Abstract
Purpose
Infusion of HER2 specific T-cells, derived from vaccine-primed patients and expanded with IL-2/IL-12, has induced tumor regression in a minority of patients with metastatic treatment-refractory HER2+ breast cancer. We questioned whether alteration of cytokine growth factors used to culture vaccine-primed T-cells could improve anti-tumor activity.
Experimental Design
Using the TgMMTV-neu murine mammary tumor model we cultured T-cells derived from mice immunized with a previously defined neu class II peptide, p98–114 (neu p98), and evaluated different cytokine combinations for expansion.
Results
Infusion of neu p98 specific T-cell lines derived from all cytokine conditions evaluated resulted in significant anti-tumor activity compared to infused naïve splenocytes (p<0.05). T-cells cultured with IL-2/IL-21 could uniquely mediate complete regression of spontaneous mammary tumors. IL-2/IL-21 cultured neu specific T-cells demonstrated a different cytokine secretion pattern as compared to other cultured T-cells; secreting high levels of TNF-alpha and IL-17 (p<0.05). Moreover, tumor infiltrating CD8+ cells were significantly increased after the infusion of IL-2/IL-21 cultured T-cells as compared to tumors treated with T-cells expanded under other cytokine conditions (p<0.001). The anti-tumor effect of the infusion of IL-2/IL-21 cultured cells was mediated by CD8 T-cells. Depletion of TNF-alpha or IL-17, but not IFN-gamma, abrogated the tumor growth inhibition induced by the IL-2/IL-21 T-cells and markedly decreased the influx of CD8 into tumors. Finally, IL-2/IL-21 cultured human antigen specific T-cells also displayed a similar polyfunctional Th1/Th17 phenotype.
Conclusion
Expansion of HER2 vaccine-primed T-cells with IL-2/IL-21 may have the potential to effectively mediate tumor regression when used in adoptive transfer.
Keywords: polyfunctional T-cell, adoptive T-cell therapy, IL-17, HER2, breast cancer, vaccine
Introduction
Adoptive T-cell therapy has shown clinical efficacy in patients with advanced melanoma, but has not been as effective in other solid tumors. Efforts to improve the clinical efficacy of T-cell transfer have focused on chimeric antigen receptor (CAR) engineered T-cells which have been enhanced to improve recognition of and interaction with tumor. Several hurdles have been identified in the clinical application of CAR to the treatment of solid tumors; the T-cells can be short-lived, high avidity CAR can interact with normal tissue resulting in off-target effects and autoimmunity, and the T-cells may become inactivated in the tumor microenvironment (1).
An alternative method of adoptive T-cell transfer is to use the patients’ autologous T-cells. Studies performed using autologous tumor infiltrating lymphocytes (TIL) in melanoma demonstrated a 50% response rate in patients with metastatic disease (2). Often, TIL are not available due to inability to access metastatic sites, for this reason vaccine-primed T-cells can be generated via active immunization, collected, and expanded ex vivo for therapeutic infusion. Antigen specific T-cells have been expanded after immunization to increase specificity for hTERT, survivin, MAGE-3 and HER2 and have shown some clinical benefit (3–5). The clinical efficacy of autologous T-cell infusions is hampered by the generation of lower avidity T-cells which slowly expand in vivo and also become inactivated in the immunosuppressive tumor microenvironment.
We questioned whether the anti-tumor efficacy of ex vivo expanded autologous vaccine-primed T-cells could be modulated via the cytokine culture conditions employed for expansion. A focus on CD4+ tumor specific Th1 offers several advantages over other T-cell populations. First, tumor antigen-specific CD4+ Th1 cells may home to the tumor and the inflammatory cytokines they secrete, such as IFN-gamma, may modulate the tumor microenvironment. Th1 cytokines enhance the function of local antigen presenting cells (APCs) and augment endogenous antigen presentation (6). Increased processing of endogenous tumor cells results in epitope spreading, the development of an immune response to the multiple immunogenic proteins expressed in the tumor (7). In addition, by providing a robust CD4+ Th1 T cell response, tumor-specific CD8+ T cells will be elicited and proliferate endogenously(8). Finally, antigen specific CD4+ T cells would provide the environment needed to enhance and sustain tumor specific T cell immune responses over time.
We evaluated a variety of cytokine combinations, all previously shown to have utility in T-cell culture, to determine whether specific cytokines could impact the phenotype and anti-tumor function of tumor specific T-helper-cells suitable for therapeutic infusion.
Methods and Materials
Mice and syngeneic tumor cell line
TgMMTV-neu mice (strain name, FVB/N-TgN(MMTVneu)-202Mul), 6–10 weeks of age, were obtained from Charles River Laboratory (Bar Harbor, ME) and bred under pathogen-free conditions at the University of Washington in compliance with Institutional Animal Care and Use Committee guidelines. The neu-expressing mouse mammary carcinoma (MMC) cell line has been previously described (9). MMC cells were maintained in RPMI/L-glutamine/HEPES medium (Mediatech, Manassas, VA), supplemented with 10% FCS (Gemini, CA), 1% Penicillin/Streptomycin (Mediatech, Inc.), and 55 μM beta-mercaptoethanol (Gibco, NY).
Generation of neu antigen specific T-cells
Female TgMMTV-neu mice (6–8 weeks) without palpable tumors were immunized s.c. 3 times (7–10 d apart) with 100 μg of neu peptide 98–114 (RLRIVRGTQLFEDKYAL; neu p98) (Genemed Synthesis Inc., San Antonio, TX). neu p98 has been shown to be a native MHC II epitope of the rat neu protein in TgMMTV-neu mice (10). Complete and incomplete freunds were used as adjuvants as previously described (11). Splenocytes were harvested 7–10 days after the last vaccine. For T-cell expansion, splenocytes were stimulated with 10 μg/ml of neu p98 at 3×106 cells/ml in culture media (RPMI/L-glutamine/HEPES medium supplemented with 10% FBS, % Penicillin/Streptomycin and 55 μM beta-mercaptoethanol). The stimulated cells were treated with IL-2 (10 U/ml) on Day 4 and re-stimulated with 10 μg/ml of neu p98 on Day 7. Respective cytokines were added on Days 9 and 13. Recombinant cytokines and their final concentrations were as follows: IL-2 (10 U/ml), IL-4 (50 U/ml), IL-7 (10 ng/ml), IL-12 (5 ng/ml), IL-15 (5 ng/ml), IL-18 (100 ng/ml), or IL-21 (100 ng/ml). All cytokines, except IL-2 (Hoffman-La Roche, Nutley, NJ) and IL-18 (MBL International, Woburn, MA), were purchased from PeproTech (Rocky Hill, NJ). T-cells were stimulated with soluble anti-CD3 antibody (50 ng/ml; eBioscience, San Diego, CA) on Day 19 and IL-2 (30 U/ml) was added every 2–3 days afterward. For in vivo studies, T-cells were infused 2–5 days after anti-CD3 activation.
Flow cytometry
Cultured T-cell lines were stained with fluorochrome-conjugated monoclonal antibodies against CD3 (1 μg), gamma-delta TCR, CD4, CD8, CD19, NK1.1 (0.5 μg each) for subset analysis (all antibodies from BD Bioscience, San Diego, CA). Cells were stained with FOXP3 Alexa488 (1 μg), CD3 PerCP (1 μg) and CD4 APC (0.5 μg) for T-regulatory cells (Treg) analysis according to FOXP3 staining protocol (eBioscience, San Diego, CA). Data acquisition was performed on a FACS Canto flow cytometer (BD Biosciences) and was analyzed using the FlowJo software (Tree Star, OR).
IFN-gamma ELISPOT
Cultured T-cell lines were plated at 2×105 per well into IFN-gamma pre-coated ELISPOT plates. neu p98 (10 μg/ml) was added into the cells in the presence of naïve splenocytes from TgMMTV-neu mice as antigen presenting cells. CD3 antibody (100 ng/ml; clone 145-2C11; eBioscience) was used as positive control. IGFBP-2 p251–265 (GPLEHLYSLHIPNCD; irrelevant peptide, 10 μg/ml) and medium only served as negative controls. The plates were cultured at 37°C/5%CO2 for 3 days, and colored IFN-gamma spots were developed and counted as previously described (11). Data was reported as corrected neu p98 antigen specific spots (number of mean spots in antigen wells minus spots in irrelevant peptide wells) per 2×105 T-cells (4–6 replicates). The IFN-gamma spots in medium only were similar to that in irrelevant antigen wells (p>0.05), and each positive control gave maximal levels of spots.
Adoptive T-cell transfer
T-cells (5×107 cells) were injected i.v. in TgMMTV-neu mice bearing palpable (100-mm3) spontaneous mammary tumors. Tumor growth was monitored every 2 to 3 days with Vernier calipers, and calculated as the product of length × width × height × 0.5236. Data on tumor growth were presented as the mean tumor size ± SEM of 5 mice per group. For other experiments, each cultured T-cell line (107) was admixed with live MMC cells (106) and implanted s.c. into the dorsal flank of TgMMTV-neu mice. Control (naïve) T-cells were derived from spleen preparations and infused at the same dose as neu p98 T-cells.
Cytokine analysis
Supernatants from cultured mouse T-cells were collected at the end of expansion and stored at −80°C. Quantification of secreted cytokines (IFN-gamma, TNF-alpha, IL-17, IL-5, IL-10 and IL-13) was performed using a Milliplext kit (Millipore, Billerica, MA) on a Luminex instrument (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Using the instrument software, the concentrations for the standards and samples were determined by a 5-parameter logistic method based on the mean fluorescent intensity. Experiments were repeated twice. Cytokine concentrations are reported in pg/ml.
Immunohistochemistry (IHC) staining
To evaluate tumor infiltrating CD8+ T-cells, tumors were frozen in Tissue-Tek OCT and stored at −80°C. Frozen tumors were stained with rat anti-mouse CD8 (clone KT15; AbD Serotec, Raleigh, NC) using previously described methods (12). CD8+ positive cells and DAPI stained nuclei were counted with a fluorescent microscope (Zeiss LSM 510 META, Carl Zeiss Microimaging Inc., Thornwood, NY). Five to 10 random microscopic fields per mouse were counted and the mean of positive cells per mouse obtained. The data were expressed as the mean number (± SEM) of positive cells per field, or % of positive cells over the total number of nuclei in the field for 5 mice/group.
in vivo cell subset depletions
Depletion of CD4, CD8, and B cells were performed by i.p. injection of monoclonal antibodies. Anti-CD8 (150 μg; Clone 2.43), anti-CD4 (250 μg; clone GK1.5), or anti-CD22 (100 μg; clone CY34.1.2) antibodies (UCSF) were injected into tumor-bearing mice which had received infusion of IL-2/IL-21 expanded T-cells. The mice received three consecutive injections of appropriate antibody in the first week followed thereafter by two injections weekly until the end of experiment. Tumor growth was measured as described above and presented as mean tumor growth (± SE) of 4 mice per group.
in vivo cytokine depletions
TgMMTV-neu mice bearing spontaneous tumors (100 mm3) were infused i.v. with 4×107 T-cells (day 0) and then injected with 100 μg neutralizing antibodies against IFN-gamma (clone 37895), or TNF-alpha (clone MP6-XT22), or IL-17 (clone 50104) i.p. on the following day and then every other day (days 1, 3, 5, 7, 9). Respective isotype control antibodies (IgG2a, clone 54447, for IL-17 and IFN-gamma; IgG1a, clone 43414, for TNF-alpha) were administered to control mice in an identical fashion. All antibodies were purchased from R&D systems (Minneapolis, MN), except for TNF-alpha (BioLegend, San Diego, CA). The same number of naive splenocytes was used as controls. Tumor growth was measured as described above and presented as mean tumor growth (± SE) of 3 mice per group. Experiments were repeated twice.
Gene expression analysis
To evaluate Granzyme B mRNA expression, spontaneous tumors were obtained from the mice 21 days after the infusions of neu p98 specific and naïve T-cells. Total RNA was extracted from the expanded T-cells using a RNA Easy Mini Extraction Kit (Qiagen, Valencia, CA) and the integrity of the RNA was confirmed using an Agilent BioAnalyzer (Foster City, CA). cDNA synthesis and real-time RT-PCR were performed as previously described (13) using primer and probes from Applied Biosystems (Carlsbad, CA). The levels of Granzyme B mRNA expression were normalized to b-actin using the delta Ct method (13).
To evaluate Th1/Th17 gene expression, CMV specific human T cells were expanded ex vivo with the different cytokine conditions of IL-2 (Hoffman-La Roche), IL-2/IL-12 (R&D System, Minneapolis, MN), and IL-2/IL-21 (Peprotech) using previously published methods (14). Total RNA was extracted and real-time RT-PCR was performed as above. The levels of mRNA expression of Th1/Th17 cytokine (IFN-gamma, TNF-alpha, IL-17, IL-21) and Th17-differentiation genes RORc and IFN-gamma regulatory gene IRF-4 were normalized to b-actin.
Human IL-17 and IFN-gamma ELISPOT
PBMC derived from a breast cancer patient after HER2 peptide vaccination were stimulated with three MHC class II epitopes (p369–384, p688–703, and p971–984; 10 μg/ml) (15) and cultured with IL-2/IL-21 as described above. The expanded T-cells were plated at 1×105/well in human IL-17 and IFN-gamma pre-coated ELISPOT plates (Mabtech, Mariemont, OH). The cells were re-stimulated with HER2 peptides loaded on autologous antigen presenting cells with no-antigen wells as control. After 48 hour culture, IFN-gamma spots were developed and counted as described (14) and IL-17 spots were developed using a biotinylated IL-17 antibody (Mabtech). Data are reported as mean spots/well of 5 replicates.
Statistical analyses
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA). The unpaired, two-tailed Student’s t-test was used to evaluate differences in tumor cell growth, T-cell responses in ELISPOT, cytokine production, numbers of tumor infiltrated CD8+ lymphocytes, and gene expression. The two-way analysis of variance (ANOVA) test was used to compare the tumor growth in TgMMTV-neu mice among experimental groups. A value of p<0.05 was considered statistically significant.
Results
Specific cytokines influence the number of antigen-specific T-cells and anti-tumor activity generated
We evaluated several common cytokines based on their potential benefit for ex vivo T-cell expansion including interleukin (IL)-2 alone (2, 16, 17) or IL-2 combined with IL-4 (18), IL-7 (19, 20), IL-15 (20, 21), IL-7/IL-15 (20), IL-12 (22), IL-18 (23), or IL-21 (24). The T-cell lines generated from all cultures were predominantly CD3+ (Supplemental Table 1A). Most T-cell lines had a higher percentage of CD8+ cells than CD4+ cells among the CD3+ T-cells except the IL-2 and IL-2/IL-12 cultured cells which were predominantly CD4+. CD4+FOXP3+ Treg were rarely expanded in all cytokine conditions (Supplemental Table 1B). The cytokines evaluated had a minimal impact on the overall fold expansion of CD3+ T-cells compared to IL-2 alone except for the IL-2/IL-7 culture condition (p<0.05) (Fig. 1A). All cytokine conditioned T-cells demonstrated a specific IFN-gamma response to neu p98 as compared to an irrelevant peptide derived from IGFBP-2 (p<0.05, Supplemental Fig. 1). After correcting for the irrelevant peptide responses, only IL-2/IL-15 conditioned T-cells generated significantly greater antigen specific IFN-gamma responses relative to IL-2 alone (p<0.01) (Fig. 1B). The culture condition using IL-2/IL-4 was the least successful in expanding antigen specific T-cells.
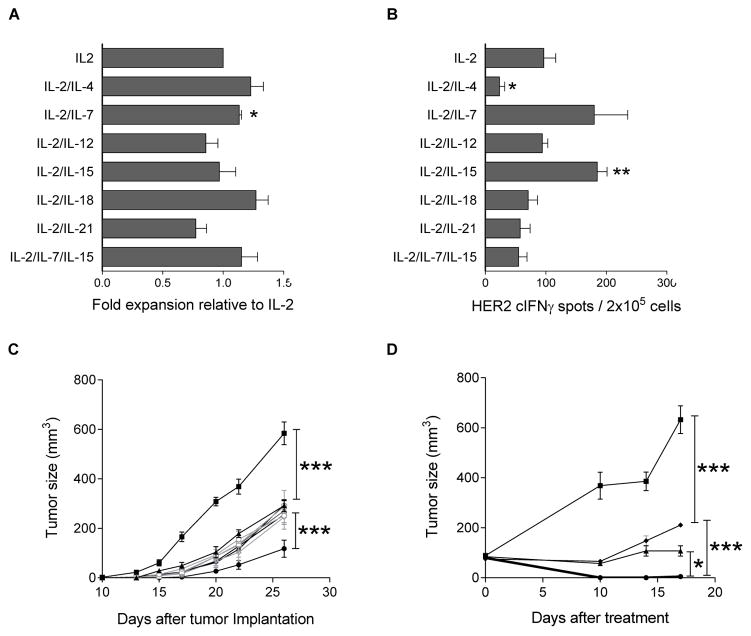
Figure 1. Specific cytokines influence the number of antigen-specific T-cells and anti-tumor activity generated.
(A) Cell growth under different cytokine conditions. Bars show mean fold expansions relative to IL-2 condition for all cytokine conditions. * indicates p<0.05. (B) neu p98 specific IFN-gamma secretion from T-cells cultured under different cytokine conditions. * indicates p<0.05; ** p<0.01 vs. IL2 only. Data is representative of two experiments. (C) Tumor growth (y-axis) over time (x-axis) in TgMMTV-neu mice implanted with MMC admixed with naïve splenocytes (-■-) or with T cell lines expanded in IL-2 (-▲-), or combined with IL-4 (-○□-), IL-7 (-□-), IL-12 (-△-), IL-15 (-▼□-), IL-18 (-◇□-), IL-21 (-●-), or IL-7/IL-15 (-◆-). Data points are the mean (± SE) of tumor size from five mice. (D) Tumor growth (y-axis) over time (x-axis) of spontaneous tumors developed in TgMMTV-neu mice treated with naïve splenocytes (■) or with T-cell lines expanded in IL-2 (▲), or combined with IL-21 (●), or IL-7/IL-15 (◆). Data points are the mean (± SE) of tumor size from 3 mice. * indicates p<0.05, *** p<0.001.
To test the effects of various cytokine culture conditions on the anti-tumor efficacy of neu p98 specific T-cells in vivo, the cultured T-cells from all conditions were admixed with live MMC cells and administered to TgMMTV-neu mice. As shown in Fig. 1C, the T-cell lines from all cytokine cultured conditions significantly inhibited the tumor growth (p<0.001; n=5) as compared to naïve splenocytes. Notably, only the lines cultured with IL-2/IL-21 induced a significant tumor inhibition greater than IL-2 alone and all other lines (p<0.001). We then infused the cultured T-cells into mice that had developed spontaneous mammary tumors. Similarly, the cultured T-cells from selected cytokine conditions tested significantly inhibited the tumor growth (p<0.001) as compared to naïve splenocytes. However, IL-2/IL-21 cultured T-cells were significantly more effective at mediating tumor inhibition as compared to IL-2 only (p<0.05) and IL-2/IL-7/IL-15 (p<0.001, n=3; Fig. 1D). Indeed, neu specific T-cells cultured in IL-2/IL-21 induced complete tumor regression.
T-cells expanded with IL-2/IL-21 demonstrate a polyfunctional Th1/Th17 phenotype
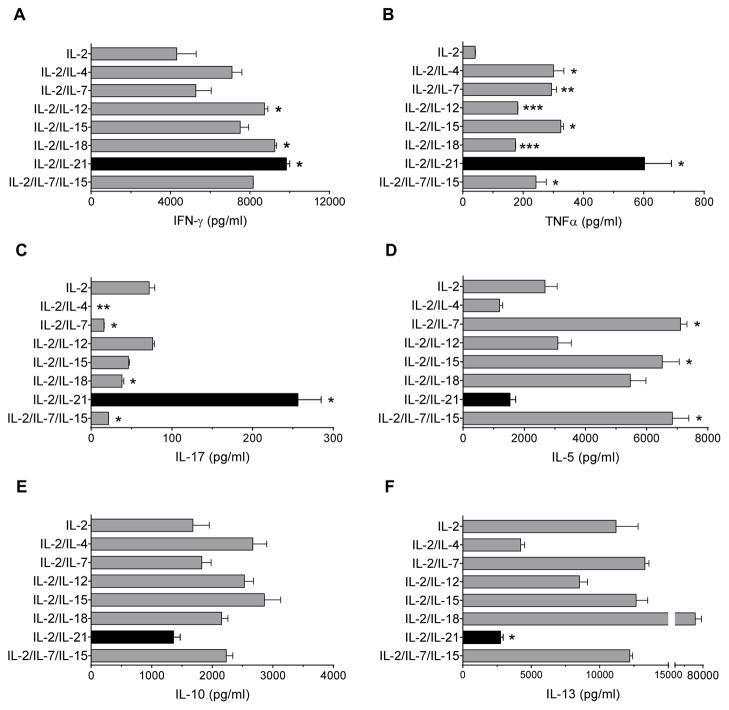
To further explore the potential differences in the T-cell lines that could be contributing to the variation in anti-tumor efficacy, we evaluated antigen specific cytokine secretion. As shown in Fig. 2A, the levels of IFN-gamma from IL-2/IL-12, IL-2/IL-18, and IL-2/IL-21 conditions were significantly increased as compared with IL-2 alone (p<0.05). The levels of TNF-alpha from all cytokine conditions were significantly greater than IL-2 alone (p<0.05). Notably, the level from the IL-2/IL-21 culture condition was strongly enhanced nearly 2-fold above all other conditions (Fig. 2B). Only IL-2/IL-21 cultured T-cells showed significantly greater levels of IL-17 than IL-2 alone (p<0.05) and all other culture conditions (vs. IL-2/IL-12, IL-2/IL-15, IL-2/IL-18, IL2/IL-7/IL-15, IL-2/IL-7, or IL-2/IL-4, p<0.05; Fig. 2C). Moreover, IL-2/IL-21 conditioned T-cells secreted significantly less IL-5 than IL-2/IL-7, IL2/IL-15, IL-2/IL-18, and IL-2/IL-7/IL-15 (p<0.05; Fig. 2D), and secreted significantly less IL-10 than IL-2/IL-4, IL2/IL-12, IL-2/IL-15, IL-2/IL-18, and IL-2/IL-7/IL-15 (p<0.05; Fig. 2E), although these were not significantly less than IL-2 alone. In addition, IL-2/IL-21 cultured T-cells secreted significantly less IL-13 than IL-2 alone (p<0.05) and all other cytokine conditions (vs. IL-2/IL-12, or IL-2/IL-18, p<0.05; vs. IL-2/IL-7, IL-2/IL-15, or IL-2/IL-7/IL-15, p<0.01) except vs. IL-2/IL-4 (Fig. 2F). Thus, T-cells expanded in IL-2/IL-21 exhibited a unique Th1/Th17 cytokine profile when compared to other culture conditions.
Figure 2. T cells expanded in IL-2/IL-21 condition demonstrate a polyfunctional Th1/Th17 phenotype.
Bars are the mean (± SE) of duplicates for all culture conditions. (A) IFN-gamma, (B) TNF-alpha, (C) IL-17, (D) IL-5, (E) IL-10, (F) IL-13. * p<0.05, ** p<0.01, *** p<0.001 vs. IL-2. Results shown are representative of two experiments.
The anti-tumor efficacy of IL-2/IL-21 neu-specific T-cells is mediated by CD8+ T-cells
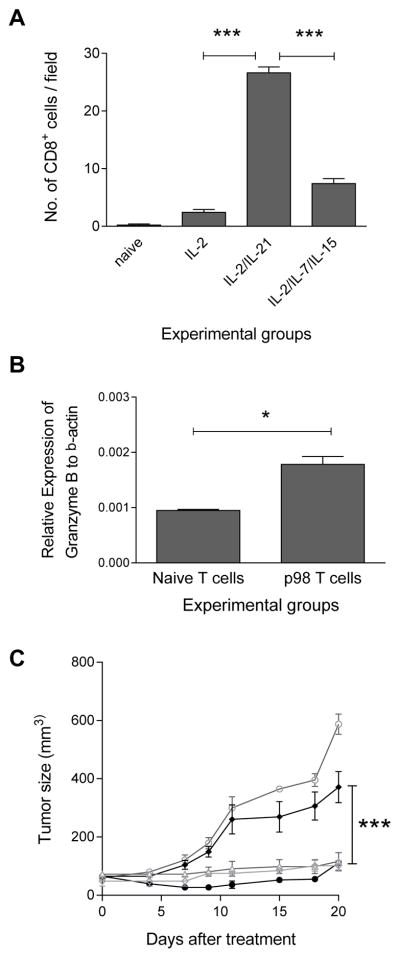
We evaluated the frequencies of tumor infiltrating CD8+ T cells after the infusion of IL-2, IL-2/IL-21, IL-2/IL-7/IL-15 cultured and naïve T-cells in mice bearing spontaneous tumors. As shown in Fig. 3A, the number of CD8+ T-cells in all cytokine cultured T-cell infused were significantly higher than that in naïve T-cell infused (mean ± SE: naïve, 0.2 ± 0.2; IL-2, 2.4 ± 0.5, p<0.01; IL-2/IL-21, 26.6 ± 1.0, p<0.001; IL-2/IL-7/IL-15, 7.4 ± 0.9, p<0.001; n=5). However, the tumor infiltrating CD8+ cells in mice infused with IL-2/IL-21 cultured T-cells were significantly increased compared to that in mice infused with IL-2 cultured T-cells (p<0.001) and IL-2/IL-7/IL-15 cultured T-cells (p<0.001). Supplemental Figure 2 shows a representative IHC staining for intratumoral CD8+ cells. We further analyzed the mRNA expression of Granzyme B within the spontaneous tumors after the T-cell infusions. As shown in Fig. 3B, the expression was significantly higher in p98 specific T-cells infused vs. naïve T-cells infused mice (p98 T-cells, 0.002 ± 0.000; naïve T cells, 0.001 ± 0.000; n=2; p<0.05).
Figure 3. The anti-tumor efficacy of IL-2/IL-21 neu-specific T-cells is mediated by CD8+ T-cells.
(A) Quantitation of CD8+ cells in tumors after infusion of IL-2, IL-2/IL-21, or IL-2/IL-7/IL-15 cultured p98 T-cells, or naïve T-cells. Columns and bars represent the mean (± SE) of 5 mice per group. ** indicates p<0.01; *** p<0.001. (B) Relative mRNA expression of Granzyme B to B-actin. Bars are the mean (± SE) of 2 mice per group, 3 replicates per mouse. *p<0.05. (C) Spontaneous tumors developed in TgMMTV-neu mice treated with naïve splenocytes (-○-), IL-2/IL-21 cultured T-cells only (-●-), or with anti-CD8 (-◆-), or with anti-CD4 (-▲-), or anti-CD22 antibody (-◇-). Data points are the mean of tumor size (± SE) from 4 mice. *** indicates p<0.001.
To determine which immune cell subsets mediated the antitumor effect of IL-2/IL-21 cultured T-cells, we then selectively depleted CD4+, CD8+ T cells or CD22+ cells after the infusion of the T-cells into TgMMTV-neu mice with spontaneous tumors. As shown in Fig. 3C, the elimination of CD8+ T-cells in mice significantly hindered the anti-tumor efficacy of IL-2/IL-21 cultured neu p98 specific T-cells (mean ± SE: T-cells only, 110±10, vs. with CD8 depletion, 371 ± 53; p<0.001), whereas the depletion of either CD4+ T-cells (116 ± 31; p>0.05) or CD22+ B-cells (101±19, p>0.05) demonstrated no impact.
The anti-tumor efficacy of IL-2/IL-21 cultured neu-specific T-cells is IL-17 and TNF-alpha dependent
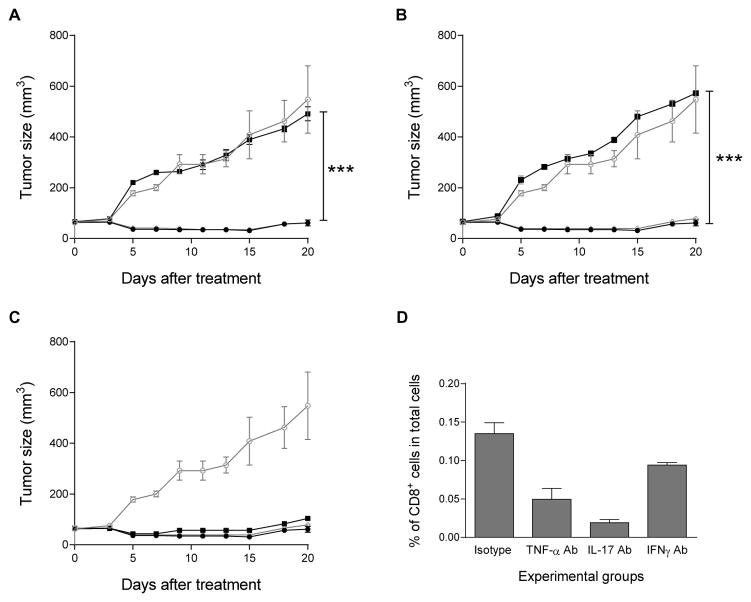
As IL-2/IL-21 cultured neu p98 specific T-cells demonstrated a unique cytokine profile and secreted high levels of IFN-gamma, TNF-alpha and IL-17, we questioned whether the therapeutic efficacy of the T-cells was diminished when these cytokines were blocked in vivo. The respective neutralizing cytokine antibodies or isotype controls were administered to TgMMTV-neu mice with spontaneous breast tumors 24 hours after the infusion of IL-2/IL-21 cultured T-cells. We found that neutralization of TNF-alpha significantly impaired T-cell efficacy, resulting in increased tumor volumes compared to control (mean ± SE of tumor size: IL-2/IL-21 only, 61 ± 12; vs. with TNF-alpha Ab, 491± 28; p<0.001; Fig 4A). Furthermore, the neutralization of IL-17 abolished the anti-tumor efficacy elicited by IL-2/IL-21 cultured T-cells (572 ± 7; p<0.001; Fig 4B). However, the neutralization of IFN-gamma had little impact on the anti-tumor effect elicited by the T-cells (104 ± 6; p>0.05; Fig. 4C). As expected, all isotype controls had no effect on the anti-tumor response (p>0.05 Fig 3A–3C). To determine whether the unique cytokine secretion pattern of neu specific T-cells cultured in IL-2/IL-21 contributed to enhanced CD8+ T-cell egress into the tumors, we examined the number of tumor infiltrating CD8+ T-cells after the neutralization of TNF-alpha, IL-17, or IFN-gamma respectively. Blocking any cytokine secretion resulted in a significant reduction in the number of tumor-infiltrating CD8+ T-cells compared to isotype control (mean ± SE of % of CD8+ T cells: control: 0.14 ± 0.00, vs. TNF-alpha, 0.05 ± 0.00, p<0.01; vs. IL-17, 0.02 ± 0.00, p<0.001; and vs. IFN-gamma, 0.09 ± 0.01. p<0.05). However, both TNF-alpha and IL-17 neutralization resulted in a significantly greater reduction of CD8+ T-cells within the tumor compared to IFN-gamma neutralization (p<0.01 and p<0.001 respectively; Fig. 4D).
Figure 4. The anti-tumor efficacy of IL-2/IL-21 cultured neu-specific T-cells is IL-17 and TNF-alpha dependent.
(A–C) Spontaneous tumors developed in TgMMTV-neu mice after infusion with naïve splenocytes (-○-), or with IL-2/IL-21 cultured T-cells alone (-●-), or with isotype control (-◇), or neutralizing anti-IL-17 (A), or TNF-alpha (B), or IFN-gamma (C) antibodies (-■-). Data points are the mean tumor size (± SE) of three mice. Data is representative of 2 separate experiments. *** indicates p<0.001. (D) Number of CD8+ cells % of total cells (y-axis) in isotype control, and TNF-alpha, IL-17 or IFN-gamma depleted mice after T-cell infusion (x-axis). Columns and bars represent the mean (± SE) of 5 mice per group. * p<0.05; ** p<0.01; *** p<0.001.
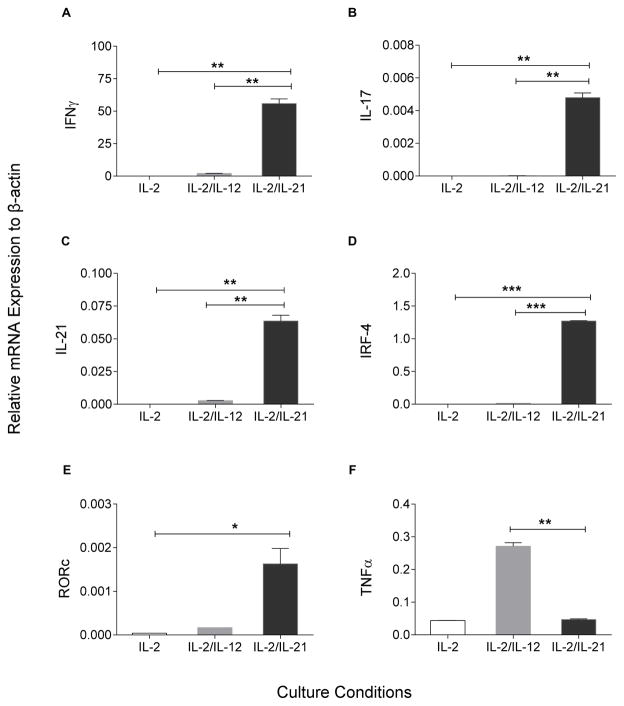
The expression of Th1/Th17 differentiation genes is increased in IL-2/IL-21 cultured CMV-specific T-cells
We cultured human CMV specific T-cells with IL-2, IL-2/IL-12, and IL-2/IL-21 to determine whether a similar phenotype could be induced. T-cells from a CMV+ donor cultured in IL-2/IL-21 showed a significant increase not only in IFN-gamma (Fig. 5A, p<0.01) but also in IL-17 (Fig. 5B, p<0.01), IL-21 (Fig. 5C, p<0.01) and IRF-4 (Fig. 5D, p<0.001) gene expression as compared to the other two culture conditions. In addition, expression of RORc was significantly increased vs. IL2, p<0.05; vs. IL-2/12, p=0.053 (Fig. 5E). Human T-cells cultured in IL-2/IL-21, however, demonstrated less TNF-alpha expression than IL-2/IL-12 cultured cells (Fig. 5F, p<0.01).
Figure 5. The expression of Th1/Th17 differentiation genes is increased in IL-2/IL-21 cultured CMV-specific T-cells.
Relative mRNA expression to B-actin (y-axis) in IL-2 (white columns), IL-2/IL-12 (grey), and IL-2/IL-21 (black) cultured condition (x-axis). (A) IFN-gamma, (B) IL-17; (C) IL-21, (D) IRF-4, (E) RORc, and (F) TNF-alpha. Bars are the mean (± SE) of duplicates for all culture conditions. * p<0.05; **p<0.01. and *** p<0.001.
We further examined whether polyfunctional human HER2 specific T-cells can be generated from a patients who had received a HER2 peptide based vaccine (15). The IL-2/IL-12 cultured T-cells were re-stimulated with HER2 peptide antigens with no added antigen as control. As shown in Supplemental Figure 3, both antigen specific IFN-gamma (HER2, 49 ± 4, no antigen, 1 ± 1; n=5; p<0.001) and IL-17 (HER2, 30 ± 4; no-antigen, 9 ± 2; n=5; p<0.01) secretion were significantly higher than controls.
Discussion
We used a single class II restricted epitope derived from the tumor antigen neu, p98–114, to vaccinate mice and generate neu specific T-cells to expand under different culture conditions. Our goal was to determine the role of cytokines in influencing the phenotype and enhancing the anti-tumor efficacy of autologous vaccine-primed tumor specific T-cells. Studies described here demonstrate that IL-2/IL-21, when used to culture either murine or human antigen specific T-cells, will induce a polyfunctional T-cell phenotype; Th1/Th17. Moreover, the IFN-gamma, TNF-alpha, IL-17 secreting cells generated in mice demonstrate potent anti-tumor activity mediated by CD8 T-cells. Finally, TNF-alpha and IL-17 were necessary for the anti-tumor response and secretion of these cytokines was associated with enhanced CD8 T-cell egress into mammary tumors.
Polyfunctional T-cells are more effective in eradicating tumors or viruses than T-cells that secrete a single dominant cytokine (25, 26). Antigen specific polyfunctional T-cells can amplify inflammation in the tumor microenvironment and, via diverse cytokine secretion, stimulate cross-priming by activating antigen presenting cells. The development of polyfunctional T-cells is associated with clinical response after cancer immunotherapy. The combination of a TLR5 ligand with a PI3K inhibitor resulted in the generation of polyfunctional T-cells secreting IFN-gamma, IL-17 and IL-2 (27). In multiple mouse models, the anti-tumor effect of this drug combination was associated with increased levels of these polyfunctional T-cells. Polyfunctional T-cells induced by CTLA-4 blockade and secreting IFN-gamma, MIP-1beta, and TNF-alpha were found in increased frequency in responding patients with metastatic melanoma as compared to non-responders (28). Most cytokine conditions we tested generated a neu p98 specific T-cell population with a mixed phenotype of Type I and Type II cells. Although IFN-gamma and TNF-alpha were secreted at greater levels than cells generated with IL-2 alone, only the cells cultured with IL-21 significantly secreted IL-17. Moreover, secretion of Type II cytokines was markedly diminished with this culture condition. IL-21 is an autocrine growth factor for Th17 cells (29). We show that human Th1/Th17 T-cells can also be elicited with IL-2/IL-21 culture. Not only is the specific cytokine gene expression enhanced after culture, but the Th17 transcription factor, RORc and IFN-gamma regulatory gene, IRF-4, were also increased. Although TNF-alpha expression was not as elevated as in the mice, further refinement of the cytokine culture conditions could enhance TNF-alpha secretion. The ability to recapitulate the polyfunctional phenotype with human antigen specific T-cells provides the basis for the potential therapeutic infusion of Th1/Th17 tumor specific T-cells. Th17 cells have been shown to have “stem cell-like” properties [(30) and have increased potential for proliferation and self-renewal (31). Thus, polyfunctional IL-17 secreting T-cells may persist as effector memory cells much longer that other forms of adoptively transferred cells, such as CAR, which can be short-lived (32). The ability of these cells to induce the same type of response across other murine models has yet to be determined. For example, in highly inflammatory cancer, such as colon cancer arising from colitis, IL-17 secretion can induce further proliferation of the tumor and increase cell motility thus enhancing tumor growth (33, 34).
CD8 T-cells are important effectors in the elimination of human malignancy. High levels of tumor infiltrating CD8 T-cells have been found to be associated with a survival benefit in many different types of cancers (35–37). Especially in breast cancer, CD8 TIL have been shown to be an independent predictor of pathologic complete response after chemotherapy (38). Most breast cancers, however, have low to no CD8 TIL. This is most likely due to the Th2 bias that occurs early in oncogenesis resulting in significant antibody generation but low Type I T-cell expansion (39–41). We show that Th1/Th17 secreting T-cells generated with IL-2/IL-21 drive significantly more CD8 T-cells into the tumor after infusion than cells cultured with more standard cytokine combinations. These data support the notion that the cytokine phenotype of CD4 T-cells is a critical factor in the effective function of CD8 cytotoxic T-cells. Investigations in both infectious disease and cancer have underscored the role of CD4 Th. Interferon-gamma secreting CD4+ T-cells have been shown to activate dendritic cells resulting in the generation of CMV specific CD8+ T-cells (42). In vitro studies demonstrate antigen specific CD4 are required to effectively stimulate functional CMV-specific cytotoxic T-cells. In cancer, specifically in the neu model, Th1 cells secreting IFN-gamma were essential for mediating tumor regression by inducing MHC expression on tumor cells thereby enhancing direct recognition by both CD8 and CD4 T-cells (43). The neutralization of IL-17 and TNF-alpha markedly reduced CD8 TIL. This observation suggests these cytokines are uniquely associated with tumor CD8 T-cell recruitment.
Increasing CD8 T-cell influx into tumors has been a barrier to successful adoptive T-cell therapy in solid tumors, including breast cancer. The ability of antigen specific T-cells to home to tumors is dependent on a network of receptors and secreted proteins; selectins, integrins, and chemokines expressed both on the T-cell as well as on or by tumor cells or other immune system cells (44). Type I cytokines have been shown to induce the up-regulation of integrin receptors or the production of chemokines. Thus, the generation of antigen specific T-cells secreting specific cytokines can allow direct modulation of the tumor microenvironment to enhance CD8 T-cell influx into the tumor. Recent studies have demonstrated that IL-17 and TNF-alpha act synergistically to induce the expression of both P- and E-selectin on endothelium (45). The two cytokines also enhance endothelial expression of specific chemokines such as CXCL2 and CXCL5 significantly increasing T-cell transmigration into inflammatory sites (45). Th17 cells have been shown to be able to recruit effector T-cells to the tumor via stimulation of CXCL9 and CXCL10 as well as directly activate CD8 T-cells by recruitment of specific dendritic cell populations (46, 47). The importance of IL-17 on T-cell recruitment is underscored by experiments demonstrating the anti-tumor effects of Th17 cells are abrogated in CCR6 deficient mice (47). CCR6 regulates the recruitment of T-cells to sites of an active immune response. Polyfunctional tumor specific T-cells that secrete both IL-17 and TNF-alpha may be ideally suited for use in adoptive T-cell transfer for the treatment of malignancy. Moreover, whether polyfunctional T-cells will impact the expression of PD-L1 on tumor and antigen presenting cells over time could influence the role of immune checkpoint inhibitors in further augmenting the anti-tumor immunity induced by these cells.
In summary, the choice of cytokine combination to expand autologous tumor specific T-cells is critically important in impacting the anti-tumor function of those cells. Specifically the generation of Th1/Th17 polyfunctional T-cells with IL-2 and IL-21 and other cytokines may result in a greater clinical response rate and potentially more sustained or durable responses due to the impact of these cytokines on CD8 T-cell tumor infiltration.
Supplementary Material
Translational Relevance.
Adoptive T-cell therapy with tumor infiltrating lymphocytes, vaccine-primed T-cells, or chimeric antigen receptor engineered T-cells has shown some evidence of clinical efficacy in a minority of solid tumor patients treated. Methods to optimize different modes of T-cell transfer are under exploration. Data shown here demonstrates that variation in the cytokines used to expand autologous vaccine-primed tumor specific T-cells impacts the phenotype as well as anti-tumor activity of the cells. IL-2/IL-21 when used in culture will generate polyfunctional Type I T-cells that enhance the egress of CD8 T-cells into the tumor via TNF-alpha and IL-17 secretion and result in complete resolution of established tumors in TgMMTV-neu mice. IL-21, when used in the culture of human antigen specific T-cells will induce a similar phenotype. Polyfunctional Th1/Th17 cells may have the potential to effectively induce tumor regression when used in adoptive transfer.
Acknowledgments
Financial Support: This work was supported in part by DOD Breast Cancer Research Program Multidisciplinary Postdoctoral Award W81XWH-06-1-0724 (V.P.L.), Ruth L. Kirschstein NIH Training grant T32 CA138312 (V.P.L.), and R01CA136632, CA136632, and CA101190 (M.L.D.). MLD is also supported by a Komen Leadership Grant as well as the Athena Distinguished Professorship for Breast Cancer Research. Human samples were collected through the Clinical Research Center Facility at the University of Washington (NIH grant UL1TR000423).
Footnotes
Conflict of Interest: MLD is a stockholder in Epithany and VentiRx and receives grant support from Celgene, EMD Serono, VentiRx and Seattle Genetics. The remaining authors have no COI.
References
- 1.Magee MS, Snook AE. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discovery Medicine. 2014;18:265–71. [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of Clinical Oncology. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M, et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clinical Cancer Research. 2014;20:1355–65. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–97. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disis ML, Dang Y, Coveler AL, Marzbani E, Kou ZC, Childs JS, et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunology, Immunotherapy. 2014;63:101–9. doi: 10.1007/s00262-013-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nature Immunology. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 7.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nature Reviews Immunology. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 8.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annual Review of Immunology. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 9.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Research. 2004;64:1146–51. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 10.Lai VPGE, Saddoughi S, Waisman Z, DeLong J, Disis ML. Targeting HER2-specific CD4+ Th1 cells to tumors to induce CD8+ T cell-mediated tumor rejection. Journal for Immunotherapy of Cancer. 2007 Abstract. [Google Scholar]
- 11.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Research. 2008;68:8400–9. doi: 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disis ML, Gad E, Herendeen DR, Lai VP, Park KH, Cecil DL, et al. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prevention Research. 2013;6:1273–82. doi: 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor-bearing neu transgenic mice predicts human tumor antigens. Cancer Research. 2006;66:9754–61. doi: 10.1158/0008-5472.CAN-06-1083. [DOI] [PubMed] [Google Scholar]
- 14.Dang Y, Knutson KL, Goodell V, dela Rosa C, Salazar LG, Higgins D, et al. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clinical Cancer Research. 2007;13:1883–91. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- 15.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. The Journal of Clinical Investigation. 2001;107:477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu SY, Chou T, Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. Journal of Immunology. 1989;143:740–8. [PubMed] [Google Scholar]
- 17.Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. Journal of Clinical Oncology. 2013;31:2152–9. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stack RM, Thompson CB, Fitch FW. IL-4 enhances long-term survival of CD28-deficient T cells. Journal of Immunology. 1998;160:2255–62. [PubMed] [Google Scholar]
- 19.Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–19. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande P, Cavanagh MM, Le Saux S, Singh K, Weyand CM, Goronzy JJ. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. Journal of Immunology. 2013;190:1416–23. doi: 10.4049/jimmunol.1201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Gallardo HF, Ku GY, Li H, Manukian G, Rasalan TS, et al. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy. 2009;11:912–22. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson KL, Disis ML. IL-12 enhances the generation of tumour antigen-specific Th1 CD4 T cells during ex vivo expansion. Clinical and Experimental Immunology. 2004;135:322–9. doi: 10.1111/j.1365-2249.2004.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9971–6. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, et al. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood. 2012;120:2229–39. doi: 10.1182/blood-2011-12-398321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd A, Almeida JR, Darrah PA, Sauce D, Seder RA, Appay V, et al. Pathogen-specific T cell polyfunctionality is a correlate of T cell efficacy and immune protection. PloS One. 2015;10:e0128714. doi: 10.1371/journal.pone.0128714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Research. 2012;72:581–91. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 30.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–85. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Science Translational Medicine. 2011;3:104ra0. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N, Barrett DM. Donor-derived CD19 chimeric antigen receptor T cells. Current Opinion in Hematology. 2015;22:503–8. doi: 10.1097/MOH.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 33.Martin M, Kesselring RK, Saidou B, Brunner SM, Schiechl G, Mouris VF, et al. RORgammat(+) hematopoietic cells are necessary for tumor cell proliferation during colitis-associated tumorigenesis in mice. The European Journal of Immunology. 2015;45:1667–79. doi: 10.1002/eji.201444915. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–63. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. Journal of Clinical Oncology. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunology, Immunotherapy. 2009;58:449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. British Journal of Cancer. 2013;109:2705–13. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. Journal of Experimental Medicine. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama H, Boldt C, Ladd JJ, Johnson MM, Chao T, Capello M, et al. An autoimmune response signature associated with the development of triple-negative breast cancer reflects disease pathogenesis. Cancer Research. 2015;75:3246–54. doi: 10.1158/0008-5472.CAN-15-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ladd JJ, Chao T, Johnson MM, Qiu J, Chin A, Israel R, et al. Autoantibody signatures involving glycolysis and splicesome proteins precede a diagnosis of breast cancer among postmenopausal women. Cancer Research. 2013;73:1502–13. doi: 10.1158/0008-5472.CAN-12-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flinsenberg TW, Spel L, Jansen M, Koning D, de Haar C, Plantinga M, et al. Cognate CD4 T-cell licensing of dendritic cells heralds anti-cytomegalovirus CD8 T-cell immunity after human allogeneic umbilical cord blood transplantation. Journal of Virology. 2015;89:1058–69. doi: 10.1128/JVI.01850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mortenson ED, Park S, Jiang Z, Wang S, Fu YX. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clinical Cancer Research. 2013;19:1476–86. doi: 10.1158/1078-0432.CCR-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abastado JP. The next challenge in cancer immunotherapy: controlling T-cell traffic to the tumor. Cancer Research. 2012;72:2159–61. doi: 10.1158/0008-5472.CAN-11-3538. [DOI] [PubMed] [Google Scholar]
- 45.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. Journal of Immunology. 2012;188:6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.