Abstract
Inflammatory and immune responses in the brain can shape the clinical presentation and outcome of stroke. Approaches for effective management of acute stroke are sparse and many measures for brain protection fail, but our ability to modulate the immune system and modify the disease progression of multiple sclerosis is increasing. As a result, immune interventions are currently being explored as therapeutic interventions in acute stroke. In this Review, we compare the immunological features of acute stroke with those of multiple sclerosis, identify unique immunological features of stroke, and consider the evidence for immune interventions. In acute stroke, microglia activation and cell death products trigger an inflammatory cascade that damages vessels and the parenchyma within minutes to hours of the ischaemia or haemorrhage. Immune interventions that restrict brain inflammation, vascular permeability and tissue oedema must be administered rapidly to reduce acute immune-mediated destruction and to avoid subsequent immunosuppression. Preliminary results suggest that the use of drugs that modify disease in multiple sclerosis might accomplish these goals in ischaemic and haemorrhagic stroke. Further elucidation of the immune mechanisms involved in stroke is likely to lead to successful immune interventions.
Introduction
Over the past 20 years, progress has been made in the management of patients with acute stroke, including acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH). Intravenous administration of tissue plasminogen activator (tPA) to induce intravenous thrombolysis has become the only FDA-approved medication for AIS, and can salvage dying cells from the ischaemic penumbra, but must be administered within 4.5 h of symptom onset to be beneficial.1,2 ‘Door-to-needle’ times have reduced dramatically so that more patients are eligible for tPA.3,4 Furthermore, six trials support the use of intra-arterial strategies as an alternative or supplement to intravenous thrombolysis in patients who are eligible to receive tPA.5–10 Nevertheless, a significant hiatus exists during which no means of effective medical management is available for patients with acute stroke. Furthermore, over 250 clinical trials, which have included more than 1,000 brain-protective molecules, have failed, showing a critical need for new approaches to developing therapies for acute stroke.11
Inflammation and immune responses have emerged as important elements in the onset and progression of stroke. Several reviews have discussed how individual lymphocyte populations and inflammatory mediators contribute to the development of brain lesions and neurological deficits, mostly in experimental models of stroke.12–16 In this Review, we focus on how the immune system as a whole participates in acute stroke, and the mechanisms involved. We compare the characteristics of stroke, including the sites of immune action and the dynamics and spectrum of inflammation, with those of multiple sclerosis (MS), which is a classic inflammatory and autoimmune disorder of the CNS. These comparisons are made in the context of how disease-modifying drugs control MS. By identifying similarities and differences between the immune mechanisms involved in stroke and MS, we aim to provide insight into how MS disease-modifying drugs could be used to attenuate inflammation and improve clinical outcomes for patients with acute stroke. Results from proof-of-concept clinical trials of fingolimod in AIS and ICH,17,18 together with ongoing studies of natalizumab in AIS, suggest that this approach is feasible.
Immunological features of stroke and MS
The immune response can contribute to the pathogenesis of AIS and ICH at stroke onset, as multiple lymphocyte populations and the proteins that these cells produce have an important role in cell death and the enlargement of brain lesions that result from stroke.13,14 In AIS, the immune response can also contribute to pathogenesis before the onset of stroke: aberrant immune responses can induce inflammation within and around vessel walls, thereby promoting thrombosis, altering vascular reactivity, and encouraging atherosclerosis.19,20 Leukocytes contribute to the growth of atherosclerotic plaques, leading to inflammation, instability and rupture, and occlusion of arteries by atherosclerotic plaques leads to ischaemic events.21 Identifying the immunological features of stroke that are distinct from those of MS provides insight that could be crucial to the design of immunotherapy for stroke. Most of the following discussion focuses on AIS and assumes that ICH shares some of these characteristics (see section Immune interventions in ICH). Discussion of immunomodulation (Box 1) and unique immune mechanisms (relative to MS) apply to AIS and ICH.
Box 1. Key considerations for immune intervention in stroke.
Timing: fast-acting medication for early anti-inflammatory effects during the acute stage and cessation of action after the last dose
Interventions that simultaneously target multiple cellular and soluble components of the immune system
Intravenous formulations are preferable to oral formulations
Baseline immune functions should be determined before the initiation of immunomodulatory treatment; avoid drug treatment if immune function is compromised.
Cellular and humeral immune function should be monitored during therapy
Initiation of disease
At the initiation of disease, the triggers of the inflammatory–immune response differ substantially between stroke and MS (Table 1). Pathogens are thought to initiate inflammation and immune-mediated pathology in MS, although specific triggers cannot be identified in most individuals. The current view is that peripheral activation of the immune system is followed by migration of the myelin-reactive T cells and other antigen-specific or non-specific immune cells into the CNS. Myelin-reactive T cells then undergo in situ expansion after encountering neuroantigens, a process that is aided by antigen-presenting cells that migrate into the CNS (dendritic cells, macrophages, B cells) or that are brain-resident (astrocytes and microglia).22, 23 Consequent primary and secondary immune-mediated destruction of the myelin sheath and axons drives progression of disease in the early phases of MS.24 In the subsequent chronic stage, as the intensity of inflammation diminishes, axonal damage and degeneration (in the cerebral cortex and other neural structures) dominate the pathology (Table 1).24
Table 1.
Contrasting features of immunity and inflammation in stroke and multiple sclerosis
| Features of inflammation and disease stages | Stroke | Multiple sclerosis |
|---|---|---|
| Initiation | ||
| Aetiology | Hypertension, diabetes, hyperlipidaemia | Genetic and environmental factors |
| Triggering events | Cell death products, microglia activation | Mostly unidentified |
| Location of activation signals | Brain and cerebral vessels | Periphery |
| Time kinetics of immunity | ||
| Initiation | Minutes to hours | Years |
| Peak of disease | 3–5 days for AIS, 7–10 days for ICH | Uncertain, several years |
| Chronic stage | Autoimmunity after acute disease | Intensity of inflammation diminishes after 3–5 years |
| Antigen specificity | ||
| Initiation | Minimal or absent | Multiple antigenicity, determinant might change from pathogen to myelin |
| Peak of disease | Antigen presentation and emergence of T cell and B cell reactivity to myelin and neuronal antigens |
Multiple clones of myelin-reactive T cells and B cells |
| Chronic stage | Autoimmunity to myelin, neurons and blood vessels | Relatively constant |
| Immune effector cells | ||
| Initiation | Neutrophils, NK cells, macrophages | Myelin-reactive T cells and B cells |
| Peak of disease | Neutrophils, NK cells, macrophages, T cells and B cells | Myelin-reactive T cells and B cells |
| Chronic stage | Persistent autoimmunity | Reduced role of T cells and B cells, degeneration |
| Role of inflammatory mediators | ||
| Any stage | Probably many, including IL-1, IL-17, MMP-9 | Prrobably many, including TNF-α, IFN-γ, IL-17 |
| Peripheral immune phenotype | ||
| Initiation | Many arms of immunity activated | Persistent myelin reactive T cells, no global alteration |
| Peak of disease | Immune suppression | Immune activation |
| Chronic stage | Persistent autoimmunity | Diminished inflammation in the brain, no change in the periphery |
| Role of inflammation in brain pathology | ||
| Initiation | Circulating leukocytes cross the blood–brain barrier and participate in inflammation in the brain parenchyma that leads to microthrombosis formation |
Lymphocytes cross the blood–brain barrier and drive inflammation |
| Peak of disease | Substantial cell death | Demyelination and axonal changes |
| Chronic stage | Clearance of dead cells, neurogenesis, angiogenesis and/or autoimmunity that can cause cerebral atrophy or recurrence |
Remyelination, axonal loss and neural degeneration, including cortical atrophy |
| Use of immune modulation | ||
| Initiation | Earlier intervention is more efficacious for retaining neural functions | Earlier treatment is more efficacious for retaining neural functions |
| Peak of disease | Caution is required owing to immune suppression, optimal treatment is different for AIS and ICH |
High-dose glucocorticoid hormone or plasma exchange |
| Chronic stage | Unknown, possibilities include targeting autoimmunity or promoting neural regeneration by manipulating inflammation |
Aggressive disease-modifying drugs |
By contrast, the immune–inflammatory response in stroke begins within the brain and its vessels. The cessation of blood supply or direct or indirect effect of hemorrhage quickly induces primary irreversible tissue damage. Secondary processes, such as excitotoxicity, oxidative stress and mitochondrial disturbances, then extend this damage to the partially preserved peri-infarct area (the penumbra). The first immune cells that respond to these events seem to be brain-intrinsic microglia, followed by leukocytes that enter the brain from the periphery through the compromised endothelial cell lining of the blood–brain barrier. The entry of these leukocytes is presumably guided by chemokines that derive from dying neurons.25 Endogenous damage-associated molecular pattern molecules, are also released from dying cells, and favour upregulation of inflammatory mediators.26
Timing
The ways in which the timing of the immune–inflammatory response contributes to the pathogenesis of disease is also crucial for the design of immunomodulatory therapies (Table 1). In MS, the time interval between cell sensitization in the periphery and tissue destruction in the brain or spinal cord can be years, whereas in stroke, the cascade of inflammatory events occurs within minutes or hours of cerebral ischaemia followed by transiently compromised immune functions. Furthermore, cross-talk between lymphocytes and ischaemic neurons can affect the window of time 27, 28 in which immune intervention will be successful (Figure 1).
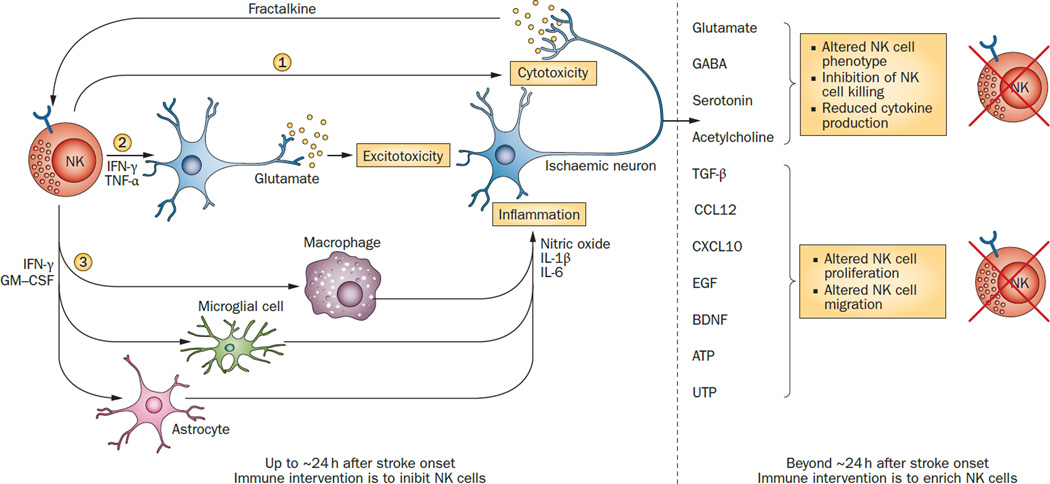
Figure 1. The effects of cross-talk between lymphocytes and ischaemic neurons on the time window for immune intervention.
NK cells are used as an example of lymphocytes. In the early stages of stroke (less than ~24h after onset), fractalkine from ischaemic neurons recruits NK cells to the ischaemic areas.27 These NK cells affect ischaemic neurons in three ways. They directly kill neurons that have lost immunological identity through loss of MHC Ib (1).27 They release cytokines, mainly IFN-γ and TNF-α, that promote glutamate release and lead to neuronal hyperactivity and excitotoxicity (2).27 Finally, they secrete cytokines such as IFN-γ and GM-CSF that activate microglia and macrophages and condition astrocytes, which in turn release inflammatory mediators such as IL-1β, IL-6, and NO (3). At times more than ~24 h after stroke onset, signals from ischaemic neurons can turn off NK cells.98, 116, 117 Peripheral NK cells are also downregulated by the effects of ischaemic brain injury on the sympathetic, parasympathetic and/or hypothalamic–pituitary–adrenal axis systems. Abbreviations: BDNF, brain-derived neurotrophic factor; CCL12, chemokine ligand 12; CXCL10, C–X–C motif chemokine 10; EGF, epidermal growth factor; GABA, γ-aminobutyric acid; GM-CSF, granulocyte macrophage colony-stimulating factor; NK, natural killer; NO, nitrogen oxide; TGF-β, transforming growth factor β; TNF, tumour necrosis factor. Whether this scenario applies to ICH is not known. It is presumed that some features, such as cell trafficking, and the impact of NK cells on neural structures, are shared by ICH.
Immune cells
Autoreactive T cells in the CNS are considered to be important in MS, as they coordinate a number of immune effectors that are detrimental to myelin and other neural structures.24 By contrast, no specific cell population has been identified as a dominant pathogenetic effector in stroke. The kinetics of lymphocyte invasion into the brain immediately after ischaemia in experimental models of stroke, suggest that within hours to days of the initial injury, neutrophils migrate into the brain parenchyma, followed by macrophages and natural killer cells.15,27–29 T and B lymphocytes arrive later.15 Therefore, an immune intervention should be initiated in the very early stages of stroke if it is to be effective (Box 1).
One difficulty in identifying targets for immunotherapy in stroke is that the interactions in the brain between innate immune cells, adaptive immune cells and brain-intrinsic cells are not well understood. In the early stages of brain ischaemia (until 3–5 days after onset), the innate and adaptive immune systems act synergistically in the CNS to promote nonspecific inflammation and antigen-specific immune responses that are analogous to those seen in the periphery. Evidence for the roles of individual immune cell populations (macrophages, natural killer cells and T cells) comes from animal models of brain ischaemia.27,30,31 Inflammatory cytokines, such as IL-1β, IFN-γ, IL-17, MMP-9 and C–C motif chemokine 2, that are produced by these cells damage neural structure directly or indirectly.32–34
Cell death and enlargement of the lesion in stroke are probably sequential events to which multiple cell types, and the soluble molecules they release, contribute. However, ischaemic lesions stabilize in a matter of days, which is insufficient time for an adaptive immune response to develop. Therefore, the ways in which T cells, B cells and regulatory T cells might affect stroke outcomes are unclear. One proposed explanation for the involvement of T cells and B cells in stroke is that cerebrovascular dysfunction and prothrombotic events, promoted by proteins released by these cells after ischaemia lead to microvascular occlusion during the acute stage.35 Subsequently, T cells and B cells can acquire neuroantigen specificity via presentation of released cell death products and become autoreactive, particularly in patients with large lesions, massive cell death and antigen release.36,37 The role of these autoreactive cells is controversial. Some studies suggest that they contribute to post-stroke cognitive decline,36,37 whereas others have not demonstrated a substantial impact of autoimmunity on the outcome of stroke.38 Irrespective of the role of individual lymphocyte subpopulations, treatment with fast-acting agents that target multiple immune cell types could be a reasonable approach after stroke (Box 1).
A salient feature of the neuroimmunological response to stroke that does not occur in MS is immune suppression, which occurs for 3–5 days after stroke onset.39 This phenomenon is discussed in more detail below.
Interventions in ischaemic stroke
Although the immune system has not generally been considered as a target for therapy in stroke, several retrospective observations show that patients with AIS benefit from anti-inflammatory therapy. For example, the platelet inhibitor dipyridamole, which is used for the prevention of stroke, has anti-inflammatory and antioxidative properties that might prevent hypoxia-induced endothelial cytotoxicity and therefore protect neurons.40 Similarly, administration of statins to inpatients and outpatients after AIS is associated with improved survival, whereas statin withdrawal is associated with poorer survival and functional outcomes.41 However, statins act on multiple targets, so identifying the mechanisms that underlie their benefits in stroke is difficult. Indeed, statins have numerous pleiotropic effects owing to their anti-inflammatory, vasodilatory and antithrombotic properties; a combination of these effects could underlie the benefits of statins in patients with acute cerebral vascular disease. Several controlled trials have assessed the efficacy of various immunomodulatory drugs in patients with stroke (Table 2).
Table 2.
Immune interventions in acute stroke
| Drug | Mode of action | Time of first dose after stroke onset |
Duration of treatment |
Phase of trial |
No. of patients |
Clinical and imaging outcomes |
Comments |
|---|---|---|---|---|---|---|---|
| Ischaemic stroke | |||||||
| Enlimomab43 | Anti-ICAM-1 monoclonal antibody |
0–6 h | 5 days | III | 625 | Significant adverse events, increased mRS score, increasedmortality, increased infarct volume |
Negative outcome attributed to the use of a mouse antibody |
| rhIL-1ra45 | IL-1 receptor antagonist | 0–6 h | 3 days | II | 34 | Safe, well tolerated, decreased mRS score | Safe and effective for ischaemic stroke |
| E-selectin47 | Induction of mucosal tolerance |
1–4 months | 30 days | II | 60 | NA | Terminated |
| Minocycline50 | Multiple, including modulation of early inflammatory signalling |
6–24 h | 5 days | Open label |
152 | Safe, well tolerated, decreased mRS score | Effective for experimental stroke, but terminated in a clinical study |
| Fingolimod18 | Multiple, including inhibition of lymphocyte egress from the lymph node into systemic circulation |
4.5–72 h | 3 days | Open label |
23 | Safe, well tolerated, did not promote infections, decreased mRS, limited lesion enlargement, decreased microvascular permeability |
Safe and effective for ischaemic Stroke |
| Fingolimod58 | Multiple, including inhibition of lymphocyte egress from the lymph node into systemic circulation |
No more than 4.5h, combined with alteplase |
3 days | Open label |
47 | Safe, well tolerated, limited haemorrhage transformation and lesion enlargement, decreased mRS |
Combined fingolimod and alteplase attenuated reperfusion injury and improved clinical outcomes |
| Natalizumab62 | Blocking of α4 integrin | 0–9 h | Single dose | II | 200 | NA | Ongoing |
| Haemorrhagic stroke | |||||||
| Celecoxib84 | Selective cyclo-oxygenase 2 inhibitor |
0–24 h | 14 days | Open label |
44 | Safe, well tolerated, mRS score not improved at 90 days, decreased PHO volume |
Functional outcome not improved |
| Rosiglitazone85 | PPAR-γ agonist, promotes phagocytosis of RBCs by microglia |
0–24 h | 3 days | II | 84 | NA | Ongoing |
| Minocycline89 | Multiple, including modulation of early inflammatory signalling |
0–12 h | 5 days | Open label |
24 | NA | Ongoing |
| Fingolimod17 | Multiple, including inhibition of lymphocyte egrees from the lymph node into systemic circulation |
4.5–72 h | 3 days | Open label |
22 | Safe, well tolerated, did not promote infections; decreased mRS scores, decreased PHO volume |
Safe and effective for haemorrhagic stroke |
Abbreviations: mRS, modified Rankin scale; NA, not available; RBC, red blood cell; rhIL-1ra, recombinant human IL-1 receptor antagonist.
Anti-ICAM-1 antibody
Early attempts to treat stroke with anti-ICAM-1 antibody were unsuccessful. Trials in rat models of AIS initially indicated that infusion of anti-ICAM-1 antibody led to significant improvements in neurological outcomes,42 but in a prospective phase III study, greater infarct volumes and higher mortality were seen in patients who received murine anti-ICAM-1 monoclonal antibody (enlimomab) within 6 h of stroke onset than in patients who received a placebo.43 This failed trial is representative of others that, in retrospect, led to an understanding that use of murine antibodies can activate neutrophils through complement-dependent mechanisms.44
IL-1 receptor antagonist
Blockade of IL-1 receptors with the antagonist IL-1ra has also been attempted for treatment of AIS. A randomized phase IIa trial that included 34 patients with acute stroke45 showed that recombinant human IL-1ra was safe, readily crossed the blood–brain barrier and seemed to provide some benefits, particularly to patients with cortical infarcts. Scores on the NIH stroke scale reduced by a median of four points for at least 3 months after treatment among patients who received IL-1ra and by just one point among patients who received a placebo. Furthermore, 30% of patients who received IL-1ra, compared with just 7% of patients who received a placebo, had a modified Rankin scale (mRS) score of 0–1 at 3 months after treatment. More information on the pharmacokinetics of IL-1ra, particularly the timing of its entry to the brain, is now required. A dose-range (phase IIb) study is also needed to identify the most effective dose of IL-1ra.
E-selectin
The observation of unregulated E-selectin expression on the endothelium systemically within a few hours of focal cerebral ischaemia in experimental stroke prompted further investigation E-selectin as a possible therapy, despite the fact that serum levels of E-selectin are not elevated in patients after stroke.46 Evidence suggests that transnasal E-selectin tolerization attenuates cerebral ischaemic damage in experimental stroke, and studies are underway to prepare for clinical trials of E-selectin for secondary prevention of stroke.47,48
Minocycline
In an open-label clinical trial, treatment with oral minocycline resulted in mRS scores that were significantly lower after 90 days in patients with AIS than in untreated patients.49 A phase IIb clinical study established drug safety, dose range and feasibility (when minocycline was used in combination with tissue plasminogen activator,50 but a phase IV clinical trial to determine the efficacy of minocycline in stroke and long-term recovery was recently deemed to be futile and consequently terminated.51
Fingolimod
Fingolimod, in its phosphorylated form, is a high-affinity agonist of four sphingosine-1-phosphate (S1P) receptors (S1P1, S1P3, S1P4 and S1P5) The drug was the first to be approved by the FDA for the treatment of relapsing–remitting MS, and affects the numbers, trafficking and apoptosis of lymphocytes.
In stroke, fingolimod reduces the number of circulating lymphocytes by preventing their egress from lymph nodes, and might help to prevent early infiltration of lymphocytes to the brain and local activation of microglia and/or macrophages.52, 53 Fingolimod can cross the blood–brain barrier and can therefore directly affect the CNS.54,55
The pharmacodynamics of fingolimod were characterized in the context of reversible transient lymphopaenia.56 Within 6 h of the first dose, lymphocyte numbers had fallen to their lowest level at 42% of the baseline numbers; the lymphocyte count returned to baseline within 72 h of the last dose. Oral single dose of 0.25–3.50 mg fingolimod are well tolerated.56 Fingolimod’s elimination half-life is 89–157 h.
The benefits of targeting S1P receptors in brain ischaemia have been demonstrated in preclinical study. 57 In an open-label trial, oral fingolimod was administered to patients with anterior cerebral circulation occlusion and an onset of stroke that exceeded 4.5 h.18 The first dose (0.5 mg) was administered within 72 h of the ictus, and treatment continued for 3 days. As early as 24 h from the first dose, the counts of CD4+, CD8+ and CD19+ B cells were lower in patients who received fingolimod than in patients who received standard management. Between baseline and day 7, fingolimod restricted enlargement of the infarct volume and reduced microvascular permeability. The treatment was also associated with short-term neurological improvements, and no safety concerns arose. In a multi-center study, combination of t-PA with fingolimod appeared reduce the hommorghic transformation induced by t-AP in AIS patients within 4.5 hours of disease onset.58 Collectively, the fast action of fingolimod on multiple lymphocyte subsets, which ceased promptly after the last dose, might be instructive in future selection of immunomodulatory drugs in stroke trials. Medications with similar properties would be considered as appropriate candidates for future trials
Natalizumab
The antibody natalizumab is currently one of the most effective therapies for relapsing–remitting MS. The antibody blocks α4-integrin, which normally mediates the invasion of lymphocytes into the CNS. In experimental AIS, natalizumab has shown a similarly protective effect through acute blockade of T cell infiltration into the brain.59 Another study, however, showed no protection.60 An international consortium is currently co-ordinating a preclinical phase III trial of natalizumab in mouse models of stroke to determine whether specific inhibition of T cell trafficking into the ischaemic brain improves outcomes.61 Furthermore, enrolment is complete for a phase II clinical trial to determine how natalizumab affects infarct volume in AIS.62
Cell transplantation
Cell transplantation could modulate inflammation and directly promote tissue repair in stroke.63 A single-blind controlled phase I–II trial of this approach was conducted in patients with subacute middle cerebral artery ischaemic stroke.64 In this study, patients who received intra-arterial injection of autologous bone marrow mononuclear cells at 5–9 days after stroke had higher levels of plasma β-nerve growth factor than did untreated patients. The treated patients also exhibited non-significant improvements in neurological outcomes. Multipotent adherent bone marrow cells (MultiStem®) are now being tested in patients with cortical cerebral ischaemic stroke.65 MultiStem® will be administered intravenously at 24–36 h after the ischaemic event to determine its effects on the recovery of motor function after brain ischemia.65
In summary, a common thread in the above listed controlled trials in patients with ischaemic stroke indicates is the targeting of immune pathways that include adhesion molecules essential for lymphocyte trafficking (e.g., anti-ICAM1, fingolimod, and α4- integrin) or key inflammatory mediators (IL-1). Ongoing studies, and some that have been published within the past year,17, 18 target pathways of cell migration, activation and other effector functions that are shared by many lymphocyte subsets. The development of compounds with improved pharmacodynamics and precise targeting are anticipated to bolster new trials of this kind.
Interventions in ICH
ICH accounts for 10–15% of all strokes and for the most fatalities across all stroke subtypes. Mortality for patients with ICH is 30–50% within the first year, and 74% among survivors after the first year.66 No effective therapy has been established beyond general critical management of the acute event.67,68
In contrast to AIS, no evidence exists for a role of inflammation in the aetiology of ICH. ICH is frequently associated with hypertensive cerebral microangiopathy in the basal ganglia and brain stem, with cerebral amyloid angiopathy in cortical arteriolar and vennular microvessels in the elderly, and with the use of oral anticoagulants.69,70 However, inflammation is triggered by ICH, and secondary brain injury that contributes to the clinical presentation and outcome of ICH is at least partly caused by this inflammation.14,16 The overall process is similar to that in AIS, but with a longer period of inflammation and oedema.
The first cells that respond to the insults of ICH are the brain-resident microglia. In experimental ICH, microglia activation occurs as early as 1 h after collagenase injection.74 Activated microglia release cytokines (including tumour necrosis factor-α [TNF-α] and IL-1β),75,76 chemokines (including C–X–C motif chemokine 2)77 and reactive oxygen species. These inflammatory mediators, together with products of cell death (e.g. heme and iron), cause breakdown of the blood–brain barrier and consequent influx of neutrophils, monocytes and macrophages, followed by T cells and possibly other lymphocyte populations.28,30 Activation of resident and migrant cells fuels the inflammatory process surrounding the haematoma, which causes perihaematomal oedema (PHO).
The extent of PHO correlates with the activity of inflammatory cytokines and matrix metalloproteinase.78 Inflammation-associated upregulation of specific ion channels leads to ion and water perturbations, PHO and lymphocyte infiltration. PHO exacerbates the mass effect of intracerebral blood and catalyzes secondary brain tissue damage and neurological deterioration through secondary ischaemic and inflammatory insults.79,80 Attenuation of brain swelling is, therefore, a plausible approach to preventing the destructive effects of PHO and the resultant secondary brain injury. Evidence that supports the use of this approach comes from retrospective studies that showed statin use to decrease PHO and improve outcomes of ICH.81,82 Several studies have tested other approaches to reducing brain swelling.
Celecoxib
Treatment with celecoxib, a selective inhibitor of cyclo-oxygenase 2, after ICH reduced inflammatory cell infiltration, brain oedema and subsequent perihaematomal cell death.83 A multicentre trial of celecoxib that included 44 patients also showed that administration of celecoxib in the acute stage of ICH was associated with less expansion of PHO.84
PPAR-γ
PPAR-γ is a ligand-dependent transcription factor that regulates the expression of CD36, which is itself a scavenger receptor that is important for phagocytic activity. In a mouse model of ICH, treatment with PPAR-γ agonists, such as rosiglitazone, increased CD36 expression and promoted phagocytosis of red blood cells by microglia and/or phagocytes.71 These findings suggest that CD36-mediated phagocytosis is critical in the mechanism by which PPAR-γ agonists lead to haematoma resolution.85 The PPAR-γ agonists rosiglitazone and pioglitazone are approved by the FDA for glycaemic control in type 2 diabetes mellitus, and an ongoing phase II dose escalation trial is assessing the safety of pioglitazone use for haematoma resolution after ICH.85
Minocycline
Minocycline seems to be beneficial in a rat model of ICH, even when administered up to 6 h after the insult is induced. 3 days after intracerebral blood injection, the extent of brain oedema was lower in rats that received minocycline than in those that received vehicle, and neurological deficits were also reduced.86 Minocycline also reduced the number of microglia and macrophages around the haematoma at 5 days after ICH,87 preserved microvessels, reduced brain water content, and lowered levels of TNF-α and MMP-12.88 A randomized, single-blinded clinical trial of minocycline in ICH is underway.89
Fingolimod
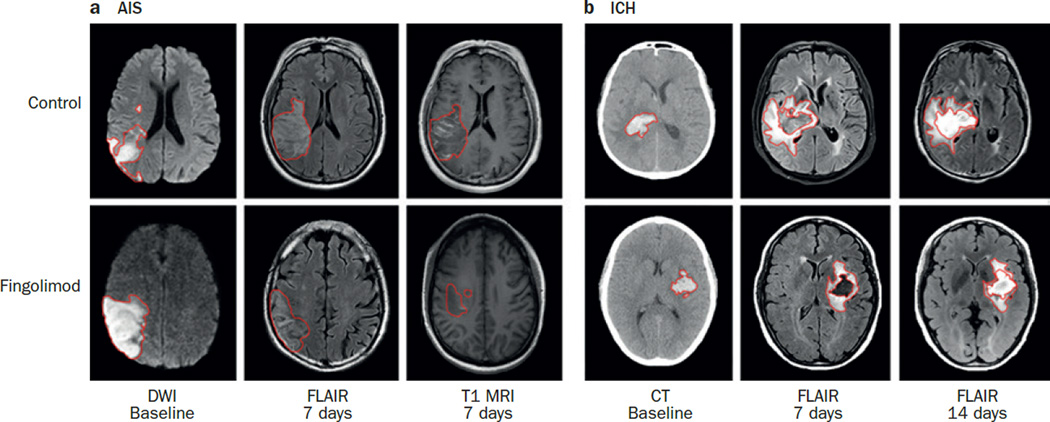
Preclinical experiments have indicated that fingolimod can reduce oedema, apoptosis and brain atrophy in animal models of ICH.90,91 In a two-arm proof-of-concept study in humans, 11 participants were treated with 0.5 mg oral fingolimod daily for 3 days after ICH; the first dose was administered within 72 h of the ictus. 17 Short-term and long-term neurological functions were better in participants who received fingolimod than in participants who did not receive fingolimod. Soon after administration, fingolimod also lowered the number of circulating macrophages, natural killer cells, CD4+ cells and CD8+ cells, and reduced levels of MMP-9. These effects suggest that fingolimod reduces the migration of these cells and inflammatory mediators to the brain after ICH.92 The drug also suppressed the increase in PHO that normally occurs in the first week after ICH (Figure 2) and protected the vascular barrier. Collectively, the immune modulation of fingolimod seemed to improve clinical outcomes.
Figure 2. The effect of fingolimod in patients with AIS and ICH.
Red outlines indicate the sizes of lesions. a | Representative MRI images from patients with AIS who were treated with fingolimod or with standard management.18 Infarct locations and occlusion of arteries were similar at baseline in the patients shown (left). Fingolimod treatment led to a significant reduction in lesion size after 7 days (middle). CET1 imaging showed that the acute ischaemic lesion was smaller in patients who received fingolimod than in controls (right). b | Representative CT and MRI images from patients with ICH who were treated with fingolimod or with standard management.17 Both patients had basal ganglia region haemorrhage and haematoma volumes were similar at baseline. Fingolimod led to a marked resolution of oedema and no midline shift, whereas in control patients, oedema persisted during days 7–14 and was accompanied by a midline shift. Abbreviations: AIS, acute ischaemic stroke; CET, contrast-enhanced T1-weighted; DWI, diffusion-weighted imaging; ICH, intracerebral haemorrhage.
Verification of these results is needed in large-scale studies, but a more immediate question regarding the optimal timing of fingolimod treatment must be addressed. After ICH, the volume of PHO increases by 75% in the first 24 h, peaks 5–6 days later, and lasts for up to 14 days. The window for effective immune intervention might, therefore, be wider in ICH than in AIS (Box 2). Treatment that is started within 72 h and includes three doses—as used in studies to date—seem to fall within this time-frame,17 but now we need to determine whether starting treatment earlier and continuing for 5 days would further improve clinical outcomes.
Box 2. Outstanding questions in immune interventions in stroke.
Unique immune mechanisms in stroke
What can we learn from models of stroke about the nature of disease and management of patients?
How can we study the contributions of inflammation and immunity to stroke?
What is the temporal relationship between immune-mediated damage and immune deficiency in stroke?
How are immune function and immune surveillance within the brain altered during stroke?
How do brain-intrinsic cells and migrant lymphocytes interact?
Which inflammatory elements promote neurorepair? How and when do they promote neurorepair?
How is the immune response to pneumonia or urinary tract infections altered by stroke?
Practical challenges in designing immunotherapies for stroke
How can immune-mediated cell death be limited without exacerbating immune deficiency?
Can anti-inflammatory therapy reduce the vasogenic oedema and reperfusion injury that are associated with tissue plasminogen activator?
Can anti-inflammatory therapy extend the therapeutic time window for tissue plasminogen activator?
Can anti-inflammatory therapy promote neuroprotection?
Does the size of an infarct or haematoma predict the benefit of immune modulation?
Is the time window for immunomodulation longer in ICH than in ischaemic stroke?
Post-stroke immunodeficiency
Severe, acute insults to the CNS (such as traumatic brain or spinal cord injury and stroke) have a marked impact on the immune system. Within days of AIS, patients develop lymphopaenia, and in animals and humans, the spleen size is reduced,93,94 although both subsequently recover. The volume of the brain infarct in AIS has been directly associated with the extent of lymphocytopaenia and monocyte dysfunction.95 This association can be explained by activation of the sympathetic, parasympathetic (cholinergic anti-inflammatory) and hypothalamo–pituitary–adrenal axis pathways,96–98 which leads to increased circulating levels of noradrenaline, acetylcholine and glucocorticoids. Abrupt elevation of these mediators in combination impairs development, trafficking and effector functions of lymphocytes, and ultimately induces apoptosis and consequent atrophy of lymphoid organs such as the spleen.
This downregulation of immune responses that originates in the injured brain avoids autoimmunity against brain antigens that are released during cell death. However, immunosuppression varies in its extent according to the volume of the infarct,95 and can manifest as high rates of systemic infection in the immediate post-stroke period. A meta-analysis of 87 studies that included 137,817 patients showed that infections, including pneumonia and urinary tract infection, complicated AIS in ~30% of patients, and were associated with subsequent death in this context.99
Stroke-induced immunosuppression poses a considerable challenge in the use of immune intervention during the acute phase of stroke, particularly with approaches that are designed to limit immune-mediated brain damage. Immune-based intervention should end within days, i.e. two or three days of stroke onset, ideally before immunosuppression occurs. Early-stage intervention could also improve the options for later immune intervention by boosting immunity and promoting neurorepair (Figure 1).
Therapies to prevent post-stroke immunodeficiency can target three mechanisms. First, antagonists of neurosteroid receptors or adrenergic receptors could counteract lymphocyte apoptosis, reduce the rate of infection, decrease mortality and improve functional outcomes by increasing CNS antigen-specific autoreactivity in experimental stroke.38,97 Use of β-blockers seems to lower the incidence of pneumonia and decrease mortality after stroke,100 although this approach requires verification. The challenge in developing such therapies is to identify compounds that target only excess circulating neurotransmitters to avoid unwanted effects on heart rate, vascular tone and blood supply to the brain.
The second approach is prophylactic administration of antibiotics to protect against infection. This approach is particularly relevant to individuals with severe stroke who are more prone to infectious complications.95 In a double-blind, randomized controlled trial (The Early Systemic Prophylaxis of Infection after Stroke [ESPIAS] study), prophylactic levofloxacin failed to improve outcomes over those achieved with optimal care for the prevention of infections.101 However, another double-blind, randomized controlled trial (The Preventive Antibacterial Therapy in Acute Ischemic Stroke, PANTHERIS) in patients with severe middle cerebral artery stroke suggests that moxifloxacin can reduce post-stroke infections.102 A prospective meta-analysis revealed that preventative antibiotic therapy reduced the incidence of infection from 36% to 22% without significantly affecting mortality,103 although differences between study populations and designs meant the meta-analysis had insufficient power to draw a definitive conclusion about differences in mortality.
The Preventive Antibiotics in Stroke Study (PASS) attempted to overcome this problem. In this trial, 2,550 patients were randomized to receive either standard care alone or in combination with 2 g ceftriaxone by continuous intravenous infusion over 4 days, followed by 3 months of monitoring to assess functional outcomes.104 The results suggest that preventative ceftriaxone does not improve functional outcomes at 3 months in adults with acute ischaemic stroke. However, subgroup analysis suggested that preventative antibiotic treatment reduces the risk of disability in patients who received intravenous thrombolysis treatment. Another trial that used antibiotics to prevent infection in stroke has been completed in the UK and the results are eagerly waited.105
The third, and possibly the most physiological, approach is to boost immune function in patients with stroke, but this approach has not been attempted in experimental models or patients. The principle is to use engineered human cytokines to promote recovery of specific lymphocyte populations that are depressed by stroke. When to initiate this therapy and which types of cells or immune components to target are unclear.
Designing an effective immune intervention for acute stroke might only be possible when the timing of immunodeficiency in stroke has been pinpointed and we have a greater understanding of the cells that play a major role in the immune response to infection after stroke, and the cells and factors that promote neurorepair. For example, early immune intervention could block a neuroprotective effect of microglia,106,107 so immune intervention must balance the prevention of immune-mediated damage with allowing inflammatory responses to promote neurorepair.
Practical considerations
Patient selection
Theoretically, patients with the largest infarct volumes or haematomas are most likely to benefit from immune modulation, because inflammation is more pronounced in these patients (Box 2). However, no controlled clinical trials have tested this hypothesis. One study showed that eight patients with total or partial occlusion of the anterior circulation had much better outcomes than three patients with lacunar syndrome that resulted from occlusion of deep branches in the anterior circulation. 18 Similarly, a study of patients with ICH showed that fingolimod had a greater benefit in 6 patients with haematoma volumes of 15–30 ml than in 5 patients with haematoma volumes of 5–15 ml.17 Larger haematoma volumes were associated with greater changes in NIHSS score from baseline to day 7 and day 90.
Timing
The time window in which immune modulation could reverse post-stroke immune deficiency and reduce the associated complications is largely determined by the time at which immune deficiency develops (Figure 1). Given that inflammation develops within hours of stroke onset, an earlier immune intervention should better prevent brain damage. In studies to date, immunomodulatory drugs have typically been administered at 6–72 h after stroke onset. The time window might be narrower in ICH than in AIS because PHO persists for ~2 weeks after ICH, but this hypothesis needs verification (Box 2).
Drug formulation
Intravenous administration is probably the optimal route of delivery for immunomodulatory drugs in patients with stroke, particularly severe stroke, because compromised cognitive function and ability to swallow means oral administration might not be feasible. Altered perfusion of the gastrointestinal tract in bed-bound patients or in patients with unstable blood pressure can also affect the absorption of medications, making oral administration an unreliable way to ensure the correct dosage.
Safety
Long-term use of the latest generation of disease-modifying drugs, such as natalizumab and fingolimod, in MS has been associated with rare but fatal adverse effects, including progressive multifocal leukoencephalopathy (PML) and herpesviral encephalitis.108 The incidence of these adverse effects was significantly higher in patients who had previously been treated with other immunotherapy. 109 Compromised immune surveillance as a result of using these disease-modifying drugs is believed to allow the development of PML.110
The adverse effects that are associated with these drugs when used in MS are less likely to occur when they are used in stroke because they are not used for as long, but a better understanding of the immune environment in the CNS after stroke is imperative to understand the risks.
Conclusions and future perspectives
Evidence suggests that targeting inflammation and immune responses could be a viable approach to rescuing brain tissue and improving outcomes after stroke. However, multiple failed attempts to develop such therapies for stroke cast doubt on new attempts. Targeting of the highly dynamic events that occur during inflammation in the relatively inaccessible brain microenvironment is challenging, and an incomplete understanding of the interactions between the immune system and the brain during stroke limits progress.
Animal models and phase I and II human studies have enabled progress towards understanding immunity in stroke, but relatively little work has been done in this area. The variation between commonly used models of stroke offers an opportunity to investigate the spectrum of neuroinflammation that occurs in this highly diverse disease. Animal models represent only a segment of disease pathogenesis, so what we can learn from them might be limited. However, emerging tools, such as molecular imaging enable particular cell populations to be followed in vivo during the course of stroke. For example, activated microglia can be visualized in humans by using a PET ligand that binds to microglia translocator protein or by using a PET tracer (l-deuteriodeprenyl) to reveal reactive astrocytes during brain inflammation.111,112 Ultrasmall superparamagnetic iron oxide contrast agent-enhanced MRI can be used to study the role of macrophages in the development of ischaemic lesions in experimental ischaemia and human stroke.113–115 This approach can shed light on the roles of these cells after onset of ischaemia, and could determine when immune status is altered after disease onset by revealing morphological features of the cells.
Use of high-output approaches, such as proteomic analysis, gene expression microarrays and quantitative imaging, in future trials that include large cohorts of patients could provide valuable information on the relationship between immunity and stroke subtype, localization, time course and comorbidities. Successful immune system intervention with drugs such as fingolimod or natalizumab also provides an opportunity to understand their effects on cellular interactions and on disease outcomes.
Clinical trials in acute stroke require the expertise of specialized neurologists and neuroradiologists to interpret the clinical, immunological and neuroimaging readouts, which can delay the initiation of necessary therapy, i.e. tPA infusion and therefore pose a risk to patients. Better coordination of these elements and streamlining the efforts contributed by these parties would likely minimize the risk of delaying administration of therapy to patients.
To date, only seven clinical trials have assessed the effects of immune modulators in AIS, and one trial in ICH. Most of those trials were early-phase, proof-of-concept studies, but gave promising preliminary results. Since clinical trials of enlimommab failed 20 years ago because of unwanted immune activation by murine antibodies, techniques have advanced and many questions about immune mechanisms and therapeutic design in stroke are being addressed with new intensity (Box 2). The combination of improved understanding about immune factors in the disease and improved abilities to manipulate immune responses without adverse effects has given rise to cautious optimism in the exploration of new modalities to reduce inflammatory responses and immune-mediated damage in stroke.
Review criteria.
We searched PubMed for English-language articles that were published between January 1950 and June 2015. We used the terms “inflammation in cerebral h(a)emorrhagic stroke”, “immunity in cerebral h(a)emorrhagic stroke”, “inflammation in cerebral isch(a)emic stroke”, “immunity in cerebral isch(a)emic stroke”, “sensitization”, “immune and brain”, “ innate immunity and adaptive immunity in stroke”, “immune therapies in stroke”, “immune suppression after stroke”, “post-stroke infection”, “specific antigenic recognition in stroke”, “autoimmunity in stroke” and inflammation and neural repair in stroke”. We selected articles reporting all clinical and preclinical findings on immune modulations in stroke.
Acknowledgments
The authors would like to thank members of their laboratories and clinical teams for their passionate work in immunology and stroke. They would also like to thank D. Huang (Neurology and Neuroscience Associates, Unity Health Network, Akron, OH), T. Vollmer (Department of Neurology, University of Colorado School of Medicine, Aurora, CO) and A. La Cava (Department of Medicine, University of California, Los Angeles, Los Angeles, CA) for fruitful discussions and advice and P. Minick and K. Wood (Department of Neurology, Barrow Neurological Institute, Phoenix, AZ) for editorial assistance. The authors’ research programs are supported in part by the NIH (R01AI083294, R01NS34179 and R01NS081179), the American Heart Association (14GRNT18970031), the Foundation Leducq, the National Basic Research Program of China (2013CB966900), the National Natural Science Foundation of China (81230028) and the National Key Clinical Specialty Construction Project of China. This paper is dedicated to our families.
Footnotes
Competing interests
- Recognition that immune mechanisms contribute to stroke and an increasing ability to manipulate the immune system suggests that immunomodulation maybe a feasible therapy for acute stroke
- Immune interventions, including nonspecific anti-inflammatory drugs and approaches that target immune cells, inflammatory mediators and adhesion molecules, have been tested in patients with acute ischaemic stroke, with mixed outcomes
- Proof-of-concept studies have demonstrated that fingolimod can attenuate brain inflammation and improve neurological outcomes in acute stroke; a large international trial of natalizumab is nearly complete
- Trials have shown that drugs that target multiple elements of the immune system and act quickly could be viable candidates
- Future success of immunomodulation as a therapy for stroke depends on elucidation of immune interactions during stroke, and on the ability to limit immune-mediated tissue damage and promote tissue repair
References
- 1.Albers GW, et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 2.Lees KR, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313:1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 7.Kidwell CS, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broderick JP, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccone A, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkhemer OA, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 11.O’Collins VE, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 12.Macrez R, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamorro A, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 16.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkind MS. Inflammatory mechanisms of stroke. Stroke. 2010;41:S3–S8. doi: 10.1161/STROKEAHA.110.594945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marnane M, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke. 2014;45:801–806. doi: 10.1161/STROKEAHA.113.003657. [DOI] [PubMed] [Google Scholar]
- 21.Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 2014;71:233–236. doi: 10.1001/jamaneurol.2013.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao J, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao J, et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann Neurol. 2011;69:721–734. doi: 10.1002/ana.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- 25.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;1(147 Suppl):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan Y, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A. 2014;111:2704–2709. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 29.Hammond MD, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond MD, Ambler WG, Ai Y, Sansing LH. alpha4 integrin is a regulator of leukocyte recruitment after experimental intracerebral hemorrhage. Stroke. 2014;45:2485–2487. doi: 10.1161/STROKEAHA.114.005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 32.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 33.Lambertsen KL, et al. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63:942–955. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- 34.Seifert HA, et al. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol. 2014;9:679–689. doi: 10.1007/s11481-014-9560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 36.Doyle KP, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci. 2015;35:2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker KJ, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romer C, et al. Blocking stroke-induced immunodeficiency increases CNS antigen-specific autoreactivity but does not worsen functional outcome after experimental stroke. J Neurosci. 2015;35:7777–7794. doi: 10.1523/JNEUROSCI.1532-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meisel C, Meisel A. Suppressing immunosuppression after stroke. N Engl J Med. 2011;365:2134–2136. doi: 10.1056/NEJMcibr1112454. [DOI] [PubMed] [Google Scholar]
- 40.Sacco RL, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarenco P, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 42.Zhang RL, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 43.Enlimomab Acute Stroke Trial, I. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 44.Furuya K, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 45.Emsley HC, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shyu KG, Chang H, Lin CC. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischaemic stroke. J Neurol. 1997;244:90–93. doi: 10.1007/s004150050055. [DOI] [PubMed] [Google Scholar]
- 47.E-Selectin Nasal Spray to Prevent Stroke Recurrence. [accessed May 2010]; clinicaltrials.gov/ct2/show/NCT00012454.
- 48.E-Selectin Nasal Instillation to Prevent Secondary Stroke. [accessed May 2014]; clinicaltrials.gov/ct2/show/NCT00069069.
- 49.Lampl Y, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 50.Fagan SC, et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuroprotection With Minocycline Therapy for Acute Stroke Recovery Trial (NeuMAST) [accessed May 2013]; clinicaltrials.gov/ct2/show/NCT00930020.
- 52.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 53.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. N Engl J Med. 2006;355:1088–1091. doi: 10.1056/NEJMp068159. [DOI] [PubMed] [Google Scholar]
- 54.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109:15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 56.Budde K, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13:1073–1083. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Z, et al. Combination of an immune modulator fingolimod with alteplase in acute ischemic stroke: a randomized multi-center study. Circulatin. in press doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liesz A, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 60.Langhauser F, et al. Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45:1799–1806. doi: 10.1161/STROKEAHA.114.005000. [DOI] [PubMed] [Google Scholar]
- 61.Schabitz WR, Dirnagl U. Are we ready to translate T-cell transmigration in stroke? Stroke. 2014;45:1610–1611. doi: 10.1161/STROKEAHA.114.005294. [DOI] [PubMed] [Google Scholar]
- 62.Effect of Natalizumab on Infarct Volume in Acute Ischemic Stroke (ACTION) [accessed June 2014]; clinicaltrials.gov/ct2/show/NCT01955707.
- 63.Liu X, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moniche F, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 65.Hess DC, et al. A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke. 2014;9:381–386. doi: 10.1111/ijs.12065. [DOI] [PubMed] [Google Scholar]
- 66.van Asch CJ, Oudendijk JF, Rinkel GJ, Klijn CJ. Early intracerebral hematoma expansion after aneurysmal rupture. Stroke. 2010;41:2592–2595. doi: 10.1161/STROKEAHA.110.589291. [DOI] [PubMed] [Google Scholar]
- 67.European Stroke Initiative Writing, C et al. Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 68.Trabert J, Steiner T. [Deep vein thrombosis and lung embolisms in patients with stroke: prevention and therapy] Nervenarzt. 2014;85:1315–1325. doi: 10.1007/s00115-014-4031-9. [DOI] [PubMed] [Google Scholar]
- 69.Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 2005;4:662–672. doi: 10.1016/S1474-4422(05)70195-2. [DOI] [PubMed] [Google Scholar]
- 70.Sutherland GR, Auer RN. Primary intracerebral hemorrhage. J Clin Neurosci. 2006;13:511–517. doi: 10.1016/j.jocn.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- 72.Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J Neurotrauma. 2004;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- 73.Qureshi AI, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 75.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 76.Lin S, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsushita H, et al. Suppression of CXCL2 upregulation underlies the therapeutic effect of the retinoid Am80 on intracerebral hemorrhage in mice. J Neurosci Res. 2014;92:1024–1034. doi: 10.1002/jnr.23379. [DOI] [PubMed] [Google Scholar]
- 78.Li N, et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke. 2013;44:658–663. doi: 10.1161/STROKEAHA.112.673590. [DOI] [PubMed] [Google Scholar]
- 79.Schellinger PD, et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke. 2003;34:1674–1679. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- 80.Zazulia AR, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–810. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Tapia-Perez H, et al. Use of statins for the treatment of spontaneous intracerebral hemorrhage: results of a pilot study. Cent Eur Neurosurg. 2009;70:15–20. doi: 10.1055/s-0028-1082064. [DOI] [PubMed] [Google Scholar]
- 82.Flint AC, et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 2014;71:1364–1371. doi: 10.1001/jamaneurol.2014.2124. [DOI] [PubMed] [Google Scholar]
- 83.Chu K, et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24:926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- 84.Lee SH, et al. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. Eur J Neurol. 2013;20:1161–1169. doi: 10.1111/ene.12140. [DOI] [PubMed] [Google Scholar]
- 85.Safety of Pioglitazone for Hematoma Resolution In Intracerebral Hemorrhage (SHRINC) [accessed May 2013]; doi: 10.1111/j.1747-4949.2011.00761.x. clinicaltrials.gov/ct2/show/NCT00827892. [DOI] [PubMed]
- 86.Wu J, et al. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:147–150. doi: 10.1007/978-3-211-98811-4_26. [DOI] [PubMed] [Google Scholar]
- 87.Szymanska A, Biernaskie J, Laidley D, Granter-Button S, Corbett D. Minocycline and intracerebral hemorrhage: influence of injury severity and delay to treatment. Exp Neurol. 2006;197:189–196. doi: 10.1016/j.expneurol.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–237. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 89.A Pilot Study of Minocycline in Intracerebral Hemorrhage Patients (MACH) [accessed January 2015]; clinicaltrials.gov/ct2/show/NCT01805895.
- 90.Rolland WB, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rolland WB, 2nd, et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li YJ, et al. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci Bull. 2015 doi: 10.1007/s12264-015-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ajmo CT, Jr, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahota P, et al. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke. 2013;8:60–67. doi: 10.1111/ijs.12022. [DOI] [PubMed] [Google Scholar]
- 95.Hug A, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 96.Mracsko E, et al. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 98.Prass K, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dziedzic T, Slowik A, Pera J, Szczudlik A. Beta-blockers reduce the risk of early death in ischemic stroke. J Neurol Sci. 2007;252:53–56. doi: 10.1016/j.jns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Chamorro A, et al. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36:1495–1500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- 102.Harms H, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS One. 2008;3:e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van de Beek D, et al. Preventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch Neurol. 2009;66:1076–1081. doi: 10.1001/archneurol.2009.176. [DOI] [PubMed] [Google Scholar]
- 104.Westendorp WF, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–1526. doi: 10.1016/S0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]
- 105.Antibiotics to Prevent Infections in Stroke. [accessed July 2007]; ISRCTN37118456. [Google Scholar]
- 106.Zhang L, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neumann J, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yousry TA, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bloomgren G, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 110.Trampe AK, et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology. 2012;78:1736–1742. doi: 10.1212/WNL.0b013e3182583022. [DOI] [PubMed] [Google Scholar]
- 111.Ribeiro MJ, et al. Could (18) F-DPA-714 PET imaging be interesting to use in the early post-stroke period? EJNMMI Res. 2014;4:28. doi: 10.1186/s13550-014-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santillo AF, et al. In vivo imaging of astrocytosis in Alzheimer’s disease: an (1)(1)C-L-deuteriodeprenyl and PIB PET study. Eur J Nucl Med Mol Imaging. 2011;38:2202–2208. doi: 10.1007/s00259-011-1895-9. [DOI] [PubMed] [Google Scholar]
- 113.Doerfler A, et al. MR contrast agents in acute experimental cerebral ischemia: potential adverse impacts on neurologic outcome and infarction size. J Magn Reson Imaging. 2000;11:418–424. doi: 10.1002/(sici)1522-2586(200004)11:4<418::aid-jmri10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 114.Rausch M, et al. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage. Magn Reson Med. 2001;46:1018–1022. doi: 10.1002/mrm.1290. [DOI] [PubMed] [Google Scholar]
- 115.Saleh A, et al. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- 116.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 117.Hao J, et al. Nicotinic receptor beta2 determines NK cell-dependent metastasis in a murine model of metastatic lung cancer. PLoS One. 2013;8:e57495. doi: 10.1371/journal.pone.0057495. [DOI] [PMC free article] [PubMed] [Google Scholar]