Abstract
Objectives:
The aim of this study was to investigate the association and to estimate the crude absolute risk of seizure among patients exposed to fluoroquinolones (FQs) in Hong Kong and the United Kingdom.
Methods:
A self-controlled case series study was conducted. Data were collected from the Hong Kong Clinical Data Analysis and Reporting System database and the Clinical Practice Research Datalink. Patients who were prescribed any oral FQ and had an incident seizure diagnosis from 2001 to 2013 were included. The risk windows were defined as pre-FQ start, FQ-exposed, and post-FQ completion. Incidence rate ratios were estimated in all risk windows and compared with baseline periods. A post hoc subgroup analysis was conducted to examine the effect of patients with a history of seizure.
Results:
An increased incidence rate ratio was found in the pre-FQ start periods and no association was found in the post-FQ completion periods in both databases. The crude absolute risk of an incident seizure in 10,000 oral FQ prescriptions was 0.72 (95% confidence interval 0.47–1.10) in the Clinical Data Analysis and Reporting System and 0.40 (95% confidence interval 0.30–0.54) in the Clinical Practice Research Datalink. The rate ratio during treatment was not higher than pre-FQ start periods among patients with a history of seizure, therefore the results did not raise serious concerns.
Conclusions:
This study does not support a causal association between the use of oral FQs and the subsequent occurrence of seizure. An increased risk before the FQ exposure period suggests that the clinical indication for which FQ was prescribed may have contributed to the development of seizure rather than the drug itself.
Fluoroquinolones (FQs) are a class of broad-spectrum antibiotics. Convulsion is listed as one of the potential side effects of FQs.1 Numerous case reports on the association between FQs and seizure have also been published.2–11 Concerns were raised about the safety of FQs on the central nervous system. Although some studies have explored the potential mechanisms of FQ-induced seizure,12–15 comprehensive epidemiologic data from population-based studies are needed. The primary objective of this study was to investigate the relationship between FQs and incident seizures using data from Hong Kong (HK) and the United Kingdom (UK). The objective of the post hoc subgroup analysis was to investigate this relationship in patients with a history of seizure. Furthermore, we aimed to estimate the crude absolute risk of incident seizure among patients prescribed oral FQs.
METHODS
We conducted a self-controlled case series (SCCS) study with the data retrieved from the Clinical Data Analysis and Reporting System (CDARS) in HK and the Clinical Practice Research Datalink (CPRD) in the UK to investigate the association between the use of oral FQs and seizures.
Data sources.
Clinical data analysis and reporting system.
The CDARS is a computerized clinical management system developed by the HK Hospital Authority that contains electronic patient records. The health services provided by the Hospital Authority (primary care, emergency room, hospital outpatient and inpatient) are available to all 7.2 million HK citizens. CDARS includes information on demographics, diagnoses, procedures, prescription details, laboratory tests, and hospitalization details. Patient records in CDARS are deidentified, i.e., name, identification card number, and contact information are not available to ensure patient confidentiality. A unique reference key was generated and assigned to each patient to facilitate data retrieval and further analysis. CDARS has been used in previous epidemiologic studies.16–21
Clinical practice research datalink.
The CPRD contains the anonymized electronic primary care records for approximately 8.5% of the UK population with more than 5 million currently active patients and more than 13 million records dating back to 1987. The CPRD contains information on patient demographics, consultations, prescribed medication, and diagnoses. The crude death rates in the CPRD population are representative of the national rates.22 Numerous high-quality studies have been published using data from CPRD, which affirms the validity and credibility of this database.23,24
Study design.
The SCCS method conducts within-person comparisons of individuals who have both the exposure and outcome of interest.25 An incidence rate ratio (IRR) is estimated by comparing the rate of events during the exposure and nonexposure periods. This study method has been used frequently in pharmacoepidemiologic studies.24,26
However, to apply SCCS correctly, several assumptions should be met. First, the occurrence of the event should not affect the occurrence of subsequent events. In this study, since patients who experienced the first seizure would have a higher chance of a recurrent event, only patients with their incident seizure recorded within the study period were considered in the analyses. A post hoc subgroup analysis was also conducted to examine the effect of oral FQs on the risk of the subsequent seizure in patients with a history of seizure. Second, the occurrence of the event should not permanently influence the chance of exposure. In this case, seizure is not a contraindication for the use of FQs. Therefore, the occurrence of the seizure would not influence the exposure to FQs. Third, occurrence of the outcome should not censor the observation period, for example, in the event of death. Although we believe such censoring to be unlikely in this study, we conducted sensitivity analysis with a SCCS extension, which is not vulnerable to this assumption.27
The SCCS extension is applied if the censoring of the observation period is event-dependent. In this case, the event is seizure and the potentially seizure-related death may lead to censoring of the observation period. The SCCS extension was conditioned on the age at censoring and also involved weighting cases with the density of intervals from the event to censoring of observation periods. It corrects for event-dependent censoring, which may otherwise result in bias if the standard SCCS method was used.
In addition to the SCCS analyses, we calculated the crude absolute risk of a seizure during current oral FQ use to estimate how often this event occurred in a general clinical setting.
Patient identification.
Clinical data analysis and reporting system.
The study period was from January 1, 2001, to December 31, 2013. Patients of any age and sex who were prescribed oral FQs from an outpatient setting during the study period were identified from the CDARS database. Patients who had received at least one FQ prescription and also had a diagnosis of incident seizure (ICD-9-CM) (table e-1 on the Neurology® Web site at Neurology.org) during the study period were defined as the cases. Date of the incident seizure recorded in the database was defined as the index date.
Clinical practice research datalink.
Study period and patient identification criteria were similar to that in CDARS. All patients with an incident seizure (Read codes) (table e-1) and at least one FQ prescription during the same period as defined in CDARS were included in the SCCS. The recorded seizure date was defined as the index date.
Exclusion criteria.
In both databases, patients with unknown date of birth or sex were excluded. Those who had a previous seizure(s) or history of posttraumatic seizure or febrile convulsion since the beginning of the database (CDARS: 1993; CPRD: 1987) were excluded (table e-1). In CPRD, patients with less than 12 months of continuous registration or temporary registration were excluded. Patients with any FQ prescription or seizure diagnosis in the first 12 months of registration during the observation period were also excluded, to ensure only incident events were considered in the analysis.
Exposure definition.
Prescriptions 7 days or less apart were combined as they were probably prescribed for the same indication. The prescription duration was calculated by using the quantity dispensed, frequency, and dosage information in the database. Prescriptions with missing duration or insufficient information to estimate prescription duration were imputed with the median duration of the other prescriptions among the study population in each database.
Statistical analysis.
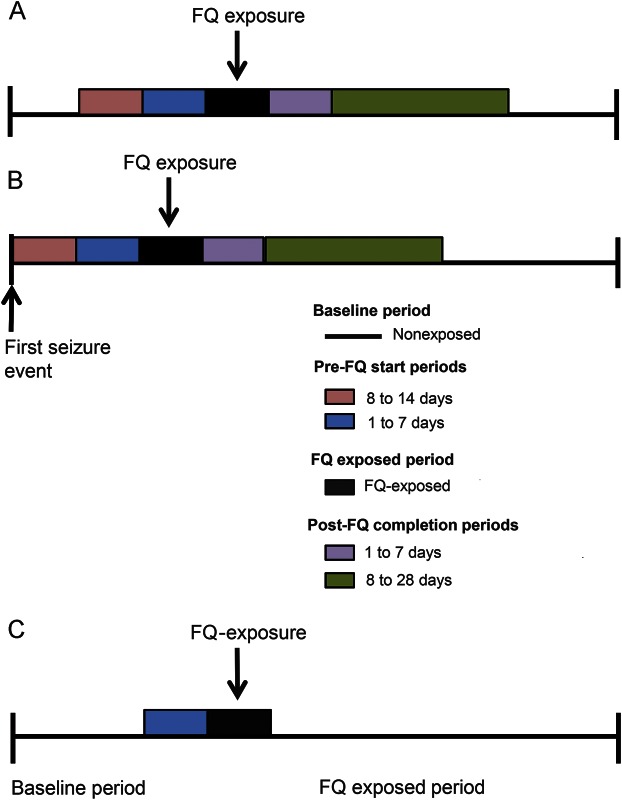
The observation period for each patient was defined as follows: in CDARS, a patient's observation period began on January 1, 2001, or the first record in the database, whichever was latest. It was then censored at the end of the study period or registered date of death if this was earlier than the end of study period. In CPRD, the observation period began on January 1, 2001, or 12 months after the first record in the database, whichever was latest. It was censored on transfer out, death, or last data collection date of practice, whichever came first. Risk periods were defined as follows: 8 to 14 days before FQ start (8–14 days pre-FQ), 1 to 7 days before FQ start (7 days pre-FQ), the FQ exposed period, 1 to 7 days after FQ completion (7 days post-FQ), and 8 to 28 days after FQ completion (8–28 days post-FQ) (figure 1A). The preexposure period serves to measure whether the occurrence of incident seizure may itself be temporarily associated with the probability of being prescribed an FQ. The postexposure period allowed us to determine whether any increased risk observed during FQ exposure would decline after FQ treatment is ceased. This approach would detect seizures potentially induced by the underlying disease that required a prescription of oral FQ, if an increased IRR was observed before the FQ prescription period. Any delayed effect would also be captured in the post-FQ completion period. The IRRs estimated in CDARS and CPRD were meta-analyzed with a random-effects model to obtain the summary measure of effect. I2 statistics were used to test for heterogeneity. If no heterogeneity was found, then a fixed-effects model was used.
Figure 1. Typical observation period of a patient.
(A) In the primary analysis; (B) in the post hoc subgroup analysis; and (C) in the sensitivity analysis comparing the 7 days pre-FQ (baseline) and the first 7 days of the FQ-exposed period. FQ = fluoroquinolone.
Conditional Poisson regression was used to estimate the IRR and 95% confidence interval (CI). Age effect was adjusted in a 1-year band. A significance level of 5% was used in all statistical analyses.
Sample size calculation.
A total of approximately 3,224 cases are needed to detect an IRR of 2.0 with 95% confidence and 90% power.28
Post hoc subgroup analysis.
The risk of a seizure subsequent to oral FQ exposure among patients with a history of seizure is a clinically important question to be addressed. Therefore, a post hoc subgroup analysis was conducted to investigate this further. Patients with a history of seizure were defined as those with at least 2 seizure events with the incident seizure recorded after the beginning of the study period. The follow-up period began on the day after their incident seizure event to ensure that the baseline risk of recurrent seizure among the cases was standardized (figure 1B). The rate ratios for each risk period in the 2 databases were meta-analyzed as for the primary analysis.
Crude absolute risk.
The crude absolute risk for incident seizure was estimated using the method in the previous study,16 presented as per 10,000 oral FQ prescriptions.
Sensitivity analysis.
In SCCS extension sensitivity analysis, the distribution of time from event to the censoring of observation periods was estimated for each patient in both CDARS and CPRD. To determine whether the extension was needed, we plotted the interval from event to censor of the observation period in a bar chart with a bin-width of 1 year. If clustering had been observed shortly after the seizure (≤1 year), this would suggest the event might cause censoring of the observation period. This would violate a key assumption of the standard SCCS design, that event timing is independent of the observation period. Therefore, we applied the SCCS extension to account for event-dependent censoring.
As infection can lead to seizure and is subsequently treated with antibiotics, another sensitivity analysis was conducted by comparing seizure rates in the 7 days before the FQ prescription start date and the first 7 days of the FQ-exposed period. The purpose of this comparison was to determine whether increased risk of incident seizure was associated with the FQ prescription or the infection. The 7 days before the FQ prescription start date is assumed to be the period when manifestation of infection began but before FQs were commenced. This served as a baseline and was compared with the period after FQ was prescribed (figure 1C).
Microsoft Excel, RevMan 5.3 (Cochrane Collaboration, 2012), R 3.1.2 (R Development Core Team, 2008), and SAS 9.3 (SAS Inc., Cary, NC) were used for data manipulation and analyses.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (institutional review board reference number: UW 13-458) and the Independent Scientific Advisory Committees for Medicines and Healthcare Products Regulatory Agency database research and the London School of Hygiene and Tropical Medicine ethics committee.
RESULTS
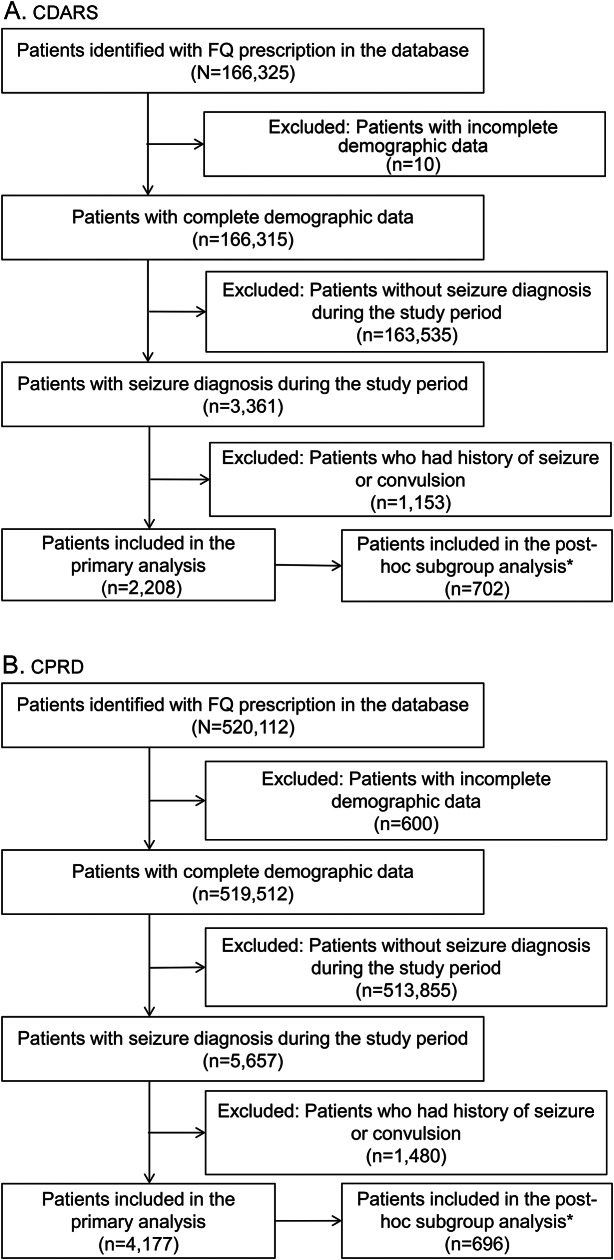
There were 2,208 patients from CDARS and 4,177 patients from CPRD who had both oral FQ and incident seizure during the study period and were included in the primary analysis (table 1). The inclusion and exclusion criteria are illustrated in figure 2.
Table 1.
Patient characteristics
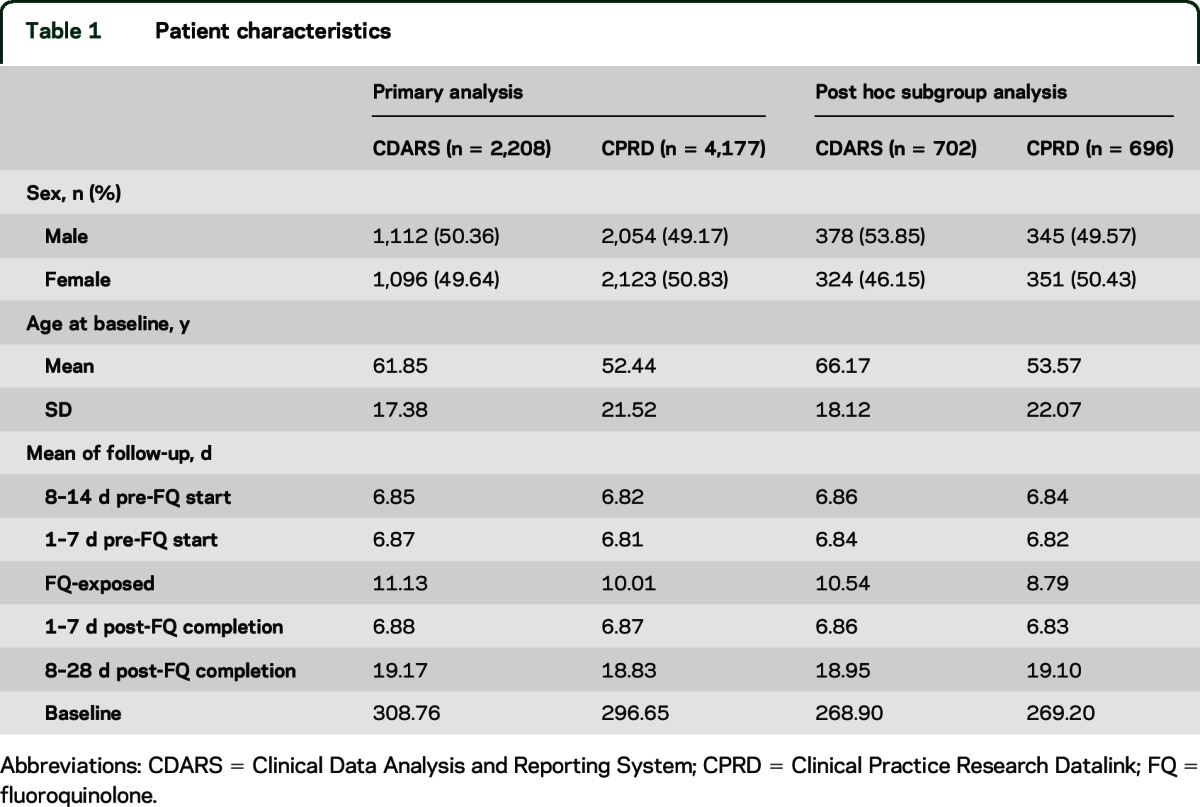
Figure 2. Flowchart of inclusion/exclusion of patients.
Patient count for each stage of inclusion and exclusion in (A) CDARS and (B) CPRD. *Post hoc subgroup analysis: patients with history of seizure. CDARS = Clinical Data Analysis and Reporting System; CPRD = Clinical Practice Research Datalink; FQ = fluoroquinolone.
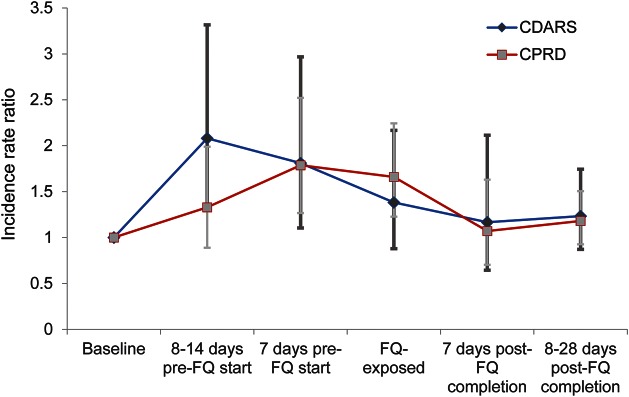
A total of 21 cases were found during the FQ-exposed period with an IRR of 1.38 (95% CI 0.88–2.17) in CDARS. Increased IRR was found in all pre-FQ start periods: 8–14 days pre-FQ (2.08 [95% CI 1.31–3.32]) and 7 days pre-FQ (1.81 [95% CI 1.10–2.97]). No association was observed in the time periods post-FQ. In CPRD, 47 cases were observed during the FQ exposed period with an IRR of 1.66 (95% CI 1.23–2.24). An increased IRR was also found during the 7 days pre-FQ (1.79 [95% CI 1.27–2.52]). No association was observed 8 to 14 days pre-FQ, or during the post-FQ periods. Meta-analyses of the IRRs for the 7 days pre-FQ and FQ-exposed periods also gave similar results (table 2). Overall, similar trends were observed in both databases (figure 3).
Table 2.
Model details of self-controlled case series of the association between oral FQs and seizure
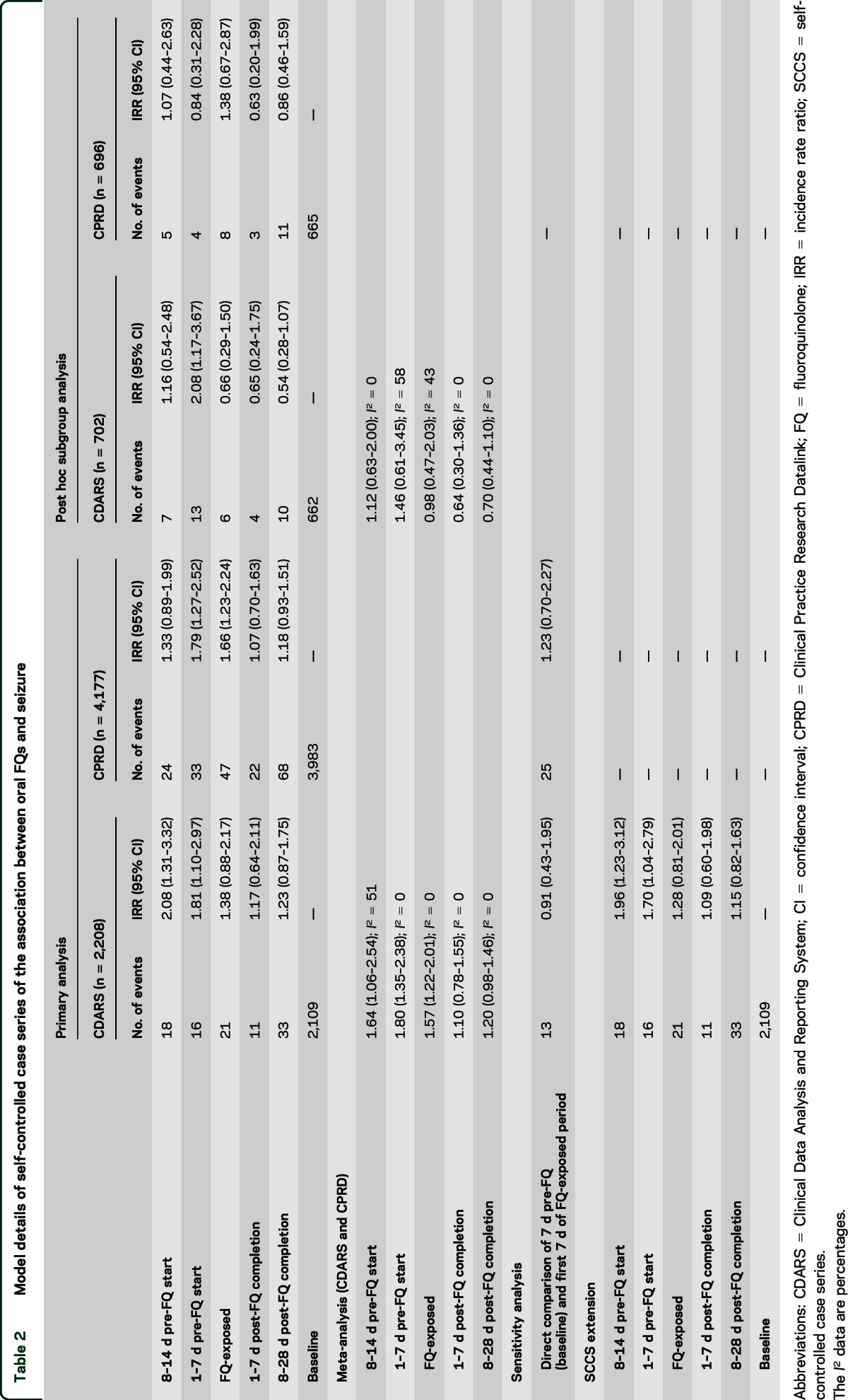
Figure 3. Incidence rate ratio trends of CDARS and CPRD (all risk periods).
Incidence rate ratios and their corresponding confidence intervals from the primary analysis were used. CDARS = Clinical Data Analysis and Reporting System; CPRD = Clinical Practice Research Datalink; FQ = fluoroquinolone.
Post hoc subgroup analysis.
A total of 702 and 696 patients from CDARS and CPRD, respectively, who had at least 2 seizure events and with the incident seizure recorded after the beginning of the observation period, were included in the analysis (table 1). A slightly increased IRR was observed during 7 days pre-FQ in CDARS only (2.08 [95% CI 1.17–3.67]). The rate ratios of the 2 databases are presented in table 2. The meta-analyzed rate ratios for all risk periods were not statistically significant (table 2).
Crude absolute risk.
A total of 291,751 and 1,166,213 oral FQ prescriptions were identified from CDARS and CPRD, respectively. The absolute risk of developing incident seizure during the FQ-exposed period in 10,000 oral FQ prescriptions was 0.72 (95% CI 0.47–1.10) in CDARS and 0.40 (95% CI 0.30–0.54) in CPRD.
Sensitivity analysis.
Data from both databases were tested for the need for SCCS extension. Figure e-1 shows the time from event to censoring of the observation period with each bin representing a 1-year interval. In the CDARS data, a clustering of observation period censoring can be seen shortly after incident seizure, leading to the potential violation of an SCCS assumption similar to the example demonstrated in a previous study.27 The analysis was then repeated with the use of SCCS extension. The estimated IRRs of all risk windows were similar to those in the primary analysis (table 2), suggesting the results of the primary analysis were not biased by this censoring. Clustering of observation period censoring was not observed in the CPRD dataset; therefore, analysis with the SCCS extension was not performed.
In the sensitivity analysis comparing the first 7 days of FQ exposure against the 7 days before FQ initiation, 27 patients were included from CDARS and 45 were included from CPRD. Comparing the first 7 days of FQ exposure with the 7 days before FQ exposure, the IRR estimated in CDARS was 0.91 (95% CI 0.43–1.95) and 1.23 (95% CI 0.70–2.27) in CPRD (table 2).
DISCUSSION
This study shows an association between the use of oral FQs and the development of incident seizure. However, an increased risk of incident seizure is seen shortly before starting FQ treatment in both databases. If an association between the use of oral FQs and seizure exists, the seizure would be captured subsequent to the beginning of FQ prescription, thus an increased IRR in the FQ-exposed or post-FQ periods would be expected. An increased IRR observed in the pre-FQ periods and no further increased risk after the start of FQ treatment suggests that it is not a causal effect of FQs. The increased IRR in the sensitivity analysis also suggested no causal effect but the clinical indication for which FQ was prescribed may have contributed to the development of incident seizure. Such a phenomenon is consistently seen in both CDARS and CPRD.
A possible reason for the increased risk of seizure before starting FQ treatment is the occurrence of infection-induced seizure. Patients may have presented with signs of infection such as fever or febrile infection–related seizures29 before they were prescribed an oral FQ. Therefore, an increased risk of seizure was found before the FQ exposure. Although patients with febrile convulsion were excluded in the analysis, we cannot rule out the fact that the increased risk was associated with infection-related complications attributable to the limitations of data recorded in the CDARS and CPRD.
Although several case reports described a potential association between FQs and the occurrence of seizure,3,10,30 most reported seizure as a subsequent event to FQ prescription. The result of this study contradicts speculation from the case reports. The findings of this study will not be detected in a classic cohort study where incident events before the exposure will not be typically considered. In addition, such a study design may exclude patients with pre-FQ seizure. The risk before the FQ exposure would not be observed and an association between the use of FQs and subsequent seizure may be concluded depending on the comparator chosen. A similar phenomenon had also been reported in previous literature.24
The findings of this study do not support a causal relationship between the use of oral FQs and the development of seizure. In addition, the crude absolute risk estimated in this study was very low in both databases. Seizure is a rare adverse event of FQs if there was an association. Clinicians should weigh the risk and benefit of FQ and its use should not be precluded in patients with the appropriate indication.
The SCCS study design enabled within-person comparison of the FQ-exposed and nonexposed periods. Therefore, it controlled for time-invariant factors including genetic factors that could not be completely accounted for in classic epidemiologic study designs.
The result of the SCCS extension is similar to that of the primary analysis in this study. This suggests that the occurrence of the event is independent of the censoring of the observation period. Hence, we are confident that SCCS is an appropriate study design.
The meta-analyzed findings of the 2 databases did not show any statistically significant increased risk of seizure before, during, or after the use of oral FQ among patients with a history of seizure. The observed differences between the primary and post hoc subgroup analysis may be attributable to the small sample size of the post hoc subgroup analysis, hence reduced power in detecting small increases in seizure risk. It is worth noting that the rate ratio during treatment is not higher than pre-FQ start periods, thus the results do not support FQ-induce seizure in patients with a history of seizure.
This study does not support a causal relationship between the use of oral FQ and the development of incident seizure. The consistent results found in the 2 databases further enhance the credibility of the findings. We believe that the clinical indication for which oral FQ was prescribed may have contributed to the development of incident seizure rather than the drug itself. In patients with a history of seizure, we did not find evidence of higher risk of seizure during FQ treatment than pre-FQ treatment; however, we cannot exclude the possibility of type 2 errors because of limited sample size. Therefore, further investigation with a larger sample size is warranted to confirm the findings of our post hoc subgroup analysis.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Hong Kong Hospital Authority for access to data, Dr. Ruth Brauer from London School of Hygiene and Tropical Medicine for statistical advice, and Ms. Jody K.P. Chu from the University of Hong Kong for clinical advice. The authors also thank Ms. Lisa Wong for editing and proofreading.
GLOSSARY
- CDARS
Clinical Data Analysis and Reporting System
- CI
confidence interval
- CPRD
Clinical Practice Research Datalink
- FQ
fluoroquinolone
- HK
Hong Kong
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IRR
incidence rate ratio
- SCCS
self-controlled case series
- UK
United Kingdom
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
C.S.L.C., E.W.C., A.Y.S.W., A.R., I.J.D., and I.C.K.W. had the original idea for this study and contributed to the development of the idea and the study design. C.S.L.C. reviewed the literature. C.S.L.C., E.W.C., and I.J.D. undertook the primary analysis. A.Y.S.W. performed independent analysis for quality control. C.S.L.C., E.W.C., A.Y.S.W., A.R., I.J.D., and I.C.K.W. contributed to the interpretation of the analysis. C.S.L.C. and E.W.C. wrote the first draft of the manuscript. C.S.L.C., E.W.C., A.Y.S.W., A.R., I.J.D., and I.C.K.W. critically reviewed the manuscript. E.W.C., I.J.D., and I.C.K.W. provided oversight of all aspects of this project. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
STUDY FUNDING
This study was supported by the University of Hong Kong Small Project Funding 201309176167.
DISCLOSURE
C. Chui reports no disclosures relevant to the manuscript. E. Chan has received research support from Bristol-Myers Squibb, Janssen, a division of Johnson & Johnson, the Research Grants Council (RGC, Hong Kong), and the Health and Medical Research Fund (HMRF, Hong Kong). A. Wong and A. Root report no disclosures relevant to the manuscript. I. Douglas is funded by a Medical Research Council Methodology Fellowship. He received personal fees from GlaxoSmithKline and Gilead outside the submitted work. I. Wong reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary, 70th ed London: British Medical Journal Publishing Group; 2015. [Google Scholar]
- 2.Kushner JM, Peckman HJ, Snyder CR. Seizures associated with fluoroquinolones. Ann Pharmacother 2001;35:1194–1198. [DOI] [PubMed] [Google Scholar]
- 3.Darwish T. Ciprofloxacin-induced seizures in a healthy patient. N Z Med J 2008;121:104–105. [PubMed] [Google Scholar]
- 4.Quigley CA, Lederman JR. Possible gatifloxacin-induced seizure. Ann Pharmacother 2004;38:235–237. [DOI] [PubMed] [Google Scholar]
- 5.Traeger SM, Bonfiglio MF, Wilson JA, Martin BR, Nackes NA. Seizures associated with ofloxacin therapy. Clin Infect Dis 1995;21:1504–1506. [DOI] [PubMed] [Google Scholar]
- 6.Tattevin P, Messiaen T, Pras V, Ronco P, Biour M. Confusion and general seizures following ciprofloxacin administration. Nephrol Dial Transpl 1998;13:2712–2713. [DOI] [PubMed] [Google Scholar]
- 7.Marinella MA. Myoclonus and generalized seizures associated with gatifloxacin treatment. Arch Intern Med 2001;161:2261–2262. [DOI] [PubMed] [Google Scholar]
- 8.Bird SB, Orr PG, Mazzola JL, Brush DE, Boyer EW. Levofloxacin-related seizure activity in a patient with Alzheimer's disease: assessment of potential risk factors. J Clin Psychopharmacol 2005;25:287–288. [DOI] [PubMed] [Google Scholar]
- 9.Koussa SF, Chahine SL, Samaha EI, Riachi MA. Generalized status epilepticus possibly induced by gatifloxacin. Eur J Neurol 2006;13:671–672. [DOI] [PubMed] [Google Scholar]
- 10.Lahmek P, Michel L, Meunier N, Aubin HJ. A seizure attributed to ofloxacine in a woman undergoing detoxification for alcohol dependence. Case Rep Med 2009;2009:705635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Famularo G, Pizzicannella M, Gasbarrone L. Levofloxacin and seizures: what risk for elderly adults? J Am Geriatr Soc 2014;62:2018–2019. [DOI] [PubMed] [Google Scholar]
- 12.Gervasoni C, Cattaneo D, Falvella FS, et al. Levofloxacin-induced seizures in a patient without predisposing risk factors: the impact of pharmacogenetics. Eur J Clin Pharmacol 2013;69:1611–1613. [DOI] [PubMed] [Google Scholar]
- 13.Schmuck G, Schurmann A, Schluter G. Determination of the excitatory potencies of fluoroquinolones in the central nervous system by an in vitro model. Antimicrob Agents Chemother 1998;42:1831–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akahane K, Sekiguchi M, Une T, Osada Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with gamma-aminobutyric acid receptor sites. Antimicrob Agents Chemother 1989;33:1704–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christ W. Central nervous system toxicity of quinolones: human and animal findings. J Antimicrob Chemother 1990;26(suppl B):219–225. [DOI] [PubMed] [Google Scholar]
- 16.Chui CS, Man KK, Cheng CL, et al. An investigation of the potential association between retinal detachment and oral fluoroquinolones: a self-controlled case series study. J Antimicrob Chemother 2014;69:2563–2567. [DOI] [PubMed] [Google Scholar]
- 17.Man KK, Ip P, Hsia Y, et al. ADHD drug prescribing trend is increasing among children and adolescents in Hong Kong. J Atten Disord Epub 2014 Jul 3. [DOI] [PubMed]
- 18.He Y, Chan EW, Man KK, et al. Dosage effects of histamine-2 receptor antagonist on the primary prophylaxis of non-steroidal anti-inflammatory drug (NSAID)-associated peptic ulcers: a retrospective cohort study. Drug Saf 2014;37:711–721. [DOI] [PubMed] [Google Scholar]
- 19.Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology 2015;149:586–595.e3. [DOI] [PubMed] [Google Scholar]
- 20.Man KK, Chan EW, Coghill D, et al. Methylphenidate and the risk of trauma. Pediatrics 2015;135:40–48. [DOI] [PubMed] [Google Scholar]
- 21.Wong AYS, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 22.Campbell J, Dedman DJ, Eaton SC, Gallagher AM, Williams TJ. Is the CPRD GOLD population comparable to the UK population? Pharmacoepidemiol Drug Saf 2013;22:280–281. [Google Scholar]
- 23.Rodriguez LAG, Gutthann SP. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol 1998;45:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas IJ, Langham J, Bhaskaran K, Brauer R, Smeeth L. Orlistat and the risk of acute liver injury: self controlled case series study in UK Clinical Practice Research Datalink. BMJ 2013;346:f1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768–1797. [DOI] [PubMed] [Google Scholar]
- 26.Douglas IJ, Evans SJ, Pocock S, Smeeth L. The risk of fractures associated with thiazolidinediones: a self-controlled case-series study. PLoS Med 2009;6:e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrington CP, Anaya K, Whitaker H, Hocine MN, Douglas IJ, Smeeth L. Self-controlled case series analysis with event-dependent observation periods. J Am Stat Assoc 2010;106:417–426. [Google Scholar]
- 28.Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med 2006;25:2618–2631. [DOI] [PubMed] [Google Scholar]
- 29.Serrano-Castro PJ, Quiroga-Subirana P, Payan-Ortiz M, Fernandez-Perez J. The expanding spectrum of febrile infection-related epilepsy syndrome (FIRES). Seizure 2013;22:153–155. [DOI] [PubMed] [Google Scholar]
- 30.Bellon A, Perez-Garcia G, Coverdale JH, Chacko RC. Seizures associated with levofloxacin: case presentation and literature review. Eur J Clin Pharmacol 2009;65:959–962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.