The transcription factor ZAT6 coordinately activates phytochelatin synthesis-related gene expression and directly targets GSH1 to positively regulate Cd accumulation and tolerance in Arabidopsis.
Abstract
Cadmium (Cd) is an environmental pollutant with high toxicity to animals and plants. It has been established that the glutathione (GSH)-dependent phytochelatin (PC) synthesis pathway is one of the most important mechanisms contributing to Cd accumulation and tolerance in plants. However, the transcription factors involved in regulating GSH-dependent PC synthesis pathway remain largely unknown. Here, we identified an Arabidopsis (Arabidopsis thaliana) Cd-resistant mutant xcd2-D (XVE system-induced cadmium-tolerance2) using a forward genetics approach. The mutant gene underlying xcd2-D mutation was revealed to encode a known zinc-finger transcription factor, ZAT6. Transgenic plants overexpressing ZAT6 showed significant increase of Cd tolerance, whereas loss of function of ZAT6 led to decreased Cd tolerance. Increased Cd accumulation and tolerance in ZAT6-overexpressing lines was GSH dependent and associated with Cd-activated synthesis of PC, which was correlated with coordinated activation of PC-synthesis related gene expression. By contrast, loss of function of ZAT6 reduced Cd accumulation and tolerance, which was accompanied by abolished PC synthesis and gene expression. Further analysis revealed that ZAT6 positively regulates the transcription of GSH1, GSH2, PCS1, and PCS2, but ZAT6 is capable of specifically binding to GSH1 promoter in vivo. Consistently, overexpression of GSH1 has been shown to restore Cd sensitivity in the zat6-1 mutant, suggesting that GSH1 is a key target of ZAT6. Taken together, our data provide evidence that ZAT6 coordinately activates PC synthesis-related gene expression and directly targets GSH1 to positively regulate Cd accumulation and tolerance in Arabidopsis.
Cadmium (Cd) is one of the most dangerous environmental pollutants with high toxicity to animals and plants (Raskin et al., 1997; Lanphear, 1998; Kim et al., 2007; Shim et al., 2009; Lin and Aarts, 2012; Clemens et al., 2013; Dias et al., 2013). Cd has been demonstrated to inactivate or denature proteins by binding to sulfhydryl groups, which causes cellular damages by displacing cofactors from a variety of proteins, including transcription factors and enzymes (Goyer, 1997; Sharma et al., 2000; Schützendübel et al., 2001; Lin and Aarts, 2012). Moreover, Cd also induces oxidative stress, which in turn mediates cellular damage in various plants and animals (Shim et al., 2009; Gallego et al., 2012).
Plants have evolved a number of potential mechanisms to act in detoxification and, thus, in tolerance to heavy metal stress (Clemens, 2001; Hall, 2002; Lin and Aarts, 2012; Gallego et al., 2012; Thapa et al., 2012). The detoxification of heavy metals in plants includes heavy metals being pumped out at the plasma membrane, chelated, or bound to various thiol compounds in the cytosol and sequestered into vacuoles (Clemens, 2001; Hall, 2002; Kim et al., 2006; Gallego et al., 2012). Other antioxidant and signaling mechanisms may also participate in the process (Clemens, 2001; Hall, 2002; Sandalio et al., 2012; Gill et al., 2013). In addition, a lot of key genes have been shown to be involved in Cd detoxification and tolerance in plants. These include ZNT1, OsHMA9, OsNRAMP5, ZntA, CAD2, AtPDR12, AtPDR8, AtATM3, ACBP1, Nramp5, EIN2, and MAN3 (Pence et al., 2000; Sharma et al., 2000; Shiraishi et al., 2000; Lee et al., 2003, 2007; Kim et al., 2006, 2007; Xiao et al., 2008; Cao et al., 2009; Lin and Aarts, 2012; Sasaki et al., 2012; Ishikawa et al., 2012; Chen et al., 2015). It was also found that Heat shock transcription factor A4a (HsfA4a) confers Cd tolerance by up-regulating MT gene expression in wheat (Triticum aestivum) and rice (Oryza sativa; Shim et al., 2009). Although these studies have unraveled some of the key players in Cd detoxification and tolerance in plants, the transcription factors that regulate the mechanisms of Cd detoxification have been poorly studied.
It is well documented that glutathione (GSH) is essential for Cd detoxification (Zhu et al., 1999; Xiang et al., 2001; Cobbett and Goldsbrough, 2002; Jozefczak et al., 2012, 2015; Sobrino-Plata et al., 2014b; Hernández et al., 2015; Flores-Cáceres et al., 2015), and GSH- or phytochelatin (PC)-conjugated vacuolar sequestration is one of the most important mechanisms contributing to Cd accumulation and tolerance in plants (Grill et al., 1989; Li et al., 1997; Cobbett and Goldsbrough, 2002; Lee et al., 2003; Lin and Aarts, 2012; Jozefczak et al., 2012). GSH is known to be synthesized from γ-Glu, with Cys and Gly being successively catalyzed by γ-glutamyl-Cys synthetase (encoded by GSH1) and glutathione synthetase (encoded by GSH2; May et al., 1998; Cobbett, 2000; Jozefczak et al., 2012), and PC is polymerized from GSH by phytochelatin synthase (PCS; encoded by PCS1 and PCS2 in Arabidopsis [Arabidopsis thaliana]), which is strongly induced by Cd stress (May et al., 1998; Noctor et al., 2002; Semane et al., 2007). The rate-limiting step of GSH biosynthesis is catalyzed by GSH1 (Jozefczak et al., 2012). Overexpression or inhibition of GSH1 expression causes Arabidopsis to have enhanced or depressed levels of glutathione, respectively (Cobbett et al., 1998; Xiang and Oliver, 1998; Xiang et al., 2001). Arabidopsis GSH-deficient cad2-1 mutant is cadmium sensitive (Cobbett et al., 1998). Thus, the regulation of GSH1 expression is fundamental to Cd detoxification. However, the transcription factors that directly regulate the transcription of GSH1 remain unknown.
The C2H2-type zinc-finger proteins (ZFPs) represent a large family of eukaryotic transcription factors. In Arabidopsis, a total of 176 proteins that contain one or more zinc-finger domain have been reported (Englbrecht et al., 2004), and many of them have been found to play important roles in plant development and stress responses (Englbrecht et al., 2004). Zinc finger of Arabidopsis thaliana 6 (ZAT6) was identified during the isolation and characterization of a diverse family of Arabidopsis two- and three-fingered C2H2 ZFPs (Meissner and Michael, 1997). ZAT6 expression has been shown to be regulated by phytohormones (Glazebrook et al., 2003). In addition, it was also found that ZAT6 is involved in regulating root development and low Pi stress responses (Devaiah et al., 2007). Recently, ZAT6 was shown to be involved in regulating responses of plants to pathogen infection, salt, drought, and freezing stresses (Shi et al., 2014). However, the possible role of ZAT6 in heavy metal detoxification remains uninvestigated in Arabidopsis.
In this study, we report a previously unknown function of ZAT6 in plant responses to Cd stress. We demonstrate that plants overexpressing ZAT6 manifested enhanced Cd tolerance, whereas loss of function of ZAT6 led to increased Cd sensitivity. Our data provide evidence that ZAT6 coordinately activates PC synthesis-related gene expression and directly targets GSH1 to regulate Cd tolerance in Arabidopsis.
RESULTS
Isolation of Gain-of-Function xcd2-D mutant
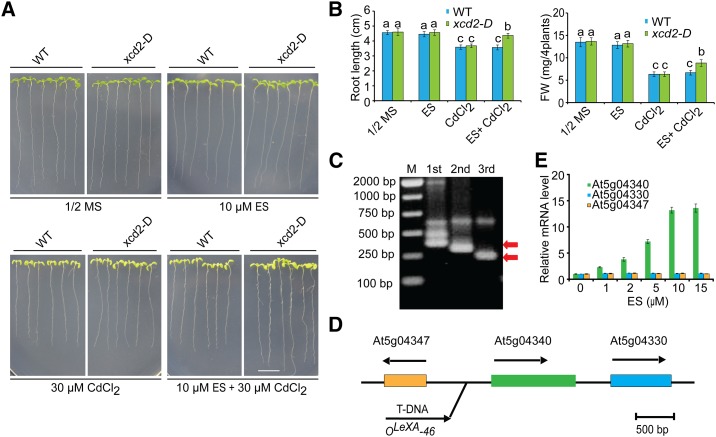
To screen for Cd-resistant mutants, we undertook a gain-of-function genetic screen (Chen et al., 2015) and isolated several putative mutants from the XVE-tagging T-DNA insertion lines (Zhang et al., 2005). One of these mutants, designated xcd2-D (XVE system-induced cadmium-tolerance2), was chosen for detailed analysis. To test their Cd tolerance, seeds of the wild type and the xcd2-D mutants were germinated on one-half-strength Murashige and Skoog (1/2 MS) agar plates for 3 d and then seedlings were transferred to 1/2 MS media with or without 30 μm CdCl2 and 10 μm estradiol for 11 d. When grown on 1/2 MS media with or without 10 μm estradiol, there was no significant difference between the wild type and the xcd2-D mutant (Fig. 1, A and B). Similarly, reduced growth was observed in both wild-type and xcd2-D mutant seedlings grown on 1/2 MS media containing 30 μm CdCl2 (Fig. 1, A and B). However, when the inducer estradiol was added to the 1/2 MS media containing 30 μm Cd, xcd2-D seedlings were more tolerant to Cd than the wild type (Fig. 1, A and B). These results suggest that the increased tolerance to Cd occurring in xcd2-D mutant is estradiol dependent.
Figure 1.
Cloning of the XCD2 gene. A, Phenotypes of the xcd2-D mutant under Cd stress. Three-day-old seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium with or without 30 μm CdCl2 and 10 μm estradiol (ES). Photographs were taken 11 d after transfer. Bar = 1 cm. B, Root length and fresh weight of the wild type and xcd2-D in A. Three independent experiments were done with similar results, each with three biological repeats. Four plants per genotype from one plate were measured for each repeat. Data are presented as means ± se, n = 3. Statistical significance was determined by ANOVA in combination with posthoc tests; significant differences (P ≤ 0.05) are indicated by different lowercase letters. B, TAIL-PCR analysis of the T-DNA insertional site in the xcd2-D mutant. Three rounds of TAIL-PCR were carried out using nested primers. These holes showed DNA markers, 1st, 2nd, and 3rd round products from left to right, respectively. DNA bands marked with red arrows were recycled for DNA sequencing. C, A schematic of the genomic region flanking the T-DNA insertion site in the xcd2-D mutant. Arrows indicate the orientation of transcription. D, qRT-PCR analysis of estradiol-induced transcription levels of neighboring genes including At5g04330, At5g04340 (XCD2), and At5g04347 in 2-week-old seedlings. ACTIN11 was used as the internal control. Data are presented as means ± se of three replicate experiments.
Using thermal asymmetric interlaced-PCR (TAIL-PCR; Liu et al., 1995), we identified a single T-DNA insertion in the xcd2-D mutant (Fig. 1C), which was located between At5g04347 and At5g04340, 540 bp upstream of the start codon of At5g04340 (Fig. 1D). To clarify which gene is dependent on β-estradiol induction in xcd2-D seedlings, we examined the expression levels of three neighboring genes in the presence of the inducer estradiol. qRT-PCR analysis showed that, in xcd2-D seedlings, the transcription level of At5g04340 was increased in an estradiol-dependent manner, whereas expression of the other two neighboring genes (At5g04347 and At5g04340) was independent on estradiol induction (Fig. 1E). Taken together, these results suggest that the Cd-tolerant phenotype of xcd2-D might be caused by the estradiol-induced expression of At5g04340, which was previously named as a known gene, ZAT6 (Devaiah et al., 2007).
Enhanced Expression of ZAT6 Is Responsible for the Increased Cd Tolerance
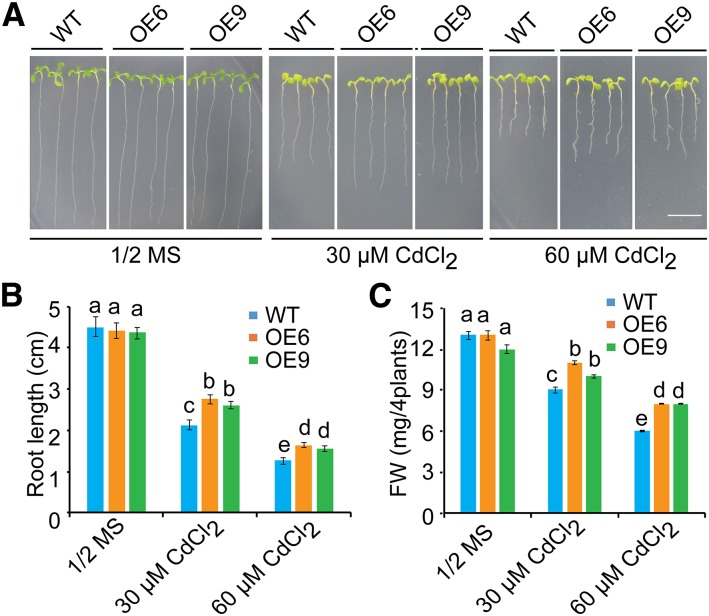
To confirm that the estradiol-induced expression of ZAT6 caused the Cd-tolerant phenotype of the xcd2-D mutant, we generated transgenic Arabidopsis overexpressing ZAT6 driven by the cauliflower mosaic virus 35S promoter and obtained more than 10 transgenic lines. In accordance with previous studies (Devaiah et al., 2007; Shi et al., 2014), overexpression of ZAT6 retards plant growth in lines with strong ZAT6 expression (Supplemental Fig. S1). Therefore, two lines, OE6 and OE9, with moderate expression of ZAT6 (Supplemental Fig. S1B), which showed normal plant growth (Supplemental Fig. S1A), were chosen for further analysis in Cd tolerance. When grown on 1/2 MS media, there was no significant difference between the wild type and the ZAT6-overexpressing lines OE6 and OE9; however, when grown on 1/2 MS containing 30 or 60 μm Cd, these lines showed increased tolerance to Cd stress compared with the wild type (Fig. 2A). Quantitative analyses confirmed that the root length of ZAT-OE lines (OE6 and OE9) was significantly longer than that of the wild type in the presence of Cd (P < 0.05; Fig. 2C), and that the fresh weights of the wild type and the ZAT6-overexpressing lines were similar in the 1/2 MS media, but in the Cd-containing media, the fresh weight of ZAT-OE lines was significantly higher than that of the wild type (P < 0.05; Fig. 2D). These results suggest that overexpression of ZAT6 is responsible for the enhanced Cd tolerance phenotype.
Figure 2.
Cd tolerance in the ZAT6 overexpression lines. A, Growth of the wild type and ZAT6 overexpression lines (OE6 and OE9) on 1/2 MS media with or without 30 or 60 μm CdCl2. Three-day-old seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium without or with 30 or 60 μm CdCl2. Photographs were taken 11 d after transfer. Bar = 1 cm. B and C, Root length (B) and fresh weight (C) of plants described in A. Three independent experiments were done with similar results, each with three biological repeats. Four plants per genotype from one plate were measured for each repeat. Data are presented as means ± se, n = 3. Statistical significance was determined by ANOVA in combination with posthoc tests; significant differences (P ≤ 0.05) are indicated by different lowercase letters.
Loss of Function of ZAT6 Led to Increased Cd Sensitivity
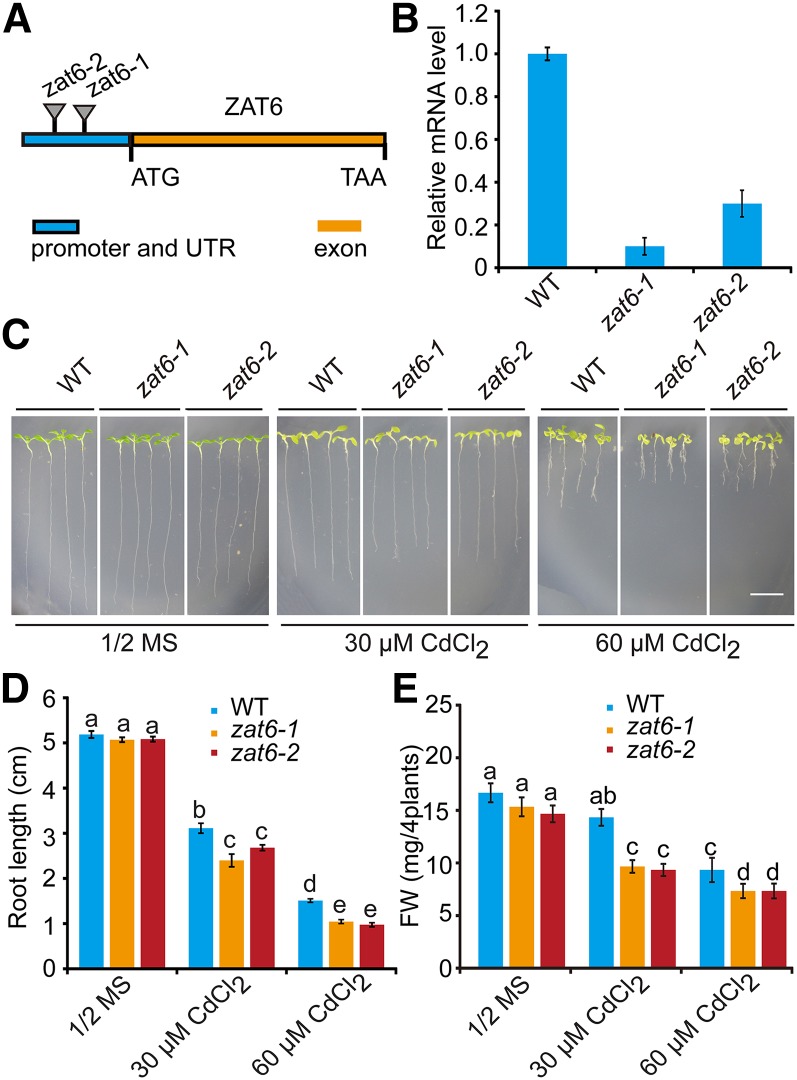
To further investigate the function of ZAT6 in vivo, we isolated two T-DNA insertional mutants, zat6-1 (SALK_061991C) and zat6-2 (SALK_050196), from the SALK T-DNA collection (Alonso et al., 2003). A single T-DNA is inserted at 359 or 427 bp upstream of ZAT6 coding region in zat6-1 or zat6-2, respectively (Fig. 3A). The two insertions resulted in significant reduction of ZAT6 transcripts (Fig. 3B). To test their Cd tolerance, seeds of the wild type and the zat6 mutants were germinated on 1/2 MS agar plates for 3 d and then seedlings were transferred to 1/2 MS media with or without 30 or 60 μm CdCl2 for 11 d. We found that both zat6-1 and zat6-2 mutants showed increased sensitivity to Cd stress compared with the wild type (Fig. 3C). Quantitative analyses further confirmed that, in the Cd-containing medium, both root length and fresh weight of zat6 mutant seedlings were significantly (P < 0.05) lower than those of wild-type seedlings (Fig. 3, D and E). These results suggest that ZAT6 plays an important role in regulating Cd tolerance in Arabidopsis.
Figure 3.
Cd hypersensitivity in the zat6 mutants. A, Schematic of T-DNA insertion sites on the locus of ZAT6 gene in the zat6 mutants. B, qRT-PCR analysis of the transcription level of ZAT6 in the wild type and the zat6 mutants. C, Phenotypes of the zat6 mutants under Cd stress. Three-day-old seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium without or with 30 or 60 μm CdCl2. Photographs were taken 11 d after transfer. Bar = 1cm. D and E, Root length (D) and fresh weight (E) of plants described in C. Three independent experiments were done with similar results, each with three biological repeats. Four plants per genotype from one plate were measured for each repeat. Data are presented as means ± se, n = 3. Statistical significance was determined by ANOVA in combination with posthoc tests; significant differences (P ≤ 0.05) are indicated by different lowercase letters.
To further determine whether ZAT6 is also involved in the regulation of other heavy metal and oxidative stresses, including Na3AsO4, Pb(NO3)2, ZnSO4, CuSO4, and H2O2, seeds of the wild type and the zat6 mutants were germinated on 1/2 MS agar plates for 3 d and then seedlings were transplanted to vertically placed 1/2 MS agar plates in the presence of heavy metals for 11 d. However, there was no significant difference between wild-type and zat6 mutant plants in response to Na3AsO4, Pb(NO3)2, ZnSO4, CuSO4, HgCl2, and H2O2 (Supplemental Fig. S2). Together, these results suggest that ZAT6 might be specifically involved in regulating Cd tolerance in our tested experiments.
Inducible Expression of ZAT6 by Cd Treatment
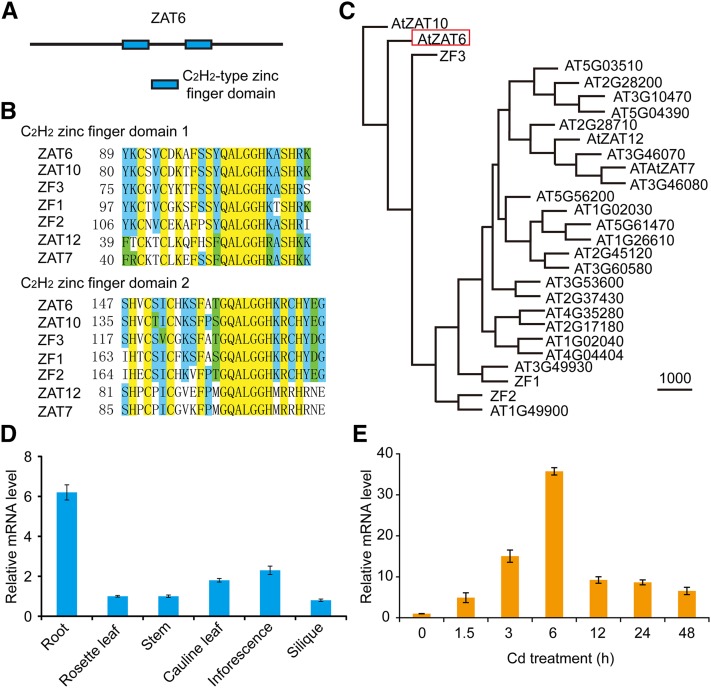
The ZAT6 gene contains only one exon and encodes a 238-amino acid protein with two C2H2-type zinc-finger domains (Fig. 4A), both of which share ∼80% sequence similarity with other zinc-finger proteins, such as ZF1, ZF2, ZF3, ZAT7, ZAT10, and ZAT12 (Fig. 4, B and C). The ZAT6 transcripts were detected in all organs tested, but the highest level was in roots (Fig. 4D). To examine its role in plant defense against Cd stress, we analyzed its expression in response to Cd treatment. qRT-PCR analysis shows that the transcript level of ZAT6 was enhanced as early as 1.5 h after Cd treatment, and it rose up to 30-fold after 6 h under Cd pressure (Fig. 4E). Cd-induced expression of ZAT6 further supports its involvement in the regulation of Cd tolerance.
Figure 4.
Expression patterns of ZAT6 gene. A, Predicted motifs in ZAT6. Rectangles mean C2H2-type zinc-finger domains in ZAT6. None of other motifs with annotation was found. B, The similarity in amino acid sequences of zinc-finger domains between ZAT6, ZAT7, ZAT10, ZAT12, ZF1, ZF2, and ZF3. C, Phylogenic tree of ZAT6 and its homologs in Arabidopsis. ZAT6 is marked by a red rectangle. D, qRT-PCR analysis of ZAT6 transcription in different tissues of wild-type plants. mRNAs were isolated from roots, rosette leaves, cauline leaves, inflorescences, stems, and siliques of 6-week-old wild-type plants. ACTIN11 was used as the internal control. E, Expression of ZAT6 in response to Cd stress. Two-week-old wild-type seedlings grown on 1/2 MS media were treated with CdCl2 (60 μm) for 0, 1.5, 3, 6, 12, 24, and 48 h and then the tissues were harvested for qRT-PCR analysis. ACTIN11 was used as the internal control. Data are presented as means ± se of three replicate experiments.
ZAT6 Positively Regulates Cd Tolerance through the GSH-Dependent Pathway
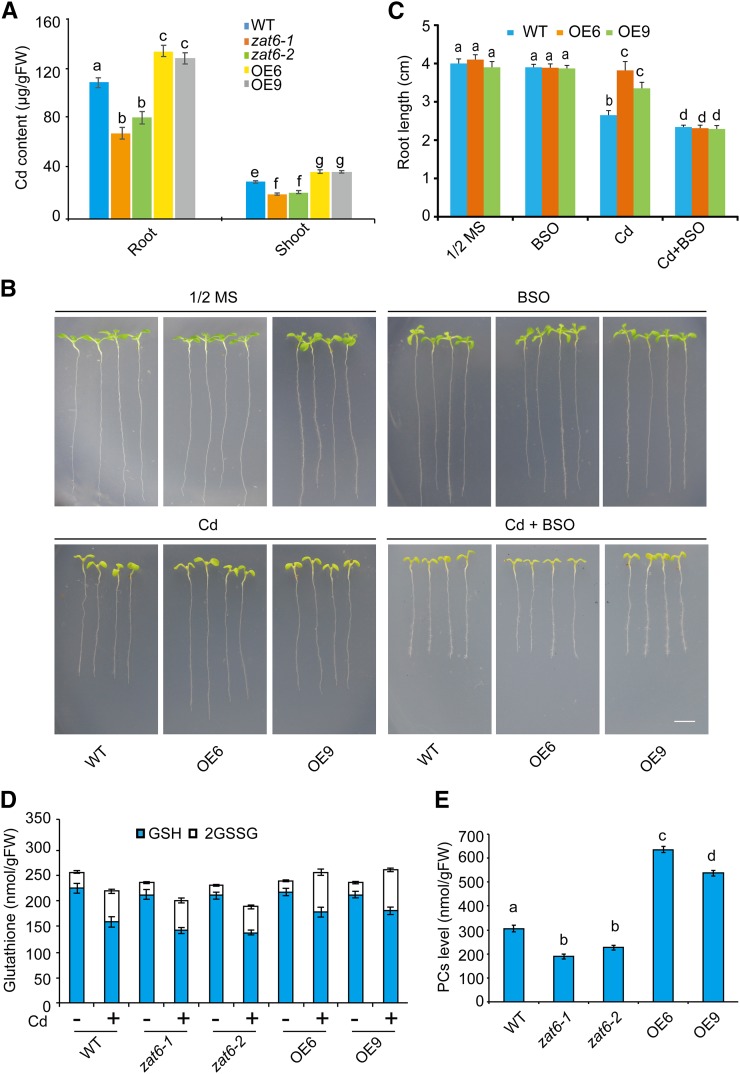
To test whether ZAT6-mediated Cd tolerance is associated with alternation of Cd content, we measured Cd content in wild-type, ZAT6-OE, and zat6 mutant seedlings under Cd stress and found that Cd content was significantly increased in ZAT6-OE lines (∼23% in OE6 and ∼17% in OE9, P < 0.05) but was decreased in zat6 mutant plants under Cd stress (∼38% in zat6-1 and ∼25% in zat6-2, P < 0.05; Fig. 5A). These results suggest that enhanced expression of ZAT6 accelerates Cd accumulation in plants.
Figure 5.
ZAT6 regulates Cd tolerance through the GSH-dependent pathway. A, Measurements of Cd contents in the wild type, zat6 mutants, and ZAT6-OE lines. These plants were grown on 1/2 MS media with 60 μm CdCl2 for 2 weeks, and roots and shoots of these samples were collected respectively for Cd content measurements. B, Effect of BSO on growth of wild-type and ZAT6-OE plants. Three-day-old seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium with or without 60 μm CdCl2 or 0.1 mm BSO for 7 d. Bar = 5 mm. C, Effect of BSO on root length of wild-type and ZAT6-OE plants described in B. Three independent experiments were done with similar results, each with three biological repeats. Four plants per genotype from one plate were measured for each repeat. Data are presented as means ± se, n = 3. D and E, Glutathione (D) and PC (E) contents in the ZAT6-OE lines and the zat6 mutant plants. The wild type, the ZAT6-OE lines, and the zat6 mutant plants were grown on 1/2 MS media for 2 weeks and then treated with (+) or without (−) 60 μm CdCl2 for 24 h, and their GSH (D) and PC (E) contents were quantified. Data are presented as means ± se of three replicate experiments. Statistical significance was determined by ANOVA in combination with posthoc tests; significant differences (P ≤ 0.05) are indicated by different lowercase letters.
An important pathway of heavy metal detoxification in plants is the GSH-dependent PC synthesis pathway that confers plants to tolerable Cd accumulation (Grill et al., 1989; Li et al., 1997; Noctor et al., 2002; Lee et al., 2003; Kim et al., 2006; Verbruggen et al., 2009a, 2009b). Therefore, we analyzed the growth of wild-type and ZAT6-OE plants in 1/2 MS media containing buthionine sulfoximine (BSO), an inhibitor of GSH synthesis (Kim et al., 2006). As shown in Figure 5B, in the BSO-containing media, similar growth in wild-type and ZAT6-OE plants was observed. When grown in media containing CdCl2, ZAT6-OE plants showed increased Cd tolerance than the wild type; however, when BSO was added to the media containing CdCl2, the improved growth rate of the ZAT6-OE plants over wild-type plants to Cd stress disappeared (Fig. 5B), representing as parameters of root length of these plants (Fig. 5C). These results suggest that the GSH-dependent PCs synthesis pathway is bona fide involved in regulation of ZAT6-mediated Cd tolerance.
Next, we measured the intracellular levels of glutathione in wild-type, ZAT6-OE, and zat6 mutant plants treated with or without Cd. There was no significant difference in total glutathione (GSH plus 2GSSG) between the wild type, zat6 mutants, and ZAT6-OE lines without Cd treatment (P > 0.05; Fig. 5D). When challenged by Cd stress, GSH concentrations decreased significantly in the wild type, ZAT6-OE, and zat6 mutant plants; however, a reduced decrease was observed in ZAT6-OE lines than in wild-type plants (P < 0.05; Fig. 5D). The total PC content was also measured in the wild type, zat6 mutants, and ZAT6-OE lines under Cd treatment. There were significantly reduced PC contents in zat6 mutants (∼62% in zat6-1 and ∼74% in zat6-2, P < 0.05), while there were elevated PC contents in ZAT6-OE lines (∼2.1-fold in OE6 and ∼1.8-fold in OE9, P < 0.05; Fig. 5E) compared to the wild type. These data support the notion that ZAT6 positively regulates Cd tolerance through the GSH-dependent PC synthesis pathway.
ZAT6 Positively Regulates the Expression of PCs Synthesis-Related Genes
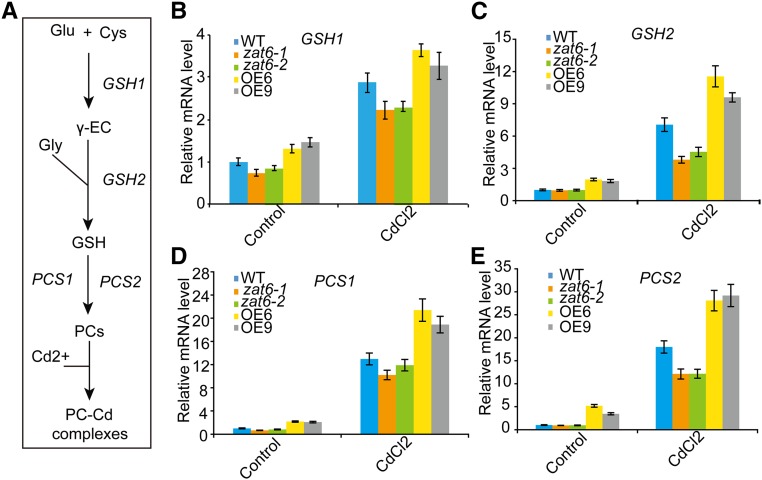
To examine whether the alteration of ZAT6 expression levels could affect the transcription of PC synthesis genes (Fig. 6A), we isolated total RNA from wild-type, ZAT6-OE, and zat6 mutant seedlings and performed qRT-PCR analyses. Our results clearly indicated that the expression levels of GSH1, GSH2, PCS1, and PCS2 were elevated in the ZAT6-OE seedlings but reduced in the zat6 mutant seedlings in both the absence and presence of Cd (Fig. 6B). These results support the notion that ZAT6 coordinately regulates the transcription of PC synthesis genes.
Figure 6.
qRT-PCR analysis of the genes involved in PC synthesis. A, Scheme of GSH/PC synthesis and genes involved in this process. B to E, Quantitative analysis of transcription of genes involved in GSH/PC synthesis in the zat6 mutants and ZAT6-OE lines, including GSH1 (B), GSH2 (C), PCS1 (D), and PCS2 (E). The wild type, the zat6 mutants, and ZAT6-OE lines were grown on 1/2 MS media for 2 weeks, treated with 60 μm CdCl2 for 6 h, and then their mRNAs were isolated for qRT-PCR analysis. ACT11 was used as internal control. Data are presented as means ± se of three replicate experiments.
In addition, we also analyzed expression pattern of other Cd stress-related genes, including GR1, GR2, ABCC1, ABCC2, PDR8, and ATM3 (Xiang and Oliver, 1998; Kim et al., 2006, 2007; Song et al., 2010). qRT-PCR results showed that transcription of GR1 and PDR8 was upregulated in ZAT6-OE lines and downregulated in zat6 mutants (Supplemental Fig. S3). There was no significant difference (P > 0.05) in the transcript level of GR2, ABCC1, ABCC2, and ATM3 between the wild type, ZAT6-OE lines, and zat6 mutant plants treated with or without Cd (Supplemental Fig. S3).
GSH1 Is a Direct Target of ZAT6
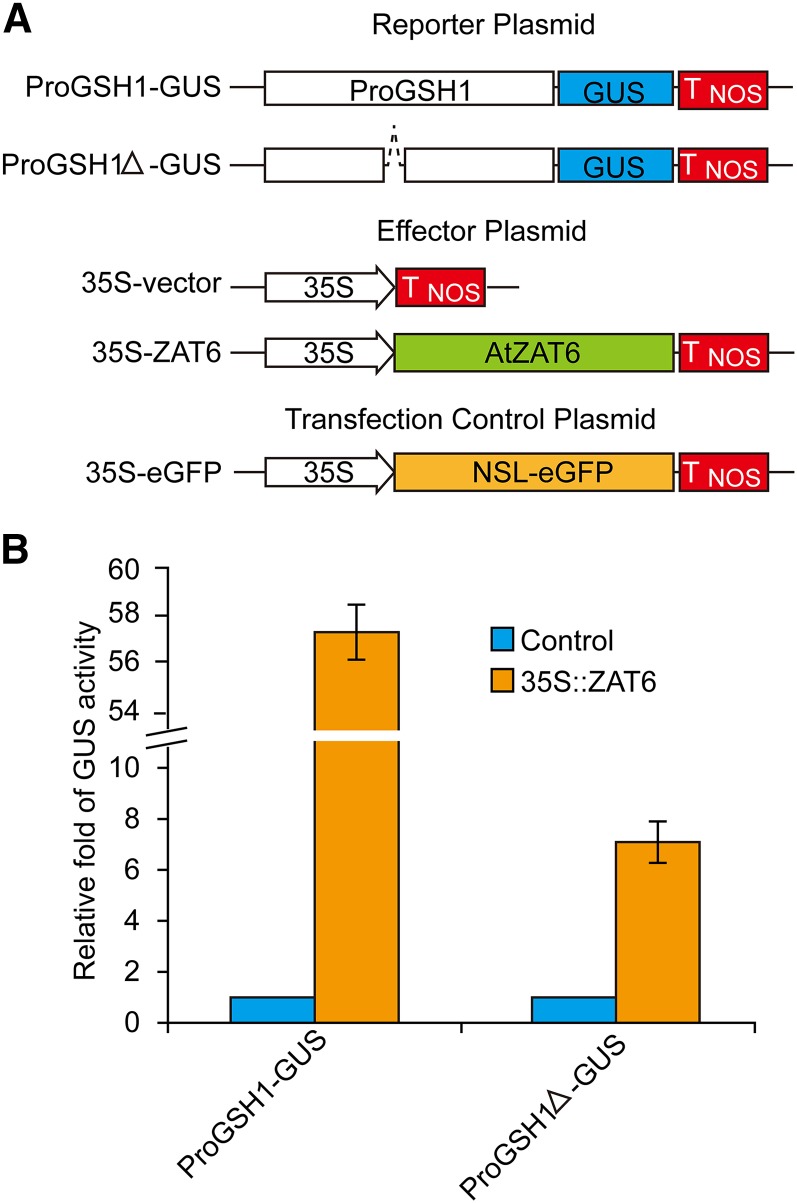
To further test whether ZAT6 activates transcription of GSH1, GSH2, PCS1, PCS2, GR1, and PDR8, transient expression analysis was carried out in Nicotiana benthamiana based on the reported procedure (Yang et al., 2000). As shown in Figure 7, A and B, GUS expression driven by GSH1 promoter was significantly enhanced after coexpression of ZAT6. However, the expression of ProGSH2::GUS, ProPCS1::GUS, ProPCS2::GUS, ProGR1::GUS, and ProPDR8::GUS was not changed by coexpressing of ZAT6 (Supplemental Fig. S4). These results suggest that GSH1 promoter could be specifically activated by ZAT6.
Figure 7.
Transient expression of GSH1-promoter reporters in N. benthamiana. A, Schematic of the GSH1-promoter reporter constructs, the effector plasmids, and the transfection control plasmid. ProGSH1-GUS or ProGSH1Δ-GUS plasmids represent GUS reporters driven by full-length or TACAAT-box-deleted GSH1-promoter, respectively. B, Relative folds of GUS activity of ProGSH1-GUS or ProGSH1Δ-GUS reporters after coexpression of ZAT6 protein. The 35S-empty vector was used as effector plasmid control. Data are presented as means ± se of three replicate experiments.
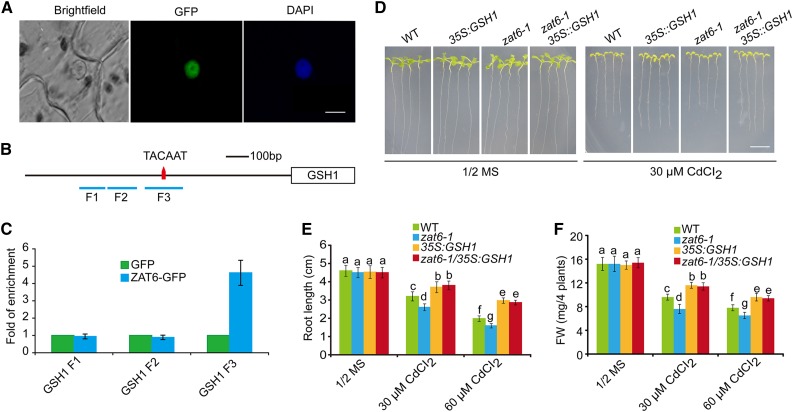
It has been showed that ZAT6 can directly bind to TACAAT box (Shi et al., 2014). Therefore, we searched ZAT6-binding cis-element in the promoters of its candidate target genes and found that GSH1, GSH2, and PCS1 each contains a TACAAT box in its promoter (Fig. 8; Supplemental Fig. S5). To test whether ZAT6 can bind directly to the promoters of GSH1, GSH2, and PCS1 in vivo, we performed a chromatin immunoprecipitation (ChIP; Kaufmann et al., 2010) assay using 35S::ZAT6:GFP transgenic plants. Consistent with previous report (Devaiah et al., 2007), this fusion protein was localized in the nucleus (Fig. 8A). Enrichments of three fragments within GSH1 promoter region, with fragments F1 and F2 located upside the TACAAT box and fragment F3 around it (Fig. 8B), were measured using qRT-PCR. We found that only fragment F3 is significantly enriched with GFP antibody (P < 0.05; Fig. 8C). Other fragments within GSH2 and PCS1 promoter regions were also measured but no significant enrichment was detected (Supplemental Fig. S5). These data support the hypothesis that ZAT6 directly binds to the promoter of GSH1.
Figure 8.
ZAT6 directly binds to the promoter region of GSH1. A, Nuclear localization of ZAT6-GFP in epidermal cells. Bar = 5 μm. B, Schematic diagram of the promoter region of GSH1. Red triangle represents the locus of TACAAT box. Blue lines, including F1, F2, and F3, show the region of ChIP-qPCR primer pairs. C, Quantitative real-time PCR assay of DNA after ChIP. Input DNAs were used as internal control. D, GSH1 overexpression restores the Cd-hypersensitive phenotype in zat6-1 mutant. Three-day-old wild-type, zat6-1, 35S::GSH1, and zat6-1/35S::GSH1 seedlings grown on 1/2 MS medium were transferred to 1/2 MS medium without or with 30 μm CdCl2. Photographs were taken 11 d after transfer. Bar = 1 cm. E and F, Root length (E) and fresh weight (F) of plants described in D. Three independent experiments were done with similar results, each with three biological repeats. Four plants per genotype from one plate were measured for each repeat. Data are presented as means ± se, n = 3. Statistical significance was determined by ANOVA in combination with posthoc tests; significant differences (P ≤ 0.05) are indicated by different lowercase letters.
To examine the importance of ZAT6-binding sequence in the GSH1 promoter, the TACAAT box in the F3 fragment was removed from the GSH1 promoter region to build up the ProGSH1Δ::GUS construct (Fig. 7A). The ProGSH1Δ::GUS reporter activity was significantly decreased than the ProGSH1::GUS reporter after coexpression of ZAT6 (P < 0.05; Fig. 7B), indicating that TACAAT box contributes to transcriptional activation of GSH1 promoter by ZAT6.
To genetically verify these results, we overexpressed GSH1 in the background of zat6-1 and found that the Cd-sensitive phenotype of the zat6-1 mutant was rescued by overexpression of GSH1 (Fig. 8D; Supplemental Fig. S6). This result was also confirmed by quantitative analyses of both root length and fresh weight (Fig. 8, E and F). These results demonstrate that ZAT6 indeed acts upstream of GSH1.
DISCUSSION
In this study, we cloned and characterized a zinc-finger transcription factor, ZAT6, that acts as a positive regulator in Cd tolerance in Arabidopsis. C2H2 zinc-finger proteins have been shown to be required for key cellular processes including transcriptional regulation, development, pathogen defense, and stress responses (Laity et al., 2001; Englbrecht et al., 2004; Ciftci-Yilmaz and Mittler, 2008; Zhou et al., 2011). ZAT6 contains a typical C2H2 domain and has been shown to be induced by cold and osmotic stress (Vogel et al., 2005). It was also found that ZAT6 is involved in regulating root development and Pi stress responses (Devaiah et al., 2007) and responses of pathogen infection, salt, drought, and freezing stresses (Shi et al., 2014). In this study, we found that ZAT6 is involved in Cd tolerance in Arabidopsis. The expression level of ZAT6 was strongly induced by Cd stress, which is consistent with the results of previous studies (Herbette et al., 2006; Weber et al., 2006). Moreover, transgenic plants overexpressing ZAT6 showed enhanced Cd tolerance, while zat6 mutant plants displayed increased Cd sensitivity, suggesting that ZAT6 plays an important role in Cd tolerance. Interestingly, it was previously shown that putative ZAT6 RNAi mutants showed the nongerminating phenotype (Devaiah et al., 2007). However, we identified T-DNA insertion mutants, zat6-1 and zat6-2, from the Salk T-DNA collection (Alonso et al., 2003) and found that zat6 mutants showed increased Cd sensitivity. One possible explanation is that ZAT6 homologous genes essential for seed germination might be off-targets suppressed by RNAi-mediated silencing pathway.
A GSH-dependent pathway is one of the most important mechanisms contributing to heavy metal tolerance (Lee et al., 2003; Sobrino-Plata et al., 2014a, 2014b; Hernández et al., 2015; Flores-Cáceres et al., 2015; Jozefczak et al., 2015). GSH, as the metabolic precursor of the heavy metal chelating PCs, plays an important role in the heavy metal detoxification in plants (Cobbett and Goldsbrough, 2002; Ball et al., 2004; Kim et al., 2006). An Arabidopsis mutant with a reduced capacity to produce GSH, cad2, is hypersensitive to Cd (Howden et al., 1995a, 1995b; Cobbett et al., 1998), while overexpression of GSH1 results in enhanced Cd tolerance and accumulation (Zhu et al., 1999). In this study, we observed that, under Cd stress, ZAT6-overexpressing and zat6 mutant plants displayed similar phenotypes as GSH1-overexpressioning (Zhu et al., 1999) and cad2-1 mutant (Howden et al., 1995a, 1995b; Cobbett et al., 1998) plants, respectively, and that overexpression of GSH1 restored normal Cd sensitivity in the zat6-1 mutant (Fig. 8), suggesting that ZAT6 acts as upstream of GSH1. Further analysis showed that BSO treatment completely eliminates the enhanced Cd tolerance phenotype caused by overexpression of ZAT6 (Fig. 5), suggesting that ZAT6-mediated enhanced Cd tolerance is GSH dependent. qRT-PCR analysis showed that the expression of GSH1 is positively regulated by ZAT6, and transient expression experiments showed that ZAT6 activates GSH1 promoter activity, suggesting that ZAT6 transcriptionally targets GSH1 to regulate Cd tolerance. Furthermore, although we failed in EMSA and yeast one-hybrid experiments possibly due to lack of protein phosphorylation, which is necessary for ZAT6 (Liu et al., 2013), ChIP assays showed that the ZAT6 protein can specifically bind to the promoter of GSH1 in plants. These results collectively demonstrate that GSH1 is a key target gene under transcriptional control of ZAT6. Thus, ZAT6 positively regulates Cd tolerance directly by activating GSH1 transcription.
Glutathione metabolic genes have been demonstrated to coordinately respond to heavy metals (Xiang and Oliver, 1998). Furthermore, overexpression of GSH1, PCS1, or PCS2 is effective in improving plant tolerance upon Cd stress (Zhu et al., 1999; Cazalé and Clemens, 2001; Pomponi et al., 2006; Gasic and Korban, 2007; Brunetti et al., 2011), and simultaneous expression of PC synthesis genes leads to higher Cd accumulation and tolerance (Wawrzyński et al., 2006). In our study, we found that ZAT6 functions in Cd accumulation and tolerance, and up-regulation of ZAT6 leads to elevated expressions of GSH1, GSH2, PCS1, and PCS2 (Fig. 6), demonstrating that ZAT6 may coordinately regulate PC synthesis genes to effectively improve Cd tolerance. In contrast with relationships between ZAT6 and GSH1, transient expression assay showed that coexpression of ZAT6 didn’t elevate transcriptions of GSH2, PCS1, and PCS2 (Supplemental Fig. S4); in accordance with these results, ChIP-qPCR results showed that DNA fragments around the TACAAT box were not enriched in promoter regions of GSH2 and PCS1 (Supplemental Fig. S5), which was possibly due to differences in flanking sequence (Supplemental Table S1). These results suggested that transcriptions of GSH2, PCS1, and PCS2 might be indirectly regulated by ZAT6.
PCs are known to play an important role in Cd accumulation and detoxification through PC-conjugated vacuolar sequestration (Grill et al., 1989; Li et al., 1997; Cobbett, 2000; Cobbett and Goldsbrough, 2002; Lee et al., 2003; Lin and Aarts, 2012). PCs are also known to be synthesized by GSH1, GSH2, PCS1, and PCS2 in Arabidopsis (May et al., 1998; Cobbett, 2000; Noctor et al., 2002; Semane et al., 2007; Jozefczak et al., 2012). In our study, we found that ZAT6 directly regulates GSH1 and coregulates GSH2, PCS1, and PCS2 (Fig. 6), leading to reduced PCs in zat6 mutants and elevated PCs in ZAT6 overexpression lines (Fig. 5, D and E). Interestingly, we also found that PDR8, a known Cd pump in Arabidopsis (Kim et al., 2007), was regulated by ZAT6 (Supplemental Fig. S3). As PDR8 leads to pump-out of Cd from the cytosol (Kim et al., 2007), there would be less Cd accumulation in plants. However, although AtZAT6 overexpression lines exerted significant Cd tolerance, they showed similar growth in comparison with wild-type plants when they were treated with BSO (Fig. 5), suggesting that ZAT6-mediated Cd tolerance is GSH dependent. Moreover, transient expression assay showed that GSH1 is a direct target of ZAT6, while PDR8 is indirectly regulated by ZAT6 (Fig. 7; Supplemental Fig. S4). Therefore, ZAT6 acts mainly through regulation of GSH/PC synthesis, leading to more contribution of Cd chelation than through Cd detoxification. That might explain the phenomenon of reduced/elevated Cd accumulation in zat6 mutants and ZAT6 overexpression lines (Fig. 5A), respectively.
Plant responses to Cd stress involve the transcriptional regulation of numerous genes to establish an adaptive mechanism (Herbette et al., 2006; Weber et al., 2006; Shim et al., 2009). Several Arabidopsis transcription factors have been shown to be induced by heavy metal stress in plants; however, their physiological roles in heavy metal tolerance have not been clearly demonstrated (Herbette et al., 2006; Shim et al., 2009). A few transcriptional factors, such as CaPF1 and HsfA4a, were identified functioning in the Cd stress response (Tang et al., 2005; Shim et al., 2009). Overexpression of CaPF1, an ERF/AP2 pepper (Capsicum annuum) transcription factor, in transgenic VA pine (Pinus virginiana) confers tolerance to heavy metals Cd, Cu, and Zn, to heat, and to pathogens Bacillus thuringiensis and Staphylococcus epidermidis (Tang et al., 2005). The CaPF1-mediated Cd tolerance was mediated by protection from oxidative damage, which is implicated in general stress tolerance (Tang et al., 2005). In contrast, HsfA4a regulates Cd tolerance by upregulating metallothionein gene expression (Shim et al., 2009). Metallothionein is a potential target gene of HsfA4a because it is a good chelator of Cd and Cu but is not effective in the detoxification of other heavy metals (Shim et al., 2009). Thus, HsfA4a greatly enhanced Cd tolerance in yeast and rice but did not significantly enhance their tolerance to the other heavy metals, namely, Pb, Zn, Co, Mn, Ag, Hg, and Fe (Shim et al., 2009). In this study, we found that zat6 mutant plants showed enhanced sensitive to Cd but not to Pb, Cu, As, Zn, and H2O2 (Supplemental Fig. S2). To further confirm the results, we also test tolerance of the wild type and the ZAT6-overexpressing lines OE6 and OE9 to Na3AsO4, ZnSO4, Pb(NO3)2, and HgCl2. However, there was no significant difference between the wild type and the ZAT6-overexpressing lines in response to Na3AsO4, ZnSO4, Pb(NO3)2, and HgCl2 (Supplemental Fig. S7). In addition, we also found that the expression of ZAT6 was highly induced by CdCl2, but only slightly induced by other heavy metals (Supplemental Fig. S8). Together, these results suggest that ZAT6 might be specifically involved in regulating Cd tolerance in our tested experiments. Significantly, however, our data showed that GSH1 is a key target gene under transcriptional control of ZAT6. One possible explanation for this phenomenon is that plant responses to Pb, As, Zn, Hg, Cu, and H2O2 are regulated by some other regulatory factors. Another possible explanation is that translational and posttranslational regulation of GSH1 may exist in controlling GSH concentration because GSH can engage in thiol-disulphide exchange reactions that may be a key process in linking the regulation of gene expression to the redox state of cells or specific subcellular compartments (Schafer and Buettner, 2001; Noctor et al., 2002). In plants, the regulatory processes are known to be potentially influenced by the levels or redox state of cellular GSH pools (May et al., 1998; Vernoux et al., 2000).
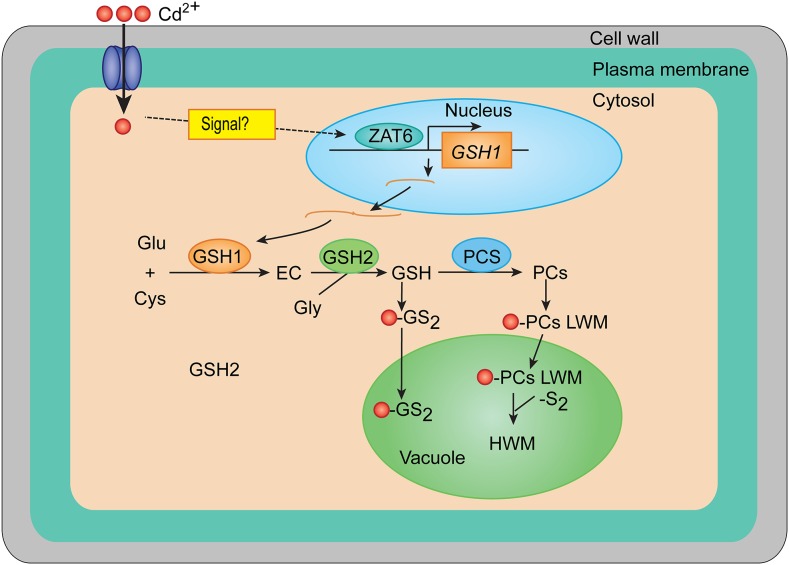
In summary, we cloned and characterized a C2H2 zinc-finger transcription factor, ZAT6, which is induced by Cd stress and plays a key role in regulating Cd stress response by directly targeting GSH1 expression, leading to Cd tolerance and accumulation in Arabidopsis (Fig. 9). The identification of ZAT6 provides a first step in the elucidation of mechanisms underlying GSH biosynthesis in response to Cd stress. Identifying and characterizing other target genes and their promoters should further shed light on the molecular mechanisms of the action of ZAT6. Moreover, it will also be important to study the upstream signaling pathways that connect Cd stress to activation of ZAT6.
Figure 9.
Working model for the role of ZAT6 in regulating Cd accumulation and tolerance. Cd stress quickly induces the expression of ZAT6, which directly binds to the promoter of GSH1 and actives its expression, thereby triggering Cd-activated PC synthesis, which results in enhanced Cd accumulation and tolerance.
MATERIALS AND METHODS
Plant Materials
Plant materials used in this study include Arabidopsis (Arabidopsis thaliana) wild-type Columbia-0 (Col-0) and the zat6 mutants (zat6-1, Salk_061991C; zat6-2, Salk_050196; Alonso et al., 2003). The xcd2-D mutant was screened from T3 populations (18,000 lines) of the XVE-tagging T-DNA insertional lines (Zhang et al., 2005); the pooled seeds of XVE lines were kindly provided by Dr. Jianru Zuo.
Screening, Identification, and Phenotype Analysis of Mutants
The xcd2-D mutant was screened as reported before (Chen et al., 2015). The XVE T-DNA-tagged genomic sequence in xcd2-D was identified by TAIL-PCR (Liu et al., 1995; Zuo et al., 2000; Sun et al., 2003) and DNA sequencing. The xcd2-D and zat6 mutant plants were individually identified through genetic methods using primer pairs of ZAT6-LB and ZAT6-RB (Supplemental Table S2).
All plants were vernalized for 3 d in the dark at 4°C and then were grown in a growth chamber maintained at 22°C, with a light intensity of ∼100 µmol m−2 s−1 and a 16-h daylength. Seeds of the wild type and mutants or transgenic plants were germinated on 1/2 MS agar plates for 3 d, and then seedlings were transplanted to grow on vertically placed 1/2 MS agar plates in the absence or presence of heavy metals or other supplements for 11 d. To reduce variation due to the precipitation of heavy metals, wild-type and mutant or transgenic plants were grown side-by-side in the same plate and their growth was compared. After the indicated days of growth, plants were sampled for root growth assays and measurement of fresh weight.
Vector Construction and Transformation
The GSH1, GSH2, PCS1, PCS2, GR1, and PDR8 full-length promoters were amplified by PCR from Arabidopsis genomic DNA. These promoters were cloned into the transformation vector pXB93 (pART27 with expanded restriction sites and GUS reporter) at the KpnI and XhoI restriction sites using specific primers (Supplemental Table S2). The ProGSH1-GUS construct was built through overlap-extension PCR (Ho et al., 1989) using primer pair of GSH1Δ-P1/GSH1Δ-P2 together with GSH1pro-S/GSH1pro-AS.
ZAT6 and GSH1 cDNA was amplified from Arabidopsis with primer pairs of ZAT6OE-S/ZAT6OE-AS, ZAT6GFP-S/ZAT6GFP-AS, or GSH1OE-S/GSH1OE-AS by RT-PCR. These products were cloned into the KpnI and XbaI sites of pXB94 (pART27 with expanded restriction sites, 35S promoter and GFP reporter) or pCambia1301. The ZAT6 and GSH1 overexpression constructs were generated by ligation with the KpnI-XbaI fragment from pPXB94 or pCambia1301. All primer sequences used for vector constructions are listed in Supplemental Table S2.
The 35S::ZAT6, 35S::ZAT6:GFP, and 35S::GSH1 constructs were introduced into the Agrobacterium tumefaciens GV3101 strain, which was then used to transform the wild-type Col-0 or the zat6-1 mutant using the flower infiltration method (Clough and Bent, 1998). Subcellular localization of fluorescent fusion protein was visualized using confocal microscopy (Leica TCS-SP8; Wetzlar). All transgenic lines used in this study are T3 homozygous plants with single-copy insertion. More than 10 lines of GSH1 overexpression were obtained and four of these lines (lines 1, 2, 3, and 4) were quantified using qRT-PCR (Supplemental Fig. S6). GSH1 overexpression line 4 was chosen to cross with the zat6-1 mutant and homozygous lines of double mutant were isolated for further analysis.
RNA Extraction and qRT-PCR Analysis
Six-week-old plants were used for quantification of ZAT6 transcription in different tissues, and 2-week-old seedlings were used for analysis of expression of ZAT6 in mutants or overexpression lines. Fresh samples were collected for RNA extraction. Total RNA was extracted using Trizol reagent (Invitrogen). cDNA was synthesized from total RNA by SuperScript II RNase H2 reverse transcriptase (Invitrogen) using Random Hexamer Primer (Promega). Quantitative real-time PCR was performed according to the instructions provided for the Bio-Rad iCycler iQ system (Bio-Rad Laboratories) with platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). The fold change of transcripts was calculated based on an efficiency calibrated model (Yuan et al., 2006) and compared with the transcript level under normal condition. Statistical differences between samples were evaluated by Student’s t test or ANOVA in combination with posthoc test using delta Ct values (Yuan et al., 2006). The ACTIN11 transcript was used to quantify the relative transcript level of each target gene in each tissue type. The primers used are listed in Supplemental Table S2.
Measurement of Cd and GSH/GSSG/PC Content
Plants were grown on 1/2 MS media (Murashige and Skoog, 1962) for 2 weeks, and their seedlings were treated with 60 µm Cd for 24 h and then sampled for analysis of Cd and GSH/GSSG/PC content. Cd content was determined according to the method described by Lee et al. (2003). Digested samples were analyzed using an atomic absorption spectrometer (Solaar M6; Thermo Fisher). GSH/GSSG/PC were extracted from seedlings and quantitated according to previous report (Chen et al., 2015).
Transient Expression Assays in Nicotiana benthamiana
Transient expression assays were performed according to the method described previously (Sparkes et al., 2006; Chen et al., 2009). A. tumefaciens cells were harvested by centrifugation and suspended in the solutions containing 50 mm MES, 5 g/L d-Glc, 2 mm Na3PO4, and 0.1 mm acetosyringone to an optical density (600 nm) of 0.1. A. tumefaciens cells were incubated at room temperature for 4 h and then used to infiltrate leaves of N. benthamiana using a needle-free syringe. The GUS staining and GUS activity measurements were performed at 48 h after injection as described previously (Xu et al., 2006).
ChIP
ChIP experiments were performed according to published protocols (Kaufmann et al., 2010). About 3 g of 7-d-old 35S::ZAT6:GFP plants and 35S::GFP control plants were harvested and then sonicated with ultrasonic cell disruption (JY96-II; output 3, 6 × 10 s). After that, the solution was divided into three parts: One was saved as input DNA, and the two other parts were incubated with anti-GFP antibody (Abmart). The relative concentrations of the DNA fragments were analyzed by quantitative real-time PCR in triplicates using the ACTIN11 gene as the reference, and enrichment fold was calculated as previously described (Jun et al., 2010; Kaufmann et al., 2010). Primers used are listed in Supplemental Table S2.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GeneBank/EMBL databases under the following accession numbers: ZAT6 (At5g04340), GSH1 (At4g23100), GSH2 (At5g27380), PCS1(At5g44070), PCS2 (At1g03980), GR1 (At3g24170), GR2 (At3g54660), ABCC1 (At1g30400), ABCC2 (At2g34660), PDR8 (At1g59870), ATM3(At5g58270), and ACTIN11 (At3g12110).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Growth of ZAT6 overexpression lines.
Supplemental Figure S2. Growth of the wild type and zat6 mutants under other abiotic stress conditions.
Supplemental Figure S3. qRT-PCR of other Cd stress-responsive genes.
Supplemental Figure S4. Transient expression of other Cd stress-responsive genes.
Supplemental Figure S5. ChIP-qPCR of GSH2 and PCS1.
Supplemental Figure S6. The transcript levels of GSH1 in GSH1-OE lines.
Supplemental Figure S7. Growth of wild-type and ZAT6-overexpression lines under other heavy metal stresses.
Supplemental Figure S8. Expression of ZAT6 in response to other heavy metal stresses.
Supplemental Table S1. Flanking sequences around TACAAT box.
Supplemental Table S2. Primers used for cloning, RT-PCR, and ChIP assay.
Supplementary Material
Acknowledgments
We thank the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, for providing the XVE-inducible activation lines. We also thank Bin Yu, Li Jiang, Lingxia Guan, Weiping Li, Na Li, Ju Gu, and Wenjia Ma for their technical assistances. We thank Mengxiang Sun and Xiongbo Peng for providing the pXB93 and pXB94 vectors.
Glossary
- PC
phytochelatin
- GSH
glutathione
- 1/2 MS
one-half-strength Murashige and Skoog
- TAIL-PCR
thermal asymmetric interlaced-PCR
- BSO
buthionine sulfoximine
- ChIP
chromatin immunoprecipitation
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 31370295, 31300989, and 31571250), the Research Fund for Doctoral Program of Higher Education of China (20120111110009), the Fundamental Research Fund for the Central Universities (2014HGCH0001), and the Anhui Provincial Natural Science Foundation (1308085QC56).
Articles can be viewed without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, Karpinski S, Mullineaux PM (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti P, Zanella L, Proia A, De Paolis A, Falasca G, Altamura MM, Sanità di Toppi L, Costantino P, Cardarelli M (2011) Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. J Exp Bot 62: 5509–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Chen Z, Liu G, Jiang L, Yuan H, Ren G, Bian X, Jian H, Ma X (2009) The Arabidopsis Ethylene-Insensitive 2 gene is required for lead resistance. Plant Physiol Biochem 47: 308–312 [DOI] [PubMed] [Google Scholar]
- Cazalé AC, Clemens S (2001) Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett 507: 215–219 [DOI] [PubMed] [Google Scholar]
- Chen J, Yang L, Gu J, Bai X, Ren Y, Fan T, Han Y, Jiang L, Xiao F, Liu Y, Cao S (2015) MAN3 gene regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis thaliana. New Phytol 205: 570–582 [DOI] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21: 3554–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S. (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18: 92–99 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett CS. (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG (2007) Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol 145: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35: 1281–1289 [Google Scholar]
- Englbrecht CC, Schoof H, Böhm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Cáceres ML, Hattab S, Hattab S, Boussetta H, Banni M, Hernández LE (2015) Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci 233: 165–173 [DOI] [PubMed] [Google Scholar]
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83: 33–46 [Google Scholar]
- Gasic K, Korban SS (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64: 361–369 [DOI] [PubMed] [Google Scholar]
- Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63: 254–261 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang H-S, Nawrath C, Métraux J-P, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34: 217–228 [DOI] [PubMed] [Google Scholar]
- Goyer RA. (1997) Toxic and essential metal interactions. Annu Rev Nutr 17: 37–50 [DOI] [PubMed] [Google Scholar]
- Grill E, Löffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL. (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53: 1–11 [PubMed] [Google Scholar]
- Herbette S, Taconnat L, Hugouvieux V, Piette L, Magniette MLM, Cuine S, Auroy P, Richaud P, Forestier C, Bourguignon J, et al. (2006) Genome-wide transcriptome profiling of the early cadmium response of Arabidopsis roots and shoots. Biochimie 88: 1751–1765 [DOI] [PubMed] [Google Scholar]
- Hernández LE, Sobrino-Plata J, Montero-Palmero MB, Carrasco-Gil S, Flores-Cáceres ML, Ortega-Villasante C, Escobar C (2015) Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J Exp Bot 66: 2901–2911 [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59 [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995a) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS (1995b) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA 109: 19166–19171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczak M, Bohler S, Schat H, Horemans N, Guisez Y, Remans T, Vangronsveld J, Cuypers A (2015) Both the concentration and redox state of glutathione and ascorbate influence the sensitivity of Arabidopsis to cadmium. Ann Bot (Lond) 116: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13: 3145–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Ha CM, Fletcher JC (2010) BLADE-ON-PETIOLE1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating ASYMMETRIC LEAVES2. Plant Cell 22: 62–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Østerås M, Farinelli L, Krajewski P, Angenent GC (2010) Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat Protoc 5: 457–472 [DOI] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Kushnir S, Noh EW, Martinoia E, Lee Y (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140: 922–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50: 207–218 [DOI] [PubMed] [Google Scholar]
- Laity JH, Lee BM, Wright PE (2001) Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 11: 39–46 [DOI] [PubMed] [Google Scholar]
- Lanphear BP. (1998) The paradox of lead poisoning prevention. Science 281: 1617–1618 [DOI] [PubMed] [Google Scholar]
- Lee S, Kim YY, Lee Y, An G (2007) Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol 145: 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131: 656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA 94: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Aarts MG (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69: 3187–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Nguyen XC, Kim KE, Han HJ, Yoo J, Lee K, Kim MC, Yun DJ, Chung WS (2013) Phosphorylation of the zinc finger transcriptional regulator ZAT6 by MPK6 regulates Arabidopsis seed germination under salt and osmotic stress. Biochem Biophys Res Commun 430: 1054–1059 [DOI] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8: 457–463 [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver CJ, Van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J Exp Bot 49: 649–667 [Google Scholar]
- Meissner R, Michael AJ (1997) Isolation and characterisation of a diverse family of Arabidopsis two and three-fingered C2H2 zinc finger protein genes and cDNAs. Plant Mol Biol 33: 615–624 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA 97: 4956–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomponi M, Censi V, Di Girolamo V, Paolis AD, Toppi LS, Aromolo R, Costantino P, Cardarelli M (2006) Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 223: 180–190 [DOI] [PubMed] [Google Scholar]
- Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8: 221–226 [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Rodríguez-Serrano M, Gupta DK, Archilla A, Romero-Puertas MC, Río LA (2012) Reactive oxygen species and nitric oxide in plants under cadmium stress: from toxicity to signaling. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. Ahmad, P and Prasad, MNV, eds, Springer, New York, pp 199–215. [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24: 2155–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212 [DOI] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semane B, Cuypers A, Smeets K, Opdenakker K, Keunen E, Remans T, Horemans N, Vanhoudt N, et al. (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129: 519–528 [Google Scholar]
- Sharma R, Rensing C, Rosen BP, Mitra B (2000) The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J Biol Chem 275: 3873–3878 [DOI] [PubMed] [Google Scholar]
- Shi H, Wang X, Ye T, Chen F, Deng J, Yang P, Zhang Y, Chan Z (2014) The cysteine2/histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and C-REPEAT-BINDING FACTOR genes in Arabidopsis. Plant Physiol 165: 1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim D, Hwang JU, Lee J, Lee S, Choi Y, An G, Martinoia E, Lee Y (2009) Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 21: 4031–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi E, Inouhe M, Joho M, Tohoyama H (2000) The cadmium-resistant gene, CAD2, which is a mutated putative copper-transporter gene (PCA1), controls the intracellular cadmium-level in the yeast S. cerevisiae. Curr Genet 37: 79–86 [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino-Plata J, Carrasco-Gil S, Abadía J, Escobar C, Álvarez-Fernández A, Hernández LE (2014a) The role of glutathione in mercury tolerance resembles its function under cadmium stress in Arabidopsis. Metallomics 6: 356–366 [DOI] [PubMed] [Google Scholar]
- Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014b) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377: 369–381 [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1: 2019–2025 [DOI] [PubMed] [Google Scholar]
- Sun J, Niu QW, Tarkowski P, Zheng B, Tarkowska D, Sandberg G, Chua NH, Zuo J (2003) The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that is involved in de novo cytokinin biosynthesis. Plant Physiol 131: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Charles TM, Newton RJ (2005) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol Biol 59: 603–617 [DOI] [PubMed] [Google Scholar]
- Thapa G, Sadhukhan A, Panda SK, Sahoo L (2012) Molecular mechanistic model of plant heavy metal tolerance. Biometals 25: 489–505 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H (2009a) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12: 364–372 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H (2009b) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181: 759–776 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, May MJ, Sung ZR (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41: 195–211 [DOI] [PubMed] [Google Scholar]
- Wawrzyński A, Kopera E, Wawrzyńska A, Kaminska J, Bal W, Sirko A (2006) Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. J Exp Bot 57: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Weber M, Trampczynska A, Clemens S (2006) Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd(2+)-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ 29: 950–963 [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Oliver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Gao W, Chen QF, Ramalingam S, Chye ML (2008) Overexpression of membrane-associated acyl-CoA-binding protein ACBP1 enhances lead tolerance in Arabidopsis. Plant J 54: 141–151 [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22: 543–551 [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN Jr (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu JX, Kong YZ, Ji ZD, Wang XC, An FY, Li C, Sun JQ, Zhang SZ, Yang XH, et al. (2005) [Generation of chemical-inducible activation tagging T-DNA insertion lines of Arabidopsis thaliana]. Yi Chuan Xue Bao 32: 1082–1088 [PubMed] [Google Scholar]
- Zhou ZJ, An LJ, Sun LL, Zhu SJ, Xi WY, Broun P, Yu H, Gan YB (2011) Zinc finger protein5 is required for the control of trichome initiation by acting upstream of zinc finger protein8 in Arabidopsis. Plant Physiol 157: 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121: 1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH (2000) Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.