Abstract
Elevated whole blood serotonin levels are observed in more than 25 % of children with autism spectrum disorder (ASD). Co-occurring gastrointestinal (GI) symptoms are also common in ASD but have not previously been examined in relationship with hyperserotonemia, despite the synthesis of serotonin in the gut. In 82 children and adolescents with ASD, we observed a correlation between a quantitative measure of lower GI symptoms and whole blood serotonin levels. No significant association was seen between functional constipation diagnosis and serotonin levels in the hyperserotonemia range, suggesting that this correlation is not driven by a single subgroup. More specific assessment of gut function, including the microbiome, will be necessary to evaluate the contribution of gut physiology to serotonin levels in ASD.
Keywords: Serotonin, 5-HT, Gastrointestinal (GI), IL-6, Medical comorbidities, Autism Treatment Network
Introduction
Elevated whole blood serotonin (5-hydroxytryptamine, 5-HT), termed hyperserotonemia, occurs in approximately 30 % of children with autism spectrum disorder (ASD) (Gabriele et al. 2014). Blood 5-HT is synthesized in the gut, where it plays a critical role in regulating motility and inflammation (Gershon 2013). Gastrointestinal (GI) symptoms are more prevalent in children with ASD than in controls, with constipation most commonly reported (Aldinger et al. 2015; Chaidez et al. 2014; Chandler et al. 2013; Gorrindo et al. 2012; Ibrahim et al. 2009; McElhanon et al. 2014).
To our knowledge, no previous study examined the relationship between GI symptoms and 5-HT in ASD. Based upon the enteric 5-HT system’s impact on gut motility (Gershon 2013; Li et al. 2011), we hypothesized that children with hyperserotonemia have reduced motility and resulting functional constipation. We also explored potential association with behavioral symptoms based upon previous, inconsistent findings (Kolevzon et al. 2010; McBride et al. 1998; Mulder et al. 2004; Sacco et al. 2010), as well as with IL-6 levels following a recent report of correlation (Yang et al. 2015). Finally, we examined non-verbal status as a potential confounder based upon its previous association with functional constipation in ASD (Gorrindo et al. 2012).
Methods
Participants
This study was approved by the Institutional Review Boards at the University of Missouri and Vanderbilt University. Consents, and, when appropriate, assents were obtained from participants and/or parents. As previously described (Ferguson et al., submitted [supplemental]), 120 six to eighteen-year-olds were recruited through the Autism Speaks Autism Treatment Network and affiliated clinics. Parents of potential participants were screened over the phone to attempt to recruit a similar number of participants with and without a GI disorder. All participants were diagnosed with ASD based on the DSM-IV criteria (American Psychiatric Association 2000), using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 1989) to verify. Thirty-eight participants were excluded because they were taking medications that affect 5-HT, including serotonin reuptake inhibitors, psychostimulants, and neuroleptics.
Assessment of Gastrointestinal Symptoms
The Questionnaire on Pediatric Gastrointestinal Symptoms- Rome III (QPGS) (Baber et al. 2008) was used to assess GI symptoms. Parent-report forms were administered to most families. In four higher-functioning individuals >16 years of age, where the parent indicated the subject would give the most reliable response, the self-report form was completed by the participant. Standard criteria were used to assign functional GI diagnoses for categorical analyses (Baber et al. 2008). To allow the analysis of GI symptoms on a continuum, the QPGS was also scored using a previously described scoring rubric (Ferguson et al., submitted [supplemental]) to assess relative severity of lower GI symptoms.
Behavioral Measures
Sensory symptoms, interfering behaviors, repetitive speech, and anxiety were assessed using caregiver-reported measures: Sensory Over-Responsivity Inventory (SensOR) (Schoen et al. 2008), Aberrant Behavior Checklist (ABC) (Aman 1994), and Repetitive Behavior Scale-Revised (RBS-R) (Bodfish et al. 2000). Participants were defined as non-verbal if fewer than five words were used in the ADOS (module 1, item A1) (Gorrindo et al. 2012).
Serotonin
Fasting whole blood 5-HT was measured by high-performance liquid chromatography, as previously described (Anderson et al. 1987; McBride et al. 1998). To identify a “hyperserotonemia” subgroup for categorical analyses, whole blood 5-HT levels were compared to previously published levels in controls performed within the same laboratory (McBride et al. 1998), corrected for race, age, and sex.
Cytokines
IL-6 was measured using R&D ELISA kits (Minneapolis, MN), in duplicate, quantified on a Spectramax M3 plate reader (Molecular Devices, Sunnyvale, CA) according to manufacturer’s instructions.
Data Analysis
Categorical analyses were used to compare participants with hyperserotonemia versus normoserotonemia, functional constipation versus no GI diagnosis, and verbal versus non-verbal groups using Fisher’s exact tests for categorical variables and one-way analysis of variance for quantitative variables. Since significant skewness was observed, 5-HT levels were log-transformed for quantitative analyses. Pearson’s and Spearman’s correlations were applied in the Caucasian-only population with correction for age and sex (McBride et al. 1998). All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and figures created in R version 3.1.2.
Results
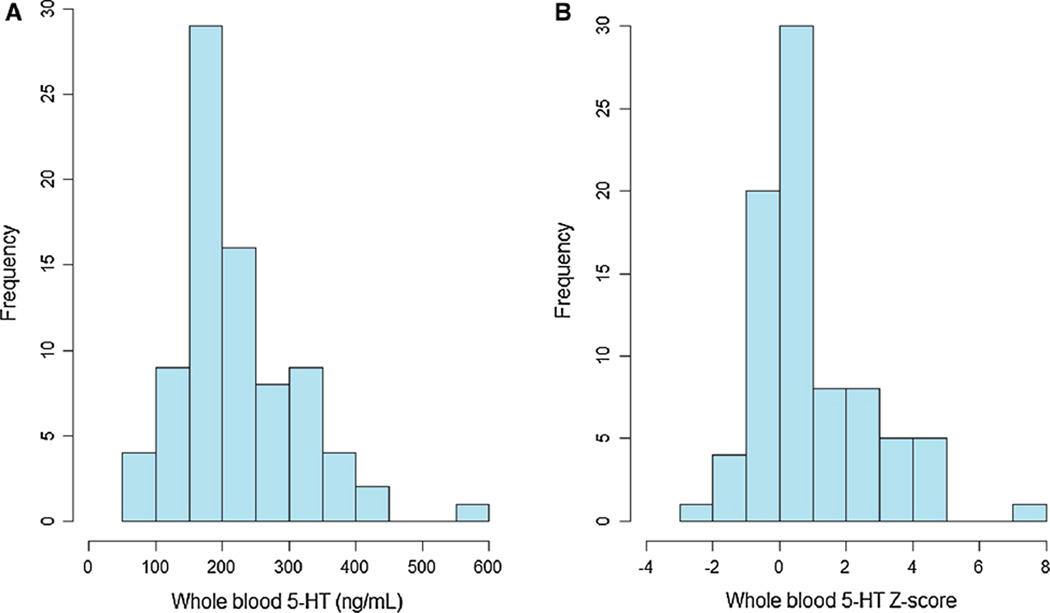
Comparing to published norms (McBride et al. 1998), 23 % of the participants had 5-HT levels two standard deviations above the mean for their race and pubertal status. The distribution of whole blood 5-HT levels showed significant skewness, with an extended tail toward elevated levels (Fig. 1). Participants with hyperserotonemia did not differ significantly from the rest of the participants on demographic characteristics, ADOS severity, or IQ (Table 1).
Fig. 1.
Distribution of whole blood serotonin (5-HT). Frequency distribution of 5-HT levels, in absolute values (ng/mL) and in Z scores. 23 % of the participants had 5-HT levels two standard deviations above the mean for their race and pubertal status. N = 82
Table 1.
Sample characteristics and relationship with 5-HT levels
| Total N | Normoserotonemia (n = 63) | Hyperserotonemia (n = 19) | p value | |
|---|---|---|---|---|
| Sex | 82 | 1.0 | ||
| Male (%) | 74 (90.2) | 57 (90.5) | 17 (89.5) | |
| Female (%) | 8 (9.8) | 6 (9.5) | 2 (10.5) | |
| Age: Mean (SD) | 82 | 11.2 (4.1) | 11.7 (3.6) | 0.61 |
| Race | 82 | 0.66 | ||
| Caucasian (%) | 75 (91.5) | 58 (92.1) | 17 (89.5) | |
| Other race (%) | 7 (8.5) | 5 (7.9) | 2 (10.5) | |
| Ethnicity | 82 | 1.0 | ||
| Non Hispanic/Latino (%) | 79 (96.3) | 60 (95.2) | 19 (100) | |
| Hispanic/Latino (%) | 3 (3.7) | 3 (4.8) | 0 (0.0) | |
| Household income | 71 | 0.58 | ||
| Less than 50k (%) | 31 (43.7) | 25 (46.3) | 6 (35.3) | |
| 50k or greater (%) | 40 (56.3) | 29 (53.7) | 11 (64.7) | |
| ADOS severity score: Mean (SD) | 65 | 7.1 (2.3) | 6.6 (2.0) | 0.46 |
| IQ: Mean (SD) | 68 | 83.9 (24.4) | 83.8 (21.0) | 0.99 |
Functional constipation (FC) was the most common Rome III diagnosis, occurring in 39 % of the sample. Participants with FC did not differ from those with no GI diagnosis on demographic characteristics, ASD severity, or IQ (Table 2). Lower GI symptoms were not significantly higher in the non-verbal subgroup (20.3 ± 15.6, n = 6) in comparison to the verbal subgroup (15.7 ± 11.2, p = 0.341).
Table 2.
Sample characteristics and relationship with GI symptoms
| Total N | No GI diagnosis (n = 44) |
Functional constipation (n = 32) |
Non-retentive fecal incontinence (n = 2) |
Other Rome-III diagnoses (n = 4) |
p value | |
|---|---|---|---|---|---|---|
| Sex | 82 | 0.40 | ||||
| Male (%) | 74 (90.2) | 41 (93.2) | 28 (87.5) | 2 (100) | 3 (75.0) | |
| Female (%) | 8 (9.8) | 3 (6.8) | 4 (12.5) | 0 (0) | 1 (25.0) | |
| Age: Mean (SD) | 82 | 12.0 (4.3) | 10.6 (3.4) | 10.0 (4.2) | 10.8 (4.4) | 0.41 |
| Race | 82 | 0.42 | ||||
| Caucasian (%) | 75 (91.5) | 38 (86.4) | 31 (96.9) | 2 (100) | 4 (100) | |
| Other race (%) | 7 (8.5) | 6 (13.6) | 1 (3.1) | 0 (0) | 0 (0) | |
| Ethnicity | 82 | 0.66 | ||||
| Non-Hispanic/Latino (%) | 79 (96.3) | 43 (97.7) | 30 (93.8) | 2 (100) | 4 (100) | |
| Hispanic/Latino (%) | 3 (3.7) | 1 (2.3) | 2 (6.3) | 0 (0) | 0 (0.0) | |
| Household income | 71 | 1.0 | ||||
| Less than 50k (%) | 31 (43.7) | 16 (42.1) | 13 (44.8) | 1 (50.0) | 1 (50.0) | |
| 50k or greater (%) | 40 (56.3) | 22 (57.9) | 16 (55.2) | 1 (50.0) | 1 (50.0) | |
| ADOS severity score: Mean (SD) | 65 | 7.1 (2.6) | 6.7 (1.9) | 7.5 (2.1) | 7.0 (1.0) | 0.86 |
| Verbal status | 73 | 1.00 | ||||
| Verbal | 67 | 38 (90.5) | 24 (92.3) | 2 (100) | 3 (100) | |
| Nonverbal | 6 | 4 (9.5) | 2 (7.7) | 0 (0) | 0 (0) | |
| IQ: Mean (SD) | 68 | 85.9 (23.5) | 80.8 (24.9) | 72.0 (5.7) | 88.3 (21.5) | 0.71 |
FC diagnosis was present in 10/19 (53 %) of the hyperserotonemia subgroup and 22/57 (39 %) of the remaining participants, excluding those with other GI diagnoses (Fisher’s exact p = 0.30). As a quantitative measure, the QPGS lower GI score differentiated the FC (27.3 ± 10.0) and no GI disorder groups (8.3 ± 4.9, p < 0.001), but it did not differ in the hyperserotonemia subgroup in comparison to the rest of the participants. Approaching the analysis from the opposite perspective, normalized whole blood 5-HT levels were not significantly higher in the FC group (Mean Z = 1.3 ± 1.9) in comparison to those with no GI diagnosis (Mean Z = 0.7 ± 1.6, p = 0.14).
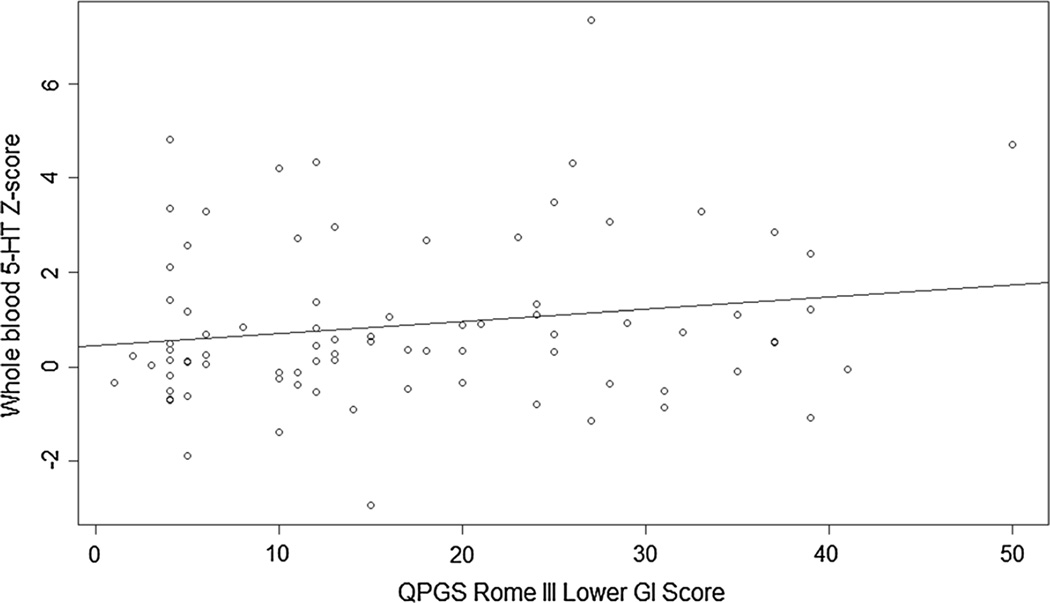
When considered as a continuous variable in the Caucasian- only sample, controlling for age and sex, a trend-level Pearson’s correlation (r = 0.21, p = 0.066) and a marginally significant Spearman’s correlation (ρ = 0.23, p = 0.048) were observed between log-transformed whole blood 5-HT levels and the QPGS lower GI score (Fig. 2). Exploratory, item-level Spearman’s correlations were identified between whole blood 5-HT levels and pain around or below the belly button (ρ = 0.36, p = 0.001), resisting bowel movements/“holding in” stool (ρ = 0.30, p = 0.011), and large bowel movements that clogged the toilet (ρ = 0.25, p = 0.030).
Fig. 2.
Correlation between QPGS Rome III lower GI scores and log-transformed whole blood serotonin raw scores. When considered as a continuous variable in the Caucasian-only sample, with age and sex as covariates, a trend-level Pearson’s correlation (r = 0.21, p = 0.066) and a marginally significant Spearman’s correlation (ρ = 0.23, p = 0.048) were observed
The hyperserotonemia group did not differ significantly on any behavioral measure, nor did whole blood 5-HT levels correlate with these measures. A trend-level association was seen with RBS-R compulsive behavior, with higher scores in the hyperserotonemia subgroup (6.4 ± 6.5 vs. 4.1 ± 4.3, p = 0.07), without correction for multiple comparisons.
IL-6 levels did not differ significantly in the hyperserotonemia subgroup (1.6 ± 1.5 vs. 1.5 ± 0.9 pg/mL, p = 0.783), and no significant correlation was observed between IL-6 and whole blood 5-HT levels.
Discussion
The study population in the present study showed a significant rightward skew for whole blood 5-HT levels, with a similar proportion in the predicted hyperserotonemia range as in previous studies (Gabriele et al. 2014; McBride et al. 1998). FC was the most common GI diagnosis in our sample, which is also consistent with previous findings in ASD (Gorrindo et al. 2012). The lack of significant association between hyperserotonemia and FC suggests that there is not a unifying subgroup of children with ASD defined by elevated blood 5-HT levels and constipation. Further, these results do not support the hypothesis that constipation somehow leads to hyperserotonemia, or, conversely, that the mechanisms underlying hyperserotonemia also cause predictable GI symptoms. We cannot rule out a weaker relationship since the observed ratio was 1.8, which would require a four-fold larger sample to test.
Quantitative analyses are a more powerful way to detect association, and we did identify a correlation between whole blood 5-HT levels and lower GI symptoms. Further, we found correlations between 5-HT levels and three QPGS items that may indicate substantial constipation, but these are exploratory analyses that need to be confirmed in a larger, independent sample. These findings suggest that whole blood 5-HT levels may relate to GI symptoms, but they do not clarify whether this association is specific to ASD.
Consistent with previous efforts to identify symptom-level associations, we did not find any significant relationship between hyperserotonemia and behavioral outcomes. We also failed to replicate the recent report of a correlation between whole blood 5-HT levels and IL-6 levels (Yang et al. 2015), with IL-6 similar to other reports (Ashwood et al. 2011; Masi et al. 2015). Finally, we did not observe the expected association between FC and minimally verbal status in this population, likely because our population only included six non-verbal children.
This study has a number of limitations. First, GI diagnoses were made based on parent report using the QPGS, which a previous study showed is predictive but underestimates constipation diagnosed by a gastroenterologist (Gorrindo et al. 2012). Direct stool assessment might provide a more valid, quantitative analysis, and would also allow examination of microbiota, which were recently shown to alter blood 5-HT levels in mice (Yano et al. 2015). Behavioral symptoms were similarly measured using well-validated parent report measures that still fall short of direct observation. As an additional concern, our sample size, while larger than most studies of this robust biomarker, is too small to detect moderate effect sizes in the subgroup of children with hyperserotonemia. Finally, a cross-sectional study without a treatment component does not allow us to make causal inferences regarding the observed, weak correlation.
In summary, our data confirm the frequency of hyperserotonemia and of FC in ASD, but there is no significant association between membership in these two subgroups. In contrast, analyses of these variables as continuous traits reveals a correlation of 5-HT levels with lower GI symptoms, including pain, stool retention, and large bowel movements. A longitudinal follow-up study with a larger sample size, including participants without ASD, would be necessary to understand whether these findings are specific to ASD and to clarify the directionality of this weak correlation.
Acknowledgments
We would like to thank the children and families who participated in this study. This research was supported by a Grant given to the Autism Treatment Network, Autism Intervention Research Network on Physical Health by the Health Resources Services Administration (HRSA Grant# UA3MC11054). This article was reviewed by the funding body prior to submission.
Dr. Veenstra-VanderWeele has served on advisory boards for Novartis and Roche Pharmaceuticals. He has received research funding from Novartis, Roche Pharmaceuticals, Seaside Therapeutics, Forest, Sunovion, and SynapDx. Dr. Beversdorf has received research funding from Seaside Therapeutics.
Abbreviations
- ABC
Aberrant Behavior Checklist
- ADOS
Autism Diagnostic Observation Schedule
- ASD
Autism spectrum disorder
- FC
Functional constipation
- GI
Gastrointestinal
- QPGS
Questionnaire on Pediatric Gastrointestinal Symptoms-Rome III
- RBS-R
Repetitive Behavior Scale-Revised
- SensOR
Sensory Over-Responsivity Inventory
- STAI
State-Trait Anxiety Inventory
Footnotes
Author Contributions SM and BF contributed to participant recruitment, data collection, data entry, data interpretation and manuscript writing. EL, BP, KW, PL, KG, DB, and JV contributed to study design, data interpretation, and manuscript writing. EM and EM performed the statistical analysis and contributed to manuscript writing. CG contributed to the immunoassays of IL-6 and manuscript writing. GA contributed to the HPLC assays of 5-HT and manuscript writing. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Ethical Standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest The other authors report no conflicts of interests.
Contributor Information
Sarah Marler, Email: sarah.marler@vanderbilt.edu.
Bradley J. Ferguson, Email: fergusonbj@health.missouri.edu.
Evon Batey Lee, Email: evon.lee@vanderbilt.edu.
Brittany Peters, Email: brittany.peters@vanderbilt.edu.
Kent C. Williams, Email: kent.williams@nationwidechildrens.org.
Erin McDonnell, Email: eimcdonnell@mgh.harvard.edu.
Eric A. Macklin, Email: emacklin@mgh.harvard.edu.
Pat Levitt, Email: plevitt@med.usc.edu.
Catherine Hagan Gillespie, Email: haganc@missouri.edu.
George M. Anderson, Email: george.anderson@yale.edu.
Kara Gross Margolis, Email: kjg2133@cumc.columbia.edu.
David Q. Beversdorf, Email: beversdorfd@health.missouri.edu.
References
- Aldinger KA, Lane CJ, Veenstra-VanderWeele J, Levitt P. Patterns of Risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research. 2015 doi: 10.1002/aur.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman M. Aberrant Behavior Checklist - Community. East Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders-text revision (DSM-IV-TR) 4th. Washington, DC: American Psychiatric Association Press, Inc; 2000. [Google Scholar]
- Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Science. 1987;40:1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. Journal of Pediatric Gastroenterology and Nutrition. 2008;47(3):299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. Retrieved from http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0011055459. [DOI] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Carcani-Rathwell I, Charman T, Pickles A, Loucas T, Meldrum D, Baird G. Parent-reported gastrointestinal symptoms in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(12):2737–2747. doi: 10.1007/s10803-013-1768-0. [DOI] [PubMed] [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. European Neuropsychopharmacology. 2014;24(6):919–929. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in Endocrinology, Diabetes, and Obesity. 2013;20(1):14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Research. 2012;5(2):101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics. 2009;124(2):680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Newcorn JH, Kryzak L, Chaplin W, Watner D, Hollander E, Silverman JM. Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry Research. 2010;175(3):274–276. doi: 10.1016/j.psychres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/ survival of enteric dopaminergic neurons. Journal of Neuroscience. 2011;31(24):8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism Diagnostic Observation Schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Molecular Psychiatry. 2015;20(4):440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, Cohen DJ. Effects of diagnosis, race, and puberty on platelet serotonin levels in autism and mental retardation. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(7):767–776. doi: 10.1097/00004583-199807000-00017. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics. 2014;133(5):872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(4):491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Sacco R, Curatolo P, Manzi B, Militerni R, Bravaccio C, Frolli A, Persico AM. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Research. 2010;3(5):237–252. doi: 10.1002/aur.151. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Green KE. Pilot study of the Sensory Over-Responsivity Scales: Assessment and inventory. American Journal of Occupational Therapy. 2008;62(4):393–406. doi: 10.5014/ajot.62.4.393. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18712002. [DOI] [PubMed] [Google Scholar]
- Yang CJ, Liu CL, Sang B, Zhu XM, Du YJ. The combined role of serotonin and interleukin-6 as biomarker for autism. Neuroscience. 2015;284:290–296. doi: 10.1016/j.neuroscience.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]