Abstract
Cerebral small vessel disease (SVD) pathophysiology is poorly understood. Cerebrovascular reactivity (CVR) impairment may play a role, but evidence to date is mainly indirect. Magnetic resonance imaging (MRI) allows investigation of CVR directly in the tissues affected by SVD. We systematically reviewed the use of MRI to measure CVR in subjects with SVD. Five studies (total n = 155 SVD subjects, 84 controls) provided relevant data. The studies included different types of patients. Each study used blood oxygen level dependent (BOLD) MRI to assess CVR but a different vasoactive stimulus and method of calculating CVR. CVR decreased with increasing white matter hyperintensities in two studies (n = 17, 11%) and in the presence of microbleeds in another. Three studies (n = 138, 89%) found no association of CVR with white matter hyperintensities. No studies provided tissue-specific CVR values. CVR decreased with age in three studies, and with female gender and increasing diastolic blood pressure in one study. Safety and tolerability data were limited. Larger studies using CVR appear to be feasible and are needed, preferably with more standardized methods, to determine if specific clinical or radiological features of SVD are more or less associated with impaired CVR.
Keywords: Blood oxygen level dependent magnetic resonance imaging, cerebral small vessel disease, cerebrovascular reactivity, hypercapnia, white matter hyperintensity
Introduction
Cerebral small vessel disease (SVD) is an important cause of morbidity and mortality worldwide. Clinically SVD has diverse presentations, including lacunar stroke, intracerebral haemorrhage, cognitive impairment, dementia and depression as well as gait and bladder dysfunction.1,2 Radiologically it is characterised by recent small subcortical (or acute lacunar) infarcts, white matter hyperintensities, lacunes, microbleeds and enlarged perivascular spaces.3 This is in contrast to the presentation of large artery, athero-thrombo-embolic or cardio-embolic stroke which typically manifests as cortical stroke syndromes clinically and cortical or large striatocapsular infarcts on neuroimaging. The diverse clinical presentations and the presence of SVD features in patients with other brain conditions, such as Alzheimer’s disease (AD),4 have made studying SVD more difficult and as a result the underlying pathophysiology is poorly understood despite its high prevalence.1
Clinically lacunar stroke and SVD are associated with some vascular risk factors such as hypertension, diabetes and smoking but have only weak associations with others such as large artery atheroma and atrial fibrillation.5,6 While some SVD imaging features and large artery stroke can occur in the same individuals, in general, recurrent stroke tends to be of the same type as the initial stroke.7 Additionally, SVD features associate more strongly with lacunar than cortical stroke.8 This suggests that SVD is not simply ‘atherosclerosis of the smaller blood vessels’.5,6
Recently it has been proposed that endothelial dysfunction may play a key role in development of SVD.1,9,10 Endothelial dysfunction may result in small blood vessels becoming stiff and unreactive, such that they cannot increase brain blood flow when needed, a feature termed cerebrovascular reactivity (CVR).
A systematic review11 showed that patients with lacunar stroke subtype had reduced CVR, assessed using transcranial Doppler (TCD), compared to age matched controls, but not compared to age plus risk factor matched controls, or to patients with cortical stroke subtype. TCD has limitations in terms of assessing vascular reactivity. It only assesses one representative artery and cannot provide information on the distribution of CVR by tissue type or brain region.11,12 It therefore remains unclear whether impairment of CVR is specific to lacunar stroke and SVD or simply reflects exposure to risk factors.
Magnetic resonance imaging (MRI) with blood oxygen level dependent (BOLD) or arterial spin labelling (ASL) techniques can now be used to measure CVR in response to a stimulus, such as breathing carbon dioxide (CO2). ASL measures blood flow directly but suffers from a low signal to noise ratio. BOLD MRI is the basis of functional MRI (fMRI) where it is used to localise the haemodynamic response to neural activity. BOLD MRI is based on the principal that when blood flow increases to a region of brain this results in a reduction in the venous concentration of paramagnetic deoxyhaemoglobin which can be detected as a small increase in signal on T2*-weighted imaging.
These MRI techniques have been used to investigate CVR in several different conditions including carotid artery stenosis and Moyamoya disease13,14 as well as in healthy volunteers. CVR measurement using MRI has the potential to investigate the pathophysiology of SVD directly in the tissues affected, and if reliable could be used as a surrogate endpoint in testing new treatments for SVD.
The purpose of this systematic review is to identify and summarise the knowledge to date from studies using MRI to assess CVR in SVD.
Materials and methods
We performed a literature review seeking full length original research articles assessing CVR using MRI in human subjects with any clinical or imaging features of SVD, published in English. We included studies of patients with AD if the results were reported in relation to SVD imaging features which are common in patients with AD.4 We excluded animal studies, studies in patients with Moyamoya disease, intracranial stenosis, extracranial carotid artery stenosis, haemorrhagic stroke or monogenetic disorders such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Studies which assessed CVR by means of TCD were also excluded as were those using Xenon CT, PET or SPECT scanning as we wanted to avoid techniques which have limited availability, are more expensive, and expose patients to ionising radiation. Studies published only as conference abstracts were also excluded.
We searched the databases Medline and Excerpta Medica Database (EMBASE) for articles published between 1990 and 7 May 2015 with the strategy shown in Appendix 1 (Supplementary Information). Additionally, to validate the search strategy, we hand searched the journals Stroke and Journal of Cerebral Blood Flow and Metabolism for relevant articles.
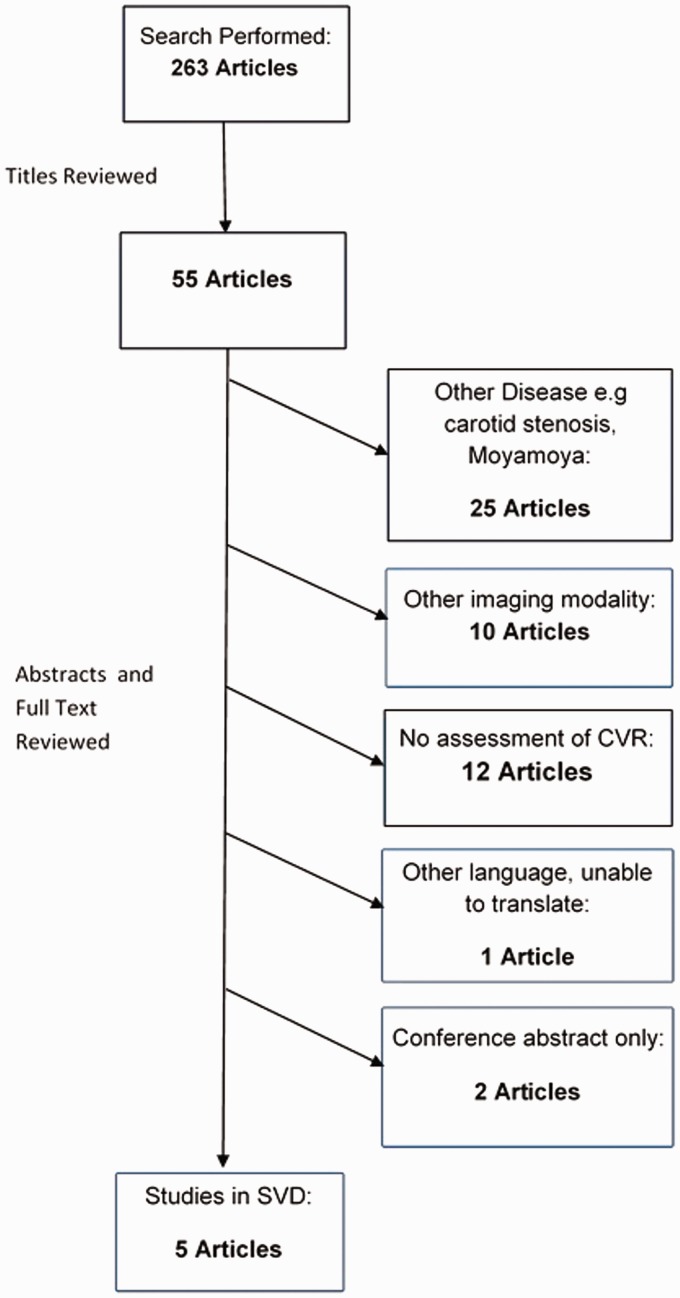
We assessed article titles and in those deemed relevant we reviewed the abstracts. We then reviewed pertinent articles in full and included them if the above criteria were met (Figure 1). We extracted data from included articles using standardised forms.
Figure 1.
Search strategy flow chart. Flow chart showing number of papers at each stage of the search and reasons for excluding papers.
We extracted data on study design including quality criteria, patient and control group demographics, the MR protocol, the vasoactive stimulus used, the CVR analysis method and the main study results including any data on tolerability or safety. We used the STROBE checklist (www.equator-network.org) to score the study methodology and reporting of findings to assess study quality. We assigned up to 22 points using this checklist (Appendix 2, Supplementary Information). This score was applied after the decision to include the study and did not influence whether a study was included in the review.
Owing to the small number of studies and the large methodological heterogeneity, it was not possible to do a formal meta-analysis to combine study data.
Results
The search strategy identified 263 potentially relevant articles. Of these, five described CVR using MRI in patients with features of SVD.15–19 Figure 1 details numbers of papers excluded and the reasons.
The five articles include 239 subjects (155 SVD subjects, 84 controls). The studies had a median STROBE criteria score of 14.5/22 (range 12.5–16). Points were lost most commonly for inadequate description of SVD diagnostic criteria, not explaining rationale for study size, inadequate description of efforts made to control for confounding factors such as age, gender and blood pressure, limited description of patient demographics, not indicating study design in the title and inadequate discussion of study limitations such as incomplete CVR examinations (Appendix 2, Supplementary Information).
Patient characteristics
Patient characteristics are shown in Table 1: these varied widely between studies. The clinical phenotype included patients with asymptomatic white matter hyperintensities (WMHs; two studies, n = 103),15,17 stroke (one study, n = 5)19 and AD (two studies, which described CVR in relation to SVD imaging features, n = 47).16,18 Four of the five studies included a healthy control group, but in only two of the four studies was the control group of similar age to the patients.16,18 One study did have an older healthy control group however there was an almost five year age difference between patients and controls which may be significant.19
Table 1.
Patient characteristics.a
| Study | Sample size | Subjects | Control group | Age |
|---|---|---|---|---|
| Gauthier et al.15 | 54 subjects 31 controls | Healthy elderly with asymptomatic WMH | Young healthy volunteers | Subjects: 63 ± 5 Controls: 24 ± 3 |
| Richiardi et al.16 | 20 AD 15 MCI 28 controls | AD and MCI | Healthy elderly | AD: 76 ± 7 MCI: 71 ± 10 Controls 73 ± 7 |
| Conijn et al.17 | 49 | Patients with history of vascular events or cardiovascular risk factors | None | 58.9 ± 10 |
| Yezhuvath et al.18 | Subjects: 12 Controls: 13 | AD | Healthy elderly | Subjects: 70.5 ± 8.3 Controls: 68.7 ± 8.4 |
| Hund-Georgiadis et al.19 | Subjects: 5 Young Controls: 6 Older Controls: 6 | Stroke and ‘cerebral microangiopathy’ (white matter MRI signal changes.) | Young healthy volunteers and older healthy volunteers | Subjects: 61.8 (54–67) Young controls: 24.8 (23–27) Older controls: 57 (51–63) |
AD: Alzheimer’s disease, MCI: mild cognitive impairment.
Patient characteristics of included studies of CVR in cerebral SVD.
MR scanner, vasodilatory stimuli and calculation of CVR
Table 2 summarises the methods used by each study to assess CVR. MR scanner magnetic field strength varied: CVR was measured at 3 or 7 T and the SVD features were assessed at 1.5, 3 or 7 T. Each study used BOLD MRI to determine CVR with a different vasodilatory stimulus, delivered over different lengths of time, although all stimuli were based on manipulation of arterial CO2 concentration to achieve a change in cerebral blood flow. Three studies used CO2 in air to increase partial pressure of carbon dioxide in arterial blood (PaCO2),15,16,18 one study used breath holding to increase CO217 and one used hyperventilation to decrease CO2.19 Each study also used a different way of calculating CVR from the CO2 and BOLD signal changes. One study17 assessed CVR and microbleed burden, assessed using visual rating with the Microbleed Anatomical Rating Scale,20 at 7 T, but compared this with WMH assessed at 1.5 T.
Table 2.
MR scanner magnetic field strength, vasoactive stimulus and CVR calculation method.a
| Study | Field strength | Vasoactive stimulus | Effect of stimulus | Imaging parameters | Method of calculating CVR |
|---|---|---|---|---|---|
| Gauthier et al.15 | 3 T | Prospective end-tidal CO2 targeting (Respiract™) – alternating periods of 2 min at each EtCO2 concentration. Total CVR imaging time 10 min | EtCO2 manipulated between 40 and 45 mm Hg | Dual-echo pseudocontinuous arterial spin labelling (used to obtain simultaneous CBF and BOLD measurements); TR/TE1/TE2 = 3000/10/30 ms; FA = 90°; parallel imaging acceleration factor = 2; 4 x 4 mm in-plane resolution with 7/8 partial Fourier sampling; 11 x 7 mm slices with 1 mm gap | Linear regression with CVR expressed as %BOLD signal change per mm Hg change in EtCO2 |
| Richiardi et al.16 | 3 T | 7% CO2 in air via nasal cannula in 2 min spells alternating with air. Total CVR imaging time 9 min | No monitoring of EtCO2 | Multi-echo EPI; TR/TE = 2970/29.5 ms; in-plane resolution 3.44 x 3.44 mm; 34 x 3.5 mm slices | Convolved the CO2 on–off timing vector with a filter to create a regressor that allowed description of ‘CVR velocity’ |
| Conijn et al.17 | 7 T to assess microbleeds and CVR 1.5 T for WMH | Breath holding – 5 periods, 4 of which were 21 s long, 5th period as long as possible, total CVR imaging time 4 min 15 s plus time of last breath hold | No monitoring of EtCO2 | Single-shot EPI; TR/TE = 3000/20 ms; parallel imaging acceleration factor = 3.5; in-plane resolution 1.5 x 1.5 mm; 45 x 1.5 mm slices with no gap | Multiple linear regression to select activated voxels and then expressed CVR as either mean % of voxels with significant signal change or mean % whole brain signal change |
| Yezhuvath et al.18 | 3 T | 5% CO2 in air in 1 min spells alternating with air. Total CVR imaging time 7 min | Mean increase in EtCO2 of 11.9 mm Hg in patients and 12.2 mm Hg in controls | Single-shot EPI; TR/TE = 3000/30 ms; FA = 90°; in-plane resolution = 1.7 x 1.7 mm; 25 x 6 mm axial slices | General linear model with EtCO2 as the regressor and expressed CVR as % BOLD signal change per mm Hg change in EtCO2 |
| Hund-Georgiadis et al.19 | 3 T | Hyperventilation for 2 min alternating with 4 min periods of normal breathing | At least 10 mm Hg decrease in EtCO2 | Single-shot EPI; TR/TE = 2000/75 ms; FA = 90°; in-plane resolution = 3 x 3 mm; 16 x 5 mm axial slices with 1 mm gap | Calculated the contrast between different conditions using the t statistic, with t values subsequently being transformed into z scores. CVR was then defined as voxels with a z score of at least −5 with ventilation |
Description of MR scanner magnetic field strength, vasoactive stimulus, imaging parameters and CVR calculation method for each included study.
CVR in SVD
Table 3 summarises the associations between SVD and CVR in the five included studies. All studies measured CVR in large brain regions (e.g. whole hemisphere or lobar measurements) and not in specific tissues such as white matter, WMH, or grey matter. Three studies assessed CVR in relation to WMH burden,15,16,18 one study assessed CVR in relation to both WMH burden and microbleed burden17 and one study assessed CVR in relation to combined burden of WMH, lacunes and perivascular spaces.19
Table 3.
Effects of SVD on CVR.
| Study | CVR in relation to SVD features | CVR in subjects vs. controls | CVR in relation to other features | Safety and tolerability |
|---|---|---|---|---|
| Gauthier et al.15 Total n = 85 | WMH volume not associated with frontal lobe CVR | Frontal lobe CVR 0.22 ± 0.06%/mm Hg in subjects vs. 0.25 ± 0.08%/mm Hg in young controls | CVR decreased with age | 2 patients excluded due to discomfort when breathing via Respiract™ device |
| Richiardi et al.16 Total n = 63 | No association between ‘CVR velocity’ and Fazekas score | Mean time to reach 90% of peak BOLD response 59 s for MCI and AD patients vs. 33 s for controls | ‘CVR velocity’ increased with increasing MMSE score | No discomfort reported |
| Conijn et al.17 Total n = 49 | Microbleeds associated with decrease in CVR. No CVR association with lacunes or WMH severity | Not applicable | CVR decreased with increasing age, female gender and increasing diastolic blood pressure | Not assessed |
| Yezhuvath et al.18 Total n = 25 | Decreased CVR associated with increased WMH volume | CVR decreased in frontal lobe, insula and anterior cingulate gyrus in subjects compared to controls | Decreased CVR associated with a lower Boston Naming Score | 5 subjects and 4 controls declined to take part in CVR scan but no reasons given |
| Hund-Georgiadis et al.19 Total n = 17 | Increased severity of SVD features (composite score of WMH, lacunes and enlarged perivascular spaces) associated with decreased CVR | Subjects had lower whole brain CVR than older controls, who in turn had lower CVR than young controls. Subjects had reduced CVR in frontal and parietal cortex vs. both control groups and in occipital cortex vs. young controls | CVR decreased with age | Not assessed |
AD: Alzheimer’s disease, MCI: mild cognitive impairment, MMSE: Mini Mental State Examination.
One study (n = 12) reported reduced CVR with worsening WMH,18 one with worsening combined WMH, lacunes and perivascular spaces (n = 5)19 and one with microbleeds but not with WMH (n = 49).17 In the study by Conjin, CVR and microbleeds were assessed at 7 T and WMH at 1.5 T.17 Two other studies (n = 54 and 35) found no association between WMH and CVR.15,16 Thus, CVR did not fall with increasing WMH in a total of 138 of 155 patients (89%) and did fall with increasing WMH or combined WMH, lacunes and perivascular spaces in 17 of 155 patients (11%). However, of the studies that did not find reduced CVR with WMH, one used breath-holding,17 one administered CO2 via nasal cannulae with no monitoring of end-tidal CO2 (EtCO2)16 and the other only included subjects without vascular risk factors (i.e. very healthy elderly).15
From the perspective of clinical classification, the four studies with control groups all showed that CVR was impaired in the patient group compared to the control groups,15,16,18,19 but in two of these studies15,19 the controls were much younger than the patients and therefore the difference between patients and controls could be due to age.
CVR decreased with age in three studies.15,17,19 Conijn et al. also showed that female gender and increasing diastolic blood pressure were associated with decreased CVR.17
Only three studies reported any safety or tolerability data for the CVR procedure. One study excluded two patients out of 83 due to discomfort during CO2 breathing.15 One study reported no discomfort in 63 participants but this was using nasal cannulae.16 One study reported that 9 of 34 participants declined to participate in the CVR examination but did not report the reasons.18
Discussion
This systematic review found only five studies that assessed CVR in any form of SVD. Collectively these studies do not provide a clear picture of whether CVR is impaired in SVD or not. Small studies found an association between reduced CVR and increased WMH or microbleeds, but three larger studies including 138 of 155 (89%) of the available SVD patients in the literature did not find an association with WMH, although the method of administering the CO2 challenge may have contributed to the lack of a CVR association. CVR was lower in subjects than controls in these studies, except that most subjects were older than controls and CVR falls with age. All studies combined provide only 155 patients with SVD and the results are difficult to compare between studies due to the patient and methodological differences.
Therefore the question of whether CVR to a CO2 challenge is reduced in SVD as detected by MRI remains unanswered.
Factors affecting measurement of CVR in patients with SVD
Patient characteristics
The type of patients differed between studies and limits the conclusions that can be drawn. The studies included patients with different manifestations of SVD or the SVD features were assessed differently: asymptomatic lesions in patients with vascular risk factors17; lacunar stroke19; WMH in mild cognitive impairment and AD16,18; and WMH in healthy elderly.15 We focused on the imaging evidence of SVD to harmonise the studies. As summarized in the introduction, SVD has several clinical and imaging manifestations which should be more clearly discriminated in future studies. It is not possible to say if specific clinical and radiological features are more associated with impaired CVR than others based on these data.
Descriptions of other patient related variables such as age, blood pressure, medical co-morbidity and medication were also limited. Age, blood pressure and gender are in themselves thought to alter CVR17,21 and therefore may have affected the results. Even where these variables were reported, the studies varied as to whether they corrected for these factors when assessing CVR.
MR protocol
Both MR scanner magnetic field strength and the scanning protocols vary significantly between the studies, so CVR values are not directly comparable. Four studies used 3 T and one used 7 T.
BOLD signal change at 3 T is reported to be 69% higher than at 1.5 T when using breath holding to achieve hypercapnia.22 One study has suggested that protocols using long TE’s may suffer from greater BOLD image distortion and that lower spatial resolution is required to detect CVR in white matter at field strengths of 1.5 T,23 highlighting the effects that the different MR parameters may have on results. All except one of the studies included here used 3 T to assess CVR.
However our review highlights a lack of direct comparisons of CVR between 1.5 and 3 T.24 Therefore, more data are required to determine the true differences in BOLD signal and artefacts due to field strength.
Vasodilatory stimuli and calculation of CVR
The comparability of the different vasodilatory stimuli (in terms of CVR response and patient tolerability) is also relatively unknown. A review of the different stimuli suggested that CO2 inhalation is a more consistent stimulus than acetazolamide, use of a thigh cuff, or lower body negative pressure.12 All the included studies used a change in CO2 as the vasoactive stimulus. We found no papers utilizing acetazolamide in combination with MRI to measure CVR in SVD despite its previous extensive use in measuring CVR with TCD methods.11 Use of acetazolamide may be declining due to problems with the inter-individual variability in response to acetazolamide and the fact that side effects are reported in 40–60% of subjects who receive the drug for CVR examination.12 However, as the studies described here illustrate, there are many methods and gas concentrations that can be used to administer CO2. The relationship between MR signal and EtCO2 is non-linear, meaning CVR results may depend on the CO2 concentration used and baseline EtCO2.25 Additionally the high oxygen concentrations used in some gas mixtures may cause further complex changes to the BOLD contrast.26 One study has suggested that there is little difference between breath holding and CO2 inhalation, although this study used ASL rather than BOLD to assess CVR.27 This study also found bigger differences between the vasodilatory stimuli it assessed when absolute change rather than percentage change CVR measures were used. This suggests the way the BOLD data are processed is another factor affecting CVR measurement and, along with the lack of an accepted definition of CVR, will hinder comparison of results between studies.
The duration of the stimulus varied between studies from 21 s to 2 min. There is known to be a significant delay between change in EtCO2 and change in BOLD signal in the white matter compared to the grey matter.21,28 It is therefore vital that the different delay between brain structures is taken into account during analysis and that the period of hypercapnia is long enough for all tissues to achieve a representative change in BOLD signal. The delay itself may be an important part of the assessment of CVR. It may be that the CO2 challenge, in terms of CO2 concentration and duration, requires maximisation of the signal change and dynamic information content, while ensuring adequate patient tolerability and consistency of stimulus.
Tolerability of CVR measurement
Safety and tolerability data are not often reported in studies that use hypercapnia achieved by breathing high percentage CO2 gas mixtures, making it difficult to assess the feasibility of using this technique in a patient population. Only three of the five studies identified here mention tolerability of the CVR technique. From these data, there appear to be few adverse effects associated with the technique, however one study excluded almost any medical comorbidity,15 one study used nasal cannulae as they believed the patient group would be unable to tolerate a more robust delivery method such as a tight face mask (that would have allowed measurement of EtCO2)16 and one study reported no adverse events but did not give the reason why more than a quarter of participants in the study declined to take part in the CVR assessment.18
One study specifically examined safety and tolerability of CVR using a hypercapnia method mainly in intracranial or carotid atherosclerosis patients with a mean age of 49.5 years. This study assessed 434 patients who underwent CVR studies using the Respiract™ prospective EtCO2 targeting system. Out of 434 patients, 48 patients (11%) reported discomfort during a CVR study. The most common complaint was breathlessness (28 patients) followed by anxiety and claustrophobia. Less commonly reported symptoms were headache, dizziness and light-headedness, conjunctival erythema, tremor, paraesthesia, hand weakness and chest tightness.29
Conclusion
Larger, more methodologically robust studies are needed to be certain of the association between SVD and CVR, to clarify if specific clinical or radiological features of SVD are more or less associated with impaired CVR and to measure CVR in specific tissues. The available data do not support an association between worsening SVD features in general and lower CVR, although do support an association between increasing age or diastolic blood pressure and falling CVR.
Future studies should clearly define the clinical features of the patient population and ensure measurement of, and controlling for, the likely confounding variables. This will allow better comparison of studies. While it is possible to measure CVR at 1.5 T, 3 T scanners yield better signal-to-noise ratio at the same spatial resolution and may be more sensitive for this technique, given the small signal changes seen in the white matter. However direct comparisons in the same patients are lacking and should be performed in future studies to determine the relative strengths and limitations of 1.5 vs. 3 T. The best method for CO2 delivery remains unclear, but avoidance of high oxygen mixtures is probably sensible so that the number of factors influencing the BOLD signal change and measured CVR is kept to a minimum. Standardisation of methods for calculating CVR is also important. Studies appear to be converging towards CVR expressed as percentage BOLD signal change per mm Hg change in EtCO2. Future studies in patients should also report specifically on tolerability and safety in this often frail patient group, and studies should aim for much larger sample sizes.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Gordon Blair is funded by the Scottish Funding Council SINAPSE (Scottish Imaging Network, A Platform for Scientific Excellence) Network as part of The Stroke Association Princess Margaret Research Development Fellowships Scheme. Michael Thrippleton is funded by NHS Lothian Research and Development Office. Fergus Doubal is funded by a Stroke Association Garfield Weston Foundation senior clinical lectureship and NHS Research Scotland Fellowship.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
GWB – performed the search for articles, extracted and analysed data and drafted the manuscript. FND, MJT, IM and JMW – interpreted the data, cross checked the data extraction and reviewed and edited the manuscript.
Supplementary information
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debette SM. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 1997; 277: 813–817. [PubMed] [Google Scholar]
- 5.Khan U, Porteous L, Hassan A, et al. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry 2007; 78: 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Allerhand M, Doubal FN, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology 2014; 82: 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain 2005; 128: 2507–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015; 36: 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey EL, Smith C, Sudlow CL, et al. Pathology of lacunar ischemic stroke in humans – a systematic review. Brain Pathol 2012; 22: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82: 126–135. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson SF, Doubal FN, Shuler K, et al. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke 2010; 41: e434–e442. [DOI] [PubMed] [Google Scholar]
- 12.Fierstra J, Sobczyk O, Battisti-Charbonney A, et al. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 2013; 591: 5809–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandell DM, Han JS, Poublanc J, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 2008; 39: 2021–2028. [DOI] [PubMed] [Google Scholar]
- 14.Ziyeh S, Rick J, Reinhard M, et al. Blood oxygen level−dependent MRI of cerebral CO2 reactivity in severe carotid stenosis and occlusion. Stroke 2005; 36: 751–756. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier CJ, Lefort M, Mekary S, et al. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging 2015; 36: 304–314. [DOI] [PubMed] [Google Scholar]
- 16.Richiardi J, Monsch AU, Haas T, et al. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2015; 36: 33–41. [DOI] [PubMed] [Google Scholar]
- 17.Conijn MM, Hoogduin JM, van der Graaf Y, et al. Microbleeds, lacunar infarcts, white matter lesions and cerebrovascular reactivity – a 7 T study. Neuroimage 2012; 59: 950–956. [DOI] [PubMed] [Google Scholar]
- 18.Yezhuvath US, Uh J, Cheng Y, et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer's disease. Neurobiol Aging 2012; 33: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hund-Georgiadis M, Zysset S, Naganawa S, et al. Determination of cerebrovascular reactivity by means of FMRI signal changes in cerebral microangiopathy: a correlation with morphological abnormalities. Cerebrovasc Dis 2003; 16: 158–165. [DOI] [PubMed] [Google Scholar]
- 20.Gregoire SM, Chaudhary UJ, Brown MM, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 2009; 73: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 21.Thomas BP, Liu P, Park DC, et al. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab 2014; 34: 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YY, Kuan WC, Lim KE, et al. Breathhold-regulated blood oxygenation level-dependent (BOLD) MRI of human brain at 3 tesla. J Magn Reson Imag 2010; 31: 78–84. [DOI] [PubMed] [Google Scholar]
- 23.Rostrup E, Larsson HBW, Toft PB, et al. Functional MRI of CO2 induced increase in cerebral perfusion. NMR Biomed 1994; 7: 29–34. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw J, Brindle W, Casado A, et al. A systematic review of the utility of 1.5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research. Eur Radiol 2012; 22: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 25.Bhogal A, Siero JC, Fisher JA, et al. Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage 2014; 98: 296–305. . [DOI] [PubMed] [Google Scholar]
- 26.Hare HV, Germuska M, Kelly ME, et al. Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. J Cereb Blood Flow Metab 2013; 33: 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tancredi FB, Hoge RD. Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab 2013; 33: 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zande FH, Hofman PA, Backes WH. Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology 2005; 47: 114–120. [DOI] [PubMed] [Google Scholar]
- 29.Spano VR, Mandell DM, Poublanc J, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology 2013; 266: 592–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.