Abstract
Objective
There is increasing attention to cardiovascular diseases in low-income countries. However, little is known about heart failure (HF) in rural areas, where most of the populations in low-income countries live. We studied HF epidemiology, care delivery and outcomes in rural Haiti.
Methods
Among adults admitted with HF to a rural Haitian tertiary care hospital during a 12-month period (2013–2014), we studied the clinical characteristics and short-term outcomes including length of stay, inhospital death and outpatient follow-up rates.
Results
HF accounted for 392/1049 (37%) admissions involving 311 individuals; over half (60%) were women. Mean age was 58.8 (SD 16.2) years for men and 48.3 (SD 18.8) years for women; 76 (41%) women were <40 years of age. Median length of stay was 10 days (first and second quartiles 7, 17), and inhospital mortality was 12% (n=37). Ninety nine (36%) of the 274 who survived their primary hospitalisation followed-up at the hospital’s outpatient clinic, and 18 (6.6%) were readmitted to the same hospital within 30 days postdischarge. Decreased known follow-up (p<0.01) and readmissions (p=0.03) were associated with increased distance between patient residence and hospital. Among the one-quarter (81) patients with echocardiograms, causes of HF included: non-ischaemic cardiomyopathy (64%), right HF (12%), hypertensive heart disease (7%) and rheumatic heart disease (5%). One-half of the women with cardiomyopathy by echocardiogram had peripartum cardiomyopathy.
Conclusions
HF is a common cause of hospitalisation in rural Haiti. Among diagnosed patients, HF is overwhelming due to non-atherosclerotic heart disease and particularly affects young adults. Implementing effective systems to improve HF diagnosis and linkage to essential outpatient care is needed to reduce long-term morbidity and mortality.
Cardiovascular diseases (CVD) are the leading causes of death and disability worldwide.1 Nearly 80% of CVD deaths occur in low-income and middle-income countries (LMIC). The United Nations and individual countries have galvanised efforts to fight the epidemic of CVDs and other non-communicable diseases to reduce premature mortality by 25% by 2025.2 Current estimates suggest that ischaemic heart disease (IHD) is a leading cause of CVD death, whereas heart failure (HF) is less common.3
However, estimates of CVD epidemiology for low-income countries, such as Haiti and those in Sub-Saharan Africa, are generally derived from urban hospitals’ data and may not reflect the experiences of the rural poor.4 Several community and hospital registries in low-income countries document the rarity of IHD and predominance of HF.5–8 The high reported burden of HF highlights the need to study current CVD prevalence and HF causes in rural LMICs to guide context-appropriate healthcare priorities. In Haiti, the reports of HF only document the high incidence and morbidity of peripartum cardiomyopathy, but do not describe other causes of HF.9 Also, health systems in LMICs, generally designed to treat acute conditions, may not be prepared to provide the longitudinal care essential to mitigate the morbidity and mortality of chronic HF. The goal of our study is to describe the characteristics of adults hospitalised for HF in rural Haiti, including demographics, HF type, provider prescribing practices, short-term retention in care and outcomes. The data in our report are needed to inform evidence-based strategies to understand HF causes and drive changes to improve HF care in Haiti and other LMICs.
METHODS
Study setting
Haiti is the poorest country in the Western hemisphere.10 The retrospective cohort study was conducted at University Hospital Mirebalais (UHM) in the rural Central Plateau of Haiti, where there are only eight doctors per 100 000 population.11 UHM is a 300-bed public academic referral hospital operated in partnership between the Haitian Ministry of Public and Population Health and the non-governmental organisations Zanmi Lasante (Haiti-based) and Partners In Health (Boston-based). Serving a regional population of 2.5 million, UHM draws patients from throughout the country. A referral structure has been designed as part of hospital planning. UHM serves the primary care needs for the 165 000 people living closest to the hospital. However, as in the rest of Haiti, most patients self-present to their chosen hospital. The private health system is concentrated in the capital, and is sparse in rural Haiti.
Patients pay a one-time registration fee of US$1 (50 Haitian Gourdes). Clinic visits, hospitalisations, diagnostic tests and medications are provided for free to facilitate access to care. Cardiology services include electrocardiography and basic echocardiography with remote and intermittent on-site clinical supervision by a visiting cardiologist. There is no specific cardiology ward. An electronic medical record (EMR) (OpenMRS, Grandville, Michigan, USA) collects demographic, inpatient and outpatient encounter data, including service dates and clinical diagnoses.
Study population
We included adults (≥18 years old) admitted to UHM’s internal medicine ward with any diagnosis of HF during a 12-month period (October 2013 through September 2014). We excluded patients admitted to the surgical or obstetric wards. HF was operationalised as one of the EMR diagnoses shown in online supplementary table S2. Physicians assigned clinical HF diagnoses based on combinations of symptoms (ie, dyspnoea), signs (ie, oedema, pulmonary rales) and diagnostics (chest X-ray, echocardiogram). During the study period, 927 unique patients (1049 total admissions) were admitted to UHM.
The ethical review boards of Zanmi Lasante and Boston University Medical Center approved the study. Informed consent was not obtained as we retrospectively reviewed routinely collected clinical data.
Data collection
Data were combined from three routinely collected clinical sources: (1) EMR export, (2) paper chart and (3) clinical echocardiography database. Data for age, sex and address were recorded for all study subjects. Full data extraction procedures are in the online supplementary material.
HF diagnostic categories
As part of UHM specialty care, a Boston-based cardiologist (GFK) visited six times for approximately 18 total weeks. During these visits, one care component included performing formal echocardiograms on all patients admitted with HF. Some patients received an echocardiogram during subsequent clinical encounters.
A single probable primary HF type was assigned for each patient based on echocardiographic findings using similar criteria to the Heart of Soweto study, summarised here.12 Cardiomyopathy was diagnosed in patients with left ventricle ejection fraction ≤50%. Peripartum cardiomyopathy was diagnosed in women with cardiomyopathy and symptom onset ‘towards the end of pregnancy or in the 6 months following delivery’.13 Rheumatic heart disease (RHD) was diagnosed based on functional and morphological abnormalities of the mitral and aortic valve.14 Patients with clinical HF, preserved left ventricle systolic function and a history of hypertension were considered to have hypertensive heart disease.12 HF from IHD was diagnosed in the presence of regional left ventricular wall motion abnormalities. Patients without major echocardiographic abnormalities and thought to not have HF by the cardiologist were categorised as ‘normal’.
Clinical care and outcomes
We ascertained short-term outcomes including (1) inhospital death, (2) known linkage to outpatient care—defined as a UHM outpatient visit with a physician ≤30 days after discharge, (3) known retention in outpatient care—defined as a second UHM outpatient clinic visit ≤30 days after the initial outpatient visit and (4) UHM readmission ≤30 days after discharge. The 30-day time frame was chosen as medications are generally dispensed in 30-day supplies. Patients who did not return to UHM for care were not contacted.
Data analysis
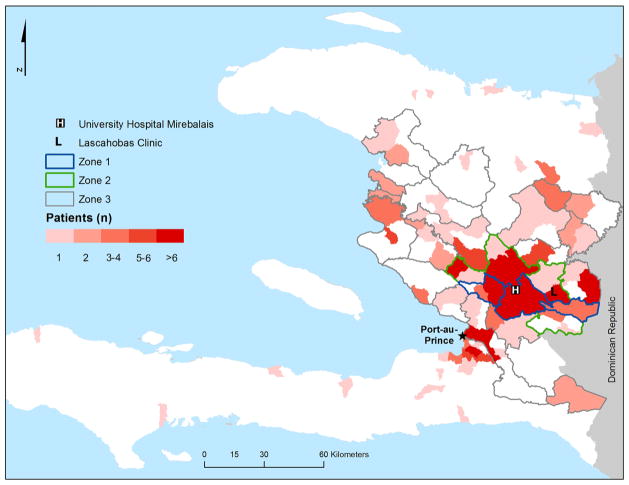
Patients were grouped into proximity-based geographic zones based on self-reported addresses and UHM’s regional referral structure (figure 1 and see online supplementary table S1). Patients from zone 1 live closest to the hospital, while those from zone 4 live outside the standard referral area.
Figure 1.
Map of Haiti. The red shade indicates the number of patients with heart failure admitted from each communal section. Blue, green and grey outlines show catchment zones 1, 2 and 3, respectively. All other areas are part of zone 4.
The numbers of patients with available data are shown in the tables. We tested differences in means with the two-sample t test. We tested differences of medians across several groups with the Kruskal–Wallis test. We summarised categorical data as proportions and the difference of proportions test (Pearson χ2). We used the two-sided Cochran–Armitage test for trend to evaluate the linear trend in outcome proportions with increasing zone. Two-sided p values of ≤0.05 were considered statistically significant. Analyses were performed using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Characteristics of the patients with HF are shown in table 1. There were 1049 (927 unique individuals) adult non-surgical internal medicine admissions to UHM; 392 (37.3%) of the admissions were for HF, representing 311 (33.5%) unique patients. Among these 311 patients, women (n=186, 59.8%) outnumbered men (n=125, 40.2%). Compared with men, women on average were younger (mean age 48.3 vs 58.8 years, p<0.01), or very young: 40.9% of women were ≤40 years old compared with 12.0% of men (p<0.01). Among those with non-missing data, many had a history of hypertension (50.3% of women; 43.4% of men), while fewer women (14.4%) than men (43.0%) had ever used tobacco (p<0.01).
Table 1.
Patient demographic and clinical presentation*
| Characteristic N (%) |
n with available data† | All patients 311 |
Women 186 (59.8) |
Men 125 (40.2) |
p Value‡ |
|---|---|---|---|---|---|
| Age at admission, years | 311 | ||||
| Mean±SD | 52.5±18.5 | 48.3±18.8 | 58.8±16.2 | <0.01 | |
| ≤40 years, n (%) | 91 (29.3) | 76 (40.9) | 15 (12.0) | <0.01 | |
| Catchment area, n (%) | 311 | 0.12 | |||
| Zone 1 (primary) | 100 (32.2) | 68 (36.6) | 32 (25.6) | ||
| Zone 2 (secondary) | 49 (15.8) | 28 (15.5) | 21 (16.8) | ||
| Zone 3 (tertiary) | 99 (31.8) | 59 (31.7) | 41 (32.0) | ||
| Zone 4 (outside referral zones) | 63 (20.3) | 31 (16.7) | 32 (25.6) | ||
| Co-morbidities (self-reported) | |||||
| Hypertension, n (%) | 263 | 125 (47.5) | 79 (50.3) | 46 (43.4) | 0.31 |
| Diabetes, n (%) | 267 | 22 (8.3) | 17 (10.6) | 5 (4.7) | 0.11 |
| HIV, n (%) | 269 | 7 (2.6) | 5 (3.1) | 2 (1.9) | 0.71 |
| Habits | |||||
| Smoking, current or past, n (%) | 267 | 69 (25.8) | 23 (14.4) | 46 (43.0) | <0.01 |
| Alcohol, current or past, n (%) | 209 | 43 (20.7) | 6 (4.8) | 37 (44.1) | <0.01 |
| Vital signs | |||||
| Systolic blood pressure, mm Hg, mean±SD | 275 | 142±36 | 142±35 | 139±37 | 0.27 |
| Diastolic blood pressure, mm Hg, mean±SD | 275 | 90±24 | 91±24 | 87±23 | 0.18 |
| Heart rate, bpm, mean±SD | 275 | 108±26 | 110±29 | 106±21 | 0.26 |
| Oxygen saturation, %, mean±SD | 273 | 94±8 | 94±9 | 95±6 | 0.16 |
| ECG | |||||
| Atrial fibrillation, n (%) | 232 | 14 (6.0) | 4 (2.8) | 10 (11.2) | 0.01 |
| Prior infract (Q wave), n (%) | 232 | 15 (6.5) | 6 (4.2) | 9 (10.1) | 0.10 |
| Laboratory | |||||
| Sodium, mmol/L, mean±SD | 175 | 140±7 | 140±7 | 139±7.1 | 0.51 |
| Potassium, mmol/L, mean±SD | 182 | 4.7±1.1 | 4.5±1.0 | 4.9±1.3 | 0.01 |
| Blood urea nitrogen, mg/dL, median [Q1, Q3] | 176 | 16 [11, 30] | 13 [9, 24] | 21 [13, 40] | <0.01 |
| Creatinine, mg/dL, median [Q1,Q3] | 211 | 1.0 [0.7, 1.6] | 0.8 [0.7, 1.2] | 1.2 [0.9, 2.2] | <0.01 |
| Haemoglobin, g/dL, mean±SD | 269 | 10.6±2.7 | 10.4±2.3 | 10.8±3.2 | 0.26 |
| Length of stay, days, median [Q1, Q3] | 311 | 10 [7, 17] | 10 [7, 16] | 12 [7, 18] | 0.14 |
Data shown are N (%), mean±SD or median [Q1, Q3].
Characteristics obtained through review of paper charts were either not available or incompletely obtained for some patients. Data are computed from non-missing.
p Values shown are from a comparison of women and men: χ2 exact test (comparison of proportions), t test (comparison of means) or Wilcoxon rank sum test (comparison of medians).
Q1, 25th percentile; Q3, 75th percentile.
Patients admitted to UHM with HF came from throughout the country, but many came from the hospital’s primary catchment area (zone 1, (32.2%, n=100)) or zone 2 (15.8%, n=49) (table 1 and figure 1). One-fifth of the patients (20.3%, n=63) were from outside the hospital’s official referral area (zone 4).
New York Heart Association (NYHA) class was formally documented in 156 (50.2%) of the patients. Of those with recorded NYHA class, 114 (73.1%) had class IV symptoms (most severe) and 39 (25.0%) had class III symptoms. Of the 232 patients with available ECGs, only 14 (6.0%) had atrial fibrillation and 15 (6.5%) had Q waves.
Causes of HF
Of the 311 patients with HF, 81 (26.0%) received a formal cardiologist echocardiogram: 50 women and 31 men. Patients who had echocardiograms were overall similar to those without echocardiograms (see online supplementary table S3). Cardiomyopathy was the leading cause of HF among both women (70%) and men (55%) with an echocardiogram (table 2). Other leading causes of HF were right HF (n=10, 12%) and hypertensive heart disease (n=6, 7%). Other less frequent causes included RHD (n=4, 5%), pericardial disease (n=2, 2%) and congenital heart disease (n=1, 1%). Among the 50 women with echocardiograms, peripartum cardiomyopathy was diagnosed in 17 (34%). Only one patient had echocardiographic evidence of IHD. Mean left ventricular ejection fraction for patients with cardiomyopathy was 22% (SD 7.6%). Full echocardiographic measures are shown in online supplementary table S4. After cardiologist evaluation, only three patients (4%) had normal echocardiograms and were thought to not have clinical HF. For the clinical diagnosis of HF by local providers, the positive predictive value compared with cardiologist evaluation was 96% (n=78/81).
Table 2.
HF diagnostic categories of 81 patients (26%) with cardiologist echocardiogram; n (%)
| Diagnostic category | Total | Women | Men |
|---|---|---|---|
| Cardiomyopathy | 35 (43) | 18 (36) | 17 (55) |
| Peripartum cardiomyopathy | 17 (21) | 17 (34) | – |
| Right HF | 10 (12) | 4 (8) | 6 (19) |
| Hypertensive heart disease | 6 (7) | 4 (8) | 2 (6) |
| Rheumatic heart disease | 4 (5) | 3 (6) | 1 (3) |
| Pericardial disease | 2 (2) | 1 (2) | 1 (3) |
| Congenital heart disease | 1 (1) | 0 (0) | 1 (3) |
| Other* | 3 (4) | 2 (4) | 1 (3) |
| Normal—not HF | 3 (4) | 1 (2) | 2 (6) |
| Total | 81 | 50 (62) | 31 (38) |
Other=Endocarditis (1), ischaemic cardiomyopathy (1), possible infiltrative cardiomyopathy (1).
HF, heart failure.
Medications
A total of 80% (220 of 274 discharged patients) had discharge medication lists available in the paper chart. Among these patients, furosemide was prescribed most frequently (66.4% of patients), followed by ACE inhibitors (55.0%), antiplatelet agents (47.7%) and β-blockers (35.5%) (table 3). Of the 44 discharged patients with echocardiogram-confirmed cardiomyopathy and available data, only nine (20.5%) were prescribed a combination of (1) furosemide, (2) β-blocker and (3) afterload-reducing medicines (ACE inhibitor, aldosterone antagonist or combination of hydralazine and isosorbide dinitrate).
Table 3.
Medications prescribed at discharge; n (%)*
| Medication | All patients (n=220) | Cardiomyopathy by echocardiogram (n=44) |
|---|---|---|
| Furosemide | 146 (66.4) | 31 (70.5) |
| ACE inhibitor | 121 (55.0) | 28 (63.6) |
| Antiplatelet | 105 (47.7) | 20 (45.5) |
| β-Blocker | 78 (35.5) | 18 (40.9) |
| Nitrate | 48 (21.8) | 7 (15.9) |
| Spironolactone | 48 (21.8) | 9 (20.5) |
| Calcium channel blocker | 47 (21.4) | 6 (13.6) |
| Hydrochlorothiazide | 39 (17.7) | 7 (15.9) |
| Digoxin | 17 (7.7) | 5 (11.4) |
| Warfarin | 14 (6.4) | 3 (6.8) |
| Statin | 12 (5.5) | 0 (0) |
| Hydralazine | 4 (1.8) | 0 (0) |
| α-Methyldopa | 2 (0.9) | 0 (0) |
| Penicillin | 1 (0.5) | 0 (0) |
Patients with available discharge medication data: n=220 (80.3%) of the 274 discharged patients; n=44 (88%) of the 50 discharged patients with echocardiogram-based cardiomyopathy.
Outcomes
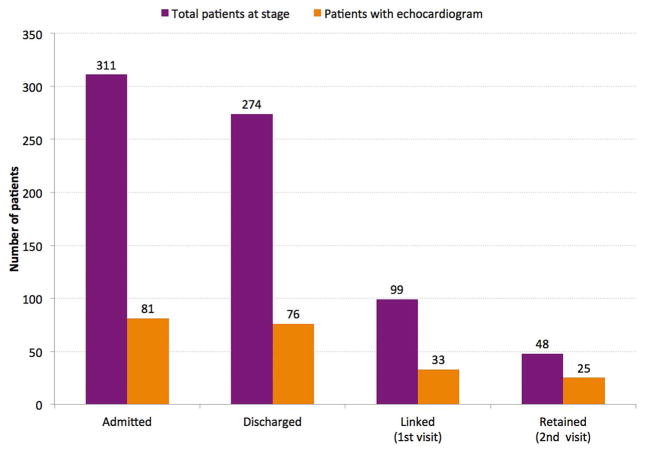
Of the 311 patients admitted, 37 (11.9%) died during the primary admission (table 4, figure 2). Median (25th percentile, 75th percentile) length of stay was 10 (7, 17) days. Of the 274 patients discharged, 99 (36.1%) were successfully linked to outpatient clinic evaluation at UHM within 30 days of discharge. Only 48 patients (17.5%) were retained and came back for a second appointment at UHM; 18 patients (6.6%) were readmitted to UHM within 30 days.
Table 4.
Short-term outcomes by catchment area
| Outcome | Total | Zone 1 | Zone 2 | Zone 3 | Zone 4 | p Value* |
|---|---|---|---|---|---|---|
| All patients | n=311 | n=100 | n=49 | n=99 | n=63 | |
| Inhospital mortality, n (%) | 37 (11.9%) | 11 (11.0%) | 3 (6.1%) | 17 (17.2%) | 6 (9.5%) | 0.63 |
| Length of stay, days, median (Q1, Q3) | 10 (7, 17) | 9 (7, 18.5) | 10 (6, 14) | 10 (7, 17) | 13 (9, 19) | 0.20 |
| Discharged patients | n=274 | n=89 | n=46 | n=82 | n=57 | |
| Length of stay, days, median (Q1, Q3) | 10 (7, 17) | 9 (7, 18) | 9.5 (6, 14) | 11 (8, 16) | 13 (9, 19) | 0.11 |
| Readmission within 30 days, n (%) | 18 (6.6%) | 9 (10.1%) | 4 (8.7%) | 4 (4.9%) | 1 (1.8%) | 0.03 |
| Linkage: 30-day clinic visit, n (%) | 99 (36.1%) | 40 (44.9%) | 18 (39.1%) | 27 (32.9%) | 14 (24.6%) | <0.01 |
| Retention: of linked patients, those with a second clinic visit within 30 days, n (%) | 48 (48.5%) | 20 (50%) | 9 (50%) | 11 (40.7%) | 8 (57.1%) | 0.96 |
Denominators shown are for patients eligible for the outcome of interest. For example, only discharged patients are eligible for linkage to outpatient care.
p Values for categorical data across zones 1–4 are from the Cochran–Armitage test of trend. p Values for length of stay are from the Kruskal–Wallis test.
Q1, 25th percentile; Q3, 75th percentile.
Figure 2.
Cascade of care for patients with heart failure admitted to University Hospital Mirebalais (UHM) (October 2013–September 2014). The total number of patients (darker bars) and number with echocardiograms (lighter bars) at each stage of care are shown. Linked patients had a UHM clinic visit ≤30 days after hospital discharge. Retained patients had a second clinic visit ≤30 days after the initial postdischarge clinic visit.
There was a significant trend associating farther zones and lower outcomes of known linkage (p<0.01) and readmission (p=0.03) (figure 3 and table 4). Patients from zone 1 had the highest linkage and readmission rates (44.9% and 10.1%, respectively) with stepwise reductions of outcomes as distance increased (24.6% and 1.8%, respectively, for zone 4). None of the patients were seen at the nearby clinic in Lascahobas (zone 2, 15 miles away) within 30 days (see online supplementary material).
Figure 3.
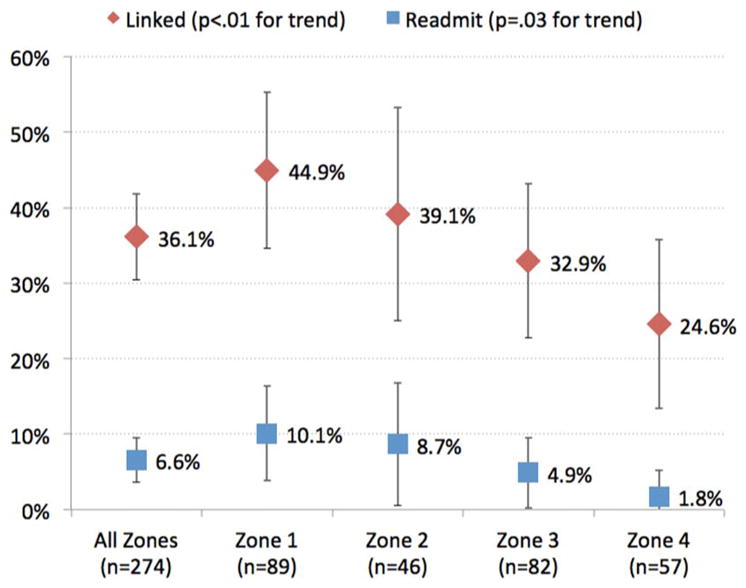
Proportion of patients linked to care (diamond) and readmitted (square) by zone. 95% CI bars are shown. Two-sided p values for Cochran–Armitage trend test across zones 1–4 are shown.
DISCUSSION
Endemic causes of HF: diagnosis and treatment
In a hospital in rural Haiti, we found that HF is a very common cause of morbidity among hospitalised adults, representing one-third of non-surgical/non-obstetric admissions during the study period. Non-ischaemic cardiomyopathies were the major cause of HF, followed by right HF, hypertensive heart disease and RHD. Peripartum cardiomyopathy was a major cause of HF among women of childbearing age in Haiti, where the incidence is the highest reported in the world.9 Our overall findings are consistent with studies from other rural low-income countries,6,7–15,16 and contrast with the main cause of HF in high-income countries: IHD.17 Though there has been considerable focus on the growing epidemic of atherosclerotic CVD in LMICs, specifically myocardial infarction and stroke,2 our study shows specific attention to the HF burden presently affecting communities in rural Haiti is needed.
HF among rural low-income patients has a unique set of risk factors—different from the traditional causes of atherosclerotic heart disease—which must be addressed to reduce the HF burden.18 Cardiomyopathies may be attributed to micronutrient deficiencies19 or viral infections.20 Genetic polymorphisms among people of African ancestry have been associated with familial cardiomyopathies.21 Right HF is caused by increased exposure to indoor and outdoor air pollution, schistosomiasis, tuberculosis and sickle cell disease.22 Such risk factors are exacerbated by using solid cooking fuels, over-crowding, poor hygiene and lack of access to basic healthcare.23 Consistent with community-based surveys,24 self-reported prevalence of hypertension was high, and any prior smoking was also high in men, but less in women.
Encouragingly, we found that doctors without subspecialty training can correctly identify patients with HF based on symptoms and signs with a high positive predictive value compared with a cardiologist. In our study, only one-quarter of patients received echocardiograms based on the availability of the visiting cardiologist. There are only four Haitian cardiologists currently performing echocardiograms in Haiti—all in the capital. Improved availability of diagnostic echocardiography is needed for local providers to diagnose and manage patients with HF. Simplified basic echocardiography protocols have been developed and studied in other LMICs.15 In assigning patients to HF categories—such as cardiomyopathy or suspected valvular heart disease—providers can initiate evidence-based therapies while referring specific patients for cardiologist confirmation. In our study, an estimated two-thirds of patients could be identified as having cardiomyopathy by basic echocardiography. Telemedicine involving remote review of digitally transmitted echocardiograms also may improve access.
Evidence-based therapies to reduce morbidity and mortality of HF are well described. For patients with systolic HF, treatments with β-blockers, ACE inhibitors, aldosterone antagonists and vasodilators have proven efficacy in reducing mortality and are recommended at the highest level by professional cardiology associations.25 However, there remains a gap in translating efficacious HF treatment into the delivery of effective care, particularly in LMICs. For CVDs, such evidence-practice gaps are noted in the low rates of hypertension treatment and control,26 and low use of medicines for IHD secondary prevention.27 In our study, a high proportion of patients with apparent non-ischaemic HF were treated with antiplatelet agents—some of which may have been prescribed unnecessarily for presumed IHD. Similar observations were noted in urban Sub-Saharan Africa.7 Programmes to improve provider behaviour by targeting the medication prescription-effectiveness gap are needed to enhance the quality of care in rural Haiti.
Health systems to care for patients with HF
Our study highlights several issues regarding the interaction between patients and health system. First, patients admitted with HF presented with advanced symptoms. Second, known linkage to outpatient clinic postdischarge was low. Even among patients living closest to the hospital where UHM represented their designated primary care facility, less than half were seen in clinic within 30 days. Additionally, none of the patients in our study had timely follow-up in one of the close regional clinics (Lascahobas). Despite the distance, however, one-quarter of patients who live far from the hospital still returned for follow-up. Third, known readmission rates to the hospital within 30 days were low at only 6.6% overall and 10.1% for the closest patients. For comparison, the 30-day HF readmission rate is 15% in Massachusetts.28 However, we do not know if patients living further from the hospital presented to other facilities for follow-up or readmission, and if the other facilities have HF management capacity.
The late presentation and low linkage in Haiti may be related to the structure and function of the current health system. In rural LMICs, the unique needs of the poor must be incorporated into health system design to promote equity. In Haiti, 78% of people earn <US$2/day.10 Patients must choose between spending their limited time and money to attend clinic versus working, or buying medications instead of food. Lessons can be learned from HIV care in Haiti—another chronic disease —where lower income,29 long or costly transportation, low formal education, poor social support and food insecurity30 contribute to non-adherence. In our study, despite the very low financial barrier at UHM, known linkage rates were still low.
HF is a chronic condition requiring long-term medical therapy, patient empowerment and lifestyle changes. Strong outpatient care is necessary to facilitate earlier case finding and initiate and maintain chronic treatment to control symptoms. Effective communication linking hospitals and clinics are needed to improve interfacility care coordination. Every down-step along the chronic care cascade represents attrition of patients and requires thoughtful assessment of the causes of loss-to-follow-up. Patient-centred studies among patients living in extreme poverty are needed to better understand personal health agency and the many competing priorities affecting the choices that influence CVD health.
Limitations
The current study is the first to report the HF types and short-term outcomes affecting patients in impoverished rural communities of Haiti. However, our study has a number of specific limitations. Using routinely collected clinical data posed several challenges. Chart documentation was incomplete. For example, NYHA class was not formally documented for all patients. Also, some paper records were not located. Subsequently, complete baseline and longitudinal data were available in only 62 (20%) patients. Similarly, we could not confirm the accuracy of EMR-coded clinical diagnoses. Comorbidities were assessed by self-report and were likely underestimates as awareness of chronic diseases is low in LMICs.26
A major limitation in estimating linkage and readmission was our inability to ascertain outcomes for all patients. Patients from distant zones may follow-up or be rehospitalised at other facilities—our observed linkage and readmission rates were likely underestimated. However, even among patients for whom UHM was their primary care source, linkage at UHM or the nearest alternative clinic was low.
Cardiologist echocardiograms were performed in only one-quarter of the patients, which may have affected the reported HF epidemiology. Patients who had more interaction with the health system—such as longer hospital stay, more clinic visits or readmissions—had a greater likelihood of receiving an echocardiogram, potentially over-representing patients with advanced disease. IHD was defined in patients with regional wall motion abnormalities on echocardiogram as more specific diagnostics, such as angiography and stress testing, are not available in Haiti. Thus, we may have underdiagnosed IHD as an underlying cause. Because cardiomyopathy due to prolonged severe hypertension may appear echocardiographically similar to other causes, and history of hypertension was missing for some patients, it is possible that there may be misclassification of hypertension-related HF as cardiomyopathy. Additionally, echocardiograms were not performed on patients without a diagnosis of HF and we, therefore, could not measure the sensitivity or specificity of local clinical diagnoses.
We conducted our study in a unique clinical and socioeconomic setting in rural Haiti. Though the overall results are similar to reports from other rural LMICs, broad generalisability is uncertain. Additional studies in rural LMICs are needed to evaluate CVD epidemiology and health system performance.
CONCLUSION
HF in our referral hospital in rural Haiti was a major cause of hospitalisation and was predominantly due to endemic causes including cardiomyopathies and right HF; diagnosed IHD was rare. Patients presented with advanced symptoms and even among patients with close proximity to the hospital, there was incomplete follow-up. Effective interventions are needed to increase local diagnostic capacity, diminish evidence-practice gaps, address patient-level barriers to care seeking for initial and follow-up care and strengthen the health system to care for poor patients with chronic diseases in low-income countries.
Supplementary Material
Key messages.
What is already known on this subject?
The majority of cardiovascular disease morbidity is in low-income and middle-income countries. However, little is known about heart failure epidemiology and care delivery in rural regions of low-income countries.
What might this study add?
In a rural academic referral hospital in Haiti, heart failure is a major cause of non-obstetric/non-surgical hospitalisations for adults. Non-ischaemic cardiomyopathy, right heart failure, hypertensive heart disease and rheumatic heart disease are the major causes of heart failure. Ischaemic cardiomyopathy is rare. Medical therapy and linkage from inpatient to outpatient care need improvement.
How might this impact on clinical practice?
Policy-makers in rural Haiti can expand the focus on the burden of heart failure. Interventions are needed to improve prescribing patterns of medicines for heart failure. Improved healthcare delivery systems are needed to better coordinate care for patients with chronic heart failure.
Acknowledgments
We thank Ricard Pognon, Jimmy Jean Baptiste, and Brittany Eddy of UHM for extraction of the EMR dataset; and Ermyas Birru of Partners In Health for generating the map. We also thank the healthcare workers of Zanmi Lasante, who diligently work under difficult circumstances. We finally thank the patients in Haiti whom we serve as they motivate us to strive for equity through improved care delivery.
Funding Support for the clinical care delivered jointly from The Haitian Ministry of Public and Population Health and Partners In Health/Zanmi Lasante. GFK was supported in part by the National Heart, Lung and Blood Institute (K12 HL083781). EJB was supported in part by 2R01HL092577 and 1R01HL128914.
Footnotes
GB and LRH contributed equally to this study.
Contributors GFK conceived the study and led the writing of the paper. All the authors contributed to the analysis and interpretation of data and provided critical revisions of the paper. All the authors read and approved the final manuscript.
Competing interests None declared.
Ethics approval Boston University Medical Campus, Boston; Zanmi Lasante, Haiti.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Sixty-Fifth World Health Assembly Resolutions, Decisions, Annexes. 2012 WHA65/2012/REC/1. http://apps.who.int/gb/or/e/e_wha65r1.html.
- 3.Moran A, Forouzanfar M, Sampson U, et al. The epidemiology of cardiovascular diseases in sub-saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog Cardiovasc Dis. 2013;56:234–9. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukhman G. Heart failure in Africa: continuity or change? Heart. 2014;100:1223–4. doi: 10.1136/heartjnl-2014-305936. [DOI] [PubMed] [Google Scholar]
- 5.Kwan G, Bukhman A, Miller AC, et al. Heart Failure in Rural Sub-Saharan Africa: Etiology and Severity. Abstract presented at American Heart Association Epidemiology and Prevention and Nutrition, Physical Activity and Metabolism 2013 Scientific Sessions. Circulation. 2013;127:AP110. [Google Scholar]
- 6.Makubi A, Hage C, Lwakatare J, et al. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study. Heart. 2014;100:1235–41. doi: 10.1136/heartjnl-2014-305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–94. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 8.Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–22. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 9.Fett JD, Carraway RD, Dowell DL, et al. Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. Am J Obstet Gynecol. 2002;186:1005–10. doi: 10.1067/mob.2002.122423. [DOI] [PubMed] [Google Scholar]
- 10.Development Data Group, The World Bank. [accessed 10 Feb 2015];Haiti at a Glance. http://siteresources.worldbank.org/INTHAITI/Resources/Haiti.AAG.pdf.
- 11.Ministère de la Santé Publique et de la Population. Rapport Statistique 2014. Port au Prince; Haiti: 2015. [Google Scholar]
- 12.Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. 2008;118:2360–7. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- 13.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 14.Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan GF, Bukhman AK, Miller AC, et al. A simplified echocardiographic strategy for heart failure diagnosis and management within an integrated noncommunicable disease clinic at district hospital level for sub-Saharan Africa. JACC Heart Fail. 2013;1:230–6. doi: 10.1016/j.jchf.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Tantchou Tchoumi JC, Butera G. Profile of cardiac disease in Cameroon and impact on health care services. Cardiovasc Diagn Ther. 2013;3:236–43. doi: 10.3978/j.issn.2223-3652.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women. Arch Intern Med. 2001;161:996. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 18.Gersh BJ, Sliwa K, Mayosi BM, et al. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31:642–8. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 19.Seftel HC, Metz J, Lakier JB. Cardiomyopathies in Johannesburg Bantu. I. Aetiology and characteristics of beriberi heart disease. S Afr Med J. 1972;46:1707–13. [PubMed] [Google Scholar]
- 20.Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart. 2013;99:1146–53. doi: 10.1136/heartjnl-2012-303177. [DOI] [PubMed] [Google Scholar]
- 21.Ntusi NBA, Wonkam A, Shaboodien G, et al. Frequency and clinical genetics of familial dilated cardiomyopathy in Cape Town: Implications for the evaluation of patients with unexplained cardiomyopathy. South African Med J. 2011;101:394–8. [PubMed] [Google Scholar]
- 22.Bloomfield GS, Lagat DK, Akwanalo OC, et al. Waiting to inhale: An exploratory review of conditions that may predispose to pulmonary hypertension and right heart failure in persons exposed to household air pollution in low- and middle-income countries. Glob Heart. 2012;7:249–59. doi: 10.1016/j.gheart.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidder A, Kwan GF, Cancedda C, et al. Partners in health guide to chronic care integration for endemic non-communicable diseases: Rwanda edition. Boston: Partners In Health; 2011. [Google Scholar]
- 24.Jean-Baptiste E, Larco P, Charles-Larco N, et al. Glucose intolerance and other cardiovascular risk factors in Haiti (PREDIAH) Diabetes Metab. 2006;32:443–51. doi: 10.1016/s1262-3636(07)70302-6. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 26.Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–68. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–43. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 28.Center for Health Information and Analysis. Hospital-Wide Adult All-Payer Readmissions in Massachusetts: 2011–2013. Boston, MA: 2015. [Google Scholar]
- 29.Noel E, Esperance M, McLaughlin M, et al. Attrition from HIV testing to antiretroviral therapy initiation among patients newly diagnosed with HIV in Haiti. J Acquir Immune Defic Syndr. 2013;62:e61–9. doi: 10.1097/QAI.0b013e318281e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivers LC, Chang Y, Gregory Jerome J, et al. Food assistance is associated with improved body mass index, food security and attendance at clinic in an HIV program in central Haiti: a prospective observational cohort study. AIDS Res Ther. 2010;7:33. doi: 10.1186/1742-6405-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.