Abstract
Methylmercury (MeHg) and polychlorinated biphenyls (PCBs) are seafood contaminants known for their adverse effects on neurodevelopment. This study examines the relation of developmental exposure to these contaminants to information processing assessed with event-related potentials (ERPs) in school-aged Inuit children from Nunavik (Arctic Québec). In a prospective longitudinal study on child development, exposure to contaminants was measured at birth and 11 years of age. An auditory oddball protocol was administered at 11 years to measure ERP components N1 and P3b. Multiple regression analyses were performed to examine the associations of levels of the contaminants to auditory oddball performance (mean reaction time, omission errors and false alarms) and ERP parameters (latency and amplitude) after control for potential confounding variables. A total of 118 children provided useable ERP data. Prenatal MeHg exposure was associated with slower reaction times and fewer false alarms during the oddball task. Analyses of the ERP parameters revealed that prenatal MeHg exposure was related to greater amplitude and delayed latency of the N1 wave in the target condition but not to the P3b component. MeHg effects on the N1 were stronger after control for seafood nutrients. Prenatal PCB exposure was not related to any endpoint for sample as a whole but was associated with a decrease in P3b amplitude in the subgroup of children who had been breast-fed for less than 3 months. Body burdens of MeHg and PCBs at 11 years were not related to any of the behavioural or ERP measures. These data suggest that prenatal MeHg exposure alters attentional mechanisms modulating early processing of sensory information. By contrast, prenatal PCB exposure appears to affect information processing at later stages, when the information is being consciously evaluated. These effects seem to be mitigated in children who are breast-fed for a more extended period.
Keywords: Children, Event-related potentials, Methylmercury, Neurotoxicity, P3b, Polychlorinated biphenyls
1. Introduction
Methylmercury (MeHg) and polychlorinated biphenyls (PCBs) are widespread environmental pollutants contaminating the marine food web. Both contaminants are known to be neurotoxic to humans. Vulnerability of the central nervous system to these substances is increased during early development, especially during the prenatal period [1]. MeHg derives from methylation of inorganic mercury (Hg), a toxic metal that is ubiquitous in the environment, by aquatic micro-organisms. The effects of acute MeHg intoxication have been documented following large-scale poisoning episodes that occurred in Japan (1953 and 1964–1965) and Iraq (1971–1972). The Japanese episodes resulted from industrial Hg releases in waters supplying the population with fish and shellfish [2]. Consumers of MeHg-contaminated seafood displayed symptoms, such as ataxia, constriction of the visual fields and hearing impairments [3]. The Iraqi contamination was caused by the intake of bread made from wheat treated with a MeHg contaminated fungicide [4]. In all cases, the most disastrous effects--mental retardation, seizures, cerebral palsy, blindness and death--were found in children exposed prenatally [5]. Several epidemiological studies have been conducted within fish-eating populations to examine the consequences of chronic exposure to lower doses of MeHg on cognitive development using behavioural assessments. The most consistent findings include impairment in visuomotor function [6,7; cf. 8] and alterations in early auditory processing [9] in relation to prenatal exposure. Verbal, attention and learning deficits have also been reported but somewhat less consistently [7,10,11,12; cf. 13,14].
PCBs are synthetic organochlorine compounds widely used in industry during the mid-20th century, notably as lubricating oils and hydraulic fluids [15]. Developmental neurotoxicity of PCBs was documented following large-scale Japanese and Taiwanese poisoning episodes in 1968 and 1979, respectively, due to the intake of rice oil accidentally contaminated with PCBs and dioxins during production processes. Many children born to women exposed prior to or during pregnancy displayed symptoms, such as growth retardation, nail malformation, delays in cognitive development and behavioural problems [16,17]. Subsequent birth-cohort studies have reported detrimental PCB effects on neuropsychological functioning apparent in infancy [18,19,20] and continuing through school age [e.g. 21,22], notably on IQ and executive and attentional function (reviewed in [23]). Some of these effects, however, were only present in children who had not been breast-fed [24,25].
Despite the existence of a few large, well designed cohort studies documenting neurobehavioural outcomes following prenatal exposure to MeHg and PCBs, the nature of the effects of these contaminants on brain function remains unclear. The neurobehavioural tasks typically used in epidemiological studies depend on a broad range of cognitive processes making difficult the identification of specific aspect(s) of brain function impaired by a given contaminant. Sensory electrophysiological assessment, such as brainstem-auditory evoked potentials (BAEPs) and visual evoked potentials (VEPs), have successfully identified sub-clinical alterations associated with exposure to seafood contaminants [9,26]. Further insights into brain processing can be obtained with cognitive electrophysiological assessment using event-related potentials (ERPs), which can reveal subtle and specific effects of these neurotoxicants on cognitive and attention processing [27].
One of the most frequently used ERP protocols is the oddball detection task, in which the participant is asked to respond to targets randomly distributed among a series of standard stimuli. The average EEG waveforms obtained from oddball protocols show several ERP components including the N1, a wave of negative voltage peaking around 100 ms at the vertex after stimulus onset [28], and the P3b, a positive wave peaking around 300 ms at centro-parietal electrodes, which is seen predominant in the target condition [29]. The N1 wave is composed of several overlapping subcomponents [28,30]. Some are automatically elicited by the occurrence of any detectable stimulus and are modality-specific. However, the vertex-recorded N1 obtained during an active task (e.g., oddball detection) appears to reflect a non-modality-specific subcomponent, which is significantly affected by the vigilance state of the participant, as its amplitude increases with the level of attention to task [31,32,33,34]. In this context, the vertex-recorded N1 has been proposed to reflect cerebral excitability during attention, or coarse mechanisms of selective attention to the attended input [32,35,36,37]. The P3b wave is elicited by the active detection of an anticipated and unpredictable stimulus, and has been attributed to working memory processing [38,39,40] or post-decision closure mechanisms [41,42,43]. P3b latency is thought to reflect information categorization speed [44,45] and is inversely associated with behavioural performance in speeded attention tasks and on digit span tasks [46,47]. P3b amplitude is used as an index of allocation of attentional resources [48] and correlates with performance on tests of selective attention and verbal learning [49,50,51].
Two epidemiological studies in children assessed the P3b component in an auditory oddball paradigm to study the impact of prenatal exposure to PCBs on brain processing. In Taiwan, 7- to 12-year-old children exposed to PCBs and polychlorinated dibenzofurans in utero (n = 27) due to maternal consumption of contaminated rice oil were found to have smaller P3b amplitudes and longer P3b latencies than matched controls [52]. In a birth cohort study conducted in the Netherlands, 9-year-old children from a high PCB exposed group (n = 32) were found to have longer P3b latencies than those from a low exposed group (n = 28) [53]. However, both studies were limited by small sample sizes, and neither assessed the N1 wave preceding the P3b component, which can provide information about the contaminant’s effects on earlier stages of information processing. Furthermore, no study conducted to date has used ERPs to assess MeHg neurotoxicity in children. Findings from the behavioural assessments of attention and memory in the Faroe Islands study [7,10] suggest that ERPs associated with the domains of cognitive function assessed in the auditory oddball paradigm might also be affected by prenatal exposure to MeHg. We have previously reported an association between prenatal lead (Pb) exposure and increased P3b amplitude on a visual oddball task in Inuit children at 5 years, as well as an association of Pb exposure during the preschool period with delayed P3b latency [54]. However, neither prenatal Pb nor 11-year Pb body burden was related to auditory oddball performance at 11 years.
The present study examined the association of prenatal and current exposures to MeHg and PCBs with ERP measures of information processing elicited during an auditory oddball task in a cohort of Inuit children in Arctic Quebec, who were exposed to these contaminants through marine mammal and fish consumption. The Inuit are among the most highly exposed populations on earth due to long-range transport of these compounds via atmospheric and ocean currents and their bioaccumulation in fish and sea mammals that are staples of the Inuit diet [55]. It is hypothesized that higher prenatal exposure to PCBs and MeHg will be related to increased latencies and decreased amplitudes of N1 and P3b.
2. Materials and Methods
2.1 Participants
This ERP study is part of an 11-year-old follow-up assessment of a group of Inuit children from Nunavik (Arctic Québec, Canada) recruited at birth. For most of the participants, umbilical cord blood samples were obtained under the auspices of the Cord Blood Monitoring Program, conducted between 1993 and 1998 [56]. Between September 2005 and November 2008, three groups of Inuit mothers and their children were invited to participate in this assessment: (1) children who had participated in the Environmental Contaminants and Child Development Study as infants [55,57], (2) children for whom cord blood samples were available and who had participated in the Nunavik Preschool Study [26,54,58], and (3) children for whom cord blood samples were available but had not been previously tested1. Assessments were conducted in the three largest Nunavik villages. Participants who resided in other communities were transported by plane to one of the larger villages for assessment. Written informed consent was obtained from a parent of each participant; oral assent, from each child. The research was approved by Laval University and Wayne State University ethics committees. A detailed interview was conducted with the child’s mother (or other principal caregiver) to document potential confounding variables.
The following inclusion criteria were used for this ERP study: children between 10.0 and 13.0 years of age, birth weight ≥ 2500 g, gestation duration ≥ 36 weeks, and no known neurological or clinically significant developmental disorder. Participants with a medical condition that could affect ERP recording, such as a history of epilepsy (n = 2) or on medication at testing time (n = 1) were excluded. Pure-tone audiometry (1000 and 2000 Hz) was performed to check for hearing impairment before ERP testing. Children with a hearing threshold ≥ 35 dB at best ear on either frequency were excluded (n = 3). Of the 222 children who were invited to participate, 20 refused to perform the task and 13 others could not complete it due to technical problems during the EEG recording.
2.2 ERP protocol, recording and analyses
ERPs were recorded during an auditory oddball protocol. The child was asked to sit still in front of a computer screen and to press the space key on the computer keyboard as quickly and accurately as possible to the target stimulus (2000 Hz tone; 20% probability) but not to press to the standard stimulus (1000 Hz tone; 80% probability). Stimuli (sound pressure level: 70 dB; duration: 50 ms; rise/fall time: 5 ms; inter-stimulus interval: 2 s) were presented binaurally through E-A-RTONE audiometric insert earphones (Auditory Systems) and generated by Presentation Software (Neurobehavioural Sciences). The total protocol included four trials of 100 stimuli each, for a total of 13 minutes of stimulus presentation. Data acquisition was performed with InstEP® v5.17, and data were stored on-line for later averaging. The electro-oculogram (EOG) was recorded for both eyes with tin electrodes placed at the supra- and infra-orbital ridges. ERPs were recorded with 29 Ag-AgCl electrodes (Fz, F3, F4, F7, F8, AF3, AF4, AF7, AF8, FCz, FC1, FC2, Cz, C3, C4, T3, T4, T5, T6, Pz, P3, P4, Oz, O1, O2, M1, M2, A1, and A2 according to the international 10–20 system), but ERP scoring was performed only at two locations (Cz for N1, Pz for P3b). Electrodes were referenced to linked ear lobes, with forehead ground. The impedance was kept below 10 kΩ. EOG and EEG gain were amplified with a gain of 7500 and 15000, respectively, with Grass Model 15A54 amplifiers. The digitization rate was 512 Hz.
ERP analyses were performed using Analyzer 1.05 (Brain Vision©) hardware. EOG correction was performed from the vertical EOG electrode. High and low pass filters were set at 0.16 and 30 Hz, respectively. Artefact rejection was performed with a ± 100 µV criterion. Baseline correction was applied using a 100 ms pre-stimulus baseline. Trials with omission errors (false negative response), false alarms and impulsive responses (<200 ms post-stimuli) were excluded from averaging. ERPs were identified according to the following definitions: N1, most negative peak (Cz) ranging in the 90–150 ms post-stimulus interval; P3b, most positive peak (Pz) in the 250–500 ms interval. Latency for each component was determined from stimulus onset to maximal peak, and peak amplitude was measured from baseline. Peaks were manually identified by a trained doctoral student unaware of exposure levels (OB). Peak validity was ensured by performing co-validation of P3b ratings (target condition) by a trained research assistant. Inter-rater validity was high. Pearson correlations between both scorings were r = 0.86 for latency (p < 0.001) and r = 0.97 for amplitude (p < 0.001). In case of disagreement, an experienced neurophysiologist (CB) resolved the issue.
Among the 189 children from whom data were obtained, the data were considered reliable for 118 (62.4% of the sample). Reasons for exclusion were: noise in data due to alpha waves or eye movement (n = 27), omission errors on ≥ 50% of the target stimuli (n = 6), false alarms on ≥ 15% of standard stimuli (n = 2), < 20 target stimuli in average wave [59] (n = 1) and inability to identify a clear P3b wave (n = 35). An exclusion rate of this magnitude is not uncommon in ERP studies with children [e.g. 60,61]. T-tests showed that the children with reliable data were representative of the sample as a whole; the Hg and PCB 153 concentrations in the cord and 11-year blood samples of the participants with reliable data did not differ from those of the excluded subjects (all p’s > 0.20).
2.3 Contaminant and nutrient analyses in biological samples
PCB, Hg, Pb and selenium (Se) analyses were performed at the Laboratoire de Toxicologie INSPQ, which is accredited by the Canadian Association for Environmental Analytical Laboratories. Concentrations in umbilical cord blood were used as indicators of prenatal exposure. Detailed analytical and quality control procedures are described elsewhere [54,55,62]. The 14 most prevalent PCB congeners (IUPAC nos. 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187) were measured in purified plasma extracts using high-resolution gas chromatography (Hewlett-Packard HP5890A), with two capillary columns (Hewlett-Packard Ultra I and Ultra II) and dual Ni-63 electron capture detectors. Total Hg concentrations were determined in umbilical cord blood samples using cold vapor atomic absorption spectrometry (Pharmacia Model 120). Blood Pb levels were determined by graphite furnace atomic absorption with Zeeman background correction (Perkin Elmer model ZL 4100). Se concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS) on a Perkin Elmer Sciex Elan 6000 instrument. The limits of detection (LODs) were 0.2 µg/L for blood Hg, 0.2 µg/dL for Pb, 0.1 µmol/L for Se, and 0.02 µg/L for all PCB congeners in plasma. Docosahexaenoic acid (DHA), an omega-3 fatty acid, was measured in plasma phospholipids at the University of Guelph Lipid Analytical Laboratory (B.J. Holub) as described in Jacobson et al. [57].
In addition, venous blood samples (20 mL) obtained from each participant were used to document exposure of children at time of testing. Concentrations of 13 PCB congeners (IUPAC nos. 99, 101, 105, 118, 128, 138, 153, 156, 163, 170, 180, 183, 187) were measured in plasma extracts by gas chromatography (HP 5890 Series II Plus) equipped with a 30-m DB-5 (J&W Scientific) and HP 5890B mass spectrometer (Agilent) according to the method described by Dallaire et al. [63]. Compounds are automatically extracted from the aqueous matrix using solid phase extraction. LODs were less than 0.05 µg/L for all PCB congeners except for PCB-52 (LOD = 0.15 µg/L). Total Hg, Pb and Se concentrations were determined in children whole blood samples by ICP-MS (Perkin Elmer Sciex Elan 6000 ICP-MS instrument for Pb and Se; PE DRC II instrument for Hg). LODs were 0.002 µg/dL for Pb, 0.10 µg/L for Hg and 0.09 µmol/L for Se. DHA was measured in plasma phospholipids with the same procedure as in cord blood.
For the present study, PCB congener 153, expressed on a lipid basis, was used as an indicator of total PCB exposure since it is highly correlated with other PCB congeners and is considered an adequate marker of exposure to environmental PCB mixtures in the Arctic [55,64]. A value equal to half the limit of detection of the analytical method was entered in the database whenever a substance was not detected.
2.4 Confounding variables
Variables used as covariates in previous studies on the effects of exposure to seafood contaminants and situational and biological factors known to influence ERPs were selected as potential confounding variables. The potential confounding variables were age and gender of child; whether the child was a local resident or had to travel by plane from a remote village same day of testing; time when testing took place (morning or afternoon); maternal age, education (years), and parity; breast-feeding status (yes/no) and duration (number of months); maternal tobacco smoking (yes/no), regular use of marijuana (at least once/month; yes/no) and binge drinking (at least one episode of ≥ 5 alcohol drinks; yes/no) during pregnancy; socioeconomic status (SES) [65] of the primary caregiver; and maternal non-verbal reasoning abilities (Raven Progressive Matrices) [66]. We also considered as potential confounders the haemoglobin status (assessed from child’s blood sample at testing) as well as newborn and child Pb and nutrients (DHA and Se) body burdens. Number of trials retained for computing the ERP average was also considered a potential confounding variable.
2.5 Statistical analyses
The normality of each variable’s distribution was subjected to visual inspection and checked for skewness/kurtosis values (normality range: −2.0 to 2.0). Log transformations were conducted on Hg, PCB 153, Pb and Se concentrations (both prenatal and current); rates of omission errors and false alarms; and breast-feeding duration and parity since they follow log-normal distributions. Extreme values (>3 standard deviations from the mean) for normally distributed variables were recoded to one point greater than the highest observed non-outlying value following the procedure recommended by Winer [67]. The following variables were corrected with this procedure: maternal age, SES, haemoglobin level, cord Se, child Se, cord DHA, rate of false alarms (log), N1 latency in standard and target conditions, target-N1 amplitude and target-P3b latency. For most of these variables, there was only one outlying value, with the exceptions of child Se (2 outliers), target-N1 amplitude (3 outliers) and rate of false alarms (log; 9 outliers).
The associations among the contaminants and nutrients were examined with Pearson correlation analyses. Hierarchical multiple regression analyses were conducted to assess the relation between each of the contaminants (cord and 11-year blood levels) and each of the following behavioural and ERP outcomes after controlling for potential confounders: hit reaction time, rate of omission errors, rate of false alarms, and latencies and amplitudes of N1 and P3b in both the standard and target conditions. Selection of confounders was determined using a two-phase strategy combining significance test and change-in-estimate criteria: 1) each variable related at p < 0.20 to the outcome measure being examined was initially selected from the set of potential confounders; 2) the contaminant variable was entered in the first step of the regression analysis; 3) each confounder that met the p < 0.20 criterion was entered hierarchically starting with the one showing the highest correlation with outcome, and a confounder was retained if its inclusion altered the association (Std β coefficient) between contaminant exposure and the outcome by at least 10% at the step of entry (see [57]). The 0.20 alpha level and 10% change in value criteria were based on the work of Greenland and associates [68–69] and are based on the premise that the p < 0.20 criterion will ensure that all potential confounders are assessed but that only those that alter the relation of exposure and outcome by at least 10% affect this association in a meaningful fashion. Effects were considered significant when the contaminant measure was associated with the dependent variable at p < 0.05 after control for confounders. Pearson correlations relating two important seafood nutrients (DHA and Se levels in cord and 11-year blood) to the outcome measures are also reported.
Based on previous studies reporting a moderating role of breast-feeding on the effects of prenatal PCB exposure [24,25], analyses of the effects of prenatal exposure to cord PCB 153 and cord Hg were rerun separately for two groups of children: those who had not been breast-fed or were breast-fed for less than 3 months vs. those who were breast-fed for at least 3 months. The same control variables as those used for analyses involving the entire sample were included in these additional regression analyses. The median value of breast-feeding duration for the entire sample (3 months) was used as the cut-point in order to create two subsamples of sufficient size, optimising the statistical power.
3. Results
3.1 Descriptive statistics
Descriptive data for the participants included in the ERP analyses are summarized in Table 1. The final sample includes a higher proportion of girls than boys (χ2 = 11.0, p = 0.001). Table 1 shows that most children were breast-fed, and the duration of breast-feeding was typically long relative to Southern Canadian and U.S. norms (more than 1 year for 39.3 % of breast-fed infants).
Table 1.
Descriptive data for participants with reliable ERP data
| N | Mean | Median | S.D. | Range | % | |
|---|---|---|---|---|---|---|
| Child characteristics | ||||||
| Age at assessment (years) | 118 | 11.3 | 11.4 | 0.57 | 10.2 – 12.9 | |
| Gender (% girls) | 118 | 65.3 | ||||
| Tested in their home village a | 118 | 56.8 | ||||
| Time of testing (% morning) | 118 | 59.3 | ||||
| Maternal/other principal caregiver characteristics | ||||||
| Mother’s age (years) | 117 | 39.2 | 37.6 | 9.0 | 22.3 – 71.6 | |
| Education (years) | 117 | 8.3 | 8.0 | 2.6 | 0 – 16 | |
| Socioeconomic status (SES score)b | 118 | 28.1 | 27.5 | 12.5 | 8 – 66 | |
| Nonverbal intelligence c | 116 | 35.5 | 38.0 | 9.6 | 10 – 56 | |
| Parity (number of children) | 109 | 2.2 | 2.0 | 1.9 | 0 – 8 | |
| Breast-feeding status (% yes) | 68.7 | |||||
| Duration (months) d | 79 | 17.9 | 10.0 | 20.4 | 0.1 – 108.0 | |
| Maternal consumption during pregnancy | ||||||
| Alcohol, binge drinking (% yes) | 95 | 26.2 | ||||
| Marijuana, monthly use (% yes) | 93 | 12.9 | ||||
| Tobacco (% yes) | 108 | 82.4 | ||||
| Seafood contaminants and other biological variables | ||||||
| Cord PCBs (ng/g fat)e | % detected | |||||
| Congener 28 | 116 | 0.9 | ||||
| Congener 52 | 116 | 52.1 | ||||
| Congener 99 | 116 | 27.6 | 24.3 | 20.3 | 3.6 – 132.8 | 94.9 |
| Congener 101 | 116 | 49.6 | ||||
| Congener 105 | 116 | 34.2 | ||||
| Congener 118 | 116 | 23.5 | 18.7 | 17.9 | 3.6 – 121.0 | 93.2 |
| Congener 128 | 116 | 2.6 | ||||
| Congener 138 | 116 | 85.3 | 73.8 | 60.5 | 12.3 – 385.6 | 100.0 |
| Congener 153 | 116 | 129.7 | 103.0 | 97.4 | 19.5 – 653.6 | 100.0 |
| Congener 156 | 116 | 46.2 | ||||
| Congener 170 | 116 | 18.6 | 13.5 | 14.6 | 3.4 – 74.0 | 82.9 |
| Congener 180 | 116 | 53.7 | 39.6 | 39.2 | 9.1 – 187.7 | 100.0 |
| Congener 183 | 113 | 43.9 | ||||
| Congener 187 | 116 | 25.5 | 22.0 | 16.6 | 4.1 – 97.0 | 97.4 |
| Child PCBs (ng/g fat) e | ||||||
| Congener 99 | 113 | 14.6 | 10.3 | 13.6 | 1.0 – 83.3 | 96.4 |
| Congener 101 | 112 | 53.6 | ||||
| Congener 105 | 113 | 54.0 | ||||
| Congener 118 | 114 | 12.3 | 9.5 | 9.1 | 1.1 – 42.9 | 97.3 |
| Congener 128 | 113 | 6.2 | ||||
| Congener 138 | 114 | 39.5 | 25.1 | 40.6 | 2.6 – 261.9 | 100.0 |
| Congener 153 | 114 | 84.6 | 44.7 | 107.3 | 4.1 – 809.5 | 100.0 |
| Congener 156 | 114 | 50.0 | ||||
| Congener 163 | 114 | 13.3 | 6.7 | 16.1 | 1.0 – 97.6 | 90.4 |
| Congener 170 | 114 | 11.6 | 5.4 | 16.2 | 0.8 – 121.4 | 83.3 |
| Congener 180 | 114 | 37.7 | 17.5 | 54.0 | 1.0 – 404.8 | 95.6 |
| Congener 183 | 114 | 62.3 | ||||
| Congener 187 | 114 | 16.7 | 9.4 | 18.9 | 0.9 – 109.8 | 91.2 |
| Cord Hg (µg/L) | 116 | 21.5 | 14.2 | 18.8 | 1.8 – 99.3 | |
| Child Hg (µg/L) | 115 | 4.69 | 2.8 | 4.9 | 0.2 – 28.1 | |
| Haemoglobin level at testing (g/L) | 115 | 130.3 | 129.0 | 9.4 | 98.0 – 149.0 | |
| Cord lead (µg/dL) | 116 | 5.0 | 4.1 | 3.5 | 1.0 – 22.8 | |
| Child lead (µg/dL) | 115 | 2.3 | 1.9 | 2.2 | 0.4 – 12.8 | |
| Cord DHA (% of fatty acids) | 115 | 3.7 | 3.5 | 1.4 | 1.1 – 7.7 | |
| Child DHA (% of fatty acids) | 113 | 2.4 | 2.2 | 1.0 | 0.6 – 5.0 | |
| Cord selenium (µmol/L) | 109 | 4.4 | 3.7 | 2.5 | 2.0 – 20.0 | |
| Child selenium (µmol/L) | 115 | 2.5 | 2.2 | 1.2 | 0.9 – 9.5 | |
The remaining children are those from smaller, more remote villages who traveled by plane to a larger village for testing;
Hollingshead index for the mother and her partner or, if she was not self-supporting, for her primary source of support [65];
Raven Progressive Matrices [66];
For breast-fed children only;
Full descriptive statistics are provided only for PCB congeners detected in at least 70% of plasma samples.
Associations between the blood levels of the contaminants and nutrients are presented in Table 2. As expected, the Hg, PCB 153, Pb, DHA and Se concentrations were moderately associated in both cord and child blood samples since all these substances are found at relatively high concentrations in traditional Inuit food [55]. Moderate associations were found between cord and 11-year blood concentrations for Hg and PCB 153, presumably because a child born to a mother with a traditional diet is likely to consume more traditional foods during childhood. It is noteworthy that breast-feeding duration was associated with higher child PCB 153 levels at testing (r = 0.52, p < 0.001), presumably because breast milk provides a major source of postnatal PCB exposure [64,70]. Breast-feeding was not included as a covariate in the child PCB models due to multicollinearity.
Table 2.
Intercorrelations among contaminants and nutrients in cord and child blood samples
| Hg |
PCB |
Pb |
DHA |
Se |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hg | Cord | Child | Cord | Child | Cord | Child | Cord | Child | Cord | Child | |
| Cord | 1.00 | 0.42** | 0.44** | 0.49** | 0.39** | 0.07 | 0.28** | 0.12 | 0.43** | 0.26** | |
| Child | 1.00 | 0.09 | 0.56** | 0.29** | 0.21* | 0.18# | 0.38** | 0.15 | 0.63** | ||
| Cord | 1.00 | 0.41** | 0.25** | 0.04 | 0.24* | 0.07 | 0.27** | 0.06 | |||
| Child | 1.00 | 0.32** | 0.27** | 0.22* | 0.21* | 0.27** | 0.42** | ||||
| Pb | |||||||||||
| Cord | 1.00 | 0.16 | 0.23* | −0.05 | 0.06 | 0.18# | |||||
| Child | 1.00 | 0.05 | 0.04 | −0.05 | 0.24 | ||||||
| DHA | |||||||||||
| Cord | 1.00 | 0.39** | 0.13 | 0.18# | |||||||
| Child | 1.00 | 0.05 | 0.26** | ||||||||
| Se | |||||||||||
| Cord | 1.00 | 0.25** | |||||||||
| Child | 1.00 | ||||||||||
Abbreviations. DHA: docosahexaenoic acid; Hg: mercury; Pb: lead; PCB: polychlorinated biphenyl congener IUPAC 153; Se: selenium.
Note. Log transformations were performed for Hg, PCB 153, Pb and Se concentrations.
p < 0.01;
p < 0.05;
p < 0.10.
3.2 Auditory oddball results
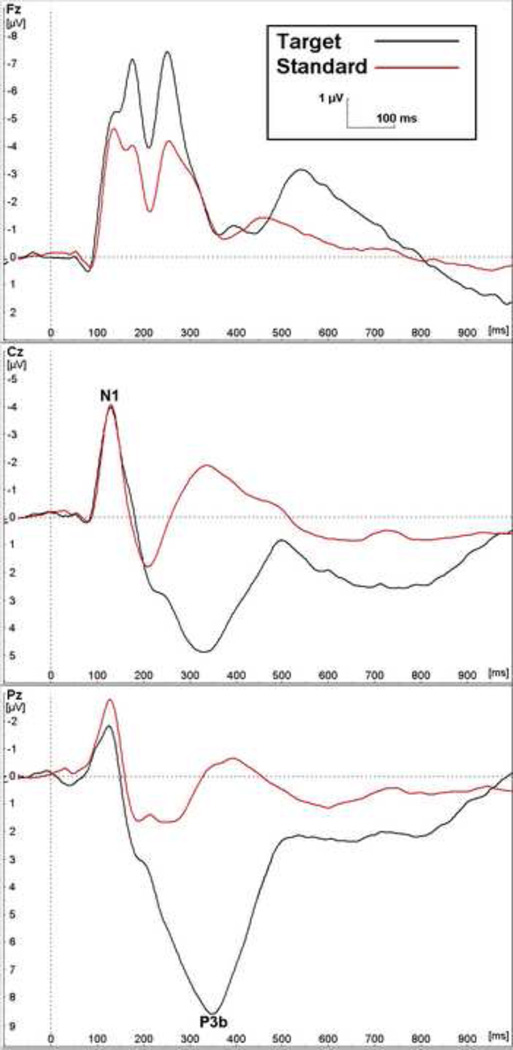
The weighted grand averages at three midline electrode locations for both the standard and target conditions of the auditory oddball task are shown in Figure 1. As expected, the N1 is more clearly manifest at Cz, while the P3b is more posterior and is much larger in the target condition. The waveform is very similar to those observed in previous studies using auditory oddball protocols with children of this age [e.g., 71,72]. Behavioural and ERP results from participants with reliable ERP data are reported in Table 3. False alarm errors were much less frequent than omission errors. Pearson correlations relating the behavioural to the ERP data (not shown in the tables) reveal, as expected, that longer hit reaction times are associated with more prolonged P3b latencies (r = 0.29, p < 0.01) and smaller P3b amplitudes (r = −0.23, p = 0.01) in the target condition, while higher rates of omission errors are related to longer P3b latencies (r = 0.28, p < 0.01) in the target condition and smaller N1 amplitudes in the standard condition (r = 0.20, p = 0.03). False alarms are not related to any ERP parameter.
Figure 1.
Grand average for auditory oddball task at midline (Fz = frontal, Cz = central, Pz = parietal) scalp positions (N = 118).
Table 3.
Behavioural and ERP parameters obtained from the auditory oddball task
| N | Mean | S.D. | Range | |
|---|---|---|---|---|
| Behavioural performance | ||||
| Mean hit reaction time (ms) | 118 | 542.0 | 75.7 | 345.0 – 748.6 |
| Omission errors rate (%) | 118 | 8.7 | 8.3 | 0.0 – 42.5 |
| False alarms rate (%) | 118 | 0.7 | 0.9 | 0.0 – 5.0 |
| ERP parameters a,b | ||||
| Standard condition | ||||
| N1 latency (ms) | 113 | 129.8 | 8.5 | 103.5 – 148.4 |
| N1 amplitude (µV) | 113 | −4.7 | 2.3 | −10.0 – 0.12 |
| P3b latency (ms) | 51 | 316.4 | 43.4 | 255.9 – 451.2 |
| P3b amplitude (µV) | 51 | 3.1 | 1.7 | −1.1 – 7.0 |
| Target condition | ||||
| N1 latency (ms) | 99 | 127.1 | 9.5 | 97.7 – 148.4 |
| N1 amplitude (µV) | 99 | −4.7 | 3.0 | −17.0 – 3.0 |
| P3b latency (ms) | 118 | 346.0 | 39.5 | 267.6 – 484.4 |
| P3b amplitude (µV) | 118 | 10.9 | 3.8 | 4.3 – 21.1 |
N1 at Cz and P3b at Pz;
Sample sizes vary because not all ERP components were identifiable in all the averaged waveforms.
3.2.1 Associations between contaminants and behavioural parameters
The relations of the contaminants to the behavioural parameters within the final study sample are shown in Table 4. After statistical control for confounders, none of the contaminants are related to any of the behavioural performance measures from the auditory oddball task. To optimise statistical power, these analyses were rerun with the initial sample of 189 children who provided reliable behavioural data. Within this larger sample, higher cord blood Hg levels are associated with longer hit reaction times and with lower rates of false alarms. There is no association with other contaminants. One of the seafood nutrient parameters, DHA levels at 11 years, was associated with increased rates of false alarms (r = 0.16, p = 0.03), but including this variable in the regression model had no influence on the cord Hg-false alarms relationship.
Table 4.
Regression analyses for the relations between contaminants and behavioural performance parameters
| Cord Hg |
Cord PCB 153 |
11-year Hg |
11-year PCB 153 |
|||||
|---|---|---|---|---|---|---|---|---|
| r | Std β | r | Std β | R | Std β | r | Std β | |
| ERP sample (N = 118) | ||||||||
| Mean hit reaction time | 0.12 | 0.06 a,f | 0.09 | 0.06 a | 0.17 # | 0.11 a,f | 0.08 | 0.02 f |
| Rate of omission errors | −0.00 | −0.03 c | 0.02 | 0.00 c | 0.05 | 0.02 c | 0.11 | 0.08 c |
| Rate of false alarms | −0.14 | −0.17 #c,e | −0.18* | −0.16 c,e | −0.05 | −0.07 c | −0.06 | −0.11 c,e |
| Total sample (N = 189) | ||||||||
| Mean hit reaction time | 0.18* | 0.15*a,f,b | 0.13# | 0.10 a,b | 0.08 | 0.00 a,f,b,d | 0.11 | 0.10 a |
| Rate of omission errors | 0.06 | 0.02f,a,b,c,g | 0.10 | 0.08f,c,g | 0.05 | −0.05f,a,b,c,g | 0.07 | −0.01f,a,b,c,g |
| Rate of false alarms | −0.13# | −0.21* h,c | −0.07 | −0.13 h,c | −0.01 | −0.06 i,h,c | −0.05 | −0.11 h,c |
Abbreviations: Hg: mercury; PCB 153: polychlorinated biphenyl congener IUPAC 153.
Note. Confounding variables included in the regression analyses:
child age
child gender
testing location
breast-feeding status
maternal binge drinking during pregnancy
haemoglobin status
cord DHA
cord Pb
time of testing
p < 0.01;
p < 0.05;
p < 0.10.
3.2.2 Associations between contaminants and ERP parameters
Multiple regression analyses examining PCB and Hg exposures to latencies and amplitudes of the N1 and P3b components are presented in Table 5. After control for confounders, cord Hg concentrations are significantly associated with longer N1 latencies and larger N1 amplitudes (i.e., higher Hg levels result in more negative voltage) in the target condition. Cord Hg concentrations are not related to P3b parameters, and child Hg is not related to any ERP component. Neither cord nor child PCB 153 at testing is related to the outcomes examined.
Table 5.
Regression analyses for the relations between contaminants and ERP parameters
| Cord Hg |
Cord PCB 153 |
11-year Hg |
11-year PCB 153 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | r | Std β | r | Std β | R | Std β | r | Std β | |
| Standard condition | |||||||||
| N1 latency | 113 | 0.06 | 0.04 d,o,e,f,b,c,g | 0.01 | −0.02 d,o,e,f,b,c,g | −0.01 | 0.04 d,o,e,f,b,c,g | 0.04 | −0.00 o,e,f,b,c,g |
| N1 amplitude | 113 | −0.18# | −0.13 i,g | 0.01 | 0.01 i,g | −0.08 | −0.04 i,g | −0.02 | 0.03 i,g |
| P3b latency | 51 | −0.02 | −0.07 k,c,b | −0.10 | −0.12 k,c,b | −0.03 | 0.11 k,c,b | 0.03 | 0.06 k,c |
| P3b amplitude | 51 | −0.10 | −0.06 k,h,d | −0.04 | −0.08 k,h,d | −0.04 | −0.06 k,d | −0.06 | 0.09 f |
| Target condition | |||||||||
| N1 latency | 99 | 0.17# | 0.29**d,m | 0.08 | 0.08 d,m,f | −0.09 | 0.02 d,c,l,f | −0.04 | 0.03 c,l,f |
| N1 amplitude | 99 | −0.21* | −0.32**p,k | −0.07 | −0.12 p,k,i | −0.11 | −0.14 k,n | −0.06 | −0.17 p,k |
| P3b latency | 118 | −0.07 | −0.16#j | 0.03 | 0.05 j,d,a | 0.14 | 0.10 j,d | 0.14 | 0.07 j,a |
| P3b amplitude | 118 | 0.01 | 0.10 c,g | −0.09 | −0.08 c | 0.01 | 0.08 c,g | 0.00 | 0.08 c,g |
Abbreviations: Hg: mercury; PCB 153: polychlorinated biphenyl congener IUPAC 153.
Note. Because N1 has negative voltage, negative (−) associations between contaminants and N1 amplitude mean greater (more negative) amplitude, while positive (+) associations mean reduced (more positive) amplitude; confounding variables included in the final regression analyses:
child age
child gender
testing location
breast-feeding status
maternal age
maternal binge drinking during pregnancy
Hollingshead SES score
maternal Raven score
number of trials retained in the averaged waveform
haemoglobin status
cord DHA
child DHA
child Se
time at testing
maternal regular use of marijuana during pregnancy
parity
p < 0.01;
p < 0.05;
p < 0.10.
Cord blood DHA was associated with smaller N1 amplitude (r = 0.20, p = 0.05) and its inclusion in the regression model increases the standardised regression coefficient for the cord Hg-N1 amplitude relation from −0.29 (p = 0.007) to −0.32 (p = 0.003). Child blood Se levels at 11 years are related to shorter N1 latency (r = −0.21, p = 0.04), and the standardised regression coefficient for the cord Hg-N1 latency relation increases from 0.20 (p = 0.06) to 0.29 (p = 0.005) after including this confounder in the model, indicating that it too acts as a suppressor.
We underline that Pb exposure, which was considered as a potential confounder, was not associated with any auditory oddball ERP measure and was thus not included in the final regression models.
3.2.3 Associations between contaminants and ERP parameters as a function of breastfeeding duration
Independent-samples t-tests show that children with longer breast-feeding duration have higher cord Hg concentrations (mean: 24.9 µg/L) compared to those with shorter breast-feeding duration (mean: 17.0 µg/L) (t (114) = −2.72, p < 0.01). However, these groups do not differ in terms of cord PCB 153 concentrations (mean: 127.9 ng/g fat in children breast-fed < 3 months vs 132.1 ng/g fat in those breast-fed ≥ 3 months; t(114) = 0.20, p = 0.85). Non-verbal intelligence scores are moderately (although not significantly) higher in mothers who breast-fed for a longer duration (raw score mean: 36.8 vs 33.9 on the Raven Progressive Matrices; t(114) = −1.65, p = 0.10), and there is no significant difference for maternal education or socioeconomic status (both p ‘s > 0.20).
Regression analyses examining the associations of cord Hg and PCBs with the ERP parameters separately for the children who were breast-fed less than 3 months vs. those who were breast-fed for a longer period of time are presented in Table 6. The relations of cord Hg to N1 latency and amplitude to the target stimulus are markedly stronger for the children who received little or no breast-feeding when compared with the data for the sample as a whole (Table 5), and an association between cord Hg and larger N1 amplitude in the standard condition emerges in this group that was not seen in Table 5. By contrast, none of these associations are seen in the children who were breast-fed for at least 3 months. An association between cord Hg and shorter P3b latency in the target condition is seen only in the minimally breast-fed group. In addition, an association between cord PCB 153 and reduced target P3b amplitude emerges solely in the minimally breast-fed group.
Table 6.
Regression analyses relating seafood contaminants in cord blood to ERP parameters in children, comparing children with shorter vs. longer duration of breast-feeding
| Cord Hg |
Cord PCB 153 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast-fed < 3 months |
Breast-fed ≥ 3 months |
Breast-fed < 3 months |
Breast-fed ≥ 3 months |
|||||||||
| N | r | Std β | N | r | Std β | N | R | Std β | N | r | Std β | |
| Standard condition | ||||||||||||
| N1 latency | 49 | 0.26 # | 0.12 | 62 | 0.05 | 0.04 | 49 | 0.13 | 0.09 | 62 | −0.07 | −0.03 |
| N1 amplitude | 49 | −0.45** | −0.37* | 62 | 0.07 | 0.11 | 49 | −0.08 | −0.16 | 62 | 0.07 | 0.11 |
| P3b latency | 19 | −0.16 | −0.22 | 30 | 0.07 | 0.03 | 19 | −0.25 | −0.23 | 30 | −0.03 | −0.06 |
| P3b amplitude | 19 | −0.36 | −0.20 | 30 | −0.12 | −0.16 | 19 | 0.08 | 0.02 | 30 | −0.20 | −0.15 |
| Target condition | ||||||||||||
| N1 latency | 42 | 0.38* | 0.43** | 55 | 0.10 | 0.16 | 42 | 0.30* | 0.15 | 55 | −0.08 | 0.09 |
| N1 amplitude | 42 | −0.43** | −0.50** | 55 | 0.04 | −0.07 | 42 | −0.23 | −0.23 | 55 | 0.05 | −0.01 |
| P3b latency | 52 | 0.03 | −0.05 | 65 | −0.17 | −0.26* | 52 | 0.17 | 0.16 | 65 | −0.08 | −0.07 |
| P3b amplitude | 52 | −0.09 | 0.08 | 65 | 0.11 | 0.15 | 52 | −0.34* | −0.32* | 65 | 0.11 | 0.12 |
Note. Confounding variables included in the models were the same as in Table 5 for analyses involving cord PCB 153 and cord MeHg as an independent variables.
p < 0.01;
p < 0.05;
p < 0.10.
The PCB-153 analyses were re-run twice—once using congener 118 (the only congener among those measured in this study with dioxin-like properties) as the exposure measure and then using the sum of four PCB congeners (118, 138, 153 and 180), the exposure measure used in the Dutch cohort study [e.g., 53]. These three indexes of prenatal PCB exposure provided virtually identical results than those reported with PCB 153 (see Table 7).
Table 7.
Associations between cord PCB and P3b parameters (target condition) according to the exposure variable and breast-feeding duration
| Cord PCB153 |
Cord PCB118 |
Cord ∑PCB118,138,153,180 |
|||||
|---|---|---|---|---|---|---|---|
| N | r | Std β | r | Std β | r | Std β | |
| Total sample | |||||||
| P3b latency | 118 | 0.03 | 0.05 | 0.06 | 0.06 | 0.03 | 0.05 |
| P3b amplitude | 118 | −0.04 | −0.08 | −0.11 | −0.06 | −0.09 | −0.07 |
| Breast-fed < 3 months | |||||||
| P3b latency | 52 | 0.17 | 0.16 | 0.22 | 0.17 | 0.17 | 0.15 |
| P3b amplitude | 52 | −0.34* | −0.32* | −0.36* | −0.32* | −0.33* | −0.31* |
| Breast-fed ≥ 3 months | |||||||
| P3b latency | 65 | −0.08 | −0.07 | −0.06 | −0.02 | −0.08 | −0.05 |
| P3b amplitude | 65 | 0.11 | 0.12 | 0.06 | 0.13 | 0.11 | 0.13 |
Note. Confounding variables included in the models were the same as in Table 5 for analyses involving cord PCB 153 as an independent variable.
p < 0.01;
p < 0.05;
p < 0.10.
4. Discussion
This study examined the associations between developmental exposure to Hg and PCBs and information processing in 11-year-old children using ERPs during an auditory oddball task. We found that cord blood Hg concentration, a well-recognized surrogate for prenatal MeHg exposure in fish-eating populations [73], was related to larger N1 amplitude and delayed N1 latency in the target condition. These effects were particularly salient in the subgroup of children who had been breast-fed for less than 3 months, a group in which cord Hg was also related to greater N1 amplitude in the standard condition. Although cord Hg levels did not relate significantly to behavioural performance on this task for the 118 children for whom reliable ERP data were obtained, cord Hg was associated with slower behavioural reaction time and fewer false alarms when all 189 children who completed the task were included in the analyses. Cord PCB 153 concentrations were associated with reduced P3b amplitude only within the subgroup of participants who had been breast-fed for less than 3 months. Current blood MeHg and PCB 153 levels were not associated with any of the outcomes assessed in this study.
The observed associations between cord Hg and the N1 parameters are indicative of subtle alterations in attentional mechanisms modulating early processing of sensory information. The longer N1 latency found in association with prenatal Hg exposure confirms our initial hypothesis and is consistent with the slower behavioural reaction time that was observed in the sample as a whole. Although the N1 component in the auditory oddball paradigm relates more directly to vigilance than sensory processing per se, it is of interest that prenatal Hg exposure was related to longer sensory evoked potential latencies in the auditory [7,74,75] and visual [26] modalities in previous studies. Delayed latencies for the auditory N1 may also be caused by general prolongation of nerve conduction velocity [e.g., 76].
We had not predicted that prenatal Hg would be associated with larger N1 amplitude, which suggests greater brain responsiveness. This surprising finding may reflect a state of ―overarousal‖ or hypervigilance caused by MeHg exposure, possibly resulting from a GABA-glutamate imbalance. On the one hand, MeHg is known to selectively alter GABA receptor function, leading to a decrease in inhibitory neurotransmission [77,78]. A loss of inhibition in the brain may thus alter ERP amplitude. For instance, N1 amplitude in the visual domain increases drastically following pharmacologically blocking of GABA receptors [79]. On the other hand, it is also known that MeHg inhibits glutamate uptake by astrocytes [80,81]. The resulting accumulating glutamate concentrations might account for our apparently paradoxical relation of Hg to N1 amplitude as N1 amplitude is sensitive to glutamatergic activity [82]. These explanations are indirect, and further experimental studies are needed to account for this result.
Recent research has demonstrated the importance of considering the potential beneficial effects of fish consumption when examining the association between seafood contaminants and cognitive development [83,84,85]. This was done in the present study by documenting Se and DHA concentrations in cord and child blood samples as potential confounders or suppressors of the relations between seafood contaminants and ERP parameters. This approach was justified by experimental and epidemiological evidence of beneficial and/or protective effects of these nutrients on child development [57,86]. In our study, child Se was related to shorter N1 latency in the target condition, and controlling for this nutrient variable strengthened the association between of cord Hg to N1 latency. Statistical control for cord DHA also strengthened the association between Hg and N1 amplitude. Failure to control for these nutritional influences would have obscured or understated the neurotoxicity of the MeHg exposure. These findings, therefore, give further support for the importance of measuring these nutritional variables when attempting to detect neurotoxic effects of MeHg exposure within fish-eating populations.
We recently reported significant associations between auditory oddball P3b parameters and behavioural performance on neuropsychological tests of processing speed, selective attention and learning in the children in this study cohort [49]. Because prenatal exposures to both MeHg and PCBs have been related to poorer performance in these domains in previous studies of children exposed to comparable levels of these contaminants [7,10,23,87], we expected that both pollutants would affect the P3b by prolonging its latency and/or by reducing its amplitude. However, only PCB exposure affected the P3b component in the expected direction and this, only within the subgroup of children who were breast-fed for less than 3 months. This finding is consistent with the effects of prenatal PCB exposure on infant recognition memory and school-age selective attention and working memory observed in the Michigan and Oswego cohorts [18,19,20,22,24]. The effects of cord Hg on the N1 component suggest that cord Hg affects earlier stages of information processing, those when the initial mechanisms of attention modulate sensory signals. Other processes which are not reflected in either the N1 or P3b, such as those involved in motor response programming and execution, may also be specifically affected by prenatal MeHg exposure [e.g., 6,7] and could account for the observed relation between cord Hg and slower mean reaction time on the auditory oddball task.
The auditory oddball protocol that was used in this study was designed to be easy to perform in order to maximise the number of artefact-free trials and to generate well-defined ERP components associated with different stages of information processing. The absence of a PCB effect on the behavioural parameters assessed during this task might be explained by the low demands it puts on higher-order cognitive processes, and is thus not inconsistent with other studies using more challenging, traditional neuropsychological assessments. For instance, in the Michigan study, prenatal PCB exposure did not affect the children’s response to a single target during a continuous performance task, but it did impair their performance when more complex processes were involved, such as mental rotation and behavioural inhibition [24]. The sensitivity of the P3b component to prenatal PCB exposure in the present study is consistent with previous ERP studies from Taiwan [52] and the Netherlands [53], although we did not replicate their finding of delayed P3b latency.
The finding of reduced P3b amplitude only in children who were either not breast-fed or were breast-fed for a short period of time is consistent with two previous studies reporting PCB effects on cognitive performance that were limited to non-breast-fed children [23,24]. Moreover, this study is the first to find that adverse effects associated with prenatal MeHg exposure are also more salient in children who received only minimal breast-feeding. Although our findings support a moderating role of breast-feeding on the adverse effects of prenatal exposure to seafood contaminants, further research is needed to understand the mechanisms responsible for this effect. It is possible that certain nutrients contained in breast milk, such as DHA, counteract the adverse effects of perinatal brain injury [57,88]. Alternatively, Jacobson and Jacobson [89] have suggested that breast-feeding mothers might offer more optimal intellectual stimulation to their children, which might help compensate for subtle teratogenic effects.
5. Conclusions
This study was the first to use cognitive ERPs in a birth-cohort of children exposed to Hg during prenatal development and the third to find effects using this methodology in PCB-exposed children. Our data suggest specific associations between Hg exposure and N1-mediated attentional mechanisms modulating early processing of sensory information but not with the later cognitive processes that have been linked to the P3b component. By contrast, P3b amplitude was sensitive to prenatal PCB exposure in children who were not breast-fed or breast-fed only minimally, a result compatible with findings from studies in other cohorts. Previous studies have linked prenatal exposure to MeHg and PCBs to poorer child performance on standard neuropsychological tests. Our findings illustrate the utility of ERPs for going beyond neurobehavioral assessments to identify specific stages of information processing that may be impaired by a given contaminant. These data also provide additional evidence that two sets of variables can protect against the adverse effects of these environmental exposures: 1) nutrients, such as DHA and Se, which are found in traditional Inuit food and 2) breast-feeding.
Acknowledgments
We are grateful to the Nunavik population and to all people involved in this study. We thank Renee Sun, Line Roy, Brenda Tuttle, Jocelyne Gagnon, Alacie Puv, Neil Dodge, François Doré and Philip Jackson for their valuable contribution. This research was supported by the NIH/National Institute of Environmental Health and Sciences, Health Canada, Indian and Northern Affairs Canada, and Joseph Young, Sr., Fund of Michigan. O. Boucher was supported by doctoral grants from the Canadian Institutes of Health Research and from the Nasivvik Centre for Inuit Health and Changing Environments.
Footnotes
In the third group, participants within the highest and lowest quartiles for cord blood Hg, PCB 153, and docosahexaenoic acid (DHA) concentrations were given priority for recruitment. However, because the Hg, PCB153, and DHA levels were moderately intercorrelated, the distributions of contaminants in this group of children was very similar to those in Groups 1 and 2, which were not selected according to any exposure level criteria.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 2.Tsubaki T, Irukayama K. Minamata disease: methylmercury poisoning in Minamata and Niigata. New York: Japan, Elsevier; 1977. [Google Scholar]
- 3.Eto K. Minamata disease. Neuropathology. 2000;20:S14–S19. doi: 10.1046/j.1440-1789.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 4.Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 5.Choi BH. The effects of methylmercury on the developing brain. Prog. Neurobiol. 1989;32:447–470. doi: 10.1016/0301-0082(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 6.Chevrier C, Sullivan K, White RF, Comtois C, Cordier S, Grandjean P. Qualitative assessment of visuospatial errors in mercury-exposed Amazonian children. Neurotoxicology. 2009;30:37–46. doi: 10.1016/j.neuro.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 8.Davidson PW, Sloane-Reeves J, Myers GJ, Hansen ON, Huang LS, Georger LA, et al. Association between prenatal exposure to methylmercury and visuospatial ability at 10.7 years in the Seychelles Child Development Study. Neurotoxicology. 2008;29:453–459. doi: 10.1016/j.neuro.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata K, Grandjean P, Dekeishi M. Neurophysiological evidence of methylmercury neurotoxicity. Am. J. Ind. Med. 2007;50:765–771. doi: 10.1002/ajim.20471. [DOI] [PubMed] [Google Scholar]
- 10.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandjean P, White RF, Nielsen A, Cleary D, Santos ECO. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ. Health Perspect. 1999;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjellström T, Kennedy P, Wallis S, Stewart A, Friberg L, Lind B, et al. Physical and mental development of children with prenatal exposure to mercury from fish. Stage 2. Interviews and psychological tests at age 6. Solna, Sweden: National Swedish Environmental Protection board Report 3642; 1989. [Google Scholar]
- 13.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles child development study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 14.Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 15.Brinkman UAT, De Kok A. Production, properties and usage. In: Kimbrough RD, editor. Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products. Topics in environmental health. Vol. 4. New York: Elsevier; 1980. pp. 1–40. [Google Scholar]
- 16.Guo YL, Lambert GH, Hsu CC, Hsu MML. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occup. Environ. Health. 2004;77:153–158. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- 17.Hsu ST, Ma CI, Hsu SKH, Wu SS, Hsu NHM, Yeh CC, et al. Discovery and epidemiology of PCB poisoning in Taiwan: a four-year followup. Environ. Health Perspect. 1985;59:5–10. doi: 10.1289/ehp.59-1568088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56:853–860. [PubMed] [Google Scholar]
- 19.Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to PCBs and infant performance on the Fagan Test of Infant Intelligence. Neurotoxicology. 2000;21:1029–1038. [PubMed] [Google Scholar]
- 20.Ko H, Yao B, Chang F-M, Hsu C-C, Jacobson SW, Jacobson JL. Preliminary evidence of recognition memory deficits in infants born to Yu-cheng exposed women. In: Fiedler H, Hutzinger O, Birnbaum L, Lambert G, Needham L, Safe S, editors. Dioxin '94. Kyoto: Kyoto University; 1994. pp. 505–508. [Google Scholar]
- 21.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 22.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ. Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- 25.Patandin S, Lanting CI, Mulder PGH, Boersma ER, Sauer PJJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 1999;134:33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- 26.Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, et al. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Otto DA. The assessment of neurotoxicity in children. Electrophysiological methods. Monogr. Am. Assoc. Ment. Defic. 1987;8:139–158. [PubMed] [Google Scholar]
- 28.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 29.Hansenne M. Le potential évoqué cognitif P300 (I): aspects théorique et psychobiologique [in French] Neurophysiol. Clin. 2000;30:191–210. doi: 10.1016/s0987-7053(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 30.Woods DL. The component structure of the N1 wave of the human auditory evoked potential. Electroencephalogr. Clin. Neurophysiol. 1995;(Suppl. 44):102–109. [PubMed] [Google Scholar]
- 31.García-Larrea L, Lukaszewicz AC, Mauguière F. Revisiting the oddball paradigm. Non-target vs neutral stimuli and the evaluations of ERP attentional effects. Neuropsychologia. 1992;30:723–741. doi: 10.1016/0028-3932(92)90042-k. [DOI] [PubMed] [Google Scholar]
- 32.Näätänen R. Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 33.Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 34.Campbell KB, Colrain IM. Event-related potential measures of the inhibition of information processing: II. The sleep onset period. Int. J. Psychophysiol. 2002:197–214. doi: 10.1016/s0167-8760(02)00112-5. [DOI] [PubMed] [Google Scholar]
- 35.Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- 36.Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, et al. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc. Natl. Acd. Sci. USA. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller-Gass A, Campbell K. Event-related potential measures of the inhibition of information processing: I. Selective attention in the waking state. Int. J. Psychophysiol. 2002;46:177–195. doi: 10.1016/s0167-8760(02)00111-3. [DOI] [PubMed] [Google Scholar]
- 38.Donchin E. Surprise!… Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 39.Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11:357–374. [Google Scholar]
- 40.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desmedt JE. P300 in serial tasks: an essential post-decision closure mechanism. Prog. Brain Res. 1980;54:682–686. doi: 10.1016/S0079-6123(08)61690-8. [DOI] [PubMed] [Google Scholar]
- 42.Verleger R. Event-related potentials and cognition: a critique of the context updating hypothesis and an alternative interpretation of P3. Behav. Brain Sci. 1988;11:343–427. [Google Scholar]
- 43.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol. 1988;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 44.Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- 46.Polich J, Howard L, Starr A. P300 latency correlates with digit span. Psychophysiology. 1983;20:665–669. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 47.Walhovd KB, Fjell A. The relationship between P3 and neuropsychological function in an adult life span sample. Biol. Psychol. 2002;62:65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 48.Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol. Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- 49.Boucher O, Bastien CH, Muckle G, Saint-Amour D, Jacobson SW, Jacobson JL. Behavioural correlates of the P3b event-related potential in school-age children. Int. J. Psychophysiol. doi: 10.1016/j.ijpsycho.2010.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurrera RJ, Salisbury DF, O'Donnell BF, Nestor PG, McCarley RW. Auditory P3 indexes personality traits and cognitive function in healthy men and women. Psychiatry Res. 2005;133:215–228. doi: 10.1016/j.psychres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Karis D, Fabiani M, Donchin E. “P300” and memory: individual differences in the von Restorff effect. Cogn. Psychol. 1984;16:177–216. [Google Scholar]
- 52.Chen YJ, Hsu CC. Effects of prenatal exposure to PCBs on the neurological function of children: a neuropsychological and neurophysiological study. Dev. Med. Child Neurol. 1994;36:312–20. doi: 10.1111/j.1469-8749.1994.tb11851.x. [DOI] [PubMed] [Google Scholar]
- 53.Vreugdenhil HJI, Van Zanten GA, Brocaar MP, Mulder PGH, Weisglas-Kuperus N. Prenatal exposure to polychlorinated biphenyls and breastfeeding: opposing effects on auditory P300 latencies in 9-year-old Dutch children. Dev. Med. Child Neurol. 2004;46:398–405. doi: 10.1017/s0012162204000647. [DOI] [PubMed] [Google Scholar]
- 54.Boucher O, Muckle G, Saint-Amour D, Dewailly É, Ayotte P, Jacobson SW, et al. The relation of lead neurotoxicity to the event-related potential P3b component in Inuit children from Arctic Québec. NeuroToxicology. 2009;30:1070–1077. doi: 10.1016/j.neuro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ. Health Perspect. 2001;109:1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muckle G, Dewailly E, Ayotte P. Prenatal exposure of Canadian children to polychlorinated biphenyls and mercury. Can. J. Public Health. 1998;89:S20–S25. [PubMed] [Google Scholar]
- 57.Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J. Pediatr. 2008;152:356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Després C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, et al. Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol. Teratol. 2005;27:245–257. doi: 10.1016/j.ntt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J, Polich J. On the number of trials needed for P300. Int. J. Psychophysiol. 1997;25:249–255. doi: 10.1016/s0167-8760(96)00743-x. [DOI] [PubMed] [Google Scholar]
- 60.Czernochowski D, Mecklinger A, Johansson M, Brinkmann M. Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adults. Cogn. Affect. Behav. Neurosci. 2005;5:417–433. doi: 10.3758/cabn.5.4.417. [DOI] [PubMed] [Google Scholar]
- 61.Rueda MR, Posner MI, Rothbart MK, Davis-Stober CP. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5:39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhainds M, Levallois P, Dewailly E, Ayotte P. Lead, mercury, and organochlorine compound levels in cord blood in Québec, Canada. Arch. Environ. Health. 1999;54:40–47. doi: 10.1080/00039899909602235. [DOI] [PubMed] [Google Scholar]
- 63.Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ. Health Perspect. 2009;117:1014–1020. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit cohort study. Environ. Health Perspect. 2003;111:1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollingshead AB. Four Factor Index of Social Status, Yale University Department of Sociology. New Heaven: 1975. Unpublished Manuscript. [Google Scholar]
- 66.Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary scales: standard progressive matrices, Psychologists. Oxford: 1992. [Google Scholar]
- 67.Winer BJ. Statistical principles in experimental design. second. McGraw-Hill; NewYork: 1971. [Google Scholar]
- 68.Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. Lippincott: Williams, & Wilkins, Philadephia; 1998. pp. 253–79. [Google Scholar]
- 69.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am. J. Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 70.Jacobson JL, Humphrey HEB, Jacobson SW, Schantz SL, Mullin MD, Welch R. Determinants of polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), and dichlorodiphenyl trichloroethane (DDT) levels in the sera of young children. Am. J. Public Health. 1989;79:1401–1404. doi: 10.2105/ajph.79.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnstone SJ, Barry RJ, Anderson JW, Coyle SF. Age-related changes in child and adolescent event-related potential component morphology, amplitude and latency to standard and target stimuli in an auditory oddball task. Int. J. Psychophysiol. 1996;24:223–238. doi: 10.1016/s0167-8760(96)00065-7. [DOI] [PubMed] [Google Scholar]
- 72.Mueller V, Brehmer Y, von Oertzen T, Li S-C, Lindenberger U. Electrophysiological correlates of selective attention: a lifespan comparison. BMC Neurosci. 2008;9 doi: 10.1186/1471-2202-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am. J. Epidemiol. 1999;150:301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- 74.Murata K, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Grandjean P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J. Pediatr. 2004;144:177–183. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 75.Murata K, Weihe P, Renzoni A, Debes F, Vasconcelos R, Zino F, et al. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotoxicol. Teratol. 1999;21:343–348. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- 76.Papp A, Nagymajtényi L, Vezér T. Subchronic mercury treatment of rats in different phases of ontogenesis: functional effects on the central and peripheral nervous system. Food Chem. Toxicol. 2005;43:77–85. doi: 10.1016/j.fct.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol. Sci. 2005;26:549–557. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and γ-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol. Appl. Pharmacol. 2005;204:343–354. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Zemon V, Kaplan E, Ratliff F. Bicuculline enhances a negative component and diminishes a positive component of the visual evoked cortical potential in the cat. Proc. Natl. Acad. Sci. 1980;77:7476–7478. doi: 10.1073/pnas.77.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albrecht J, Talbot M, Kimelberg HK, Aschner M. The role of sulfhydryl groups and calcium in the mercuric chloride-induced inhibition of glutamate uptake in rat primary astrocyte cultures. Brain Res. 1993;607:249–254. doi: 10.1016/0006-8993(93)91513-r. [DOI] [PubMed] [Google Scholar]
- 81.Aschner M, Yaho CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem. Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 82.Watson TD, Petrakis LI, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int. J. Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ. Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–75. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol. Teratol. 1999;21:83–88. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson JL, Jacobson SW, Humphrey HEB. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J. Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka D, Kon N, Ohkawa N, Yoshikawa N, Shimizu T. Does breastfeeding in the neonatal period influence the cognitive function of very-low-birth-weight infants at 5 years of age? Brain Dev. 2009;31:288–293. doi: 10.1016/j.braindev.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Jacobson JL, Jacobson SW. Breast-feeding and gender as moderators of teratogenic effects on cognitive development. Neurotoxicol. Teratol. 2002;24:349–358. doi: 10.1016/s0892-0362(02)00197-6. [DOI] [PubMed] [Google Scholar]