55 (m, 1H), 2.39-1.93 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ: 171.1, 158.1, 148.0, 137.0, 134.1, 129.7, 129.5, 129.3, 128.8, 127.2, 126.7, 125.0, 114.1, 112.0, 111.6, 110.5, 109.6, 57.9, 56.3, 56.1, 55.5, 52.0, 41.2, 39.6, 38.9, 35.7, 32.1, 29.2, 27.9. HRMS calcd. for C27H30NO4, 432.21694 [M + H]+; found, 432.21737 [M + H]+. Anal. (C27H29NO4): C, H, N.
(3-chlorophenyl)(6,7-dimethoxy-1-(4-methoxyphenethyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (161)
Compound 161 was prepared according to Procedure VII using 150 (0.20 g, 0.6 mmol) and 3-chlorobenzoyl chloride (0.13 g, 0.73 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (0.21 g, 74%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.40-7.23 (m, 4H), 2H [7.16, 6.95 (d, J = 8.4 Hz; d, J = 8.4 Hz)], 2H (d, J = 8.4 Hz; d, J = 8.4 Hz), 1H [6.63, 6.59 (s, s)], 1H [6.55, 6.32 (s, s)], 1H [5.77, 4.73 (dd, J1 = 4.8 Hz, J2 = 10 Hz; m)], 3.88-3.70 (m, 10H), 1H [3.49, 3.31 (m, m)], 3.15-2.21 (m, 4H), 2.25-1.95 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.5, 158.1, 148.1, 147.9, 138.5, 134.9, 133.8, 130.3, 129.9, 129.4, 129.2, 126.9, 124.7, 114.1, 111.5, 110.3, 56.2, 56.1, 55.5, 52.2, 41.2, 38.7, 32.1, 29.0. HRMS calcd. for C27H29NO4Cl, 466.17796 [M + H]+; found, 466.17859 [M + H]+. Anal. (C27H28NO4Cl): C, H, N.
(3-bromophenyl)(6,7-dimethoxy-1-(4-methoxyphenethyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (162)
Compound 162 was prepared according to Procedure VII using 150 (0.20, 0.6 mmol) and 3-bromobenzoyl chloride (0.16 g, 0.73 mmol, 1.2 equiv). The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–60% EtOAc/hexanes gradient) to afford the title compound as an off-white amorphous solid (0.18 g, 58%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.59-7.48 (m, 2H), 7.31-7.22 (m, 2H), 2H [7.18, 6.97 (d, J = 8.4 Hz, 2H; d, J = 8.4 Hz)], 2H [6.85, 6.78 (d, J = 8.8 Hz; d, J = 8.8 Hz)], 1H [6.66, 6.62 (s, s)], 1H [6.58, 6.35 (s, s)], 1H [4.82, 4.71 (m, m)], 3.87-3.76 (m, 10H), 3.54 (m, 1H), 2.93-2.80 (m, 2H), 2.80-2.70 (m, 1H), 2.68-2.53 (m, 1H), 2.21-2.59 (m, 2H). 13C NMR (CDCl3, 400 MHz) δ: 169.3, 158.1, 148.1, 148.0, 147.7, 138.8, 138.6, 133.8, 132.9, 132.7, 130.5, 130.1, 129.7, 129.5, 129.2, 128.9, 125.9, 125.7, 125.2, 124.8, 123.0, 114.1, 112.0, 111.5, 110.4, 109.5, 56.2, 56.1, 55.5, 52.2, 41.2, 38.7, 32.1. 29.1. HRMS calcd. for C27H29NO4Br, 510.12745 [M + H]+; found, 510.12812 [M + H]+. Anal. (C27H28NO4Br): C, H, N.
((6,7-dimethoxy-1-(4-methoxybenzyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone) (171)
Compound 167 was prepared via Procedure IV using 165 (1.2 g, 3.7 mmol, 1.0 equiv). The crude solid was filtered and carried on without further purification. Compound 171 was prepared via Procedure VI using dihydroisoquinoline 167 (0.88 g, 2.6 mmol, 1.0 equiv.). The crude residue was subjected to flash column chromatography (ISCO, Redisep 24 g column, 0–10% MeOH/DCM gradient) to afford 169 (0.36 g, 41 %) in an impure form. The impurities were inseparable by chromatography. The product was visible by LCMS and was carried on without further purification. HRMS calcd. for C19H23NO3, 314.17507 [M + H]+; found, 314.17543 [M + H]+. Compound 171 was prepared via Procedure VII with tetrahydroisoquinoline 169 (0.18 g, 0.59 mmol, 1.0 equiv.) and benzoyl chloride (0.08 mL, 0.71 mmol, 1.2 equiv.). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 10 – 80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.13 g, 53 % mixture of two rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.67; 1H NMR (CDCl3, 400 MHz) δ: 7.41-7.34 (m, 2H), 7.29-6.25 (m, 3H) 7.15-7.13 (d, J = 8.8 Hz, 1H), 6.88-6.67 (m, 3H), 6.57-6.37 (m, 1H), 1H [6.10, 5.87 (s,m)], 1H [4.86, 4.75 (dd, J1 = 5.6 Hz, J2 = 13 Hz; t, J1 = 7.2 Hz)], 3.85-3.64 (m, 10H), 3.36-3.25 (m, 1H), 3.16-3.03 (m, 2H), 2.86-2.49 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ: 171.3, 170.6, 158.8, 158.6, 148.3, 147.9, 147.3, 136.9, 131.1, 130.9, 130.2, 129.8, 129.5, 129.3, 128.7, 128.4, 128.1, 126.7, 126.7, 126.3, 125.5, 114.2, 113.9, 111.8, 111.3, 110.7, 110.1, 59.9, 56.1, 56.0, 55.9, 55.6, 55.5, 42.5, 41.9, 41.5, 35.7, 29.2, 28.1 HRMS calcd. for C26H28NO4, 418.20179 [M + H]+; found, 418.20165 [M + H]+.
((3-chlorophenyl)(6,7-dimethoxy-1-(4-methoxybenzyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (172)
Compound 172 was prepared according to Procedure VII with tetrahydroisoquinoline 169 (0.18 g, 0.59 mmol, 1.0 equiv.) and 3-chlorobenzoyl chloride (0.09 mL, 0.71 mmol, 1.2 equiv.). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 10 – 80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.38 g, 64 % mixture of two rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.61; 1H NMR (CDCl3, 400 MHz) δ: 7.34-7.08 (m, 4H), 6.86-6.80 (m, 3H), 6.85-6.36 (m, 2H), 1H [6.26, 5.81 (s, m)], 1H [4.85, 4.66 (dd, J1 = 6.1 Hz, J2 = 13 Hz; dd, J1 = 4.9 Hz, J2 = 9.5 Hz)], 3.84-3.69 (m, 9H), 3.61-3.56 (m, 1H), 3.37-3.28 (m 1H), 3.14-3.12 (d, J = 6.8, 1H), 3.07-3.01 (m, 1H), 2.85-2.52 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 168.9, 158.9, 158.7, 148.0, 147.5, 138.5, 138.0, 134.7, 134.5, 131.0, 130.9, 130.2, 129.9, 129.7, 129.6, 129.5, 128.2, 127.9, 126.9, 126.2, 125.2, 124.8, 124.5, 114.4, 113.9, 111.8, 11.4, 110.6, 109.9, 60.2, 56.2, 56.1, 55.9, 55.5, 55.4. 42.4, 41.9, 41.5, 25.6, 29.1, 28.1. HRMS cald. For C26H27NO4Cl, 452.16231 [M + H]+; found, 452.16270 [M + H]+.
((6,7-dimethoxy-1-(3-methoxybenzyl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone) (173)
Compound 168 was prepared according to Procedure IV using 166 (2.0 g, 6.19 mmol, 1.0 equiv). The crude solid was filtered and carried on without further purification. Compound 170 was prepared according to Procedure VI using dihydroisoquinoline 168 (2.6 g, 8.4 mmol, 1.0 equiv.). The crude residue was subjected to flash column chromatography (ISCO, Redisep 24 g column, 0–10% MeOH/DCM gradient) to afford 170 (0.5 g, 18 %) in an impure form. The impurities were inseparable by chromatography. The product was visible by LCMS and was carried on without further purification. HRMS calcd. for C19H23NO3, 314.17507 [M + H]+; found, 314.17484 [M + H]+. Compound 173 was prepared according to Procedure VII using tetrahydroisoquinoline 171 (0.23 g, 0.74 mmol, 1.0 equiv.) and benzoyl chloride (0.10 mL, 0.89 mmol, 1.2 equiv.). The crude residue was purified by silica gel chromatography (ISCO, Silicycle 12 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.12 g, 32 %, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.63; 1H NMR (CDCl3, 400 MHz) δ: 7.38 -7.32 (m, 2H), 7.26-7.24 (m, 3H), 7.21-7.12 (m, 1H), 6.88-6.76 (m, 2H), 6.64-6.34 (m, 2H), 1H [6.06, 5.89 (s,m)], 4.89-4.79 (m, 1H), 3.86-3.63 (m, 9H), 3.37-3.29 (m, 2H), 3.21-3.07 (m, 2H), 2.89-2.76 (m, 2H); 13C NMR (100 MHz) δ: 171.2, 170.6, 160.0, 159.8, 148.3, 147.9, 147.4, 139.7, 139.2, 136.8, 136.5, 129.8, 129.6, 129.5, 129.3, 128.7, 128.4, 128.3, 127.9, 126.3, 125.3, 122.5, 122.3, 115.4, 115.1, 112.7, 112.6, 111.7, 111.3, 110.6, 110.0, 59.8, 56.1, 56.0, 55.9, 55.4, 43.5, 42.5, 41.9, 35.8, 29.1, 28.1. HRMS calcd. for C26H28NO4, 418.20072 [M + H]+; found, 418.20045 [M + H]+.
((3-chlorophenyl)(6,7-dimethoxy-1-(3-methoxybenzyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (174)
Compound 174 was prepared according to Procedure VII using tetrahydroisoquinoline 170 (0.23 g, 0.740 mmol, 1.0 equiv.) and 3-chlorobenzoyl chloride (0.11 mL, 0.89 mmol, 1.2 equiv.). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white amorphous solid (0.09 g, 31%, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.59; 1H NMR (CDCl3, 400 MHz) δ: 7.36–7.09 (m, 4H), 6.84-6.77 (m, 2H), 6.70–6.62 (m, 1H), 6.55–6.37 (m, 2H), 1H [6.23, 5.87 (s,m)], 1H [4.87, 4.74 (dd, J1 = 6.0 Hz, J2 = 13 Hz; dd, J1 = 5.2 Hz, J2 = 8.8 Hz)], 3.86–3.69 (m, 9H), 3.64–3.59 (m, 1H), 3.42–3.30 (m, 1H), 3.17 (d, J = 6.8 Hz, 1H), 3.13–3.04 (m, 1H), 2.81–2.53 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.6, 169.1, 160.1, 159.8, 148.4, 148.1, 147.5, 139.0,138.5, 137.9,134.8, 134.5, 130.2, 129.9, 129.7, 129.6, 129.5, 128.1, 127.8, 126.9, 126.2, 125.1, 124.7, 124.6, 122.5, 122.3, 115.5, 115.4, 112.6, 112.5, 111.8, 111.3, 110.5, 109.8, 60.1, 56.1, 55.9, 55.4, 55.4, 43.4, 42.5, 41.9, 35.7, 29.1, 28.1. HRMS calcd. for C26H27NO4Cl, 452.16165 [M + H]+; found, 452.16148 [M + H]+.
((3-bromophenyl)(6,7-dimethoxy-1-(3-methoxybenzyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone) (175)
Compound 175 was prepared according to Procedure VII using tetrahydroisoquinoline 170 (0.20 g, 0.62 mmol, 1.0 equiv) and 3-bromobenzoyl chloride (0.10 mL, 0.75 mmol, 1.2 equiv). The crude residue was purified by silica gel chromatography (ISCO, Redisep 12 g column, 10–80% EtOAc/hexanes gradient) to afford the title compound as a white solid (0.09 g, 31%, mixture of rotamers) TLC (EtOAc: hexanes, 1:1, v/v) Rf = 0.54; 1H NMR (CDCl3, 400 MHz) δ: 7.55-7.08 (m, 4H), 6.88-6.75 (m, 3H), 6.68-6.40 (m, 2H), 1H [6.24, 5.89 (s, m)], 1H [4.89, 4.77 (dd, J1 = 6.1 Hz, J2 = 13 Hz; dd, J1 = 5.2, J2 = 9.2)], 3.88-3.72 (m, 9H), 3.66-3.62 (m, 1H), 3.45-3.33 (m, 1H), 3.20 (d, J = 7.2 Hz, 1H), 3.19-3.08 (m, 1H), 2.91-2.57 (m, 2H). 13C NMR (CDCl3, 100 MHz) δ: 169.5, 168.9, 160.1, 159.8, 148.5, 147.5, 147.5, 139.5, 139.5, 138.9, 138.7, 138.1, 132.7, 132.5, 130.4, 130.0, 129.8, 129.5, 129.3, 128.5, 128.1, 127.8, 126.2, 125.5, 125.2, 125.1, 122.9, 122.7, 122.5, 122.2, 115.5, 115.4, 112.6, 112.5, 111.8, 111.3, 110.5, 109.9, 76.9, 56.1, 55.9, 55.5, 43.4, 42.5, 41.9, 35.7, 29.1, 28.0. HRMS calcd. for C26H27NO4Br, 496.11180 [M + H]+; found, 496.11228 [M + H]+.
(R)-(3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (2-R)
Compound 63-R (0.10 g, 0.30 mmol) was dissolved in dry DCM (3 mL). Triethylamine (0.06 g, 0.08 mL, 0.6 mmol, 2.0 equiv) was added followed by dropwise addition of 3-chlorobenzoyl chloride (0.06 g, 0.04 mL, 0.33 mmol, 1.1 equiv). The reaction was allowed to stir at room temperature for 1 hour. Water was added to quench and the reaction was extracted with DCM (3×). The combined organics were washed with brine and dried over MgSO4, filtered and concentrated in vacuo. The crude material was purified by silica gel chromatography (ISCO, Redisep 4 g column, 0–70% EtOAc/hexanes gradient) to afford the final product as a white amorphous solid (0.04 g, 28%). 1H NMR (CDCl3, 400 MHz) δ: 7.62-7.28 (m, 4H), 6.89-6.80 (m, 4H), 1H [6.68, 6.62 (s, s)], 6.48-5.96 (m, 1H), 1H [5.10, 4.87 (dd, J1 = 9.6 Hz, J2 = 4 Hz, dd, J1 = 9.6 Hz, J2 = 4 Hz)], 4.37-3.95 (m, 2H), 3.88-3.76 (m, 9H), 3.70-2.70 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ: 170.3, 169.6, 154.4, 154.3, 152.9, 152.5, 148.8, 148.4, 148.x0, 147.8, 138.3, 134.9, 134.5, 130.2, 129.9, 129.8, 128.2, 127.3, 127.0, 126.3, 125.9, 125.2, 124.9, 124.0, 115.9, 115.5, 115.0, 114.9, 112.0, 111.5, 110.4, 109.9, 71.1, 70.2, 57.4, 56.2, 56.1, 55.9, 51.9, 42.9, 35.7, 29.2, 27.8. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15728 [M + H]+; Anal. (C26H26NO5Cl): C, H, N. HPLC: Reverse Phase Chiral OD-RH column. Conditions: 75% MeCN/25% water plus 0.1% formic acid isocratic over 20 minutes, read at λmax = 285 nm, RT = 9.192 minutes, 95%. (S-enantiomer, RT = 8.141 minutes, 5%). [α]D20 = −88 (c 0.1, dry DMSO).
(S)-(3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (2-S)
Compound 2-S was synthesized as described above with the crude material from 63-S (0.4 g, 1.2 mmol). After purification, the title compound was obtained as a white amorphous solid (0.4 g, 70%, mixture of rotamers). 1H NMR (CDCl3, 400 MHz) δ: 7.63-7.29 (m, 4H), 6.90-6.81 (m, 4H), 1H [6.69, 6.64 (s, s)], 6.50-5.96 (m, 1H), 1H [5.10, 4.88 (dd, J1 = 9.6 Hz, J2 = 4 Hz, dd, J1 = 9.6 Hz, J2 = 4 Hz)], 4.38-3.96 (m, 2H), 3.88-3.77 (m, 9H), 3.71-2.68 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ: 170.2, 169.8, 154.1, 154.3, 152.9, 152.5, 148.9, 148.4, 148.1, 147.8, 138.3, 135.1, 134.6, 130.2, 129.9, 129.9, 128.2, 127.2, 127.0, 126.4, 125.9, 125.2, 124.9, 123.8, 115.7, 115.5, 115.0, 114.9, 112.1, 111.7, 110.4, 110.0, 71.4, 70.1, 57.4, 56.2, 56.0, 55.8, 51.9, 42.7, 35.6, 29.2, 27.6. HRMS calcd. for C26H27NO5Cl, 468.15723 [M + H]+; found, 468.15857 [M + H]+; Anal. (C26H26NO5Cl): C, H, N. HPLC: Reverse Phase Chiral OD-RH column. Conditions: 75% MeCN/25% water plus 0.1% formic acid isocratic over 20 minutes, read at 254 nm, RT = 8.140 minutes, 100%. [α]D20 = +121 (c 0.1, dry DMSO).
Supplementary Material
Acknowledgments
This work was supported by the NIH (NS065371, SFT; DA015040, KKO; GM008602, KKO) and a research grant from Lundbeck (SFT). We thank Kimberly Vellano, Phuong Le, and Sara Dawit for excellent technical assistance. We gratefully acknowledge Garrick Paul Smith, Henrik Pedersen, and Valentina Lauritzen for separation of the enantiomers of compound 2.
Abbreviations Used
- ATD
amino-terminal domain
- LBD
ligand-binding domain
- PCP
phencyclidine
- BHK
baby hamster kidney
- BAPTA-AM
(1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester)
- K-BAPTA
potassium 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid
- SEM
standard error of the mean
Footnotes
Supporting Information Available: Supporting information including the experimental detail for the synthesis of all intermediates as well as off-target activity of compound 83 is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Citri A, Malenka RC. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 3.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillman BG, Gupta SC, Stairs DJ, Buonanno A, Dravid SM. Behavioral analysis of NR2C knockout mouse reveals deficit in acquisition of conditioned fear and working memory. Neurobiol. Learn. Mem. 2011;95:404–414. doi: 10.1016/j.nlm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA Receptor Ablation on Parvalbumin-Positive Interneurons Impairs Hippocampal Synchrony, Spatial Representations, and Working Memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortum F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tonnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010;42:1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 7.Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. J. Physiol.-London. 2010;588:93–99. doi: 10.1113/jphysiol.2009.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan HJ, Myers SJ, Dingledine R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyle JT. NMDA Receptor and Schizophrenia: A Brief History. Schizophrenia Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, Grp MS. Memantine in moderate-to-severe Alzheimer's disease. New Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 13.Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol. Therapeut. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An Innovative Design to Establish Proof of Concept of the Antidepressant Effects of the NR2B Subunit Selective N-Methyl-D-Aspartate Antagonist, CP-101,606, in Patients With Treatment-Refractory Major Depressive Disorder. J. Clin. Psychopharm. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 16.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and Efficacy of Repeated-Dose Intravenous Ketamine for Treatment-Resistant Depression. Biol. Psychiat. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 17.Wu LJ, Zhuo M. Targeting the NMDA Receptor Subunit NR2B for the Treatment of Neuropathic Pain. Neurotherapeutics. 2009;6:693–702. doi: 10.1016/j.nurt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CK, Nehls DG, Graham DI, Teasdale GM, McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann. Neurol. 1988;24:543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- 19.Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa E, Mori H, Kiyama Y, Mishina M, Asano T, Kirino T. Attenuation of focal ischemic brain injury in mice deficient in the epsilon1 (NR2A) subunit of NMDA receptor. J. Neurosci. 1998;18:9727–9732. doi: 10.1523/JNEUROSCI.18-23-09727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 22.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 23.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JAH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- 24.Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J. Physiol. 1998;510(Pt 1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat. Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- 26.Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J. Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 28.Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJA, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol. Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- 29.Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O'Toole KK, Le P, Lee CJ, Snyder JP, Traynelis SF, Wyllie DJ. Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J. Physiol. 2008;586:227–245. doi: 10.1113/jphysiol.2007.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential Expression of 5 N-Methyl-D-Aspartate Receptor Subunit Messenger-RNAs in the Cerebellum of Developing-Rats and Adult-Rats. J. Comp. Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- 31.Jones S, Gibb AJ. Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J. Physiol.-London. 2005;569:209–221. doi: 10.1113/jphysiol.2005.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and Regional Expression in the Rat-Brain and Functional-Properties of 4 NMDA Receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacol. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menniti FS, Lindsley CW, Conn PJ, Pandit J, Zagouras P, Volkmann RA. Allosteric Modulators for the Treatment of Schizophrenia: Targeting Glutamatergic Networks. Curr. Topics Med. Chem. 2013;13:26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heresco-Levy U. N-Methyl-D-aspartate (NMDA) receptor-based treatment approaches in schizophrenia: the first decade. Int. J. Neuropsychopharmacol. 2000;3:243–258. doi: 10.1017/S1461145700001978. [DOI] [PubMed] [Google Scholar]
- 37.Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol. Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- 38.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz TL, Sachdeva S, Stahl SM. Glutamate neurocircuitry: theoretical underpinnings in schizophrenia. Front. Pharmacol. 2012;3:195. doi: 10.3389/fphar.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacol. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat. Neurosci. 1998;1:451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- 42.Sakurada K, Masu M, Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. J. Biol. Chem. 1993;268:410–415. [PubMed] [Google Scholar]
- 43.Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K, Williams K. Channel blockers acting at N-methyl-D-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol. Pharmacol. 2002;61:533–545. doi: 10.1124/mol.61.3.533. [DOI] [PubMed] [Google Scholar]
- 44.Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anson LC, Chen PE, Wyllie DJ, Colquhoun D, Schoepfer R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J. Neurosci. 1998;18:581–589. doi: 10.1523/JNEUROSCI.18-02-00581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 48.Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Brauner-Osborne H, Liotta DC, Traynelis SF. Quinazolin-4-one Derivatives: A Novel Class of Noncompetitive NR2C/D Subunit-Selective N-Methyl-D-aspartate Receptor Antagonists. J. Med. Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen KB, Traynelis SF. Structural and Mechanistic Determinants of a Novel Site for Noncompetitive Inhibition of GluN2D-Containing NMDA Receptors. J. Neurosci. 2011;31:3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC, Traynelis SF. Mechanism for Noncompetitive Inhibition by Novel GluN2C/D N-Methyl-D-Aspartate Receptor Subunit-Selective Modulators. Mol. Pharmacol. 2011;80:782–795. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and Characterization of Novel NMDA Receptor Antagonists Selective for NR2A-over NR2B-Containing Receptors. J. Pharmacol. Exp. Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 53.Hansen KB, Ogden KK, Traynelis SF. Subunit-Selective Allosteric Inhibition of Glycine Binding to NMDA Receptors. J. Neurosci. 2012;32:6197–6208. doi: 10.1523/JNEUROSCI.5757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa BM, Irvine MW, Fang GY, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. A Novel Family of Negative and Positive Allosteric Modulators of NMDA Receptors. J. Pharmacol. Exp. Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa BM, Irvine MW, Fang GY, Eaves RJ, Mayo-Martin MB, Laube B, Jane DE, Monaghan DT. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacol. 2012;62:1730–1736. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durand GM, Bennett MVL, Zukin RS. Splice Variants of the N-Methyl-D-Aspartate Receptor NR1 Identify Domains Involved in Regulation by Polyamines and Protein-Kinase-C. Proc. Natl. Acad. Sci. USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Traynelis SF, Hartley M, Heinemann SF. Control of Proton Sensitivity of the Nmda Receptor by Rna Splicing and Polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 58.Williams K, Dawson VL, Romano C, Dichter MA, Molinoff PB. Characterization of polyamines having agonist, antagonist, and inverse agonist effects at the polyamine recognition site of the NMDA receptor. Neuron. 1990;5:199–208. doi: 10.1016/0896-6273(90)90309-4. [DOI] [PubMed] [Google Scholar]
- 59.Masuko T, Kuno T, Kashiwagi K, Kusama T, Williams K, Igarashi K. Stimulatory and inhibitory properties of aminoglycoside antibiotics at N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 1999;290:1026–1033. [PubMed] [Google Scholar]
- 60.Wu FS, Gibbs TT, Farb DH. Pregnenolone Sulfate - a Positive Allosteric Modulator at the N-Methyl-D-Aspartate Receptor. Mol. Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 61.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. A novel family of negative and positive allosteric modulators of NMDA receptors. J. Pharmacol. Exp. Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irvine MW, Costa BM, Volianskis A, Fang G, Ceolin L, Collingridge GL, Monaghan DT, Jane DE. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-D-aspartate receptors. Neurochem. Int. 2012;61:593–600. doi: 10.1016/j.neuint.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacol. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 64.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Vellano K, Snyder JP, Traynelis SF. Structural Determinants of D-Cycloserine Efficacy at the NR1/NR2C NMDA Receptors. J. Neurosci. 2010;30:2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen KB, Mullasseril P, Dawit S, Kurtkaya NL, Yuan HJ, Vance KM, Orr AG, Kvist T, Ogden KK, Le P, Vellano KM, Lewis I, Kurtkaya S, Du YH, Qui M, Murphy TJ, Snyder JP, Brauner-Osborne H, Traynelis SF. Implementation of a Fluorescence-Based Screening Assay Identifies Histamine H3 Receptor Antagonists Clobenpropit and Iodophenpropit as Subunit-Selective N-Methyl-D-Aspartate Receptor Antagonists. J. Pharmacol. Exp. Ther. 2010;333:650–662. doi: 10.1124/jpet.110.166256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan HJ, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC, Traynelis SF. A subunit-selective potentiator of NR2C-and NR2D-containing NMDA receptors. Nat. Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Traynelis SF, Liotta DC, Santangelo RM, Garnier EC. Subunit Selective NMDA Receptor Potentiators For The Treatment Of Neurological Conditions. 2012/0028977. US. 2012;A1
- 68.Suetake-Koga S, Shimazaki T, Takamori K, Chaki S, Kanuma K, Sekiguchi Y, Suzuki T, Kikuchi T, Matsui Y, Honda T. In vitro and antinociceptive profile of HON0001, an orally active NMDA receptor NR2B subunit antagonist. Pharmacol. Biochem. Behav. 2006;84:134–141. doi: 10.1016/j.pbb.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 69.Bellamy FD, Ou K. Selective Reduction of Aromatic Nitro-Compounds with Stannous Chloride in Non-Acidic and Non-Aqueous Medium. Tetrahedron Lett. 1984;25:839–842. [Google Scholar]
- 70.Ono M, Watanabe R, Kawashima H, Kawai T, Watanabe H, Haratake M, Saji H, Nakayama M. F-18-labeled flavones for in vivo imaging of beta-amyloid plaques in Alzheimer's brains. Bioorg. Med. Chem. 2009;17:2069–2076. doi: 10.1016/j.bmc.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 71.Polniaszek RP, Mckee JA, Kaufman CR. Stereoselective Nucleophilic-Addition Reactions of Chiral Iminium Ions. Abstr. Pap. Am. Chem. Soc. 1989;197 126-ORGN. [Google Scholar]
- 72.Youte J-J, Barbier D, Al-Mourabit A, Gnecco D, Marazano C. An Enantioselective Access to 1-Alkyl-1,2,3,4-tetrahydroisoquinolines. Application to a New Synthesis of (−)-Argemonine. J. Org. Chem. 2004;69:2737–2740. doi: 10.1021/jo0357869. [DOI] [PubMed] [Google Scholar]
- 73.Komori K, Takaba K, Kunitomo J. Asymmetric synthesis of (R)-(+)-noranicanine. Heterocycles. 1996;43:1681–1686. [Google Scholar]
- 74.Polniaszek RP, McKee JA. Stereoselective reductions of chiral iminium ions. Tetrahedron Lett. 1987;28:4511–4514. [Google Scholar]
- 75.Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J. Neurosci. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC, Traynelis SF. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol. Pharmacol. 2011;80:782–795. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.