Abstract
Background:
Female genital schistosomiasis (FGS) is a tissue reaction to lodged ova of Schistosoma haematobium in the genital mucosa. Lesions can make the mucosa friable and prone to bleeding and discharge. Women with FGS may have an increased risk of HIV acquisition, and FGS may act as a cofactor in the development of cervical cancer.
Objectives:
To explore cytology as a method for diagnosing FGS and to discuss the diagnostic challenges in low-resource rural areas. The correlation between FGS and squamous cell atypia (SCA) is also explored and discussed. Cytology results are compared to Schistosoma polymerase chain reaction (PCR) in vaginal lavage and urine and in urine microscopy.
Materials and Methods:
In a clinical study, 394 women aged between 16 and 23 years from rural high schools in KwaZulu-Natal, South Africa, underwent structured interviews and the following laboratory tests: Cytology Papanicolaou (Pap) smears for S. haematobium ova and cervical SCA, real-time PCR for Schistosoma-specific DNA in vaginal lavage and urine samples, and urine microscopy for the presence of S. haematobium ova.
Results:
In Pap smears, S. haematobium ova were detected in 8/394 (2.0%). SCA was found in 107/394 (27.1%), seven of these had high-grade squamous intraepithelial lesion (HSIL). Schistosoma specific DNA was detected in 38/394 (9.6%) of vaginal lavages and in 91/394 (23.0%) of urines. Ova were found microscopically in 78/394 (19.7%) of urines.
Conclusion:
Schistosoma PCR on lavage was a better way to diagnose FGS compared to cytology. There was a significant association between S. haematobium ova in Pap smears and the other diagnostic methods. In low-resource Schistosoma-endemic areas, it is important that cytology screeners are aware of diagnostic challenges in the identification of schistosomiasis in addition to the cytological diagnosis of SCA. Importantly, in this study, three of eight urines were negative but showed Schistosoma ova in their Pap smear, and one of them was also negative for Schistosoma DNA in urine. In this study, SCA was not significantly associated with schistosomiasis. HSIL detected in this young population might need future consideration.
Keywords: Cervical atypia, cytology, female genital schistosomiasis, gynecology, laboratory diagnostics, real-time polymerase chain reaction
INTRODUCTION
In terms of the public health impact, schistosomiasis (bilharzia) is globally second only to malaria among parasitic diseases. It affects approximately 262 million people worldwide.[1] The disease is most commonly found in sub-Saharan Africa.
Schistosoma haematobium infection is the most common schistosome infection in KwaZulu-Natal, South Africa,[2] with reported focal prevalences between 40% and 98%.[3] A recent study performed in coastal, Southern KwaZulu-Natal showed a prevalence of S. haematobium of 32% among girls aged 10–12 years.[4]
Schistosomal lesions can be seen in the urinary and gastrointestinal tracts. It is, however, well known that the ova also can be trapped in a variety of sites within the human definitive host including the brain, pancreas, kidneys, testes, the vagina, and uterine cervix.[5] This partly random distribution of schistosomal lesions makes it difficult to identify a diagnostic gold standard for a specific region where infections can occur. It has recently been suggested that schistosomal lesions in the vagina and cervix uteri, i.e., female genital schistosomiasis (FGS), may increase the risk of HIV acquisition.[6] FGS has also been suggested a possible cofactor in the development of precancerous lesions and cervical cancer.[7,8] FGS lesions are caused by the human host's response to living and dead parasite ova that are lodged or trapped in the submucosa of the uterine cervix and vagina. Visual inspection by colposcopy and targeted biopsies are considered a more accurate way of diagnosing FGS; however, they are expensive, and the latter is not feasible in populations at risk for HIV.[9,10]
Papanicolaou (Pap) smears are commonly used in low-resource areas by doctors and nurses for the diagnosis of cervical cancer. The aim of this study was to explore the possibilities and limits of diagnosing FGS using Pap smear cytology. The cytology results were compared to Schistosoma polymerase chain reaction (PCR) in vaginal lavage and urine, and in urine microscopy in a young, female population who resided in a rural area. We also wanted to explore a possible correlation between schistosomiasis and squamous cell atypia (SCA) of the cervix.
MATERIALS AND METHODS
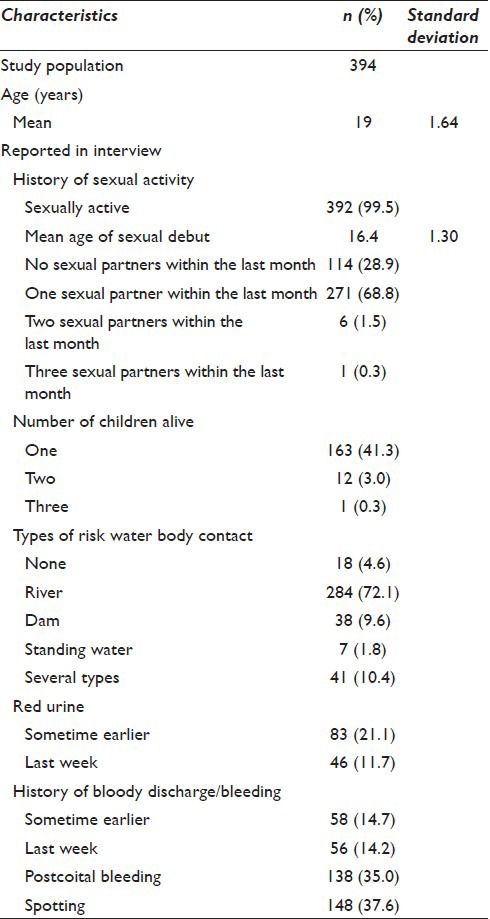
The study area in coastal KwaZulu-Natal South of Durban is endemic for schistosomiasis. This area had a population of 710,000 people, 51% below the age of 20 years of which 55% are female. In these mostly rural communities, women do their laundry and children play in Schistosoma infested rivers. As part of a school-based, clinical study of FGS, females from 42 randomly selected high schools were included. Included in this cross-sectional study were 394 consenting young women aged between 16 and 23 years (median age 19 years); all were examined in the period 2010–2012. Pregnant women, virgins, and those who were not ready for gynecological examination were excluded.
The study was initially discussed with the relevant stakeholders including the KwaZulu-Natal Department of Health, Department of Education, school staff, parents, and pupils. A team of trained field assistants and school nurses visited the schools to give general information and recruit young women for laboratory investigations, treatment, and gynecological examinations. In the first meetings, the focus of the project, its importance, benefits, and possible negative consequences were explained to the stakeholders. Written informed consent was sought at this time. It was explained that the research samples would be investigated in batches and hence, an extended waiting period for results could occur. Participants were encouraged to visit local health care facilities should they experience any problems in the meantime. Logistical arrangements at suitable times to transport the young women to the clinic for their gynecological examinations and interviews were discussed with school principals and other relevant staff. According to practice in rural clinics, a syndromic protocol was used to diagnose and treat findings at the point of investigation. When laboratory results became available, patients were contacted and asked if they had been treated and helped with further management of the disease.
Permission was also obtained from the Biomedical Research Ethics Administration, University of KwaZulu-Natal (Ref BF029/07), the Departments of Health, and Education of KwaZulu-Natal (Ref HRKM010-08), the Norwegian Ethics Committee, Regional Committee for Medical and Health Research Ethics (REC), South Eastern Norway (Ref 469- 7066a1.2007.535), and the European Group on Ethics in Science and New Technologies (Ref IRSES-2010:269245).
Consenting participants underwent a private face-to-face structured interview, conducted by research assistants in isiZulu, the local language, including their history of water contact, observed red urine and genital symptoms. In addition, the research assistants asked questions about their sexual history and parity as these variables could have had a bearing on the risk of cervical atypia.
During the gynecological examination, the surfaces of the vulva, vagina, and cervix were examined visually and colposcopically in accordance with the protocol from a previous FGS study.[11] Thereafter, cervicovaginal lavage samples were collected by spraying 10 ml saline on the vaginal wall and cervix 4 times, which was then drawn back into the syringe and divided into six sets of cryotubes and stored in a −80 freezer with CO2 and generator backup systems. The Pap smears were then collected with a wooden spatula or a cytobrush. The Pap smears were preserved by using a commercial cytological spray fixative. Urine samples for PCR analysis were stored as described for vaginal lavage samples. The urine samples for microscopy were stored in dark cooler boxes to ensure sample quality.
For cytological analyses, the smears were Pap stained[12] and examined by a cytologist using an Olympus B×43 microscope and viewed at magnification with objectives 10 and 40. The smears were examined for all cytological entities in addition to Schistosoma ova and SCA. The diagnosis of S. haematobium was based on finding intact living or dead ova within oval chitinous shells with a terminal spine [Figure 1a].[13]
Figure 1.
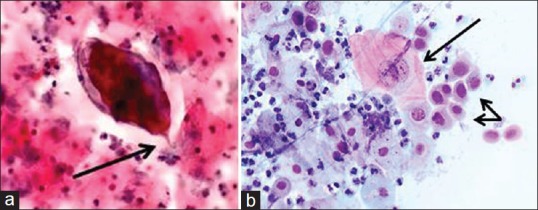
(a) Cervical smear, (Papanicolaou stain, ×40). Schistosoma haematobium ovum with an embryo miracidium. Note the diagnostic terminal spine(arrow). (b) Cervical smear, (Papanicolaou stain, ×40). Cytological changes consistent with high grade squamous intraepithelial lesion including human papillomavirus infection. Long arrow: Human papillomavirus cytopathic changes including nuclear and cellular enlargement, and koilocytosis. Double arrow: Cells with increased nuclear to cytoplasmic ratios, nuclear membrane irregularities and marked hyperchromasia consistent with high-grade squamous intraepithelial lesion. The background contains neutrophil granulocytes denoting inflammation
Epithelial dysplasias [Figure 1b] were graded according to the Bethesda system.[14] In short, the SCA was stratified into atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), a category that encompasses mild dysplasia/cervical intraepithelial neoplasia (CIN) 1 and human papillomavirus (HPV), and high-grade squamous intraepithelial lesion (HSIL). High-grade lesions encompass moderate dysplasia/CIN 2, severe dysplasia/CIN 3, and carcinoma in situ. To ensure quality control, 10% of all negative smears were re-screened.
Internal transcribed spacer (ITS)-based real-time PCR with quantification of Schistosoma-specific DNA in cervicovaginal lavages and urines were performed as described previously.[4,15,16] Cycle threshold (Ct) values were used as the output of the PCR, reflecting the parasite-specific DNA load in the sample tested. Ct-values were arbitrarily categorized in the following infection intensity groups: High-intensity (Ct <30), medium-intensity (30≤ Ct <35), low-intensity (35≤ Ct <50), or negative (Ct = 50).[15]
Urine microscopy samples were preserved with 1 ml of 2% tincture of merthiolate in 5% formalin solution.[4,15] The samples were spun for 10 min at 4000 rounds/min and the sediment examined microscopically by trained fieldworkers. The samples underwent quality control by an independent microscopist on 10% of randomly chosen samples, and any discrepancies were discussed with fellow field workers.
Data were analyzed using IBM SPSS version 23.0 (Armonk, NY, USA) Ct-values were not normally distributed and therefore described by median and range and compared using a nonparametric statistical method. Inferential statistics was done using the Fisher's exact test. The odds and risks ratios (OR and RR) and the corresponding 95% confidence intervals (CIs) were determined for 2 × 2 cross-tabulations. All P values were two-tailed and P < 0.05 considered significant.
RESULTS
The flowchart of the study population is presented in Figure 2. The population characteristics are presented in Table 1 and show that 376/394 (95%) of the participants had a prior risk of water contact.
Figure 2.
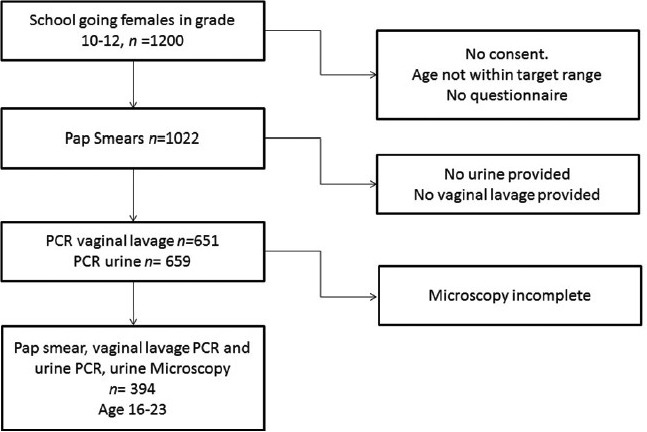
Flowchart of the study participation
Table 1.
Participant characteristics
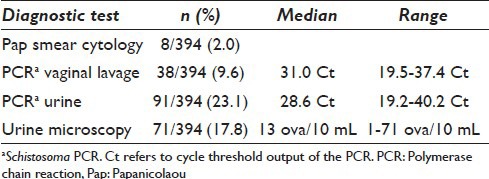
In the Pap smears, S. haematobium ova were detected in 8/394 (2.0%). Schistosoma-specific DNA was found in 38/394 (9.6%) of vaginal lavages including the 8 Pap smears with S. haematobium eggs. Significantly lower Schistosoma DNA loads were seen in the lavages of Pap-smear negatives (median Ct-value 32.9, range 20.4–37.4). In 78/394 (19.8%), Schistosoma ova were detected microscopically in the urines while Schistosoma DNA was detected in 91/394 (23.1%) of the urines [Table 2]. There was a significant association between S. haematobium eggs in Pap smears and the presence of Schistosoma DNA in lavage (OR: 12.87, P < 0.001; 95% CI 9.13–18.14), Schistosoma DNA in urine (OR: 25.1, P < 0.001; 95% CI 3.1–207.4) as well as eggs in urine determined by microscopy (OR: 7.1, P = 0.009; 95% CI 1.7–30.6).
Table 2.
Schistosoma prevalence using cytology, vaginal lavage, and urine PCR or urine microscopy
Between 95% and 97% of the corresponding Pap smears in which Schistosoma ova were identified either in vaginal lavage or urines were deemed suboptimal [Table 3]. Smears were deemed suboptimal or “inadequate” due to technical interpretability or inadequate cellular composition or incorrect sampling. In poorly preserved and bloodstained smears, it was not often possible microscopically to identify the cellular characteristics of epithelial cells, Schistosoma ova; however, they were identifiable [Figure 3a and b].
Table 3.
Summary of cytology results. For definitions of suboptimal Pap smears, ASCUS, LSIL, HISL, HSIL with possible invasion and SCA
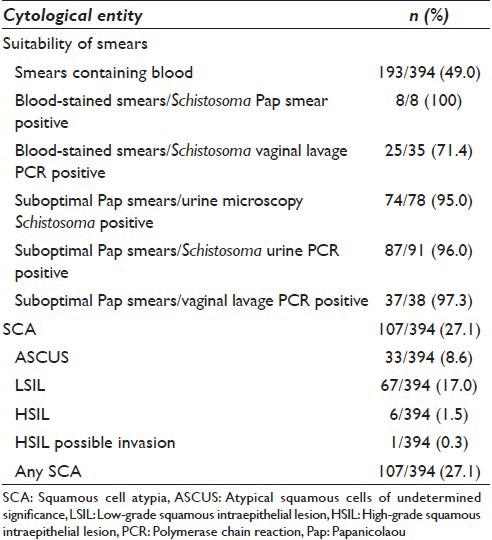
Figure 3.
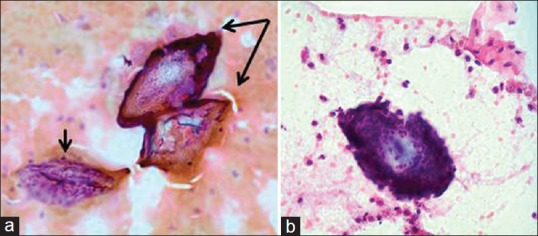
(a) Cervical smear, magnification (Papanicolaou stain, ×40). Poorly preserved smears with cellular detail obscured by blood and inflammatory cells. Small arrow: Non-diagnostic, poorly preserved epithelial cells. Double arrow: Two Schistosoma haematobium ova, identifiable by their refractile shells and characteristic terminal spines. (b) Cervical smear, magnification (Papanicolaou stain, ×40). Schistosoma ovum surrounded by many neutrophil granulocytes. Terminal spine detected on fine focus, however, not seen clearly in this image
Ova of S. haematobium typically range in size from 80 to 170 μm in length and are 30–70 μm wide and have a diagnostic terminal spine. Within the ova embryo, miracidia were seen, under-developed, or well-developed, or as emergent miracidia with a finely ciliated outline [Figure 4a]. Empty, refractile, folded, and crumpled shells with no visible internal structure were also seen and read as ova with signs of degeneration [Figure 4b]. Partially or completely blackened and opaque, nonviable forms were also seen.
Figure 4.
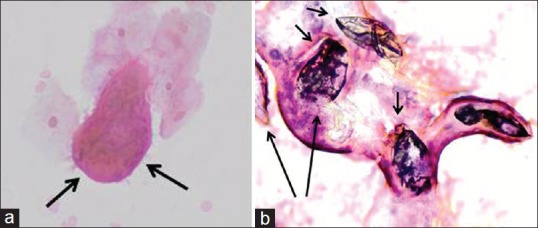
(a) Cervical smear, magnification (Papanicolaou stain, ×40). Arrows: emergent miracidium with a finely ciliated outline. (b) Cervical smear, magnification (Papanicolaou stain, ×40). Numerous calcified, dead ova (four calcified ova - short arrows and two non-calcified ova - long arrows) of Schistosoma haematobium
The local inflammatory reaction in the cytological smears with S. haematobium ova varied from sparse to marked or chronic inflammatory changes. The heavily blood-stained smears might reflect the clinical finding of abnormal mucosal blood vessels seen in the FGS lesions prone to contact bleeding [Figure 3b]. All Pap smears where Schistosoma ova were identified, 8/8 (100%), included blood, as did 25/35 (71.4%) of the Schistosoma positive PCR vaginal lavage samples [Table 3].
On comparing the diagnostic methods used in this study, Schistosoma PCR lavage analysis was 6 times more sensitive than Pap smears in detecting schistosomiasis. All Schistosoma positive Pap smears were also positive for Schistosoma PCR in vaginal lavage [Table 4]. There were, however, three cases with Schistosoma positive Pap smears and Schistosoma PCR lavage that had microscopy negative urine samples; one of them also negative for Schistosoma DNA in the urine. The distribution of SCA is shown in Table 3. HSIL was diagnosed in 6/394 (1.5%). One additional case was reported as HSIL with possible invasion. SCA was not significantly associated with any tests for schistosomiasis used in this study. The SCA was found across the whole age range examined [Figure 5].
Table 4.
Eight cases positive for Schistosomiasis by cytology compared to Schistosoma PCR in vaginal lavage, Schistosoma PCR in urine and urine microscopy
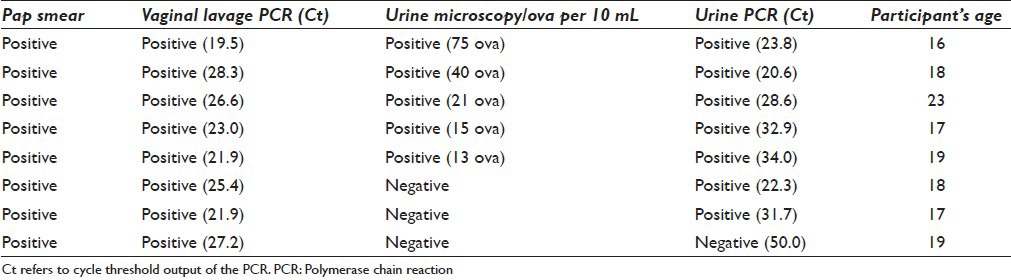
Figure 5.
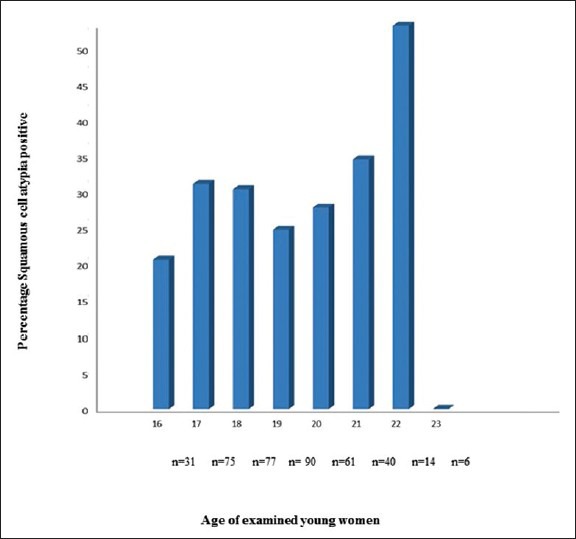
Percentage of squamous cell atypia positive in relation to age of the 394 examined young women. The number of examined women per year of age is indicated
DISCUSSION
With emerging knowledge of possible serious lifelong complications of FGS, there is an increasing demand for better diagnostic tools. It is important to find ways of diagnosing FGS that can be applied in rural areas with low resources. An increased awareness of the symptoms and signs of FGS is also needed among gynecologists and other health care workers that encounter women from Schistosoma-endemic areas.[10] FGS mucosal lesions can clinically mimic sexually transmitted infections and cervical neoplasias.[7,8,9,17]
The anti-schistosomal drug praziquantel is effective in killing adult worms responsible for laying eggs. The effect that the drug has on FGS lesions has not been fully explored. Treatment given to adults apparently did not change their FGS lesions.[18] Previous studies using histopathological reports indicate that the inflammatory reactions also around dead ova, presumably PCR negative, continue to pose a clinical problem.[19,20] This is in contrast to another study among adults with urinary schistosomiasis, showing that anti-schistosomal treatment could contribute to the resolution of bladder lesions.[21] All the young women in the study were offered anti-schistosomal treatment. The effect of treatment on FGS will be followed and discussed in future publications.
A targeted biopsy is a method that has been used to diagnose FGS; however, this procedure is invasive and unethical in a young population that is also prone to HIV. Alternatively, diagnosis of FGS can be achieved directly by identification of parasite ova or parasite DNA in material from the vagina. In addition, the diagnosis can be made or suspected by visual inspection at a gynecologic examination through finding typical FGS lesions with the naked eye, by colposcopy, or by a camera with a magnification lens.[9] The latter has, however, never been tested. FGS should also be considered high on the list of differential diagnosis, once sexual abuse and sexually-transmitted infections have been precluded. The typical FGS lesions have been described and discussed extensively in recent publications and will be addressed in a forthcoming WHO FGS pocket Atlas with posters.[10] These mucosal lesions include two types of sandy patches, abnormal blood vessels, and rubbery papules.
In a recent study, primary school girls reported similar symptoms as adults with genital schistosomiasis There was also an association between genital symptoms and urinary schistosomiasis.[22] An unresolved question relates to diagnosing FGS in pre-pubertal young girls, who may have severe genital symptoms with bloody discharge, genital pain, and spotting. In our study, we had young women with negative urine tests, but positive tests from the genital tract [Table 3], in line with the seemingly random submucosal allocation of the ova-producing parasites to epithelial linings generally in the body, including the genital or urinary tracts. This is in line with a recent FGS study from Madagascar where women between 15 and 35 years with Schistosoma negative urines had Schistosoma DNA in genital samples as well as cases in our study.[23] The important notion of this is that testing only urine samples for schistosomiasis may be insufficient to diagnose FGS.
In our study, the diagnostic yield of Schistosoma infection in Pap smears was low which is consistent with previous findings from mainland Africa.[24,25] The number of positive results for schistosomiasis in cytology Pap smears in this study was small (8/394); hence, deductions need to be interpreted with caution. The Pap smear yield in our study might be low due to the sampling techniques as vaginal lavage samples were collected prior to the cervical smears. This might have removed Schistosoma ova. The lavage additionally possibly altered the cervical mucosal surface, resulting in suboptimal or unsatisfactory smears. Poor fixation could also contribute to the suboptimal smears. Furthermore, it might be that the ova load was low either proportional with a low parasite load or proportional with a different genotype that did not produce as many ova as found in, for example, Madagascan population.[23]
A crucial aspect of the cytology report is the issue of adequacy. According to the Bethesda system, adequacy is expressed in terms of an adequate number of well-preserved epithelial cells including cells from the transformation zone.[14] Smears which do not meet the criteria but at the same time, enabled a diagnosis are reported as “satisfactory for evaluation but limited by” followed by whichever criteria was lacking, for example, inadequate endocervical cells, inflammation, etc., Smears deemed “unsatisfactory for evaluation” may reveal obscuring blood; excessive inflammation; thick, multicellular areas; poor preservation; air drying artifact; or contaminants. In this study, the interpretation was compromised in approximately 75% of the smears.
Furthermore, in this study, all cytology Pap smear S. haematobium cases identified were also positive for vaginal lavage Schistosoma PCR. Some of the smears included nonviable ova. They are; however, significant as they indicate lesions where inflammation continues to develop.[9] Pitfalls exist, when contaminants (foreign bodies) introduced into smears either during sampling, transportation or processing, with similar oval shapes as seen in Figure 6a are encountered. The depicted structure; however, lacks the diagnostic terminal spine, essential for the cytological diagnosis of S. haematobium. It is important to view such cases carefully since ova can be hidden among the contaminants and easily overlooked [Figure 6b]. Schistosoma mansoni can be differentiated from S. haematobium by its lateral spine.
Figure 6.
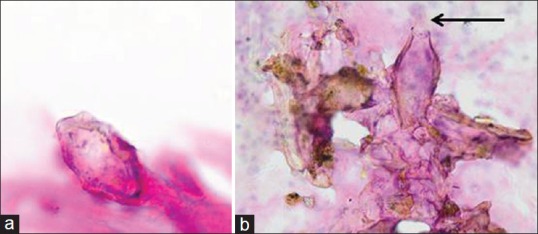
(a) Cervical smear, magnification (Papanicolaou stain, ×40). A contaminant easily misdiagnosed for Schistosoma haematobium ova. Note the lack of a diagnostic terminal spine. (b) Cervical smear, magnification (Papanicolaou stain, ×40). Arrow: Schistosoma ovum with a terminal spine found among contaminants
The diagnostic yield in our study could probably have been higher with an alternative to conventional Pap smears by using liquid-based cytology (LBC) samples. In a population-based study among Costa Rican women who also have a high prevalence of cervical cancer, LBC was compared with conventional Pap smears. The samples processed via LBC were significantly more sensitive in detecting HSIL and other abnormalities.[26] Specimens from LBC testing could possibly also be tested for Schistosoma PCR and HPV subtypes.
In our study, the inclusion criteria required that all of the participants were or had been sexually active at some point in their lifetime to undergo the gynecological examination. The mean age of sexual debut was 16 and 163/394 (41.0%) had at least one child. SCA occurred across the age spectrum; the majority was ASCUS and LSIL 100/107 (94.3%). Seven cases of HSIL occurred between ages of 17 and 22 years, including one case of HSIL with possible invasion, which occurred at age 19. While studies have shown that at least low grade 96% of abnormalities in young women tend to regress within 36 months,[27] in contrast to this it has been shown that progression of squamous lesions occurs more rapidly in HIV-positive women.[28] It is not known if this will occur in individuals with schistosomiasis. In South Africa, cervical cancer is the second most prevalent cancer affecting women. A screening program does exist for women at the ages of 30, 40, and 50. Much of the screening is done in family planning clinics or antenatal clinics on symptomatic women.[29] Many women are unaware of the routine screening program.[29] As a consequence, women usually reach healthcare facilities when they have advanced or terminal cervical cancer, which could have been prevented by early diagnosis.
The long-term outcome of HSIL in a young population who are at risk for contracting HIV and schistosomiasis raises public health issues. The young age of women with HSIL makes it important for policy makers to consider increasing efforts to educate women on the dangers of cervical cancer and to screen young women in Schistosoma-endemic areas for SCA.
CONCLUSION
Pap smear cytology is a simple diagnostic test, that is, relatively cheap and noninvasive. It can be widely used for the detection of SCA and may also detect FGS. In our study, the “high tech” real-time PCR detects Schistosoma DNA, reflecting FGS, in cervical lavage almost 6 times more often than Pap smears. In this limited sample size, there was no significant association between cervical atypia and the tests which were positive for genital schistosomiasis. It is, however, important to note that SCA consistent with at least HPV infection was detected in 27% of this young population who are not conventionally screened for cervical cancer, some of them with advanced atypia. A shift in cytology sampling to LBC could open for a triage of cytology, HPV DNA testing, and Schistosoma PCR; this will be considered in future.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
PP, LvL, MT, MS, SGZ, EK, BR, and EFK have all participated in this original research and the process of writing the manuscript. They all concur with its submission and subsequent revisions by the corresponding author, Pavitra Pillay. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from and permission from the Biomedical Research Ethics Administration, University of KwaZulu-Natal (Ref BF029/07), the Departments of Health, and Education of KwaZulu-Natal (Ref HRKM010-08), the Norwegian Ethics Committee, Regional Committee for Medical and Health Research Ethics, South Eastern Norway (Ref 469-07066a1.2007.535), and the European Group on Ethics in Science and New Technologies (Ref IRSES-2010:269245). Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
CIN - Cervical Intraepithelial Neoplasia
CIs - Confidence Intervals
Ct - Cycle Threshold
FGS - Female Genital Schistosomiasis
HPV - Human Papillomavirus
HSIL - High-Grade Squamous Intraepithelial Lesion
ITS - Internal Transcribed Spacer
LBC - Liquid-Based Cytology
LSIL - Low-Grade Squamous Intraepithelial Lesion
Pap - Papanicolaou
PCR - Polymerase Chain Reaction
SCA - Squamous Cell Atypia.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
The authors would like to thank the study community district including the KwaZulu-Natal, Department of Health and Department of Education. Recruitment and data collection would not have been possible without the tremendous support from the BRIGHT Project Team South Africa, mentioning Svein Gunnar Gundersen, Birgitte Jyding Vennervald, Jane Kvalsvig, Ingrid EA Hegertun, and Kristin MS Gundersen. The following people assisted with clinical or laboratory work and discussions on ethical implications: Andile Mtshali, Kristine Lillebo, Marc FD Baay, and Mathias Onsrud. Research assistants and nurses assisted with the clinical investigations, there were recruitment team, information, and consent teams. We acknowledge Dr. Jaco J Verweij for devising and implementing the ITS-based real-time Schistosoma PCR assay used in this study and Eric AT Brienen for his assistance with PCR analyses. We are most grateful to Roy Manyaira for data management.
Contributor Information
Pavitra Pillay, Email: pillayp@dut.ac.za.
Lisette van Lieshout, Email: e.a.van_lieshout@lumc.nl.
Myra Taylor, Email: taylormyra@gmail.com.
Motshedisi Sebitloane, Email: sebitloanem@ukzn.ac.za.
Siphosenkosi Gift Zulu, Email: siphogzulu@gmail.com.
Elisabeth Kleppa, Email: elisabethkleppa@gmail.com.
Borghild Roald, Email: b.b.h.roald@medisin.uio.no.
Eyrun Floerecke Kjetland, Email: e.f.kjetland@medisin.uio.no.
REFERENCES
- 1.World Health Organization. WHO Global Health Observatory (GHO) Data. Schistosomiasis Situation and Trends Published. 2015. [Last accessed on 2015 Aug 09]. Available from: http://www.who.int/gho/neglected_diseases/schistosomiasis/en/
- 2.Appleton CC, Kvalsvig JD. A school-based helminth control programme successfully implemented in KwaZulu-Natal. [Last accessed on 2015 Aug 01];South Afr J Epidemiol Infect. 2006 21:55–67. Available from: http://www.reference.sabinet.coza/sa_epublication_article/mp_sajei_v21_n2_a7 . [Google Scholar]
- 3.Saathoff E, Olsen A, Magnussen P, Kvalsvig JD, Becker W, Appleton CC. Patterns of Schistosoma haematobium infection, impact of praziquantel treatment and re-infection after treatment in a cohort of schoolchildren from rural KwaZulu-Natal/South Africa. BMC Infect Dis. 2004;4:40. doi: 10.1186/1471-2334-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillay P, Taylor M, Zulu SG, Gundersen SG, Verweij JJ, Hoekstra P, et al. Real-time polymerase chain reaction for detection of Schistosoma DNA in small-volume urine samples reflects focal distribution of urogenital schistosomiasis in primary school girls in KwaZulu Natal, South Africa. Am J Trop Med Hyg. 2014;90:546–52. doi: 10.4269/ajtmh.13-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edington GM, Nwabuebo I, Junaid TA. The pathology of schistosomiasis in Ibadan, Nigeria with special reference to the appendix, brain, pancreas and genital organs. Trans R Soc Trop Med Hyg. 1975;69:153–6. doi: 10.1016/0035-9203(75)90027-9. [DOI] [PubMed] [Google Scholar]
- 6.Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis. 2011;5:e1170. doi: 10.1371/journal.pntd.0001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moubayed P, Lepère JF, Mwakyoma H, Neuvians D. Carcinoma of the uterine cervix and schistosomiasis. Int J Gynaecol Obstet. 1994;45:133–9. doi: 10.1016/0020-7292(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 8.Kjetland EF, Ndhlovu PD, Mduluza T, Deschoolmeester V, Midzi N, Gomo E, et al. The effects of genital schistosoma haematobium on human papillomavirus and the development of cervical neoplasia after five years in a Zimbabwean population. Eur J Gynaecol Oncol. 2010;31:169–73. [PubMed] [Google Scholar]
- 9.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28:58–65. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Norseth HM, Ndhlovu PD, Kleppa E, Randrianasolo BS, Jourdan PM, Roald B, et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl Trop Dis. 2014;8:e3229. doi: 10.1371/journal.pntd.0003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjetland EF, Ndhlovu PD, Mduluza T, Gomo E, Gwanzura L, Mason PR, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72:311–9. [PubMed] [Google Scholar]
- 12.Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942;95:438–9. doi: 10.1126/science.95.2469.438. [DOI] [PubMed] [Google Scholar]
- 13.Berry A. Multispecies schistosomal infections of the female genital tract detected in cytology smears. Acta Cytol. 1976;20:361–5. [PubMed] [Google Scholar]
- 14.Solomon D, Nayar R. New York: Springer Science & Business Media; 2004. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. [Google Scholar]
- 15.Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–33. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- 16.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. New diagnostic tools in schistosomiasis. Clin Microbiol Infect. 2015;21:529–542. doi: 10.1016/j.cmi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Kjetland EF, Hove RJ, Gomo E, Midzi N, Gwanzura L, Mason P, et al. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2009;81:1050–5. doi: 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- 18.Samuel MI, Taylor C. A case of female urogenital schistosomiasis presenting as viral warts. Int J STD AIDS. 2015;26:599–601. doi: 10.1177/0956462414544723. [DOI] [PubMed] [Google Scholar]
- 19.Kjetland EF, Mduluza T, Ndhlovu PD, Gomo E, Gwanzura L, Midzi N, et al. Genital schistosomiasis in women: A clinical 12-month in vivo study following treatment with praziquantel. Trans R Soc Trop Med Hyg. 2006;100:740–52. doi: 10.1016/j.trstmh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am J Trop Med Hyg. 2011;85:1060–4. doi: 10.4269/ajtmh.2011.11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magak P, Chang-Cojulun A, Kadzo H, Ireri E, Muchiri E, Kitron U, et al. Case-control study of posttreatment regression of urinary tract morbidity among adults in Schistosoma haematobium – Endemic communities in Kwale County, Kenya. Am J Trop Med Hyg. 2015;93:371–6. doi: 10.4269/ajtmh.15-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegertun IE, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, et al. S. haematobium as a common cause of genital morbidity in girls: A cross-sectional study of children in South Africa. PLoS Negl Trop Dis. 2013;7:e2104. doi: 10.1371/journal.pntd.0002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randrianasolo BS, Jourdan PM, Ravoniarimbinina P, Ramarokoto CE, Rakotomanana F, Ravaoalimalala VE, et al. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: A cross-sectional study in Madagascar. J Infect Dis. 2015;212:275–84. doi: 10.1093/infdis/jiv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poggensee G, Sahebali S, Van Marck E, Swai B, Krantz I, Feldmeier H. Diagnosis of genital cervical schistosomiasis: Comparison of cytological, histopathological and parasitological examination. Am J Trop Med Hyg. 2001;65:233–6. doi: 10.4269/ajtmh.2001.65.233. [DOI] [PubMed] [Google Scholar]
- 25.Poggensee G, Feldmeier H. Female genital schistosomiasis: Facts and hypotheses. Acta Trop. 2001;79:193–210. doi: 10.1016/s0001-706x(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson ML, Zahniser DJ, Sherman ME, Herrero R, Alfaro M, Bratti MC, et al. Utility of liquid-based cytology for cervical carcinoma screening. [Last accessed on 2015 Aug 01];Cancer Cytopathol. 1999 87:48–55. doi: 10.1002/(sici)1097-0142(19990425)87:2<48::aid-cncr2>3.0.co;2-d. Available from: http://www.onlinelibrary.wiley.com/doi/101002/(SICI) 1097-0142 (19990425) 87:2%3C48:AID-CNCR2%3E30CO;2-D/epdf . [DOI] [PubMed] [Google Scholar]
- 27.Szarewski A, Sasieni P. Cervical screening in adolescents – At least do no harm. Lancet. 2004;364:1642–4. doi: 10.1016/S0140-6736(04)17366-2. [DOI] [PubMed] [Google Scholar]
- 28.Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, Sanne IM, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control. 2010;21:433–43. doi: 10.1007/s10552-009-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pillay P, Knight S, Rmaih WN. Cervical cancer screening in urban clinics in eThekwini municipal area. [Last accessed on 2015 Aug 01];South Afr J Epidemiol Infect. 2009 24:18–20. Available from: http://www.reference.sabinet.coza/webx/access/electronic_journals/mp_sajei/mp_sajei_v24_n2_a5.pdf . [Google Scholar]


