Abstract
Metastasis is the underlying cause of death for the majority of breast cancer patients. Despite significant advances in recent years in basic research and clinical development, therapies that specifically target metastatic breast cancer remain inadequate, and represents the single greatest obstacle to reducing mortality of late-stage breast cancer. Recent efforts have leveraged genomic analysis of breast cancer and molecular dissection of tumor-stromal cross-talk to uncover a number of promising candidates for targeted treatment of metastatic breast cancer. Rational combinations of therapeutic agents targeting tumor-intrinsic properties and microenvironmental components provide a promising strategy to develop precision treatments with higher specificity and less toxicity. In this review, we discuss the emerging therapeutic targets in breast cancer metastasis, from tumor-intrinsic pathways to those that involve the host tissue components, including the immune system.
Keywords: Breast cancer, Metastasis, Targeted therapy, Tumor microenvironment, Immunotherapy
1. Introduction
The overall 5-year survival rate for breast cancer currently stands at 90% — a dramatic improvement over the 63% survival rate in the early 1960s. When stratified by stage, the 5-year survival rates have increased to 99% for localized disease and 85% for regional advanced disease, a trend that can be attributed to early diagnoses and better treatment regimens. However, for patients with advanced or metastasized breast cancer at the time of diagnosis (abbreviated as mBC in this review), the 5-year survival rate remains at only 26% (American Cancer Society, 2013), reflecting a need for both new therapies and insights into the metastatic process. This disparity in survival between early and late stage breast cancer represents the principle obstacle in breast cancer management.
To date, the FDA has approved over 25 oncology drugs to treat breast cancer (Table 1). These drugs fall into three major categories: 1) Cytotoxic chemotherapies, including mitotic inhibitors, antimetabolites, and various DNA-damaging reagents; 2) Endocrine therapies, including selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), and estrogen receptor downregulators (ERDs); and 3) Targeted therapies, which target certain dysregulated pathways in mBC or tumor microenvironment. The majority of these drugs belong to cytotoxic chemotherapies (44%), followed by endocrine therapies (24%) and HER2-targeting therapies (16%). Only 4 drugs (16%) target tumor- and microenvironment-specific molecules beyond the EGFR family, namely mTOR, CDK4/6, RANKL, and bisphosphonates.
Table 1.
FDA approved oncology drugs suitable for treatment of mBC.
| Category | Generic name | Brand name | Class | Company | Patient population | Approval date |
|---|---|---|---|---|---|---|
| Cytotoxic chemotherapy | Capecitabine | Xeloda | Antimetabolite | Roche | mBC | 1998 |
| Cyclophosphamide | Cytoxan | DNA-damaging reagent | Roxane, Baxter Healthcare | mBC | 1959 | |
| Docetaxel | Taxotere | Mitotic inhibitor | Rhone Poulenc Rorer | mBC | 1996 | |
| Eribulin mesylate | Halaven | Mitotic inhibitor | Eisai | mBC | 2010 | |
| Fluorouracil | Adrucil | Antimetabolite | N/A | N/A | N/A | |
| Gemcitabine hydrochloride | Gemzar | Antimetabolite | Eli Lilly | mBC | 2004 | |
| Ixabepilone | Ixempra | Mitotic inhibitor | Bristol-Meyers Squibb | mBC | 2007 | |
| Paclitaxel | Taxol | Mitotic inhibitor | Bristol-Myers Squibb | BC | 1994 | |
| Protein-bound paclitaxel | Abraxane | Mitotic inhibitor | Celgene | mBC | 2005 | |
| Thiotepa | Thiotepa | DNA-damaging reagent | American Cyanamid | mBC | 1950s | |
| Vinblastine Sulfate | Velban | Mitotic inhibitor | N/A | N/A | N/A | |
| Endocrine therapy | Anastrozole | Arimidex | Aromatase inhibitor | AstraZeneca | HR+ mBC | 1996 |
| Exemestane | Aromasin | Aromatase inhibitor | Pharmacia & Upjohn | HR+ mBC | 1999 | |
| Fulvestrant | Faslodex | Estrogen receptor antagonist | AstraZeneca | HR+ mBC | 2002 | |
| Letrozole | Femara | Aromatase inhibitor | Novartis | HR+ mBC | 2001 | |
| Tamoxifen | Nolvadex | Selective estrogen receptor modulator | AstraZeneca | HR+ mBC | 1998 | |
| Toremifene | Fareston | Selective estrogen receptor modulator | GTx | HR+ mBC | 2009 | |
| HER2-targeted therapy | Lapatinib | Tykerb | Dual HER1/HER2 inhibitor | GlaxoSmithKline | HER2+ mBC | 2007 |
| Pertuzumab | Perjeta | HER2 inhibitor | Genentech | HER2+ mBC | 2012 | |
| Trastuzumab | Herceptin | HER2 inhibitor | Genentech | HER2+ mBC | 1998 | |
| Trastuzumab emtansine (TDM-1) | Kadcyla | HER2- antibody-drug conjugate | Genentech | HER2+ mBC | 2013 | |
| Other targeted therapy | Denosumab | Xgeva | RANKL inhibitor | Amgen | Bone mBC | 2010 |
| Everolimus | Afinitor | mTOR inhibitor | Novartis | HR+, HER2-mBC | 2012 | |
| Palbociclib | Ibrance | Inhibitor of CDK4 and CDK6 | Pfizer | HR+, HER2-mBC | 2015 | |
| Pamidronate, Zoledronic acid | Aredia, Zometa | Bisphosphonate | Chiron, Novartis | Bone mBC | 1996, 2002 |
The paucity of targeted therapies is further compounded by several critical issues in the management of mBC. The most crucial of these is the disparity in response rates to systemic chemotherapy, as only 50% of metastatic cancers respond in contrast to 90% of primary tumors (Gonzalez-Angulo et al., 2007). Lower response rate in mBC is also accompanied by a stronger likelihood of acquired therapeutic resistance during the course of treatment (Coley, 2008; Gonzalez-Angulo et al., 2007). Finally, the development of targeted agents for the highly metastatic triple-negative (TNBC) subtype has been largely hindered by a relative lack of understanding into its heterogeneous molecular nature (Cleator et al., 2007; Criscitiello et al., 2012). While targeting tumor-intrinsic factors remains the traditional approach to therapeutic intervention (i.e. targeting aberrant driver events in breast cancer cells), developing research on the tumor microenvironment suggests that a new paradigm of stroma-targeting may yield many novel opportunities. In particular, the use of immunotherapies to target mBC is a potentially promising field (Korkaya et al., 2011; Mao et al., 2013; Place et al., 2011).
Here we review the current status of pharmacological management and focus on the landscape of emerging therapeutics for mBC. These include tumor-intrinsic targets such as the PI3K/ATK/mTOR pathway, PARP enzymes, activated growth factor receptors (e.g. EGFRs and FGFRs), cancer stem cell network (e.g. the Notch and Wnt pathways), androgen receptor, as well as therapies targeting communications between the tumor and host (e.g. immunotherapies). We discuss the mechanisms of action and biological implications of these targets with analysis of data from mechanistic and clinical studies. Finally, we address the need for the development of combinatorial therapies, in the context of targeting both the drivers of metastasis as well as the associated stromal components.
2. Status quo in the management of mBC
Clinical management of breast cancer has greatly improved in recent decades through the diagnostic classification of breast cancer into different subtypes, mainly on the basis of the hormone receptors (ER/PR) and the epidermal growth factor receptor HER2. Accordingly, breast cancer treatment is divided into three main classes: 1) hormone receptor-positive (ER+ and/or PR+), 2) ErbB2/HER2/neu-overexpressing (i.e. HER2+), and 3) the remaining TNBC marked by the absence of ER/PR and HER2, which are poorly characterized and the most difficult to manage.
Hormone receptor-positive (HR+) BC patients are primarily treated with endocrine therapy (e.g. letrozole or tamoxifen), either alone or in combination with a cytotoxic chemotherapy drug. These patients generally have a favorable prognosis and are associated with better overall survival compared to the other subtypes. However, HR+ patients also exhibit a higher risk of late recurrence following 5 years of adjuvant endocrine therapy, likely due to the low proliferation of HR+ tumor cells (Bosco et al., 2009; Voduc et al., 2010). Extended adjuvant endocrine therapy beyond 5 years has been shown to significantly reduce risk of recurrence and mortality in HR+ patients, and has been applied in clinical practice (Davies et al., 2013; Jin et al., 2012). The recent identification of activating mutations in the estrogen receptor (ESR1) demonstrates a central role of acquired endocrine resistance in HR+ mBC, suggesting that second-line ER antagonists may be of substantial therapeutic benefit (Robinson et al., 2013; Toy et al., 2013).
The more aggressive HER2+ BC is characterized by high expression of HER2/neu. These patients are primarily treated with trastuzumab (Herceptin), a landmark success in BC-targeted therapy. However, not all HER2+ tumors respond to anti–HER2 therapy. Although tumors resolve in many patients, others show no response or become resistant (Lee-Hoeflich et al., 2008; Nahta et al., 2006). Therefore, a multitude of HER2-targeting drugs exploiting different targeting mechanisms have been developed, such as lapatinib (HER2 and EGFR dual tyrosine kinase inhibitor) and pertuzumab (HER2 dimerization inhibitor) (see Table 1). Many other HER2-targeting strategies have been proposed and are currently under active investigations (Murphy and Morris, 2012; Nielsen et al., 2009).
TNBC represents 15–20% of newly diagnosed breast cancer cases. These patients have a high risk of recurrence, especially in the brain and viscera. This high propensity to metastasize contributes to the worst rates of overall and disease free survival for TNBC patients compared to other subtypes (Onitilo et al., 2009). There are currently no targeted therapies available for these patients, leaving cytotoxic chemotherapies as the only standard of care. Treatment of TNBC remains one of the most pressing challenges in today’s clinical practice. Addressing this need, gene expression profiling of over 3000 TNBCs has revealed a deeper stratification of six different TNBC subtype (Lehmann et al., 2011). Both this and other genomics-based studies continue to shed light on the molecular understanding of TNBC heterogeneity and may provide critical insights in developing TNBC-targeted therapies (Huebschman et al., 2015; Kreike et al., 2007; Lehmann et al., 2011; Turner et al., 2010).
For breast cancer patients, metastasis to the bone is a common site of recurrence (Coleman, 2006). This type of metastasis is characterized by a viscous cycle of bone resorption and accelerated tumor growth (Ren et al., 2015). Osteoclast inhibitors such as the bisphosphonates (e.g. pamidronate, zoledronic acid) and the RANKL-targeting denosumab have been repurposed for breast cancer patients with bone metastases, representing the only organ-tropic anti-metastatic treatment strategy currently available (Esposito and Kang, 2014). Bisphosphonates are analogs of pyrophosphate that bind to the bone matrix and are internalized by osteoclast inhibitors. Once inside the cell, they inhibit osteoclast activity through a variety of means, such as acting as farnesyl-transferase inhibitors (Drake et al., 2008; Fleisch, 1998). Denosumab is a mAb that binds and inhibits receptor activator of nuclear factor kappa-B ligand (RANKL), the principle ligand responsible for the maturation of bone-resorbing osteoclasts (Baron et al., 2011). Both classes of drug effectively reduce the risk of skeletal-related events (SREs) in breast cancer patients with bone metastases, while denosumab has been shown superior to bisphosphonates in terms of both efficacy and tolerability (Lipton et al., 2012; Martin et al., 2012; Stopeck et al., 2010).
Targeted drug delivery to cancer cells, exemplified by the antibody drug conjugate (ADC), has recently emerged as a novel and promising strategy in treating mBC. ADCs link potent chemotherapy drugs with monoclonal antibodies (mAbs) targeting cancer-specific antigens (Sassoon and Blanc, 2013; Sievers and Senter, 2013). Administration of ADCs leads to high intratumoral drug concentrations, while non-target tissues are largely spared from chemotherapeutic exposure (Alley et al., 2010). Ado-trastuzumab emtansine (Kadcyla, TDM-1), a HER2-targeting mAb trastuzumab conjugated with the microtubule-inhibitory agent DM1, was approved in 2013 for treating HER2+ mBC. Since, multiple ADCs have been developed to target various subtypes of breast cancer and are currently being tested in clinical trials (Table 2).
Table 2.
Breast cancer-targeting ADCs in clinical development
| Target antigen | ADC designation | Application | Patient population | Development Stage | Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* | Reference |
|---|---|---|---|---|---|---|---|
| HER2 | MM-302 | Combo | HER2+ mBC | Phase I, II, III | Manageable safety profile, evidence of antitumor activity | NCT02213744, NCT01304797 | (Geretti et al., 2015; LoRusso et al., 2015a) |
| SYD985 | Mono | mBC | Phase I | Preclinical antitumor activity | NCT02277717 | (Dokter et al., 2014; Elgersma et al., 2015; van der Lee et al., 2015) | |
| XMT-1522 | N/A | mBC | Preclinical | Preclinical antitumor activity | N/A | (Bergstrom et al., 2015) | |
| gpNMB | CDX-011 | Mono | gpNMB+ mBC | Phase I, II | Acceptable safety profile, evidence of antitumor activity | NCT01997333, NCT01156753, NCT00704158 | (Bendell et al., 2014a; Vaklavas and Forero, 2014; Yardley et al., 2015) |
| Trop-2 | IMMU-132 | Mono | mBC | Phase I, II | Acceptable safety profile, evidence of antitumor activity | NCT01631552 | (Goldenberg et al., 2015; Sharkey et al., 2015; Starodub et al., 2015) |
| Mesothelin (MSLN) | BMS-986148 | Mono | Advanced solid tumor | Phase I, II | Preclinical antitumor activity | NCT02341625 | (Gupta et al., 2014; Lamberts et al., 2015; Scales et al., 2014) |
| BAY94-9343 | Mono | Advanced solid tumor | Phase I | Preclinical antitumor activity | NCT01439152, NCT02485119 | ||
| CA6 | SAR566658 | Mono | CA6+ breast cancers | Phase I | Favorable safety profile, evidence of antitumor activity | NCT01156870 | (Boni et al., 2014) |
| Ephrin-A4 (EFNA4) | PF-06647263 | Mono | Advanced solid tumor | Phase I | Preclinical antitumor activity | NCT02078752 | (Damelin et al., 2015) |
| LIV-1 (SLC39A6) | SGN-LIV1A | Mono | LIV-1-Positive mBC | Phase I | Preclinical antitumor activity | NCT01969643 | (Sussman et al., 2014) |
| Ly6E | DLYE5953A | Mono | Advanced solid tumor | Phase I | Preclinical antitumor activity | NCT02092792 | (Asundi et al., 2015) |
| c-RET | Y078-DM1/DM4 | N/A | HER2+ and basal breast cancer | Preclinical | Preclinical antitumor activity | N/A | (Nguyen et al., 2015) |
| B7-H4 | Anti-B7-H4 ADC | N/A | B7-H4+ breast cancer | Preclinical | Preclinical antitumor activity | N/A | (Leong et al., 2015) |
| CD138 | BT-062 | N/A | mBC | Preclinical | Preclinical antitumor activity | N/A | (Ibrahim et al., 2013; Rapraeger, 2013) |
Phase III, II and I trials are highlighted in red, blue and black respectively
3. Tumor-intrinsic targeting
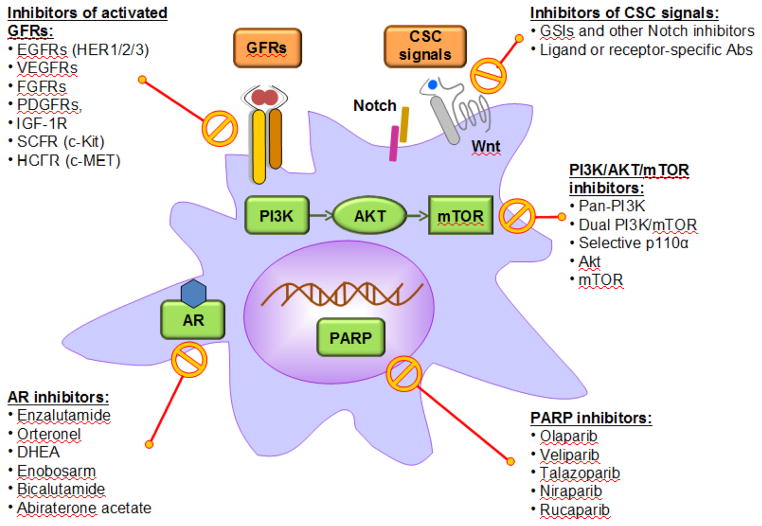
Genomics studies have identified numerous genetic and pathway alterations in breast cancer cells, however, only a few have been validated as viable targets in clinical studies. Therapies against ER and HER2 highlight the landmark successes in targeted breast cancer treatment, suggesting that a tumor-intrinsic targeting approach is of significant value. In this section, we discuss several promising therapeutic candidates supported by strong clinical evidence (Figure 1).
Figure 1. Therapeutic candidates targeting tumor-intrinsic pathways of mBC.
This schematic diagram highlights several tumor-intrinsic pathways and the therapeutic agents currently available or being developed to target them.
3.1. Inhibition of PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR pathway mediates multiple cellular processes such as proliferation, migration, invasion, survival, metabolism, and angiogenesis (Fresno Vara et al., 2004; Fruman and Rommel, 2014; Martini et al., 2014). PIK3CA is one of the most frequently mutated genes in all subtypes of breast cancer (The Cancer Genome Atlas Network, 2012). Its most prevalent activating mutation PIK3CAH1047R has recently been shown to play key effects in tumor initiation and cell fate determination in the pre-neoplastic mammary gland (Van Keymeulen et al., 2015; Koren et al., 2015). In addition, loss of PTEN and INPP4B are also frequently observed in breast cancer malignancies (Fedele et al., 2010; Gewinner et al., 2009; Liu et al., 2009; López-Knowles et al., 2010). Moreover, breast cancers that have constantly active Akt signaling will rely on Akt for survival and are associated with therapeutic resistance (Bose et al., 2006; Kim et al., 2005; Tokunaga et al., 2006). Finally, crosstalk between the PI3K/AKT/mTOR and the Ras-MAPK pathways have been reported, creating a more complex network of signaling (Aksamitiene et al., 2012; Carracedo and Pandolfi, 2008; Mendoza et al., 2011).
Disabling the PI3K/AKT/mTOR pathway provides a compelling and rational approach to improve the antitumor effects of existing chemotherapies. Activation of the PI3K/AKT/mTOR pathway is frequently implicated in resistance to endocrine (Cavazzoni et al., 2012; Creighton et al., 2010; Miller et al., 2009), HER2-targeted (Berns et al., 2007; Nagata et al., 2004; Yakes et al., 2002) and cytotoxic (Kolasa et al., 2009) chemotherapies. Everolimus, a rapamycin analog that inhibits mTOR kinase activity, is the first and so far the only FDA-approved compound targeting this pathway. Small molecules designed to specifically target different components in the PI3K/AKT/mTOR pathway have been developed and are under active investigations in clinical trials (Table 3).
Table 3.
mBC-targeting PI3K/AKT/mTOR pathway inhibitors in clinical development.
| Category | Agent | Application | Patient population |
Development stage |
Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* |
Reference |
|---|---|---|---|---|---|---|---|
| Pan-PI3K inhibitor | Buparlisib (BKM120) | Mono, combo | mBC | Phase I, II, III | Acceptable safety profile, evidence of antitumor activity | NCT01633060, NCT01610284, NCT01572727, NCT02000882, NCT02404844, NCT01629615, NCT01923168, NCT02088684 | (Geuna et al., 2015; Sirohi et al., 2015) |
| Pictilisib (GDC-0941) | Combo | mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT01740336, NCT01437566, NCT01740336, NCT01918306, | (Krop et al., 2015; Sarker et al., 2015) | |
| Pilaralisib (SAR245408, XL147) | Combo | mBC | Phase I, II | Acceptable safety profile, evidence of antitumor activity | NCT01042925, NCT01082068 | (Soria et al., 2015; Tolaney et al., 2015) | |
| Dual PI3K/mTOR inhibitor | BEZ235 (NVP-BEZ235) | Combo | mBC | Phase I, II | Poorly tolerated, evidence of antitumor activity | NCT01495247 | (Ahnert et al., 2014; Wise-Draper et al., 2015) |
| XL765 (SAR245409) | Combo | HR+ BC refractory to AI | Phase I, II | Manageable safety profile, associated with clinically relevant stable disease | NCT01082068 | (Papadopoulos et al., 2014) | |
| Apitolisib (GDC-0980, RG7422) | Combo | mBC | Phase I, II | Acceptable safety profile, preclinical antitumor activity | NCT01437566 | (Lehmann et al., 2014; Sun et al., 2015; Wagner et al., 2011) | |
| Selective p110α inhibitor | Alpelisib (BYL719) | Mono, combo | mBC | Phase I, II, III | Acceptable safety profile, evidence of antitumor activity | NCT02437318, NCT02506556, NCT01872260, NCT02379247, NCT02088684, NCT01923168 | (Matulonis et al., 2015; Shah et al., 2015) |
| Taselisib (GDC-0032) | Combo | mBC | Phase I, II, III | Well tolerated, promising preliminary activity in HR+ mBC, especially in patients with PIK3CA mutant tumors | NCT02340221, NCT02457910, NCT02390427 | (Juric et al., 2014; Saura et al., 2015) | |
| MLN1117 (INK1117) | Mono | Advanced solid tumors | Phase I | Acceptable safety profile, evidence of antitumor activity | NCT01449370 | (Juric et al., 2015) | |
| Akt inhibitor | MK2206 | Mono, combo | mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT01923168, NCT01277757, NCT01042379, | (Gonzalez-Angulo et al., 2015; Hudis et al., 2013; Molife et al., 2014) |
| Ipatasertib (GDC-0068) | Combo | mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT02162719, NCT01362374 | (Isakoff et al., 2015) | |
| mTOR inhibitor | Temsirolimus (CCI-779) ** | Mono, combo | mBC | Phase I, II, III | Minimal activity as monotherapy or combined with letrozole in mBC | NCT00083993, NCT02456857, NCT01111825, NCT00376688 | (Fleming et al., 2012; Wolff et al., 2013) |
| Ridaforolimus (Deforolimus, MK-8669, AP23573) | Combo | mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT01605396, NCT00736970, NCT01234857 | (Di Cosimo et al., 2015; Gupta et al., 2015; Rugo et al., 2015; Seiler et al., 2015) | |
| MLN0128 (INK128) | Combo | mBC | Phase I, II | Preclinical antitumor activity | NCT02049957, NCT01058707, NCT02142803, NCT02159989, NCT02327169 | (De et al., 2015; Gökmen-Polar et al., 2012) |
Phase III, II and I trials are highlighted in red, blue and black respectively
Approved for advanced renal cell carcinoma (RCC), Phase I, II in mBC
Alpelisib (also known as BYL719, PI3Kα inhibitor), buparlisib (also known as BKM120, pan-PI3K inhibitor), taselisib (also known as GDC-0032, selective inhibitor of Class I PI3K α,β,γ isoforms), ipatasertib (also known as GDC-0068, ATP-competitive small molecule inhibitor of all three isoforms of Akt) have emerged as promising drug candidates. Alpelisib, buparlisib and taselisib demonstrate significant antitumor effects when combined with endocrine therapy (Mayer et al., 2014; Saura et al., 2015; Shah et al., 2014). Combination of trastuzumab, LJM716 (HER3 mAb) and alpelisib has antitumor activity in pre-treated HER2+, PIK3CA-mutated mBC (Shah et al., 2015). The LOTUS (NCT02162719) trial is an ongoing phase II clinical trial to evaluate the efficacy of ipatasertib combined with paclitaxel in treatment of metastatic TNBC.
3.2. Inhibition of PARP
PARP inhibitors represent another emerging class of targeted therapeutics for mBC. PARP1, the first described enzyme of the PARP family, plays a key role in the repair of DNA single-strand breaks via base excision repair (BER); it has also been implicated in other pathways resolving or generating single-strand breaks, such as the nucleotide excision repair (NER) and mismatch repair (MMR) mechanisms (Feng et al., 2015). PARP1 inhibitors function by inhibiting the covalent attachment of ADP-ribose to PARP1 substrates, such that DNA repair enzymes cannot be recruited to the site of DNA damage (Kummar et al., 2012; Rouleau et al., 2010). Studies indicate that PARP1 inhibitors have the most potent antitumor effects in cancers with BRCA-mutations, as double strand break repair via homologous recombination (HR) is not available in BRCA-defective cells (Drost and Jonkers, 2014; Weil and Chen, 2011). This creates a situation of synthetic lethality in which only the BRCA-mutated tumor cells are affected (Ashworth, 2008).
Olaparib is the only PARP inhibitor approved by the FDA, as a single-agent treatment for advanced ovarian cancer driven by defective BRCA genes. Olaparib has been tested in breast cancer as either a single agent or in combination with other cytotoxic drugs. The current clinical trials involving Olaparib primarily enrolled patients with BRCA-mutated mBC (Gelmon et al., 2011; Kaufman et al., 2014; Tutt et al., 2010). Second-generation PARP inhibitors have also been developed and are being evaluated in multiple clinical trials (Table 4). Among the agents, veliparib (ABT888) and talazoparib (BMN 673) are the two most promising candidates that have entered phase III clinical trials.
Table 4.
mBC-targeting PARP inhibitors in clinical development.
| Agent | Application | Patient population | Development stage | Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* | Reference |
|---|---|---|---|---|---|---|
| Olaparib (AZD-2281) | Mono, combo | Phase I, II, III | Acceptable safety profile, durable response in mBC treated with olaparib monotherapy after combination with carboplatin and paclitaxel | NCT02000622, NCT00494234, NCT01078662, NCT02208375, NCT01116648, NCT02498613, NCT02299999, NCT00679783, NCT00516724 | (Dent et al., 2013; Kaufman et al., 2014; Lee et al., 2014; van der Noll et al., 2015) | |
| Veliparib (ABT888) | Combo | mBC with BRCA1/2 mutations | Phase I, II, III | Acceptable safety profile, promising antitumor activity | NCT02163694, NCT01009788, NCT01306032, NCT01042379, NCT01149083, NCT01827384, NCT01506609, NCT02158507 | (Isakoff et al., 2015b; Pahuja et al., 2015; Puhalla et al., 2014; Somlo et al., 2014) |
| Talazoparib (BMN 673) | Mono | Phase I, II, III | Well tolerated, promising antitumor activity | NCT01945775, NCT02034916, NCT02282345, NCT01989546, NCT02286687, NCT02401347, NCT02358200 | (Shen et al., 2013; Wainberg et al., 2014) | |
| Niraparib (MK-4827) | Mono | Phase I, II, III | Well tolerated, promising antitumor activity | NCT01905592 | (Sandhu et al., 2010; Sandhu et al., 2013; Wang et al., 2015) | |
| Rucaparib (CO-338) | Mono | Phase I, II | Acceptable safety profile, efficacy warrants further investigation | NCT01482715, NCT00664781, NCT01074970 | (Durmus et al., 2015; Dwadasi et al., 2014; Shapiro et al., 2014) |
Phase III, II and I trials are highlighted in red, blue and black respectively
Veliparib is a PARP inhibitor targeting both PARP1 and PARP2. Potential synergistic interactions between veliparib and cisplatin or the EGFR inhibitor lapatinib were reported to suppress TNBC cell lines (Chuang et al., 2012; Nowsheen et al., 2012). A phase III trial (NCT02163694) has been initiated in 2014 to assess efficacy and toxicity of veliparib in BRCA-associated mBC (Puhalla et al., 2015). Talazoparib is a dual-mechanism PARP inhibitor that potently inhibits the PARP enzyme and effectively traps PARP on DNA. It selectively targets tumor cells with BRCA1, BRCA2, or PTEN gene defects with a high potency in cell lines and xenograft models (Andrei et al., 2015; Hopkins et al., 2015; Murai et al., 2012; Shen et al., 2013). EMBRACA (NCT01945775) is an ongoing phase III trial evaluating the safety and efficacy of Talazoparib in mBC patients with BRCA mutations.
The use of synthetic lethality has been mostly studied in the context of BRCA1/2-deficient breast and ovarian cancers (Sonnenblick et al., 2014). Nevertheless, studies suggest that PARP1 inhibitors may also have positive effects in patients that are non-BRCA mutation carriers (Gelmon et al., 2011; Pothuri, 2013), possibly due to the less-defined roles of PARP in double strand-break repair, transcriptional regulation, and hormone-dependent cancers (Feng et al., 2015).
3.3. Inhibition of activated growth factor receptors
Increased expression of growth factor receptors and activation of their tyrosine kinase activities are frequently observed in breast cancers (Gschwind et al., 2004; Hynes, 2000; Templeton et al., 2014). Besides HER2, other members of the EGFR family (e.g. EGFR/HER1/ErbB1, HER3/ErbB3) (Lee-Hoeflich et al., 2008; Masuda et al., 2012), vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) (Goel and Mercurio, 2013; Guo et al., 2010), fibroblast growth factor (FGF) and it receptors (FGFRs) (Penault-Llorca et al., 1995; Tenhagen et al., 2012), platelet-derived growth factor (PDGF) and its receptors (PDGFRs) (Carvalho et al., 2005; Coltrera et al., 1995), insulin-like growth factor-1 (IGF-1) and its receptor (IGF-1R) (Pollak, 2008; Yang and Yee, 2012), mast/stem cell growth factor receptor (SCFR, also known as c-Kit) (Kashiwagi et al., 2013; Regan et al., 2012), and MET and hepatocyte growth factor receptor (HGFR, also known as c-MET) (Ho-Yen et al., 2015; Raghav et al., 2012; Sierra and Tsao, 2011) have been found dysregulated (by amplification, translocations, or mutations) in breast tumors and are associated with poor patient outcomes.
Therapeutic agents targeting these growth factor-responsive tumors include blockade of individual receptors with mAbs and inhibition of tyrosine kinase activities with small molecule inhibitors. The clinical testing of the long-heralded VEGFR inhibitors failed in Phase II and III clinical trials (see section 4.1.2). Of the myriad growth factors, FGFR is an emerging target of promise. The FGF-FGFR signaling axis plays essential functions in regulating cell proliferation, survival, migration and differentiation in both normal and cancer development (Turner and Grose, 2010; Wesche et al., 2011). Activation of FGFR signaling may lead to increased tumor angiogenesis and play a role in tumor resistance to antiangiogenic agents and other chemotherapies (Kono et al., 2009; Lieu et al., 2011). In breast cancer, upregulation of the FGFR pathway activity could occur through multiple mechanisms. Amplification of FGFR1 (10% of all BC), FGFR2 (4% of TNBC) and FGFR4 genes (10% of all BC), and germline single nucleotide polymorphisms (SNPs) of FGFR2 and FGFR4 have been reported and are associated with poor prognosis (Jaakkola et al., 1993; Penault-Llorca et al., 1995; The Cancer Genome Atlas Network, 2012; Turner et al., 2010). Therapeutic agents targeting aberrant FGFR activation are now being evaluated in multiple phase II/III clinical trials (Table 5).
Table 5.
mBC-targeting FGFR inhibitors in clinical development.
| Category | Agent | Application | Patient population | Development stage |
Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* |
Reference |
|---|---|---|---|---|---|---|---|
| Non-selective FGFR TKI | Dovitinib (TKI258) | Mono, combo | mBC, solid tumors | Phase I, II, III | Acceptable safety profile, evidence of antitumor activity | NCT02116803, NCT01484041, NCT01262027, NCT01528345, NCT00958971 | (Andre et al., 2011; André et al., 2013) |
| JNJ-42756493 | Mono | Advanced solid tumors | Phase I | Manageable safety profile, evidence of antitumor activity | NCT01962532, NCT01703481 | (Dienstmann et al., 2014; Tabernero et al., 2015) | |
| Lucitanib (E-3810) | Mono | mBC (FGF-FGFR aberrant) | Phase I, II | Manageable safety profile, evidence of antitumor activity | NCT02202746, NCT02053636, NCT01283945 | (Soria et al., 2014) | |
| Nintedanib (BIBF1120) | Mono, combo | mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT02389764, NCT01658462 | (Lee et al., 2015; Quintela-Fandino et al., 2014) | |
| Selective FGFR TKI | AZD4547 | Mono, combo | mBC | Phase I, II | Acceptable safety profile, evidence of antitumor activity | NCT01791985, NCT01202591, NCT02299999 | (Andre et al., 2014; Smyth et al., 2015) |
| BAY1163877 | Mono | Advanced solid tumors | Phase I | Preclinical antitumor activity | NCT01976741 | (Heroult et al., 2014) | |
| BGJ398 | Mono | Advanced solid tumors | Phase I, II | Acceptable safety profile, single-agent activity in patients with FGFR-genetically altered solid tumors | NCT02160041, NCT01928459, NCT01004224 | (Sequist et al., 2014) | |
| FGF ligand trap and FGFR mAb | FP-1039 (GSK3052230) | Combo | Solid malignancies with deregulated FGF signaling | Phase I | Preclinical antitumor activity | NCT01868022 | (Harding et al., 2013) |
| MGFR1877S (RG744) | Mono | Advanced solid tumors | Phase I | N/A | NCT01363024 | N/A |
Phase III, II and I trials are highlighted in red, blue and black respectively
3.4. Targeting cancer stem cell signaling
TNBC represents a highly diverse group of cancers (Lehmann et al., 2011; Metzger-Filho et al., 2012; Montagna et al., 2013), and certain subtypes within this classification show a strong enrichment of cancer stem cell–like features (Lehmann et al., 2011). The cancer stem cell theory hypothesizes that a small fraction of the cells in a tumor have characteristics of somatic stem cells with highly tumorigenic and chemo-resistant potential, and could be responsible for the majority of metastatic relapse (Balic et al., 2006; Velasco-Velázquez et al., 2012). As such, the treatment of specific stem cell features may offer a curative approach to cancer therapy. The Notch and Wnt pathways are the two most extensively studied pathways that have been implicated in both normal and cancer stem cells of the mammary gland, and may be potential targets in TNBC (Farnie and Clarke, 2007; Howe and Brown, 2004; Stylianou et al., 2006).
3.4.1. Targeting the Notch pathway
Upregulated Notch signaling has been reported in TNBC, and is usually associated with the down-regulation of Numb (Pece et al., 2004) or activating fusion mutations found in NOTCH1 or NOTCH2 (5–10% of TNBC cases) (Robinson et al., 2011; Stoeck et al., 2014). Early attempts to target the Notch pathway focused on gamma secretase inhibitors (GSIs) (Table 6). Gamma secretase is a multi-protein integral membrane complex that catalyzes the proteolytic processing of Notch receptors and liberates the Notch intracellular domain (NCID); NCID subsequently translocates to the nucleus and regulates transcription of Notch downstream target genes (Shih and Wang, 2007). Clinical trials involving GSIs demonstrate the feasibility of targeting aberrant Notch signaling in treating mBC (Table 6). However, a low specificity for tumor cells induced high toxicity in GSI-treated patients (Imbimbo, 2008; Olsauskas-Kuprys et al., 2013).
Table 6.
mBC-targeting Notch pathway inhibitors in clinical development.
| Category | Agent | Application | Patient population | Development stage |
Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* |
Reference |
|---|---|---|---|---|---|---|---|
| Pan-Notch inhibitor | BMS-906024 | Mono, combo | Advanced solid tumors | Phase I | Preclinical antitumor activity | NCT01653470, NCT01292655 | (Gavai et al., 2015) |
| DLL4-specific antibody | Demcizumab (OMP-21M18) | Mono | Advanced solid tumors | Phase I | Well tolerated with encouraging early clinical activity in pancreatic cancer and metastatic NSCLC; clinical trial in mBC ongoing | NCT00744562 | (Hidalgo et al., 2015; McKeage et al., 2014) |
| MEDI0639 | Mono | Advanced solid tumors | Phase I | Manageable safety profile, evidence of antitumor activity | NCT01577745 | (Falchook et al., 2015) | |
| Anti-Notch 1 | OMP-52M51 | Mono | Advanced solid tumors | Phase I | Acceptable toxicity profile, evidence of antitumor activity | NCT01778439 | (Cancilla et al., 2015; Davis et al., 2014) |
| Anti-Notch2/3 | OMP-59R5 (tarextumab) | Mono | Advanced solid tumors | Phase I | Acceptable toxicity profile, evidence of antitumor activity | NCT01277146 | (Tolcher et al., 2012; Yen et al., 2015) |
| Notch inhibitor | Not stated | Mono | Advanced cancer | Phase I | N/A | NCT01158404 | N/A |
| Gamma secretase inhibitors | MK0752 | Combo | mBC | Phase I, II | Acceptable toxicity profile, evidence of antitumor activity | NCT00645333, NCT00106145, NCT01295632 | (Brana et al., 2014; Krop et al., 2012; Schott et al., 2013) |
| PF-03084014 | Mono | Advanced solid tumors | Phase I, II | Acceptable toxicity profile, efficacy warrants further investigation | NCT02338531, NCT02299635 | (Messersmith et al., 2012, 2015) | |
| RO4929097 | Mono | mBC | Phase I, II | Acceptable toxicity profile, evidence of antitumor activity | NCT01151449, NCT01217411 | (Sahebjam et al., 2013; Tolcher et al., 2012) |
Phase III, II and I trials are highlighted in red, blue and black respectively
Other Notch-targeting strategies to interfere with Notch signaling show promise in improving therapeutic specificity (Table 6). Antibodies against specific Notch ligands or receptors have been heavily studied in many cancer systems, and will provide an attractive alternative in treating mBC (Andersson and Lendahl, 2014). For example, tumor-derived Jagged1, one of the five Notch ligands, has been shown to promote osteolytic bone metastasis in breast cancer by engaging Notch signaling in the bone cells (Sethi et al., 2011). It is anticipated that more specific Notch-targeting approaches will rapidly expand as we gain increasing understanding of the Notch signaling network in breast cancer.
3.4.2. Targeting the Wnt pathway
Similar to Notch pathway, activated Wnt signaling has also been implicated in breast cancer tumorigenesis and recurrence (Ahmad, 2013; Anastas and Moon, 2013). Canonical β catenin-mediated WNT signaling supports self-renewal of both normal and malignant mammary stem cells (Anastas and Moon, 2013). Activated canonical Wnt signaling has been observed in both the primary tumor and lung metastasis of TNBC/basal-like subtype, and is associated with poor clinical outcomes (DiMeo et al., 2009; Geyer et al., 2011; Lin et al., 2000).
Somatic mutations in the Wnt pathway are uncommon in breast cancer; however, dysregulation of proteins in the signaling cascade have been reported. In breast cancer patients, WNT1 protein expression was increased in the tumor tissue compared with non-cancerous adjacent tissue (Wong et al., 2002). In this study, Wnt-1 was shown to be markedly elevated in low-grade tumors, but expression levels were reduced as the tumors progressed, suggesting a functional role of Wnt1 at early stages of tumorigenesis. Inhibition of Wnt1 expression reduces the enrichment of cancer stem cells in a mouse model of breast cancer (Choi et al., 2012). Receptor-tyrosine-kinase-like orphan receptor 1 (ROR1), a receptor in the non-canonical Wnt pathway, is found to be highly expressed in primary tumors of TNBC patients (Zhang et al., 2012). ROR1 expression increases in TNBC samples relative to their corresponding normal controls, and correlates with decreased patient survival (Borcherding et al., 2014a). ROR1-positive TNBC has been associated with EMT and stem cell-like phenotypes, suggesting that ROR1 might be a potential target for TNBC therapy (Borcherding et al., 2014a, 2014b). Together these studies suggest a potential for Wnt-targeted treatment in breast cancers, but the design of clinical trials should be carefully considered for specific subtypes and/or tumor grades.
3.5. Androgen receptor (AR)
The androgen receptor (AR) is a nuclear receptor activated by the binding of androgenic hormones in the cytoplasm and then translocating into the nucleus (Gelmann, 2002). Activated AR regulates gene transcription by binding to specific promoter sequences of target genes (Roy et al., 1998). Androgen contributes to the growth of certain types of cancers, most notably prostate cancer (Garay and Park, 2012). Enzalutamide, an androgen receptor antagonist and potent inhibitor of AR signaling, has been approved for the treatment of metastatic castration-resistant prostate cancer. Although androgens are often considered to be male sex hormones, they also play important physiologic roles in females (Burger, 2002).
The prognostic power and clinical relevance of AR expression in breast cancer remains a topic of investigation, and is likely to be subtype-dependent. Some breast cancers express AR, and higher expression is more commonly seen in women with ER+ (67–95%) breast cancer than in women with the HER2+ (63%) or TNBC (10–43%) subtypes (Agoff et al., 2003; Farmer et al., 2005; Gilmore et al., 2015; Gucalp et al., 2013; Hu et al., 2011; Loibl et al., 2011; Luo et al., 2010; Niemeier et al., 2010; Park et al., 2011). A systematic review and meta-analysis of AR expression and outcomes in early breast cancer concluded that the expression of AR in women with breast cancer is associated with better overall survival (OS) and disease-free survival (DFS) irrespective of ER status (Vera-Badillo et al., 2014). Other studies indicated that AR expression is most significantly associated with a favorable prognosis in ER+ BC patients (Hu et al., 2011; Park et al., 2011). While many retrospective histology-based analysis suggest that the presence of AR in breast cancer correlates with better survival outcome (Agoff et al., 2003; Gonzalez et al., 2008; Hu et al., 2011; McGhan et al., 2014; Rakha et al., 2007; Sutton et al., 2012), other preclinical and clinical studies implicate that AR antagonists may improve the survival of AR+ TNBC patients (Barton et al., 2014, 2015; Gucalp et al., 2013; Lehmann et al., 2011). A subclass of TNBC was identified as the luminal androgen receptor (LAR) subtype, as characterized by expression of AR and AR gene targets; LAR cell lines were shown to be uniquely sensitive to AR antagonist bicalutamide (Lehmann et al., 2011).
Investigational trials using AR antagonists, either as monotherapy or combined with other drugs, in patients with AR+ breast cancer are currently ongoing (Table 7). Most of these clinical trials focus on AR+ metastatic TNBC, and a few others target AR+/HR-/HER2+ or AR+/HR+/HER2- mBC subtypes. The discrepancy between the prognostic value of AR and the anti-AR treatment efficacy in clinical trials requires further translational investigations. Such studies will enable a better understanding of the biological functions of androgen and AR, and the mechanisms of action of anti-AR treatment in breast cancer patients.
Table 7.
mBC-targeting AR inhibitors in clinical development.
| Agent | Application | Patient population | Development stage | Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* | Reference |
|---|---|---|---|---|---|---|
| Enzalutamide (MDV3100) | Mono, combo | AR+ mBC | Phase I, II | Acceptable toxicity profile, preliminary activity observed in AR+ TNBC | NCT02007512, NCT02457910, NCT02091960, NCT01889238, NCT01597193, NCT01362374 | (Cochrane et al., 2014; Traina et al., 2015) |
| Orteronel (TAK-700) | Mono | AR+ mBC | Phase I, II | Well tolerated, clinical benefit observed in heavily pretreated HR+ mBC | NCT01990209, NCT01808040 | (Rampurwala et al., 2014) |
| DHEA (Dehydroepiandrosterone) | Mono | AR+ mBC | Phase I, II | N/A | NCT02000375, NCT01990209 | N/A |
| AZD5312 (ISIS-ARRx) | Mono | AR+ advanced solid tumors | Phase I | Preclinical pharmacology reported | NCT02144051 | (Davies et al., 2014) |
| Enobosarm (GTx-024) | Mono | AR+ mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT02368691, NCT02463032, NCT01616758 | (Dalton et al., 2011; Overmoyer et al., 2015) |
| Bicalutamide | Mono | AR+ mBC | Phase I, II | Well tolerated, evidence of antitumor activity | NCT02348281, NCT00468715, NCT02299999 | (Gucalp et al., 2013) |
| CR1447 (4-OH-testosterone) | Mono | AR+ mBC | Phase I/II | N/A | NCT02067741 | N/A |
| Abiraterone Acetate (CB7630) | Mono, combo | AR+/ER+ mBC | Phase I, II, III | Manageable toxicity profile, efficacy warrants further investigation | NCT01517802, NCT01381874, NCT01842321, NCT00755885 | (O’Shaughnessy et al., 2014) |
Phase III, II and I trials are highlighted in red, blue and black respectively
4. Stroma-centric targeting
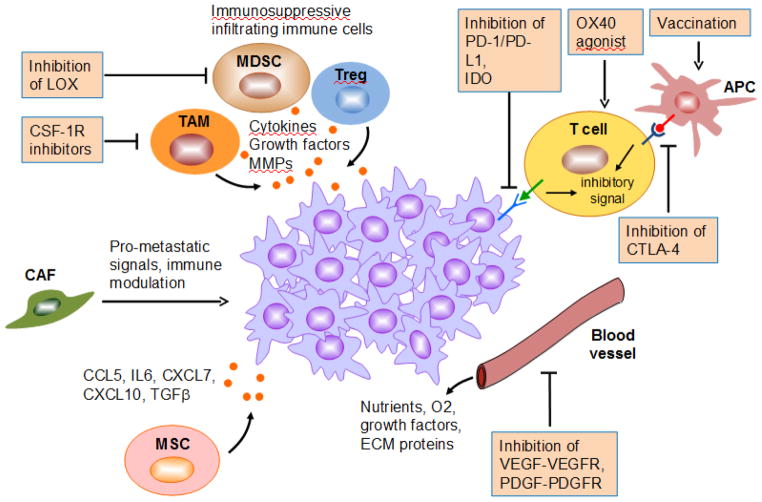
Our knowledge of the stroma’s contribution to cancer has rapidly grown in the past decade. Stromal cells in the tumor microenvironment (TME) play pivotal roles in facilitating multiple steps during cancer progression and metastasis (Whiteside, 2008). Therapeutics targeting tumor-stromal interactions and enhancing host immune response represents an attractive alternative to treat human breast cancer (Place et al., 2011) (Figure 2).
Figure 2. Therapeutic candidates targeting tumor-stromal interactions and enhancing host immune response of mBC.
This schematic diagram illustrates severeal key stromal cell populations important for regulating the growth of mBC, as well as therapeutic agents available or being developed to target the molecular pathways that mediate tumor-stromal interactions.
4.1. Contributions of stromal cells in creating a favorable tumor microenvironment
Myriad evidence has highlighted the importance of the crosstalk between tumor cells and their surrounding microenvironment (Langley and Fidler, 2011; Pietras and Ostman, 2010). Cancer progression and metastasis are enabled, sustained, and co-evolved through interactions with the stromal cells. While these stromal cells are far from being fully characterized, they include infiltrating immune cells, endothelial cells, cancer-associated fibroblasts (CAFs), and mesenchymal stem/stromal cells (MSCs). (Hanahan and Coussens, 2012). Cytokines and extracellular matrix (ECM) proteins provided by both the stromal and tumor cells facilitate breast cancer progression through a complex network of tumor-stromal communication (Landskron et al., 2014; Lu et al., 2012). Heterogeneous cell types within the TME can also actively influence therapeutic responses and shape resistance (Junttila and de Sauvage, 2013).
4.1.1. Infiltrating immune cells
Myeloid lineage-derived macrophages can be recruited and educated by tumor cells and the TME to become tumor-associated macrophages (TAMs) or metastasis-associated macrophages (MAMs) (Kitamura et al., 2015; Qian and Pollard, 2010). These TAMs and MAMs are immunosuppressive and contribute to tumor progression via numerous macrophage-derived signaling factors, such as cytokines, growth factors, matrix metalloproteinases (MMPs), and hypoxia response proteins (Baay et al., 2011; Hao et al., 2012; Luo et al., 2006; Noy and Pollard, 2014). The tumor microenvironment of metastasis (TMEM), characterized by direct interaction between perivascular TAMs, endothelial cells and tumor cells, has been found in human breast cancer specimens (Robinson et al., 2009). A high TMEM score is associated with increased risk of distant metastasis (Robinson et al., 2009; Rohan et al., 2014). Expression of Mena (INV), an invasive isoform of Mena, correlates with a high TMEM score and enhances transendothelial migration of breast cancer cells (Roussos et al., 2011). This process has been shown to be mediated through paracrine and autocrine activation of colony-stimulating factor-1 receptor (CSF-1R) (Pignatelli et al., 2014; Roussos et al., 2011).
Preclinical and clinical observations suggest that macrophage-targeting therapies could be a feasible approach to treat breast cancer metastasis. CSF-1/CSF-1R is one of the most important signaling axes during the differentiation of myeloid progenitors to monocytes, macrophages, dendritic cells and bone-resorbing osteoclasts (Stanley and Chitu, 2014). Blocking macrophage recruitment using CSF-1R inhibitors, in combination with chemotherapy, decreases breast cancer progression and metastasis, and improves survival through CD8+ T-cell-dependent mechanisms in tumor-bearing mice (DeNardo et al., 2011). TH2/M2-type macrophage-derived IL-10 was shown to mediate this CD8+ T-cell-dependent responses (Ruffell et al., 2014). Antibodies against CSF-1R reduced TAM numbers and translated into clinical responses in patients with diffuse-type giant cell tumor (Dt-GCT), a type of cancer that overexpresses CSF-1 (Cassier et al., 2015; Ries et al., 2014). Small molecule inhibitors and mAbs against CSF-1R are currently evaluated in patients with advanced solid tumors (Table 8).
Table 8.
Stroma and immune targeting agents in clinical development.
| Category | Agent | Application | Patient population | Development stage | Main results | Representative clinical trials (ClinicalTrials.gov Identifier)* | Reference |
|---|---|---|---|---|---|---|---|
| CSF1R inhibitor | AMG 820 | Mono | Advanced solid tumors | Phase I | N/A | NCT01444404 | N/A |
| ARRY-382 | Mono | Advanced cancer | Phase I | Well tolerated, CSF1R significantly inhibited, efficacy not revealed | NCT01316822 | (Bendell et al., 2014b) | |
| IMC-CS4 (LY3022855) | Mono | mBC | Phase I | N/A | NCT02265536, NCT01346358 | N/A | |
| PLX3397 (PLX108-01) | Combo | Advanced solid tumors | Phase I, II | N/A | NCT02452424, NCT01596751, NCT01042379, NCT01004861, NCT01525602 | N/A | |
| anti–PD-1 | MEDI0680 (AMP-514) | Mono, combo | Advanced solid tumors | Phase I | N/A | NCT02118337, NCT02013804 | N/A |
| Nivolumab | Combo | mBC | Phase I | N/A | NCT02309177 | N/A | |
| Pembrolizumab (MK-3475) | Mono, combo | mBC | Phase I, II | Acceptable safety profile, promising antitumor activity including durable responses in PD-L1 metastatic TNBC | NCT02129556, NCT02395627, NCT02331251, NCT02318901, NCT02178722, NCT02447003, NCT02411656, NCT02513472, NCT02452424, NCT02331251, | (Buisseret et al., 2015; Nanda et al., 2015) | |
| anti-PD-L1 | Avelumab (MSB0010718C) | Mono | Advanced solid tumors | Phase I | Acceptable safety profile, activity not revealed | NCT01772004, NCT01943461 | (Kelly et al., 2015) |
| MEDI4736 | Mono, combo | Advanced solid tumors | Phase I, II | Acceptable safety profile, efficacy warrants further investigation | NCT02484404, NCT02318277, NCT01693562, NCT02403271, NCT02221960, NCT01938612, NCT02261220, NCT02301130, NCT01975831, NCT02141347 | (Iguchi et al., 2015; Lutzky et al., 2014; Segal et al., 2014) | |
| MPDL3280A (atezolizumab) | Mono, combo | Advanced solid tumors | Phase I, II, III | Well tolerated, promising efficacy in pretreated metastatic PD-L1 IHC 2 or 3 TNBC patients | NCT02425891, NCT02530489, NCT02543645, NCT02458638, NCT02478099, NCT02174172, NCT02471846, NCT02350673, NCT02304393, NCT02410512, NCT01988896, NCT02323191 | (Emens et al., 2015) | |
| anti-CTLA-4 | Tremelimumab | Combo | Advanced solid tumors | Phase I, II | Acceptable safety profile, efficacy warrants further investigation | NCT02205333, NCT02301130, NCT02261220, NCT02141347, NCT01975831 | (Grosso and Jure-Kunkel, 2013; Millward et al., 2013) |
| OX40 agonist | anti-OX40 | Mono, combo | mBC | Phase I | N/A | NCT02221960 | N/A |
| MEDI0562 | Mono | Advanced solid tumors | Phase I | N/A | NCT02318394 | N/A | |
| MEDI6469 | Mono, combo | Advanced solid tumors | Phase I, II | N/A | NCT02205333, NCT01862900 | N/A | |
| IDO pathway inhibitor | Indoximod (1-methyl-d-trypto phan) | Combo | mBC | Phase I, II | Acceptable safety profile, evidence of antitumor activity | NCT01792050, NCT01042535 | (Soliman et al., 2014, 2015) |
| Agonist anti-CD27 antibody | Varlilumab (CDX-1127) | Combo | Advanced solid tumors | Phase I, II | Well tolerated, promising signs of clinical activity | NCT02543645, NCT02270372 | (Bullock et al., 2014; Thomas et al., 2015) |
| Therapeutic vaccine | Multiple | Mono, combo | mBC | Phase I, II | ONT-10, a MUC1 liposomal vaccine: acceptable safety profile with evidence of antitumor activity | NCT00266110, NCT02018458, NCT00971737, NCT02479230, NCT01730118, NCT01837602, NCT01061840, NCT01660529, NCT01376505, NCT01526473, NCT02157051, NCT02270372, NCT01556789 | (Nemunaitis et al., 2014; Pestano et al., 2014) |
Phase III, II and I trials are highlighted in red, blue and black respectively
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immune cells from the myeloid lineage. MDSCs possess strong immunosuppressive activities, primarily through suppressing CD8+ T-cell cytotoxicity (Gabrilovich and Nagaraj, 2009; Talmadge and Gabrilovich, 2013). MDSCs contribute to the establishment of an immunosuppressive pre-metastatic niche via tumor-derived factors such as GM-CSF, IL-6 and lysyl oxidase (LOX) (Erler et al., 2006, 2009; Morales et al., 2010; Oh et al., 2013). Inhibition of LOX activity in tumor cells block myeloid cell recruitment to pre-metastatic niche and reduce breast cancer lung and bone metastasis (Cox et al., 2015; Erler et al., 2009), suggesting a possible strategy for therapeutic intervention.
Regulatory T (T-reg) cell is another class of immunosuppressive cell that primarily functions to inhibit induction and proliferation of effector T cells. A significant increase of T-reg cells was observed in the peripheral blood and TME of invasive breast and pancreas cancer patient (Liyanage et al., 2002). Tumor-infiltrating T-reg cells, recruited by tumor-derived CCL5, could stimulate mammary cancer metastasis through RANKL–RANK signaling (Tan et al., 2011). In a breast cancer xenograft model, attenuation of a glycan-binding protein galectin-1 (Gal1) reduced the frequency of T-reg cells within the tumor and reduced lymph node, spleen, and lung metastases, suggesting the possibility of targeting Gal1 in breast cancer metastasis (Dalotto-Moreno et al., 2013). Understanding the specific roles of T-reg cells in cancer is the key for development of therapeutics against this class of immune cells (Mougiakakos et al., 2010)
4.1.2. Angiogenic vasculature
Vascular endothelial cells and their supporting pericytes together form the angiogenic vasculature (Armulik et al., 2005). The induction of angiogenesis (also known as angiogenic switch) promotes cancer cell proliferation (Hanahan and Coussens, 2012). The angiogenic vasculature promotes tumor progression not only by supplying nutrients and oxygen, but also through an “angiocrine” mechanism by producing stem and progenitor cell-active factors, ECM components etc. to generate a tumor-favorable microenvironment (Butler et al., 2010).
VEGF is one of the most well-known angiogenic factors and has an established role in breast cancer (Goel and Mercurio, 2013). VEGFRs are tyrosine kinase receptors primarily expressed on endothelial cells, and are responsible for binding with VEGF to initiate signal cascades that stimulate angiogenesis (Carmeliet, 2005) and lymphangiogenesis (Karnezis et al., 2012; Skobe et al., 2001). VEGFRs are up-regulated in multiple cancer types which have a high need for nutrients and oxygen, including breast cancer (Guo et al., 2010; Srabovic et al., 2013). Since early in this century, clinical trials involving mAbs or small molecule inhibitors that interrupt VEGF-VEGFR signaling have been conducted extensively in various cancer types. Despite the promising results from other cancer types (e.g. lung cancer, colorectal cancer, renal cell carcinoma (RCC), glioblastoma), most trials have failed to demonstrate a statistically significant survival advantage in mBC patients (Meadows and Hurwitz, 2012). This could be explained by several reasons, for example: drug resistance through activation of alternative angiogenic pathways, and lack of biomarkers to stratify patients that could benefit most from anti-VEGF therapy (Sledge, 2015). Nevertheless, several recent studies shed light on potentially new directions for anti-VEGF therapies. For example, it was demonstrated that blocking lipid synthesis could overcome tumor regrowth and metastasis after antiangiogenic therapy withdrawal (Sounni et al., 2014). Moreover, PD-L1 was shown to crosstalk with VEGFR2 during angiogenesis, and a combination of anti-VEGF and immunotherapeutic strategies may improve treatment efficacy in mBC (Jin et al., 2011; Voron et al., 2014).
In addition to the VEGF-VEGFR axis, PDGF-PDGFR signaling has also been implicated in breast cancer tumor-stromal interaction. PDGFRs are expressed on breast cancer stromal cells and tumor-derived PDGFs stimulate the stromal cells to further promote tumorigenesis (Heldin, 2013; Paulsson et al., 2009). PDGFRs are also expressed in invasive breast carcinomas, which correlate with tumor aggressiveness, higher chance of metastasis and shortened survival (Carvalho et al., 2005; Jechlinger et al., 2006). Inhibition of PDGFR signaling has proven effective in patients with certain rare tumors (Malhotra and Schuetze, 2012; Stacchiotti et al., 2012), yet it remains to be examined whether this treatment will be beneficial for mBC patients.
4.1.3. Cancer-associated fibroblasts (CAFs)
CAFs are a heterogeneous population of fibroblasts residing within the TME and can facilitate malignant transformation and growth of cancer cells (Madar et al., 2013). CAFs secrete pro-proliferation signals (e.g. TGF-α), pro-angiogenic signals (e.g. VEGF, FGF and PDGF) and pro-EMT signals (e.g. TGF-β) to facilitate tumor growth, angiogenesis and metastasis (Gao et al., 2013; Yu et al., 2014). CAFs can also modulate immune polarization in the TME and inhibit the anti-tumor activities of cytotoxic T cells and natural killer (NK) cells (Baginska et al., 2013; Balsamo et al., 2009; Ohshio et al., 2015). CAFs in the primary breast TME were shown to select for bone specific metastatic traits in tumor cells via CAF-secreted CXCL12 and IGF1 (Zhang et al., 2013). A small population of breast cancer stem cells induces periostin (POSTN) expression in resident fibroblasts at the secondary target organ in order to initiate metastasis colonization (Malanchi et al., 2012). Although CAFs are primarily studied as pro-tumorigenic stromal cells, recent studies suggested that tumor-resident fibroblasts could also have tumor-inhibitory effects, suggesting a degree of plasticity in this stromal cell type (Augsten, 2014).
4.1.4. Mesenchymal stem/stromal cells (MSCs)
MSCs are multipotent stromal cells that can differentiate into a variety of cell types such as osteoblasts, chondrocytes, adipocytes, myocytes, and cardiomyocytes (Nombela-Arrieta et al., 2011). They are traditionally found in the bone marrow, but can also be isolated from cord and peripheral blood (Malgieri et al., 2010). Bone-marrow-derived MSCs have been shown to enhance breast cancer cell motility, invasion and metastasis, dependent on CCL5-CCR5 signaling between cancer cells and MSCs (Karnoub et al., 2007). Other studies also confirm that MSCs are able to supply contextual signals (e.g. CCL5, IL6, CXCL7, CXCL10, TGFβ1) to the tumor-associated stroma, creating an immunosuppressive microenvironment and promoting metastatic progression (Chaturvedi et al., 2013; Liu et al., 2011; Mi et al., 2011; Patel et al., 2010). These signaling events have been observed between MSCs and breast cancer cells as well as between MSCs and other stromal cell types. Recent studies also highlighted the roles of MSC-associated microRNAs in regulating breast cancer progression (Cuiffo et al., 2014; Ono et al., 2014). The therapeutic potential of targeting MSCs in mBC warrants further investigations as MSCs could be either tumor-promoting or tumor-suppressing depending on different environmental inflammatory conditions (Kim and Cho, 2013).
4.2. Immunotherapies
Historically, breast cancer has been considered poorly immunogenic, thus less responsive to immunotherapies. A few mechanisms may contribute to this. First, the percentages of both type1 (IL-2, IFNγ or TNFα) and type 2 (IL-4) cytokines produced by T lymphocytes were found significantly lower in the peripheral blood of patients with breast cancer compared to healthy controls, suggesting a general immune dysfunction in these patients (Campbell et al., 2005). Second, myeloid suppressor cells, Th2 CD4+T cells and T-reg cells function together to create an immune-suppressive TME and suppress CD8+ cytotoxicity via cytokine signaling (e.g. IL-4, IL-13, IL-10, IL-6 and TGFβ) (DeNardo and Coussens, 2007). Third, many cancer cells, including breast cancer, downregulate MHC molecules and/or co-stimulatory molecules and upregulate immunosuppressive factors such as PD-L1, resulting in immune escape (Dushyanthen et al., 2015; Pardoll, 2012).
Despite the less immunogenic nature, a small population of breast cancer do induce stronger cytotoxic T- and NK-cell responses, and tend to have a more favorable prognosis with a better response to chemotherapy (Denkert et al., 2010; Finak et al., 2008; Mahmoud et al., 2011). Recent studies suggest that significant immune cell infiltration via tumor-infiltrating lymphocytes (TIL) in early breast cancer predicts higher responses to chemotherapy, reduced risk of distant metastasis, and better overall survival, particularly in the TN and HER2+ subtypes (Dieci et al., 2015; Salgado et al., 2015). These observations suggest that immunotherapies could have potential to elicit strong immune response in at least a subset of mBC.
4.2.1. Therapeutic vaccine
Therapeutic vaccines are designed to elicit an immune response against tumor-associated antigens (TAAs) and enhance the host immune system to attack TTA-bearing cancer cells. Vaccination usually takes a relatively a long time (i.e. weeks to months) to produce enough antigen-specific cytotoxic T cell for an effective immune response (David et al., 2009; Gonzalez et al., 2003; Melero et al., 2014), thus may not be the most appropriate treatment for patients in an advanced-disease setting. Most breast cancer vaccine clinical trials are studied in an adjuvant setting in early stage patients with minimal residual tumor cells. Vaccination in combination with other targeted therapies or immunotherapies in patients with high risk of recurrence provide an opportunity to boost and maintain the host immune response in order to eradicate the fewer number of isolated tumor cells before they have a chance to establish larger metastases (Cimino-Mathews et al., 2015; Page et al., 2014) (Table 8).
4.2.2. Immune checkpoint blockade
After decades of incremental progresses, researchers have started to appreciate the therapeutic power of modulating activities of immune checkpoints, such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) (Pardoll, 2012; Sharma and Allison, 2015). Both CTLA-4 and PD1 belong to the CD28 family receptors and they transmit inhibitory signals to prevent T-cell activation (Parry et al., 2005). Blocking the functions of these checkpoint proteins liberates tumor-specific T cells to exert their effector function against tumor cells and subsequently induces a strong immune response (Pardoll, 2012). Immune checkpoint blockade have demonstrated promising activities in a variety of malignancies, including melanoma, RCC, head and neck, lung, bladder and prostate cancers (Pardoll, 2012; Postow et al., 2015; Topalian et al., 2015). To date, the FDA has approved three such drugs for the treatment of metastatic melanoma and squamous non-small cell lung cancer: ipilimumab (Yervoy), pembrolizumab (Keytruda), and nivolumab (Opdivo). The first targets the CTLA-4 pathway and the latter two target PD-1.
PD-1 ligand (PD-L1) expression was observed in 30% of breast cancer patients, and positively associated with HR-negative and triple-negative (TN) status and high levels of TILs (Wimberly et al., 2015). Clinical studies indicate that immune checkpoint blockade, in combination with cytotoxic drugs, have the potential to improve clinical outcomes for patients with mBC, especially metastatic TN and HER2+ breast cancer with lymphocytic tumor infiltrates at diagnosis (Singh et al., 2014; Stagg and Allard, 2013). mAb targeting PD-1 (Pembrolizumab/MK-3475) or PD-L1 (MPDL3280A) demonstrated promising therapeutic activities in heavily pretreated metastatic TNBC (Emens et al., 2015; Nanda et al., 2015). Multiple clinical trials, including a phase III trial of MPDL3280A, have been recently initiated in patients with mBC (Table 8).
Several other immunomodulatory agents which have the potential to enhance anti-tumor immune response were also reported. For example, indoleamine-pyrrole 2, 3-dioxygenase (IDO) is an immune checkpoint molecule expressed on various stromal cells such as macrophages (Prendergast et al., 2014). IDO is also expressed by a number of tumor types that possess an immune-escape strategy, and is correlated with poor prognosis (Iversen et al., 2015; Katz et al., 2008; Prendergast, 2008). Another example is OX40, a potent TNF receptor family co-stimulatory receptor that can potentiate T cell receptor signaling. Ligation of OX40 with an agonistic anti-OX40 mAb has been shown to enhance anti-tumor immunity (Chen et al., 2014; Curti et al., 2013; Redmond et al., 2014). Phase II trials of IDO inhibitor and anti-OX40 mAb are currently being tested in mBC patients (Table 8). Additional therapeutic targets involved in anti-cancer immune response include T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) (Ngiow et al., 2011; Sakuishi et al., 2010), lymphocyte-activation gene 3 (LAG-3) (Nguyen and Ohashi, 2014; Woo et al., 2012), and the costimulatory receptor of the TNF receptor family CD137 (Kohrt et al., 2012; Narazaki et al., 2010; Palazón et al., 2011).
A small subset of HER2+ and TNBC patients that have relatively high levels of TILs might benefit most from current immune checkpoint inhibitors. Those patients that have no or relatively low TILs would require additional strategies to increase TILs prior to immune checkpoint blockade (Bellone and Calcinotto, 2013). Cancer vaccine can activate and expand tumor-specific CD8+ T cells but itself usually yields limited success in controlling cancer progression (McGray et al., 2014). Vaccine priming before or concurrent with one or several immune checkpoint blockade may potentiate the effector T-cell functions and finally translate into long-term disease control of mBC.
5. Conclusions and future directions
Advances in cancer genomics have identified a large number of potential therapeutic targets, many of which have been studied extensively in preclinical and clinical models. Better classification of breast cancer subtypes and in depth characterization of druggable signaling pathways using new biotechnologies like next-generation sequencing (NGS) will enable selection of the most appropriate drugs to target specific genomic alterations. Mutations of BRCA, PIK3CA, AKT1, etc., as well as deregulated signaling such as the FGF and AR pathways, provide promising targets to individualize therapy in metastatic breast cancer. Tumor-intrinsic targeted therapies are likely to yield higher specificity and less toxicity, but may develop drug resistance as the treatment continues. This could be due to secondary mutations that abrogate contact points for drug binding, and/or activation of alternative compensatory signaling mechanisms (Garraway and Jänne, 2012; Holohan et al., 2013). On the other hand, the stroma-centric targeting approach could elicit a broader impact against cancerous cells. This strategy has been proved successful in other tumor types such as melanoma, and has also demonstrated promising preliminary results in a subset of mBC patients. Targeting the tumor-promoting elements of the TME and harnessing host immune mechanisms to eradicate cancer cells represent an emerging treatment paradigm. However, while the signaling molecules between the tumor and immune-suppressive stromal cells are potential drug targets, the similarities between tumor-associated stroma and normal stromal cells may potentially cause severe adverse effects (Postow et al., 2015).
Our knowledge of breast cancer metastasis has advanced significantly in recent years, with particular emphasis on an integrated understanding of the tumor and its associated microenvironment. Most of current clinical studies are still at early stages of evaluating single or double agent toxicity and efficacy. As a step forward, it is important to start combining drugs using biologically informed translational studies to guide trial design and improve patient outcomes. Although most current approaches of precision medicine aim at targeting driver events, additional applications such as identification of DNA repair deficiencies and mechanisms of immune suppression could be developed in the future. The ultimate goal is to attack the tumor cells as well as the TME, and at the same time preserve the normal function of organs not involved. Rational combinations of surgery/radiotherapy/cytotoxic chemotherapy, with tumor-intrinsic and/or stroma-centric targeted therapies, are likely to improve the treatment efficacy without causing severe toxic effects.
Acknowledgments
We thank the members of our laboratories for helpful discussions and M. Esposito, W. Lu, Y. Wei, and H.A. Smith for critical reading of the manuscript. We also apologize to the many investigators whose important studies could not be cited directly here owing to space limitations. The work was supported by Howard Hughes Medical Institute International Student Research Fellowship to Z.L. and grants from the Brewster Foundation, the Breast Cancer Research Foundation and the U. S. Department of Defense (BC123187) to Y.K.
Abbreviations
- ADC
antibody drug conjugate
- AR
androgen receptor
- CAF
cancer associated fibroblasts
- GSI
gamma secretase inhibitor
- HR
hormone receptor
- mBC
metastatic breast cancer
- MSC
mesenchymal stem/stromal cells
- TAM
tumor-associated macrophages
- TIL
tumor-infiltrating lymphocyte
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
- T-reg
regulatory T cell
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–731. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- Ahmad A. Pathways to breast cancer recurrence. ISRN Oncol. 2013;2013:290568. doi: 10.1155/2013/290568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnert JR, Schuler MH, Machiels JH, Hess D, Paz-Ares L, Awada A, et al. Phase lb study of BEZ235 plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC) J Clin Oncol. 2014;32:5s. suppl; abstr 621. [Google Scholar]
- Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc; 2013. [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- Andre F, Bachelot TD, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. A multicenter, open-label phase II trial of dovitinib, an FGFR1 inhibitor, in FGFR1 amplified and non-amplified metastatic breast cancer. ASCO Meet Abstr. 2011;29:508. [Google Scholar]
- Andre F, Ranson M, Dean E, Varga A, van der Noll R, Stockman PK, et al. Abstract LB-145: Results of a phase I study of AZD4547, an inhibitor of fibroblast growth factor receptor (FGFR), in patients with advanced solid tumors. Cancer Res. 2014;73 LB – 145 – LB – 145. [Google Scholar]
- André F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19:3693–3702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]
- Andrei AZ, Hall A, Smith AL, Bascuñana C, Malina A, Connor A, et al. Increased in vitro and in vivo sensitivity of BRCA2-associated pancreatic cancer to the poly(ADP-ribose) polymerase-1/2 inhibitor BMN 673. Cancer Lett. 2015;364:8–16. doi: 10.1016/j.canlet.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- Asundi J, Crocker L, Tremayne J, Chang P, Sakanaka C, Tanguay J, et al. An Antibody-Drug Conjugate Directed against Lymphocyte Antigen 6 Complex, Locus E (LY6E) Provides Robust Tumor Killing in a Wide Range of Solid Tumor Malignancies. Clin Cancer Res. 2015;21:3252–3262. doi: 10.1158/1078-0432.CCR-15-0156. [DOI] [PubMed] [Google Scholar]
- Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginska J, Viry E, Paggetti J, Medves S, Berchem G, Moussay E, et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013;4:490. doi: 10.3389/fimmu.2013.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Ferrari S, Russell RGG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Barton V, D’Amato N, Richer J. Abstract A047: Targeting androgen receptor decreases proliferation of triple-negative breast cancer. Mol Cancer Res. 2014;11:A047–A047. [Google Scholar]
- Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell J, Saleh M, Rose AAN, Siegel PM, Hart L, Sirpal S, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2014a;32:3619–3625. doi: 10.1200/JCO.2013.52.5683. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Tolcher AW, Jones SF, Beeram M, Infante JR, Larsen P, et al. Abstract A252: A phase 1 study of ARRY-382, an oral inhibitor of colony-stimulating factor-1 receptor (CSF1R), in patients with advanced or metastatic cancers. Mol Cancer Ther. 2014b;12:A252–A252. [Google Scholar]
- Bergstrom DA, Bodyak N, Yurkovetskiy A, Park PU, DeVit M, Yin M, et al. Abstract LB-231: A novel, highly potent HER2-targeted antibody-drug conjugate (ADC) for the treatment of low HER2-expressing tumors and combination with trastuzumab-based regimens in HER2-driven tumors. Cancer Res. 2015;75 LB – 231 – LB – 231. [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Boni V, Rixe O, Rasco D, Gomez-Roca C, Calvo E, Morris JC, et al. Abstract A73: A Phase I first-in-human (FIH) study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs) ( NCT01156870) Mol Cancer Ther. 2014;12:A73–A73. [Google Scholar]
- Borcherding N, Ameka M, Kolb R, Xie Q, Zhang W. Abstract 2083: WNT5a/ROR1 axis in triple-negative breast cancer progression and potential therapy. Cancer Res. 2014a;74:2083–2083. [Google Scholar]
- Borcherding N, Kusner D, Liu GH, Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014b;5:496–502. doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco JLF, Lash TL, Prout MN, Buist DSM, Geiger AM, Haque R, et al. Breast cancer recurrence in older women five to ten years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2979–2983. doi: 10.1158/1055-9965.EPI-09-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br J Cancer. 2014;111:1932–1944. doi: 10.1038/bjc.2014.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret L, Specht J, Dees EC, Berger R, Gupta S, Geva R, et al. 14P * KEYNOTE-012: A phase Ib study of pembrolizumab (MK-3475) in patients (pts) with metastatic triple-negative breast cancer (mTNBC) Ann Oncol. 2015;26:iii6–iii6. [Google Scholar]
- Bullock T, McClintic H, Jeong S, Smith K, Olson W, Ramakrishna V, et al. Immune correlates of Varlilumab treated cancer patients are consistent with CD27 costimulatory activity. J Immunother Cancer. 2014;2:P100. [Google Scholar]
- Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91:163–171. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- Cancilla B, Tam R, Zhang C, Anderson S, Lewicki J, Hoey T, et al. Abstract 1549: Development and validation of a biomarker for prospective selection of Notch1 activation in patients with certain advanced solid tumors in a first-in-human phase1 study of the cancer stem cell targeting antibody OMP-52M51 (anti-Notch1) Cancer Res. 2015;75:1549–1549. [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005;7:R788–R795. doi: 10.1186/bcr1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- Cavazzoni A, Bonelli MA, Fumarola C, La Monica S, Airoud K, Bertoni R, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 2012;323:77–87. doi: 10.1016/j.canlet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Gilkes DM, Wong CCL, Luo W, Zhang H, Wei H, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Ouyang H, Zhou S, Li J, Ye Y. PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cell Immunol. 2014;287:91–99. doi: 10.1016/j.cellimm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Choi AR, Park JR, Kim RJ, Kim SR, Cho SD, Jung JY, et al. Inhibition of Wnt1 expression reduces the enrichment of cancer stem cells in a mouse model of breast cancer. Biochem Biophys Res Commun. 2012;425:436–442. doi: 10.1016/j.bbrc.2012.07.120. [DOI] [PubMed] [Google Scholar]
- Chuang HC, Kapuriya N, Kulp SK, Chen CS, Shapiro CL. Differential anti-proliferative activities of poly(ADP-ribose) polymerase (PARP) inhibitors in triple-negative breast cancer cells. Breast Cancer Res Treat. 2012;134:649–659. doi: 10.1007/s10549-012-2106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park) 2015;29:375–385. [PubMed] [Google Scholar]
- Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s– 6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Coltrera MD, Wang J, Porter PL, Gown AM. Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- Di Cosimo S, Sathyanarayanan S, Bendell JC, Cervantes A, Stein MN, Braña I, et al. Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial. Clin Cancer Res. 2015;21:49–59. doi: 10.1158/1078-0432.CCR-14-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscitiello C, Azim HA, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi13–vi18. doi: 10.1093/annonc/mds188. [DOI] [PubMed] [Google Scholar]
- Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014;15:762–774. doi: 10.1016/j.stem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damelin M, Bankovich A, Park A, Aguilar J, Anderson W, Santaguida M, et al. Anti-EFNA4 Calicheamicin Conjugates Effectively Target Triple-Negative Breast and Ovarian Tumor-Initiating Cells to Result in Sustained Tumor Regressions. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0695. [DOI] [PubMed] [Google Scholar]
- David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol. 2009;115:S1–S6. doi: 10.1016/j.ygyno.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Davies BR, Thomason A, Ellston R, Campbell H, D’Cruz C, Mazzola AM, et al. 457 Preclinical pharmacology of AZD5312, a generation 2.5 antisense oligonucletotide targeting the androgen receptor with differentiated activity from enzalutamide. Eur J Cancer. 2014;50:150. [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SL, LoRusso P, Xu L, Kapoun AM, Dupont J, Munster P, et al. Abstract B48: A first-in-human Phase I study of the novel cancer stem cell (CSC) targeting antibody OMP-52M51 (anti-Notch1) administered intravenously to patients with certain advanced solid tumors. Mol Cancer Ther. 2014;12:B48–B48. [Google Scholar]
- De PK, Sun Y, Carlson JH, Lin X, Dey N, Leyland-Jones B. Abstract 1940: Dual inhibition of mTORC1/C2 and HER2 results in maximal antitumor efficacy in preclinical model of HER2+ breast cancer resistant to trastuzumab therapy. Cancer Res. 2015;75:1940–1940. [Google Scholar]
- DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- Dent RA, Lindeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15:R88. doi: 10.1186/bcr3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstmann R, Bahleda R, Adamo B, Rodon J, Varga A, Gazzah A, et al. Abstract CT325: First in human study of JNJ-42756493, a potent pan fibroblast growth factor receptor (FGFR) inhibitor in patients with advanced solid tumors. Cancer Res. 2014;74 CT325–CT325. [Google Scholar]
- DiMeo TA, Anderson K, Phadke P, Fan C, Feng C, Perou CM, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13:2618–2629. doi: 10.1158/1535-7163.MCT-14-0040-T. [DOI] [PubMed] [Google Scholar]
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost R, Jonkers J. Opportunities and hurdles in the treatment of BRCA1-related breast cancer. Oncogene. 2014;33:3753–3763. doi: 10.1038/onc.2013.329. [DOI] [PubMed] [Google Scholar]
- Durmus S, Sparidans RW, van Esch A, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) restrict oral availability and brain accumulation of the PARP inhibitor rucaparib (AG-014699) Pharm Res. 2015;32:37–46. doi: 10.1007/s11095-014-1442-z. [DOI] [PubMed] [Google Scholar]
- Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. doi: 10.1186/s12916-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwadasi S, Tong Y, Walsh T, Danso MA, Ma CX, Silverman P, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple-negative breast cancer (TNBC): Hoosier Oncology Group BRE09-146. J Clin Oncol. 2014;32:5s. suppl; abstr 1019. [Google Scholar]
- Elgersma RC, Coumans RGE, Huijbregts T, Menge WMPB, Joosten JAF, Spijker HJ, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: Toward Selection of HER2-Targeting Antibody-Drug Conjugate SYD985. Mol Pharm. 2015;12:1813–1835. doi: 10.1021/mp500781a. [DOI] [PubMed] [Google Scholar]
- Emens LA, Braiteh FS, Cassier P, Delord JP, Eder JP, Fasso M, et al. Abstract 2859: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC) Cancer Res. 2015;75:2859–2859. [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther. 2014;141:222–233. doi: 10.1016/j.pharmthera.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook GS, Dowlati A, Naing A, Gribbin MJ, Jenkins DW, Chang LL, et al. Phase I study of MEDI0639 in patients with advanced solid tumors. J Clin Oncol. 2015;33(suppl):abstr 3024. [Google Scholar]
- Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- Farnie G, Clarke RB. Mammary Stem Cells and Breast Cancer—Role of Notch Signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- Fedele CG, Ooms LM, Ho M, Vieusseux J, O’Toole SA, Millar EK, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci U S A. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng FY, de Bono JS, Rubin MA, Knudsen KE. Chromatin to Clinic: The Molecular Rationale for PARP1 Inhibitor Function. Mol Cell. 2015;58:925–934. doi: 10.1016/j.molcel.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- Fleming GF, Ma CX, Huo D, Sattar H, Tretiakova M, Lin L, et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2012;136:355–363. doi: 10.1007/s10549-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MQ, Kim BG, Kang S, Choi YP, Yoon JH, Cho NH. Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-α cleavage by ADAM17. Cancer Lett. 2013;336:240–246. doi: 10.1016/j.canlet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Garay JP, Park BH. Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012;2:434–445. [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Jänne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- Gavai AV, Quesnelle C, Norris D, Han WC, Gill P, Shan W, et al. Discovery of Clinical Candidate BMS-906024: A Potent Pan-Notch Inhibitor for the Treatment of Leukemia and Solid Tumors. ACS Med Chem Lett. 2015;6:523–527. doi: 10.1021/acsmedchemlett.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- Geretti E, Leonard SC, Dumont N, Lee H, Zheng J, De Souza R, et al. Cyclophosphamide-Mediated Tumor Priming for Enhanced Delivery and Antitumor Activity of HER2-Targeted Liposomal Doxorubicin (MM-302) Mol Cancer Ther. 2015;14:2060–2071. doi: 10.1158/1535-7163.MCT-15-0314. [DOI] [PubMed] [Google Scholar]
- Geuna E, Milani A, Martinello R, Aversa C, Valabrega G, Scaltriti M, et al. Buparlisib, an oral pan-PI3K inhibitor for the treatment of breast cancer. Expert Opin Investig Drugs. 2015;24:421–431. doi: 10.1517/13543784.2015.1008132. [DOI] [PubMed] [Google Scholar]
- Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, et al. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- Gilmore H, Varadan V, Williams N, Thompson CL, Kim S, Hsu P, et al. Abstract P3-04-07: Androgen receptor expression in triple negative breast cancer. Cancer Res. 2015;75 P3–P04 – 07–P3 –04–07. [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökmen-Polar Y, Liu Y, Toroni RA, Sanders KL, Mehta R, Badve S, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136:673–682. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015 doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461–466. doi: 10.1093/annonc/mdg102. [DOI] [PubMed] [Google Scholar]
- Gonzalez LO, Corte MD, Vazquez J, Junquera S, Sanchez R, Alvarez AC, et al. Androgen receptor expresion in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. 2008;8:149. doi: 10.1186/1471-2407-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Krop I, Akcakanat A, Chen H, Liu S, Li Y, et al. SU2C phase Ib study of paclitaxel and MK-2206 in advanced solid tumors and metastatic breast cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim Biophys Acta - Rev Cancer. 2010;1806:108–121. doi: 10.1016/j.bbcan.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Solis WA, Fuji RN, Oldendorp A, Pacheco G, Luis E, et al. Abstract 4502: Nonclinical characterization and tolerability of a surrogate anti-mesothelin-MMAE antibody-drug conjugate. Cancer Res. 2014;74:4502–4502. [Google Scholar]
- Gupta S, Argiles G, Munster PN, Hollebecque A, Dajani O, Cheng J, et al. A Phase I Trial of Combined Ridaforolimus and MK-2206 in Patients With Advanced Malignancies. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0180. 1078–0432.CCR – 15–0180 –. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin Dev Immunol. 2012;2012:1–11. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding TC, Long L, Palencia S, Zhang H, Sadra A, Hestir K, et al. Blockade of nonhormonal fibroblast growth factors by FP-1039 inhibits growth of multiple types of cancer. Sci Transl Med. 2013;5:178ra39. doi: 10.1126/scitranslmed.3005414. [DOI] [PubMed] [Google Scholar]
- Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroult M, Ellinghaus P, Sieg C, Brohm D, Gruenewald S, Collin MP, et al. Abstract 1739: Preclinical profile of BAY 1163877 - a selective pan-FGFR inhibitor in phase 1 clinical trial. Cancer Res. 2014;74:1739–1739. [Google Scholar]
- Hidalgo M, Cooray P, Jameson MB, Carrato A, Parnis F, Jeffery M, et al. A phase Ib study of the anti-cancer stem cell agent demcizumab (DEM) & gemcitabine (GEM) +/- paclitaxel protein bound particles (nab-paclitaxel) in pts with pancreatic cancer. J Clin Oncol. 2015;33(suppl):abstr 4118. [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, Digiammarino EL, et al. Mechanistic Dissection of PARP1 Trapping and the Impact on in vivo Tolerability and Efficacy of PARP Inhibitors. Mol Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-15-0191-T. [DOI] [PubMed] [Google Scholar]
- Howe LR, Brown AMC. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- Ho-Yen CM, Jones JL, Kermorgant S. The clinical and functional significance of c-Met in breast cancer: a review. Breast Cancer Res. 2015;17:52. doi: 10.1186/s13058-015-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. doi: 10.1186/bcr3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebschman ML, Lane NL, Liu H, Sarode VR, Devlin JL, Frenkel EP. Molecular heterogeneity in adjacent cells in triple-negative breast cancer. Breast Cancer (Dove Med Press. 2015;7:231–237. doi: 10.2147/BCTT.S87041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE. Tyrosine kinase signalling in breast cancer. Breast Cancer Res. 2000;2:154–157. doi: 10.1186/bcr48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Hassan H, Vilardo L, Kumar SK, Kumar AV, Kelsch R, et al. Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS One. 2013;8:e85737. doi: 10.1371/journal.pone.0085737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi H, Nogami N, Kozuki T, Matsumoto T, Tamura K, Yamamoto N, et al. Phase I study to evaluate the safety and tolerability of MEDI4736, an anti-programmed cell death ligand-1 (PD-L1) antibody, in Japanese patients with advanced solid tumors. J Clin Oncol. 2015;33(suppl):abstr 3039. [Google Scholar]
- Imbimbo BP. Therapeutic potential of gamma-secretase inhibitors and modulators. Curr Top Med Chem. 2008;8:54–61. doi: 10.2174/156802608783334015. [DOI] [PubMed] [Google Scholar]
- Isakoff SJ, Bendell JC, Cervantes A, Soria JC, Molife LR, Sanabria-Bohorquez SM, et al. Abstract P6-12-02: Phase Ib dose-escalation study of an Akt inhibitor ipatasertib (Ipat) in combination with docetaxel (Doc) or paclitaxel (Pac) in patients (pts) with metastatic breast cancer (MBC) Cancer Res. 2015;75 P6–P12 – 02–P6 – 12–02. [Google Scholar]
- Isakoff SJ, Overmoyer B, Tung NM, Gelman RS, Giranda VL, Bernhard KM, et al. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. J Clin Oncol. 2010;28:15s. suppl; abstr 1019. [Google Scholar]
- Iversen TZ, Andersen MH, Svane IM. The targeting of indoleamine 2,3 dioxygenase -mediated immune escape in cancer. Basic Clin Pharmacol Toxicol. 2015;116:19–24. doi: 10.1111/bcpt.12320. [DOI] [PubMed] [Google Scholar]
- Jaakkola S, Salmikangas P, Nylund S, Partanen J, Armstrong E, Pyrhönen S, et al. Amplification of fgfr4 gene in human breast and gynecological cancers. Int J Cancer. 1993;54:378–382. doi: 10.1002/ijc.2910540305. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Tu D, Zhao N, Shepherd LE, Goss PE. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA. 17 trial: analyses adjusting for treatment crossover. J Clin Oncol. 2012;30:718–721. doi: 10.1200/JCO.2010.34.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Chauhan SK, El Annan J, Annan JEI, Sage PT, Sharpe AH, et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am J Pathol. 2011;178:1922–1929. doi: 10.1016/j.ajpath.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Juric D, Saura C, Cervantes A, Kurkjian C, Patel M, Sachdev J, et al. Abstract PD1-3: Ph1b study of the PI3K inhibitor GDC-0032 in combination with fulvestrant in patients with hormone receptor-positive advanced breast cancer. Cancer Res. 2014;73 PD1–PD3–PD1–PD3. [Google Scholar]
- Juric D, De Bono JS, LoRusso P, Nemunaitis JJ, Heath EI, Kwak EL, et al. First-in-human, phase I, dose-escalation study of selective PI3Kα isoform inhibitor MLN1117 in patients (pts) with advanced solid malignancies. J Clin Oncol. 2015;33(suppl):abstr 2501. doi: 10.1158/1078-0432.CCR-16-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Kawajiri H, Ogawa Y, et al. c-Kit expression as a prognostic molecular marker in patients with basal-like breast cancer. Br J Surg. 2013;100:490–496. doi: 10.1002/bjs.9021. [DOI] [PubMed] [Google Scholar]
- Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J Clin Oncol. 2014;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Patel MR, Infante JR, Iannotti N, Nikolinakos P, Leach P, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or locally advanced solid tumors: assessment of safety and tolerability in a phase I, open-label expansion study. J Clin Oncol. 2015;33(suppl):abstr 3044. [Google Scholar]
- Van Keymeulen A, Lee MY, Ousset M, Brohée S, Rorive S, Giraddi RR, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525:119–123. doi: 10.1038/nature14665. [DOI] [PubMed] [Google Scholar]
- Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kolasa IK, Rembiszewska A, Felisiak A, Ziolkowska-Seta I, Murawska M, Moes J, et al. PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther. 2009;8:21–26. doi: 10.4161/cbt.8.1.7209. [DOI] [PubMed] [Google Scholar]
- Kono SA, Marshall ME, Ware KE, Heasley LE. The fibroblast growth factor receptor signaling pathway as a mediator of intrinsic resistance to EGFR-specific tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat. 2009;12:95–102. doi: 10.1016/j.drup.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S, Reavie L, do Couto JP, De Silva D, Stadler MB, Roloff T, et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature advance on. 2015 doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- Krop I, Johnston S, Mayer IA, Dickler M, Ganju V, Forero-Torres A, et al. Abstract S2-02: The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant vs fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer – Part I results. Cancer Res. 2015;75 S2–02 – S2–02. [Google Scholar]
- Kummar S, Chen A, Parchment RE, Kinders RJ, Ji J, Tomaszewski JE, et al. Advances in using PARP inhibitors to treat cancer. BMC Med. 2012;10:25. doi: 10.1186/1741-7015-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts LE, de Groot DJA, Bense RD, de Vries EGE, Fehrmann RSN. Functional genomic mRNA Profiling of a large cancer data base demonstrates mesothelin overexpression in a broad range of tumor types. Oncotarget. 2015 doi: 10.18632/oncotarget.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Taylor NJ, Attard G, Pacey S, Nathan PD, de Bono JS, et al. Phase I study of nintedanib incorporating dynamic contrast-enhanced magnetic resonance imaging in patients with advanced solid tumors. Oncologist. 2015;20:368–369. doi: 10.1634/theoncologist.2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lee MMC, Groothuis PG, Ubink R, van der Vleuten MAJ, van Achterberg TA, Loosveld EM, et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther. 2015;14:692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SR, Liang WC, Wu Y, Crocker L, Cheng E, Sampath D, et al. An anti-B7-H4 antibody-drug conjugate for the treatment of breast cancer. Mol Pharm. 2015;12:1717–1729. doi: 10.1021/mp5007745. [DOI] [PubMed] [Google Scholar]
- Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Loibl S, Müller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130:477–487. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- López-Knowles E, O’Toole SA, McNeil CM, Millar EKA, Qiu MR, Crea P, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- LoRusso P, Krop I, Miller K, Ma C, Siegel BA, Shields AF, et al. Abstract CT234: A phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015a;75 CT234–CT234. [Google Scholar]
- LoRusso PM, Tolaney SM, Wong S, Parchment RE, Kinders RJ, Wang L, et al. Abstract CT325: Combination of the PARP inhibitor veliparib (ABT888) with irinotecan in patients with triple negative breast cancer: Preliminary activity and signature of response. Cancer Res. 2015b;75 CT325–CT325. [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer. 2010;29:585–590. doi: 10.5732/cjc.009.10673. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzky J, Antonia SJ, Blake-Haskins A, Li X, Robbins PB, Shalabi AM, et al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;32:5s. suppl; abstr 3001. [Google Scholar]
- Madar S, Goldstein I, Rotter V. “Cancer associated fibroblasts”--more than meets the eye. Trends Mol Med. 2013;19:447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- Malhotra B, Schuetze SM. Dermatofibrosarcoma protruberans treatment with platelet-derived growth factor receptor inhibitor: a review of clinical trial results. Curr Opin Oncol. 2012;24:419–424. doi: 10.1097/CCO.0b013e328353d78d. [DOI] [PubMed] [Google Scholar]
- Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18:4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136:331–345. doi: 10.1007/s10549-012-2289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulonis UA, Wulf G, Barry W, Birrer M, Westin S, Spagnoletti T, et al. Abstract CT324: Phase I of oral BKM120 or BYL719 and olaparib for high-grade serous ovarian cancer or triple-negative breast cancer: Final results of the BKM120 plus olaparib cohort. Cancer Res. 2015;75 CT324–CT324. [Google Scholar]
- Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32:1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhan LJ, McCullough AE, Protheroe CA, Dueck AC, Lee JJ, Nunez-Nateras R, et al. Androgen receptor-positive triple negative breast cancer: a unique breast cancer subtype. Ann Surg Oncol. 2014;21:361–367. doi: 10.1245/s10434-013-3260-7. [DOI] [PubMed] [Google Scholar]
- McGray AJR, Hallett R, Bernard D, Swift SL, Zhu Z, Teoderascu F, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22:206–218. doi: 10.1038/mt.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeage MJ, Kotasek D, Markman B, Hidalgo M, Millward M, Jameson MB, et al. A phase 1b study of the anti-cancer stem cell agent demcizumab (DEM), pemetrexed (PEM) & carboplatin (CARBO) in pts with 1st line non-squamous NSCLC. | 2015 ASCO Annual Meeting | Abstracts | Meeting Library. J Clin Oncol 32:5s. 2014;(suppl):abstr 2544. [Google Scholar]
- Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012;2:a006577. doi: 10.1101/cshperspect.a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith WA, LoRusso PM, Cleary JM, Dasari A, Huang B, Shaik NM, et al. 588 A First-in-patient Phase I Study of the Novel Gamma Secretase Inhibitor PF-03084014 in Patients with Advanced Solid Tumor Malignancies. Eur J Cancer. 2012;48:180. [Google Scholar]
- Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, et al. A Phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res. 2015;21:60–67. doi: 10.1158/1078-0432.CCR-14-0607. [DOI] [PubMed] [Google Scholar]
- Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30:1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Pérez-Torres M, Narasanna A, Guix M, Stål O, Pérez-Tenorio G, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward M, Underhill C, Lobb S, McBurnie J, Meech SJ, Gomez-Navarro J, et al. Phase I study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br J Cancer. 2013;108:1998–2004. doi: 10.1038/bjc.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molife LR, Yan L, Vitfell-Rasmussen J, Zernhelt AM, Sullivan DM, Cassier PA, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J Hematol Oncol. 2014;7:1. doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna E, Maisonneuve P, Rotmensz N, Cancello G, Iorfida M, Balduzzi A, et al. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer. 2013;13:31–39. doi: 10.1016/j.clbc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- Murai J, Huang SN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CG, Morris PG. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anticancer Drugs. 2012;23:765–776. doi: 10.1097/CAD.0b013e328352d292. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Abstract S1-09: A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Cancer Res. 2015;75 doi: 10.1200/JCO.2015.64.8931. S1–S09 – S1–S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki H, Zhu Y, Luo L, Zhu G, Chen L. CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood. 2010;115:1941–1948. doi: 10.1182/blood-2008-12-192591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis JJ, Adams N, Bedell C, Klucher K, Taylor J, Whiting SH, et al. Tolerability, humoral immune response, and disease control in phase 1 patients receiving ONT-10, a MUC1 liposomal vaccine. J Clin Oncol. 2014;32:5s. suppl; abstr 3091. [Google Scholar]
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nat Rev Immunol. 2014;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Miyakawa S, Kato J, Mori T, Arai T, Armanini M, et al. Preclinical Efficacy and Safety Assessment of an Antibody Drug Conjugate Targeting the c-RET Proto-oncogene for Breast Carcinoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0468. [DOI] [PubMed] [Google Scholar]
- Nielsen DL, Andersson M, Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35:121–136. doi: 10.1016/j.ctrv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- Van der Noll R, Marchetti S, Steeghs N, Beijnen JH, Mergui-Roelvink MWJ, Harms E, et al. Long-term safety and anti-tumour activity of olaparib monotherapy after combination with carboplatin and paclitaxel in patients with advanced breast, ovarian or fallopian tube cancer. Br J Cancer. 2015;113:396–402. doi: 10.1038/bjc.2015.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowsheen S, Cooper T, Stanley JA, Yang ES. Synthetic lethal interactions between EGFR and PARP inhibition in human triple negative breast cancer cells. PLoS One. 2012;7:e46614. doi: 10.1371/journal.pone.0046614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy J, Campone M, Brain E, Neven P, Hayes DF, Bondarenko I, et al. Randomized phase 2 study of abiraterone acetate (AA) with or without exemestane (E) in postmenopausal patients (pts) with estrogen receptor–positive (ER+) metastatic breast cancer (MBC) J Clin Oncol. 2014;32:5s. doi: 10.1093/annonc/mdv487. suppl; abstr 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshio Y, Teramoto K, Hanaoka J, Tezuka N, Itoh Y, Asai T, et al. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci. 2015;106:134–142. doi: 10.1111/cas.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- Overmoyer B, Sanz-Altimira P, Partridge AH, Extermann M, Liu J, Winer E, et al. Abstract P1-13-04: Enobosarm for the treatment of metastatic, estrogen and androgen receptor positive, breast cancer. Final results of the primary endpoint and current progression free survival. Cancer Res. 2015;75 P1–P13 – 04–P1 –13–04. [Google Scholar]
- Page DB, Naidoo J, McArthur HL. Emerging immunotherapy strategies in breast cancer. Immunotherapy. 2014;6:195–209. doi: 10.2217/imt.13.166. [DOI] [PubMed] [Google Scholar]
- Pahuja S, Beumer JH, Appleman LJ, Tawbi HA, Stoller RG, Lee JJ, et al. A phase I study of veliparib (ABT-888) in combination with weekly carboplatin and paclitaxel in advanced solid malignancies and enriched for triple-negative breast cancer (TNBC) J Clin Oncol. 2015;33(suppl):abstr 1015. [Google Scholar]
- Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- Papadopoulos KP, Tabernero J, Markman B, Patnaik A, Tolcher AW, Baselga J, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2445–2456. doi: 10.1158/1078-0432.CCR-13-2403. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Koo JS, Kim MS, Park HS, Lee JS, Kim SI, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22:1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- Paulsson J, Sjöblom T, Micke P, Pontén F, Landberg G, Heldin CH, et al. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol. 2009;175:334–341. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penault-Llorca F, Bertucci F, Adélaïde J, Parc P, Coulier F, Jacquemier J, et al. Expression of FGF and FGF receptor genes in human breast cancer. Int J Cancer. 1995;61:170–176. doi: 10.1002/ijc.2910610205. [DOI] [PubMed] [Google Scholar]
- Pestano LA, Christian B, Koppenol S, Millard J, Christianson G, Klucher K, et al. Abstract 762: ONT-10, a liposomal vaccine targeting hypoglycosylated MUC1, induces a potent cellular and humoral response and suppresses the growth of MUC1 expressing tumors. Cancer Res. 2014;71:762–762. [Google Scholar]
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, et al. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci Signal. 2014;7:ra112. doi: 10.1126/scisignal.2005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.59.4358. JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuri B. BRCA1- and BRCA2-related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;24(Suppl 8):viii22–viii27. doi: 10.1093/annonc/mdt307. [DOI] [PubMed] [Google Scholar]
- Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhalla S, Han HS, Dieras V, Friedlander M, Somlo G, Arun B, et al. Phase 3 randomized, placebo-controlled trial of carboplatin (C) and paclitaxel (P) with/without veliparib (ABT-888) in HER2-BRCA-associated locally advanced or metastatic breast cancer (BC) ASCO Meet Abstr. 2015;33:TPS1102. [Google Scholar]
- Puhalla S, Beumer JH, Pahuja S, Appleman LJ, Tawbi HA, Stoller RG, et al. Final results of a phase 1 study of single-agent veliparib (V) in patients (pts) with either BRCA1/2-mutated cancer (BRCA+), platinum-refractory ovarian, or basal-like breast cancer (BRCA-wt) J Clin Oncol. 2014;32:5s. suppl; abstr 2570. [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela-Fandino M, Urruticoechea A, Guerra J, Gil M, Gonzalez-Martin A, Marquez R, et al. Phase I clinical trial of nintedanib plus paclitaxel in early HER-2-negative breast cancer (CNIO-BR-01-2010/GEICAM-2010-10 study) Br J Cancer. 2014;111:1060–1064. doi: 10.1038/bjc.2014.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghav KP, Wang W, Liu S, Chavez-MacGregor M, Meng X, Hortobagyi GN, et al. cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res. 2012;18:2269–2277. doi: 10.1158/1078-0432.CCR-11-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- Rampurwala MM, Burkard ME, Wisinski KB, Chimeh SE, Zeal J, Koehn T, et al. Phase 1b study of orteronel in postmenopausal women with hormone-receptor positive (HR+) metastatic breast cancer. J Clin Oncol. 2014;32:5s. doi: 10.1007/s10637-016-0403-2. suppl; abstr 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC. Synstatin: a selective inhibitor of the syndecan-1-coupled IGF1R-αvβ3 integrin complex in tumorigenesis and angiogenesis. FEBS J. 2013;280:2207–2215. doi: 10.1111/febs.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JL, Kendrick H, Magnay FA, Vafaizadeh V, Groner B, Smalley MJ. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene. 2012;31:869–883. doi: 10.1038/onc.2011.289. [DOI] [PubMed] [Google Scholar]
- Ren G, Esposito M, Kang Y. Bone metastasis and the metastatic niche. J Mol Med (Berl) 2015 doi: 10.1007/s00109-015-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor Microenvironment of Metastasis in Human Breast Carcinoma: A Potential Prognostic Marker Linked to Hematogenous Dissemination. Clin Cancer Res. 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med. 2011;17:1646–1651. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan TE, Xue X, Lin H-M, D’Alfonso TM, Ginter PS, Oktay MH, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011;124:2120–2131. doi: 10.1242/jcs.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, et al. Regulation of Androgen Action. Vitam Horm. 1998;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, et al. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo HS, Tredan O, Ro J, Morales S, Musolino A, Afonso N, et al. Abstract PD5-1: Results from the phase 2 trial of ridaforolimus, dalotuzumab, and exemestane compared to ridaforolimus and exemestane in advanced breast cancer. Cancer Res. 2015;75 doi: 10.1007/s10549-017-4375-5. PD5–1 – PD5–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Br J Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Wenham RM, Wilding G, McFadden M, Sun L, Toniatti C, et al. First-in-human trial of a poly(ADP-ribose) polymerase (PARP) inhibitor MK-4827 in advanced cancer patients (pts) with antitumor activity in BRCA-deficient and sporadic ovarian cancers. J Clin Oncol. 2010;28:15s. suppl; abstr 3001. [Google Scholar]
- Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon I, Blanc V. Antibody-drug conjugate (ADC) clinical pipeline: a review. Methods Mol Biol. 2013;1045:1–27. doi: 10.1007/978-1-62703-541-5_1. [DOI] [PubMed] [Google Scholar]
- Saura C, Sachdev J, Patel MR, Cervantes A, Juric D, Infante JR, et al. Abstract PD5-2: Ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. Cancer Res. 2015;75 PD5–2 – PD5–2. [Google Scholar]
- Scales SJ, Gupta N, Pacheco G, Firestein R, French DM, Chuh J, et al. Abstract 4494: A clinical candidate anti-mesothelin-MMAE antibody-drug conjugate (ADC) for therapy of mesothelin-expressing cancers. Cancer Res. 2014;74:4494–4494. [Google Scholar]
- Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol. 2014;32:5s. suppl; abstr 3002. [Google Scholar]
- Seiler M, Ray-Coquard I, Melichar B, Yardley DA, Wang RX, Dodion PF, et al. Oral ridaforolimus plus trastuzumab for patients with HER2+ trastuzumab-refractory metastatic breast cancer. Clin Breast Cancer. 2015;15:60–65. doi: 10.1016/j.clbc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Cassier P, Varga A, Tabernero J, Schellens JH, Delord J-P, et al. Abstract CT326: Phase I study of BGJ398, a selective pan-FGFR inhibitor in genetically preselected advanced solid tumors. Cancer Res. 2014;74 CT326–CT326. [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PD, Moynahan ME, Modi S, Hamilton N, Caravella BA, Zamora S, et al. Abstract CT330: Phase I study of PI3Kα inhibitor BYL719 + aromatase inhibitor (AI) in patients (pts) with hormone receptor-positive (HR+) metastatic breast cancer (MBC) Cancer Res. 2015;75 CT330–CT330. [Google Scholar]
- Shah PD, Chandarlapaty S, Dickler MN, Ulaner G, Zamora SJ, Sterlin V, et al. Phase I study of LJM716, BYL719, and trastuzumab in patients (pts) with HER2-amplified (HER2+) metastatic breast cancer (MBC) J Clin Oncol. 2015;33(suppl):abstr 590. [Google Scholar]
- Shah PD, Modi S, Datko FM, Moynahan ME, Zamora S, D’Andrea G, et al. Phase I trial of daily PI3Kα inhibitor BYL719 plus letrozole (L) or exemestane (E) for patients (pts) with hormone receptor-positive (HR+) metastatic breast cancer (MBC) J Clin Oncol. 2014;32:5s. suppl; abstr 2605. [Google Scholar]
- Shapiro G, Kristeleit R, Middleton M, Burris H, Molife LR, Evans J, et al. Abstract A218: Pharmacokinetics of orally administered rucaparib in patients with advanced solid tumors. Mol Cancer Ther. 2014;12:A218–A218. [Google Scholar]
- Sharkey RM, McBride WJ, Cardillo TM, Govindan SV, Wang Y, Rossi EA, et al. Enhanced Delivery of SN-38 to Human Tumor Xenografts with an Anti-Trop-2-SN-38 Antibody Conjugate (Sacituzumab Govitecan) Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0670. [DOI] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science (80- ) 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–5015. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3:S21–S35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–1898. doi: 10.1038/bjc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi B, Rastogi S, Dawood S. Buparlisib in breast cancer. Future Oncol. 2015;11:1463–1470. doi: 10.2217/fon.15.56. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Sledge GW. Anti-vascular endothelial growth factor therapy in breast cancer: game over? J Clin Oncol. 2015;33:133–135. doi: 10.1200/JCO.2014.58.1298. [DOI] [PubMed] [Google Scholar]
- Smyth EC, Turner NC, Peckitt C, Pearson A, Brown G, Chua S, et al. Phase II multicenter proof of concept study of AZD4547 in FGFR amplified tumours. J Clin Oncol. 2015;33(suppl):abstr 2508. [Google Scholar]
- Soliman HH, Jackson E, Neuger T, Dees CE, Harvey DR, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–8146. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman HH, Minton SE, Ismail-Khan R, Han HS, Vahanian NN, Link CJ, et al. Abstract P2-15-04: A phase 1/2 study of Ad.p53 DC vaccine with indoximod immunotherapy in metastatic breast cancer. Cancer Res. 2015;75 P2–P15 – 04–P2 – 15–04. [Google Scholar]
- Somlo G, Frankel PH, Luu TH, Ma C, Arun B, Garcia A, et al. Phase II trial of single agent PARP inhibitor ABT-888 (veliparib [vel]) followed by postprogression therapy of vel with carboplatin (carb) in patients (pts) with stage BRCA-associated metastatic breast cancer (MBC): California Cancer Consortium trial PHII. J Clin Oncol. 2014;32:5s. suppl; abstr 1021. [Google Scholar]
- Sonnenblick A, de Azambuja E, Azim HA, Piccart M. An update on PARP inhibitors-moving to the adjuvant setting. Nat Rev Clin Oncol. 2014;12:27–41. doi: 10.1038/nrclinonc.2014.163. [DOI] [PubMed] [Google Scholar]
- Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dienstmann R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25:2244–2251. doi: 10.1093/annonc/mdu390. [DOI] [PubMed] [Google Scholar]
- Soria JC, LoRusso P, Bahleda R, Lager J, Liu L, Jiang J, et al. Phase I dose-escalation study of pilaralisib (SAR245408, XL147), a pan-class I PI3K inhibitor, in combination with erlotinib in patients with solid tumors. Oncologist. 2015;20:245–246. doi: 10.1634/theoncologist.2014-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Srabovic N, Mujagic Z, Mujanovic-Mustedanagic J, Softic A, Muminovic Z, Rifatbegovic A, et al. Vascular endothelial growth factor receptor-1 expression in breast cancer and its correlation to vascular endothelial growth factor a. Int J Breast Cancer. 2013;2013:746749. doi: 10.1155/2013/746749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacchiotti S, Longhi A, Ferraresi V, Grignani G, Comandone A, Stupp R, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. 2012;30:914–920. doi: 10.1200/JCO.2011.35.3656. [DOI] [PubMed] [Google Scholar]
- Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5:169–181. doi: 10.1177/1758834012475152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6:a021857. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starodub AN, Ocean A, Shah MA, Guarino MJ, Picozzi VJ, Vahdat LT, et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C, et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4:1154–1167. doi: 10.1158/2159-8290.CD-13-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Sun Y, De P, Carlson JH, Friedman L, Dey N, Leyland-Jones B. Abstract 4717: In vitro potency of dual PI3K/mTORC1/C2 inhibitor, GDC-0980 in ER+ and HER2 overexpressing breast cancer cells. Cancer Res. 2015;75:4717–4717. [Google Scholar]
- Sussman D, Smith LM, Anderson ME, Duniho S, Hunter JH, Kostner H, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13:2991–3000. doi: 10.1158/1535-7163.MCT-13-0896. [DOI] [PubMed] [Google Scholar]
- Sutton LM, Cao D, Sarode V, Molberg KH, Torgbe K, Haley B, et al. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinoma. Am J Clin Pathol. 2012;138:511–516. doi: 10.1309/AJCP8AVF8FDPTZLH. [DOI] [PubMed] [Google Scholar]
- Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton AJ, Diez-Gonzalez L, Ace O, Vera-Badillo F, Seruga B, Jordán J, et al. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: a meta-analysis. Cancer Treat Rev. 2014;40:1048–1055. doi: 10.1016/j.ctrv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Tenhagen M, van Diest PJ, Ivanova IA, van der Wall E, van der Groep P. Fibroblast growth factor receptors in breast cancer: expression, downstream effects, and possible drug targets. Endocr Relat Cancer. 2012;19:R115–R129. doi: 10.1530/ERC-12-0060. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LJ, He LZ, Wasiuk A, Gergel LE, Boyer JM, Round SM, et al. Abstract 253: Synergistic antitumor activity of PD-1 signaling blockade and CD27 costimulation correlates with enhanced ratio of effector to regulatory T cells at the tumor site. Cancer Res. 2015;75:253–253. [Google Scholar]
- Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- Tolaney S, Burris H, Gartner E, Mayer IA, Saura C, Maurer M, et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;149:151–161. doi: 10.1007/s10549-014-3248-4. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, Chugh R, Chambers G, Thorpe V, Dupont J, Hill D, et al. A first-in-human phase I study to evaluate the fully human monoclonal antibody OMP-59R5 (anti-Notch2/3) administered intravenously to patients with advanced solid tumors. J Clin Oncol. 2012;30(suppl):abstr 3025. [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina TA, Miller K, Yardley DA, O’Shaughnessy J, Cortes J, Awada A, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) J Clin Oncol. 2015;33(suppl):abstr 1003. [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Turner N, Lambros MB, Horlings HM, Pearson A, Sharpe R, Natrajan R, et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29:2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet (London, England) 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- Vaklavas C, Forero A. Management of metastatic breast cancer with second-generation antibody-drug conjugates: focus on glembatumumab vedotin (CDX-011, CR011-vcMMAE) BioDrugs. 2014;28:253–263. doi: 10.1007/s40259-014-0085-2. [DOI] [PubMed] [Google Scholar]
- Velasco-Velázquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44:573–577. doi: 10.1016/j.biocel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:djt319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- Voduc KD, Cheang MCU, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AJ, Bendell JC, Dolly S, Morgan JA, Ware JA, Fredrickson J, et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. ASCO Meet Abstr. 2011;29:3020. [Google Scholar]
- Wainberg ZA, de Bono JS, Mina L, Sachdev J, Byers LA, Chugh R, et al. Abstract C295: Update on first-in-man trial of novel oral PARP inhibitor BMN 673 in patients with solid tumors. Mol Cancer Ther. 2014;12:C295–C295. [Google Scholar]
- Wang Y, Cairo S, Deas O, Hartman AR, Jones J, Gutin A, et al. Abstract P5-06-04: The PARP inhibitor niraparib demonstrated activity in patient-derived triple-negative breast cancer xenograft models with high homologous recombination deficiency (HRD) score. Cancer Res. 2015;75 P5–P06 – 04–P5 – 06–04. [Google Scholar]
- Weil MK, Chen AP. PARP Inhibitor Treatment in Ovarian and Breast Cancer. Curr Probl Cancer. 2011;35:7–50. doi: 10.1016/j.currproblcancer.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213. doi: 10.1042/BJ20101603. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol Res. 2015;3:326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Draper T, Moorthy G, Salkeni M, Thomas H, Mercer C, Kozma S, et al. P1.14 * A Dose Escalation Single Arm Phase Ib Combination Study of BEZ235 with Everolimus in Patients with Advanced Solid Malignancies. Ann Oncol. 2015;26:ii19–ii19. [Google Scholar]
- Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SCC, Lo SFE, Lee KC, Yam JWP, Chan JKC, Wendy Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yang Y, Yee D. Targeting insulin and insulin-like growth factor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2012;17:251–261. doi: 10.1007/s10911-012-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, et al. EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol. 2015;33:1609–1619. doi: 10.1200/JCO.2014.56.2959. [DOI] [PubMed] [Google Scholar]
- Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015;21:2084–2095. doi: 10.1158/1078-0432.CCR-14-2808. [DOI] [PubMed] [Google Scholar]
- Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XHF, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]