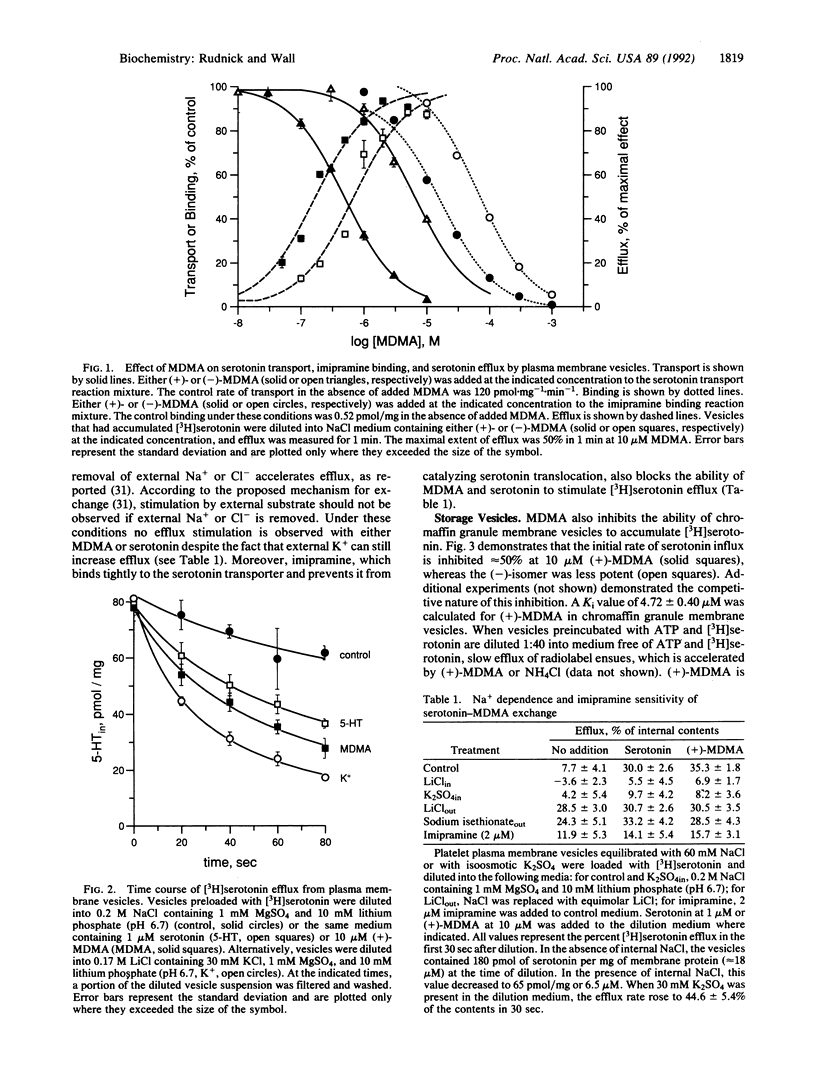
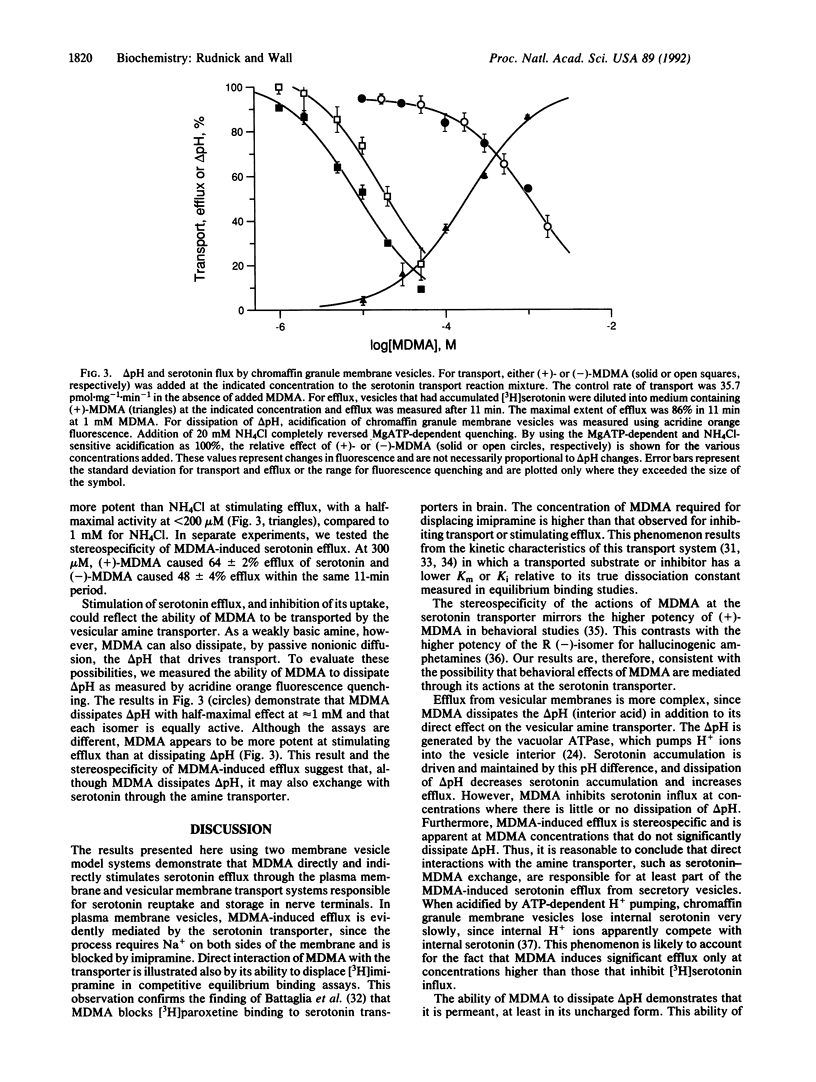
Abstract
MDMA ("ecstasy") has been widely reported as a drug of abuse and as a neurotoxin. This report describes the mechanism of MDMA action at serotonin transporters from plasma membranes and secretory vesicles. MDMA stimulates serotonin efflux from both types of membrane vesicle. In plasma membrane vesicles isolated from human platelets, MDMA inhibits serotonin transport and [3H]imipramine binding by direct interaction with the Na(+)-dependent serotonin transporter. MDMA stimulates radiolabel efflux from plasma membrane vesicles preloaded with [3H]serotonin in a stereo-specific, Na(+)-dependent, and imipramine-sensitive manner characteristic of transporter-mediated exchange. In membrane vesicles isolated from bovine adrenal chromaffin granules, which contain the vesicular biogenic amine transporter, MDMA inhibits ATP-dependent [3H]serotonin accumulation and stimulates efflux of previously accumulated [3H]serotonin. Stimulation of vesicular [3H]serotonin efflux is due to dissipation of the transmembrane pH difference generated by ATP hydrolysis and to direct interaction with the vesicular amine transporter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzaro A. J., Ziance R. J., Rutledge C. O. The importance of neuronal uptake of amines for amphetamine-induced release of 3H-norepinephrine from isolated brain tissue. J Pharmacol Exp Ther. 1974 Apr;189(1):110–118. [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Bartholini G., Pletscher A. Two types of 5-hydroxytryptamine release from isolated blood platelets. Experientia. 1964 Jul 15;20(7):376–378. doi: 10.1007/BF02147970. [DOI] [PubMed] [Google Scholar]
- Battaglia G., Brooks B. P., Kulsakdinun C., De Souza E. B. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988 Apr 27;149(1-2):159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Bönisch H. The transport of (+)-amphetamine by the neuronal noradrenaline carrier. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):267–272. doi: 10.1007/BF00506235. [DOI] [PubMed] [Google Scholar]
- Clineschmidt B. V., Totaro J. A., McGuffin J. C., Pflueger A. B. Fenfluramine: long-term reduction in brain serotonin (5-hydroxytryptamine). Eur J Pharmacol. 1976 Jan;35(1):211–214. doi: 10.1016/0014-2999(76)90318-6. [DOI] [PubMed] [Google Scholar]
- Dean G. E., Nelson P. J., Rudnick G. Characterization of native and reconstituted hydrogen ion pumping adenosinetriphosphatase of chromaffin granules. Biochemistry. 1986 Aug 26;25(17):4918–4925. doi: 10.1021/bi00365a029. [DOI] [PubMed] [Google Scholar]
- FULLER R. W., HINES C. W., MILLS J. LOWERING OF BRAIN SEROTONIN LEVEL BY CHLORAMPHETAMINES. Biochem Pharmacol. 1965 Apr;14:483–488. doi: 10.1016/0006-2952(65)90221-2. [DOI] [PubMed] [Google Scholar]
- Fishkes H., Rudnick G. Bioenergetics of serotonin transport by membrane vesicles derived from platelet dense granules. J Biol Chem. 1982 May 25;257(10):5671–5677. [PubMed] [Google Scholar]
- Fuller R. W. Mechanism by which uptake inhibitors antagonize p-chloroamphetamine-induced depletion of brain serotonin. Neurochem Res. 1980 Mar;5(3):241–245. doi: 10.1007/BF00964612. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Perry K. W., Molloy B. B. Reversible and irreversible phases of serotonin depletion by 4-chloroamphetamine. Eur J Pharmacol. 1975 Aug;33(1):119–124. doi: 10.1016/0014-2999(75)90145-4. [DOI] [PubMed] [Google Scholar]
- Humphreys C. J., Levin J., Rudnick G. Antidepressant binding to the porcine and human platelet serotonin transporters. Mol Pharmacol. 1988 Jun;33(6):657–663. [PubMed] [Google Scholar]
- Johnson M. P., Hoffman A. J., Nichols D. E. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol. 1986 Dec 16;132(2-3):269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Rudnick G. Coupling of transmembrane proton gradients to platelet serotonin transport. J Biol Chem. 1982 Feb 10;257(3):1172–1176. [PubMed] [Google Scholar]
- Knoth J., Peabody J. O., Huettl P., Njus D. Kinetics of tyramine transport and permeation across chromaffin-vesicle membranes. Biochemistry. 1984 Apr 24;23(9):2011–2016. doi: 10.1021/bi00304a020. [DOI] [PubMed] [Google Scholar]
- Knoth J., Zallakian M., Njus D. Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts. Biochemistry. 1981 Nov 10;20(23):6625–6629. doi: 10.1021/bi00526a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOTA I., BERALDO W. T., FERRI A. G., JUNQUEIRA L. C. Intracellular distribution of histamine. Nature. 1954 Oct 9;174(4432):698–698. doi: 10.1038/174698a0. [DOI] [PubMed] [Google Scholar]
- Mamounas L. A., Molliver M. E. Evidence for dual serotonergic projections to neocortex: axons from the dorsal and median raphe nuclei are differentially vulnerable to the neurotoxin p-chloroamphetamine (PCA). Exp Neurol. 1988 Oct;102(1):23–36. doi: 10.1016/0014-4886(88)90075-1. [DOI] [PubMed] [Google Scholar]
- Marcusson J. O., Bäckström I. T., Ross S. B. Single-site model of the neuronal 5-hydroxytryptamine uptake and imipramine-binding site. Mol Pharmacol. 1986 Aug;30(2):121–128. [PubMed] [Google Scholar]
- Maron R., Kanner B. I., Schuldiner S. The role of a transmembrane pH gradient in 5-hydroxy tryptamine uptake by synaptic vesicles from rat brain. FEBS Lett. 1979 Feb 15;98(2):237–240. doi: 10.1016/0014-5793(79)80190-8. [DOI] [PubMed] [Google Scholar]
- Maron R., Stern Y., Kanner B. I., Schuldiner S. Functional asymmetry of the amine transporter from chromaffin granules. J Biol Chem. 1983 Oct 10;258(19):11476–11481. [PubMed] [Google Scholar]
- Molliver D. C., Molliver M. E. Anatomic evidence for a neurotoxic effect of (+/-)-fenfluramine upon serotonergic projections in the rat. Brain Res. 1990 Mar 12;511(1):165–168. doi: 10.1016/0006-8993(90)90237-6. [DOI] [PubMed] [Google Scholar]
- Nelson P. J., Rudnick G. Coupling between platelet 5-hydroxytryptamine and potassium transport. J Biol Chem. 1979 Oct 25;254(20):10084–10089. [PubMed] [Google Scholar]
- Nelson P. J., Rudnick G. The role of chloride ion in platelet serotonin transport. J Biol Chem. 1982 Jun 10;257(11):6151–6155. [PubMed] [Google Scholar]
- Nichols D. E., Lloyd D. H., Hoffman A. J., Nichols M. B., Yim G. K. Effects of certain hallucinogenic amphetamine analogues on the release of [3H]serotonin from rat brain synaptosomes. J Med Chem. 1982 May;25(5):530–535. doi: 10.1021/jm00347a010. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3-4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- O'Hearn E., Battaglia G., De Souza E. B., Kuhar M. J., Molliver M. E. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988 Aug;8(8):2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLETSCHER A., BURKARD W. P., BRUDERER H., GEY K. F. DECREASE OF CEREBRAL 5-HYDROXYTRYPTAMINE AND 5-HYDROXYINDOLACETIC ACID BY AN ARYLALKYLAMINE. Life Sci. 1963 Nov;11:828–833. doi: 10.1016/0024-3205(63)90094-8. [DOI] [PubMed] [Google Scholar]
- Ricaurte G., Bryan G., Strauss L., Seiden L., Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985 Sep 6;229(4717):986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. J Biol Chem. 1977 Apr 10;252(7):2170–2174. [PubMed] [Google Scholar]
- Rudnick G., Fishkes H., Nelson P. J., Schuldiner S. Evidence for two distinct serotonin transport systems in platelets. J Biol Chem. 1980 Apr 25;255(8):3638–3641. [PubMed] [Google Scholar]
- Rudnick G., Nelson P. J. Platelet 5-hydroxytryptamine transport, an electroneutral mechanism coupled to potassium. Biochemistry. 1978 Oct 31;17(22):4739–4742. doi: 10.1021/bi00615a021. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Levin J. A., Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharmacol. 1987 Mar 1;36(5):747–755. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H., Kanner B. I. Role of a transmembrane pH gradient in epinephrine transport by chromaffin granule membrane vesicles. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3713–3716. doi: 10.1073/pnas.75.8.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvenheimo J., Fishkes H., Nelson P. J., Rudnick G. The serotonin transporter-imipramine "receptor". J Biol Chem. 1983 May 25;258(10):6115–6119. [PubMed] [Google Scholar]
- Talvenheimo J., Nelson P. J., Rudnick G. Mechanism of imipramine inhibition of platelet 5-hydroxytryptamine transport. J Biol Chem. 1979 Jun 10;254(11):4631–4635. [PubMed] [Google Scholar]


