Abstract
Background
Triatomines are hematophagous insects that act as vectors of Chagas disease. Rhodnius neglectus is one of these kissing bugs found, contributing to the transmission of this American trypanosomiasis. The saliva of hematophagous arthropods contains bioactive molecules responsible for counteracting host haemostatic, inflammatory, and immune responses.
Methods/Principal Findings
Next generation sequencing and mass spectrometry-based protein identification were performed to investigate the content of triatomine R. neglectus saliva. We deposited 4,230 coding DNA sequences (CDS) in GenBank. A set of 636 CDS of proteins of putative secretory nature was extracted from the assembled reads, 73 of them confirmed by proteomic analysis. The sialome of R. neglectus was characterized and serine protease transcripts detected. The presence of ubiquitous protein families was revealed, including lipocalins, serine protease inhibitors, and antigen-5. Metalloproteases, disintegrins, and odorant binding protein families were less abundant.
Conclusions/Significance
The data presented improve our understanding of hematophagous arthropod sialomes, and aid in understanding hematophagy and the complex interplay among vectors and their vertebrate hosts.
Author Summary
Chagas disease is caused by the Trypanosoma cruzi protozoan, which is transmitted to vertebrates through the feces of infected triatomines during blood sucking. The vascular injury caused by the bite triggers mechanisms capable of preventing the association with hosts, such as immune response, inflammation and haemostasis. However, hematophagous insects are able to counteract these defenses through a complex repertoire of salivary molecules that have specific targets in the host. Our results show that R. neglectus salivary glands express different protein gene families, possessing multi-functional features directly related to different anti-haemostatic activities. For instance, lipocalins are proteins possessing anti-coagulant and vasodilator functions. Saliva contents have evolved to adapt to blood-feeding habit, ensuring the maintenance of blood flow, the success of the meal, and transmission of diseases.
Introduction
Blood-sucking triatomines (Hemiptera: Reduviidae) feed exclusively on blood in all life stages. They obtain their blood meal from venules or arterioles of their vertebrate hosts. The steps during feeding include piercing of the host skin, followed by a probing period, and finally engorgement [1]. In support of this habit, these arthropods have evolved effective mechanisms to counteract host responses, such as haemostasis, inflammation and immunological reactions. While biting, their salivary glands (SG) release potent pharmacological substances, including vasodilator, anti-inflammatory, antiplatelet, anticlotting and immunomodulatory molecules, to enable the arthropod to obtain a successful blood-meal [2, 3]. These bioactive salivary components represent a promising source of molecules with therapeutic potential for treating circulatory disorders [4, 5].
In the 1990s, multinational control programs against Chagas disease led to a significant reduction of acute cases in many endemic regions of Latin America, mainly through a reduction of domestic vectors [6]. However, factors such as the wide geographical distribution of triatomine species and the availability of different infection reservoirs remain multifactorial obstacles in the control of the disease. Nowadays, there is constant concern regarding the sporadically or progressive (re)invasion and (re)colonization of human dwellings by wild secondary vectors [7, 8]. Rhodnius neglectus is found in the Brazilian Savanna (Cerrado) in association with different wild palms, playing an important role in the sylvatic maintenance of T. cruzi and Trypanosoma rangeli [9–11]. In nature, R. neglectus feeds mainly on birds and much less on rodents, and rarely on opossum [12]. This species is able to act as a secondary vector, being observed in both intra and peridomestic environments in five Brazilian states [13–17], a possible result of deforestation and wild ecotope invasion. These anthropogenic environmental changes favor vector dispersion, bridging sylvatic/domestic cycles of the disease.
Sialome studies (from the Greek sialo = saliva) have been developed for many species of bloodsucking insects, which are frequently vectors of human and animal diseases. Sanger automated sequencing technology has been used to investigate the salivary transcriptome for almost two decades. However, Next Generation Sequencing (NGS) is capable of providing much more sequence data in a single run, with a higher resolution than that from the Sanger technique, allowing for deeper analysis of the transcripts. One important application of NGS is RNA sequencing (RNA-seq), used to describe transcriptomes of cells and tissues. Deep sequencing increases the possibilities of finding new biological molecules in the saliva of bloodsucking insects, offering a new array of substances to be further investigated and functionally characterized.
The aim of this report is to catalog the transcripts of R. neglectus SGs with probable function in hematophagy using RNAseq and mass spectrometry. This strategy was used to describe the bioactive molecules in triatomine saliva and improve our understanding on the dynamics of the blood-feeding process, vector-host interaction and disease transmission. The data is available at the National Center for Biotechnology Information (NCBI) and can be used in different scientific research projects.
Methods
Insects and Transcriptome Salivary Gland Preparation
R. neglectus triatomines originating from insects collected in 1982 at Itambaracá, in Paraná State, Brazil, were reared in the insectarium at the University of Brasília (Brazil). They were kept at 27±1°C, a relative humidity of 70–75%, under a 12 h/12 h light/dark cycle. The blood source of these insects was Gallus gallus domesticus. The SGs of 5th instar nymphs and adults were dissected at 5, 12, and 24 days post blood meal in cold Trizol reagent (Invitrogen, Carlsbad, CA, USA). A pool of thirty SG pairs was stored at -80°C prior to RNA extraction.
Salivary Gland RNA Isolation, Library Preparation and Sequencing
Total RNA was extracted following the Trizol manufacturer’s instructions. RNA integrity and concentration were checked by lab-on-chip analysis using an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). A RNA sample was sent to the Federal District High-Performance Genome Center (DF, Brazil) for Illumina cDNA library construction and next generation sequencing. A Library was prepared with standard protocols using TruSeq RNA kit, v2 (Illumina, San Diego, CA). To generate paired-end reads of 300 nucleotides in length, the sequencing of cDNA libraries was performed on an Illumina MiSeq sequencer (Illumina, USA). One lane of the MiSeq machine was used for sequencing this and another library, distinguished by bar coding. The RNA-seq sequencing generated a total of 12,049,305 reads. The nominal length of the sequences was 301 nt. Following trimming of low quality bases (quality 20 or lower), the average length was 248.07, the median was 301 and L50 was 296 nt. Sequences smaller than 25 nt or with average quality < 20 were rejected.
Bioinformatic Analysis
Bioinformatic analyses were conducted as previously described [18]. As there was no reference genome to map, the strategy was to perform a de novo assembly with Abyss [19] and Soapdenovo Trans [20] assemblers using different kmer (k) values (from 20 to 90). The resulting assemblies were joined by an iterative BLAST and cap3 assembler [21]. Sequence contamination between bar-coded libraries were identified and removed when their sequence identities were over 98%. Coding sequences (CDS) were extracted based on the existence of a signal peptide and on similarities to other known proteins [22]. Coding and protein sequences were mapped into a hyperlinked Excel spreadsheet. Reads were mapped into contigs using blastn [23] with a word size of 25, masking homonucleotide decamers and allowing mapping to up to five different CDS if the BLAST results had the same scores. Mapping of the reads was also included in the Excel spreadsheet. CDS were automatically annotated a program written by JMCR that searched a vocabulary of nearly 250 words for matches various databases, including Swissprot, Gene Ontology, KOG, PFAM, and SMART, and a subset of the non-redundant protein database containing proteins from vertebrates (NCBI). Further manual annotation was done as required. Alignment analysis were done with Bioedit software [24] after sequence alignment performed using ClustalW [25]. Phylogenetic analysis and statistical neighbor-joining bootstrap tests of the phylogenies were done with Mega package [26]. The sequences used in alignments with R. neglectus CDS were obtained from the non-redundant protein database of the NCBI and are represented by six letters followed by the NCBI GI number. The letters derive from the first three letters of the genus and the first three letters of the species name.
Data Availability
The raw reads were deposited at the Sequence Read Archive (SRA) in NCBI under bioproject PRJNA292130. A total of 4,230 coding sequences were deposited in DDBJ/EMBL/GenBank through the Transcriptome Shotgun Annotation portal under the accession GDKW00000000.
LC-MS/MS Protein Identification
The SGs were dissected from 5th instar nymphs and adults at 5, 12 and 24 days post blood meal and carefully punctured at 4°C. Following centrifugation (16.000 × g, 15 min, 4°C), the soluble protein fraction from fifteen pairs of SG homogenates was ethanol/acetone precipitated. Resuspended proteins were consecutively alkylated, reduced, digested by trypsin, and subjected to LC-MS/MS analysis as previously described [27]. Briefly, the tryptic peptides were loaded onto a 2 cm fused silica trap column (150 μm inner diameter) packed in-house with reverse phase capillary column ReproSil-Pur C18-AQ 5 μm resin (Dr. Maisch GmbH, Germany) and separated using a DIONEX 3000 nanoUPLC system coupled to an LTQ-Orbitrap Elite mass spectrometer (Thermo Scientific, Waltham, USA). MS1 spectra were recorded in the Orbitrap mass analyzer with 120,000 resolution. After ion fragmentation, MS/MS spectra of the 15 most intense ions were acquired. Raw files were generated and used for protein identification using Proteome Discoverer v.1.3 (Thermo Scientific, Waltham, USA) with in-house SequestHT algorithm for R. neglectus SG transcriptome and human keratins, BSA and porcin trypsin. The false discovery rate was less than 1%, with peptide rank of 1 and at least 2 peptides per protein.
Results and Discussion
General Description of the Sialome of R. neglectus
The assembly of R. neglectus SG transcriptome enabled the extraction of 5,705 CDS. These CDS mapped a total of over 11 million reads. Following automated and manual annotation, the CDS were classified into putative secreted, housekeeping, unknown, transposable element, and viral product. The CDS of the housekeeping class comprised the largest class (Table 1). They were further characterized into 24 subclasses, according to their possible function, summarized in Table 2.
Table 1. Classification and abundance of coding sequences extracted from the salivary gland transcriptome of R. neglectus.
| Class | No. of CDS | % Total | No. of reads | % Total |
|---|---|---|---|---|
| Secreted | 636 | 11.15 | 2,978,414 | 25.44 |
| Housekeeping | 4,739 | 83.07 | 8,320,391 | 71.07 |
| Unknown product | 242 | 4.24 | 367,035 | 3.13 |
| Transposable element | 86 | 1.51 | 41,406 | 0.36 |
| Viral product | 2 | 0.03 | 208 | 0.00 |
| Total | 5,705 | 100 | 11,707,454 | 100 |
Table 2. Classification and abundance of coding sequences of putative housekeeping function extracted from the sialotranscriptome of R. neglectus.
| Subclass | No. of CDS | No. of reads | % Total |
|---|---|---|---|
| Unknown conserved | 805 | 1,219,305 | 14.65 |
| Protein export | 271 | 1,151,192 | 13.84 |
| Protein synthesis machinery | 287 | 779,314 | 9.37 |
| Signal transduction | 604 | 725,961 | 8.73 |
| Protein modification | 153 | 595,681 | 7.16 |
| Transcription machinery | 468 | 566,268 | 6.81 |
| Lipid metabolism | 217 | 477,020 | 5.73 |
| Nucleotide metabolism | 87 | 426,894 | 5.13 |
| Cytoskeletal protein | 190 | 297,617 | 3.58 |
| Transporter and Channel | 272 | 293,911 | 3.53 |
| Protein modification, protease | 91 | 244,360 | 2.94 |
| Carbohydrate metabolism | 150 | 230,497 | 2.77 |
| Immunity | 78 | 177,405 | 2.13 |
| Extracellular matrix | 92 | 175,813 | 2.11 |
| proteasome | 191 | 157,781 | 1.90 |
| Energy metabolism | 166 | 143,638 | 1.73 |
| Amino acid metabolism | 79 | 127,157 | 1.53 |
| Nuclear Export | 26 | 120,662 | 1.45 |
| Nuclear Regulation | 218 | 116,946 | 1.41 |
| Transcription factor | 100 | 99,589 | 1.20 |
| Detoxification | 93 | 77,717 | 0.93 |
| Storage | 15 | 56,888 | 0.68 |
| Intermediary metabolism | 57 | 30,227 | 0.36 |
| Signal Transduction, apoptosis | 28 | 28,548 | 0.34 |
| Total | 4739 | 8,320,391 | 100 |
Putative Secreted Proteins
The secreted class was organized in subclasses that include previously known gene families present in hematophagous saliva, such as lipocalin, nitrophorin, antigen-5, as well as gene families not commonly reported in triatomine saliva, such as serine protease and disintegrin (Table 3). The following section describes the putative secreted proteins present in R. neglectus sialome, highlighting the remarkable finding of serine proteases in this group.
Table 3. Classification and abundance of coding sequences of putative secretory function extracted from the sialotranscriptome of R. neglectus.
| Subclass | No. of CDS | No. of reads | % Total |
|---|---|---|---|
| Hypothetical secreted protein | 198 | 976,296 | 32.78 |
| Serine protease | 33 | 820,619 | 27.55 |
| Conserved secreted protein | 89 | 529,615 | 17.78 |
| Lipocalin—Triabin | 120 | 471,408 | 15.83 |
| Disintegrin | 2 | 62,933 | 2.11 |
| Others | 64 | 52,690 | 1.77 |
| Lipocalin—Nitrophorin | 31 | 13,737 | 0.46 |
| Mucin related | 11 | 11,410 | 0.38 |
| Antigen-5/SCP | 8 | 8,718 | 0.29 |
| Lipid metabolism | 16 | 6,028 | 0.20 |
| Major royal jelly protein | 2 | 5,019 | 0.17 |
| Juvenile hormone related | 8 | 4,328 | 0.15 |
| Protease inhibitor | 14 | 3,164 | 0.11 |
| Immunity related | 4 | 2,829 | 0.09 |
| Insect pheromone-binding | 6 | 2,769 | 0.09 |
| Protease inhibitor Kazal—type | 7 | 1,993 | 0.07 |
| OBP | 11 | 1,837 | 0.06 |
| Toxin | 1 | 1,542 | 0.05 |
| Nucleotid metabolism | 6 | 735 | 0.02 |
| 5’ nucleotidase | 3 | 477 | 0.02 |
| Hemolysin-like | 1 | 242 | 0.01 |
| Metalloprotease | 1 | 35 | 0.00 |
| Total | 636 | 2,978,424 | 100 |
Lipocalins
Lipocalins comprised one of the most abundant groups of transcripts, with 16.29% of putatively secreted reads. These include a large group of extracellular proteins that usually bind to small hydrophobic molecules, cell surface receptors or other proteins. The members of this family have little similarity in peptide sequence, however share a conserved three-dimensional structure, comprised of a single eight-stranded antiparallel β-barrel [28]. In blood-sucking insect and tick saliva the lipocalins are abundantly expressed, but not in Diptera or fleas. In ticks, their function is associated with binding to histamine and serotonin [29]. Triabin and nitrophorin, the two major groups found here, are discussed below.
Lipocalins of the triabin family
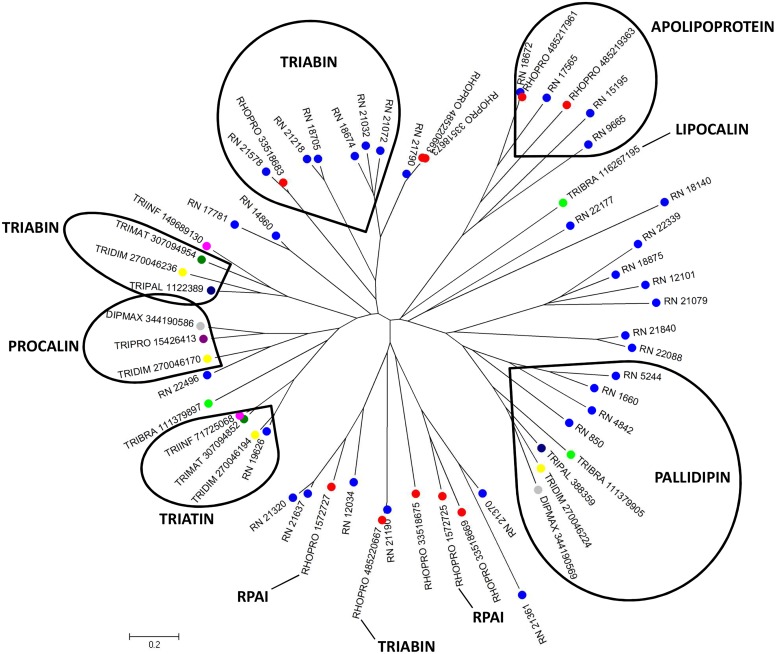
First isolated from the saliva of the Triatoma pallidipennis kissing bug [30], triabin is a lipocalin-like thrombin inhibitor, which inhibits thrombin-induced platelet aggregation, and prolongs thrombin clotting time through the formation of a noncovalent complex with thrombin at a 1:1 molar ratio. Previous analysis revealed that triabin is a compact one-domain molecule essentially consisting of an eight-stranded β-barrel and inhibits thrombin exclusively via its fibrinogen-recognition exosite [31]. Thrombin is the ultimate serine protease formed during activation of the blood coagulation cascade, which catalyzes the polymerization of fibrinogen to fibrin, the solid fibrillar component of the blood clot, thereby being a fundamental promoter of blood clotting. Thus, the triabin-like lipocalins may function as thrombin inhibitors in R. neglectus saliva. The library analysis shows 120 different CDS from lipocalin family containing the triabin conserved domain, such as triabin, pallidipin, apolipoprotein, procalin and triatin. The alignment of these members with lipocalins already described in triatomines resulted in a phylogram containing different clades (Fig 1). In addition, it is possible to note two divergent clades containing only R. neglectus and Rhodnius prolixus sequences (RPAI and Apolipoprotein), which may represent additional gene members present in Rhodnius spp. The presence of different clades indicates the expansion of this gene family by gene duplication events, suggesting that, for R. neglectus, lipocalins exert a crucial role in success feeding.
Fig 1. Phylogram of lipocalin containing triabin domain from R. neglectus SG transcriptome.
Phylogenetic tree derived from the alignment of R. neglectus CDS and other triatomine lipocalin sequences as described in Methods section. The bar at the bottom represents 20% amino acid substitution. The colored circles indicate each species whose sequences were used: blue, R. neglectus sequences from SG transcriptome; red, R. prolixus; yellow, Triatoma dimidiata; green, Triatoma brasiliensis; dark green, Triatoma matogrossensis; dark blue, T. pallidipennis; purple, Triatoma protacta; magenta, Triatoma infestans; gray, Dipetalogaster maxima.
Lipocalins of the nitrophorin family
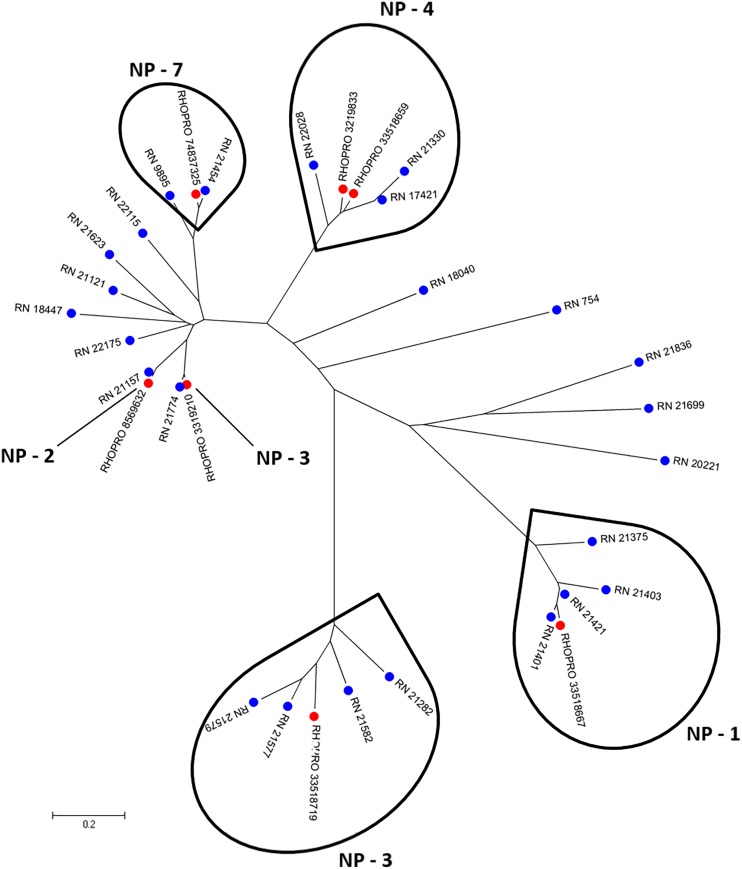
Rhodnius spp. show a characteristic red coloration in their saliva due to the presence of haemoproteins called nitrophorins (NPs). These molecules form a stable complex with nitric oxide (NO), which is sensitive to pH variation, being stabilized by low pH in the lumen of the SGs (pH ~5), and released at neutral pH in the host (pH ~7.5) [32]. The secretion of NO is an efficient way to counteract haemostasis, acting both as a potent vasodilator and as an antagonist of platelet activation. NPs 1–4 can additionally sequester histamine released by host mast cells, reducing inflammation and immune response [33, 34]. NP 2 inhibits clotting in a mechanism independent of NO or histamine binding, acting as a specific inhibitor of the intrinsic factor X-(FX)-activating complex [35]. As well as reversibly binding to NO or histamine, NP 7 also inhibits prothrombin activation by blocking phospholipid binding sites for the prothrombinase complex on the surfaces of vesicles and activated platelets through binding to phosphatidylserine [36]. The current sialotranscriptome identified 13,737 reads related to the diversity of NPs. The NPs of R. neglectus also appear to be a gene family that expanded during evolutionary processes, as inferred by the phylogenetic tree (Fig 2). Notice that there are several sequences homolog to NP1-4 and 7, NPs described in R. prolixus saliva.
Fig 2. Phylogram of lipocalin containing nitrophorin domain from R. neglectus SG transcriptome.
Phylogenetic tree derived from the alignment of R. neglectus CDS and R. prolixus nitrophorin sequences as described in Methods section. The bar at the bottom represents 20% amino acid substitution. The colored circles indicate each species whose sequences were used: blue, R. neglectus sequences from SG transcriptome and red, R. prolixus sequences from NCBI.
The mean number of nitrophorins in salivary electrophoretic profiles varies among Rhodnius species, with R. neglectus showing the fewest. The high polymorphism of NPs may help in the identification of Rhodnius species [37]. The lower proportion of nitrophorin content in the saliva compared to those found in the saliva of other Rhodnius spp. might not, by itself, explain the reduced feeding performance of R. neglectus on mammals. For instance, although R. neglectus shows lower amounts of nitrophorins, it feeds more efficiently than R. robustus [37]. It is important to note that the exact contribution of each class of saliva molecules on the feeding process is unknown.
Antigen-5 Family
The CAP superfamily members [Cysteine-Rich Secretory Proteins (CRISPS), Antigen 5 (Ag5), and Pathogenesis-Related 1 (Pr-1)] are found in a wide range of organisms, most often as secreted proteins [38]. Ag5, present in the venom of wasps and ants, are considered potent allergens to mammals [39, 40]. This superfamily can also block smooth muscle contraction when present in snake venom [41] and act in the defense response in plants [42]. They have been described in the saliva of some hematophagous, including mosquitoes [43, 44] and sand flies [45]. Among triatomines, Ag5 genes have been reported in the sialotranscriptomes of R. prolixus [46], T. infestans [47], D. maxima [48], T. matogrossensis [49] and Triatoma rubida [50]. Their functions in blood-feeder saliva remained unexplored for a long time, but a recent report revealed salivary Ag5 of D. maxima and T. infestans as Cu+2-dependent antioxidant enzymes that inhibit neutrophil oxidative burst and platelet aggregation induced by collagen [51].
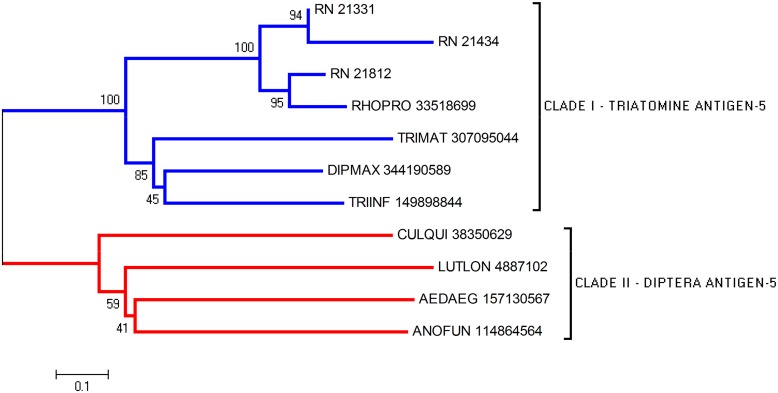
The sialotranscriptome analysis revealed eight CDS related to the Ag5 family. The alignment of R. neglectus Ag5 with other triatomine Ag5 sequences showed some conserved motifs (S1 Fig). Phylogenetic analysis offers support for the formation of clades I and II comprising triatomine and Diptera sequences, respectively (Fig 3).
Fig 3. Phylogram of Antigen-5 proteins from R. neglectus SG transcriptome.
Phylogenetic tree derived from the alignment of R. neglectus CDS and other insect antigen-5 sequences as described in Methods section. The bar represents 10% amino acid substitution.
Serine Protease Inhibitors
For blood-feeders, targeting components of the coagulation cascade is essential to attenuate the haemostatic response of their hosts. All enzymes participating in this cascade are serine proteases associated with complement activation [52, 53]. The R. neglectus sialotranscriptome exhibited a variety of transcripts coding for proteins with serine protease inhibitory function, comprising 14 CDS and 3,164 reads. Based on their Pfam signature, kazal, pacifastin and serpin families were extracted.
Kazal family
Kazal-type domain-containing proteins are serine protease inhibitors playing important functions in invertebrates, mainly having vasodilation, antimicrobial, and thrombin inhibition effects. These protease inhibitors are single or multidomain proteins that share a conserved sequence motif, a distinctive cysteine distribution pattern and highly similar three-dimensional structure [54]. Rhodniin is a kazal-type thrombin inhibitor isolated from R. prolixus [55, 56]. Dipetalogastin from D. maxima [57], infestin from T. infestans [58] and brasiliensin from T. brasiliensis [59] are thrombin inhibitors located in the intestines. From the horse fly Hybomitra bimaculata (Diptera, Tabanidae) SGs, a vasodilator named vasotab was identified as a member of Kazal-type protease inhibitor family acting through ion channel inhibition and vasodilation [60].
Seven CDS in R. neglectus sialotranscriptome possessed the typical sequence of nonclassical Kazal domains characterized by a shorter distance between the first and second cysteine residue, unlike the seven or eight spacer residues found in the classical configuration [55, 57]. The alignment showed a low degree of conserved amino acids, but confirmed the presence of the six cysteine residues responsible for the formation of disulfide bridges (Fig 4). The relative positions of cysteine residues were the same in the compared sequences.
Fig 4. Kazal-type members from R. neglectus SG transcriptome.
ClustalW alignment of Kazal-type domain-containing members from R. neglectus salivary transcriptome (RN_5563 and RN_549) and other insect kazal-type sequences, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background, the six conserved cysteines (boxes) and the blue bar indicates the signal peptide indicative of secretion.
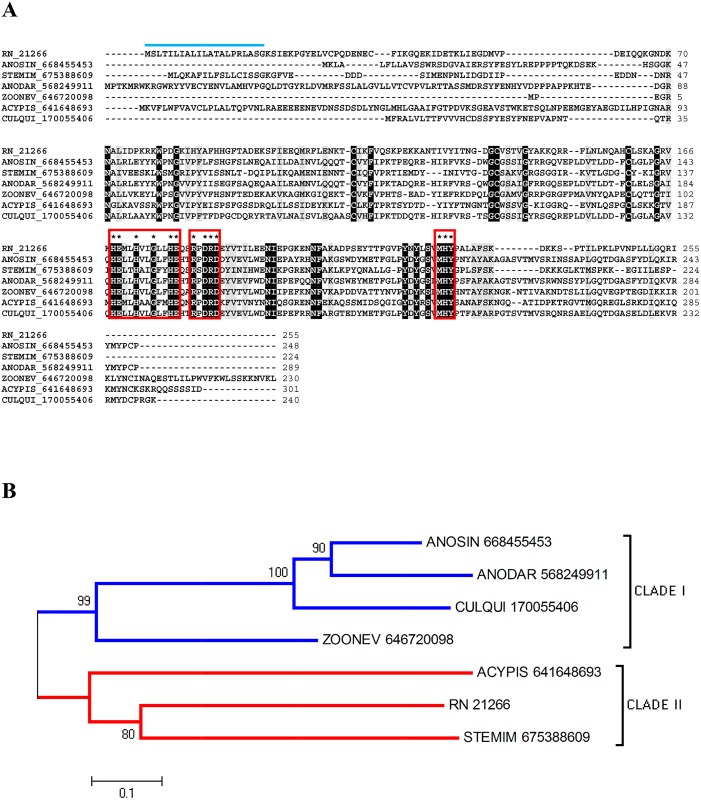
Additionally, one contig was identified as dipetalogastin due to the cysteine residues distribution and the presence of the conserved motif CGXDXXTYXNXC, a distinguishing repeat of Kazal-type inhibitors [57]. This transcript is full length and possesses the signal peptide indicative of secretion. The alignment with other protein sequences with the same features revealed a high degree of conserved amino acids (S2 Fig).
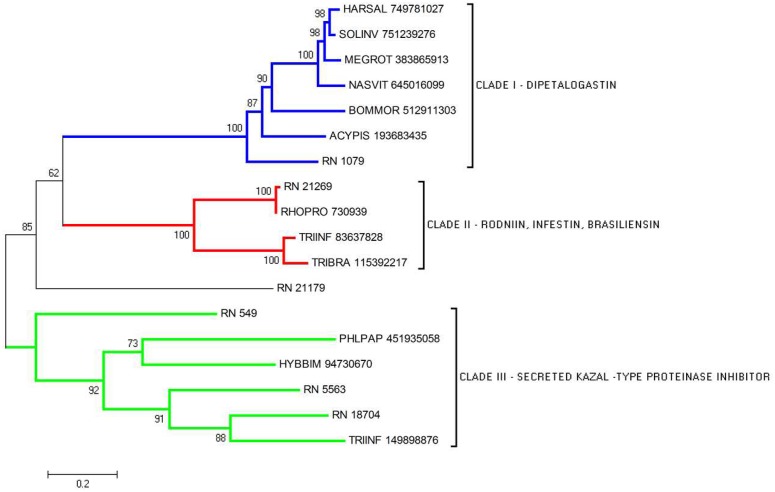
The phylogram of serine protease inhibitor members clearly shows the formation of three clades, with a good bootstrap support, each one representing a different family of serine protease inhibitor discussed above (Fig 5). The CDS RN_21179 is notably distinct from the clades, suggesting the presence of a divergent gene. The different clades may represent sequences differentially expressed sharing the same function regarding haemostasis inhibition.
Fig 5. Phylogram of Serine Protease inhibitors from R. neglectus SG transcriptome.
Phylogenetic tree derived from the alignment of R. neglectus CDS and other insect sequences as described in Methods section. The bar at the bottom represents 20% amino acid substitution.
Pacifastin family
Pacifastin is a family of serine protease inhibitors, mostly multi-domain proteins, first isolated from the plasma of the crayfish Pacifastacus leniusculus. The protein is heterodimeric, comprising both a transferrin chain (heavy chain, PHC) and a protease inhibitor chain (light chain, PLC) [61, 62]. Insect pacifastins may have multiple functions, acting as regulators of a wide variety of serine peptidase-dependent processes such as immunity and reproduction [63]. In Hemiptera, two pacifastin-like protease inhibitors from T. infestans eggs were functionally characterized, suggesting a role in insect immune response [64]. Here, two CDS are related to pacifastin, RN_17301 and RN_20047, and their alignment with other members of the pacifastin family reveals four conserved domains, containing the cysteine-rich inhibitory pattern of PLC comprised of a triple-stranded antiparallel beta-sheet connected by three disulfide bridges (S3 Fig). This is the first time pacifastin members are identified in triatomine SGs, their function in this organ is still unknown but it might be related to insect immunity.
Serpin family
Serpins are a large family of structurally related proteins found across taxa, showing diverse activities not limited to inhibition of serine proteases [65]. In vertebrates, serpins play crucial control in blood coagulation, fibrinolysis and inflammation. Dysfunction, deficiencies or over-expression of serpins can cause either abnormal bleeding or thrombosis [66]. The function of this protein in saliva of mosquitoes is related to host haemostasis regulation, seeming to act as a potent reversible inhibitor of the host factor Xa [67]. In Ixodes ricinus ticks, the molecule was also associated with inhibition of blood coagulation and fibrinolysis of the vertebrate host [68–70]. The consensus three-dimensional fold of serpins is comprised of a bundle of 8–9 α-helices and a β-sandwich composed of three β-sheets [71].
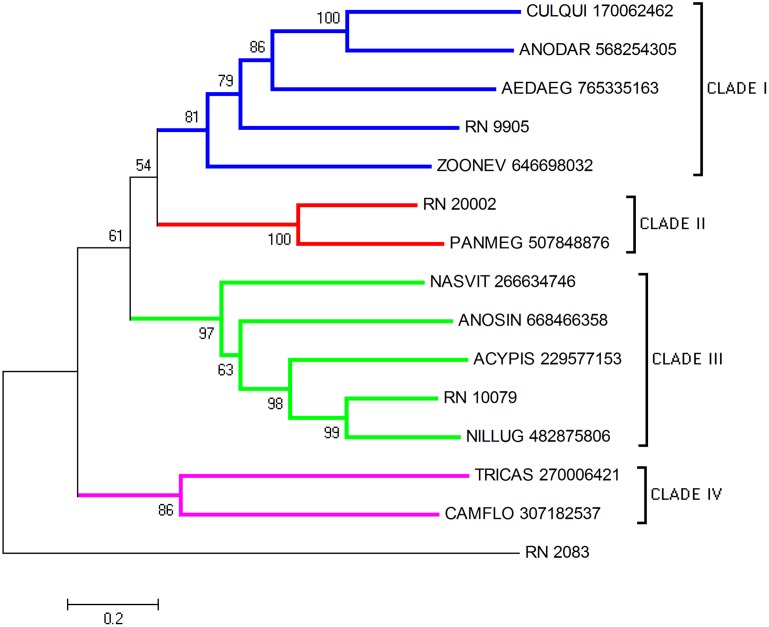
Here, four CDS from R. neglectus sialotranscriptome were classified as serpins. The phylogram showed four clades with a good bootstrap support (Fig 6). Transcripts RN_9905, RN_20002 and RN_10079 grouped each one in a separate clade while the fourth CDS, RN_2083, seemed to be a distant divergent gene. In R. neglectus saliva this inhibitor might also function in the modulation of coagulation cascade.
Fig 6. Phylogram of serpin proteins from R. neglectus SG transcriptome.
Phylogenetic tree derived from the alignment of R. neglectus CDS and other insect sequences as described in Methods section. The bar represents 20% amino acid substitution.
Proteases
Metalloprotease
One CDS found in the R. neglectus sialotranscriptome is related to the zinc-dependent metalloproteases from the astacin-like metalloproteases, a family of the metzincins superfamily. There are three conserved regions in proteins from this family. The first one is the distinguishing family signature sequence HEXXHXXGXXHE, which is the zinc-binding active site. The second region, RXDRD, is a hydrophilic region, and the third highly conserved region, MXY, is the methionine-containing turn (the Met-turn) [72–74].
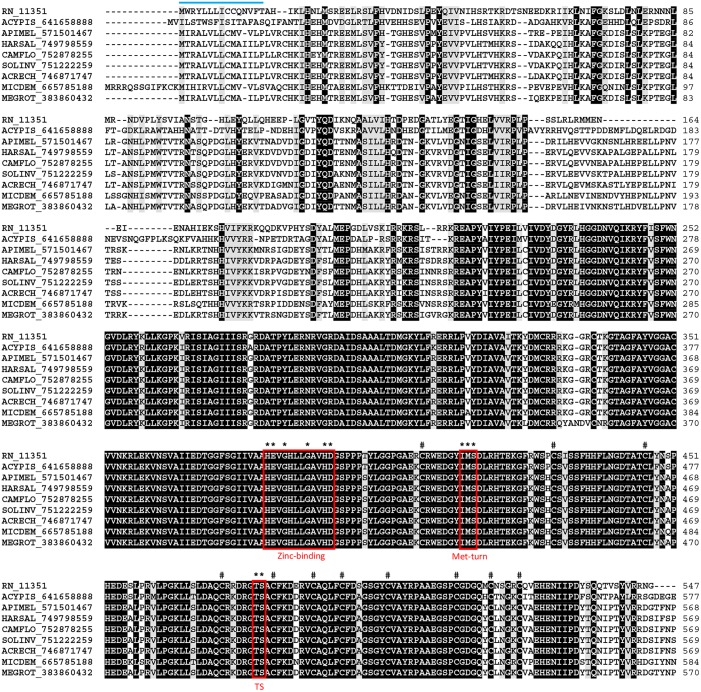
This gene family comprises many proteins from diverse species. In the venom of different spider species, there is a common toxin with the ability to hydrolyze fibrinogen and fibronectin [75–77], suggesting a relationship between this proteolytic activity with local hemorrhage, since fibronectin plays a role in platelet aggregation, blood vessel stability and wound healing [78]. Therefore, the biologic function of astacin-like proteases in triatomine saliva could be related to the maintenance of blood flow at the bite site. Astacin domain metalloproteases were already reported in T. matogrossensis sialotranscriptome [49]. The CDS RN_21266 is a full-length sequence containing a signal peptide indicative of secretion. Its alignment with other metalloproteases revealed the three conserved motifs of the family (Fig 7A). The phylogenetic tree suggests, with a good bootstrap support, that the secreted metalloproteases are closely related proteins (Fig 7B).
Fig 7. The secreted metalloprotease from R. neglectus SG transcriptome.
(A) ClustalW alignment of the secreted metalloprotease from R. neglectus salivary transcriptome (RN_21266) and other metalloproteases sequences, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The blue bar indicates the signal peptide indicative of secretion. The boxes limit the family signature sequences showing the determinant residues (black asterisk). (B) Phylogenetic tree derived from the alignment of R. neglectus CDS and other metalloproteases sequences as described in Methods section. The bar represents 10% amino acid substitution.
ADAMTS (ADAM with thrombospondin motifs)/Disintegrins
Two further members of the metzincin metalloprotease superfamily were identified in R. neglectus transcriptome and are related to the adamalysin/reprolysin family, which includes ADAM (A Disintegrin And Metalloproteinase domain) and ADAMTS (A Disintegrin And Metalloproteinase with Thrombospondin motifs). ADAMTS is a group of secreted, extracellular and multidomain proteases that have diverse roles in both mammals and invertebrates [79, 80]. They are cysteine-rich molecules that selectively block the function of integrin receptors on the cell membrane surface [81, 82], exhibiting a thrombospondin-like (TS) repeat and a cysteine-rich domain typical of disintegrins [79]. In this family, the third histidine in the family signature sequence containing three zinc ligands is followed by a conserved aspartic acid, HEXXHXXGXXHD. Moreover, it lacks the fifth zinc ligand and the methionine residue of the consensus Met-turn is placed within the sequence V/I-M-A/S [74, 79].
Together with snake venom metalloproteinases (SVMP), ADAM and ADAMTS are a group of versatile molecules in viper venom that affects different elements in haemostasis [83]. The disintegrins can bind to platelets and act as potent inhibitors of platelet aggregation [84–86]. The molecule can also bind to endothelial cells [87, 88], as well as neutrophils and phagocytes [89]. Rhodostomin is a disintegrin that inhibits activity of LPS-treated monocytes via αvβ3 integrin affecting haemostasis, cell-cell interaction and suppresses tumor growth [90]. In hematophagous organisms, the disintegrins have been described in tick and leech saliva [91]. Here, one CDS related to disintegrins was identified in the R. neglectus SG transcriptome. The alignment exhibited a high degree of homology between R. neglectus disintegrin sequence and others of the same family (Fig 8). The identification of ADAMTS is one of the main findings from the sialotranscriptome of R. neglectus.
Fig 8. The ADAMTS sequence from R. neglectus SG transcriptome.
ClustalW alignment of the ADAMTS sequence from R. neglectus salivary transcriptome (RN_11351) and other insect ADAMTS sequences, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The bar indicates the signal peptide indicative of secretion. The boxes limit the family signature sequences showing the determinant residues (black asterisk). The symbols (black hash) above indicate the conserved cysteines.
Serine proteases and trypsin-like proteins
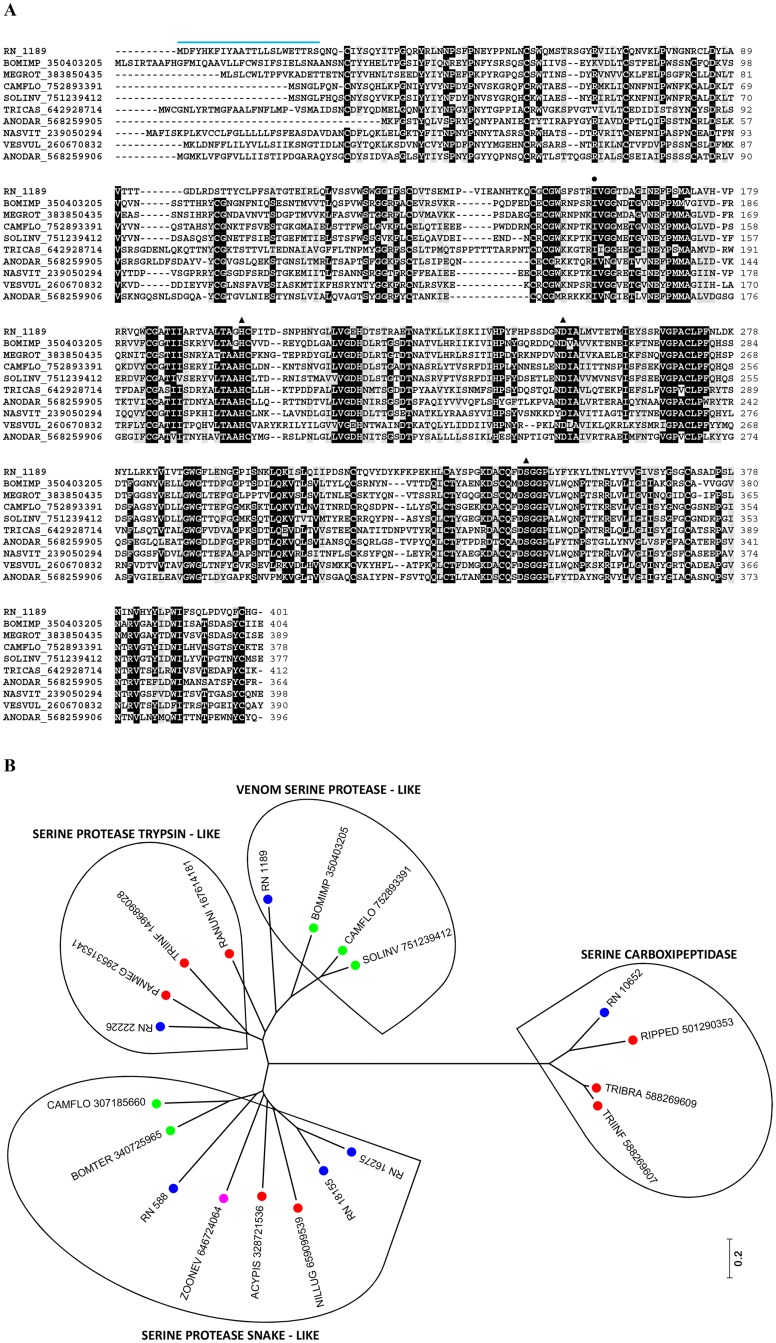
The R. neglectus SG transcriptome revealed serine proteases reads as the second most abundant group in the secreted class, comprising 820,619 reads. The majority of the sequences identified as serine proteases exhibited the trypsin domain (Tryp-SPc) of the CDD and Smart databases. RN_1189 was assembled from 768,048. Its alignment with serine proteases from other organisms revealed conserved residues located around the cleavage and active sites (Fig 9A), and the presence of a CUB (complement C1r/C1s, Uegf, Bmp1) domain, a structural motif of approximately 110 residues found almost exclusively in extracellular and plasma membrane-associated proteins. This domain is also present in honeybee allergens Api SI and Api SII, which are probably components of the honeybee defense system [92, 93].
Fig 9. Serine proteases from R. neglectus SG transcriptome.
(A) ClustalW alignment of a serine protease from R. neglectus SG transcriptome (RN_1189) and other serine proteases members, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The blue bar indicates the signal peptide indicative of secretion. The symbols above the residues indicate (black circle) cleavage site and (black triangle) active site showing the HDS triad. (B) Phylogenetic tree was built from the alignment of R. neglectus CDS and other insect sequences as described in Methods section The bar at the bottom represents 20% amino acid substitution. The colored circles identify the sequences used: blue, R. neglectus sequences from SG transcriptome; red, Hemiptera order; green, Hymenoptera order; magenta, Dictyoptera order.
Some serine proteases can function as regulators of coagulation. Thrombin can participate in this regulation by binding to thrombomodulin, a membrane protein present in host endothelial cells. This complex is able to activate Protein C (a serine protease), which acts as a potent anticoagulant enzyme by inactivating factors V and VIII, impairing thrombus progression [94, 95]. In snake venom, blockage of thrombus formation by serine proteases has also been reported. SPSV (Serine Protease Snake Venom) releases a unique fibrinopeptide that produces only instable monomers of fibrin, leading to clots that are rapidly dispersed [96]. Although the specific role in hematophagous saliva is still unknown, an active serine protease was described in T. infestans [97], as well as in horse fly Tabanus yao saliva, which functions as a fibrinogenolytic enzyme [98]. RN_22226, RN_21634, RN_19989, RN_17969, and RN_10652 were matched by blastp to serine proteases of T. infestans, T. braziliensis, Panstrongylus megistus, and R. prolixus. Serine proteases also play important roles in fertilization, embryonic development, and in the processes of molting and metamorphosis of insects [99, 100]. In our sample, the triatomines did not show any sign of larval molting at SG dissection.
Sequences containing CLIP, LDLa and SUSHI domains, which are cysteine-stabilized structures for molecular recognition, were also identified. The CLIP domain is restricted to the Arthropoda and was found N-terminally to the Tryp-SPc domain of RN_16275, RN_18155, and RN_7118. Both domains belong to the serine proteases of the trypsin-like S1 family, that are typically secreted enzymes associated with extracellular proteolysis [101]. CLIP domain has been suggested to be important for dimerization, mediating specific protein-protein interactions involved in the regulation of serine protease activities. The LDLa domain was identified in RN_12992, RN_12776, RN_12432, and RN_21634. The last two sequences also presented the SUSHI motif of smart database which is known as CCP (Complement Control Protein) module, containing approximately 60 amino acid residues identified in several proteins of the complement system. These R. neglectus putative secreted serine proteases may play critical roles in many key biological processes as blood coagulation and immunity. In the vertebrate hosts, allergenicity may reinforce the toxic effect of serine proteases, independently of their catalytic activity, as proposed by Georgieva and colleagues [93].
The phylogram clearly showed four different groups (Fig 9B), indicating the expression of at least four genes related to serine proteases in the saliva of R. neglectus. In regard to the large amount of reads, the results observed here suggests that, to R. neglectus, the serine proteases arise as an important salivary secreted gene family, a probably evolutionary adaptation where the protein could gain a new function as a result of selective pressure for the blood-feeding behavior success. These proteases could act in the vertebrate host, as well as in the insect, on pathogens that were ingested with blood. Further experiments are necessary to address the possible roles of those genes on the biology of R. neglectus.
OBP
The odorant-binding protein (OBP) family is a chemosensory protein ubiquitous in insects commonly associated with solubilizers and carriers of odorants and pheromones. Although associated with chemosensory organs, in recent times this family has been related to other roles such as hydrophobic chemical transportation [102]. The OBPs are characterized by a variable amino acid sequence, but conserve a pattern of six conserved cysteines residues paired to form three disulfide bridges [103]. The folding is a typical six α-helices assembled in a compact and stable structure [104, 105]. Eleven CDS containing protein sequences related to OBPs were recognized in our transcriptome analysis, all possessing signal peptide prediction. The conserved cysteine residues of R. neglectus CDS (Fig 10) were seen during alignment. Phylogenetic analysis with good bootstrap support shows Clade I containing most R. neglectus sequences grouped with R. prolixus OBP. However, RN_3440 was grouped in Clade III, suggesting this is a more distant OBP (S4 Fig).
Fig 10. The secreted OBP family from R. neglectus SG transcriptome.
ClustalW alignment of secreted OBPs from R. neglectus SG transcriptome (RN_5213, RN_3440 and RN_18954) and other members from the OBP family, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The blue bar indicates the signal peptide indicative of secretion and the boxes, the six conserved cysteines.
The Proteome of R. neglectus Saliva
R. neglectus saliva content was tryptic digested and subjected to mass spectrometry to validate the analysis of the transcripts possibly associated with secreted products. Among the 73 identified secreted proteins groups, 48 were from the lipocalin family, including triabin, pallidipin and nitrophorin proteins, reaffirming their abundance (Table 4). Other soluble proteins, predicted as being secreted by these arthropods, were: secreted metalloprotease, antigen-5, serpin and trypsin-like protease, each with at least one observation. It is intriguing that only one serine protease was detected by proteomic analysis, regardless the high number of transcripts reads assigned to this subclass of putative secreted proteins (Table 3). There are several possible explanations for this observation. First, the proteins are expressed in a such a small amount not detectable by our proteomic approach; second, the proteins are not secreted; third and most likely, these proteins present in SGs are expressed upon specific physiological conditions, such as during stimulation of salivation (feeding). In addition, it is also possible that those enzymes have both intracellular and extracellular functions as many other proteases do.
Table 4. Classification and abundance of proteins from the salivary proteome of R. neglectus based on LC-MS/MS.
| Class | No. of protein groups | % Total |
|---|---|---|
| Lipocalin–Triabin | 34 | 46.58 |
| Lipocalin–Nitrophorin | 17 | 23.29 |
| Others | 13 | 17.81 |
| Conserved secreted protein | 2 | 2.74 |
| Antigen-5/SCP | 1 | 1.37 |
| Inositol polyphosphate phosphatase | 1 | 1.37 |
| Metalloprotease | 1 | 1.37 |
| Protease inhibitor–Serpin | 1 | 1.37 |
| Chitinase-like lectin | 1 | 1.37 |
| Trypsin-like protease | 1 | 1.37 |
| Juvenile hormone related | 1 | 1.37 |
| Total | 73 | 100 |
Comparison of Protein Contents between R. neglectus and R. prolixus
A comparative blastp analysis was employed to address the similarity of the SGs proteins from R. neglectus compared to R. prolixus. The two species do not show high evolutionary divergence, presenting at least 80% identity in analyzed sequences (Table 5), suggesting both species share a common ancestral lineage. As described before, this high degree of protein similarity was also seen with R. brethesi and R. robustus in the Amazon rainforest [106].
Table 5. Identities of R. neglectus proteins compared to R. prolixus (v 3.0) proteins by blastp.
| Class | Average identity | SE* | N |
|---|---|---|---|
| Secreted | 86.46 | 1.47 | 200 |
| Immunity | 91.17 | 3.76 | 35 |
| Transporters and channels | 92.33 | 1.22 | 114 |
| Extracelular matrix | 94.07 | 1.81 | 43 |
| Transposable elements | 94.64 | 2.50 | 22 |
| Protein export | 95.09 | 0.84 | 166 |
| Signal transduction | 95.39 | 0.54 | 285 |
| Storage | 95.50 | 1.44 | 8 |
| Cytoskeletal | 95.64 | 0.96 | 88 |
| Unknown conserved | 95.99 | 0.44 | 369 |
| Protein modification | 95.99 | 0.78 | 144 |
| Metabolism | 96.28 | 0.45 | 405 |
| Transcription machinery | 96.50 | 0.50 | 250 |
| Proteasome machinery | 96.59 | 0.80 | 122 |
| Nuclear regulation | 96.65 | 0.75 | 95 |
| Nuclear export | 96.67 | 1.05 | 12 |
| Unknown | 97.04 | 1.47 | 24 |
| Detoxification | 97.15 | 1.16 | 48 |
| Transcription factor | 97.43 | 0.63 | 53 |
| Protein synthesis | 97.95 | 0.42 | 180 |
| Total | 2,663 |
*SE: standard error
Identity among sequences was greater in housekeeping class members, showing that these proteins have a lower evolutionary rate than those of the secreted class. This indicates that antihaemostatic proteins evolve faster after divergence. Different molecular mechanisms may be responsible for the variation between these closely related Rhodnius species, expanding their biological diversity patterns. The particularity of each species could be related to their different habitats, including different prey and abiotic factors.
Final Considerations
Hematophagy evolved independently at least six times in approximately 15,000 species allowing for adaptation to an existing complex host haemostatic system [5, 107]. Thus, many salivary molecules target different pathways for the insect to achieve a successful blood meal. Here, we described R. neglectus sialome in all its complexity to expand our knowledge of the salivary proteins from hematophagous triatomine bugs.
R. neglectus is considered of secondary importance in the transmission of T. cruzi, causative agent of Chagas disease. The analysis of salivary secretory products of R. neglectus that might be involved in vector-host interactions share similarity with other triatomine species, which can also be infected by and transmit the protozoan.
It is possible that the expression of putative trypsin-like serine proteases in the SGs of R. neglectus correlates with blood sources of this species of triatomine. Their role and that of other secreted class, hypothetical and conserved secreted proteins, in hematophagy should be analyzed in future works, and we accentuate that sialome study is still an open field for new discoveries.
Supporting Information
ClustalW alignment of antigen-5 members from R. neglectus salivary transcriptome (RN_21331, RN_21434 and RN_21812) and other hemiptera sequences, identified as described in Methods section. The alignment indicates conserved domains in black and similar domains in gray background.
(TIF)
ClustalW alignment of a dipetalogastin member from R. neglectus salivary transcriptome (RN_1079) and other sequences from dipetalogastin family, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The boxes indicate conserved motifs, and the blue bar indicates the signal peptide indicative of secretion.
(TIF)
ClustalW alignment of pacifastin members from R. neglectus salivary transcriptome (RN_17301 and RN_20047) and other sequences from the pacifastin family of proteins, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The bars indicate the four conserved pacifastin motifs.
(TIF)
Phylogenetic tree was built from the alignment of R. neglectus CDS and other OBP sequences as described in Methods section. The bar represents 20% amino acid substitution.
(TIF)
Acknowledgments
We thank Ana Cristina Gomes and Fabiano Bastos for technical assistance.
Data Availability
All Invertebrate sample from Rhodnius neglectus salivary glands files are available from the BioSample database (accession number SAMN03975952). http://www.ncbi.nlm.nih.gov/biosample/3975952
Funding Statement
This work was supported by grants 476252/2012-1 (http://www.cnpq.br) from the Brazilian National Council for Scientific and Technological Development (CNPq), PROAP-2013 (http://www.capes.gov.br) from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), CT-Infra 2011 (http://www.finep.gov.br) from the Financiadora de Estudos e Projetos (Finep), and Pronex 476252/2012-5 (http://www.fap.df.gov.br) from the Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) to JMS; and Z01 AI000810-18 (http://www.niaid.nih.gov/Pages/default.aspx) to Vector-Borne Diseases: Biology of Vector Host Relationship, from the National Institute of Allergy and Infectious Diseases (NIAID) to JMCR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.LAVOIPIERRE MM, DICKERSON G, GORDON RM. Studies on the methods of feeding of blood-sucking arthropods. I. The manner in which triatomine bugs obtain their blood-meal, as observed in the tissues of the living rodent, with some remarks on the effects of the bite on human volunteers. Ann Trop Med Parasitol. 1959;53:235–50. . [PubMed] [Google Scholar]
- 2.Fontaine A, Diouf I, Bakkali N, Misse D, Pages F, Fusai T, et al. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors. 2011;4:187 10.1186/1756-3305-4-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champagne DE. Antihemostatic strategies of blood-feeding arthropods. Current drug targets. 2004;4(4):375–96. . [DOI] [PubMed] [Google Scholar]
- 4.Champagne DE. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiology of haemostasis and thrombosis. 2005;34(4–5):221–7. 10.1159/000092428 . [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4(3):143–52. . [PubMed] [Google Scholar]
- 6.Steverding D. The history of Chagas disease. Parasit Vectors. 2014;7:317 10.1186/1756-3305-7-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guhl F, Pinto N, Aguilera G. Sylvatic triatominae: a new challenge in vector control transmission. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:71–5. . [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick S, Feliciangeli MD, Sanchez-Martin MJ, Monteiro FA, Miles MA. Molecular genetics reveal that silvatic Rhodnius prolixus do colonise rural houses. PLoS Negl Trop Dis. 2008;2(4):e210 10.1371/journal.pntd.0000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abad-Franch F, Monteiro FA, Jaramillo O N, Gurgel-Gonçalves R, Dias FB, Diotaiuti L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease: a multi-scale appraisal of the tribe Rhodniini (Triatominae). Acta Trop. 2009;110(2–3):159–77. 10.1016/j.actatropica.2008.06.005 . [DOI] [PubMed] [Google Scholar]
- 10.Gurgel-Goncalves R, Galvao C, Costa J, Peterson AT. Geographic distribution of chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012;2012:705326 10.1155/2012/705326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurgel-Goncalves R, Cura C, Schijman AG, Cuba CA. Infestation of Mauritia flexuosa palms by triatomines (Hemiptera: Reduviidae), vectors of Trypanosoma cruzi and Trypanosoma rangeli in the Brazilian savanna. Acta Trop. 2012;121(2):105–11. 10.1016/j.actatropica.2011.10.010 . [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues VL, Pauliquevis C Junior, da Silva RA, Wanderley DM, Guirardo MM, Rodas LA, et al. Colonization of palm trees by Rhodnius neglectus and household and invasion in an urban area, Araçatuba, São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2014;56(3):213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubens Antonio Silva PRBeDMVW. Doença de Chagas no Estado de São Paulo: comparaçãoentre pesquisa ativa de triatomíneos em domicílios enotificação de sua presença pela população emárea sob vigilância entomológica. Revista da Sociedade Brasileira de Medicina Tropical1999. [DOI] [PubMed]
- 14.Gurgel-Gonçalves R, Ramalho ED, Duarte MA, Palma AR, Abad-Franch F, Carranza JC, et al. Enzootic transmission of Trypanosoma cruzi and T. rangeli in the Federal District of Brazil. Rev Inst Med Trop Sao Paulo. 2004;46(6):323–30. /S0036-46652004000600005. . [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira A W. S., da Silva I G.. Distribuição geográfica e indicadores entomológicos de triatomíneos sinantrópicos capturados no Estado de Goiás. Revista da Sociedade Brasileira de Medicina Tropical; 2007. p. 204–8. [DOI] [PubMed] [Google Scholar]
- 16.Almeida PSd, al e. Levantamento da fauna de Triatominae (Hemiptera: Reduviidae) em ambiente domiciliar e infecção natural por Trypanosomatidae no Estado de Mato Grosso do Sul. Uberaba: Rev. Soc. Bras. Med. Trop; 2008. [DOI] [PubMed] [Google Scholar]
- 17.Gurgel-Gonçalves R, Abad-Franch F, Ferreira JB, Santana DB, Cuba CA. Is Rhodnius prolixus (Triatominae) invading houses in central Brazil? Acta Trop. 2008;107(2):90–8. 10.1016/j.actatropica.2008.04.020 . [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro JM, Chagas AC, Pham VM, Lounibos LP, Calvo E. An insight into the sialome of the frog biting fly, Corethrella appendiculata. Insect biochemistry and molecular biology. 2013. Epub 2014/02/12. 10.1016/j.ibmb.2013.10.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I. ABySS: a parallel assembler for short read sequence data. Genome Res. 2009;19(6):1117–23. 10.1101/gr.089532.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):18 10.1186/2047-217X-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS ONE. 2011;6(12):e28525 Epub 2012/01/05. 10.1371/journal.pone.0028525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen H, Brunak S, von Heijne G. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein engineering. 1999;12(1):3–9. . [DOI] [PubMed] [Google Scholar]
- 23.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT Oxford University Press: Nucleic Acids Symposium Series; 1999. p. 95–8. [Google Scholar]
- 25.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. 10.1093/bioinformatics/btm404 . [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5(2):150–63. . [DOI] [PubMed] [Google Scholar]
- 27.Queiroz RM, Charneau S, Motta FN, Santana JM, Roepstorff P, Ricart CA. Comprehensive proteomic analysis of Trypanosoma cruzi epimastigote cell surface proteins by two complementary methods. J Proteome Res. 2013;12(7):3255–63. 10.1021/pr400110h . [DOI] [PubMed] [Google Scholar]
- 28.Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318 (Pt 1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noeske-Jungblut C, Haendler B, Donner P, Alagon A, Possani L, Schleuning WD. Triabin, a highly potent exosite inhibitor of thrombin. The Journal of biological chemistry. 1995;270(48):28629–34. . [DOI] [PubMed] [Google Scholar]
- 31.Fuentes-Prior P, Noeske-Jungblut C, Donner P, Schleuning WD, Huber R, Bode W. Structure of the thrombin complex with triabin, a lipocalin-like exosite-binding inhibitor derived from a triatomine bug. Proc Natl Acad Sci U S A. 1997;94(22):11845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montfort WR, Weichsel A, Andersen JF. Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods. Biochimica et biophysica acta. 2000;1482(1–2):110–8. Epub 2000/11/04. S0167-4838(00)00165-5 [pii]. . [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro JM, Walker FA. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. The Journal of experimental medicine. 1994;180(6):2251–7. Epub 1994/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champagne DE, Nussenzveig RH, Ribeiro JM. Purification, partial characterization, and cloning of nitric oxide-carrying heme proteins (nitrophorins) from salivary glands of the blood-sucking insect Rhodnius prolixus. J Biol Chem. 1995;270(15):8691–5. . [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Ribeiro JM, Guimaraes JA, Walsh PN. Nitrophorin-2: a novel mixed-type reversible specific inhibitor of the intrinsic factor-X activating complex. Biochemistry. 1998;37(30):10681–90. [DOI] [PubMed] [Google Scholar]
- 36.Andersen JF, Gudderra NP, Francischetti IM, Valenzuela JG, Ribeiro JM. Recognition of anionic phospholipid membranes by an antihemostatic protein from a blood-feeding insect. Biochemistry. 2004;43(22):6987–94. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares RP, Sant'Anna MR, Gontijo NF, Romanha AJ, Diotaiuti L, Pereira MH. Identification of morphologically similar Rhodnius species (Hemiptera: Reduviidae: Triatominae) by electrophoresis of salivary heme proteins. Am J Trop Med Hyg. 2000;62(1):157–61. . [DOI] [PubMed] [Google Scholar]
- 38.Gibbs GM, Roelants K, O'Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocrine reviews. 2008;29(7):865–97. 10.1210/er.2008-0032 [DOI] [PubMed] [Google Scholar]
- 39.Fang KS, Vitale M, Fehlner P, King TP. cDNA cloning and primary structure of a white-face hornet venom allergen, antigen 5. Proc Natl Acad Sci U S A. 1988;85(3):895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman DR. Allergens in Hymenoptera venom. XXV: The amino acid sequences of antigen 5 molecules and the structural basis of antigenic cross-reactivity. The Journal of allergy and clinical immunology. 1993;92(5):707–16. . [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki Y, Koike H, Sugiyama Y, Motoyoshi K, Wada T, Hishinuma S, et al. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur J Biochem. 2002;269(11):2708–15. . [DOI] [PubMed] [Google Scholar]
- 42.Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, et al. Plant 'pathogenesis-related' proteins and their role in defense against pathogens. Biochimie. 1993;75(8):687–706. . [DOI] [PubMed] [Google Scholar]
- 43.Calvo E, Dao A, Pham VM, Ribeiro JM. An insight into the sialome of Anopheles funestus reveals an emerging pattern in anopheline salivary protein families. Insect biochemistry and molecular biology. 2007;37(2):164–75. Epub 2007/01/25. 10.1016/j.ibmb.2006.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect biochemistry and molecular biology. 2002;32(9):1101–22. Epub 2002/09/06. . [DOI] [PubMed] [Google Scholar]
- 45.Charlab R, Valenzuela JG, Rowton ED, Ribeiro JM. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis. Proc Natl Acad Sci U S A. 1999;96(26):15155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribeiro JM, Andersen J, Silva-Neto MA, Pham VM, Garfield MK, Valenzuela JG. Exploring the sialome of the blood-sucking bug Rhodnius prolixus. Insect Biochem Mol Biol. 2004;34(1):61–79. . [DOI] [PubMed] [Google Scholar]
- 47.Assumpcao TC, Francischetti IM, Andersen JF, Schwarz A, Santana JM, Ribeiro JM. An insight into the sialome of the blood-sucking bug Triatoma infestans, a vector of Chagas' disease. Insect biochemistry and molecular biology. 2008;38(2):213–32. 10.1016/j.ibmb.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assumpcao TC, Charneau S, Santiago PB, Francischetti IM, Meng Z, Araujo CN, et al. Insight into the salivary transcriptome and proteome of Dipetalogaster maxima. Journal of proteome research. 2011;10(2):669–79. 10.1021/pr100866h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assumpcao TC, Eaton DP, Pham VM, Francischetti IM, Aoki V, Hans-Filho G, et al. An insight into the sialotranscriptome of Triatoma matogrossensis, a kissing bug associated with fogo selvagem in South America. The American journal of tropical medicine and hygiene. 2012;86(6):1005–14. Epub 2012/06/06. 10.4269/ajtmh.2012.11-0690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro JM, Assumpcao TC, Pham VM, Francischetti IM, Reisenman CE. An insight into the sialotranscriptome of Triatoma rubida (Hemiptera: Heteroptera). Journal of medical entomology. 2012;49(3):563–72. Epub 2012/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Assumpção TC, Ma D, Schwarz A, Reiter K, Santana JM, Andersen JF, et al. Salivary antigen-5/CAP family members are Cu2+-dependent antioxidant enzymes that scavenge O2-. and inhibit collagen-induced platelet aggregation and neutrophil oxidative burst. J Biol Chem. 2013;288(20):14341–61. 10.1074/jbc.M113.466995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–70. . [DOI] [PubMed] [Google Scholar]
- 53.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement—their role in inflammation. Semin Immunopathol. 2012;34(1):151–65. 10.1007/s00281-011-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlott B, Wohnert J, Icke C, Hartmann M, Ramachandran R, Guhrs KH, et al. Interaction of Kazal-type inhibitor domains with serine proteinases: biochemical and structural studies. Journal of molecular biology. 2002;318(2):533–46. . [DOI] [PubMed] [Google Scholar]
- 55.Friedrich T, Kroger B, Bialojan S, Lemaire HG, Hoffken HW, Reuschenbach P, et al. A Kazal-type inhibitor with thrombin specificity from Rhodnius prolixus. The Journal of biological chemistry. 1993;268(22):16216–22. . [PubMed] [Google Scholar]
- 56.van de Locht A, Lamba D, Bauer M, Huber R, Friedrich T, Kroger B, et al. Two heads are better than one: crystal structure of the insect derived double domain Kazal inhibitor rhodniin in complex with thrombin. EMBO J. 1995;14(21):5149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mende K, Petoukhova O, Koulitchkova V, Schaub GA, Lange U, Kaufmann R, et al. Dipetalogastin, a potent thrombin inhibitor from the blood-sucking insect. Dipetalogaster maximus cDNA cloning, expression and characterization. European journal of biochemistry / FEBS. 1999;266(2):583–90. . [DOI] [PubMed] [Google Scholar]
- 58.Campos IT, Amino R, Sampaio CA, Auerswald EA, Friedrich T, Lemaire HG, et al. Infestin, a thrombin inhibitor presents in Triatoma infestans midgut, a Chagas' disease vector: gene cloning, expression and characterization of the inhibitor. Insect biochemistry and molecular biology. 2002;32(9):991–7. . [DOI] [PubMed] [Google Scholar]
- 59.Araujo RN, Campos IT, Tanaka AS, Santos A, Gontijo NF, Lehane MJ, et al. Brasiliensin: A novel intestinal thrombin inhibitor from Triatoma brasiliensis (Hemiptera: Reduviidae) with an important role in blood intake. International journal for parasitology. 2007;37(12):1351–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takac P, Nunn MA, Meszaros J, Pechanova O, Vrbjar N, Vlasakova P, et al. Vasotab, a vasoactive peptide from horse fly Hybomitra bimaculata (Diptera, Tabanidae) salivary glands. J Exp Biol. 2006;209(Pt 2):343–52. . [DOI] [PubMed] [Google Scholar]
- 61.Liang Z, Sottrup-Jensen L, Aspan A, Hall M, Soderhall K. Pacifastin, a novel 155-kDa heterodimeric proteinase inhibitor containing a unique transferrin chain. Proc Natl Acad Sci U S A. 1997;94(13):6682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hergenhahn HG, Aspan A, Soderhall K. Purification and characterization of a high-Mr proteinase inhibitor of pro-phenol oxidase activation from crayfish plasma. Biochem J. 1987;248(1):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breugelmans B, Simonet G, van Hoef V, Van Soest S, Broeck JV. Identification, distribution and molecular evolution of the pacifastin gene family in Metazoa. BMC Evol Biol. 2009;9:97 10.1186/1471-2148-9-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Marco R, Lovato DV, Torquato RJ, Clara RO, Buarque DS, Tanaka AS. The first pacifastin elastase inhibitor characterized from a blood sucking animal. Peptides. 2010;31(7):1280–6. Epub 2010/04/13. S0196-9781(10)00148-8 [pii] 10.1016/j.peptides.2010.03.033 . [DOI] [PubMed] [Google Scholar]
- 65.Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10(12):1845–64. . [DOI] [PubMed] [Google Scholar]
- 66.Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost. 2007;5 Suppl 1:102–15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stark KR, James AA. Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito, Aedes aegypti. J Biol Chem. 1998;273(33):20802–9. . [DOI] [PubMed] [Google Scholar]
- 68.Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, et al. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 2009;276(12):3235–46. 10.1111/j.1742-4658.2009.07038.x . [DOI] [PubMed] [Google Scholar]
- 69.Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, et al. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. The Journal of biological chemistry. 2006;281(36):26361–9. . [DOI] [PubMed] [Google Scholar]
- 70.Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, et al. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem. 2002;277(12):10083–9. 10.1074/jbc.M111391200 . [DOI] [PubMed] [Google Scholar]
- 71.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102(12):4751–804. . [DOI] [PubMed] [Google Scholar]
- 72.Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS letters. 1993;331(1–2):134–40. [DOI] [PubMed] [Google Scholar]
- 73.Jiang W, Bond JS. Families of metalloendopeptidases and their relationships. FEBS letters. 1992;312(2–3):110–4. [DOI] [PubMed] [Google Scholar]
- 74.Hooper NM. Families of zinc metalloproteases. FEBS letters. 1994;354(1):1–6. [DOI] [PubMed] [Google Scholar]
- 75.da Silveira RB, dos Santos Filho JF, Mangili OC, Veiga SS, Gremski W, Nader HB, et al. Identification of proteases in the extract of venom glands from brown spiders. Toxicon. 2002;40(6):815–22. . [DOI] [PubMed] [Google Scholar]
- 76.Feitosa L, Gremski W, Veiga SS, Elias MC, Graner E, Mangili OC, et al. Detection and characterization of metalloproteinases with gelatinolytic, fibronectinolytic and fibrinogenolytic activities in brown spider (Loxosceles intermedia) venom. Toxicon. 1998;36(7):1039–51. [DOI] [PubMed] [Google Scholar]
- 77.da Silveira RB, Wille AC, Chaim OM, Appel MH, Silva DT, Franco CR, et al. Identification, cloning, expression and functional characterization of an astacin-like metalloprotease toxin from Loxosceles intermedia (brown spider) venom. Biochem J. 2007;406(2):355–63. 10.1042/BJ20070363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trevisan-Silva D, Gremski LH, Chaim OM, da Silveira RB, Meissner GO, Mangili OC, et al. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie. 2010;92(1):21–32. 10.1016/j.biochi.2009.10.003 . [DOI] [PubMed] [Google Scholar]
- 79.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. 10.1042/BJ20040424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gould RJ, Polokoff MA, Friedman PA, Huang TF, Holt JC, Cook JJ, et al. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990;195(2):168–71. . [DOI] [PubMed] [Google Scholar]
- 82.Calvete JJ, Marcinkiewicz C, Monleon D, Esteve V, Celda B, Juarez P, et al. Snake venom disintegrins: evolution of structure and function. Toxicon. 2005;45(8):1063–74. 10.1016/j.toxicon.2005.02.024 . [DOI] [PubMed] [Google Scholar]
- 83.Huang TF. What have snakes taught us about integrins? Cell Mol Life Sci. 1998;54(6):527–40. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsu CC, Wu WB, Chang YH, Kuo HL, Huang TF. Antithrombotic effect of a protein-type I class snake venom metalloproteinase, kistomin, is mediated by affecting glycoprotein Ib-von Willebrand factor interaction. Mol Pharmacol. 2007;72(4):984–92. 10.1124/mol.107.038018 . [DOI] [PubMed] [Google Scholar]
- 85.Huang TF, Chang MC, Teng CM. Antiplatelet protease, kistomin, selectively cleaves human platelet glycoprotein Ib. Biochim Biophys Acta. 1993;1158(3):293–9. . [DOI] [PubMed] [Google Scholar]
- 86.Hsu CC, Wu WB, Huang TF. A snake venom metalloproteinase, kistomin, cleaves platelet glycoprotein VI and impairs platelet functions. J Thromb Haemost. 2008;6(9):1578–85. 10.1111/j.1538-7836.2008.03071.x . [DOI] [PubMed] [Google Scholar]
- 87.Wu WB, Chang SC, Liau MY, Huang TF. Purification, molecular cloning and mechanism of action of graminelysin I, a snake-venom-derived metalloproteinase that induces apoptosis of human endothelial cells. Biochem J. 2001;357(Pt 3):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu WB, Huang TF. Activation of MMP-2, cleavage of matrix proteins, and adherens junctions during a snake venom metalloproteinase-induced endothelial cell apoptosis. Exp Cell Res. 2003;288(1):143–57. . [DOI] [PubMed] [Google Scholar]
- 89.Tseng YL, Lee CJ, Huang TF. Effects of a snake venom metalloproteinase, triflamp, on platelet aggregation, platelet-neutrophil and neutrophil-neutrophil interactions: involvement of platelet GPIbalpha and neutrophil PSGL-1. Thromb Haemost. 2004;91(2):315–24. 10.1160/TH03-07-0426 . [DOI] [PubMed] [Google Scholar]
- 90.Yeh CH, Peng HC, Yang RS, Huang TF. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis elicited by basic fibroblast growth factor and suppresses tumor growth by a selective alpha(v)beta(3) blockade of endothelial cells. Mol Pharmacol. 2001;59(5):1333–42. . [DOI] [PubMed] [Google Scholar]
- 91.Assumpção TC, Ribeiro JM, Francischetti IM. Disintegrins from hematophagous sources. Toxins (Basel). 2012;4(5):296–322. 10.3390/toxins4050296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffman DR. Hymenoptera venom allergens. Clin Rev Allergy Immunol. 2006;30(2):109–28. . [DOI] [PubMed] [Google Scholar]
- 93.Georgieva D, Greunke K, Arni RK, Betzel C. Three-dimensional modelling of honeybee venom allergenic proteases: relation to allergenicity. Z Naturforsch C. 2011;66(5–6):305–12. . [DOI] [PubMed] [Google Scholar]
- 94.Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269(50):31869–80. . [PubMed] [Google Scholar]
- 95.Kalafatis M, Egan JO, van 't Veer C, Cawthern KM, Mann KG. The regulation of clotting factors. Crit Rev Eukaryot Gene Expr. 1997;7(3):241–80. . [DOI] [PubMed] [Google Scholar]
- 96.Stocker K, Fischer H, Meier J. Thrombin-like snake venom proteinases. Toxicon. 1982;20(1):265–73. . [DOI] [PubMed] [Google Scholar]
- 97.Amino R, Tanaka AS, Schenkman S. Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas' disease Triatoma infestans (Hemiptera: Reduviidae). Insect biochemistry and molecular biology. 2001;31(4–5):465–72. Epub 2001/02/27. . [DOI] [PubMed] [Google Scholar]
- 98.Xu X, Yang H, Ma D, Wu J, Wang Y, Song Y, et al. Toward an understanding of the molecular mechanism for successful blood feeding by coupling proteomics analysis with pharmacological testing of horsefly salivary glands. Mol Cell Proteomics. 2008;7(3):582–90. . [DOI] [PubMed] [Google Scholar]
- 99.Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30(2):95–105. . [DOI] [PubMed] [Google Scholar]
- 100.He W-Y, Zheng Y-P, Tang L, Zheng S-C, Béliveau C, Doucet D, et al. Cloning, expression and localization of a trypsin-like serine protease in the spruce budworm, Choristoneura fumiferana. Insect Science. 2009;16(6):455–64. [Google Scholar]
- 101.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428(3):325–46. 10.1042/BJ20100046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014;5:320 10.3389/fphys.2014.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leal WS, Nikonova L, Peng G. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 1999;464(1–2):85–90. . [DOI] [PubMed] [Google Scholar]
- 104.Tegoni M, Campanacci V, Cambillau C. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem Sci. 2004;29(5):257–64. 10.1016/j.tibs.2004.03.003 . [DOI] [PubMed] [Google Scholar]
- 105.Sandler BH, Nikonova L, Leal WS, Clardy J. Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem Biol. 2000;7(2):143–51. . [DOI] [PubMed] [Google Scholar]
- 106.Costa CM, Sousa MV, Ricart CA, Santana JM, Teixeira AR, Roepstorff P, et al. 2-DE-based proteomic investigation of the saliva of the Amazonian triatomine vectors of Chagas disease: Rhodnius brethesi and Rhodnius robustus. J Proteomics. 2011;74(9):1652–63. 10.1016/j.jprot.2011.02.022 . [DOI] [PubMed] [Google Scholar]
- 107.Mans BJ, Louw AI, Neitz AW. Evolution of hematophagy in ticks: common origins for blood coagulation and platelet aggregation inhibitors from soft ticks of the genus Ornithodoros. Mol Biol Evol. 2002;19(10):1695–705. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ClustalW alignment of antigen-5 members from R. neglectus salivary transcriptome (RN_21331, RN_21434 and RN_21812) and other hemiptera sequences, identified as described in Methods section. The alignment indicates conserved domains in black and similar domains in gray background.
(TIF)
ClustalW alignment of a dipetalogastin member from R. neglectus salivary transcriptome (RN_1079) and other sequences from dipetalogastin family, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The boxes indicate conserved motifs, and the blue bar indicates the signal peptide indicative of secretion.
(TIF)
ClustalW alignment of pacifastin members from R. neglectus salivary transcriptome (RN_17301 and RN_20047) and other sequences from the pacifastin family of proteins, identified as described in Methods section. The alignment indicates conserved residues in black and similar residues in gray background. The bars indicate the four conserved pacifastin motifs.
(TIF)
Phylogenetic tree was built from the alignment of R. neglectus CDS and other OBP sequences as described in Methods section. The bar represents 20% amino acid substitution.
(TIF)
Data Availability Statement
All Invertebrate sample from Rhodnius neglectus salivary glands files are available from the BioSample database (accession number SAMN03975952). http://www.ncbi.nlm.nih.gov/biosample/3975952
The raw reads were deposited at the Sequence Read Archive (SRA) in NCBI under bioproject PRJNA292130. A total of 4,230 coding sequences were deposited in DDBJ/EMBL/GenBank through the Transcriptome Shotgun Annotation portal under the accession GDKW00000000.