Summary
Sleep is an evolutionarily conserved behavioral state whose regulation is poorly understood. A classical model posits that sleep is regulated by homeostatic and circadian mechanisms. Several factors have been implicated in mediating the homeostatic regulation of sleep, but molecules underlying the circadian mechanism are unknown. Here we use animals lacking melatonin due to mutation of arylalkylamine N-acetyltransferase 2 (aanat2) to show that melatonin is required for circadian regulation of sleep in zebrafish. Sleep is dramatically reduced at night in aanat2 mutants maintained in light/dark conditions, and the circadian regulation of sleep is abolished in free-running conditions. We find that melatonin promotes sleep downstream of the circadian clock as it is not required to initiate or maintain circadian rhythms. Additionally, we provide evidence that melatonin may induce sleep in part by promoting adenosine signaling, thus potentially linking circadian and homeostatic control of sleep.
Introduction
Sleep is an evolutionarily conserved behavioral state whose regulation is poorly understood (Sehgal and Mignot, 2011). A key model postulates regulation by a homeostatic process that responds to internal cues for sleep need and a circadian process that responds to external cues (Borbely, 1982). Several lines of evidence suggest that accumulation of extracellular adenosine in specific brain regions plays an important role in the homeostatic mechanism, although it is clear that other factors are also required (reviewed in Brown et al., 2012). However, while mechanisms that regulate the circadian clock are well-characterized (Fisher et al., 2013), molecules that transmit circadian information to regulate sleep are largely unknown.
Melatonin is an attractive candidate for mediating the circadian process because the clock regulates its production (Klein, 2007) and it induces sleep in some contexts (Fisher et al., 2013). However, despite decades of study and widespread use, the role of melatonin in regulating sleep is controversial (Fisher et al., 2013). Exogenous melatonin is reported to have sleep-promoting effects in diurnal vertebrates including humans (Brzezinski et al., 2005; Zhdanova, 2005), nonhuman primates (Zhdanova et al., 2002), domesticated cats (Goldstein and Pavel, 1981), birds (Mintz et al., 1998) and zebrafish (Zhdanova et al., 2001). However, others failed to observe this effect (Buscemi et al., 2006; van den Heuvel et al., 2005), and its endogenous role in any animal is unknown. Exogenous melatonin can entrain (Lockley et al., 2000; Sack et al., 2000) and phase-shift (Lewy et al., 1992) the circadian clock in some contexts, and melatonin has been called a regulator of circadian rhythms (Elbaz et al., 2013). Indeed, melatonin has been proposed to promote sleep indirectly by phase-advancing the circadian clock (Arendt, 2003) or by inhibiting the circadian drive for wakefulness (Scheer and Czeisler, 2005). However, these hypotheses are based on exogenous melatonin. Determining melatonin’s role in sleep is complicated by its production at night in both diurnal and nocturnal animals, and because most laboratory mouse strains produce little or no melatonin (Goto et al., 1989). As a result, studies have relied on pharmacological approaches that have produced inconsistent results (Fisher et al., 2013). To determine whether endogenous melatonin regulates sleep in a diurnal vertebrate, we analyzed melatonin function in the zebrafish, whose circadian clock and sleep regulation are conserved with mammals (Elbaz et al., 2013).
Results
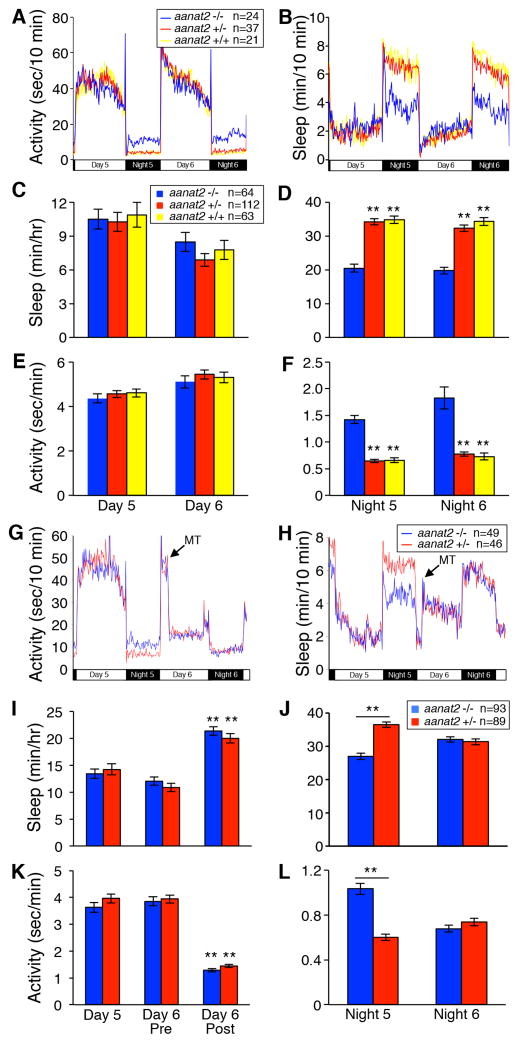
Melatonin is produced in the zebrafish pineal gland at night under control of the circadian clock (Kazimi and Cahill, 1999), as in mammals. To determine whether endogenous melatonin is required for sleep, we generated zebrafish with a predicted null mutation in arylalkylamine N-acetyltransferase 2 (aanat2) (Figure S1A), which is required for melatonin synthesis in the pineal gland (Klein, 2007). We found that wild type (WT) larvae in 14:10 hour light:dark (LD) conditions had high melatonin levels at night and low levels during the day, while aanat2 homozygous mutants (aanat2−/−) produced little or no melatonin (Figure S1B). To determine whether melatonin is required for sleep, we used a videotracking assay (Prober et al., 2006) to compare sleep/wake behaviors of aanat2−/− larvae to their aanat2+/− and WT siblings. We found that all three genotypes exhibited similar daytime amounts of sleep and activity (Figure 1A–C,E). However, at night, aanat2−/− larvae slept almost half as much and were three times more active than controls (Figure 1A,B,D,F). Decreased nighttime sleep was due mainly to a decrease in sleep bout length and corresponding increase in wake bout length, with little effect on bout number (Figure S1F,H,L). Sleep latency (time between lights-out and sleep) at night was also longer for aanat2−/− larvae (Figure S1J). These results demonstrate that endogenous melatonin promotes initiation and maintenance of nighttime sleep in a diurnal vertebrate.
Figure 1. aanat2−/− larvae sleep less at night.
(A–F) aanat2−/− larvae sleep less (B,D) and are more active (A,F) than sibling controls at night, but not during the day (A,B,C,E). (G–L) Arrow indicates addition of 10 μM melatonin on day 6. aanat2−/− sleep and locomotor activity phenotypes of night 5 are absent on night 6 (G,H,J,L). During the day, exogenous melatonin decreases locomotor activity (G,K) and increases sleep (H,I) for both genotypes. Day 6 Pre and Post refer to periods before and after melatonin addition. Total sleep amount can vary for nights 5 and 6 of development, so comparisons between different genotypes should be made on the same night. Data are from one representative experiment (A–B,G–H) or combined from two (I–L) or three (C–F) experiments. Bar graphs represent mean ± SEM. n, number of larvae. **, p<0.01 compared to aanat2−/− (C–F), each genotype Day 6 Post compared to Day 6 Pre (I,K), or the indicated comparisons (J,L) by Dunnett’s (C–F) or Tukey’s test (I–L). See also Figure S1.
Serotonin is acetylated by AANAT to form acetylserotonin, which is methylated to form melatonin. Loss of aanat2 may thus elevate serotonin levels in the pineal gland. Consistent with mammalian results (Borjigin et al., 2012), we found that serotonin levels are higher in the pineal gland at night compared to the day in aanat2+/− larvae (Figure S1C–D). Serotonin levels were even higher at night in aanat2−/− larvae compared to their aanat2+/− siblings, indicating that loss of aanat2 results in higher pineal serotonin levels. To test whether the aanat2−/− behavioral phenotype is due to loss of melatonin or increased serotonin, we added exogenous melatonin, which completely rescued the aanat2−/− phenotype (Figure 1G–J,L), indicating that it is due to loss of melatonin.
Zebrafish have a second aanat ortholog (aanat1) that is expressed in the retina (Appelbaum et al., 2006). To test whether aanat1 is partially redundant with aanat2 in regulating sleep, we generated zebrafish with a predicted null mutation in aanat1 (Figure S1A). The behavioral phenotype of aanat1−/−; aanat2−/− larvae was indistinguishable from that of aanat2−/− larvae (data not shown), suggesting that aanat1 is not required for sleep.
Since aanat2−/− larvae sleep less at night, we hypothesized that their arousal threshold might be reduced. To test this hypothesis, we applied a mechanoacoustic stimulus (Woods et al., 2014) at night at 1-minute intervals at a range of intensities. Surprisingly, we found that aanat2−/− larvae and their sibling controls exhibited similar half-maximal response probabilities (log(probability) 1.17±0.03, 1.15±0.03 and 1.16±0.04 for aanat2−/−, aanat2+/− and aanat2+/+, respectively; p=0.92 by extra sum-of-squares F test) (Figure S1M), indicating that aanat2−/− larvae have a normal arousal threshold. We next asked whether sleeping aanat2−/− larvae are more likely to awaken in response to a stimulus, since light sleep is often a feature of insomnia. To test this hypothesis, we stimulated larvae every 5 minutes, allowing larvae to re-enter sleep after each stimulus. We used a tap strength at which 50% of larvae normally respond. There was no significant difference among the three genotypes (p=0.43 by one-way ANOVA) (Figure S1N). Thus, although aanat2−/− larvae sleep less at night, their arousal threshold and sleep depth are similar to controls, suggesting that their sleep defect is not caused by hyperarousal.
As an alternative approach to deplete melatonin we ablated melatonin-producing cells. We generated transgenic zebrafish in which the aanat2 promoter drives expression of cyan fluorescent protein fused to nitroreductase (CFP-NTR), a bacterial protein that converts the inert pro-drug metronidazole (MTZ) into a potent DNA cross-linking agent that causes cell-autonomous death (Curado et al., 2007). CFP-NTR was expressed exclusively in aanat2-expressing pineal gland cells (Figure S2A,B), which developed normally but underwent cell death upon addition of MTZ (Figure S2C–E). Some pineal gland cells died within a few hours of adding MTZ, but we observed the most robust ablation without non-specific drug toxicity with treatment from 60–80 and 108–128 hpf, removing MTZ between treatments (Figure S2C–E). As observed for aanat2−/− larvae, aanat2-CFP-NTR larvae slept less and were more active at night (Figure S2F–K), although the effect was weaker, likely due to persistence of a small number of aanat2-expressing cells (Figure S2D,E). Consistent with this hypothesis, 48 hours after MTZ removal we observed a small number of new aanat2-expressing cells (Figure S2D,E) and larvae exhibited normal amounts of sleep and activity (Figure S2F–K), indicating that the aanat2-CFP-NTR phenotype is reversible and suggesting that a small number of pineal gland cells are sufficient for normal sleep.
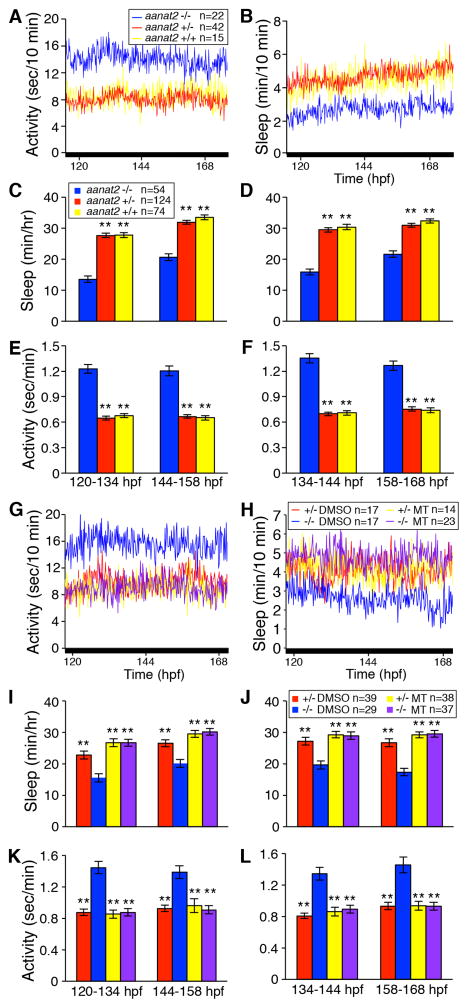
Melatonin has been proposed to promote sleep indirectly, by phase-advancing the circadian clock (Arendt, 2003) or inhibiting the circadian drive for wakefulness (Scheer and Czeisler, 2005). To determine whether the sleep-promoting role of endogenous melatonin results from effects on the circadian clock, we raised and tested aanat2−/− larvae in the dark (DD), resulting in arrhythmic animals lacking overt behavioral or molecular circadian rhythms (Figures 2A–F and S3G–H) (Kaneko and Cahill, 2005). To determine whether the arrhythmia observed in DD is due to arrested or asynchronous cellular circadian clocks (Dekens and Whitmore, 2008) we performed fluorescent in situ hybridization for the clock gene per1b (Figure S3I–Q). If the lack of overt rhythms is caused by arrested clocks, per1b should be expressed similarly at all circadian time points. However, if it results from asynchronous clocks, expression should be more heterogeneous in larvae raised in DD than in those raised in LD. Furthermore, for asynchronous clocks, the average fluorescence intensity among many cells in DD should be lower than the peak level in LD. We found that per1b expression oscillates throughout the brain in LD (Figure S3I,K,M,O) with phasing consistent with data from reverse-transcription quantitative PCR (RT-qPCR) (Figure S3G). In contrast, per1b is expressed at a similar level at all circadian time points in larvae raised in DD (Figure S3J,L,N,P). Quantification of fluorescence intensity in the hypothalamus showed that average per1b levels in DD are similar to peak levels in LD (Figure S3Q). Quantification in the forebrain and midbrain produced similar results (data not shown). These results suggest that raising larvae in DD abolishes circadian rhythms at the cellular level. Under these conditions, aanat2−/− larvae exhibited continuously decreased sleep and increased activity (Figure 2A–F). The magnitude of the difference was similar to that observed at night in LD (Figure 1A–F), and was again due to a decrease in sleep bout length and corresponding increase in wake bout length (Figure S3A–F). This phenotype was also rescued by exogenous melatonin (Figure 2G–L). These results suggest that endogenous melatonin promotes sleep directly rather than via the circadian clock in zebrafish.
Figure 2. Reduced sleep in aanat2−/− zebrafish does not require an entrained circadian rhythm.
(A,B) Larvae raised and tested in DD do not exhibit circadian locomotor activity or sleep rhythms. aanat2−/− larvae are more active (A,E,F) and sleep less (B–D) than sibling controls. (G–L) aanat2−/− larvae treated with vehicle control are more active (G,K,L) and sleep less (H–J) than aanat2+/− siblings. aanat2−/− larvae treated with 10 μM melatonin exhibit locomotor activity and sleep indistinguishable from their melatonin-treated aanat2+/− siblings (G–L). Data are from one representative experiment (A–B,G–H), or combined from two (I–L) or three (C–F) experiments. Bar graphs represent mean ± SEM for the 5th and 6th days and nights of development. **, p<0.01 compared to aanat2−/− (C–F) or aanat2−/− DMSO (I–L) by Dunnett’s test. See also Figure S3.
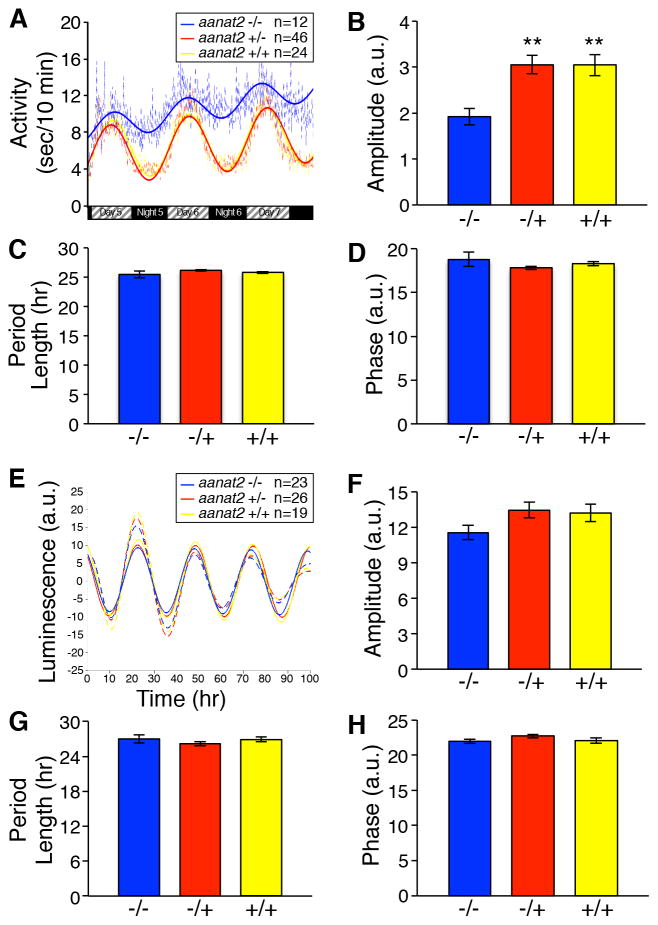
Having established that melatonin is required for sleep at night, we next asked whether melatonin is required for normal circadian rhythms. Studies in humans, nonhuman primates and rodents have shown that exogenous melatonin can phase-shift (Lewy et al., 1992) and entrain (Lockley et al., 2000; Sack et al., 2000) the circadian clock. However, loss of the pineal gland, and thus melatonin production, produces inconsistent results (Arendt, 2003). While pinealectomy in some species of fish, birds and reptiles affects circadian rhythms (Underwood, 2001), it is unclear whether this is due to loss of melatonin. We tested this using two approaches. We first asked whether melatonin is required for behavioral circadian rhythms using the videotracker assay. To monitor circadian rhythms in “free-running” conditions, we entrained larvae in LD and then shifted them to DD. In these conditions, WT larvae maintain molecular and behavioral rhythms (Figure S4A,B,K,L) (Kaneko and Cahill, 2005). While aanat2−/− larvae had smaller circadian amplitude due to increased locomotor activity during subjective night (Figures 3A,B and S4A,H), the period length and phase were similar to controls (Figure 3C,D). Second, we used transgenic fish in which the period 3 promoter regulates expression of luciferase (per3-luc), an in vivo reporter of molecular rhythms in intact larvae (Kaneko and Cahill, 2005). We found that per3-luc amplitude, period length and phase were not significantly different for any genotype (Figure 3E–H). To confirm this result, we isolated RNA from WT and aanat2−/− larvae and performed RT-qPCR for the circadian genes per1b and arntl1a. Circadian oscillation of both genes was indistinguishable between WT and aanat2−/− (Figure S4K,L), indicating that aanat2−/− larvae have normal molecular rhythms. Together the observation that aanat2−/− larvae exhibit normal locomotor activity period length and phase in LD (Figure 1A), this indicates that endogenous melatonin is not required to initiate or maintain circadian rhythms. We conclude that melatonin functions downstream of the circadian clock to promote sleep in zebrafish.
Figure 3. aanat2 is not required for behavioral or molecular circadian rhythms.
(A) Larvae raised in LD and monitored in DD maintain locomotor activity circadian rhythms. Locomotor activity data (dashed lines) was fit to damped cosine curves (solid lines) to quantify circadian amplitude (B), period length (C) and phase (D). aanat2−/− larvae have reduced amplitude due to increased locomotor activity during subjective night, but period length and phase are indistinguishable from sibling controls. Black and hatched boxes indicate subjective night and day, respectively. (E–H) per3-luc larvae were raised in LD and luminescence recorded during 100 hours in DD. (E) Dashed and solid lines indicate luminescence data and damped cosine curve fits, respectively. (F–H) Circadian rhythm amplitude, period length and phase are not significantly different for any genotype. Bar graphs represent mean ± SEM. a.u., arbitrary units. **, p<0.01 compared to aanat2−/− by Dunnett’s test. See also Figure S4.
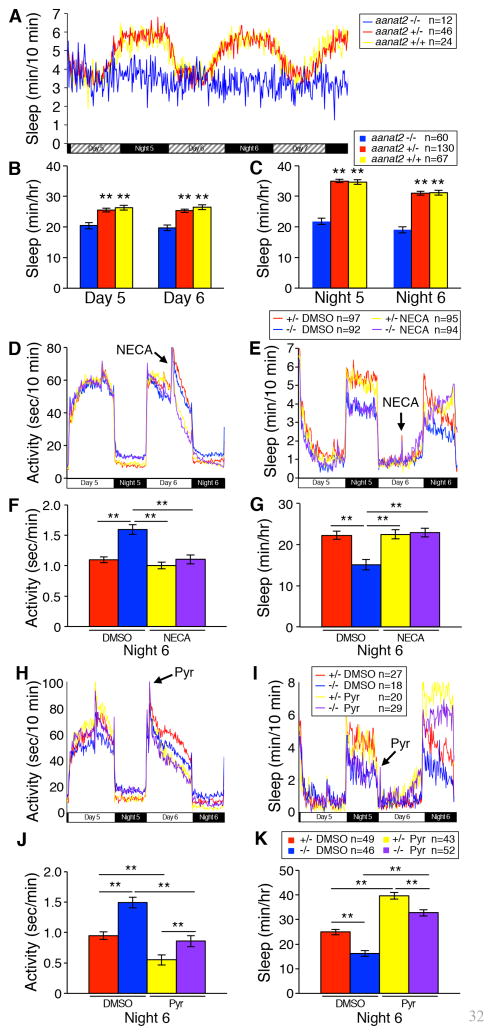
Our results suggest that melatonin may mediate process C, which determines when sleep occurs during the circadian cycle (Borbely, 1982). To address this possibility, we entrained larvae in LD and transferred them to DD to remove masking effects of light on behavior. Under these conditions, aanat2−/− larvae, but not sibling controls, exhibited similar amounts of sleep during subjective day and night (Figure 4A–C), despite the maintenance of normal molecular rhythms (Figures 3E–F, S4K,L). This indicates that melatonin is required for circadian regulation of sleep. Combined with the observation that exogenous melatonin is sufficient to induce daytime sleep (Figure 1H,I) (Zhdanova et al., 2001), this suggests that melatonin mediates process C in zebrafish larvae.
Figure 4. Melatonin is required for circadian regulation of sleep and may promote sleep via adenosine signaling.
(A–C) Larvae were raised in LD and tested in DD. aanat2−/− larvae, but not sibling controls, lack circadian sleep oscillations. Black and hatched boxes represent subjective night and day, respectively. There is no significant difference in amount of sleep for aanat2−/− larvae during each subjective day (B) and night (C) (p>0.22 by Tukey’s test). (D–G) Larvae were treated with vehicle or 50 μM NECA on day 6. On night 6, vehicle-treated aanat2−/− larvae are more active (D,F) and sleep less (E,G) than vehicle-treated aanat2+/− larvae. In contrast, NECA-treated aanat2−/− larvae exhibit the same amount of activity (D,F) and sleep (E,G) as NECA-treated aanat2+/− larvae. (H–K) Larvae were treated with vehicle control or 10 μM pyrilamine on day 6. Pyrilamine decreases locomotor activity (H,J) and increases sleep (I,K) for aanat2−/− and aanat2+/− larvae to a similar extent. Bar graphs represent mean ± SEM. **, p<0.01 compared to aanat2−/− (B,C), aanat2−/− DMSO (F,G), or for the indicated comparisons (J,K) by Dunnett’s (B,C,F,G) or Tukey’s test (J,K). Arrows indicate transient artifacts due to pipetting of compounds. See also Figure S4.
Exogenous melatonin can induce adenosine production in the mammalian forebrain (Zamorskii and Pishak, 2003). To test whether adenosine mediates sleep promotion by melatonin, we treated aanat2−/− larvae and their aanat2+/− siblings with the adenosine receptor agonist 5′-N-ethylcarboxamido-adenosine (NECA). NECA treatment decreased locomotor activity in aanat2+/− larvae during the day (Figures 4D and S4M) but had no effect on activity or sleep at night (Figure 4D–4G; arrows indicate transient artifacts caused by pipetting compounds into the plate), presumably because adenosine receptors are maximally activate at night. In contrast, NECA increased sleep and decreased activity at night for aanat2−/− larvae similarly to their aanat2+/− siblings, (Figure 4F,G), thus rescuing the mutant phenotype. As an alternative approach to modulate adenosine signaling, we treated larvae with ABT702, a small molecule inhibitor of adenosine kinase that increases extracellular adenosine levels, and thus adenosine signaling (Brown et al., 2012). Similar to NECA, ABT702 (Figure S4N) had no effect on locomotor activity or sleep of aanat2+/− larvae at night, but rescued the activity and sleep phenotypes of aanat2−/− larvae (Figure S4P–S). These results suggest that melatonin may promote sleep, at least in part, by inducing adenosine signaling. To test whether these drugs failed to increase sleep at night in aanat2+/− larvae because they were already sleeping maximally, we promoted sleep via a different mechanism. Histamine is an arousing neuromodulator via mechanisms that are not well understood(Panula and Nuutinen, 2013). Inhibition of the histamine 1 receptor (H1R) is sedating in mammals (Panula and Nuutinen, 2013) and zebrafish (Renier et al., 2007). In contrast to NECA and ABT702, the H1R antagonist pyrilamine increased sleep and decreased activity in both aanat2−/− and aanat2+/− larvae to a similar extent (Figures 4H–K and S4O). This indicates that the failure of NECA to increase nighttime sleep in aanat2+/− larvae was not because the larvae were already sleeping maximally, and suggests that histamine acts parallel to, rather than downstream of, melatonin in regulating sleep. Together, our results are consistent with a model in which adenosine acts downstream of melatonin to promote sleep at night, thus potentially linking homeostatic and circadian regulation of sleep (Figure S4T).
Discussion
Melatonin is widely used as a sleep aid and has been cited as a regulator of circadian rhythms (Elbaz et al., 2013). However, despite decades of study, the role of melatonin in regulating sleep and circadian rhythms is controversial (Fisher et al., 2013) and the function of endogenous melatonin is unknown. Here we describe the first diurnal vertebrate genetic loss of function model for melatonin. We show that aanat2−/− zebrafish larvae take twice as long to fall asleep and sleep only half as much as controls at night in LD conditions. This effect is surprisingly large since exogenous melatonin has relatively subtle sleep promoting effects in humans compared to prescribed hypnotics (Brzezinski et al., 2005; Buscemi et al., 2006), which has led some to argue that melatonin is not an important sleep regulator (van den Heuvel et al., 2005). However, most hypnotics inhibit neuronal activity throughout the brain by activating GABAA receptors, which is not a physiologically relevant mechanism of sleep promotion (Zhdanova, 2005). Indeed, such an overpowering mechanism of sleep induction would be maladaptive. Further, while exogenous melatonin may be a relatively weak sedative, it does not necessarily follow that endogenous melatonin does not play an important role in sleep. Comparing the importance of endogenous melatonin in humans and zebrafish will require more potent and specific melatonin receptor antagonists. While melatonin may play a more important role in promoting sleep in zebrafish, our results demonstrate that endogenous melatonin plays a significant role in promoting initiation and maintenance of sleep at night in a diurnal vertebrate.
It has been proposed that melatonin promotes sleep indirectly by phase-advancing the circadian clock (Arendt, 2003) or inhibiting the circadian drive for wakefulness (Scheer and Czeisler, 2005). If these hypotheses are correct, aanat2−/− larvae should have little or no sleep phenotype in the absence of entrained circadian rhythms. We tested this hypothesis by raising larvae in constant darkness, which abolished cellular circadian oscillations, at least in the brain. The aanat2−/− sleep phenotype persisted under these conditions, suggesting that endogenous melatonin does not promote sleep by modulating the circadian clock, but rather directly affects the sleep regulatory system. Indeed, while exogenous melatonin can entrain the circadian clock in free-running animals (Lockley et al., 2000; Sack et al., 2000) and phase-shift the clock in some contexts (Lewy et al., 1992), we found that endogenous melatonin is not required to initiate or maintain molecular or behavioral circadian rhythms in zebrafish. This observation does not support the hypothesis, based on exogenous melatonin, that endogenous melatonin regulates circadian rhythms (Elbaz et al., 2013). Rather, our data suggest that melatonin acts downstream of the clock to promote sleep.
A prominent model proposes that sleep is regulated by a homeostatic process responding to internal cues for sleep need (process S), and a circadian process responding to external cues (process C) (Borbely, 1982). Evidence in mammals suggests that factors such as adenosine, nitric oxide and prostaglandin D2 play important roles in mediating the homeostatic process (reviewed in Brown et al., 2012). However, while the circadian clock mechanism has been described in detail (Fisher et al., 2013), molecules that convey circadian information to regulate sleep are largely unknown. A factor mediating process C should fulfill three criteria. First, the clock should regulate the level or activity of the factor. Second, administration of the factor should induce sleep during the circadian waking period, but not the sleep period. Third, loss of the factor should abolish circadian regulation of sleep. Peptides whose expression oscillates in a circadian manner, and whose overexpression inhibits activity or promotes sleep during the circadian waking period, have been identified in nocturnal rodents, including cardiotrophin-like cytokine (Kraves and Weitz, 2006), transforming growth factor alpha (Kramer et al., 2001) and prokineticin 2 (Cheng et al., 2002). However, loss of function studies have revealed little or no effect on the circadian regulation of activity or sleep (Hu et al., 2007; Kraves and Weitz, 2006; Li et al., 2006; Roberts et al., 2006). Melatonin is an alternative candidate for mediating process C since, similar to these peptides, the circadian clock regulates its production (Klein, 2007) and it can induce sleep in some contexts (Fisher et al., 2013). Indeed, exogenous melatonin potently increases sleep and decreases locomotor activity in zebrafish larvae during the day (Figures 1G–1L) (Zhdanova et al., 2001), and circadian regulation of sleep is abolished in aanat2−/− larvae. These results suggest that melatonin mediates process C in the diurnal zebrafish animal model. This discovery may have important implications for the treatment of sleep and circadian rhythm disorders in humans.
Because melatonin is produced at night in diurnal and nocturnal animals, and administration of physiological levels of melatonin does not promote sleep in nocturnal animals (Fisher et al., 2013), nocturnal and diurnal animals likely use different mechanisms for circadian regulation of sleep (Zhdanova, 2005). This idea is supported by the observation that most nocturnal mouse strains used for genetic studies produce little or no melatonin (Goto et al., 1989) yet have circadian control of sleep. Mutation of aanat in melatonin-proficient nocturnal and diurnal mammals will clarify melatonin’s role in regulating mammalian sleep.
An open question raised by the two-process model (Borbely, 1982) is how homeostatic and circadian cues are integrated. Similar to melatonin, we found that activating adenosine signaling promotes sleep and inhibits activity during the day, but has no effect on sleep at night in WT (data not shown) and aanat2+/− larvae. In contrast, activating adenosine signaling increases sleep and decreases activity at night in aanat2−/− larvae to the same level as their aanat2+/− siblings. This result is unlikely due to a ceiling effect for sleep or parallel modulation of sleep by melatonin and adenosine because increasing nighttime sleep using a different approach, using a histamine H1R antagonist, increases nighttime sleep for both aanat2+/− and aanat2−/− larvae to a similar extent. These results suggest the sleep-promoting effect of endogenous melatonin may be mediated, at least in part, by adenosine signaling, and suggest a potential mechanism linking homeostatic and circadian regulation of sleep. This hypothesis must be further tested using genetics and measurements of adenosine levels, which will be challenging in the zebrafish due to its large number of adenosine receptor genes and its small brain size. Application of genome editing technologies to diurnal melatonin-proficient mammals would allow the use of genetics and measurement of adenosine levels using available technologies (Porkka-Heiskanen et al., 1997; Schmitt et al., 2012).
Finally, we note that reduced melatonin levels are associated with aging, mood disorders and autism (Hardeland, 2012). An improved understanding of how endogenous melatonin interacts with the sleep system and other aspects of physiology from the aanat2 mutant may lead to novel therapies for these disorders.
Experimental Procedures
Detailed methods are in Supplemental Experimental Procedures. Mutant zebrafish were generated using TALEN and CRISPR/Cas9 technologies. Videotracker behavioral experiments were performed as described (Prober et al., 2006). A period of one or more minutes with no movement was defined as sleep because it is associated with an increased arousal threshold (Prober et al., 2006). Arousal threshold was assayed by monitoring locomotor activity in response to a mechanoacoustic stimulus. Ablation of pineal gland cells (Curado et al., 2007) and per3-luc experiments (Kaneko and Cahill, 2005) were performed as described.
Supplementary Material
Highlights.
Zebrafish lacking melatonin have dramatically reduced sleep at night
Melatonin is required for circadian regulation of sleep
Melatonin is not required for normal circadian rhythms
Melatonin may promote sleep in part via adenosine signaling
Acknowledgments
We thank Jason Rihel, Kathy Tamai, Daniel Lee, Catherine Oikonomou and Chanpreet Singh for critical reading of the manuscript, and Melanie Pribisko Yen for assistance with melatonin isolation. This work was supported by a Della Martin postdoctoral fellowship (E.M), NIH grants (GO: F32NS082010; DAP: NS060996, NS070911 and DA031367), the Mallinckrodt Foundation, the Rita Allen Foundation and the Brain and Behavior Research Foundation (D.A.P.).
Footnotes
Author Contributions
A.V.G. performed all experiments except circadian rhythm and ELISA assays (E.M.) and arousal theshold assays (G.O.). A.V.G., E.M., G.O. and D.A.P. analyzed the data and wrote the manuscript.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelbaum L, Vallone D, Anzulovich A, Ziv L, Tom M, Foulkes NS, Gothilf Y. Zebrafish arylalkylamine-N-acetyltransferase genes - targets for regulation of the circadian clock. Journal of molecular endocrinology. 2006;36:337–347. doi: 10.1677/jme.1.01893. [DOI] [PubMed] [Google Scholar]
- Arendt J. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol. 2003;15:427–431. doi: 10.1046/j.1365-2826.2003.00987.x. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiological reviews. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, Ford I. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–393. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Dekens MP, Whitmore D. Autonomous onset of the circadian clock in the zebrafish embryo. EMBO J. 2008;27:2757–2765. doi: 10.1038/emboj.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz I, Foulkes NS, Gothilf Y, Appelbaum L. Circadian clocks, rhythmic synaptic plasticity and the sleep-wake cycle in zebrafish. Front Neural Circuits. 2013;7:9. doi: 10.3389/fncir.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Foster RG, Peirson SN. The circadian control of sleep. Handb Exp Pharmacol. 2013:157–183. doi: 10.1007/978-3-642-25950-0_7. [DOI] [PubMed] [Google Scholar]
- Goldstein R, Pavel S. REM sleep suppression in cats by melatonin. Brain Res Bull. 1981;7:723–724. doi: 10.1016/0361-9230(81)90126-x. [DOI] [PubMed] [Google Scholar]
- Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. ScientificWorldJournal. 2012;2012:640389. doi: 10.1100/2012/640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep. 2007;30:247–256. [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Cahill GM. Light-dependent development of circadian gene expression in transgenic zebrafish. PLoS Biol. 2005;3:e34. doi: 10.1371/journal.pbio.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimi N, Cahill GM. Development of a circadian melatonin rhythm in embryonic zebrafish. Brain Res Dev Brain Res. 1999;117:47–52. doi: 10.1016/s0165-3806(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. The Journal of endocrinology. 2000;164:R1–6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Phillips NH, Berger RJ. Daytime melatonin infusions induce sleep in pigeons without altering subsequent amounts of nocturnal sleep. Neurosci Lett. 1998;258:61–64. doi: 10.1016/s0304-3940(98)00849-0. [DOI] [PubMed] [Google Scholar]
- Panula P, Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci. 2013;14:472–487. doi: 10.1038/nrn3526. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier C, Faraco JH, Bourgin P, Motley T, Bonaventure P, Rosa F, Mignot E. Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogenet Genomics. 2007;17:237–253. doi: 10.1097/FPC.0b013e3280119d62. [DOI] [PubMed] [Google Scholar]
- Roberts RB, Thompson CL, Lee D, Mankinen RW, Sancar A, Threadgill DW. Wildtype epidermal growth factor receptor (Egfr) is not required for daily locomotor or masking behavior in mice. Journal of circadian rhythms. 2006;4:15. doi: 10.1186/1740-3391-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9:5–9. doi: 10.1016/j.smrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H. Circadian Organization in Nonmammalian Vertebrates. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks. New York: Plenum Publishers; 2001. pp. 111–140. [Google Scholar]
- van den Heuvel CJ, Ferguson SA, Macchi MM, Dawson D. Melatonin as a hypnotic: con. Sleep Med Rev. 2005;9:71–80. doi: 10.1016/j.smrv.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Woods IG, Schoppik D, Shi VJ, Zimmerman S, Coleman HA, Greenwood J, Soucy ER, Schier AF. Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J Neurosci. 2014;34:3142–3160. doi: 10.1523/JNEUROSCI.3529-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorskii I, Pishak V. Effect of Melatonin on the Intensity of Adenosine Production in the Rat Forebrain under Conditions of Acute Hypoxia and Varied Photoperiodicity. Neurophysiology. 2003;35:44–47. [Google Scholar]
- Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.