Abstract
Epithelial-to-mesenchymal transition (EMT) is a developmental process hijacked by cancer cells to leave the primary tumor site, invade surrounding tissue, and establish distant metastases. A hallmark of EMT is the loss of E-cadherin expression, and one major signal for the induction of EMT is transforming growth factor beta (TGFβ, which is dysregulated in up to 40% of hepatocellular carcinoma (HCC). We have constructed an EMT network of 70 nodes and 135 edges by integrating the signaling pathways involved in developmental EMT and known dysregulations in invasive HCC. We then used discrete dynamic modeling to understand the dynamics of the EMT network driven by TGFβ. Our network model recapitulates known dysregulations during the induction of EMT and predicts the activation of the Wnt and Sonic hedgehog (SHH) signaling pathways during this process. We show, across multiple murine (P2E and P2M) and human HCC cell lines (Huh7, PLC/PRF/5, HLE, and HLF), that the TGFβ signaling axis is a conserved driver of mesenchymal phenotype HCC and confirm that Wnt and SHH signaling are induced in these cell lines. Furthermore, we identify by network analysis eight regulatory feedback motifs that stabilize the EMT process and show that these motifs involve cross-talk among multiple major pathways. Our model will be useful in identifying potential therapeutic targets for the suppression of EMT, invasion and metastasis in HCC.
Keywords: Network modeling, systems biology, epithelial-to-mesenchymal transition, hepatocellular carcinoma, transforming growth factor beta
Introduction
Hepatocellular carcinoma (HCC) is the third most common cancer type worldwide(1). The only available cure for HCC is surgical resection, which is only an option in early stage disease. Death from HCC is primarily due to late stage disease, which is characterized by invasion, intra- and extra-hepatic metastasis, and post-surgical recurrence (2). The high mortality and frequency of recurrence in HCC make the need for novel therapeutic approaches paramount. HCC invasion is dependent upon a cellular process called epithelial-to-mesenchymal transition (EMT)(3). Therefore, targeting invasion and the EMT process could reduce HCC mortality substantially.
EMT is a crucial developmental event in embryonic tissue formation and regeneration(4). Pathological forms of EMT are hypothesized to occur during cancer progression and tissue fibrosis(5). During EMT epithelial cells lose their adhesive properties and transform into mesenchymal cells with a migratory phenotype. This provides a critical mechanism in the initial steps of metastasis, allowing cancer cells to leave the primary tumor. Loss of E-cadherin (CDH1), a cell-cell adhesion protein responsible for intercellular attachment, is widely considered the hallmark of EMT(6).
Transforming growth factor-β (TGFβ β hereafter referred to as TGFβ)βsignaling has a dual role in tumor progression; it is tumor suppressive in normal epithelia and early stage tumor cells, contrasted by its stimulation of EMT as cancers progress(7). It is thought that genetic alterations to the cell contribute to the role switch during tumor progression(8). Previous studies have demonstrated that TGFβ levels are elevated in 40% of HCC patients(9), while receptor levels are variable (10, 11).
TGFβ binds to TGFβRII, which recruits TGFβRI, leading to receptor phosphorylation and phosphorylation of SMAD2 and SMAD3. SMAD2 and SMAD3 form a complex with SMAD4 (in the canonical pathway), which translocates to the nucleus, where the complex down-regulates the transcription of epithelial junction proteins while increasing the expression of mesenchyme-related proteins(6). TGFβ can also activate PI3K/AKT, MAPK and other established oncogenic signals (non-canonical pathways)(12).
EMT and TGFβ signaling are complex biological processes that have been extensively studied using reductionist approaches. Network modeling provides a set of tools to study complex processes holistically, using an integration of individual and pairwise evidence(13). A network is a representation of a complex system; the nodes represent entities (proteins, mRNA) or concepts (outcomes) and the edges represent the relationships between nodes. Edges can be assigned a direction, representing information or signal flow from the source (upstream) node to the target (downstream) node. Furthermore, edges can be characterized as positive (activating relationship) or negative (inhibitory relationship). An additional layer of network analysis is the dynamic model, which characterizes each network node with a state variable. The biological relationships incorporated in the network can be translated into mathematical equations that summarize the regulation of each node. The dynamic model describes how the nodes’ state variables change in time, for example how a signal activates signal transduction and leads to the transcription of target genes(14).
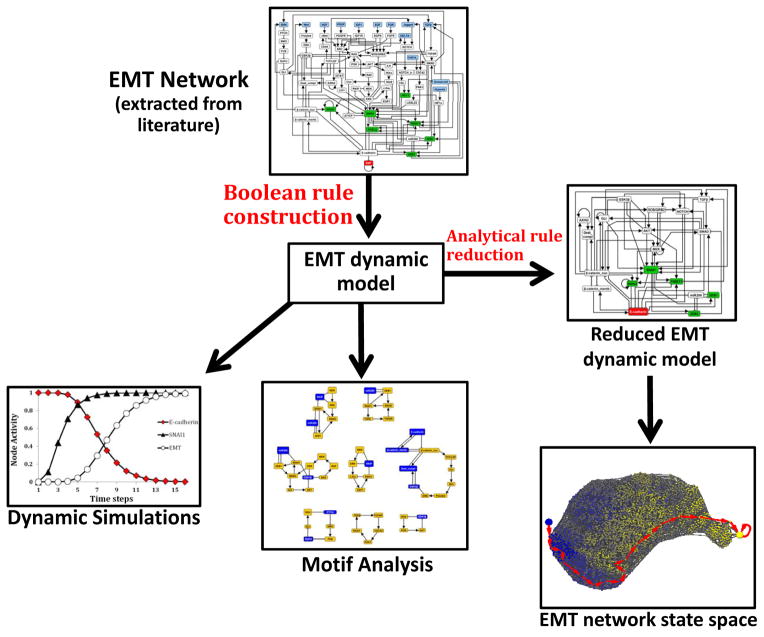
In this study, we explore TGFβ-driven EMT in HCC by an integrated experimental and computational approach (Figure 1). We found that TGFβ signaling is a conserved driver of EMT across multiple HCC models. We developed a network model of EMT signaling and then translated this network into a discrete dynamic model. The dynamic model recapitulates the known steps of TGFβ-driven EMT. The model predicts, and our experiments confirm, that joint activation of Wnt and SHH signaling by TGFβ is a conserved feature of TGFβ signaling in EMT. Our analysis suggests a putative reliance on these pathways for robust activation of the EMT program.
Figure 1. Flowchart of computational algorithms.
Our strategy was the following: we knew that TGFβ is a prominent signal driving epithelial-to-mesenchymal transition in hepatocellular carcinoma, so we started with synthesizing a network for this process from the literature. Then we added other known inducers of EMT and processes and components that are known to be deregulated during EMT. The resulting EMT network contains 70 nodes and 135 edges (EMT Network; Figure 3). We formulated a discrete dynamic model of this network by using our best estimation of the Boolean functions describing the regulation of each node. We then used simulations of the model to reveal the possible outcomes of TGFβ signaling in a cell that initially exhibits an epithelial phenotype (Dynamic simulations; Figure 4). To explore the full range of possibilities in terms of outcomes (steady states) the network can reach and ways in which these outcomes can be reached (trajectories), we performed a state space analysis. This analysis had to be preceded by network reduction to reduce the size of the network without disrupting its dynamic repertoire. State space analysis on the reduced network (Reduced EMT dynamic model; Figure 5) confirmed the results of the simulations. Furthermore, we used stable motif analysis to identify key feedback loops regulating EMT (Motif analysis; Figure 7A).
Materials & Methods
Cell culture
P2E and P2M cells were cultured in DMEM:F12 1:1 medium (Mediatech) supplemented with 10% fetal bovine serum (FBS) as described(15). Huh7 cells were provided by Dr. Harriet Isom (Penn State College of Medicine) and were cultured in DMEM + 10% FBS medium(16). Human HLE cells were acquired from Dr. Curtis Harris (National Cancer Institute) and cultured in DMEM + 10% FBS. HLF cells were acquired from Dr. Jorge Filmus (University of Toronto) and cultured in DMEM + 10% FBS(17). PLC/PRF/5 cells were acquired from the American Type Culture Collection and cultured in Eagle’s Minimum Essential Medium + 10% FBS. TGFβR inhibitor treatments were completed with LY2157299 (Selleck Chemical).
Cell Line Authentication
Huh7, HLE, and HLF cell lines were authenticated using short tandem repeat DNA profiling (Genetica DNA laboratories) on January 20th, 2014. PLC/PRF/5 cells were received directly from ATCC (July, 2013) and cultured for under three months so no authentication was deemed necessary.
Quantitative real-time polymerase chain reaction
Real-time PCR experiments were conducted as described previously(18). The housekeeping gene GAPDH was used for ΔΔ Ct calculations and relative expression was calculated using Taqman primer/probe sets (Life Technologies).
Western blot analysis
Lysates were harvested with lysis buffer as described previously(18). 50 μg of protein lysates were separated on a NuPAGE 4–12% Bis-Tris Gel (Invitrogen) and transferred to polyvinylidene difluoride (PVDF) membrane (Invitrogen). Primary antibodies for E-cadherin (Cell Signaling), pSMAD2 (Cell Signaling), pSMAD3 (Cell Signaling), SMAD2 (Cell Signaling), SMAD3 (Cell Signaling), βactin (Cell Signaling), GLI2 (Santa Cruz), and rabbit and mouse secondary antibodies (Cell Signaling) were used for this study. Signals were detected using Clarity chemiluminescence substrate on a ChemiDoc XRS+ imaging system (Bio-Rad).
Microarray gene set enrichment analysis (GSEA)
Murine HCC P2E and P2M cell line microarray datasets were acquired from NCBI Gene Expression Omnibus(15). Gene set enrichment analysis was performed to determine gene sets representing pathways and phenotypes enriched in P2M relative to P2E cell lines using datasets in the Molecular Signatures Database 4.0(19). Further details of GSEA are described in the Supplemental Text.
Patient samples
Human HCC samples were acquired from liver biopsy at Penn State Hershey Medical Center. Patients were on no cancer treatment at acquisition and were diagnosed as primary HCC. Specimens were flash frozen, and protein was extracted by homogenization of 100 mg liver tissue in 1 ml extraction buffer (150 mM NaCl, 50 mM Tris/HCl pH 7.4, 1 mM EDTA, 1% Triton 100, 0.1% SDS, 1% sodium deoxycholate, 1 mM sodium pyrophosphate, 10 mM β-glycerol phosphate, 10 mM NaF, 0.5 mM NaVO4, 1 μM Microcysteine-LR, and Protease inhibitor Cocktail Tablet without EDTA (1 tablet in 10 ml lysis buffer). Samples were incubated on ice for 30 minutes and vortexed every 5 minutes. Samples were centrifuged at 15,000 rpm at 4°C for 15 minutes. Protein (supernatant) was collected and quantified using BCA Protein Assay (Thermo Scientific).
Computational methods
An overview of the computational methodology is included in Figure 1 and in the Quick Guide to Equations and Assumptions. Network simplification was performed using analytical procedures(20) and with NET-SYNTHESIS, a signal transduction network inference and simplification tool(21). The simulations of the model and the state space analysis were performed using custom Java code and Python code (BooleanNet Python library)(22). The feedback loops responsible for the steady state of the EMT network were identified by stable motif analysis. This method is based on the principle that a certain group of nodes that forms a network motif with identifiable properties stabilizes into a single state regardless of the rest of the network. The specific criteria for identifying stable motifs were described previously (23), and adaptation to our network is described in the Supplemental Text. The stable motif analysis was implemented in a custom Java code. The networks in Figure 3, Figure 5A, Figure 5C, Figure 7A, Figure 7C and Supplemental Figure 3 were created using the yEd graph editor by yWorks (http://www.yworks.com/). The state transition network in Figure 5B was generated with Cytoscape(24) by using the Prefuse force directed layout.
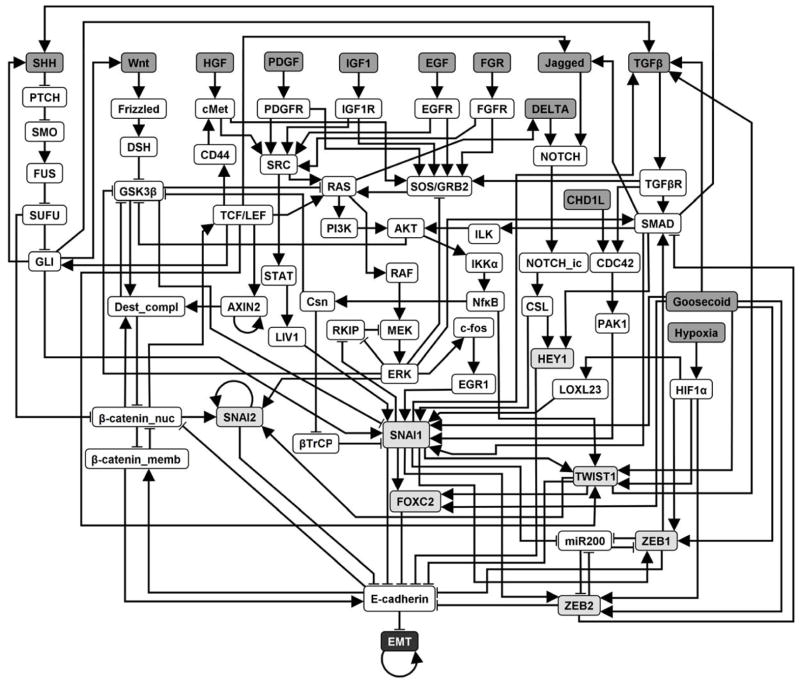
Figure 3. The 70 node, 135 edge network model representing the epithelial-to-mesenchymal transition (EMT) signaling network in HCC.
Nodes represent molecular entities (proteins, small molecules, mRNAs) and edges represent activating and inhibitory relationships between nodes. Upstream signals (black text, dark gray fill) regulate transcriptional regulators (black text, light gray fill) through signal transduction pathways, which all converge on the regulation of E-cadherin. The output of the network, “EMT”, is indicated by a symbol with black background and white text. The full names of the nodes are indicated in Supplemental Table 1. References for each edge in the network are indicated in Supplemental Table 2. The Boolean rules describing the dynamic EMT network model are given in Supplemental Table 3.
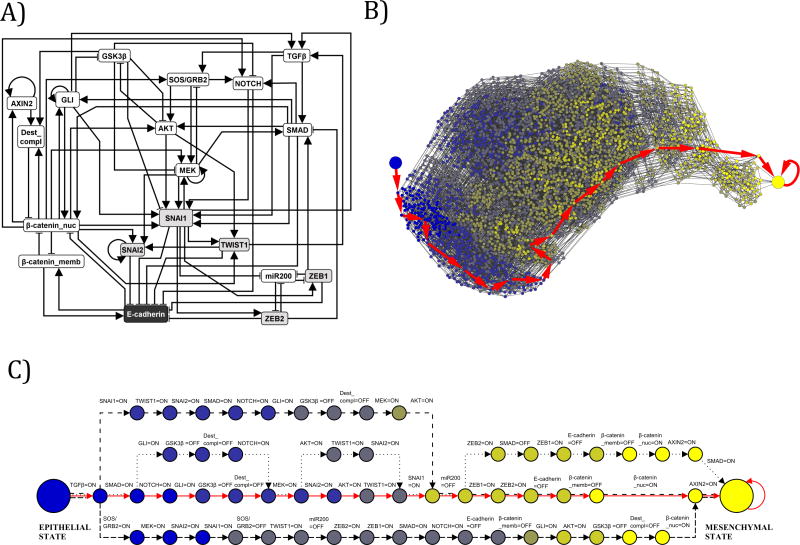
Figure 5. Network reduction and state space analysis of the EMT network.
A) Multiple network reduction techniques were applied to the 70 node, 135 edge EMT network to reduce it in size to a 19 node, 70 edge EMT network. This reduction maintains the network consistent with causal experimental observations, while permitting state space analysis that was not feasible on a larger network. The symbol shadings are the same as on Figure 3. The Boolean rules for the reduced EMT Network are included in Supplemental Table 5. B) In the reduced 19 node network, TGFβ was fixed as ON and the partial state transition network was explored from the initial epithelial condition, using a ranked asynchronous updating algorithm. These findings demonstrated that a single EMT steady state is realized through multiple trajectories, and that this steady state results from constitutive TGFβ signaling without dysregulations of any other nodes in the EMT network. This partial state transition network has 3,489 nodes (states) and 16,434 edges. Nodes are colored according to the number of stable motifs they have activated, with blue representing no stable motifs and yellow representing all of the stable motifs. The large blue node represents the initial epithelial condition just before TGFβ is turned ON, and the large yellow node represents the EMT steady state. The most probable trajectory in the state transition network is highlighted with large red arrows. All possible trajectories lead to the EMT steady state. C) The four most common trajectories in the state transition network, starting from the epithelial state (large blue node on the left) and terminating at the mesenchymal steady state (large yellow node at the right). Each node represents a network state. Each edge represents a transition between two states. Since a general asynchronous updating algorithm is implemented, a single node turns ON or OFF during each state transition; this node and its corresponding new state are indicated above the edge. For example, the second transition of all four trajectories corresponds to SMAD turning ON from an initially OFF state in the epithelial initial condition. The transitions corresponding to each of the four trajectories are identified by different line types (solid red, dashed, dash-dotted, dotted).
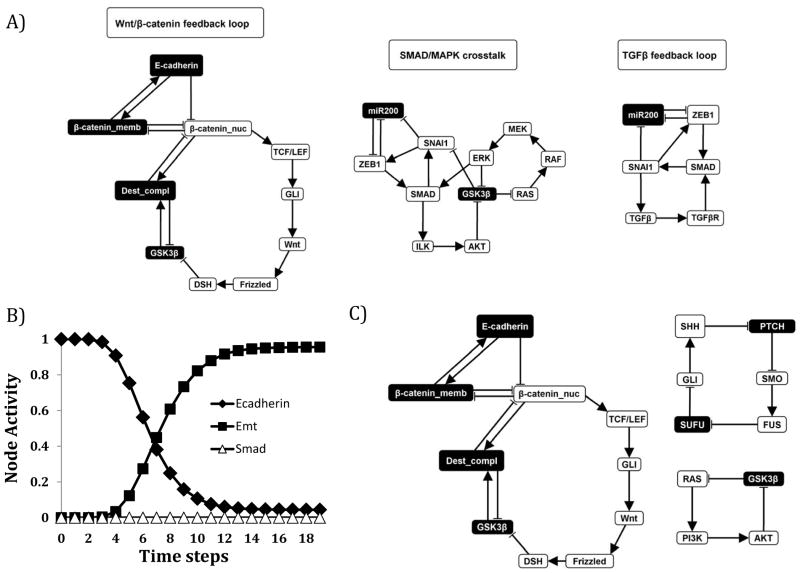
Figure 7. Network motifs that stabilize in the EMT=ON steady state.
The color of the node indicates the node’s stabilized state: Black node = OFF; White node = ON. A) The left-most motif represents a Wnt/β-catenin feedback loop in which cross-talk with GLI stabilizes low E-cadherin expression. The middle motif is a feedback loop consisting of SMAD/MAPK cross-talk, which suppresses GSK3β a negative regulator of Wnt signaling. The right-most motif is a TGFβ signaling feedback loop, which drives SMAD activity. B) Network analysis supports that SMAD-dependent and SMAD-independent mediators are important for TGFβ-driven EMT. SMAD knockout has a nominal effect on TGFβ induction of EMT (10,000 simulations, asynchronous update). C). Network motifs stabilizing the EMT steady state in the presence of SMAD knockout (when TGFβ=ON and SMAD=OFF). Targeting these motifs may lead to effective combinations with SMAD inhibition to disrupt TGFβ-driven EMT.
Results
TGFβ signaling is a conserved driver of EMT in multiple liver cancer models
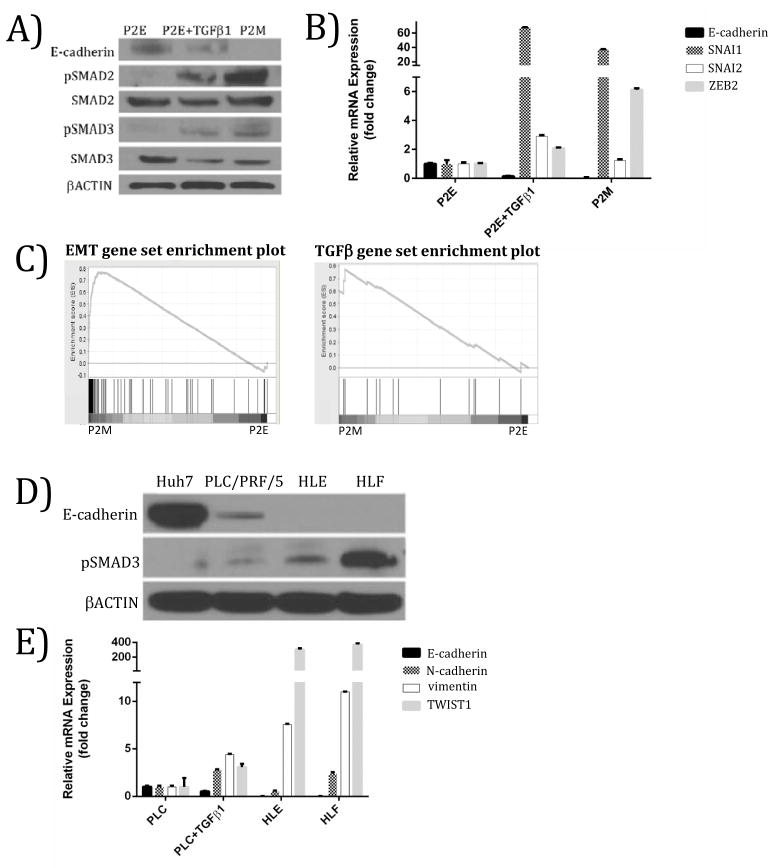
Our initial studies involved development of a novel murine model of epithelial-to-mesenchymal transition. This involved derivation of “sister” epithelial (P2E) and mesenchymal/metastatic (P2M) cell lines from a PTEN-/- mouse(15). Consistent with other carcinoma cell lines, treatment of the P2E cell line with TGFβ induced TGFβ signaling (Figure 2A) and led to induction of EMT markers (Figure 2B). These results demonstrate that activation of TGFβ signaling recapitulates the mesenchymal phenotype in a murine model of HCC. They also led us to hypothesize that the TGFβ pathway is constitutively active in P2M cells as compared to P2E cells.
Figure 2. Active TGFβ signaling correlates with mesenchymal phenotype in murine and human models of HCC.
(A) Treatment of a mouse epithelial HCC cell line (P2E) with TGFβ at 5 ng/ml for 48 hours produces a molecular phenotype similar to mouse mesenchymal HCC cell line (P2M). (B) Up-regulation of mRNA levels of EMT markers in mouse epithelial HCC with TGFβ treatment (5 ng/ml for 48 hours). (C) Gene set enrichment analysis (GSEA) supports P2M cells are enriched for mesenchymal markers relative to P2E cells (left panel). GSEA reveals that TGFβ signaling is enriched in P2M versus P2E cells, suggesting that TGFβ may be driving the EMT phenotype in this mouse model of HCC (right panel). This gene set contains genes up-regulated by TGFβ1 in MCF10A cells. (FDR = 0.031). (D) Human mesenchymal HCC cell lines HLE and HLF have low E-cadherin expression and high pSMAD3, whereas epithelial-like HCC cell lines Huh7 and PLC/PRF/5 (PLC) express high E-cadherin and low pSMAD3. (E) Relative to the PLC/PRF/5 cell line, PLC/PRF/5 treated with TGFβ (10 ng/ml, 48 hrs), HLE, and HLF cell lines exhibited lower E-cadherin and higher vimentin, and TWIST1 expression. FDR = False discovery rate.
We tested this hypothesis by gene set enrichment analysis (GSEA), a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between two phenotypes, in this case P2E and P2M cell lines(19). The primary result of GSEA is the enrichment score, which shows the degree to which a gene set is over-represented at the top or bottom of a list of genes ranked by their association with a phenotype (see Methods). An EMT gene set representing genes up-regulated during EMT induced in a mammary epithelium cell line transformed by HRAS (EpH4 line)(25) was used to probe for the EMT phenotype in P2M versus P2E cells. The use of a breast cancer reference set is reasonable because EMT is a conserved process over multiple tissue types(26). A TGFβ gene set representing genes up-regulated by TGFβ1 in MCF10A immortalized non-tumorigenic mammary gland cells(27) was used to probe for TGFβ pathway activation in P2M versus P2E cell lines. The top portion of the plot shows the running enrichment score and a maximum enrichment score of 0.78 for the EMT gene set and 0.76 for the TGFβ gene set (Figure 2C). In the bottom portion of the plot, P2M cells are represented on the left of the figure and P2E on the right. The position of the members of the gene set in the ranked list of genes demonstrates distinct genes associated with EMT and TGFβ sets. These data support up-regulation of EMT and TGFβ pathway activation gene sets in the P2M cell line relative to P2E (Figure 2C).
In order to explore the prevalence of dysregulated TGFβ signaling in HCC, we tested established human HCC cell lines for TGFβ pathway and EMT markers. Human HCC cell lines Huh7, PLC/PRF/5, HLE, and HLF cell lines were tested for epithelial and mesenchymal markers to characterize their phenotype. Huh7 and PLC/PRF/5 cells have higher E-cadherin and lower pSMAD3 compared to HLE and HLF cell lines by western blot analysis (Figure 2D). TGFβ-treated PLC/PRF/5 cells, HLE and HLF HCC cell lines have lower E-cadherin, higher vimentin (VIM), and higher TWIST1 compared to PLC/PRF/5 cells as indicated by qRT-PCR (Figure 2E). Compared to PLC/PRF/5 cells, TGFβ treated PLC/PRF/5 cells and untreated HLF cells have higher N-cadherin expression (Figure 2E). These results indicate that the Huh7 and PLC/PRF/5 cell lines have an epithelial-like phenotype, the HLE and HLF cell lines have a mesenchymal phenotype, and that TGFβ treatment induces a mesenchymal-like phenotype.
In order to study the effect of TGFβ pathway activity on the EMT phenotype of the mesenchymal-like HLE and HLF cell lines, these cell lines were treated with TGFβRI inhibitor LY2157299 and EMT markers were assessed by western blot and qRT-PCR. TGFβR1 inhibition with LY2157299 reduced SNAI1, TWIST1, N-cadherin (CDH2), and vimentin mRNA expression, while increasing E-cadherin expression in HLE cells (Supplemental Figure 1). These data support the conclusion that the mesenchymal phenotype of HLE and HLF cell lines is due to constitutive TGFβ signaling. Overall, our data demonstrate that TGFβ axis dysregulation is a conserved driver of mesenchymal phenotype in multiple models of HCC.
Constructing the signaling network that drives epithelial-to-mesenchymal transition
In order to explore the systems-level dynamics of TGFβ signaling and EMT, a network modeling approach was used. Evidence supports the concept that a conserved EMT mechanism, driven by multiple signals, occurs in development and in cancer. These signals appear as a normal part of development, playing a crucial role in formation of the body plan and in differentiation of multiple tissues and organs(6). During carcinoma progression, EMT signals (e.g. TGFβ) are aberrantly produced and hijack this developmental process(28). In order to construct our network, we performed an extensive literature search and synthesized pre-existing information representing the conserved regulation of EMT. We focused on interactions from HCC EMT; however information about EMT in other tissue types was also used when no HCC-specific information was available. The resulting network (Figure 3) spans heterogeneous molecular processes induced by growth factors, signal transduction pathways, and transcriptional regulators. This network includes the dysregulation of the TGFβ/TGFβR axis as a driver of EMT, as this is a commonly mutated pathway in HCC(9).
The information used to construct the network is summarized in Supplemental Tables 1 and 2. Proteins, mRNAs, and microRNAs were represented as nodes (Supplemental Table 1) and the pairwise relationships among nodes (Supplemental Table 2) were represented as edges. We choose as the output node of the network the concept node “EMT”, serving as the major indicator of cell fate in our model. E-cadherin plays a crucial and direct role in EMT at diverse developmental stages and during carcinoma progression, and its loss is widely considered as the hallmark of EMT(6). Furthermore, the regulation of E-cadherin expression is much better understood than that of other EMT markers(29). Thus we focus on E-cadherin as the negative regulator of the output node of the network, “EMT”. This network contains 70 nodes and 135 edges (Figure 3).
Translating the EMT signaling network into a dynamic model
To understand the dynamics of signaling abnormalities in HCC progression, invasion, and metastasis, we translated the EMT signaling network into a dynamic model. A dynamic model is used to express the behavior of a system over time by characterizing each node by a state variable (representing, e.g., its concentration or activity) and a function that describes its regulation. Dynamic models can be categorized as continuous or discrete, according to the type of node state variable used. Continuous models use a set of differential equations; however, the paucity of known kinetic details for biological interactions makes these models difficult to implement.
We employed discrete dynamic modeling because of its computational feasibility and capacity to be constructed with qualitative biological data. Specifically, we constructed a Boolean model wherein each network node is described by one of two qualitative states: ON or OFF. The ON state means an above threshold concentration of a molecular regulator whereas the OFF state means the below-threshold or inactivated form. We formulated a regulatory function for each node using its upstream regulators encapsulated in the network, evidence regarding the conditionality among these regulators, and known biological outcomes. During the construction of the regulatory functions we verified that individual modules within the network reproduced known outcomes (Supplemental Table 4). If modules did not reproduce known outcomes, we modified the rules in such a way that the known outcomes can be reproduced(30). See the Quick Guide to Equations and Assumptions and Supplemental Text for more detailed information and example rule implementations.
As in the biological system, there is a time lag between the state change of the regulators and the state change of the targets. This time lag is implemented by using discrete update events, each update meaning that the state of a node is re-calculated according to its Boolean equation. We used an updating scheme that differentiates between the two major types of biological regulation in the model: transcriptional events and signal transduction events. Although we do not know the specific kinetics of individual events, we do know that signal transduction events occur substantially faster (in seconds or faster) than transcriptional events (timescale of minutes)(31). Thus, we used a ranked asynchronous updating algorithm that updates nodes regulated by signal transduction events with a greater probability than nodes regulated by transcriptional events. This allows us to stochastically explore differences in the kinetics among molecular interactions in our network while satisfying known kinetic relationships(14). The details of the ranked asynchronous algorithm are described in the Supplemental Text.
To reproduce how a potentially diverse population of cells responds to the same signal we performed multiple simulations with the same initial conditions but different updating orders (i.e. different timing). The output of the model is the fraction of simulations in which a certain node is in the ON state, which we denote the activity of the node. We choose as time unit (step) the average number of updates needed to update a transcriptionally regulated node. This time step thus corresponds to several minutes in real time. We empirically determined that 10,000 simulations and 20 time steps in each simulation are sufficient for convergent and reproducible results (see Supplemental Text for further explanation).
The EMT network model reproduces known TGFβ-driven dysregulations during epithelial-to-mesenchymal transition
We focus on TGFβ as the prototypical inducer of EMT as the TGFβ signaling axis is disrupted in 40% of HCC(9) and TGFβ is a well-established inducer of EMT in HCC and other carcinomas(8). First, we set the states of the nodes according to their known or expected states in a prototypical epithelial cell. Specifically, known signals/pathways that could induce EMT, including TGFβ FGF, IGF, EGF, PDGF, Wnt/β-catenin, SHH/GLI, and HGF/cMet, were set to OFF. Downstream signal transduction pathways known to drive EMT, such as MAPK, AKT, SMAD, and STAT signaling, were also turned OFF. E-cadherin and miR200 were assumed to be ON and the E-cadherin transcriptional repressors were assumed to be OFF. Our model indicates that without any stimulus or dysregulation, the cell would remain in this epithelial state (listed in Supplemental Table 3).
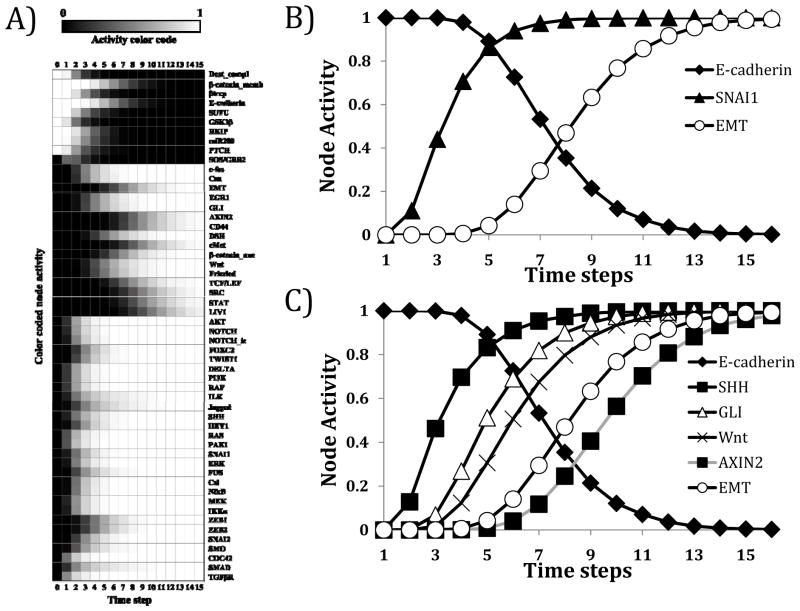
Next, in order to recapitulate a sustained TGFβ signal, the state of TGFβ was fixed in the ON state at the beginning of every simulation (see “Boolean rule over-rides” in the Supplemental Text), while the other nodes were in their epithelial initial condition. Turning TGFβ ON led to changes in the activity of nodes in the network over the time course of the simulation and ultimately led to a steady state in which the state of all nodes stabilized (Figure 4A). If the state of a node stabilized at ON at the end of the simulation, even though it was OFF at the beginning, we infer that it is activated in the steady state. If the state of a node was OFF even though it was in the ON state at the beginning of the simulation, we consider it inactivated in the steady state. Using this interpretation, the obtained steady state agrees with known markers of a mesenchymal state. The heatmap in Figure 4A and the time courses shown on Figure 4B qualitatively reflect experimental outcomes seen when starting from an epithelial condition and inducing EMT. Specifically, they reflect how TGFβ signals through TGFβRI/II and induces SMAD complex formation and non-canonical pathways including MAPK and AKT. This leads to induction of E-cadherin transcriptional repressors and ultimately to the loss of E-cadherin (Figure 4A and Figure 4B). The agreement of our network model with known experimental outcomes of TGFβ-driven EMT supports the validity of our model.
Figure 4. Network modeling reproduces established signaling abnormalities seen in TGFβ-driven epithelial-to-mesenchymal transition and predicts novel cross-talk mechanisms.
A) Heat map representing the activity (averaged state) of all nodes according to the model (10,000 simulations, asynchronous update). The initial state corresponds to a prototypical epithelial state; the final state indicates a TGFβ-driven EMT steady state. Nodes whose state did not change during the simulation are not included. Node activity level of 0 (black square) indicates that the node is inactive (OFF) in all the simulations. Node activity level of 1 (white square) indicates that the node is active (ON) in all the simulations. B) Time-lapse dynamic simulation (10,000 simulations, asynchronous update) reveals induction of SNAI1 expression and loss of E-cadherin expression during induction of EMT from an epithelial initial condition. C) Time-lapse dynamic simulations (10,000 simulations, asynchronous update) reveal induction of Sonic Hedgehog signaling and downstream GLI expression, Wnt signaling and AXIN2 expression with induction of EMT from an epithelial initial condition.
In order to explore the possibility of other steady states besides the previously described EMT steady state we performed a state space analysis of the model. The state space can be represented as a network (the state transition network), whose nodes represent the system’s states and whose edges represent every state transition that is possible for the given system. The state transition network summarizes all the possible trajectories or ways that a network state can lead to another network state. Steady states of the system are states that only have transitions leading to them but not leading out of them. The state space grows exponentially with the size of the network, making it impossible to fully map for large networks. To decrease the state space, we performed network simplification of the 70 node, 135 edge EMT network in such a way that the network remains consistent with causal experimental observations (See Supplemental Text for details) (32). This network reduction has been shown to have no effect on the permitted dynamic behaviors of a system(33). This led to a network with 19 nodes and 70 edges (Figure 5A and Supplemental Table 5).
In the reduced 19 node network, TGFβ was fixed as ON, and the part of the state space reachable from the otherwise epithelial initial condition was determined, using a ranked asynchronous updating algorithm. The resulting partial state transition network has 3,489 nodes (states) and 16,434 edges (state transitions) (Figure 5B). Only one node of the state transition network has no outgoing edges, the node corresponding to the EMT steady state. Thus this steady state is the only possible outcome for an epithelial cell in the presence of sustained TGFβ signaling; no other steady states were discovered. This steady state can be reached through many thousands of trajectories, each corresponding to a different set of state transitions. For example, the four most common trajectories (Figure 5C) include E-cadherin loss prior to nuclear localization of β-catenin, or following it. As trajectories in the model reflect the order of events possible under different kinetics, their convergence into a single outcome suggests that the TGFβ-driven EMT outcome is robust across all reasonable values of kinetic parameters. We note that none of the scenarios to reach EMT encapsulated in the state transition network involve specific dysregulations (e.g. mutational gain or loss of function) of any nodes in the EMT network. The presence of constitutive TGFβ signaling drives all dysregulations we see in our model.
Network modeling reveals that the constitutive presence of TGFβ induces Wnt and SHH signaling during epithelial-to-mesenchymal transition in hepatocellular carcinoma
Interestingly, our dynamic model of signaling induced by TGFβ activation revealed joint activation of Wnt and Sonic hedgehog (SHH) signaling pathways during induction of EMT from an epithelial initial condition (Figure 4A and Figure 4C). In order to test the predictions from our model we explored molecular readouts of Wnt and SHH signaling. AXIN2 is a well-established transcriptional marker of Wnt/β-catenin/TCF signaling and is a negative regulator of the Wnt signaling pathway(34). Furthermore, members of the GLI family of transcription factors (represented by the node GLI in our model) are major effectors of the SHH signaling pathway, and their mRNA expression can be used as a readout of SHH signaling(35). GLI2 is the major transcriptional activator of the SHH signaling pathway, with GLI2 itself undergoing transcriptional up-regulation when SHH signaling is active(36). Most evidence supports that GLI1 is activated transcriptionally by GLI2(37). Indeed in our model, simulations (10,000 simulations, asynchronous update) starting from an epithelial state in the sustained presence (ON state) of Wnt led to AXIN2 expression (Supplemental Figure 2A) and the presence of SHH in the same initial state drove GLI expression (Supplemental Figure 2B). These results affirm that our model reproduces known molecular readouts of Wnt and SHH signaling.
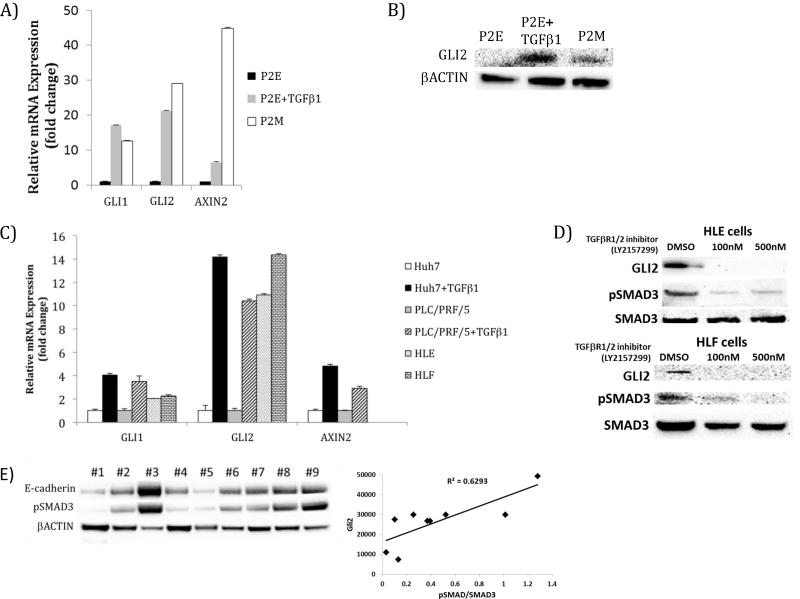
As shown in Figure 4C, TGFβ signaling induces both AXIN2 and GLI (with a delay compared to SHH) in our model. In order to experimentally explore the activation of Wnt and SHH signaling pathways by TGFβ, we tested TGFβ-driven expression of pathway markers using epithelial and mesenchymal cell lines. Treatment of the P2E cell line (murine epithelial HCC cell line) with TGFβ leads to induction of GLI2 mRNA and protein expression and induction of AXIN2, approximating the levels constitutively observed in mesenchymal P2M cells (Figure 6A and Figure 6B). TGFβ treatment drives GLI1 and GLI2 expression in human epithelial-like HCC cell lines Huh7 and PLC/PRF/5 cells. Furthermore, AXIN2 is up-regulated by TGFβ in Huh7 and PLC/PRF/5 cells after TGFβ treatment. Additionally, human mesenchymal HCC cell lines HLE and HLF exhibit up-regulated GLI expression relative to epithelial PLC/PRF/5 cells (Figure 6C). Inhibition of TGFβ signaling with a chemical inhibitor of TGFβRI/II diminished pSMAD3 and GLI2 protein expression in the mesenchymal HLE and HLF cell lines (Figure 6D). Thus our measurements validate the model prediction that Wnt and SHH signaling is induced by TGFβ during induction of EMT.
Figure 6. Network model-directed testing validates activation of Sonic hedgehog and Wnt signaling pathways by TGFβ.
A) GLI and AXIN2 transcripts are elevated in murine mesenchymal cell line P2M and by TGFβ treatment (5 ng/ml, 48 hours) of murine epithelial HCC cell line, P2E. B) GLI2 expression is elevated by TGFβ1 treatment (5 ng/ml, 48 hours) of epithelial P2E cells and in mesenchymal P2M cells relative to P2E cells. C) Human epithelial-like HCC cell line Huh7 has low expression of GLI1, GLI2, and AXIN2 and these transcripts are increased by TGFβ1 treatment (5 ng/ml, 48 hours). Epithelial-like PLC/PRF/5 cells treated with TGFβ1 (10 ng/ml, 48 hours) exhibit increased GLI1 and GLI2 expression. GLI1 and GLI2 expression were also elevated in mesenchymal phenotype cell lines with constitutively active TGFβ signaling, HLE and HLF. Treatment of PLC/PRF/5 cells with TGFβ1 (10 ng/ml, 48 hours) led to increased AXIN2 expression. D) Treatment of HLE and HLF cells with TGFβRI inhibitor, led to dose dependent loss of GLI2 levels. E) In protein extracted from nine human HCC patient biopsies, GLI2 expression correlated with pSMAD3 levels (R2= 0.6293).
To further test the joint activation of SHH signaling pathways by TGFβ, we determined the correlation between TGFβ and GLI2 expression in human HCC patient samples. Indeed, SMAD3 phosphorylation correlated positively (R2= 0.629) with GLI2 expression in these samples (Figure 6E). This information supports the network model’s prediction that induction of GLI2 occurs in human tumors with active TGFβ signaling.
For a deeper analysis of the nodes associated with steady states in the EMT network, a stable motif analysis was performed. This analysis is based on finding feedback loops that stabilize in a fixed state (23). Our findings further demonstrate the existence of a single EMT steady state (i.e. the state shown on Figure 4A). There are eight motifs associated with this steady state (Figure 7A and Supplemental Figure 3). These motifs represent interaction and node activity patterns whose sustained presence is sufficient to stabilize the mesenchymal state. The motif shown on the left panel of Figure 7A indicates that sustained Wnt and GLI signaling and a low level of GSK3β can maintain a low level of E-cadherin, the hallmark of the EMT steady state. The motif in the middle panel of Figure 7A indicates that a low level of GSK3β can be sustained by cross talk between MAPK and SMAD signaling. The motif on the right side of Figure 7A indicates that SMAD signaling can be activated by the TGFβ signal in our model, which itself can be sustained by positive feedback. Read from right to left, these motifs are consistent with the propagation of the TGFβ signal that leads to SMAD and SNAI1 activation, then to the activation of the SHH and Wnt pathways, and then to the loss of E-cadherin. However, because each stable motif is independent of the others and is sufficient to maintain the EMT steady state, the results shown on Figure 7A suggest that other, potentially TGFβ– independent, mechanisms that lead to the stabilization of a motif (e.g. MAPK signaling) can also drive EMT. Indeed, evidence for the induction of EMT through MAPK pathway activation of SNAI1 has been reported in other studies(38). In our model, MAPK signaling can drive EMT, independent of TGFβ (data not shown). Taken together, our studies demonstrate that activation of SHH and Wnt pathways are a key conserved feature of hepatocellular carcinoma EMT.
Discussion
We began our studies when we realized that TGFβ signaling mimics the molecular phenotype of a murine mesenchymal cell counterpart (P2M) to an epithelial HCC cell line (P2E). We next identified other HCC cell lines with active TGFβ signaling and demonstrated that TGFβ-active cell lines also had mesenchymal features. These results suggest TGFβ signaling is a conserved dysregulation and driver of EMT across multiple HCC models. In order to better understand the complex signaling events producing the TGFβ-driven EMT phenotype, we curated signaling pathways involved in developmental and carcinoma EMT programs in HCC and compiled them into an EMT signaling network. By formulating a Boolean dynamic model of this network, we were able to identify downstream signaling events involved in the TGFβ-specific EMT process and detect cross-talk among pathways. As EMT and TGFβ signaling are conserved features of many solid tumor types, this model (or modifications of it) may be used to study EMT in other tumor types.
TGFβ plays a dual role as a tumor suppressor and oncogene; however it remains unclear why. We argue that in the cell, network-level emergent outcomes (e.g. tumor suppression versus tumor growth) are not attributable to the TGFβ signal alone, but are dependent on the status of the entire cellular network that the signal acts upon. Network models are increasingly used to understand the organization and emergent properties of biological systems(39, 40). Network-based discrete dynamic models are particularly useful in poorly characterized biological systems, where they can be used to generate testable hypotheses(41). Boolean network models have led to new insight into signal transduction and gene regulatory networks in numerous organisms(14). Network modeling can explain why in certain differential contexts (e.g. for different states of a few nodes other than TGFβ), the TGFβ signal produces different outcomes, which can then be investigated experimentally.
Perturbations in signaling pathways that define a disease or a specific feature of a disease can result from deregulation of only a subset of these pathways. However, identifying such a subset is difficult using traditional biological approaches. In this study, we assessed this question in EMT, a mechanism that drives invasion and metastasis in solid tumors, by using a Boolean dynamic model. Through network simulations, we revealed that the TGFβ signal could percolate across multiple signaling pathways, leading to a network of perturbed proteins during the induction of EMT. Across multiple murine and human HCC models, the constitutive presence of TGFβ was shown to be sufficient to induce Wnt and SHH signaling pathways in this context. The model’s result regarding the order of induction of the Wnt and SHH pathways obtained from the model may depend on the broad classification used for the time lags (transcriptional versus signal transduction events). A future experimental determination of the order of pathway activation may support the model result or may contradict it, in which case further timing constraints could be incorporated to establish agreement. Imposing timing constraints would affect how fast the TGFβ signal propagates through the network but it would not affect the network’s steady states.
It should be noted that TGFβ, Wnt and SHH signaling pathways have individually been implicated in oncogenic processes, including EMT in carcinomas. For example, Wnt pathway induction of EMT has been demonstrated in multiple cancer types(42). Furthermore, SHH can induce EMT in brain and gastrointestinal cancer models, among others(43). It has recently been suggested that the critical interaction between TGFβ and Wnt signaling pathways deserves special attention(44). Here we present a systems level explanation of the interaction of TGFβ signaling with the Wnt and SHH signaling pathways. How these signaling pathways act as a network to integrate a signal for a specific biological process has not been realized by previous studies. Our stable motif analysis revealed that the SHH/GLI and Wnt components of the EMT network are critical nodes in feedback loops that maintain the EMT steady state (Figure 7A).
EMT is a process that occurs throughout development and is hijacked by carcinomas during solid tumor progression. Tumor metastasis is the leading cause of mortality in solid tumors. Our model provides a strong foundation for the modeling of HCC invasion. As hepatic tissue lacks a membrane barrier, it has been speculated that EMT is the major mechanism for the entry of migratory HCC cells into systemic circulation(3). To describe carcinomas of epithelia in other tissues, which do have a basement membrane, our model can be expanded by adding the pathway(s) regulating the proteolytic destruction of the basement membrane by matrix metalloproteinase proteins(45). Another possible extension is an agent-based model in which each cell is an agent with a specific morphology. Such a model could incorporate our Boolean signal transduction model, the initial epithelial architecture, as well as the reorganization of the actin cytoskeleton of each cell to enable directional motility and dynamic cell elongation(46).
Understanding the systems-level dysregulations in EMT is important not only to elucidate tumor progression and the metastatic process but also to develop therapeutic interventions to halt/suppress metastatic disease. Our analysis suggests that single interventions may not be effective. For example, SMAD proteins are the canonical downstream targets of TGFβ signaling. However, our model predicts that inhibiting SMAD proteins alone cannot completely block TGFβ-driven EMT (Figure 7B). Indeed, among the motifs that can sustain an EMT=ON state there is a TGFβ feedback loop that does not include SMAD (Supplemental Figure 3). Stable motif analysis with TGFβ=ON and SMAD=OFF (a TGFβ active network with no SMAD signaling) reveals multiple feedback loops that can maintain EMT in the absence of SMAD signaling (Figure 7C). This implies that combining SMAD inhibition with Wnt, SHH, AKT, or MAPK inhibition may more effectively suppress EMT. Similar computational analyses have been completed on smaller networks, supporting a role for feedback loops in the regulation of EMT(29), EMT plasticity(47), and tumor cell heterogeneity (non-genetic heritability)(48).
Combinatorial therapeutics is an emerging discipline in the treatment of cancer patients. Recent work indicates that targeting multiple oncogenic signals through combination of cancer therapies is superior to single therapy treatment(49). The development of effective combined therapeutics is an important goal within this field. Developing dynamic models of cancer networks to simulate single and combinatorial interventions targeting specific nodes may prove to be a rational approach to more efficiently choose single and multi-drug therapeutic regimens.
In summary, we used network analysis and Boolean modeling to investigate the signaling abnormalities that arise during EMT in the context of TGFβ signaling and confirmed our network-based results in multiple experimental liver cancer models (Figure 1). Our systems biology approach was able to maximize the use of the available pathway information and to identify putative activation of major signaling pathways during EMT and liver cancer invasion. This study confirms the ability to integrate normal and disease pathway information into a single model that is robust enough to reproduce a clinically relevant complex process yet is constructed with qualitative information.
Supplementary Material
Acknowledgments
Financial support. NIH F30 DK093234 (Steinway) NIH R01 CA018138 (Feith), NIH R01 CA098472 (Loughran), NIH P01 CA171983 (Loughran), NIH R01 CA170334 (Loughran), Leukemia and Lymphoma Society Translational Research Program 286818 (Loughran), NSF IIS 1161001 (Albert), NSF PHY 1205840 (Albert) and NSF DMS 1313115 (Albert), NSF MCB 1121612 (Albert)
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number F30DK093234. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Hien Dang (NIH/NCI) for training and technical expertise at the onset/during pilot studies. We would like to thank Dr. Xin Liu (Penn State College of Medicine) for antibodies.
Major Findings
We constructed a comprehensive cellular signaling network regulating epithelial-to-mesenchymal transition (EMT) from the literature. We used network modeling to study in silico the network level signaling events that occur during induction of EMT driven by transforming growth factor beta (TGFβ Using these methods, we identify novel pathway activation and feedback loops that regulate the EMT process. We confirm these findings experimentally in murine and human liver cancer models. Our analysis has implications for developing therapeutic interventions to halt/suppress metastatic disease.
Quick Guide to Equations and Assumptions
Major Assumptions of the Model
The Boolean modeling approach is an abstraction that aims to explain the qualitative characteristics of dynamic systems. The activation of a biological node is represented as a switch in a Boolean model; the node can be either ON or OFF and there are no intermediate levels of activation. This is a reasonable assumption when a biological node exhibits two qualitatively different states and the transition between these two states is switch-like.
The regulatory relationships among nodes can be described with the Boolean operators AND, OR, and NOT. If a node has a single regulator then the regulated node’s state is solely determined by its upstream node’s state. OR represents the combined effect of independent and individually effective upstream regulators on a downstream node, whereas AND indicates the joint requirement for multiple upstream regulators to achieve a downstream effect. NOT represents the effect of inhibitory regulators and can be combined with other regulation by using either OR or AND. Our baseline assumption is that multiple regulators of the same target node act independently of each other. However, if there is evidence of biological synergy (e.g. two proteins form a complex to perform a biological function), then an AND operator was used. We describe three Boolean equations in the section “Key Equations” below; two additional examples are given in the Supplemental Text.
We do not know the kinetics of individual events, although we do know that signal transduction events occur substantially faster (seconds or faster) than transcriptional events (timescale of minutes)(31). Thus, we employ a stochastic asynchronous updating scheme with a ranking system. We randomly select a single node and update its state, meaning that we re-calculate its state according to its Boolean equation. In order to account for signal transduction events occurring faster than transcriptional events, we update nodes regulated by signal transduction events with a greater probability than nodes regulated by transcriptional events. The results are robust to changes in the value of this probability (see Supplemental Figure 4). By performing a large number (10,000) of simulations with a stochastic update sequence, we identify the behaviors that do not depend on the detailed kinetics of the individual reactions. We define a time step as the average number of updates needed to update a transcriptionally regulated node.
Dynamic Boolean models do not require kinetic parameters, which makes them more computationally feasible than quantitative models, especially for biological systems with more than a dozen components. Boolean models assume that the structure of the biological networks they describe is more important than the kinetics of individual reactions and acquire their richness through the large number of interactions that are included in the networks they describe.
Key Equations
An explanation of the Boolean regulatory functions of two key nodes is included below. Each equation indicates the future state (shown by the use of the asterisk) of a regulated node as a function of the current states of the nodes that regulate it (i.e. nodes that are the starting points of edges incident on the regulated node). For simplicity we denote the node state by the node name, thus “node X” in a rule means “the state of node X”, and “not node X” means “the state that is the opposite of the state of node X”.
We start by a hypothetical example describing the transcriptional regulation of a target gene by a transcriptional activator (A) and a transcriptional inhibitor (I). We denote the transcript of the target gene as T.
| (Eq. 1) |
The Boolean equation indicates the condition under which the future state of the target gene’s transcript is ON. The right hand side of the equation specifies that this condition is that the transcriptional activator A is ON and simultaneously the transcriptional inhibitor I is OFF. If this condition is not satisfied, then T decays (gets turned OFF), even if it was ON before.
| (Eq. 2) |
| (Eq. 3) |
β-catenin_memb is a node representing membrane-bound β-catenin (CTNNB1). β-catenin is degraded due to an active “destruction complex” (represented by the Dest_compl node) formed by APC, AXIN1 or AXIN2, and GSK3β. The destruction complex targets free (cytoplasmic and nuclear) β-catenin for degradation(50); thus, the destruction complex can suppress nuclear β-catenin (Eq.2) (51). The pool of nuclear (β-catenin_nuc node) and membrane bound β-catenin (β-catenin_memb node) is dynamically controlled by the Wnt signal: active Wnt signaling leads to β-catenin localization to the nucleus. Hence, we assume that β-catenin cannot be simultaneously localized both to the membrane and to the nucleus and implement this assumption as a mutually inhibitory relationship between β-catenin_memb and β-catenin_nuc(52). We consider an alternative rule for β-catenin_nuc in which there is no inhibition from β-catenin_memb to β-catenin_nuc in Supplemental Figure 5. Suppresser of fused homolog (SUFU) binds β-catenin, exports it from the nucleus, and thereby represses nuclear β-catenin (Eq. 2)(50). E-cadherin has been shown to suppress β-catenin nuclear localization (Eq. 2)(51) and to stabilize membrane-bound β-catenin (Eq. 3)(52). We assume that predominant nuclear localization of β-catenin is possible if either SUFU or E-cadherin is absent, if simultaneously the destruction complex is also absent.
Footnotes
Conflicts of interest. The authors disclose no potential conflicts of interest.
Contributor Information
Steven Nathaniel Steinway, Penn State Hershey Cancer Institute, Penn State College of Medicine, Hershey, PA.
Jorge Gomez Tejeda Zañudo, Department of Physics, Pennsylvania State University, University Park, PA.
Wei Ding, Department of Pediatrics, Penn State College of Medicine, Hershey, PA.
Carl Bart Rountree, Department of Pediatric Gastroenterology, Bon Secours St. Mary’s Hospital, Richmond, VA.
David J. Feith, University of Virginia Cancer Center, University of Virginia School of Medicine, Charlottesville, VA
Thomas P. Loughran, Jr., University of Virginia Cancer Center, University of Virginia School of Medicine, Charlottesville, VA
Reka Albert, Department of Physics, Pennsylvania State University, University Park, PA.
References
- 1.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–63. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. The Journal of clinical investigation. 2010;120:1031–4. doi: 10.1172/JCI42615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ. 2008;50:755–66. doi: 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- 5.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. The Journal of clinical investigation. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Amin R, Mishra L. Liver stem cells and tgf-Beta in hepatic carcinogenesis. Gastrointest Cancer Res. 2008;2:S27–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, et al. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. American journal of surgery. 1999;177:209–15. doi: 10.1016/s0002-9610(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 10.Sue SR, Chari RS, Kong FM, Mills JJ, Fine RL, Jirtle RL, et al. Transforming growth factor-beta receptors and mannose 6-phosphate/insulin-like growth factor-II receptor expression in human hepatocellular carcinoma. Ann Surg. 1995;222:171–8. doi: 10.1097/00000658-199508000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiss A, Wang NJ, Xie JP, Thorgeirsson SS. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth Factor/c-met, TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res. 1997;3:1059–66. [PubMed] [Google Scholar]
- 12.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 13.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saadatpour A, Albert R. Boolean modeling of biological regulatory networks: a methodology tutorial. Methods. 2013;62:3–12. doi: 10.1016/j.ymeth.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Ding W, You H, Dang H, LeBlanc F, Galicia V, Lu SC, et al. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52:945–53. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer research. 1982;42:3858–63. [PubMed] [Google Scholar]
- 17.Dor I, Namba M, Sato J. Establishment and some biological characteristics of human hepatoma cell lines. Gann. 1975;66:385–92. [PubMed] [Google Scholar]
- 18.Ding W, Mouzaki M, You H, Laird JC, Mato J, Lu SC, et al. CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis. Hepatology. 2009;49:1277–86. doi: 10.1002/hep.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saadatpour A, Wang RS, Liao A, Liu X, Loughran TP, Albert I, et al. Dynamical and structural analysis of a T cell survival network identifies novel candidate therapeutic targets for large granular lymphocyte leukemia. PLoS Comput Biol. 2011;7:e1002267. doi: 10.1371/journal.pcbi.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kachalo S, Zhang R, Sontag E, Albert R, DasGupta B. NET-SYNTHESIS: a software for synthesis, inference and simplification of signal transduction networks. Bioinformatics. 2008;24:293–5. doi: 10.1093/bioinformatics/btm571. [DOI] [PubMed] [Google Scholar]
- 22.Albert I, Thakar J, Li S, Zhang R, Albert R. Boolean network simulations for life scientists. Source Code Biol Med. 2008;3:16. doi: 10.1186/1751-0473-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanudo JGT, Albert R. An effective network reduction approach to find the dynamical repertoire of discrete dynamic networks. Chaos. 2013;23:025111. doi: 10.1063/1.4809777. [DOI] [PubMed] [Google Scholar]
- 24.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–2. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jechlinger M, Grunert S, Tamir IH, Janda E, Ludemann S, Waerner T, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–69. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karakas B, Weeraratna A, Abukhdeir A, Blair BG, Konishi H, Arena S, et al. Interleukin-1 alpha mediates the growth proliferative effects of transforming growth factor-beta in p21 null MCF-10A human mammary epithelial cells. Oncogene. 2006;25:5561–9. doi: 10.1038/sj.onc.1209540. [DOI] [PubMed] [Google Scholar]
- 28.Song J. EMT or apoptosis: a decision for TGF-beta. Cell Res. 2007;17:289–90. doi: 10.1038/cr.2007.25. [DOI] [PubMed] [Google Scholar]
- 29.Shin SY, Rath O, Zebisch A, Choo SM, Kolch W, Cho KH. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer research. 2010;70:6715–24. doi: 10.1158/0008-5472.CAN-10-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RS, Saadatpour A, Albert R. Boolean modeling in systems biology: an overview of methodology and applications. Physical biology. 2012;9:055001. doi: 10.1088/1478-3975/9/5/055001. [DOI] [PubMed] [Google Scholar]
- 31.Papin JA, Hunter T, Palsson BO, Subramaniam S. Reconstruction of cellular signalling networks and analysis of their properties. Nature reviews Molecular cell biology. 2005;6:99–111. doi: 10.1038/nrm1570. [DOI] [PubMed] [Google Scholar]
- 32.Saadatpour A, Albert I, Albert R. Attractor analysis of asynchronous Boolean models of signal transduction networks. J Theor Biol. 2010;266:641–56. doi: 10.1016/j.jtbi.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Saadatpour A, Albert R, Reluga T. A Reduction Method for Boolean Network Models Proven to Conserve Attractors SIAM. J Appl Dyn Syst. 2013;12:1997–2011. doi: 10.1137/13090537X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–94. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 38.Baritaki S, Huerta-Yepez S, Sahakyan A, Karagiannides I, Bakirtzi K, Jazirehi A, et al. Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: inhibition of the metastasis-inducer Snail and induction of the metastasis-suppressor RKIP. Cell Cycle. 2010;9:4931–40. doi: 10.4161/cc.9.24.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahlem P, Birney E. Dry work in a wet world: computation in systems biology. Mol Syst Biol. 2006;2:40. doi: 10.1038/msb4100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobie EA, Lee YS, Jenkins SL, Iyengar R. Systems biology--biomedical modeling. Science signaling. 2011;4:tr2. doi: 10.1126/scisignal.2001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross L. When evidence is scant, mathematical modeling offers a roadmap for discovery. PLoS Biol. 2006;4:e323. doi: 10.1371/journal.pbio.0040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polakis P. Wnt signaling in cancer. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–50. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massague J. TGFbeta signalling in context. Nature reviews Molecular cell biology. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends in cell biology. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews Molecular cell biology. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18144–9. doi: 10.1073/pnas.1318192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Lee J, Farquhar KS, Yun J, Frankenberger CA, Bevilacqua E, et al. Network of mutually repressive metastasis regulators can promote cell heterogeneity and metastatic transitions. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E364–73. doi: 10.1073/pnas.1304840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–92. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 50.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 51.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112 ( Pt 8):1237–45. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 52.Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, et al. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011;2011:567305. doi: 10.1155/2011/567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.