Abstract
The pursuit for more sensitive NMR probes culminated with development of the cryogenic cooled NMR probe. A key factor for the sensitivity is the overall resistance of RF circuitry and sample. Lowering the coil temperature to ~25 K and the use of superconducting coil material has greatly reduced the resistance contribution of the hardware. However, the resistance of a salty sample remains the same and evolves as the major factor determining the signal-to-noise ratio. Several approaches have been proposed to reduce the resistance contribution of the sample. These range from encapsulating proteins in a water cavity formed by reverse micelles in low viscosity fluids to the optimal selection of low mobility, low conductivity buffer ions. Here we demonstrate that changing the sample diameter has a pronounced effect on the sample resistance and this results in dramatic improvements of the signal-to-noise ratio and shorter π/2 pulses. We determined these parameters for common 5 mm NMR tubes under different experimental conditions and compared them to the 2, 3 and 4 mm tubes, in addition, 5 mm Shigemi tubes were included since these are widely used. We demonstrate benefits and applicability of studying NMR samples with up to 4 M salt concentrations in cryogenic probes. Under high salt conditions, best results in terms of short π/2 pulses and high signal-to-noise ratios are obtained using 2 or 3 mm NMR tubes, especially when limited sample is available. The 4 mm tube is preferred when sample amounts are abundant at intermediate salt conditions.
Keywords: Cryogenic probe; NMR spectroscopy; High salt; Biological samples; 2, 3, 4 mm NMR tube
1. Introduction
The introduction of cryogenic probes has dramatically increased the inherent sensitivity of nuclear magnet resonance experiments [1–5]. Typically, a sensitivity gain by a factor of three to four relative to conventional room temperature probeheads [6–9] can be achieved in low conductivity organic solvents. This gain in performance is reduced for aqueous samples due to the increased conductivity and is further degraded in the presence of moderate amounts of salt [10,11]. Consequently, measurements of biomolecules in aqueous solutions are inherently compromised and are generally preferred to be studied under low salt conditions (<50–100 mM). Nature however is not limited to these “ideal” conditions and high salt environments are not uncommon. Elevated ionic conditions are often required for solubility and to prevent aggregation. Additionally, conformational transitions in nucleic acids and proteins, or folding studies, often require high ionic strength environments. Titration experiments are another example where a ligand is observed at the beginning of a titration, demanding high sensitivity, and the substrate might require a high salt concentration for solubility.
The requirement of high salt concentrations in biomolecular NMR is often not the only limitation; sample availability and limited solubility are other important considerations. This necessitates the use of methods providing the highest sensitivity offered by cryogenic probes. Unfortunately, the performance gain these probes can offer is negated by high salt concentrations, occasionally to the point where the experiment is not feasible anymore.
Wand has proposed using reversed micelles in organic solvents, where the biomolecule is encapsulated in a water cavity [12–17]. This lowers the overall dielectric contribution of the sample, which is beneficial for cryoprobe applications and addresses the slow tumbling of larger molecules [10,18–20]. The method has been successfully demonstrated but sample preparation remains challenging and its generalization is still under investigation [21].
In another approach, Dötsch et al. investigated the effect of different electrolytes on probe performance [11]. The conductivity of a sample is not only impacted by the concentration and type of buffer, but also by its ion mobility. Comparing a variety of popular buffers, substantial differences in the sample resistance were observed which result in sensitivity differences by up to a factor of 4. Nevertheless, high conductivity/mobility buffers are often unavoidable and in many cases the choice of buffer is dictated by the behavior of the biomolecule.
It has been recognized for some time that the solubility of proteins can often be maintained and improved in the presence of dipolar ions such as glycine or argenine [22,23]. Arumugam et al. [24] prepared NMR samples of lysozyme and glucose containing either 0.77 M d5-glycine or up to 0.2 M NaCl. As expected, NaCl containing samples exhibited much longer pulse widths and reduced signal to noise ratios compared to the same samples in presence of high amounts of glycine.
NMR vendors have introduced a rectangular sample tube design taking advantage of an optimized electric field (E-field) distribution [25] inside the sample [26–28]. A sensitivity gain of 20–35% has been demonstrated for moderately salty solutions. However, specialized hardware such as probes and tubes are required which may not be available.
With respect to the sample distribution in the probe, alternative distributions are possible. Here we demonstrate the effect of the sample radius on probe sensitivity under high salt conditions. We show that 3 or 4 mm NMR tubes have substantial advantages over a 5 mm tube when salty samples are measured. The susceptibility matched NMR tubes (Shigemi tubes) have also been included in this study since they are widely used and require typically only half the sample volume compared to a regular 5 mm tube. A systematic investigation on a protein sample in 0.5 M NaCl is presented, exploring two commonly occurring situations. In the first case, the sample is soluble in abundance, leading to a comparison between the different NMR tube arrangements at the same sample concentrations. The second case represents the often encountered situation where the amount of sample is limited. Therefore we compared different tube arrangements at the same, low amount of sample, e.g. varying concentration.
In addition, this NMR tube arrangement allows the use of samples with up to 4 M NaCl in the cryogenic probe while still obtaining a sensitivity advantage over the conventional room temperature probes. We also show salt effects on spin systems, which are only visible under these extreme high concentrations and now are observable even on cryogenic probes.
2. Results and discussion
2.1. Factors affecting the signal-to-noise ratio
The two main factors that impact the signal-to-noise ratio (S/N) are (1) the overall resistance/geometry of the coil and (2) the environment of the sample [29–31]. The latter is generally the only component under user control. For a cryogenically cooled probe the coil and sample temperature differ drastically which results in adjusted quality factor and temperature. Following the analysis given by Kelly et al. [11] we obtain:
| (1) |
where T denotes temperatures for RF-coil (C), preamplifier (A) and sample (S), while R stands for resistance of coil (C) and sample (S) respectively. In cryogenic probes the term RC(TC + TA) has been reduced by lowering the RF-coil temperature, use of low resistant coil material and cooling the preamplifier, directly affecting the S/N ratio. In the remaining term, the sample temperature is dictated by the measuring condition which leaves the sample resistance as a parameter to be optimized:
| (2) |
where ω is the angular frequency, r represents the conductivity, given by Σciqiλi, where c is the concentration, q the charge, and λ is the mobility of the different ionic species in the sample, and rS is the sample radius. As was shown previously, choosing buffers with low conductivity and low mobility is preferred [11,24].
This work addresses the other variable in Eq. (2), where a change in the sample radius is expected to have a major impact on the sample resistance and hence the S/N in high salt solutions.
2.2. NMR spectroscopy in high salt solution using cryogenic probes
2.2.1. Probe tuning
The salt tolerance is dependent on probe design and differs somewhat between probes. For our cryogenic probe 1H{13C,15N} we observe that for standard 5 mm sample tubes and from 1 to 4 M NaCl solutions, the probe could not be tuned and matched. By using micro tubes the total amount of salt in the probe is reduced while maintaining high ionic strength conditions. Small diameter NMR tubes were shown previously to enable high salt experiments on biomolecules utilizing regular probes [32]. With a 3 mm tube, we were able to obtain tuning/matching characteristics for 0.5–4.5 M NaCl sample that are identical to samples that did not contain any salt. Even higher salt concentrations, approaching the solubility limit, could readily be tuned/matched for 2 mm tubes.
2.2.2. 90 degree pulse
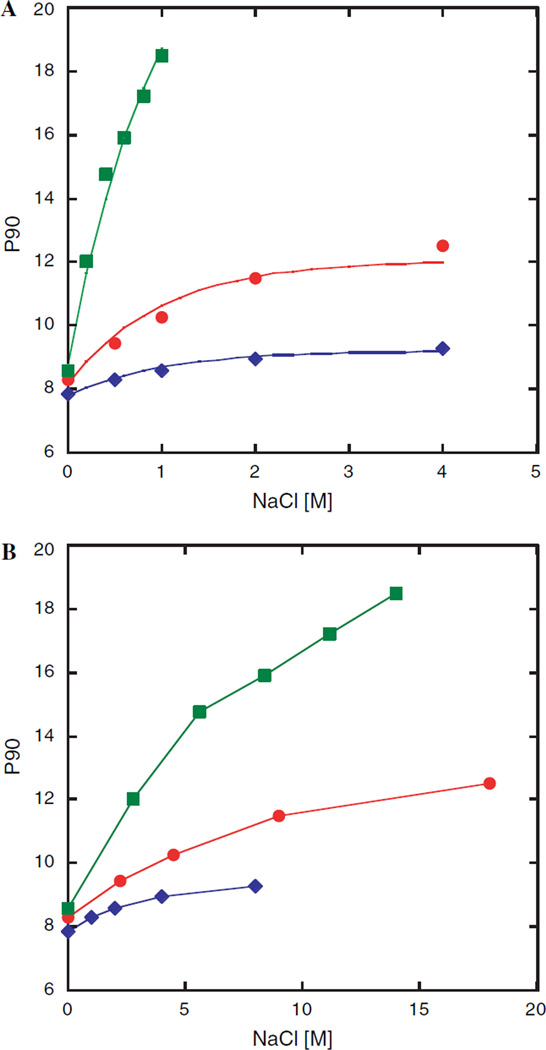
The dependence of the π/2 pulse on very salty sucrose samples with sample diameters of 2 and 3 mm versus a regular 5 mm tube is shown in Fig. 1A. For samples in 5 mm tubes the π/2 pulse rose very rapidly, more than doubling before reaching salt concentrations of 1 M; beyond which the probe could not be tuned. This severely degrades the performance of most experiments, especially those containing spin lock and decoupling elements. As anticipated, the increase of the pulse length is much less dramatic for smaller tube diameters. In the case of the 3 mm tube the pulse length increases from 8.5 µs at 0 M NaCl to 12.5 µs at 4 M NaCl, representing an increase of only 51%. An even more modest increase of only 18% is observed for the 2 mm sample tube under the same conditions. Interestingly, an initial rapid increase in pulse length is seen for 0–0.5 M NaCl while much smaller increases are observed from 1–2 to 2–4 M, respectively. Therefore the high salt behavior cannot be linearly extrapolated from data at 0–1 M NaCl. These results clearly demonstrate that it is not the salt concentration alone that determines the pulse length and signal-to-noise degradation. If the same data is plotted (Fig. 1B) to reflect the amount of salt present in the active volume, the curves are closer together. However, the larger diameter tubes still exhibit a markedly poorer performance demonstrating that the location of the salt with respect to the receiver coil is dominating. This arises from the dependence of Rs on the tube diameter in Eq. (2) and provides a rationale for the experimental observation that in very low ionic strength solutions the tube diameter has only a small effect on the signal-to-noise ratio while under very high salt conditions the size of the NMR tube is absolutely crucial.
Fig. 1.
(A) π/2 pulse length of a 10 mM sucrose test samples containing NaCl concentrations varying between 0 and 4 M. Data was recorded at 295 K using a Bruker Avance 500 for 2 [ ], 3 [
], 3 [ ] and 5 mm [
] and 5 mm [ ] NMR tubes. The solid lines correspond to the empirically determined function: P90 = P90(no salt) + 0.434d2.5(1 − e−[NaCl]), where d is the inner diameter (ID) in mm and [NaCl] is the molar salt concentration. (ID’s were 1.6, 2.4 and 4.2 mm for 2, 3 and 5 mm NMR tubes.) The 5 mm data were not extrapolated past 1 M because the probe head could not be properly tuned/matched. (B) Pulse length dependence on amount of NaCl in the active volume. The amount of salt is represented as sample tube area × salt concentration.
] NMR tubes. The solid lines correspond to the empirically determined function: P90 = P90(no salt) + 0.434d2.5(1 − e−[NaCl]), where d is the inner diameter (ID) in mm and [NaCl] is the molar salt concentration. (ID’s were 1.6, 2.4 and 4.2 mm for 2, 3 and 5 mm NMR tubes.) The 5 mm data were not extrapolated past 1 M because the probe head could not be properly tuned/matched. (B) Pulse length dependence on amount of NaCl in the active volume. The amount of salt is represented as sample tube area × salt concentration.
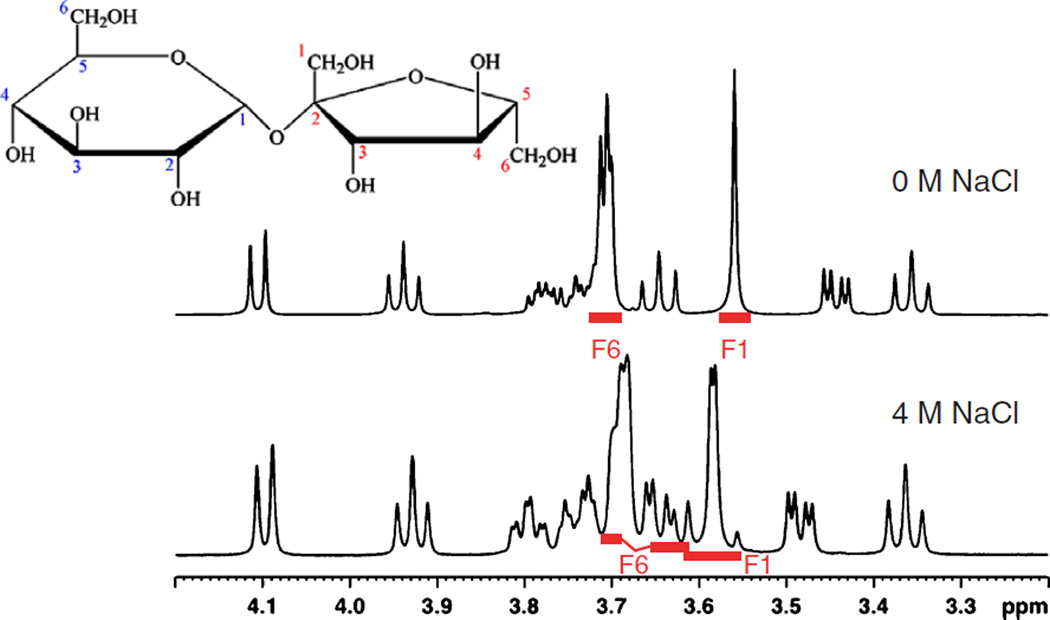
2.2.3. High salt effects observed on sucrose
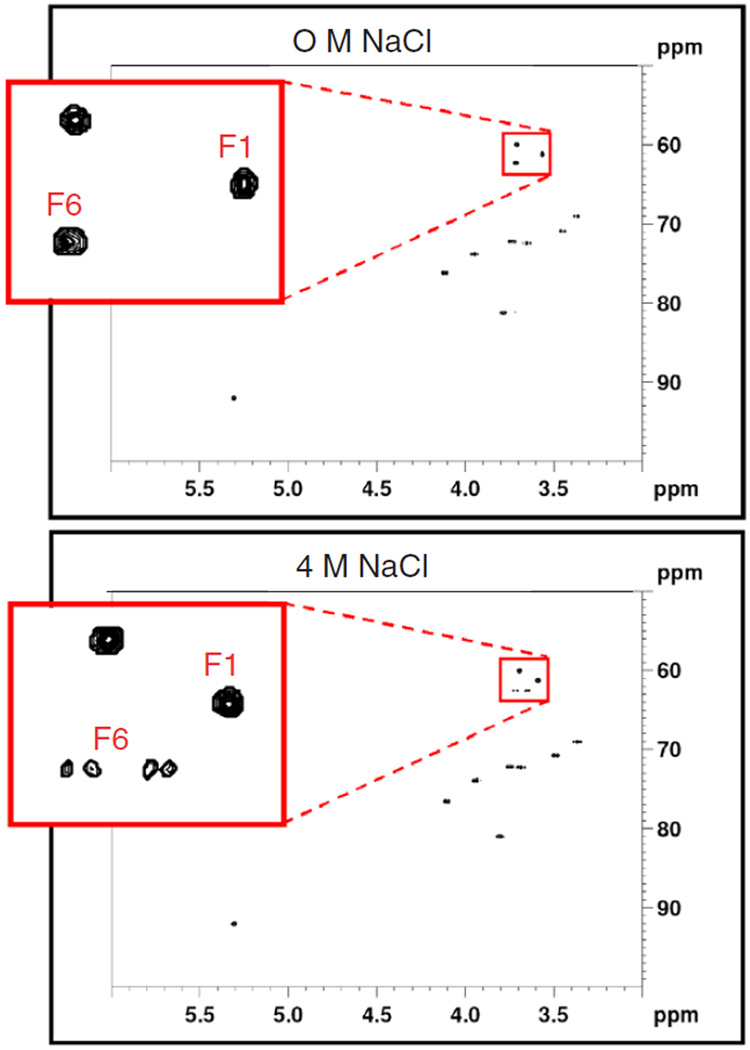
The 1D 1H spectra of 10 mM sucrose samples in either 0 or 4 M NaCl, respectively, are shown in Fig. 2. They are quite similar except for a distinct difference observed in the region between 3.6 and 3.7 ppm. This change is presumably due to the increased electrostatic screening in 4 M NaCl solutions that result in a structural change of the disaccharide. Ultimately however, we are interested in biomolecular applications of high salt NMR. A key measurement is the 1H–13C heteronuclear single quantum correlation experiment (HSQC) [33–37], which employs a large number of π/2 and π pulses on the 1H and 13C channels, particularly for the sensitivity enhanced echo/antiecho version [38]. In addition, heteronuclear decoupling is used during acquisition. These features are expected to have a negative impact on high salt solution spectra. The HSQC spectra of sucrose under different salt conditions are shown in Fig. 3. It is evident that HSQC data high salt solutions can be acquired. Interestingly, at 4 M NaCl, the fructose methylene group at 3.58 ppm (F1) exhibits an obvious AB spin pattern at 500 MHz (Fig. 3), while F6 is an AX spin system in high salt solutions. High sensitivity and high salt solution data are not mutually exclusive. Moreover, new effects may be manifested by these solution conditions.
Fig. 2.
NMR spectra of 10 mM sucrose, 100% D2O in 3 mm tubes. Top trace 0 M NaCl, bottom trace 4 M NaCl 1H–13C HSQC with water presaturation.
Fig. 3.
10 mM sucrose, 100% D2O, 295 K in 3 mm tubes. Top: 0 M NaCl, bottom 4 M NaCl. Experiment time: 33 min.
2.2.4. Signal-to-noise comparisons for a sucrose test sample
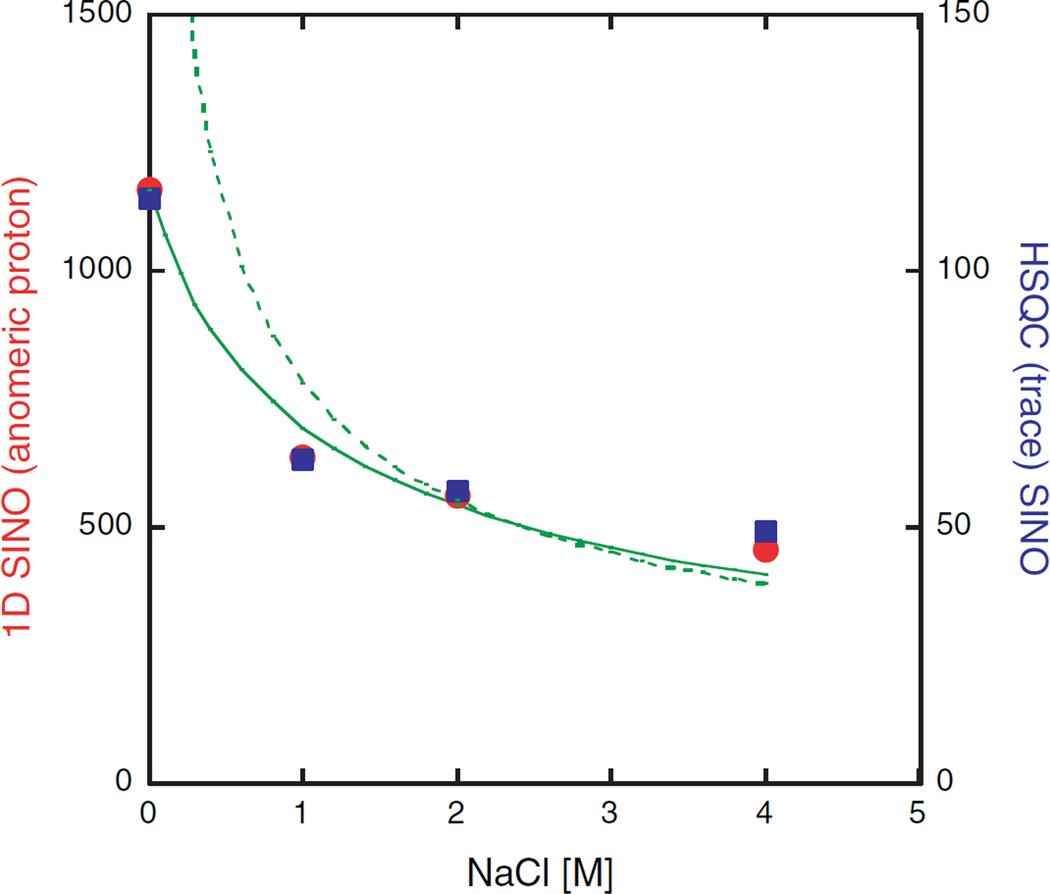
The signal-to-noise behavior for the sucrose test sample in a 3 mm NMR tube was investigated in more detail. Fig. 4 summarizes the comparative results of the S/N determination of a 1D 1H proton spectrum and a 1D trace of 1H/13C HSQC spectrum recorded at different salt concentrations. In both cases the S/N dependence on the salt concentration is similar. The drop in S/N is most pronounced between 0 and 1 M NaCl, while further increases in the salt concentration up to 4 M NaCl resulted in only a modest drop. In the case of the 1H measurement the signal-to-noise ratio was reduced by a factor of 2.55 going from 0 to 4 M NaCl solutions. A similar result was also obtained from the HSQC experiments where a reduction by a factor of 2.35 was observed, despite the larger number of pulses applied and the use of 13C decoupling. As anticipated, the 13C pulses are virtually unaffected by the salt concentration. The fact that we observe essentially the same reduction in S/N for both experiments indicates that at the end of the HSQC sequence the same amount of transverse magnetization is created; regardless of the salt concentration. This is supported by the observation that on our cryoprobe for 3 mm NMR tubes and 4 M NaCl the intensity of the 450° pulse is greater than 97% of the 90° 1H pulse.
Fig. 4.
Signal-to-noise data of 10 mM sucrose, 3 mm NMR tube as a function of the NaCl concentration. S/N analysis of 1D 1H spectra (left ordinate, ●) and HSQC trace (right ordinate, ■) for the anomeric proton (0.5 ppm noise region). The solid line depicts the predicted salt dependence for the 1D data based on Eq. (1). where [NaCl] is the salt concentration [M] and f is a proportionality constant. The sample resistance is expressed as cr4[NaCl]. c incorporates several constants and is the sample radius [mm]. The following values were obtained for the radical: . If no salt is present the S/N is primarily determined by the second term in Eq. (1), while at 2 M NaCl it is assumed that the first term dominates, therefore the S/N curve may be approximated as k*[NaCl]−0.5 where k = 792, for 3 mm tubes (dotted line). This fit provides a simple estimation of the S/N dependency at high salt concentrations. In either case, the conductivity is assumed to be proportional to the salt concentration which for high salt solutions is no longer accurate.
It is instructive to compare the observed salt dependence of the S/N ratio to the predicted behavior (Fig. 4). As a first approximation only the sample contribution in Eq. (1) is considered. This approximation, although reasonable at high salt concentration, fails as expected at low salt. A more realistic treatment includes both probe and sample contributions; this approach produces an acceptable agreement over the entire salt range shown in Fig. 4. Additionally, HSQC spectra for 3 and 1 mM sucrose samples, all at 4 M NaCl, have been recorded and represent more realistic sample concentrations. The measured signal-to-noise ratios correspond well with the expected values for each concentration even at very high salt concentrations (data not shown).
For cryogenic probes and aqueous solvents in absence of salt the sensitivity gains are typically in the range of 2.0–3.2 relative to a conventional probe. Our data obtained for 3 mm tubes shows that we incur a sensitivity loss of a factor of 2.35–2.55 compared to the same sample containing no salt. Therefore, by using 3 mm tubes our cryogenic probe exhibits, even at 4 M NaCl, comparable sensitivity to a conventional probe for a sample without salt.
Based on the assumptions inherent in the fit of the S/N data shown in Fig. 4 rough guidelines can be obtained for tube diameter selection and constant concentration samples. For this we define that when the S/N advantage offered by the larger diameter tubes drops to 20–25% of the value in absence of NaCl a smaller tube would be advantageous. At 500 MHZ a switch from 5 to 4 mm tubes should be considered at >0.2 M NaCl, from 4 to 3 mm at >0.6 M and from 3 to 2 mm at salt concentrations >2.2 M. This roughly corresponds to the salt concentrations where the 90° pulse is 40% longer than in absence of salt, providing a handy rule of thumb. It should be noted that the limits are dependent on the NMR experiment, the level of S/N loss one is willing to tolerate and the field strength. The performance degradation is more severe for higher fields (Eq. (2)), lowering the limits for switching to smaller tubes. Clearly, if the sample amount is limiting and the sample is soluble at higher concentration smaller tubes are to be preferred at elevated salt concentrations.
2.3. Systematic comparison of signal-to-noise data on a protein sample
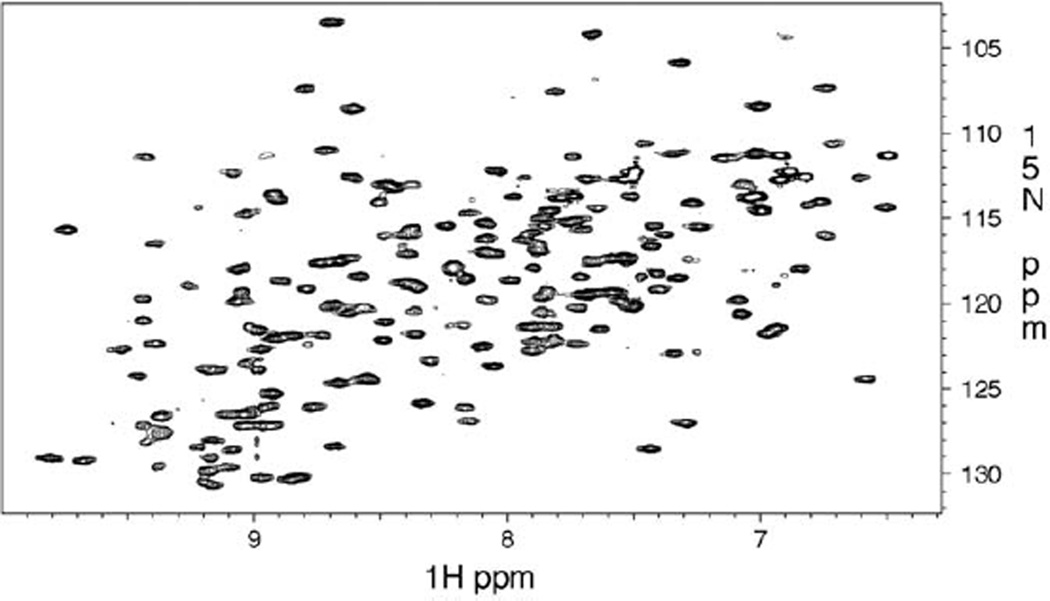
To compare the signal-to-noise effect on a larger biomolecule we recorded several HSQC experiments on a 22 kDa PolX polymerase. The 1H–15N HSQC is a key experiment in protein studies and employs a large number of π/2 and π pulses on the 1H and 15N channels, particularly for the sensitivity enhanced [39–41] echo/antiecho version [38]. The recorded spectra exhibit well-resolved peaks for most residues as shown in Fig. 5. The aim was to compare the 3, 4 and 5 mm tubes including 5 mm Shigemi tubes at either constant concentration or constant protein mass. The former situation is encountered when the protein is available in abundance; the concentration is ultimately limited by the protein solubility, which may be increased through the addition of salt. This concentration is used to assure the highest possible protein mass in the RF-coil region. It is however quite common, that only a limited amount of the biomolecule is available. Under these conditions the protein mass remains constant but the sample concentration depends on the tube diameter.
Fig. 5.
500 MHz 1H/15N HSQC of 22 kDa PolX polymerase, 1H, 13C, 15N triple resonance cryogenic probe 160 µL protein, 3 mm tube, 298 K, π/2 1H pulse = 10.1 µs, 1k*128 complex data matrix. Buffer: 20 mM Pipes, 20 mM MgCl2, 0.02% NaN3, 500 mM NaCl, and 10 mM DTT.
2.3.1. Constant protein concentration
Experiments were performed on a 0.48 mM protein sample in 0.5 M NaCl buffer. All samples were measured under identical acquisition and processing conditions. Experimental setup and results are summarized in Table 1a. This table shows, that the reduced volume and therefore the reduced amount of protein in the 3, 4 mm and Shigemi tubes does not translate into a proportional reduction of signal intensity. The intensity loss is substantially smaller than expected for the 3 and 4 mm tubes, but for the 5 mm Shigemi tube a signal increase of 15.2% is observed. The gain matches the added volume of 13.6% provided by the advanced Shigemi tube through a larger inner diameter of the sample area. It also explains the slightly larger π/2 pulse length, since the coil to sample distance is reduced, which has a major impact on the π/2 pulse length as seen above.
Table 1.
Summary of experimental conditions and results comparing 1H–15N HSQC spectra measured on a 22 kDa PolX polymerase in different NMR tubes
| (a) Tube | 5 mm | 4 mm | 3 mm | 5 mm Shigemi |
| Total sample volume (µL) | 500 | 300 | 160 | 250 |
| Sample amount in active volume (nmol) | 107.4 | 62.7 | 35.0 | 118.8 |
| Estimated sensitivity gain/loss based on the sample amount in the active volume (%) | — | −41.6 | −67.4 | +10.6 |
| π/2 1H pulse (µs) | 16.32 | 12.65 | 10.10 | 16.74 |
| Noise estimate | 233,540 | 198,630 | 156,174 | 229,692 |
| Median of sensitivity change (%) | — | −7.5 | −37.0 | +15.2 |
| (b) Tube | 5 mm | 4 mm | 3 mm | 5 mm Shigemi |
| Total sample volume (µL) | 500 | 300 | 160 | 250 |
| Sample amount in active volume (nmol) | 36.2 | 35.2 | 36.8 | 80 |
| Estimated sensitivity gain/loss based on the sample amount in the active volume (%) | — | −2.7 | 1.6 | +120 |
| π/2 1H pulse (µs) | 16.25 | 12.23 | 10.09 | 16.83 |
| Noise estimate | 207,687 | 176,990 | 151,863 | 214,687 |
| Median of sensitivity change (%) | — | +52.3 | +104.8 | +114.1 |
The inner diameters of the tubes were 4.24, 3.24, 2.42 and 4.52 mm which with a coil length of 16 mm results in active volumes of 226, 132, 73.6, and 250 µL for the 5, 4, 3 and 5 mm Shigemi tubes, respectively. (a) Constant concentrations at 0.475 mM and (b) constant protein amount (total sample 80 nmol) resulting in concentrations of 160, 267, 500 and 320 µM for the 5, 4, 3, and 5 mm Shigemi tubes, respectively. Since the active volume in a Shigemi tube is equivalent to the total volume, the amount of sample in table (b) of 80 nmol is more than twice of that in the other samples. The estimated sensitivity gain for a sample with the same amount in the active volume would be 51.3%, which is comparable to the gain in a 4 mm tube. The estimated sensitivity gain is based on the sample amount in the active volume with the 5 mm tube as a reference. The noise estimate was calculated using the NMRDraw tool. One hundred fourty three peaks were analyzed.
Overall, the Shigemi tube offers the greatest sensitivity gain in this series and is advantageous if one can tolerate the longer pulses and additional radiation damping. The 4 mm tube performs nearly as well and is easy to handle, particularly during titrations. At higher salt concentration, smaller diameter tubes are clearly preferred.
2.3.2. Constant amount of protein
Analogous to the previous measurements, results from the second case are summarized in Table 1b. Here the total protein amount in the NMR tube was held constant. Samples were prepared by taking equal volumes from a protein stock solution, diluting them with the proper amount of buffer and/or D2O as indicated. The behavior of the π/2 pulses are analogous to the previous experiment, which was expected since the sample was diluted with the sample buffer and the amount of ions in the solution did not change. In contrast to the previous experiment, the protein concentrations increase as the diameter decreases and the sample volume becomes smaller. This results in an increased sensitivity. Comparing constant sample amounts in the active volume rather than the entire sample shows that the estimated sensitivity increase of 51.3% by the 5 mm Shigemi tube is comparable to that of the 4 mm tube, however less then half of the sample amount is required. The 3 mm tube shows nearly as good a sensitivity gain as the Shigemi tube although the active and total volumes are smaller than the total/active volume of the Shigemi tube. This is a consequence of the reduced sample resistance using small diameter tubes. Clearly, a 3 mm Shigemi tube would combine both of these adantages. A regular 3 mm tube has several practical advantages; it is easy to handle and shim, has short π/2 pulse and contains only a small amount of solvent in the active volume. This is beneficial for water suppression because of reduced radiation damping; and in a dual tube arrangement (Section 4) D2O or reference materials are in the outer tube, thereby separating them from the sample of interest.
In summary, this comparison shows that when a limited amount of protein is available, one is served best by either a regular 3 or a 5 mm Shigemi tube.
3. Conclusion
Our results demonstrate that it is possible to measure samples in cryogenically cooled NMR probes at very high salt concentrations when using smaller diameter tubes. It has been shown previously that the π/2 pulse increases with more salty samples. Specifically we find that (1) the increase is not linear at very high salt concentrations and (2) the pulse length can be reduced by using the smaller 2, 3 or 4 mm tubes. By doing so, the overall amount of salt in the active volume is reduced while keeping the salt concentration in the sample at a high level and the salt is moved away from the RF coil. As a general rule we conclude that as the salt concentration is increased, smaller tube diameters are advantageous but due to the reduced volume, solubility considerations may lead to a compromise between desired diameter size and available volume.
NMR experiments recorded in high salt solutions can result in changes of the spectra. The ability to conduct experiments under high salt conditions is intrinsically relevant as biomolecular conformations and properties are dependent on it, often necessitating high salt concentrations. We have presented approaches to carry out NMR experiments utilizing smaller sample diameter tubes to take advantage of the cryogenically cooled probes without further adjustments.
The comparison of protein samples in medium salt concentration (0.5 M NaCl) at either constant protein concentration or constant amount addressed a commonly encountered situation. We find that the sensitivity of high salt samples is disproportionally enhanced in tubes with a reduced diameter compared to the 5 mm tube and these should be given preference. The Shigemi NMR tube performed well in terms of sensitivity, but requires long pulses at moderate salt concentrations and is expected to suffer the same limitations as a regular 5 mm tube at high salt concentrations. As one might expect, the 4 mm tube is most advantageous when solubility is an issue, while the 3 mm tube performs best with low sample amounts. The ease of use is another advantage offered by this arrangement for which no special hardware is required. Under either constant protein concentration or mass, we observe substantial benefits of using the smaller diameter tubes compared to the 5 mm NMR tube. In addition to an increasing signal and shorter π/2 pulses one can avoid H/D-exchange of the exchangeable protons by the dual tube arrangement when using those smaller tubes.
4. Experimental
An AVANCE spectrometer with a Triple Resonance Inverse Cryogenic Probe 1H{13C,15N} operated at 500.13 MHz from Bruker BioSpin (Rheinstetten, Germany) was used for NMR investigation. 3, 4 and 5 mm NMR tubes, part numbers 335-PP-8, 427-PP-8 and 535-PP-7, respectively were purchased from Wilmad-LabGlass (Buena, NJ). Advanced 5 mm micro tubes, susceptibility matched for D2O (Shigemi tubes), part number Z529451 were purchased from Aldrich (St. Louis, MO).
4.1. Sucrose test samples
10 mM samples were prepared in D2O in the presence or absence of 4 M NaCl. Additional 1 and 3 mM sucrose samples (4 M NaCl, D2O) were also prepared. For sucrose samples in small diameter tubes the Bruker Match™ system was utilized.
4.2. Protein samples
Purified and lyophilized PolX polymerase from the African Swine Fever Virus [42,43] was dissolved in a buffer solution containing 20 mM Pipes, 20 mM MgCl2, 10 mM DTT 0.02% NaN3, 0.5 M NaCl and adjusted to pH 6.5. For the constant concentration experiments, aliquots of 475, 285, 152 and 237.5 µL of a 0.5 mM stock solution were pipetted into the respective NMR tubes (5, 4, 3 mm and Shigemi). Twenty-five microliters of D2O was added to the 5 mm tube and 12.5 µL D2O to the Shigemi tube to provide 5% D2O for locking purposes while buffer solution was added to the 3 and 4 mm tubes to achieve the final volume. In the later arrangement, 250 and 180 µL D2O, respectively, containing 20 µM TSP-d4 was added to a 5 mm outer tube for locking and referencing purposes. The smaller tubes were inserted into the 5 mm tube. Centering the inner tube was achieved by utilizing the concave bottom of the 5 mm tube and using adhesive tape for the top portion of the 3 mm tube. This was not necessary for the 4 mm tube. Equal protein amount samples were prepared by adding a constant volume (160 µL) of the stock solution to each tube. Again 25 µL D2O was added to the 5 mm tube and 12.5 µL D2O to the Shigemi tube for lock purposes and buffer was added to obtain the final volume, 315, 140, 0, and 77.5 µL into 5, 4, 3 mm and Shigemi tubes, respectively. A standard 1H–15N HSQC measurement with flip-back pulse and watergate sequence for water suppression was used. Further parameters were: spectral width of 7003 Hz, 1024 complex points in the 1H dimension, 1622 Hz with 128 complex points in the 15N dimension and a relaxation delay of 1.2 s. The data was processed and analyzed using NMRPipe and NMRDraw software [44]. The data was zero-filled in the acquisition dimension and linear predicted followed by zero filling in the indirect dimension for a resulting data matrix of 2k × 512. Peak picking and peak intensity measurements were performed using the same processing software package.
Acknowledgments
We thank Drs. Murthy Karra, Rainer Kümmerle and Detlef Moskau for stimulating discussions. This research was supported by research and center grants from the National Institutes of Health (CA-55678) and (AI/GM-47459), the Center for Structural Biology at Vanderbilt and the Georgia Cancer Coalition.
References
- 1.Yao J, Yoon J. Low-noise electrometer and its low-noise cryogenic probe with completely guarded sample chamber. Rev. Sci. Instrum. 2000;71:1776–1780. [Google Scholar]
- 2.Styles P, Soffe NF, Scott CA, Crag DA, Row F, White DJ, White PCJ. A high-resolution NMR probe in which the coil and preamplifier are cooled with liquid helium. J. Magn. Reson. 1984;60:397–404. doi: 10.1016/j.jmr.2011.09.002. (1969) [DOI] [PubMed] [Google Scholar]
- 3.Styles P, Soffe NF, Scott Ca. An improved cryogenically cooled probe for high-resolution NMR. J. Magn. Reson. 1989;84:376–378. [Google Scholar]
- 4.Hill HDW. Improved sensitivity of NMR spectroscopy probes by use of high-temperature superconductive detection coils. IEEE Trans. Appl. Supercond. 1997;7:3750–3755. [Google Scholar]
- 5.Withers RS, Liang GC, Cole BF, Johansson M. Thin-film HTS probe coils for magnetic-resonance imaging. IEEE Trans. Appl. Supercond. 1993;3:3037–3042. [Google Scholar]
- 6.Hajduk PJ, Gerfin T, Boehlen JM, Haberli M, Marek D, Fesik SW. High-throughput nuclear magnetic resonance-based screening. J. Med. Chem. 1999;42:2315–2317. doi: 10.1021/jm9901475. [DOI] [PubMed] [Google Scholar]
- 7.Serber Z, Richter C, Moskau D, Bohlen JM, Gerfin T, Marek D, Haberli M, Baselgia L, Laukien F, Stern AS, Hoch JC, Dotsch V. New carbon-detected protein NMR experiments using CryoProbes. J. Am. Chem. Soc. 2000;122:3554–3555. [Google Scholar]
- 8.Serber Z, Richter C, Dotsch V. Carbon-detected NMR experiments to investigate structure and dynamics of biological macromolecules. Chembiochem. 2001;2:247–251. doi: 10.1002/1439-7633(20010401)2:4<247::AID-CBIC247>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Triebe R, Nast R, Marek D, Withers R, Baselgia L, Haberli MGT, Calderon P. A user-friendly system for the routine application of cryogenic NMR probes: technology and results. 40th Experimental Nuclear Magnetic Resonance Conference; Orlando, FL. 1999. p. 198. [Google Scholar]
- 10.Flynn PF, Mattiello DL, Hill HDW, Wand AJ. Optimal use of cryogenic probe technology in NMR studies of proteins. J. Am. Chem. Soc. 2000;122:4823–4824. [Google Scholar]
- 11.Kelly AE, Ou HD, Withers R, Dotsch V. Low-conductivity buffers for high-sensitivity NMR measurements. J. Am. Chem. Soc. 2002;124:12013–12019. doi: 10.1021/ja026121b. [DOI] [PubMed] [Google Scholar]
- 12.Babu CR, Flynn PF, Wand AJ. Preparation, characterization, and NMR spectroscopy of encapsulated proteins dissolved in low viscosity fluids. J. Biomol. NMR. 2003;25:313–323. doi: 10.1023/a:1023037823421. [DOI] [PubMed] [Google Scholar]
- 13.Doussin S, Birlirakis N, Georgin D, Taran F, Berthault P. Novel zwitterionic reverse micelles for encapsulation of proteins in low-viscosity media. Chemistry. 2006 doi: 10.1002/chem.200501422. [DOI] [PubMed] [Google Scholar]
- 14.Peterson RW, Lefebvre BG, Wand AJ. High-resolution NMR studies of encapsulated proteins in liquid ethane. J. Am. Chem. Soc. 2005;127:10176–10177. doi: 10.1021/ja0526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson RW, Pometun MS, Shi Z, Wand AJ. Novel surfactant mixtures for NMR spectroscopy of encapsulated proteins dissolved in low-viscosity fluids. Protein Sci. 2005;14:2919–2921. doi: 10.1110/ps.051535405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Horn WD, Simorellis AK, Flynn PF. Low-temperature studies of encapsulated proteins. J. Am. Chem. Soc. 2005;127:13553–13560. doi: 10.1021/ja052805i. [DOI] [PubMed] [Google Scholar]
- 17.Shi Z, Peterson RW, Wand AJ. New reverse micelle surfactant systems optimized for high-resolution NMR spectroscopy of encapsulated proteins. Langmuir. 2005;21:10632–10637. doi: 10.1021/la051409a. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt MR, Flynn PF, Wand AJ. Preparation of encapsulated proteins dissolved in low viscosity fluids. J. Biomol. NMR. 1999;14:75–78. doi: 10.1023/a:1008354507250. [DOI] [PubMed] [Google Scholar]
- 19.Babu CR, Flynn PF, Wand AJ. Validation of protein structure from preparations of encapsulated proteins dissolved in low viscosity fluids. J. Am. Chem. Soc. 2001;123:2691–2692. doi: 10.1021/ja005766d. [DOI] [PubMed] [Google Scholar]
- 20.Wand AJ, Ehrhardt MR, Flynn PF. High-resolution NMR of encapsulated proteins dissolved in low-viscosity fluids. Proc. Natl. Acad. Sci. USA. 1998;95:15299–15302. doi: 10.1073/pnas.95.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu WJ, Vidugiris G, Mooberry ES, Westler WM, Markley JL. Mixing apparatus for preparing NMR samples under pressure. J. Magn. Reson. 2003;164:84–91. doi: 10.1016/s1090-7807(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 22.Cohn EJ, Edsall JT. Proteins, Amino acids and Peptides as Ions and Dipolar Ions. New York: Reinhold publishing corporation; 1943. [Google Scholar]
- 23.Edsall JT. Enzymes and Enzyme Systems, their State in Nature. Cambridge: Harvard University Press; 1951. [Google Scholar]
- 24.Lane aN, Arumugam S. Improving NMR sensitivity in room temperature and cooled probes with dipolar ions. J. Magn. Reson. 2005;173:339–343. doi: 10.1016/j.jmr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.de Swiet TM. Optimal electric fields for different sample shapes in high resolution NMR spectroscopy. J. Magn. Reson. 2005;174:331–334. doi: 10.1016/j.jmr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Guclu CC. Effect of body coil electric field distribution on receive-only surface coil heating. J. Magn. Reson. Imaging. 2001;14:484–487. doi: 10.1002/jmri.1211. [DOI] [PubMed] [Google Scholar]
- 27.Mansfield P, Bowley RM, Haywood B. Controlled E-field gradient coils. Magma. 2003;16:113–120. doi: 10.1007/s10334-003-0015-7. [DOI] [PubMed] [Google Scholar]
- 28.Taylor HC, Burl M, Hand JW. Experimental verification of numerically predicted electric field distributions produced by a radiofrequency coil. Phys. Med. Biol. 1997;42:1395–1402. doi: 10.1088/0031-9155/42/7/012. [DOI] [PubMed] [Google Scholar]
- 29.Gadian DG, Robinson FNH. Radiofrequency losses in NMR experiments on electrically conducting samples. J. Magn. Reson. 1979;34:449–455. [Google Scholar]
- 30.Hoult DI, Lauterbur PC. Sensitivity of the zeugmatographic experiment involving human samples. J. Magn. Reson. 1979;34:425–433. [Google Scholar]
- 31.Hoult DI, Richards RE. Signal-to-noise ratio of nuclear magnetic-resonance experiment. J. Magn. Reson. 1976;24:71–85. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Binbuga B, Young JK. 1H, 13C and 15N backbone and side chain resonance assignments of Haloferax volcanii DHFR1. J. Biomol. NMR. 2005;33:281. doi: 10.1007/s10858-005-0459-3. [DOI] [PubMed] [Google Scholar]
- 33.Bodenhausen G, Ruben DJ. Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 1980;69:185–189. [Google Scholar]
- 34.Davis aL, Laue ED, Keeler J, Moskau D, Lohman J. Absorption- mode 2-dimensional NMR-spectra recorded using pulsed field gradients. J. Magn. Reson. 1991;94:637–644. [Google Scholar]
- 35.Hurd RE, John BK. Gradient-enhanced proton-detected heteronuclear multiple-quantum coherence spectroscopy. J. Magn. Reson. 1991;91:648–653. [Google Scholar]
- 36.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR-spectroscopy of aqueous-solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 37.Sklenar V, Piotto M, Leppik R, Saudek V. Gradient-tailored water suppression for H-1-N-15 Hsqc experiments optimized to retain full sensitivity. J. Magn. Reson. Ser. A. 1993;102:241–245. [Google Scholar]
- 38.Schleucher J, Schwendinger M, Sattler M, Schmidt P, Schedletzky O, Glaser SJ, Sorensen OW, Griesinger C. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed-field gradients. J. Biomol. NMR. 1994;4:301–306. doi: 10.1007/BF00175254. [DOI] [PubMed] [Google Scholar]
- 39.Cavanagh J, Palmer aG, Wright PE, Rance M. Sensitivity improvement in proton-detected 2-dimensional heteronuclear relay spectroscopy. J. Magn. Reson. 1991;91:429–436. [Google Scholar]
- 40.Palmer aG, Cavanagh J, Wright PE, Rance M. Sensitivity improvement in proton-detected 2-dimensional heteronuclear correlation NMR-spectroscopy. J. Magn. Reson. 1991;93:151–170. [Google Scholar]
- 41.Kay LE, Keifer P, Saarinen T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 1992;114:10663–10665. [Google Scholar]
- 42.Oliveros M, Yanez RJ, Salas ML, Salas J, Vinuela E, Blanco L. Characterization of an African swine fever virus 20-kDa DNA polymerase involved in DNA repair. J. Biol. Chem. 1997;272:30899–30910. doi: 10.1074/jbc.272.49.30899. [DOI] [PubMed] [Google Scholar]
- 43.Showalter AK, Byeon IJ, Su MI, Tsai MD. Solution structure of a viral DNA polymerase X and evidence for a mutagenic function. Nat. Struct. Biol. 2001;8:942–946. doi: 10.1038/nsb1101-942. [DOI] [PubMed] [Google Scholar]
- 44.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]