Abstract
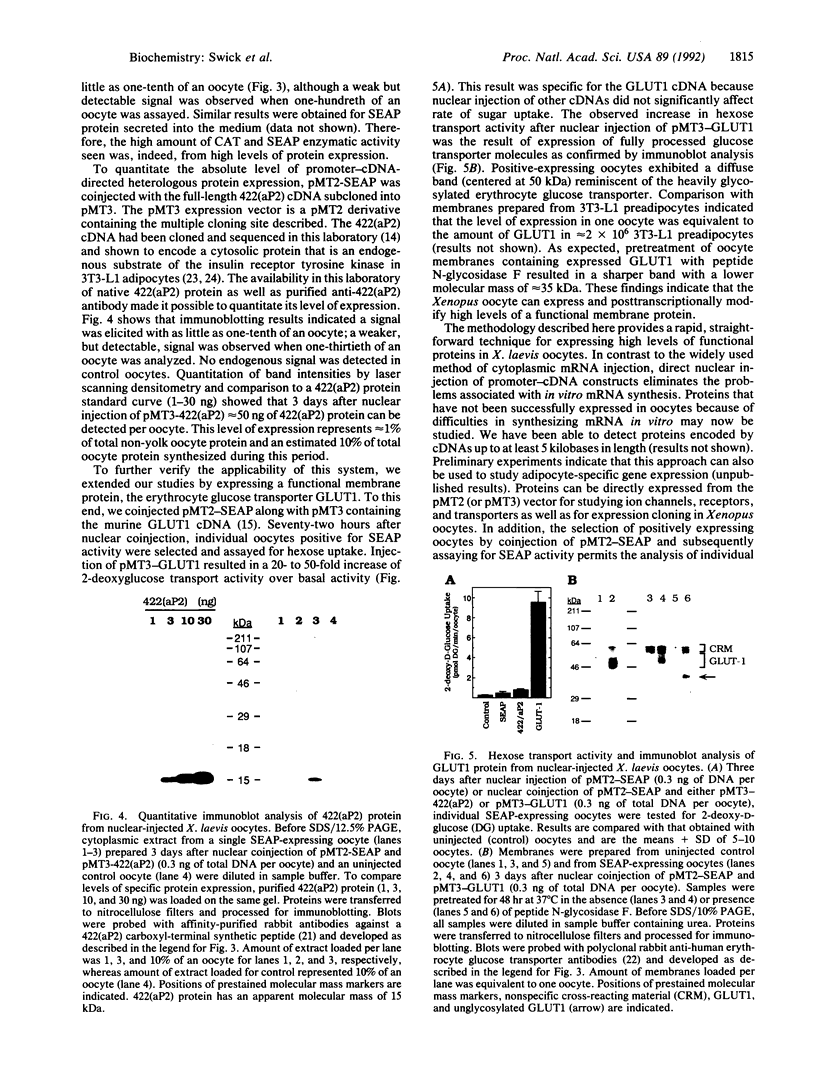
Heterologous proteins can be expressed in Xenopus laevis oocytes by cytoplasmic microinjection of mRNA. To circumvent limitations inherent in this approach we investigate direct nuclear injection of strong viral expression vectors to drive transcription and subsequent translation of cDNAs encoding cytoplasmic, secreted, and plasma membrane proteins. After several viral promoters had been tested, the pMT2 vector was found to be a superior expression vector for X. laevis oocytes capable of directing expression of high levels of functional heterologous proteins. Typically the amount of protein derived from transcription-translation of the microinjected cDNA accounts for approximately 1% of total non-yolk protein. Moreover, the inefficiency usually associated with nuclear injections was overcome by coinjection of pMT2 driving expression of a secreted alkaline phosphatase as an internal control to select positive-expressing oocytes. Using this method, we have successfully expressed high levels of chloramphenicol acetyltransferase, the adipocyte-specific cytosolic 422(aP2) protein, and the membrane-associated glucose transporter GLUT1. The system described should be applicable to a wide variety of proteins for which cDNAs are available. Hence, the cumbersome and often inefficient in vitro synthesis of mRNA for studying ion channels, receptors, and transporters as well as for expression cloning in Xenopus oocytes should no longer be necessary.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballivet M., Nef P., Couturier S., Rungger D., Bader C. R., Bertrand D., Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988 Nov;1(9):847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Dahl G., Miller T., Paul D., Voellmy R., Werner R. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science. 1987 Jun 5;236(4806):1290–1293. doi: 10.1126/science.3035715. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990 Jan 15;265(2):1124–1128. [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Bernier M., Hoffman R. D., Flores-Riveros J. R., Liao K., Laird D. M., Lane M. D. Identification of phosphorylated 422(aP2) protein as pp15, the 15-kilodalton target of the insulin receptor tyrosine kinase in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8835–8839. doi: 10.1073/pnas.85.23.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko R. C., Hoffman R. D., Flores-Riveros J. R., Lane M. D. Insulin receptor tyrosine kinase-catalyzed phosphorylation of 422(aP2) protein. Substrate activation by long-chain fatty acid. J Biol Chem. 1990 Dec 5;265(34):21075–21085. [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski R. A., Lane M. D. Homogeneous functional insulin receptor from 3T3-L1 adipocytes. Purification using N alpha B1-(biotinyl-epsilon-aminocaproyl)insulin and avidin-sepharose. J Biol Chem. 1985 Apr 25;260(8):5014–5025. [PubMed] [Google Scholar]
- Lester H. A. Heterologous expression of excitability proteins: route to more specific drugs? Science. 1988 Aug 26;241(4869):1057–1063. doi: 10.1126/science.2457947. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Smith A. A., Brooker T., Brooker G. Expression of rat mRNA coding for hormone-stimulated adenylate cyclase in Xenopus oocytes. FASEB J. 1987 Nov;1(5):380–387. doi: 10.1096/fasebj.1.5.2824269. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Urade R., Micanovic R., Gerber L., Udenfriend S. Secreted alkaline phosphatase: an internal standard for expression of injected mRNAs in the Xenopus oocyte. FASEB J. 1990 Feb 1;4(2):227–231. doi: 10.1096/fasebj.4.2.2298343. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Karschin A., Labarca C., Elroy-Stein O., Moss B., Davidson N., Lester H. A. Expression of ion channels and receptors in Xenopus oocytes using vaccinia virus. FASEB J. 1991 May;5(8):2209–2216. doi: 10.1096/fasebj.5.8.1708738. [DOI] [PubMed] [Google Scholar]