Abstract
The increased application of transcriptome-wide profiling approaches has led to an explosion in the number of documented long non-coding RNAs (lncRNAs). While these new and enigmatic players in the complex transcriptional milieu are encoded by a significant proportion of the genome, their functions are mostly unknown. Early discoveries support a paradigm in which lncRNAs regulate transcription via chromatin modulation, but new functions are steadily emerging. Given the biochemical versatility of RNA, lncRNAs may be used for various tasks, including post-transcriptional regulation, organization of protein complexes, cell-cell signalling and allosteric regulation of proteins.
Dedicated consortiums, such as the ENCODE (Encyclopedia of DNA Elements) project, have markedly expanded our knowledge of what lies in the dark recesses of the genome through their extensive annotation efforts1. These findings in conjunction with previous studies looking specifically at transcriptional outputs have underscored the pervasiveness with which genomes are transcribed2,3. An important implication of these findings is that whereas only a minuscule fraction of the human genome encodes proteins, nearly 60% is represented in processed transcripts that seem to lack protein-coding capacity4. Together with observations that more sophisticated organisms tend to have more non-coding DNA, this raises the possibility that the barren regions between genes are actually elysian fields’ rich with information5. The implications of this are undeniably intriguing, but we are still far from ascribing biological functions to the vast array of non-coding RNA (ncRNA) transcripts. With thousands of documented ncRNAs, pervasive transcription has been described in virtually all eukaryotic organisms6,7.
For the better part of the past decade, particular attention has focused on the exploding class of transcripts referred to as long non-coding RNAs (lncRNAs), arbitrarily defined as being longer than 200 nucleotides7,8. Given the prevalence of lncRNA expression, it has been posited that lncRNAs might constitute a significant fraction of the functional output of mammalian genomes7–9. Such notions have been met with considerable, and quite possibly legitimate, scepticism10. Indeed, the documentation of pervasive transcription has far outpaced the molecular characterization of the transcripts produced. Although some lncRNA transcripts may represent transcriptional noise, a small but steadily growing list has authentic biological roles6,11–13. For example, lncRNAs have been implicated in regulating imprinting, dosage compensation, cell cycle regulation, pluripotency, retro-transposon silencing, meiotic entry and telomere length, to name just a few12,13. Despite these advances, most lncRNAs remain partially uncharacterized. Additionally, there has been a heavy focus so far on the ways that lncRNAs regulate chromatin states, and this emphasis probably underrepresents the full repertoire of lncRNA function. Nonetheless, the rapidly growing lncRNA field is already changing not just our perspective of genomic content, but also the way we think about genes.
In this Review, we focus on the functional attributes of RNA and highlight the unconventional, and perhaps underappreciated, biological contributions of lncRNAs, including the diverse mechanisms through which lncRNAs participate in transcriptional regulation. We touch briefly on the roles of lncRNAs in regulating chromatin states, as this has been explored in several recent reviews (see REFS 8,9,13–15). In addition, we highlight roles beyond transcription whereby lncRNAs function in various cellular contexts, including post-transcriptional regulation, post-translational regulation of protein activity, organization of protein complexes, cell-cell signalling, as well as recombination.
A biochemically versatile polymer
RNA is a versatile molecule making it well suited for a myriad of functions. It is this feature that inspired the ‘RNA world hypothesis’ in which it was postulated that billions of years ago, RNA provided the precursors of all life16. The multifunctionality of RNA stems from several unique physiochemical properties. First, and perhaps most obvious, is its ability to base pair with other nucleic acids (FIG. 1a). RNA is, therefore, particularly adept at recognizing both RNA and DNA targets through simple one-to-one base pairing interactions. By comparison, proteins such as transcription activator-like effectors (TALEs) and PUF proteins require 100 times more genomic sequence space than an RNA to achieve sequence-specific binding17. Moreover, because two RNA transcripts can base pair at any point during the life cycle of the target mRNA, regulatory RNAs can influence transcription, processing, editing, translation or degradation of target mRNAs. Second, RNA molecules can fold into intricate three-dimensional structures that provide complex recognition surfaces (FIG. 1b). This structure expands the large variety of molecular targets that RNA can bind with high affinity and specificity. RNA structures can even be selected for in vitro to bind to anything from small molecules to proteins18. Third, in terms of both expression and structure, RNA is dynamic. More explicitly, because RNA can be rapidly transcribed and degraded, it is well suited for dynamic, transient expression (FIG. 1c). Moreover, without the need to be translated, a regulatory RNA gene could transition faster from being transcriptionally inactive to fully functional. In addition, as conformational changes can be triggered by ligand binding, RNA structures themselves can be very dynamic19. Fourth, RNA is malleable and therefore provides an excellent platform for evolutionary innovation (FIG. 1d). Specifically, unencumbered by amino acid-coding potential, regulatory RNAs are less restricted in terms of their conservation. As such, RNAs are more tolerant of mutations, which could allow for the rapid evolution of diverse cellular activities. Last, RNA-dependent events can have the capacity to be heritable. This idea is supported by the demonstration of RNA-templated modifications to the genome (FIG. 1e). For example, retroviral genomic integrations as well as the presence of thousands of processed pseudogenes suggest that information housed within mature RNA transcripts can be integrated back into the genome20,21. These instances of RNA-mediated events that have manifested in genomic change suggest it is possible for other RNA-dependent events to become heritable. Importantly, these defining properties of RNAs raise exciting possibilities as to what roles lncRNAs could have in the cell. Although various functional roles have now been attributed to lncRNAs, it is likely that as we dig deeper into the molecular biology of lncRNAs more functions will emerge.
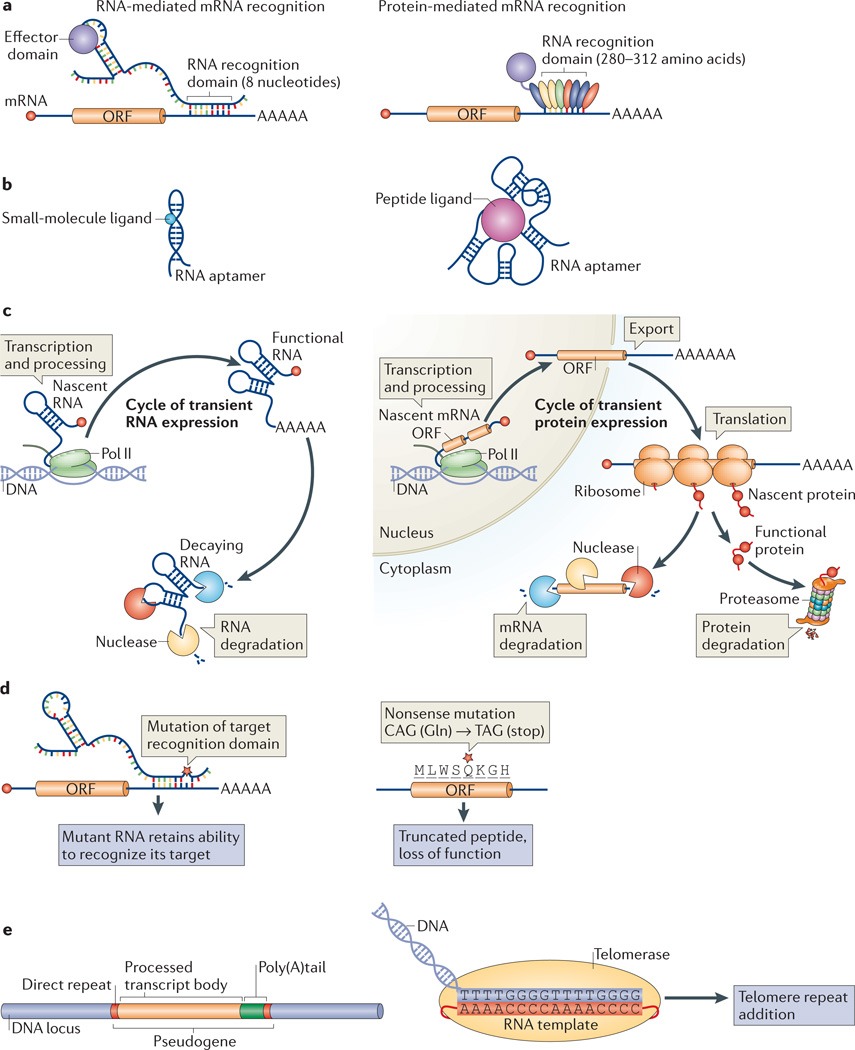
Figure 1. RNA is a biochemically versatile polymer.
a | RNA is particularly well suited for sequence-specific nucleic acid targeting through base pairing interactions over a short region (for example, eight nucleotides). By contrast, proteins require repeat motifs comprising 35–39 amino acids (105–117 base pairs of genomic sequence) to recognize a single RNA base with specificity. Therefore, to recognize eight nucleotides, 280–312 amino acids (840–936 base pairs of genomic sequence) would be required. Compared to the eight base pairs required for an RNA, protein-based nucleic acid recognition requires substantially more genomic sequence17.b | RNA can fold into complex three-dimensional structures that can specifically bind various ligands, including small molecules and peptides18.c | RNA is suitable for transient expression, because a fully functional RNA can be generated immediately following transcription and processing but can also be rapidly degraded. Together, this allows RNA effectors to be produced in quick pulses. Proteins, however, require additional steps, including mRNA export and translation, to produce a functional peptide. Likewise, both the mRNA and the protein need to be degraded to turn off expression, d | RNA is malleable and, therefore, more tolerant of mutations. Although some mutations in protein-coding genes are silent, many are deleterious such as nonsense mutations that generate truncated polypeptides. RNA, however, can tolerate mutations even within the regions responsible for target recognition. e | RNA-dependent events can be heritable. For instance, processed pseudogeneswere once RNA transcripts that have beengenomically integrated. In add it ion, telomerase uses an RNA template to add telomeric repeats to the ends of chromosomes. ORF, open reading frame; Pol II, RNA polymerase II.
lncRNAs as regulators of transcription
The number of lncRNAs with described functions is steadily increasing, and many of these reports revolve around their regulatory capacity. For example, lncRNAs often function as important cis- and trans-acting modulators of protein-coding gene expression8. A common theme has emerged in which lncRNAs regulate transcription via chromatin modulation (for reviews, see REFS 8,13,15). lncRNAs across a broad range of eukaryotes affect chromatin context, suggesting that this is a conserved function despite the fact that the transcripts themselves are often not conserved12. Numerous lncRNAs physically associate with, and potentially target, histone-modifying activities to specific loci22,23 TABLE 1). lncRNAs such as HOTAIR (HOX transcript antisense RNA), ANRIL (also known as CDKN2B anti-sense RNA 1) and KCNQ1OT1 (KCNQ1 opposite strand or antisense transcript 1) have even been shown to bind more than one histone-modifying complex. As such, a paradigm in which lncRNAs can act as scaffolds that organize the concerted actions of chromatin-modifying complexes spatially and temporally is emerging15,24–28 (FIG. 2a; TABLE 1). For example, HOTAIR physically associates not only with Polycomb repressive complex 2 (PRC2) but also with LSD1 (Lys-specific demethylase 1)24. PRC2 and LSD1 are responsible for the deposition of the repressive histone mark trimethylated Lys27 of histone H3 (H3K27me3) and removal of active H3K4me2 marks, respectively. Moreover, global analyses suggest that a large number of other lncRNAs can also bind PRC2 and LSD1 (REF. 22). In addition, other lncRNAs have been shown to bind overlapping but distinct combinations of histone-modifying complexes. For example, KCNQ1OT1 binds PRC2 and the methyltransferase G9A (also known as EHMT2), whereas ANRIL binds PRC1 and PRC2 (REFS 26–28) (TABLE 1). HOTAIR and other lncRNAs have, therefore, been proposed to function as scaffolds that coordinate the targeting of distinct repressive histone-modifying complexes to target loci25. However, within this framework, the detailed mechanism of how lncRNAs target specific DNA regions remains unclear.
Table 1.
lncRNA-mediated gene expression control
| lncRNA | Function | Mechanism | Refs |
|---|---|---|---|
| Regulation of mRNA transcription | |||
| XIST | X inactivation | Chromatin-mediated repression | 23,121 |
| HOTA1R | Repression at the HOXD locus |
Chromatin-mediated repression | 24,122 |
| HOTT1P | Activation at the HOXA locus | Chromatin-mediated activation | 123 |
| KCNQIOT1 | Imprinting at the KCNQ1 cluster |
Chromatin-mediated repression | 27 |
| ANR1L | Repression at the lNK4b ARF-INK4a locus |
Chromatin-mediated repression | 26,28 |
| AIRN | Imprinting at the IGF2R cluster |
Chromatin-mediated repression, transcription interference |
30 |
| ME4 antisense | Repression of ME4 mRNA | Transcription interference | 31 |
| IRT1 | Repression of IME1 mRNA | Chromatin-mediated repression | 32 |
| GAL10 lncRNA | Repression of GAL1 and CAL10 mRNAs |
Chromatin-mediated repression | 35 |
| PH084 antisense | Repression of PH084 mRNA | Chromatin-mediated repression | 33 |
| ICR1 | Repression of FLO11 mRNA | Modulation of transcription factor recruitment |
37,41 |
| PWR1 | Activation of FLO11 mRNA | Modulation of transcription factor recruitment |
37,41 |
| SRG1 | Repression of SER3 mRNA | Nucleosome remodelling | 38 |
| fbp1 ncRNA | Activation of fbp1 | Chromatin remodelling | 39 |
| UNOCR | Activation of lysozyme mRNA |
Nucleosome remodelling | 40 |
| Alu repeat-containing RNA | Transcriptional repression during heat shock |
Inhibition of Pol II | 47 |
| HSR1 | Activation of the HSF1 transcription factor |
Allosteric activation together with eEFIA | 49 |
| Non-coding DHFR | Transcriptional repression of DHFR |
Inhibition of pre-initiation complex formation |
48 |
| CAS5 | Repression of glucocorticoid receptor-mediated transcription |
DNA mimicry | 50 |
| EVF2 | Transcriptional activation of DLX2 targets, transcriptional repression of MeCP2 targets |
Recruitment of DLX2 or MeCP2 | 51,52 |
| CCND1 promoter RNA | Repression of CCND1 transcription |
Allosteric activation of TLS | 53 |
| NRON | Repression of NFAT-mediated transcription |
Inhibition of transcription factor nucleocytoplasmic shuttling |
54 |
| Regulation of mRNA processing | |||
| Neuroblastoma MYC (NAT) | Inhibition of neuroblastoma MYC intron 1 splicing |
Unknown mechanism involving the inhibition of splicing via RNA-RNA duplex formation |
61 |
| Rev-ErbAalpha | Inhibition of the c-ErbAalpha 2 splice isoform |
Unknown mechanism involving the inhibition of splicing via RNA-RNA duplex formation |
62 |
| ZEB2 (NAT) | Activation of ZEB2 translation |
Unknown mechanism involving regulated splicing of an IRES-containing intron |
59 |
| MALAT1 | Ser/Arg splicing factor regulation |
Scaffolding of subnuclear domains | 64 |
| Sas10 mRNA 3′UTR | Repression of Rnp4F mRNA | Unknown mechanism involving RNA editing |
66 |
| Modulation of mRNA post-transcriptional regulatory pathways | |||
| Antisense UCHL1 | Upregulation of UCHL1 protein production |
SINE2B element-mediated translational upregulation |
68 |
| KCS1 antisense | Production of truncated KCS1 protein |
Unknown mechanism involving base pairing | 69 |
| 1/2-sbsRNA1 | Down-regulation of SERPINE1 and FLJ21870 mRNAs |
Staufen-mediated decaythrough Alu element base pairing | 70 |
| BACE1AS | Up-regulation of BACE1 | Stabilization of BACE1 mRNA by blocking miRNA-induced repression |
71,72 |
| UNCMD1 | Control of muscle differentiation through upregulation of MAML1 and MEF2C transcript ion factors |
Sequestration of miRNAs | 74 |
| HULC | Downregulation of miRNA-mediated repression |
Sequestration of miRNAs | 75 |
| PTENP1 pseudogene | Upregulation of PTEN | Sequestration of miRNAs | 79 |
| IPS1 | Downregulation of miRNA-mediated repression |
Sequestration of miRNAs | 76 |
| CDR1as | Downregulation of miRNA-mediated repression |
Sequestration of miRNAs | 77,78 |
1/2-sbsRNA1, half-STAU1-binding site RNA1;AIRN, antisense of IGFR2 non-coding RNA; BACE1AS, beta-site APP-cleaving enzyme 1 antisense; CCND1, cyclin D1; CDR1as, CDR1 antisense; DHFR, dihydrofolate reductase; fbp1, fructose-l,6-bisphosphatase 1; eEF1A, eukaryotic elongation factor 1A; FLO11; GAS5, growth arrest specific 5; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA transcript at the distal tip; HOX, homeobox cluster; HSF1, heat shock factor 1; HSR1, heat shock RNA1; HULC, highly upregulated in liver cancer; IGF2R, insulin-like growth factor 2 receptor; IME, inducer of meiosis; IPS1, INDUCED BY PHOSPHATE STARVATION 1; IRES, internal ribosome entry site; IRT1, IME1 regulatory transcript 1; KCNQ1, potassium voltage-gated channel, KQT-like subfamily, member 1; KCNQ1OT1, KCNQ1 opposite strand or antisense transcript 1; LINOCR, LPS-inducible non-coding RNA; lncRNA, long non-coding RNA; MALAT1, metastasis associated lung adenocarcinoma transcript 1; MAML1, mastermind-like 1; MeCP2, methyl CpG binding-protein 2; MEF2C, myocyte enhancer factor 2C; miRNA, microRNA; NAT, natural antisense transcript; ncRNA, non-coding RNA; NFAT, nuclear factor of activated T cells; NRON, non-coding repressor of N FAT; Pol II, RNA polymerase II; PTENP1, phosphatase and tensin homologue; Rnp4F, RNA-binding protein 4F; TLS, translocated in liposarcoma; UCHL1, ubiquitin carboxyl-terminal esterase LI; UTR, untranslated region; XIST, X inactivation-specific transcript; ZEB2, zinc-finger E-box binding homeobox 2.
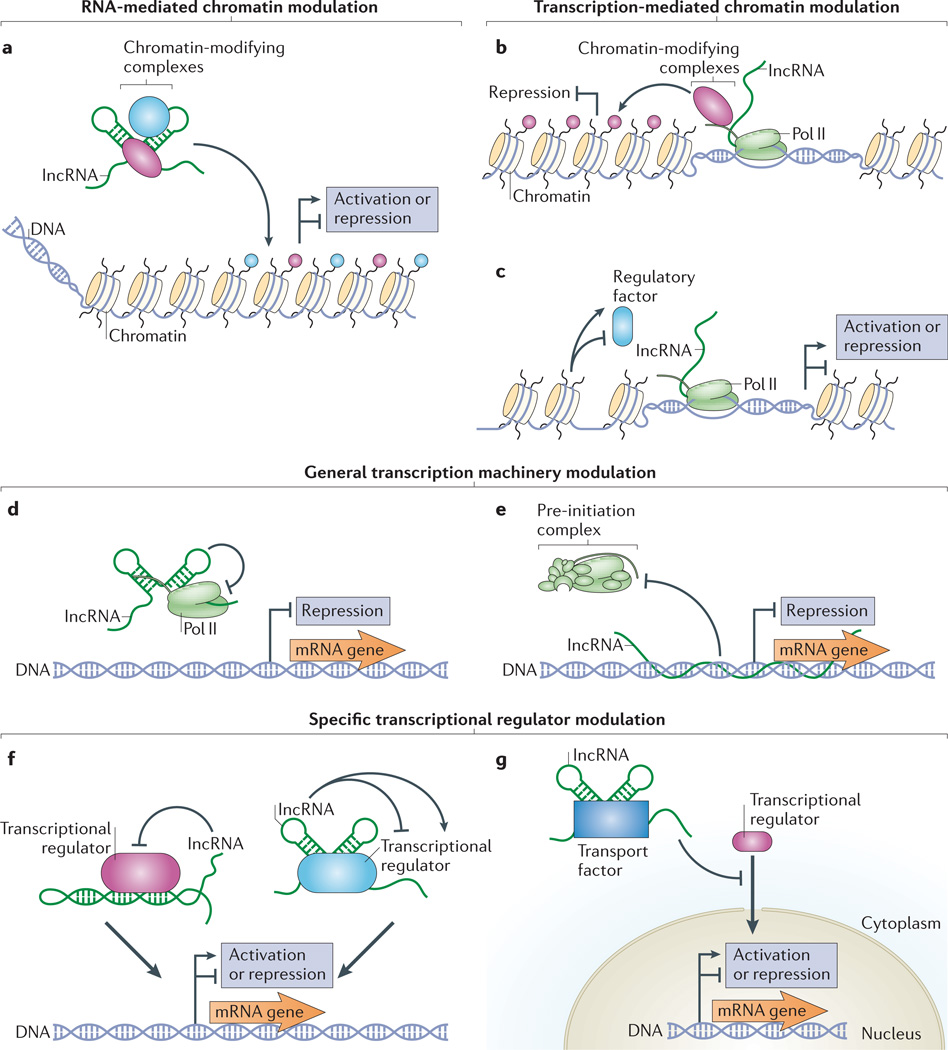
Figure 2. lncRNAs regulate transcription through several mechanisms.
a-c | Long non-coding RNAs (lncRNAs) can modulate chromatin through transcription-independent (part a) and transcription-dependent mechanisms (parts b and c). lncRNAs can bind one or more chromatin-modifying complexes and target their activities to specific DNA loci (part a). Depending on the nature of the enzymes bound, lncRNA-mediated chromatin modifications can activate or repress gene expression22,23,26,27,120. Chromatin-modifying complexes bound to the RNA polymerase II (Pol II) carboxy-terminal domain (CTD) can modify chromatin during transcription of lncRNAs33–35 (part b). Transcription of lncRNAs can also result in chromatin remodelling that can either favour or inhibit the binding of regulatory factors (part c). Depending on the nature of the factors that bind during remodelling, gene expression is activated or repressed 37–40. d–g | lncRNAs can modulate both the general transcription machinery (parts d and e) as well as specific regulatory factors (parts f and g). lncRNAs can bind Pol II directly to inhibit transcription47 (part d). Formation of lncRNA-DNA triplex structures can also inhibit the assembly of the pre-initiation complex48 (part e). lncRNAs can fold into structures that mimic DNA-binding sites (left) orthat generally inhibit or enhance the activity of specific transcript ion factors (right)50–53 (part f). lncRNAs can also regulate gene expression by binding specific transport factors to inhibit the nuclear localization of specific transcription factors54 (part g).
Additionally, at least in some cases, lncRNA expression may influence epigenetic events through transcription-dependent mechanisms29. The mammalian lncRNA Airn (antisense of Igf2r non-coding RNA) has been suggested to interfere with transcription during its regulation of Igf2r (insulin-like growth factor 2 receptor) because Airn transcription rather than the lncRNA product itself is required for silencing30 (TABLE 1). Similarly, an antisense RNA has also been postulated to repress mRNA expression at the yeast IME4 locus through transcriptional interference31 (TABLE 1). In some instances (for example, the GAL10-1, IME1 and PHO84 loci in yeast), movement of the polymerase along the DNA locus can result in the deposition of histone modifications, which in turn repress expression from nearby promoters. This may be one mechanism of transcription-dependent lncRNA regulation 32–35 (FIG. 2b; TABLE 1). Conversely, in flies non-coding transcription through Polycomb response elements is thought to counteract silencing during the switch from repressed to active states36. Moreover, lncRNA transcription in various organisms can modulate the binding of regulatory factors37–40 (FIG. 2c; TABLE 1). An interesting example is the pair of cis-acting lncRNAs, ICR1 and PWR1, which dictate the variegated expression of FLO11 mRNA in yeast. Specifically, transcription of ICR1 is thought to reset the FLO11 locus by inhibiting recruitment of the Flo8 or Sfl1 transcription factors, which promote FLO11 mRNA repression and activation, respectively. After this reset, if Fl08 binds it drives expression of PWR1, which in turn interferes with ICR1 expression in cis. ICR1 and PWR1 lncRNAs therefore represent a ‘toggle switch’, resulting in FLO11 mRNA expression when PWR1 is expressed and FLO11 mRNA repression when ICR1 is expressed37,41. By contrast, the lncRNA SRG1 exerts chromatin regulation by directing a high level of nucleosomes to the region of the phosphoglycerate dehydrogenase SER3 mRNA promoter38. In these particular cis-acting instances, it is often unclear whether the phenotype associated with the locus arises from the lncRNA itself or rather from changes in DNA-protein interactions that arise from polymerase movement.
lncRNAs have now also been implicated in transcriptional upregulation by enhancers42,43. A specific type of lncRNA, termed enhancer RNA (eRNA), displays enhancer-like activity and upregulates expression via the Mediator complex43,44 (TABLE 1). As studies suggest that classic enhancer elements are widely expressed, eRNAs may frequently be important for enhancer function at chromatin42,45,46.
Aside from modulating chromatin, lncRNAs can regulate transcription through additional mechanisms. For example, lncRNAs can influence the transcription machinery directly. During heat shock, lncRNAs generated from Alu SINE elements mediate transcriptional repression through direct contact with RNA polymerase II (Pol II) (FIG. 2d; TABLE 1). This interaction inhibits transcription of specific mRNAs during heat shock47. Furthermore, several lncRNAs can regulate the binding and/or activity of transcription factors. At the DHFR (dihydrofolate reductase) locus, expression of an upstream lncRNA impairs the assembly of the transcription pre-initiation complex in trans through the formation of an RNA-DNA triplex structure48 (FIG. 2e; TABLE 1).
Moreover, several lncRNAs act directly on specific transcription factors. For instance, during the heat shock response, heat shock factor 1 (HSF1) is activated through the combined actions of a lncRNA, HSR1 (heat shock RNA 1), and a surprising protein interaction partner and co-activator, translation elongation factor eEF1A49 (TABLE 1). In another example, the GAS5 (growth arrest specific 5) lncRNA folds into a structure that mimics the DNA-binding site of the glucocorticoid receptor, and the resulting interaction represses GR-mediated transcription50 (FIG. 2f; TABLE 1). By contrast, the lncRNA Evf2 (also known as Dlx6os1) can act either as a co-activator or co-repressor, depending on whether it recruits the transcriptional activator DLX2 or the transcriptional repressor MeCP2 (methyl-CpG binding-protein 2) to specific DNA regulatory elements51,52 (FIG. 2f; TABLE 1). Furthermore, binding of lncRNAs generated from the CCND1 (cyclin D1) promoter allosterically promotes a conformational switch in the TLS (translocated in liposarcoma) protein factor from an inactive to active form. Active TLS inhibits histone acetyltransferases, ultimately leading to repression of CCND1 transcription53 (FIG. 2f; TABLE 1). The lncRNA transcript thus indirectly promotes a repressive chromatin environment.
By contrast, the NRON (non-coding repressor of NFAT) lncRNA indirectly represses transcription by inhibiting nucleocytoplasmic shuttling of the transcription factor NFAT (nuclear factor of activated T cells)54. The transport of NFAT, which is imported from the cytoplasm into the nucleus in response to calcium-dependent signals, is inhibited by NRON. NRON binds the transport receptor importin-β, and knockdown of NRON results in nuclear accumulation of NFAT, suggesting that NRON competes with NFAT for importin-β interaction54 (FIG. 2g; TABLE 1).
Interestingly, lncRNAs have been indirectly linked to both gene activation and repression through the organization of nuclear subdomains. For instance, the lncRNAs TUG1 (taurine upregulated 1) and MALAT1 (metastasis associated lung adenocarcinoma transcript 1; also known as NEAT2) have been linked to repressive Polycomb group bodies and more active interchromatin granules, respectively (TABLE 1). Both lncRNAs bind Polycomb 2, but TUG1 binds methylated Polycomb 2 and MALAT1 binds the unmethylated protein55. The methylation status of Polycomb 2 therefore dictates a switch in both its lncRNA-binding specificity and nuclear subcompartment localization. Importantly, this switch is accompanied by movement of Polycomb 2 target genes between active and repressive nuclear domains and ultimately influences downstream gene expression55.
lncRNAs, therefore, can regulate transcription through several mechanisms (FIG. 2). Given the decades of research focused on transcriptional control from a transcription factor-centric point of view, it is interesting to speculate about the purpose of this additional layer of RNA-based regulation. Even at the yeast GAL locus, arguably one of the most extensively studied DNA loci during the past 50 years, a hidden layer of lncRNA-based regulation has now been described35,56. Indeed there has been a growing interest in such RNA-based control during the past decade57, and we and others have speculated that this extra layer of regulation reinforces the control that is imposed by protein factors at a locus. Notably, the impressive diversity of transcriptional regulatory mechanisms discussed here might just be the tip of the iceberg, with additional means of lncRNA-mediated transcriptional regulation to be uncovered in the future.
Regulators of mRNA processing
mRNA transcripts often have a complicated post-transcriptional existence58. Immediately in the wake of transcription, nascent pre-mRNAs are spliced and processed into one of potentially many isoforms. Importantly, alternative splicing and editing contribute to increasing gene isoform diversity.
In some cases, lncRNA genes that have an anti-sense orientation to known protein-coding genes, also known as natural antisense transcripts (NATs), can influence how an mRNA arising from the sense strand is processed. For example, NATs influence splicing patterns of mRNAs at the neuroblastoma MYC, c-ErbAalpha (also known as Thra) and ZEB2 (zinc-finger E-box binding homeobox 2) loci in mammalian cells59–62 (FIG. 3a; TABLE 1). In the case of neuroblastoma MYC and c-ErbAalpha, the NAT and pre-mRNA were suggested to form RNA-RNA duplexes, which then inhibit splicing61,62. At the ZEB2 locus, NAT expression inhibits splicing of an internal ribosome entry site (IRES)-containing intron. Translation of ZEB2 relies on this IRES, and therefore expression of the NAT indirectly facilitates expression of ZEB2 protein59. The mechanism by which NATs influence splicing is unclear, but it has been postulated to involve splice-site masking and a subsequent block in spliceosome recruitment63.
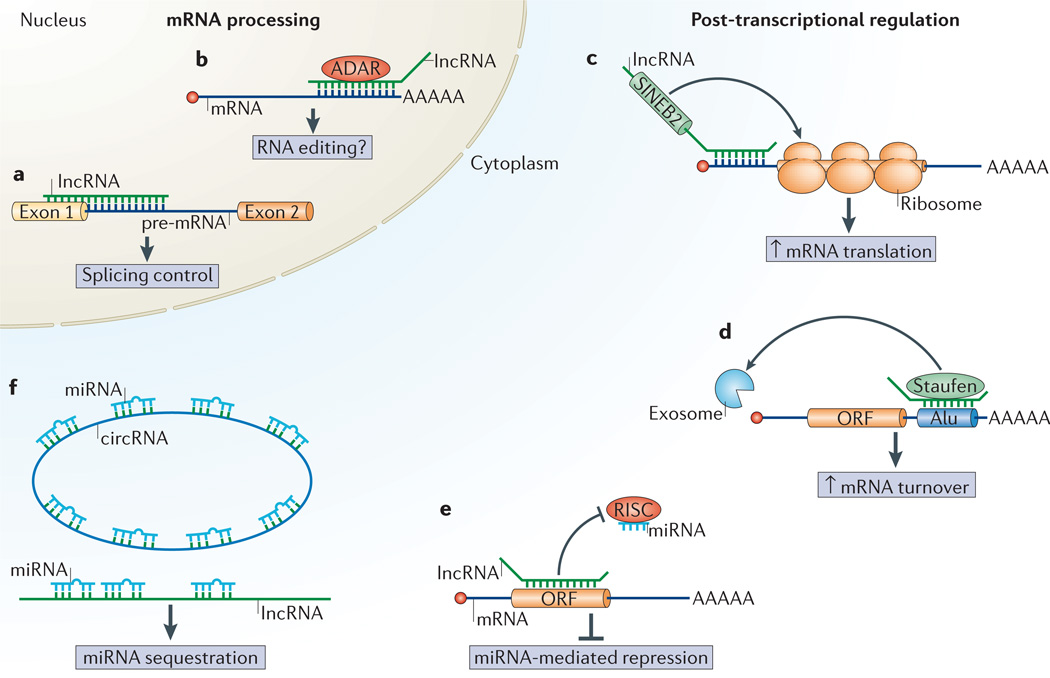
Figure 3. lncRNAs influence mRNA processing and post-transcriptional regulation.
a,b | Long non-coding RNAs (lncRNAs) can modulate mRNA processing. Splicing patterns can be influenced by lncRNAs that associate with the pre-mRNA(part a). For example, splicing of the first intron of neuroblastoma MYC mRNA is prevented by a natural antisense transcript61. Antisense lncRNAs that associate with an mRNA could direct mRNA editing, perhaps through association of the duplex with ADAR (adenosine deaminase acting on RNA) enzymes that catalyse adenosine to inosine conversion in double-stranded RNA63,66 (part b). c-f | lncRNAs modulate post-transcriptional regulatory events. lncRNAs containing SIN EB2 repeat elements can upregulate translation through association with the 5′ region of an mRNA68 (part c). lncRNAs containing Alu repeat elements associate with the Alu elements in the 3′ untranslated region (UTR)of an mRNA, and this double-stranded structure can direct Staufen-mediated decaythrough a pathwaythat is molecularly similar to nonsense-mediated decay70 (part d). lncRNAs can mask miRNA-binding sites on a target mRNA to block miRNA-induced silencing through the RNA-induced silencing complex (RISC)72 (part e). Linear or circular lncRNAs can function as miRNA decoys to sequester miRNAs from their target mRNAs74,75 (part f).
The MALAT1 lncRNA also affects splicing, but through a more indirect mechanism. This lncRNA, which is retained in the nucleus and associates with interchromatin granules, has been implicated in alternative splicing through the modulation of active Ser/Arg splicing factors, named after characteristic Ser- and Arg- rich domains. Ser/Arg proteins are important regulators of alternative splicing, and MALAT1 interacts with, and influences the nuclear distribution and levels of, phosphorylated Ser/Arg proteins. Importantly, depletion of MALAT1 changes the alternative splicing patterns of the pre-mRNAs that they target64.
In addition to modulating splicing, overlapping antisense lncRNAs have in principle the potential to direct mRNA editing (FIG. 3b). During editing, ADAR (adenosine deaminase acting on RNA) enzymes catalyse adenosine to inosine conversion in double-stranded RNA, and this conversion can influence RNA structure, splicing patterns, coding potential and targeting by microRNAs (miRNAs)65. In Drosophila melanogaster, editing of Rnp4F (RNA-binding protein 4F) mRNA depends on developmentally restricted expression of a long isoform of the partially overlapping Sas10 transcript (TABLE 1). Although, in this case, an mRNA isoform with an extended 3′ untranslated region (UTR) provides the source of an antisense RNA, lncRNAs could act in a similar manner to direct editing66. Given that many, if not most, mammalian genomic loci produce multiple RNA transcripts from both strands with at least partial overlap, the potential for double-stranded RNA editing substrates is extensive67. With many of these pervasive transcripts anticipated to be lncRNAs, lncRNAs are likely to help diversify the transcriptome and proteome through control of RNA editing.
Modulators of post-transcriptional control
Following processing and nuclear export, mRNAs are subjected to various post-transcriptional regulatory pathways that modulate gene expression levels. For example, the overall level of protein produced from an mRNA depends on translation efficiency, mRNA turnover kinetics and small RNA-mediated translational repression. A growing number of reports implicate lncRNAs in control of these post-transcriptional events.
Translation control
The mouse Uchl1AS lncRNA produced from the Uchl1 (ubiquitin carboxyl-terminal esterase L1) locus was shown to upregulate translation of Uchl1 mRNA through a repeat element (FIG. 3c; TABLE 1). In this instance, sense and antisense transcripts are oriented in a 5′ head-to-head fashion such that the mature lncRNA contains a 73-nucleotide motif complementary to the 5′ end of the Uchl1 mRNA. This sequence-specific interaction serves to position the effector domain, which is contained in the non-overlapping 3′ region of Uchl1AS and consists of a SINEB2 repeat element that upregulates protein expression without changing Uchl1 mRNA levels. Bioinformatic analysis has identified 59 other cDNAs with similar antisense orientations and SINEB2 elements, suggesting that this regulatory mechanism might be used at other loci68. lncRNA-mediated translational regulation has also been documented in yeast, in which an antisense KCS1 lncRNA was suggested to regulate translation of the inositol pyrophosphate synthase KCS1 mRNA expressed from the same locus. Through an unknown mechanism, which is thought to involve base pairing interactions between the antisense and sense RNAs, expression of KCS1 antisense RNA results in the production of truncated KCS1 protein69 (TABLE 1).
mRNA stability control
lncRNAs have also been implicated in both positive and negative regulation of mRNA stability. For instance, Alu repeat-containing lncRNAs are involved in targeting mRNA transcripts for Staufen-mediated decay (SMD)70. SMD is induced by Staufen 1 (STAU1) binding to a double-stranded structure in mRNA 3′ UTRs. Through imperfect base pairing interactions with Alu elements in the 3′ UTR, Alu repeat-containing lncRNAs create STAU1-binding sites that trans-activate SMD and destabilize the target mRNA (FIG. 3d; TABLE 1).
By contrast, BACE1AS, an antisense lncRNA that arises from the BACE1 (beta-site APP-cleaving enzyme 1) locus, increases stability of BACE1 mRNA71 (TABLE 1). BACE1AS and BACE1 mRNA form an RNA-RNA duplex, which has been suggested to stabilize the mRNA by abrogation of miRNA-induced repression. More specifically, the antisense transcript and miR-485-5p compete for binding to the same region in the BACE1 mRNA71,72 (FIG. 3e; TABLE 1). BACE1 mRNA encodes β-secretase, the rate-limiting enzyme in amyloid-β synthesis. Regulation of BACE1 expression, therefore, has important implications in Alzheimer’s disease. Intriguingly, BACE1AS levels are increased in the brains of patients with Alzheimer’s disease, which perhaps suggests that the regulation of this lncRNA might be relevant in this condition71.
miRNA sponges
Aside from competing with small RNAs for binding sites on target mRNAs, lncRNAs also can act as decoys to attenuate small RNA regulation, for example through sequestration of proteins or RNA-dependent effectors. The competing endogeneous RNA (ceRNA) hypothesis is based on this idea. It postulates that a widespread network of crosstalk exists between coding and non-coding RNAs that manifests through competition for miRNA binding73. Examples of potential ceRNAs include LINCMD1, HULC (highly upregulated in liver cancer), PTENP1 (PTEN pseudogene 1), IPS1 (INDUCED BY PHOSPHATE STARVATION 1) and CDR1as (CDR1 antisense; also known as ciRS-7)74–78 (TABLE 1). Specifically, the muscle-specific lncRNA LINCMD1 regulates muscle differentiation by binding and sequestering miR-133 and miR-135 (REF. 74). Normally, these miRNAs negatively regulate expression of the MAML1 (mastermind-like 1) and MEF2C (myocyte enhancer factor 2C) transcription factors, which drive muscle-specific gene expression. So, by sequestering these miRNAs, LINCMD1 indirectly activates MAML1 and MEF2C74. Similarly, the HULC lncRNA has been suggested to act as a ‘sponge’ that inhibits miR-372 by sequestering it away from potential mRNA targets75. This regulatory principle is shared with pseudogenes, which can also act as miRNA decoys to upregulate expression of their cognate genes. This has been shown, for example, in the case of the pseudogene PTENP1 (REF. 79).
The Arabidopsis thaliana lncRNA IPS1 also sequesters miR-399 away from its target mRNAs76. Whereas most miRNAs in plants have perfect complementarity to their targets, which results in mRNA cleavage, IPS1 contains an imperfect binding site for miR-399. Thus, miR-399 binding to IPS1 does not result in its cleavage but instead limits the levels of miR-399 available for other targets. This ability to evade cleavage is an important aspect of IPS1 regulation, because mutant IPS1 with perfect complementarity to miR-399 no longer regulates miR-399 (REF. 76).
More recently, another example of lncRNA-based miRNA sponges has been described, but these RNAs are unique in that they have a circular structure77,78. In humans, the highly stable circular RNA (circRNA) CDR1as has numerous miR-7-binding sites77,78 (FIG. 3f; TABLE 1). Importantly, a similar CDR1as genomic locus can be found across eutherian mammals, suggesting that, unlike many other lncRNAs, this RNA might be conserved77. Moreover, bioinformatic analyses indicate that there may be thousands of expressed circRNAs across a broad range of multicellular eukaryotes78.
lncRNAs can, therefore, modulate gene expression by diverse post-transcriptional regulatory pathways (FIG. 3c–f; TABLE 1). Whereas some lncRNAs seem to influence translation, others operate at the RNA level. As more and more lncRNAs are functionally characterized, we will probably see additional examples of post-transcriptional regulation by lncRNAs.
Regulators of protein activity
In addition to lncRNA-mediated modulation of gene expression events through effects on mRNAs, lncRNAs can also act at the protein level. Indeed, some of the same lncRNAs that affect mRNAs, such as GAS5, EVF2 and CCND1, alter the activity of transcription factors (TABLE 2). However, the ability of lncRNAs to bind and modulate protein activity extends beyond factors involved in transcription.
Table 2.
lncRNA-mediated regulation of proteins
| lncRNA | Function | Mechanism | Refs |
|---|---|---|---|
| Regulation of protein activity | |||
| CAS5 | Repression of glucocorticoid receptor-mediated transcription |
DNA mimicry | 50 |
| EVF2 | Transcriptional activation of DLX2 targets | Activation of DLX2 | 51,52 |
| CCND1 promoter RNA | Repression of CCND1 transcription | Allosteric activation of TLS | 53 |
| NRON | Repression of NFAT-med iated transcription | Inhibition of transcription factor nucleocytoplasmic shuttling |
54 |
| 15q11-q13sno-lncRNA | Regulation of alternative splicing | Inhibition of FOX2 function | 80 |
| rncs-1 | Inhibition of Dicer-mediated repression | Sequestration of Dicer or accessory double-stranded RNA-binding proteins |
81 |
| sfRNA | Stabilization of viral and host mRNAs | Inhibition of XRNl-mediated mRNA degradation |
82,83 |
| gadd7 | Inhibition of TDP43-mediated regulatory events |
Sequestration of TDP43 | 84 |
| Organization of protein complexes | |||
| HOTA1R | Repression at the HOXD locus | Recruitment of PRC2 and LSD1 | 24 |
| KCNQ1OT1 | Imprinting at the KCNQ1 cluster | Recruitment of PRC2 and G9A | 27 |
| ANR1L | Repression at the INK4b–ARF–INK4a locus | Recruitment of PRC1 and PRC2 | 26,28 |
| TERC | Add it ion of telomeric repeats to the ends of chromosomes |
Organizational scaffold fortelomerase components and template for repeat addition |
90 |
| SRP RNA | Directing of proteinstothe ER | Organizational scaffold for SRP components |
91 |
| NEAT1 | Assembly of paraspeckles | Nucleation of subnucleardomains | 95–97 |
CCND1, cyclin Dl; ER, endoplasmic reticulum; GAS5, growth arrest specific 5; HOTAIR, HOX transcript antisense RNA; HOXD, homeobox D cluster; KCNQ1, potassium voltage-gated channel, KQT-like subfamily, member 1; KCNQ1OT1, KCNQ1 opposite strand or antisense transcript 1; LSD1, Lys-specific demethylase 1; NFAT, nuclear factor of activated T cells; NRON, non-coding repressor of NFAT; PRC, Polycomb repressive complex; sfRNA, subgenomic flavivirus RNA; sno-lncRNA, small nucleolar long non-coding RNA; SRP, signal recognition particle; TDP43, TAR DNA-binding protein 43; TERC, telomerase RNA component; TLS, translesion DNA synthesis; XRN1, 5′ to 3′exoribonuclease 1.
For example, a new class of lncRNAs flanked by small nucleolar RNA (snoRNA) sequences, termed sno-lncRNAs, influence splicing patterns via physical interactions with an alternative splicing regulator in human cell lines80. These sno-lncRNAs are derived from introns and are nuclear-enriched. A particularly abundant member of the sno-lncRNA family, generated from the 15q11-q13 chromosomal region, directly associates with the FOX2 alternative splicing factor (FIG. 4a; TABLE 2). Importantly, sno-lncRNA knockdown results in changes in FOX2-regulated splicing, and it has been speculated that the sno-lncRNA might inhibit FOX2 function via a sequestration mechanism80. Similarly, the Caenorhabditis elegans lncRNA rncs-1 has been suggested to influence the processing of small RNAs via Dicer inhibition81. The rncs-1 lncRNA forms an extensive double-stranded helix, but is not cleaved by Dicer due to inhibitory secondary structures flanking this helix (FIG. 4b; TABLE 2). It has been suggested that rncs-1 competitively binds either Dicer or accessory double-stranded RNA-binding proteins to preclude processing of small RNAs from double-stranded RNA precursors81.
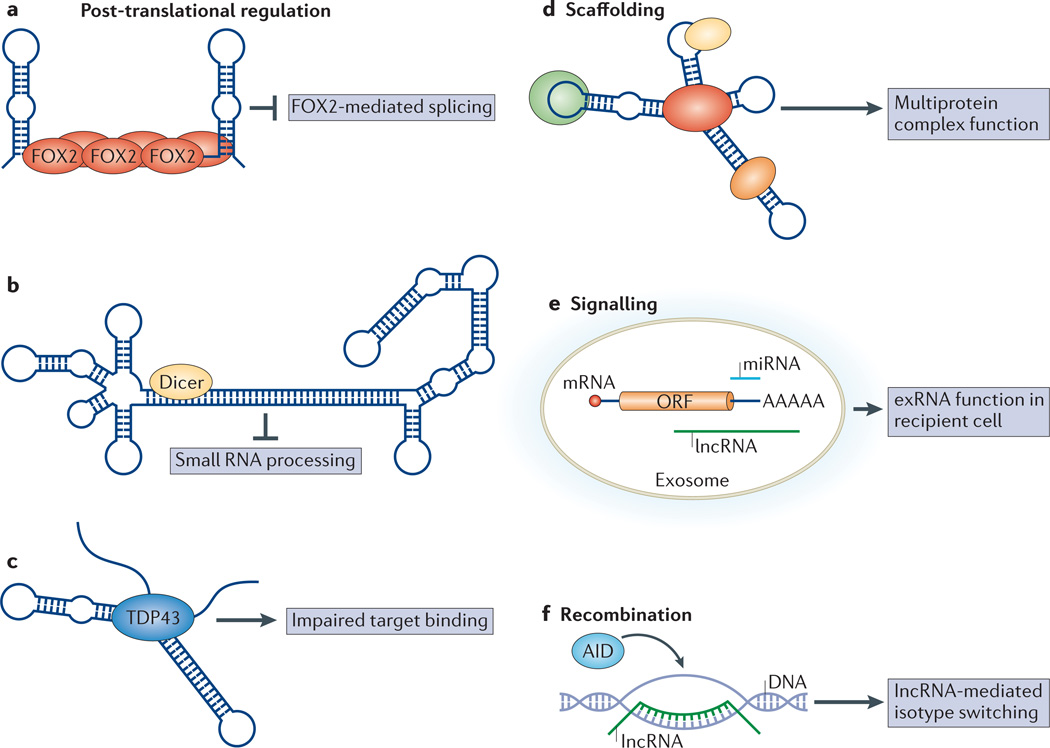
Figure 4. lncRNAsare involved in various cellular contexts.
Long non-coding RNAs(lncRNAs) modulate protein activity by post-translational mechanisms (parts a-c). a | Small nucleolar lncRNAs(sno-lncRNAs) generated from the 15qll-ql3 locus bind and modulate the activity of the FOX2 alternative splicing factor, and this can inhibit FOX2-mediated splicing80.b | The highly structured rncs-1 lncRNA binds Dicer to inhibit the processing of small RNAs81c | The gadd 7 IncRN A binds and modulates the ability of TDP43 (TAR DNA-binding protein 43) to target and process specific mRNAs84.d | lncRNAscan act as scaffolds to organize several complexes24.e | As the cargo of exosomesthat mediate transfer of material between cells, exosomal shuttle RNAs(exRNAs)may act as signalling molecules during cell-cell communication; exosomal cargo includes mRN As, microRNAs (miRNAs) and lncRNAs102.f | lncRNAs expressed from the switch region of genes encoding antibodies form R-loops to direct class switch recombinationvia activation-induced deaminase (AID) recruitment111
Flaviviruses, such as West Nile virus, also produce a highly structured lncRNA termed subgenomic flavivirus RNA (sfRNA), which is resistant to destruction by host nucleases. sfRNA is essential for pathogenicity and is thought to stall the host 5′ to 3′ exoribonuclease, XRN1, during viral RNA genome degradation82. The inhibition of XRN1 induced by sfRNA is even strong enough to stabilize host cellular mRNAs83 (TABLE 2). Although this is an example of a viral lncRNA that inhibits a host cellular enzyme, it illustrates that structured lncRNAs have the capacity to inhibit wide-ranging enzymatic activities.
A ultraviolet (UV) light-induced lncRNA, gadd7, has also been shown to influence cellular mRNA stability84. This lncRNA, however, does so by modulating the activity of the RNA-binding protein TDP43 (TAR DNA-binding protein 43). TDP43 has been implicated in pre-mRNA splicing as well as mRNA transport, translation and stability85–88. It binds 3′ UTR elements in a large number of genes, and this binding can result in either the stabilization or destabilization of mRNA targets84,86–88. The association of gadd7 with TDP43 impairs TDP43 binding to several of its targets (FIG. 4c; TABLE 2). For example, by preventing TDP43 association with cyclin-dependent kinase 6 (CDK6) mRNA, gadd7 alters the role of TDP43 in modulating mRNA stability84. Interestingly gadd7 is not the only lncRNA that TDP43 binds. TDP43 also associates the MALAT1 and NEAT1 (also known as Men ε/β) lncRNAs89. As both MALAT1 and TDP43 are implicated in control of alternative splicing, it will be interesting to further explore this interaction in future studies.
Scaffolds for higher-order complexes
RNA transcripts associate with proteins to form ribonucleoprotein particles (RNPs). Compared with other RNAs such as snRNAs and rRNAs, we know very little about the composition of RNPs formed by lncRNAs. Some specific lncRNA-protein interactions have been characterized, but the lncRNA interaction network in cells is likely to be more complicated than single lncRNAs interacting with single proteins. Indeed there are indications that lncRNAs can act as scaffolds to organize higher-order complexes.
Some of the lncRNAs involved in chromatin-dependent events (such as HOTAIR, KCNQ1OT1 and ANRIL) have been suggested to act as scaffolds that coordinate the activities of histone-modifying complexes15,25 (FIG. 2; TABLE 2). There are also notable examples of classic ncR-NAs such as the RNA component of telomerase (TERC) and signal recognition particle (SRP) RNA that can act as scaffolds at telomeres and on translating ribosomes during protein targeting to the endoplasmic reticulum (ER), respectively90,91 (TABLE 2). Although the SRP and TERC ncRNAs are not generally considered to be lncRNAs, they demonstrate that RNA is particularly adept as a scaffold and that many lncRNAs could function as scaffolds in diverse contexts.
The telomerase RNP complex is responsible for adding telomeric repeats to chromosomal ends and thereby maintains their length in replicating cells. The RNA component of telomerase is not only responsible for templating the addition of telomeric repeats but also provides a scaffold that organizes telomeric regulatory proteins90 (FIG. 4d; TABLE 2). Interestingly, other lncRNAs generated from telomeric repeats, termed TERRA, have a distinct role in telomere biology. Rather than extending telomere ends, these lncRNAs promote telomere shortening via exonuclease 1-dependent resection of chromosome ends92. lncRNA-mediated events thus serve critical functions in telomere homeostasis.
The SRP is a highly conserved RNP complex, consisting of the SRP RNA transcript and six proteins, which directs proteins to the ER. SRP co-translationally binds the signal sequence in nascent peptides, stalls translational elongation and then targets the ribosome-nascent chain complex to the SRP receptor on the ER. Whereas specific protein domains in SRP mediate peptide recognition and arrest of translational elongation, SRP RNA provides a scaffold to organize and coordinate distally occurring events at the sites of peptide exit and elongation factor binding on the ribosome91 (TABLE 2).
In addition to serving as scaffolds for specific multi-protein complexes, lncRNAs have been implicated in nuclear organization through the scaffolding of sub-nuclear domains93. Indeed, RNA, both coding and non-coding, has been implicated in the nucleation of histone locus bodies, interchromatin granules, paraspeckles and nuclear stress bodies94. Perhaps the best-studied lncRNA of this type is NEAT1, which is important for the de novo assembly of paraspeckles (subnuclear domains that may mediate retention of hyperedited mRNAs in the nucleus)95,96. Interestingly, the nascent lncRNA is important for this because ongoing NEAT1 lncRNA transcription is required for paraspeckle maintenance97.
It is enticing to speculate that other uncharacterized lncRNAs may serve as scaffolds to organize and hold together other higher-order complexes. Imagine what might have been missed through the routine treatment of protein preparations with nuclease to remove RNA contaminates before purification and identification of interacting partners. Perhaps lncRNAs could even hold together enzymes involved in fundamental metabolic processes such as glycolysis or the Krebs cycle. Indeed, the orchestration of electron transport factors on the inner lumen of the mitochondria illustrates that spatial arrangements of enzymes can partly facilitate the catalysis of reactions by overcoming the limits imposed by diffusion. Similarly to cell membranes, lncRNA might also help facilitate this purpose by bringing enzymes closer together. Perhaps this is not such a far stretch, as metabolic enzymes such as aconitase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are known to have RNA-binding activity98–100.
Signalling molecules
RNA can be transferred between cells in small vesicles known as exosomes101–103. Not to be confused with the molecular machine with the same name that mediates RNA degradation, these exosomes are membrane-bound vesicles of endosomal origin that are released from various cell types in mammals. Upon fusion with another cell, both their RNA and protein cargo can be transferred102. The RNAs that have been found in exosomes, termed exosomal shuttle RNAs (exRNAs), do not simply reflect the RNA composition of the cell of origin, suggesting that there may be selective loading of RNAs into exosomes104. Because transmitted RNAs can function in the recipient cell, it has been suggested that exRNAs might be used as a signal to change gene expression patterns in the recipient cell101–104. Although exosomes contain large amounts of exRNAs, so far miRNAs and mRNAs have been a primary focus of study103,105,106 (FIG. 4e). However, a recent report characterizing the full complement of human plasma-derived exRNA indicates that lncRNAs are indeed present in exosomes106. The presence of lncRNAs certainly raises the exciting possibility that they might provide signals that impinge on various gene expression events.
Vehicles for increasing genetic diversity
Genetic diversity is crucial for the survival of a species and, within individuals, genetic innovation is of paramount importance to the adaptive immune system. Diversity in developing lymphocytes is achieved through genomic rearrangements in the form of class switch recombination (CSR; also known as isotype switching) and V(D)J recombination events. Interestingly, non-coding transcription has been implicated in both forms of recombination107,108. Through CSR, the constant regions of antibodies are exchanged. As such, this process increases the range of effectors that a particular antibody can interface with and, therefore, increases its versatility109,110. During CSR, the non-coding switch region (S region) is transcribed, and the lncRNAs generated from this S region are likely to be important guides in dictating the locations of recombination. The nascent lncRNA forms an RNA-DNA hybrid or R-loop structure, which displaces one strand of DNA and this, in turn, is thought to facilitate targeting of activation-induced deaminase (AID), the enzyme that initiates CSR107,111,112 (FIG. 4f). Transcription through non-coding regions also has a role in V(D)J recombination, the genomic rearrangement that generates diversity in antigen receptor-binding pockets in antibodies113,114. During V(D)J recombination, chromatin accessibility has been suggested to affect recombinase targeting114, and production of non-coding transcripts from the mouse Tcra (T cell receptor alpha chain) locus can trigger changes in chromatin structure that then influence recombination108.
It is tempting to postulate that non-coding transcription might also increase genetic diversity outside the immune system. During meiosis, sites of recombination are not distributed randomly but tend to occur in discrete locations115. Intriguingly, in fission yeast these hot spots correlate with lncRNA-expressing loci116. How exactly lncRNAs contribute to recombination-site selection is currently unclear, but one possibility is that this could involve similar mechanisms to those used during recombination in lymphocytes.
Conclusions and perspectives
Amidst the exciting discoveries being made during this time of genome exploration, RNA is taking centre stage. The burgeoning lncRNA field has a strong part in this, and lncRNAs have now been demonstrated to regulate all aspects of gene expression, including transcription (FIG. 2), processing and post-transcriptional control pathways (FIG. 3). Likewise, lncRNAs have also been shown to regulate protein function and organize multiprotein complex assembly. Now with hints that lncRNAs might participate in cell-cell communication and recombination, the possible reach of lncRNA functions seems endless (FIG. 4). With most biologists trained to dissect function based on a protein-centric view of the cell, the task of functionally characterizing this new RNA world seems daunting. It is important, therefore, as we move forward, to utilize and develop more functional characterization methods that play to the strengths of RNA. Indeed technical advances are already underway that have the promise of greatly improving the in vivo functional characterization of lncRNAs. For instance, techniques to probe RNA chemical structure have often been limited to in vitro studies, but recently developed chemical probes that can be used in living cells have the promise of greatly improving our ability to determine in vivo RNA structures117. Additionally, the application of high-throughput microfluidics-based screening technologies towards the functional analysis of pre-programmed RNA libraries has the potential to streamline the process of discovering functional motifs within lncRNAs118. Last, recently developed RNA aptamers such as Spinach have adapted GFP tagging for RNA transcripts to allow RNA fusions to be imaged in living cells119.
Much like the multifunctional nature of a Swiss army knife, RNA has the biochemical diversity to function in diverse contexts. It may, however, take some time to determine in which contexts the cell uses some of the more exotic RNA tools. With eyes open to new possibilities, undoubtedly we will be surprised by what we find.
Acknowledgments
The authors are most grateful to T. Nilsen and K. Baker for insights and suggestions. The authors regret that not all contributions of their colleagues could be discussed due to space constraints. Work in the authors’ laboratory is funded by the National Institute of General Medical Sciences (NIGMS] (GM080465).
Glossary
- Chromatin
Condensed DNA structure that is associated with histone proteins and other l)NA-binding proteins
- Transcription activator-like effectors
TALEs). Naturally found in some bacteria, TALEs are proteins that bind DNA through repeat domains, and their code for sequence specificity has been elucidated allowing sequence specific TALEs to be engineered
- PUF proteins
A family of sequence-specific RNA-binding proteins, which bind 3′ untranslated regions within mRNAs to repress target mRNA translation
- Pseudogenes
Dysfunctional relatives of normal genes thought to arise from duplication or retrotransposition
- Chromatin-modifying complexes
Protein complexes that catalyse the covalent chemical modification of chromatin
- Enhancers
Short regions of DNA that enhance the expression of genes at varying distances. Effects can be mediated by transcription factor binding to these sites
- Alu SINE elements
Highly abundant retrotransposons of the short interspersed nuclear elements (SINE) family
- Nuclear subdomains
Non-membrane bound subcompartmentsof eukaryotic nuclei where factors with similar functions colocalize
- GAL locus
An inducible locus in yeast comprising the GAL1 and GAL10 genes, which are required for galactose metabolism
- Alternative splicing
An mRNA processing step whereby exons can be alternatively used to generate different isoforms of the same gene
- Internal ribosome entry sites
IRESs). Nucleotide sequence that allows cap-independent translation initiation within the middle of an mRNA transcript
- Spliceosome
The macromolecular machinery (composed of both RNA and protein) responsible for pre-mRNA splicing
- miRNAs
(miRNAs). A class of short (~ 23 nucleotides) endogenous non-coding RNAs that control gene expression post-transcriptionally through either translational repression or mRNA degradation
- Competing endogeneous RNA
(ceRNA). RNA transcripts (both coding and non-coding), which share microRNA-targeting sites and thus regulate each other via direct competition for microRNA binding
- Circular RNA
(circRNA). As opposed to conventional linear RNA transcripts, the 5′ and 3′ ends of circular RNAs are covalently linked together.
- Small nucleolar RNA
(snoRNA). A class of small RNA molecules that guide the chemical modification of other RNA transcripts
- sno-lncRNAs
(small nucleolar long non-coding RNAs). Class of intron-derived long non-coding RNA flanked by snoRNA ends
- Dicer
An RNase III family endoribonuclease responsible for the processing of pre-miRNAs into short double-stranded RNAs to be loaded into the RNA-induced silencing (RISC) complex
- Adaptive immune system
A system of specialized cells that create immunological memory via specific antibodies after an initial response to a pathogen
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Sarah Geisler, Email: sarah.geisler@bsse.ethz.ch.
Jeff Coller, Email: jmc71@case.edu.
References
- 1. The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. Provides an overview of a series of papers releasee as part of the ENCODE project in which landmarks of biochemical function (regions of transcription, transcription factor association and histone modifications, among others) were attributed to 80% of the genome
- 2.Carninci P, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 3.Koch E, Jourquin E, Ferrier P, Andrau J-C. Genome-wide RNA polymerase II: not genes only! Trends Biochem. Sci. 2008;33:265–273. doi: 10.1016/j.tibs.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Djebali S, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays. 2007;29:288–299. doi: 10.1002/bies.20544. [DOI] [PubMed] [Google Scholar]
- 6.Berretta J, Morillon A. Pervasive transcription constitutes a new level of eukaryotic genome regulation. EMBO Rep. 2009;10:973–982. doi: 10.1038/embor.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 8.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nature Struct. Mol. Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 10.Ponting CP, Belgard TG. Transcribed dark matter: meaning or myth? Hum. Mol. Genet. 2010;19:R162–R168. doi: 10.1093/hmg/ddq362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Lee J. T Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 15.Kugel JF, Goodrich JA. Non-coding RNAs: key regulators of mammalian transcription. Trends Biochem. Sci. 2012;37:144–151. doi: 10.1016/j.tibs.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson MP, Joyce G. F The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a003608. pii:a003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipovska A, Rackham O. Modular recognition of nucleic acids by PUF, TALE and PPR proteins. Mol. Biosyst. 2012;8:699–708. doi: 10.1039/c2mb05392f. [DOI] [PubMed] [Google Scholar]
- 18.Stoltenburg R, Reinemann C, Strehlitz B. SELEX — a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007;24:381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Serganov A, Patel DJ. Molecular recognition and function of riboswitches. Curr Opin. Struct. Biol. 2012;22:279–286. doi: 10.1016/j.sbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei B, et al. The GENCODE pseudogene resource. Genome Biol. 2012;13:R51. doi: 10.1186/gb-2012-13-9-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nature Rev. Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 22.Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl Acad. Sci. USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsai M-C, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. Illustrates an elegant example of a dominant theme in the lncRNA field whereby lncRNAs physically associate with histone-modifying complexes to regulate chromatin states. Importantly, shows that HOTAIR can also act as a scaffold to organize the concerted actions of two enzymatic activities
- 25.Spitale RC, Tsai M-C, Chang HY. RNA templating the epigenome: long noncoding RNAs as molecular scaffolds. Epigenetics. 2011;6:539–543. doi: 10.4161/epi.6.5.15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yap KL, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a . Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey RR, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Kotake Y, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15INK4B tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latos PA, et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–1472. doi: 10.1126/science.1228110. Provides a particularly compelling example of a mammalian lncRNA AIRN which represses target expression by transcriptional interference, as transcriptional overlap of the lncRNA with the target promoter rather than the lncRNA transcript itself is sufficient to interfere with Pol II recruitment
- 31.Hongay CF, Grisafi PL, Galitski T, Fink GR. Antisense transcription controls cell fate in Saccharomyces cerevisiae . Cell. 2006;127:735–745. doi: 10.1016/j.cell.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 32.van Werven FJ, et al. Transcription of two long noncoding RNAs mediates mating-type control of gametogenesis in budding yeast. Cell. 2012;150:1170–1181. doi: 10.1016/j.cell.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camblong J, Iglesias N, Fickentscher C, Dieppois G, Stutz F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation inS. cerevisiae . Cell. 2007;131:706–717. doi: 10.1016/j.cell.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Carrozza MJ, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Houseley J, Rubbi L, Grunstein M, Tollervey D, Vogelauer M. A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol. Cell. 2008;32:685–695. doi: 10.1016/j.molcel.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt S, Prestel M, Paro R. Intergenic transcription through a polycomb group response element counteracts silencing. Genes Dev. 2005;19:697–708. doi: 10.1101/gad.326205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bumgarner SL, et al. Single-cell analysis reveals that noncoding RNAs contribute to clonal heterogeneity by modulating transcription factor recruitment. Mol. Cell. 2012;45:470–482. doi: 10.1016/j.molcel.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hainer SJ, Pruneski JA, Mitchell RD, Monteverde RM, Martens JA. Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev. 2011;25:29–40. doi: 10.1101/gad.1975011. Demonstrates a novel mode of SER3 gene repression by the yeast lncRNA SRG1 that involves the direction of nucleosome occupancy at the SER3 promoter
- 39.Hirota K, et al. Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs. Nature. 2008;456:130–134. doi: 10.1038/nature07348. [DOI] [PubMed] [Google Scholar]
- 40.Lefevre P, Witham J, Lacroix CE, Cockerill PN, Bonifer C. The LPS-induced transcriptional upregulation of the chicken lysozyme locus involves CTCF eviction and noncoding RNA transcription. Mol. Cell. 2008;32:129–139. doi: 10.1016/j.molcel.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bumgarner SL, Dowell RD, Grisafi P, Gifford DK, Fink GR. Toggle involving cis-interfering noncoding RNAs controls variegated gene expression in yeast. Proc. Natl Acad. Sci. USA. 2009;106:18321–18326. doi: 10.1073/pnas.0909641106. Provides the first description of the pair of cis interfering lncRNAs at the FL011 locus in yeast, where a regulatory circuit toggles between two states of expression depending on the identity of the lncRNA expressed
- 42.Flynn RA, Chang HY. Active chromatin and noncoding RNAs: an intimate relationship. Curr: Opin. Genet. Dev. 2012;22:172–178. doi: 10.1016/j.gde.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ørom UA, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. Describes a novel class of lncRNAs that, instead of repressing, activates target genes to function as RNA-dependent enhancers of gene expression
- 44.Lai F, et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T-K, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mariner PD, et al. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol. Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–670. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 49.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 50.Kino I, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3 doi: 10.1126/scisignal.2000568. ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng J, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bond AM, et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nature Neurosci. 2009;12:1020–1027. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willingham AT, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. The first study to functionally screen 512 evolutionarily conserved putative lncRNAs. Identified the NRON lncRNA as a repressor of NFAT nuclear trafficking
- 55. Yang L, et al. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell. 2011;147:773–788. doi: 10.1016/j.cell.2011.08.054. Describes a particularly interesting paradigm whereby the post-translational modification status of a protein effector constitutes a switch in lncRNA-binding specificity and consequently determines the nuclear subdomain localization of target genes
- 56.Geisler S, Lojek L, Khalil AM, Baker KE, Coller J. Decapping of long noncoding RNAs regulates inducible genes. Mol. Cell. 2012;45:279–291. doi: 10.1016/j.molcel.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattick JS. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 59. Beltran M, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1 -induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769. doi: 10.1101/gad.455708. Provides an example of a NAT lncRNA that regulates splicing of the sense encoded mRNA, but with a twist in that the NAT increases protein levels of its target by preventing the splicing of a 5′ UTR IRES-containing intron
- 60.Hastings ML, Milcarek C, Martincic K, Peterson ML, Munroe SH. Expression of the thyroid hormone receptor gene, erbAα, in B lymphocytes: alternative mRNA processing is independent of differentiation but correlates with antisense RNA levels. Nucleic Acids Res. 1997;25:4296–4300. doi: 10.1093/nar/25.21.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krystal GW, Armstrong BC, Battey J. F N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol. Cell. Biol. 1990;10:4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munroe SH, Lazar MA. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA . J. Biol. Chem. 1991;266:22083–22086. [PubMed] [Google Scholar]
- 63.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nature Rev. Mol. Cell. Biol. 2009;10:637–643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tripathi V, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hundley HA, Bass BL. ADAR editing in double-stranded UTRs and other noncoding RNA sequences. Trends Biochem. Sci. 2010;35:377–383. doi: 10.1016/j.tibs.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters NT, Rohrbach JA, Zalewski BA, Byrkett CM, Vaughn JC. RNA editing and regulation of Drosophila 4f-rnp expression by sas-10 antisense readthrough mRNA transcripts. RNA. 2003;9:698–710. doi: 10.1261/rna.2120703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katayama S, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 68. Carrieri C, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457. doi: 10.1038/nature11508. Discovers and characterizes the first lncRNA of a potentially new class of partially antisense SINE2B repeat-containing lncRNAs which upregulates translation of targets
- 69.Nishizawa M, et al. Nutrient-regulated antisense and intragenic RNAs modulate a signal transduction pathway in yeast. PLoS Biol. 2008;6:2817–2830. doi: 10.1371/journal.pbio.0060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gong C, Maquat LE. lncRNAs transactivate STAU1 -mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470:284–288. doi: 10.1038/nature09701. Provides the first evidence that Alu element-containing lncRNAs can transactivate SMD by imperfectly base pairing with 3′ UTR Alu elements in target mRNAs
- 71.Faghihi MA, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feedforward regulation of β-secretase. Nature Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faghihi MA, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11:R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cesana M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franco-Zorrilla JM, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nature Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 77. Hansen TB, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013 doi: 10.1038/nature11993. http://dx.doi.org/10.1038/nature11993. References 77 and 78 provide powerful evidence that circRNAs, covalently linked by the head-to-tail splicing of exons, can function as miRNA sponges to suppress miRNA activity
- 78.Memczak S, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013 doi: 10.1038/nature11928. http://dx.doi.org/10.1038/nature11928. [DOI] [PubMed]
- 79.Poliseno L, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yin Q-F, et al. Long noncoding RNAs with snoRNA ends. Mol. Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. Describes the discovery of a new class of intron-derived lncRNAs flanked by snoRNAs and shows that one in particular associates with splicing regulators to alter splicing patterns
- 81.Hellwig S, Bass BL. A starvation-induced noncoding RNA modulates expression of Dicer-regulated genes. Proc. Natl Acad. Sci. USA. 2008;105:12897–12902. doi: 10.1073/pnas.0805118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pijlman GP, et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe. 2008;4:579–591. doi: 10.1016/j.chom.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Moon SL, et al. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA. 2012;18:2029–2040. doi: 10.1261/rna.034330.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Li D, Zhang W, Guo M, Zhan Q. Long non-coding RNA gaddl interacts with TDP-43 and regulates Cdk6 mRNA decay. EMBO J. 2012;31:4415–4427. doi: 10.1038/emboj.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buratti E, Baralle FE. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010;7:420–429. doi: 10.4161/rna.7.4.12205. [DOI] [PubMed] [Google Scholar]
- 86.Colombrita C, et al. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcription al fate in motoneuron-like cells. J. Biol. Chem. 2012;287:15635–15647. doi: 10.1074/jbc.M111.333450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strong MJ, et al. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol. Cell. Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Volkening K, Leystra-Lantz C, Yang W, Jaffee H, Strong MJ. Tar DNA binding protein of 43 kDa (TDP-43), 14-3-3 proteins and copper/zinc superoxide dismutase (SOD1) interact to modulate NFL mRNA stability. Implications for altered RNA processing in amyotrophic lateral sclerosis (ALS) Brain Res. 2009;1305:168–182. doi: 10.1016/j.brainres.2009.09.105. [DOI] [PubMed] [Google Scholar]
- 89.Tollervey JR, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nature Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zappulla DC, Cech TR. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl Acad. Sci. USA. 2004;101:10024–10029. doi: 10.1073/pnas.0403641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halic M, et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature. 2004;427:808–814. doi: 10.1038/nature02342. [DOI] [PubMed] [Google Scholar]
- 92.Pfeiffer V, Lingner J. TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet. 2012;8:e1002747. doi: 10.1371/journal.pgen.1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmo-Fonseca M, Rino J. RNA seeds nuclear bodies. Nature Cell Biol. 2011;13:110–112. doi: 10.1038/ncb0211-110. [DOI] [PubMed] [Google Scholar]
- 94.Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nature Cell Biol. 2011;13:167–173. doi: 10.1038/ncb2157. [DOI] [PubMed] [Google Scholar]
- 95.Sunwoo H, et al. MEN ε/β nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359. doi: 10.1101/gr.087775.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasaki YTF, Ideue I, Sano M, Mituyama T, Hirose T. MENε/β noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc. Natl Acad. Sci. USA. 2009;106:2525–2530. doi: 10.1073/pnas.0807899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mao YS, Sunwoo H, Zhang B, Spector DL. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nature Cell Biol. 2011;13:95–101. doi: 10.1038/ncb2140. Uses a live-cell imaging system to directly visualize paraspeckle protein recruitment and shows that NEAT1 lncRNA transcription regulates paraspeckle maintenance with the lncRNA potentially acting as an assembly platform
- 98.Ciesla J. Metabolic enzymes that bind RNA: yet another level of cellular regulatory network? Acta Biochim. Pol. 2006;53:11–32. [PubMed] [Google Scholar]
- 99.Hentze MW, Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991;19:1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochern. Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh. Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip. Rev. RNA. 2012;3:286–293. doi: 10.1002/wrna.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 104.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang X, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. Characterizes, for the first time, the RNA content of exosomes by RNA sequencing and reveals that lncRNAs are indeed present in these membrane-bound vesicles
- 107.Yu K, Chedin F, Hsieh C-L, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nature Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 108.Abarrategui I, Krangel MS. Noncoding transcription controls downstream promoters to regulate T-cell receptor-α recombination. EMBO J. 2007;26:4380–4390. doi: 10.1038/sj.emboj.7601866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pone EJ, Xu Z, White CA, Zan H, Casali P. B cell TLRs and induction of immunoglobulin class-switch DNA recombination. Front. Biosci. 2012;17:2594–2615. doi: 10.2741/4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stavnezer J, Amemiya CT. Evolution of isotype switching. Semin. Immunol. 2004;16:257–275. doi: 10.1016/j.smim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 111.Hackney JA, et al. DNA targets of AID evolutionary link between antibody somatic hypermutation and class switch recombination. Adv. Immunol. 2009;101:163–189. doi: 10.1016/S0065-2776(08)01005-5. [DOI] [PubMed] [Google Scholar]
- 112.Seising E. Ig class switching: targeting the recombinational mechanism. Curr. Opin. Immunol. 2006;18:249–254. doi: 10.1016/j.coi.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 113.Abarrategui I, Krangel MS. Regulation of T cell receptor-α gene recombination by transcription. Nature Immunol. 2006;7:1109–1115. doi: 10.1038/ni1379. [DOI] [PubMed] [Google Scholar]
- 114.Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. Accessibility control of V(D)J recombination. Adv. Immunol. 2006;91:45–109. doi: 10.1016/S0065-2776(06)91002-5. [DOI] [PubMed] [Google Scholar]
- 115.Petes TD. Meiotic recombination hot spots and cold spots. Nature Rev. Genet. 2001;2:360–369. doi: 10.1038/35072078. [DOI] [PubMed] [Google Scholar]
- 116.Wahls WP, Siegel ER, Davidson MK. Meiotic recombination hotspots of fission yeast are directed to loci that express non-coding RNA. PLoS ONE. 2008;3:e2887. doi: 10.1371/journal.pone.0002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spitale RC, et al. RNA SHAPE analysis in living cells. Nature Chem. Biol. 2013;9:18–20. doi: 10.1038/nchembio.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin L, et al. Systematic reconstruction of RNA functional motifs with high-throughput microfluidics. Nature Methods. 2012;9:1192–1194. doi: 10.1038/nmeth.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science. 2011;333:642–646. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsai M-C, Spitale RC, Chang H. Y Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao J, Sun BK, Erwin JA, Song JJ, Lee J. I Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rinn JL, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]