Abstract
A number of brain regions have been implicated in the anxiety disorders, yet none of these regions in isolation has been distinguished as the sole or discrete site responsible for anxiety disorder pathology. Therefore, the identification of dysfunctional neural networks as represented by alterations in the temporal correlation of blood-oxygen level dependent (BOLD) signal across several brain regions in anxiety disorders has been increasingly pursued in the past decade. Here, we review task-independent (e.g., resting state) and task-induced functional connectivity magnetic resonance imaging (fcMRI) studies in the adult anxiety disorders (including trauma- and stressor-related and obsessive compulsive disorders). The results of this review suggest that anxiety disorder pathophysiology involves aberrant connectivity between amygdala-frontal and frontal-striatal regions, as well as within and between canonical “intrinsic” brain networks - the default mode and salience networks, and that evidence of these aberrations may help inform findings of regional activation abnormalities observed in the anxiety disorders. Nonetheless, significant challenges remain, including the need to better understand mixed findings observed using different methods (e.g., resting state and task-based approaches); the need for more developmental work; the need to delineate disorder-specific and transdiagnostic fcMRI aberrations in the anxiety disorders; and the need to better understand the clinical significance of fcMRI abnormalities. In meeting these challenges, future work has the potential to elucidate aberrant neural networks as intermediate, brain-based phenotypes to predict disease onset and progression, refine diagnostic nosology, and ascertain treatment mechanisms and predictors of treatment response across anxiety, trauma-related and obsessive compulsive disorders.
Keywords: anxiety, fMRI, OCD, PTSD, Resting state, Functional connectivity
A neural network approach to the pathophysiology of anxiety disorders
Anxiety and stress disorders have a lifetime prevalence of nearly 30% (1); cost more than $42 billion each year - almost 1/3 of the U.S.’s annual mental health costs (2); and impose significant suffering and burden on patients (3). For more than three decades, neuroimaging work has attempted to uncover the neural basis of anxiety and other psychiatric disorders, however no study has yet discovered a single brain region responsible for psychiatric pathology as delineated in current nosological systems (4). Therefore, recent research has shifted toward the identification of disruptions between regions and in large-scale neural networks distributed throughout the brain (5; 6) to better define anxiety pathophysiology. Here, we define neural networks as collections of neural regions that function together, in the sense that they are statistically dependent on each other (7).
The field of functional connectivity magnetic resonance imaging (fcMRI) - a measure of temporally correlated fluctuations in blood oxygen-level dependent (BOLD) signal across spatially distributed brain regions - has availed a mapping tool to explore how the brain is organized and how that organization may be altered in psychopathology. This tool holds great promise; however, its application to the anxiety disorders is still in its relative infancy. As such, gaps in the literature remain: disorders and networks are inconsistently represented, discrepancies are observed across methods and there are few transdiagnostic investigations. Nonetheless, some conclusions can be drawn about common and shared neural network aberrations evident in anxiety. Below, we review key studies of task-independent (e.g., resting state) and task-induced functional connectivity - in the adult anxiety, trauma- and stressor-related and obsessive compulsive disorders1. Overall results of this review are summarized in Table 1, in which increased (i.e., more positive or less negative) connectivity for patients versus controls is indicated with a red “up” arrow and reduced (i.e., less positive or more negative) connectivity for patients versus controls is indicated with a blue “down” arrow. In the text, we have grouped the results of our review by connectivity regions/network, and below each, by anxiety disorder, where sufficient literature exists, ordered according to the size of the existing literature on each disorder (largest to smallest).
Table 1.
Summary of anxiety disorder connectivity findings.
| Task | Resting State | |
|---|---|---|
| Amygdala-frontal | SAD ; GAD, OCD ; GAD, OCD
|
SAD
|
| Frontal-striatal | OCD
|
OCD
|
| DMN | PTSD, OCD, SAD
|
|
| (DMN-SN) | PTSD
|
|
| SN | PTSD, SAD ; GAD ; GAD
|
PTSD
|
Blue down arrows indicate disorders with reduced (less positive) connectivity compared to controls; red up arrows indicate disorders with greater (more positive) connectivity compared to controls. Results reported here reflect those described in the text, where at least 2 studies were in agreement. We aggregated “frontal” regions here, but where possible, we note in the text the specific frontal subdivision (ventral, dorsal; medial, lateral) in which aberrant connectivity was observed.
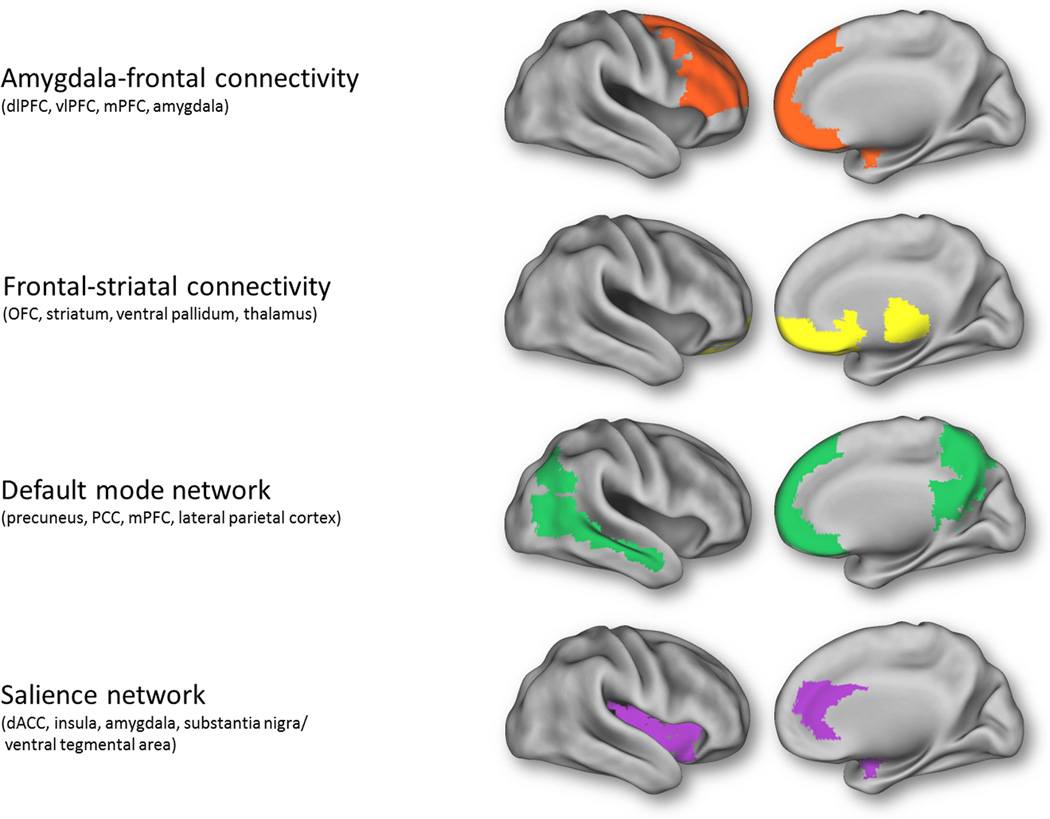
First, we summarize research on connectivity between amygdala-frontal regions and frontal-striatal regions (Fig. 1). Of note, coordinated response of these regions is not thought to reflect canonical neural network activity – i.e., initially discovered during resting state studies, and believed to reflect the inherent organization of the brain. Instead, amygdala-frontal connectivity includes regions that are frequently implicated in fear extinction and the regulation of negative affect - namely, the dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC) and medial PFC (8–10; see 11 for a meta-analysis of emotion regulation connectivity). Frontal-striatal connectivity includes nodes that are frequently implicated in reward processing, action selection, habit formation, and motor control, such as the orbitofrontal cortex (OFC), striatum (ventral and dorsal), ventral pallidum and thalamus. It is important to note that “frontal” connectivity encompasses several subdivisions (e.g., dorsal and ventral, medial and lateral), which differ in connectivity (12; functional and structural connectivity with the amygdala; 13–15). There is also some evidence that these subdivisions have distinct functions in relation to negative emotions (e.g., appraisal/expression versus regulation; 16) and that trait anxiety dissociates dorsal from ventral medial PFC functional connectivity with the amygdala at rest (17). However, no study has directly examined the differential and specific contribution of these subdivisions in the context of anxiety disorders in terms of network connectivity. Therefore, in what follows, we have more generally aggregated these subdivisions into a “frontal” grouping (“amygdala-frontal” and “frontal-striatal” and as summarized in Table 1). When specified in the original paper, details are provided as to the specific frontal subdivision in which aberrant connectivity was observed.
Figure 1.
Regions implicated in amygdala-frontal connectivity and frontal-striatal connectivity, as well as those involved in intrinsic neural networks, the default mode and salience networks. dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; vlPFC, ventrolateral prefrontal cortex.
Next, we review research on two canonical neural networks (Fig. 1) that have been characterized in the context of resting state studies of spontaneous low-frequency BOLD fluctuations (6; 18): the default mode network (DMN), which includes the precuneus, posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), and lateral parietal cortex (19) and the salience network (SN), which includes the dorsal anterior cingulate cortex (dACC), the anterior insula, amygdala and substantia nigra/ventral tegmental area (6; 20). Because coherence in the DMN and the SN can be observed both when participants are at rest and on task (21), these networks have come to be thought of as reflecting intrinsic connectivity – i.e., neural regions that “hang together” regardless of a person’s current state of mind or task-evoked changes (though resting state itself might be considered as an unconstrained and passive but nonetheless, task state; 5). Among intrinsic connectivity networks, the DMN is unique in that it is known to activate more while participants are at rest than on task; the SN is thought to be engaged by more active processes - e.g., responding to external stimuli (22; 23). Other intrinsic neural networks have been identified (e.g., the central executive network, CEN; 6), but insufficient literature exists to implicate these neural networks in the anxiety disorders – thus, they are not reviewed here.
Finally, we examine how task-based and resting-state connectivity can jointly increase understanding of anxiety disorder pathophysiology and how functional connectivity can complement our current understanding of aberrant activation in discrete nodes in the anxiety disorders (24). We explore the clinical relevance of connectivity findings in the anxiety disorders, and discuss open questions for future research.
Amygdala-frontal connectivity
Social anxiety disorder
Reduced amygdala-frontal connectivity has been observed in social anxiety disorder (SAD). For instance, in the context of an emotional and neutral word viewing task, greater SAD symptom severity was associated with less amygdala connectivity with the vmPFC and OFC (25; see also words previously associated with fearful or neutral faces 26; and resting state, 27; 28). Similarly, using an amygdala seed in a SAD group that exhibited amygdala hyperactivation to threat faces, Hahn and colleagues (27) found evidence of reduced amygdala connectivity to medial OFC at rest. On the other hand, effective connectivity work has found that SAD may be characterized by a positive connection from the OFC to the amygdala during facial emotion and object discrimination tasks, (whereas a negative feedback loop was observed in controls; 29). Anatomical connections between the OFC and the amygdala may facilitate the implementation of emotion regulation (9; 13; 16). Therefore, aberrant connectivity between these regions might underlie impaired emotion regulatory function or altered threat processing in SAD.
Abnormal functional connectivity involving the amygdala and more lateral regions of the PFC has also been observed in SAD. For instance, SAD participants viewing fearful faces showed reduced amygdala connectivity to the dlPFC (and rostral ACC, rACC; 30). Additionally, when asked to down-regulate their emotional response to negative self-beliefs, SAD participants showed fewer regulatory regions inversely related to amygdala activity - including three regions in the dlPFC and two in the vlPFC - suggesting less widespread cortical control of amygdala activation (31). Interestingly, amygdala-lateral frontal connectivity does not appear to differ between SAD and controls at rest (30; 32), indicating some functional specificity.
Generalized anxiety disorder
Etkin and colleagues (33; 34) have found blunting of the normally negative connectivity between the pregenual/ventral cingulate and the amygdala in generalized anxiety disorder (GAD) during emotional conflict adaptation (33), suggesting impaired emotion regulation (see also 35 for evidence of increased vmPFC-amygdala connectivity during rest). In addition, participants with GAD showed increased positive connectivity between the amygdala and the dlPFC (36). According to the authors, greater amygdala-dlPFC connectivity in the GAD group may serve a compensatory function – e.g., cognitive strategies to reduce or manage negative emotion. This notion was supported by a negative correlation between amygdala-dlPFC connectivity and anxiety symptoms in the GAD group, suggesting that – while amygdala-dlPFC connectivity was heightened in patients overall –patients who were the most anxious engaged this connection the least (36).
Obsessive compulsive disorder
Several studies have identified increased connectivity between the amygdala and the dlPFC during face processing and working memory tasks in obsessive compulsive disorder (OCD; 37; 38); at least one result from the resting state literature (using a graph theoretical approach) has, however, suggested reduced connectivity between the amygdala and frontoparietal executive/attention areas (39). In OCD, increased amygdala-PFC connectivity might signify an overactive cognitive control system, potentially implicated in heightened error monitoring and checking behaviors (37).
Post-traumatic stress disorder
Post-traumatic stress disorder (PTSD), which involves decreased activation in the medial PFC (implicated in fear extinction and its retention; e.g., 40). may be characterized by reduced connectivity between this region and the amygdala on task - e.g., amygdala-vmPFC/subgenual(s)ACC during a passive face-viewing task (41). Still, other work suggests that certain amygdala subregions – i.e., the basolateral amygdala complex - may show increased connectivity to the dmPFC at rest (42).
Summary
Amygdala-medial frontal connectivity is reduced during the viewing of emotional stimuli in SAD (and potentially PTSD), suggesting a neural substrate for impaired top-down control of threat-processing and impaired emotion regulation in these disorders (43–45). Similar results are also observed at rest in SAD. Altered amygdala-lateral PFC connectivity, however, may be observed only during emotional tasks and not at rest in SAD. Evidence suggests that GAD and OCD may involve increased amygdala-frontal connectivity during emotional tasks, in line with prior evidence of different disease pathologies – e.g., failures to observe amygdala hyperactivation (46–48) – in these disorders. Given differences in dorsal versus ventral frontal connectivity (12; 49), future work may wish to go farther in delineating regional distinctions in amygdala-frontal connectivity in the anxiety disorders.
Frontal-striatal connectivity
Obsessive compulsive disorder
Both task-based (induced sadness, 50; conflict processing, 51; see also symptom provocation, 52; but see risk aversion, 53) and resting state (54–61; but see 62) studies have repeatedly revealed increased functional connectivity between orbitofrontal and striatal regions in OCD. Structural abnormalities have also been observed in frontal-striatal regions in OCD, though the direction of results has been mixed (63; 64). Recent work suggests that heightened frontal-striatal connectivity at rest (e.g., between the caudate and OFC) is also evident in first degree relatives of OCD patients (65), suggesting that it may represent a marker of risk. By contrast, heightened amygdala-frontal connectivity during a working memory task is observed only in OCD patients and not their first degree relatives (38).
Social anxiety disorder and specific phobia
Though the vast majority of work on frontal-striatal connectivity in the anxiety disorders has focused on OCD, there is growing evidence of altered frontal-striatal connectivity in SAD and specific phobia. Similar to OCD, adults with SAD show greater task-induced functional connectivity between the thalamus and the medial frontal cortex, as well as between the medial frontal cortex and the basal ganglia (caudate nucleus), when perceiving scrutiny (66). Additionally, a resting state study of SAD that used several subcortical striatal seeds (caudate, putamen, globus pallidus) showed widespread increased connectivity during resting-state throughout medial, orbital, dorsolateral and cingulate regions, including nodes within the executive control network and the DMN (32; but see 67, also at rest). In dental phobics, Scharmüller and colleagues (68) found evidence of reduced connectivity of the basal ganglia (putamen and pallidum) with the ACC and dlPFC when viewing phobia-relevant pictures.
Summary
Patients with OCD show increased frontal-striatal connectivity across emotional and cognitive tasks and during resting state – results that could relate to uncontrolled and repetitive cognition and behavior (69). More work is needed to understand patterns of altered frontal-striatal connectivity in other anxiety disorders, however initial results suggest that SAD may be associated with increased frontal-striatal connectivity both on task (i.e., perceiving scrutiny) and while at rest, whereas specific phobia may be associated with reduced connectivity in these regions during symptom provocation, though no resting state studies exist for comparison.
Default mode network
Post-traumatic stress disorder
Studies of the DMN –often revealed by connectivity to a PCC seed at rest - have primarily been conducted in patients with PTSD as opposed to other anxiety disorders. The PCC is thought to be important for integration of past and present information, and its aberrant connectivity, may be related to memory impairments and difficulties with contextualization - hallmark features of PTSD. The central finding across seed-based resting state studies has been evidence of decreased connectivity involving the DMN in PTSD (e.g., PCC-mPFC, PCC-lateral parietal cortex, 70; hippocampus-PCC: hippocampus-pgACC, 71; vmPFC-rACC, PCC-hippocampus, 72) and in acutely traumatized individuals who go on to develop symptoms of PTSD (PCC-mPFC, 73). Work examining amplitude of low frequency fluctuation (ALFF; 74) and using independent component analysis (ICA; 75; see also 76) has found similar results.
Another main finding has been evidence of increased connectivity between the DMN and nodes of the SN at rest in PTSD (73; 72; but see 77 for greater negative connectivity). Along these lines, Lanius and colleagues (78) found evidence of increased connectivity between the dACC (a key node of the SN) and the PCC during script-driven symptom provocation. Furthermore, emerging evidence from the resting state literature suggests that connectivity between DMN and SN node, the amygdala, may predict the development of PTSD symptoms, though the direction of these associations is mixed (79; 80).
Obsessive compulsive disorder
As for PTSD, evidence indicates reduced connectivity within the DMN in OCD (PCC-mPFC, 81; PCC-middle frontal gyrus, 82; 39; see also 83; but see 58), suggesting a neural substrate for altered self-referential processing. Also similar to PTSD, some work has also found evidence of increased connectivity between the DMN and the SN in OCD (83; but see 39).
Social anxiety disorder
SAD has been associated with reduced connectivity of the PCC to the rest of the brain (i.e., reduced centrality; 84) and with reduced coherence (regional homogeneity, ReHo) in the medial PFC (85). In addition, SAD has been associated with reduced connectivity between nodes in the SN (amygdala) and DMN (PCC; 27).
Generalized anxiety disorder and panic disorder
In GAD, older age and increasing anxiety symptoms may be associated with less connectivity between the PCC and medial PFC (86). Findings in PD have been inconsistent (decreased, 87; and increased, 88).
Summary
Conducted primarily during resting state, research suggests that the anxiety disorders are characterized by reduced DMN connectivity, with the strongest evidence available for PTSD and OCD. Evidence also suggests that DMN-SN connectivity is increased in PTSD both during symptom provocation and while at rest. Future work may wish to determine whether reduced DMN connectivity represents a general liability for or marker of anxious psychopathology. Moreover, given evidence of DMN hyperactivity and connectivity in depression (89), the DMN might help inform understanding of anxiety- and depression-specific processes.
Salience network
Post-traumatic stress disorder
Altered SN connectivity – in particular, amygdala-insula connectivity – has been found to be decreased in PTSD when processing emotional images (90) or social signals/faces (91; see also 92) but increased at rest (93; using ALFF, 74; see 42 for increased amygdala-dACC). Furthermore, some work has found evidence of both increased and decreased connectivity, depending on which nodes of the SN are involved (increased amygdala-insula; decreased amygdala-dACC, 94). Therefore, while findings point towards SN involvement in PTSD pathophysiology, more work is needed to clarify the meaning of discrepant results.
Social anxiety disorder
Reduced SN connectivity has been observed in SAD during the presentation of aversive social stimuli (amygdala-insula and amygdala-dACC, 95; insula-dACC, 96) and during resting state (amygdala-ACC, 28; but see 32), and may underlie aberrant threat processing observed in this disorder (95).
Generalized anxiety disorder
GAD may be associated with increased SN connectivity during the processing of threatening faces (97; with comorbid SAD, 98; but see 36). Compared to other anxiety disorders, GAD has been less frequently associated with amygdala hyper-reactivity to negative stimuli (46; 47), which could explain why connectivity effects involving the amygdala may differ for GAD.
Panic disorder and obsessive compulsive disorder
PD has been associated with both increased and decreased connectivity between SN seeds (i.e., left and right dACC) and somatosensory and superior parietal brain regions (99), suggesting an altered system for interpreting bodily signals and assessing homeostasis. In OCD there is evidence of hypoconnectivity within the SN (amygdala-dACC) during a task involving risk (53).
Summary
The choice of paradigm seems to yield different results in SN connectivity for PTSD, with connectivity reduced for task-based studies involving the viewing of emotional stimuli but increased during resting state. By contrast, reduced SN connectivity is observed in SAD during both the presentation of aversive social stimuli and during resting state; GAD, however, may be associated with increased SN connectivity when viewing social stimuli (no resting state studies exist for comparison). There are few studies and results are mixed for PD and OCD. Finally, many of the studies examining the SN in anxiety have focused on connectivity with the amygdala, which is considered part of the SN by some (e.g., 6), but not all (e.g., 100) researchers. Therefore, future work may wish to determine whether anxiety-related aberrations in SN connectivity extend beyond the amygdala.
Future Directions and Conclusion
As reviewed above, growing evidence suggests altered brain connectivity across the anxiety disorders. Given that connectivity is aberrant across several neural regions (amygdala-frontal, frontal-striatal) and across several canonical neural networks (DMN, SN), a more accurate depiction of anxiety pathophysiology may therefore involve widespread distributed disturbances in functional brain organization rather than discrete nodes of over- or under-activation. However, the potential of fcMRI for understanding anxiety disorder pathophysiology should be considered in the context of its limitations and remaining unanswered questions.
First, understanding of the functional and clinical relevance of aberrant (increased or decreased) patterns of functional connectivity remains poorly defined. This is driven in part by the gap between task-independent and task-induced fcMRI approaches, which can yield discrepant results (e.g., 101). In addition to contextualizing results in terms of the paradigm and analytic approach used, evidence of shared and discrete patterns of connectivity changes on task and at rest in the same study may facilitate interpretation. For example, reduced amygdala-rACC connectivity both at rest and during threat processing in SAD (30) might explain SAD-related hypervigilance that persists even in the absence of threat-related stimuli (unprovoked state). On the other hand, reduced amygdala-dlPFC connectivity observed during threat but not rest (30; in line with 31) may explain failure to recruit the PFC when top-down control is most needed (102) – i.e., as part of exaggerated threat responding in SAD. Therefore, conducting both resting state and task-based connectivity studies on the same group of individuals will likely advance understanding of anxiety pathophysiology and may help reconcile discrepancies. Integrating task-based activation and connectivity findings at rest or on task (30; 35) can also give meaning to results. For example, increased dlPFC activity in during threat regulation in SAD (102) could at first glance suggest increased emotion regulatory engagement; however, in the context of reduced amygdala-dlPFC connectivity (30), increased dlPFC activity may signal inefficient emotion regulatory control. Linking functional connectivity in anxiety disorders with behavior (103) or environmental adversity (104) could also aid in interpretability. Likewise, growing studies point to the utility of fcMRI to elucidate treatment mechanisms (105) and to predict treatment success – e.g., at baseline - in PD (reduced SN connectivity during fear conditioning, 106), SAD (increased amygdala-frontal connectivity at rest, 107) and OCD (reduced small-world network efficiency at rest, 108). Therefore, research is beginning to expand the significance and interpretability of task-independent and task-dependent functional connectivity findings, though more work is needed in these areas.
Second, few fcMRI studies have examined network changes between anxiety-disordered adults and children or longitudinally across the lifespan, particularly during childhood development when neural plasticity and remodeling are most active (109). Among those studies that do exist, results suggest increased amygdala-insula connectivity in youth with GAD (110; 111; see also 112), as well as less negative/more positive connectivity between the amygdala and vlPFC for youth with GAD compared to controls (101) - results that are generally consistent with the adult GAD literature (97; 36). On the other hand, among patients with OCD ranging in age between 8 to 40 years old, younger patients may exhibit less positive connectivity of subcortical regions (thalamus and striatum) with the ACC during rest (113), suggesting that the pathophysiology of frontal-striatal connectivity changes with age in OCD, and consistent with negative ACC/mPFC-striatal connectivity observed in socially anxious adolescents but not adults (114). In healthy individuals, subcortical-ACC connectivity goes from positive to negative from childhood to adolescence, possibly as a result of synaptic pruning (115) and signifying greater emotion regulation capacity (116). Therefore, a more “mature” pattern of functional connectivity between these regions at an earlier age in OCD – whether due to biological or environmental (e.g., 117) factors – might indicate an aberrant trajectory linked to early disease onset. Collectively, these emerging data prompt further investigation into how aging interacts with functional network maturation in normal development and anxiety psychopathology.
Third, although similar aberrations in connectivity have been found across several anxiety disorders that may differ from patterns observed in other forms of psychopathology (e.g., depression, addiction, psychosis; 89; 118; 119), there is insufficient evidence in aggregate to point to shared or discrete connectivity abnormalities. Very few studies have incorporated multiple diagnostic categories (e.g., 71; 98; 120) or taken a transdiagnostic approach to fcMRI in the anxiety disorders. In a study examining both SAD and PD, Demenescu and colleagues (121) found that greater amygdala-rACC and amygdala-dmPFC connectivity to fearful versus neutral faces was explained not by anxiety diagnosis (SAD or PD), but by anxiety symptom severity across all patients, pointing toward a translational account of attentional biases and emotion dysregulation in the anxiety disorders (122). The growing field of fcMRI along with sufficient number of studies using comparable analytic approaches should soon avail the opportunity to aggregate findings across disparate single studies into data-driven meta-analyses examining transdiagnostic and disorder-specific findings (123). In addition, some networks such as the CEN (i.e., executive control/frontoparietal network; sometimes including the dorsal attention network; 6; 100; 124) have been less studied in anxiety (but see 85; 39) than in other disorders (e.g., depression, schizophrenia; 125). However, amygdala-frontal connectivity – discussed above – includes nodes within the SN (the amygdala) and the CEN (e.g., the dlPFC). The majority of connectivity work to-date has also focused on within-network connectivity and work examining connectivity between neural networks is needed (72; 126). Along these lines, work that combines both large-scale network connectivity analyses with more localized (e.g., seed-based) connectivity analyses might be helpful in bridging literatures and aggregating findings.
Fourth, although functional connectivity provides a unique and useful means of examining brain organization in the anxiety disorders, it can be difficult to know what fcMRI is measuring. That is, functional connectivity is constrained by but does not simply reflect anatomical connectivity (5). It depends on an indirect measure of brain activity (BOLD), is subject to confounds such as head movement (127) and respiratory and cardiac artifacts (128) and is influenced by recent experience (129) and the subjective state of participants (i.e., even while at rest; 5). Moreover, anticorrelations can be particularly difficult to interpret (130), although new methods for normalization may help overcome limitations (131). Nonetheless, despite these challenges, functional connectivity may be particularly relevant for understanding anxiety disorders, precisely because it reflects a combination of both anatomic connectivity and experience-driven changes in synaptic efficiency (132). To more thoroughly interrogate aberrant functional connectivity in the anxiety disorders, one suggestion (5) is that researchers use functional connectivity to generate hypotheses that are then followed up using other methods including electrophysiology and neurochemistry (133; 134) to better understand underlying mechanisms.
Conclusion
In sum, evidence suggests that anxiety disorder pathophysiology involves aberrant functional connectivity between disparate nodes, particularly within and between amygdala-frontal and frontal-striatal nodes, as well as within canonical default mode and salience networks. As advances in study design and analytic approaches continue to address existing gaps in knowledge, patterns of functional connectivity elucidated on task and at rest may emerge as intermediate, brain-based phenotypes to predict onset and progression of anxiety disorders, to help parse phenotypically heterogeneous disorders into more meaningful subgroups, and to elucidate mechanisms and predictors of existing and novel therapeutic interventions.
Acknowledgments
This work was supported in part by National Institute of Mental Health grants (K23MH105553 to AM and R01MH101497 to KLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Obsessive compulsive disorder and post-traumatic stress disorder were considered anxiety disorders at the time the majority of these studies were conducted; therefore, we have opted to include research on these disorders in our review.
Financial Disclosures
Drs. MacNamara, DiGangi and Phan report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JRT, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999 doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 3.Nepon J, Belik S, Bolton J, Sareen J. The relationship between anxiety disorders and suicide attempts: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Depress Anxiety. 2010;27:791–798. doi: 10.1002/da.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornito A, Harrison BJ. Brain Connectivity and Mental Illness. Front Psychiatry. 2012:3. doi: 10.3389/fpsyt.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 6.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal- component analysis of large (PET) data sets. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 8.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex. 2013:bht154. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 10.Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, et al. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex N Y N 1991. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon V. Salience Network. In: Toga AW, editor. Brain Mapp Encycl Ref (Vol. 2: Anatomy and Physiology, Systems) Waltham: Academic Press; 2015. pp. 597–611. [Google Scholar]
- 21.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body . Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 23.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laeger I, Dobel C, Radenz B, Kugel H, Keuper K, Eden A, et al. Of “Disgrace” and “Pain” - Corticolimbic Interaction Patterns for Disorder-Relevant and Emotional Words in Social Phobia. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0109949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laeger I, Keuper K, Heitmann C, Kugel H, Dobel C, Eden A, et al. Have we met before? Neural correlates of emotional learning in women with social phobia. J Psychiatry Neurosci JPN. 2014;39:E14–E23. doi: 10.1503/jpn.130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 28.Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39:2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sladky R, Höflich A, Küblböck M, Kraus C, Baldinger P, Moser E, et al. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for FMRI. Cereb Cortex N Y N 1991. 2015;25:895–903. doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold Anteraper S, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4:81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- 33.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of Anterior Cingulate Activation and Connectivity With the Amygdala During Implicit Regulation of Emotional Processing in Generalized Anxiety Disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etkin A, Schatzberg AF. Common Abnormalities and Disorder-Specific Compensation During Implicit Regulation of Emotional Processing in Generalized Anxiety and Major Depressive Disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 35.Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica-Parodi LR. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J Neurosci Off J Soc Neurosci. 2014;34:4043–4053. doi: 10.1523/JNEUROSCI.3372-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 37.Cardoner N, Harrison BJ, Pujol J, Soriano-Mas C, Hernández-Ribas R, López-Solá M, et al. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2011;12:349–363. doi: 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- 38.de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76:878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Göttlich M, Krämer UM, Kordon A, Hohagen F, Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:5617–5632. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA. Altered Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2014;39:361–369. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behav Res Ther. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 44.Jazaieri H, Morrison AS, Goldin PR, Gross JJ. The role of emotion and emotion regulation in social anxiety disorder. Curr Psychiatry Rep. 2015;17:531. doi: 10.1007/s11920-014-0531-3. [DOI] [PubMed] [Google Scholar]
- 45.New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Palm ME, Elliott R, McKie S, Deakin JFW, Anderson IM. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol Med. 2011;41:1009–1018. doi: 10.1017/S0033291710001455. [DOI] [PubMed] [Google Scholar]
- 47.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56:916–920. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Vossel S, Geng JJ, Fink GR. Dorsal and Ventral Attention Systems Distinct Neural Circuits but Collaborative Roles. The Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fontenelle LF, Harrison BJ, Pujol J, Davey CG, Fornito A, Bora E, et al. Brain functional connectivity during induced sadness in patients with obsessive-compulsive disorder. J Psychiatry Neurosci JPN. 2012;37:231–240. doi: 10.1503/jpn.110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry. 2014;75:615–622. doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banca P, Voon V, Vestergaard MD, Philipiak G, Almeida I, Pocinho F, et al. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain J Neurol. 2015;138:798–811. doi: 10.1093/brain/awu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Admon R, Bleich-Cohen M, Weizmant R, Poyurovsky M, Faragian S, Hendler T. Functional and structural neural indices of risk aversion in obsessive-compulsive disorder (OCD) Psychiatry Res. 2012;203:207–213. doi: 10.1016/j.pscychresns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 55.Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 56.Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 57.Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, López-Solà M, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:321–328. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Hou J, Song L, Zhang W, Wu W, Wang J, Zhou D, et al. Morphologic and functional connectivity alterations of corticostriatal and default mode network in treatment-naïve patients with obsessive-compulsive disorder. PloS One. 2013;8:e83931. doi: 10.1371/journal.pone.0083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung WH, Kang D-H, Kim E, Shin KS, Jang JH, Kwon JS. Abnormal corticostriatal-limbic functional connectivity in obsessive-compulsive disorder during reward processing and resting-state. NeuroImage Clin. 2013;3:27–38. doi: 10.1016/j.nicl.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang D-H, Jang JH, Han JY, Kim J-H, Jung WH, Choi J-S, et al. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:340–346. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, Fukui K. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry J Assoc Eur Psychiatr. 2011;26:463–469. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, et al. Multicenter Voxel-Based Morphometry Mega-Analysis of Structural Brain Scans in Obsessive-Compulsive Disorder. Am J Psychiatry. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 64.Eng GK, Sim K, Chen S-HA. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neurosci Biobehav Rev. 2015;52:233–257. doi: 10.1016/j.neubiorev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Hou J-M, Zhao M, Zhang W, Song L-H, Wu W-J, Wang J, et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci JPN. 2014;39:304–311. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giménez M, Pujol J, Ortiz H, Soriano-Mas C, López-Solà M, Farré M, et al. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Res. 2012;202:214–223. doi: 10.1016/j.pscychresns.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Manning J, Reynolds G, Saygin ZM, Hofmann SG, Pollack M, Gabrieli JDE, Whitfield-Gabrieli S. Altered Resting-State Functional Connectivity of the Frontal-Striatal Reward System in Social Anxiety Disorder. PLoS ONE. 2015:10. doi: 10.1371/journal.pone.0125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scharmüller W, Leutgeb V, Schöngaβner F, Hermann A, Stark R, Schienle A. Altered functional connectivity of basal ganglia circuitry in dental phobia. Soc Cogn Affect Neurosci. 2014;9:1584–1588. doi: 10.1093/scan/nst150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burguière E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci JPN. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- 71.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin L, Wang Z, Sun Y-W, Wan J-Q, Su S-S, Zhou Y, Xu J-R. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, et al. Spontan eous brain activity in combat related PTSD. Neurosci Lett. 2013;547:1–5. doi: 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 75.Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PloS One. 2014;9:e96834. doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, Lanius RA. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- 77.Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, et al. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med. 2014;44:1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- 78.Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- 79.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Wang Z, Qin L, Wan J, Sun Y, Su S, et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PloS One. 2012;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry J Ment Sci. 2014;205:376–382. doi: 10.1192/bjp.bp.113.137380. [DOI] [PubMed] [Google Scholar]
- 82.Jang JH, Kim J-H, Jung WH, Choi J-S, Jung MH, Lee J-M, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–162. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 83.Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PloS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu F, Zhu C, Wang Y, Guo W, Li M, Wang W, et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol. 2015;126:1711–1716. doi: 10.1016/j.clinph.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 85.Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, et al. Regional homogeneity changes in social anxiety disorder: A resting-state fMRI study. Psychiatry Res Neuroimaging. 2011;194:47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Andreescu C, Sheu LK, Tudorascu D, Walker S, Aizenstein H. The ages of anxiety-- differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. Int J Geriatr Psychiatry. 2014;29:704–712. doi: 10.1002/gps.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai C-H, Wu Y-T. The alterations in inter-hemispheric functional coordination of patients with panic disorder: the findings in the posterior sub-network of default mode network. J Affect Disord. 2014;166:279–284. doi: 10.1016/j.jad.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 88.Shin Y-W, Dzemidzic M, Jo HJ, Long Z, Medlock C, Dydak U, Goddard AW. Increased resting-state functional connectivity between the anterior cingulate cortex and the precuneus in panic disorder: resting-state connectivity in panic disorder. J Affect Disord. 2013;150:1091–1095. doi: 10.1016/j.jad.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitfield-Gabrieli S, Ford JM. Default Mode Network Activity and Connectivity in Psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 90.Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Res. 2013;211:93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rabinak CA, Angstadt M, Welsh RC, Kennedy A, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Neuropsychiatr Imaging Stimul. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci JPN. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ, Phan KL. Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40:278–286. doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulated cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Simmons AN, Paulus MP, Stein MB. Cognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions. J Affect Disord. 2014;169:76–85. doi: 10.1016/j.jad.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala “aversive amplification” circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry. 2014;1:294–302. doi: 10.1016/S2215-0366(14)70305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pannekoek JN, Veer IM, van Tol M-J, van der Werff SJA, Demenescu LR, Aleman A, et al. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J Affect Disord. 2013;145:29–35. doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro H, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan Y, Herrera-Melendez AL, Pestke K, Feeser M, Aust S, Otte C, et al. Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum Brain Mapp. 2014;35:5328–5339. doi: 10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lueken U, Straube B, Konrad C, Wittchen H-U, Ströhle A, Wittmann A, et al. Neural Substrates of Treatment Response to Cognitive-Behavioral Therapy in Panic Disorder With Agoraphobia. Am J Psychiatry. 2013;170:1345–1355. doi: 10.1176/appi.ajp.2013.12111484. [DOI] [PubMed] [Google Scholar]
- 107.Klumpp H, Keutmann MK, Fitzgerald DA, Shankman SA, Phan KL. Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2014:4. doi: 10.1186/s13587-014-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feusner JD, Moody T, Lai TM, Sheen C, Khalsa S, Brown J, et al. Brain connectivity and prediction of relapse after cognitive-behavioral therapy in obsessive-compulsive disorder. Front Psychiatry. 2015;6:74. doi: 10.3389/fpsyt.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol. 2015;11:361–377. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu W, Yin D, Cheng W, Fan M, You M, Men W, et al. Abnormal functional connectivity of the amygdala-based network in resting-state FMRI in adolescents with generalized anxiety disorder. Med Sci Monit Int Med J Exp Clin Res. 2015;21:459–467. doi: 10.12659/MSM.893373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, et al. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roy AK, Fudge JL, Kelly C, Perry JSA, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:938–948.e3. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jarcho JM, Romer AL, Shechner T, Galvan A, Guyer AE, Leibenluft E, et al. Forgetting the best when predicting the worst: Preliminary observations on neural circuit function in adolescent social anxiety. Dev Cogn Neurosci. 2015;13:21–31. doi: 10.1016/j.dcn.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Supekar K, Musen M, Menon V. Development of Large-Scale Functional Brain Networks in Children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal Asymmetry of Brain Connectivity in Schizophrenia. Front Hum Neurosci. 2014:8. doi: 10.3389/fnhum.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–122. doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological Signatures of Anxiety and Depression in Resting-State Functional Magnetic Resonance Imaging. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Demenescu LR, Kortekaas R, Cremers HR, Renken RJ, van Tol MJ, van der Wee NJA, et al. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 122.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, et al. A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Hum Brain Mapp. 2013;34:3392–3399. doi: 10.1002/hbm.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 127.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buckner RL. Human functional connectivity: New tools, unresolved questions. Proc Natl Acad Sci. 2010;107:10769–10770. doi: 10.1073/pnas.1005987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological low- frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]