Abstract
Recent studies have proposed that the major anti-tumor effect of DNA methylation inhibitors is induction of interferon-responsive genes via dsRNAs-containing endogenous retroviruses. Recently, a 3D culture system for stem cells known as organoid culture has been developed. Lgr5-positive stem cells form organoids that closely recapitulate the properties of original tissues. To investigate the effect of DNA demethylation on tumor organoids, we have established organoids from intestinal tumors of ApcMin/+ (Min) mice and subjected them to 5-aza-2′-deoxycytidine (5-Aza-CdR) treatment and Dnmt1 knockdown. DNA demethylation induced by 5-Aza-CdR treatment and Dnmt1 knockdown significantly reduced the cell proliferation of the tumor organoids. Microarray analyses of the tumor organoids after 5-Aza-CdR treatment and Dnmt1 knockdown revealed that interferon-responsive genes were activated by DNA demethylation. Gene ontology and pathway analyses clearly demonstrated that these genes activated by DNA demethylation are involved in the anti-viral response. These findings indicate that DNA demethylation suppresses the proliferation of intestinal tumor organoids by inducing an anti-viral response including activation of interferon-responsive genes. Treatment with DNA methylation inhibitors to activate a growth-inhibiting immune response may be an effective therapeutic approach for colon cancers.
Aberrant DNA methylation at CpG island promoters of tumor suppressor genes is frequently observed in various human malignancies1. The DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-Aza-CdR), which is an analog of cytidine, has been widely studied and was recently approved for the treatment of myelodysplastic syndrome (MDS)1,2. Other DNA methylation inhibitors are also being assessed in clinical trials. Although it is believed that the anti-tumor effect of DNA methylation inhibitors is mediated by re-activation of epigenetically silenced tumor suppressor genes in cancer cells, recent studies have proposed that the major effect of DNA methylation inhibitors is induction of interferon-responsive genes via dsRNA-containing endogenous retroviruses (ERVs)3,4. These findings may represent a major shift in our understanding of the anti-tumor mechanisms of DNA demethylating agents.
Tumor stem cells with self-renewal and multipotential capacity play critical roles in malignant tumors that show a marked capacity for metastasis and invasion. The development of chemotherapy targeting tumor stem cells has important implications for the management of various cancers. However, little is known about the effect of DNA demethylating agents on the proliferation of tumor stem cells. Lgr5, a member of the Wnt signaling pathway, has been identified as a new molecular marker of stem cells in the small intestine, colon, stomach, liver and pancreas5,6,7,8,9,10. A newly developed 3D culture system allows Lgr5-positive stem cells to form budding cyst-like structures (organoids) that resemble the properties of original tissues5. This type of 3D culture uses serum-free medium that includes only specifically defined factors such as R-spondin 1 (Rspo1), epidermal growth factor (EGF) and noggin. Rspo1 has been identified as a ligand for Lgr5 and an essential factor for activation of the Wnt signaling pathway11,12. In addition, a recent study has clearly demonstrated that tumor organoids closely recapitulate the properties of original tumors13, suggesting that patient-derived organoids may allow high-throughput drug screening and personalized cancer therapy. To investigate the effect of DNA demethylation on tumor organoids, we established intestinal tumor organoids derived from tumors of ApcMin/+ (Min) mice and subjected them to treatment with 5-Aza-CdR and knockdown of Dnmt1.
Materials and Methods
Animals and experimental design
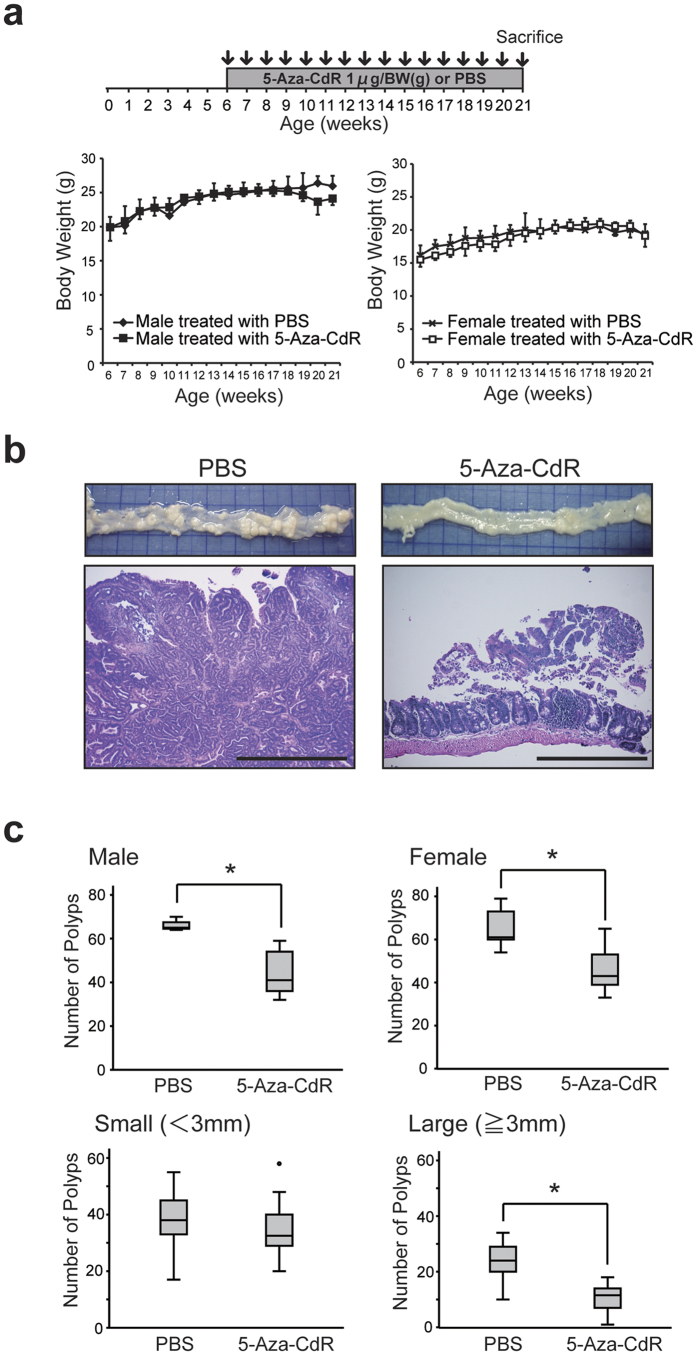
The experimental design for our animal study is shown in Fig. 1a. All experiments and procedures were approved by the Keio University Animal Research Committee, and all methods were carried out in accordance with the approved guidelines. Min mice were maintained in a temperature-controlled specific pathogen-free animal facility and given free access to water and food. They were treated with 5-Aza-CdR (Sigma-Aldrich, 1 μg/body weight, n = 12) or phosphate-buffered saline (PBS, n = 11) by subcutaneous weekly injection. The injections were started at 6 weeks of age and continued for 100 days (15 injections). The weights of the mice were monitored weekly. At 21 weeks of age, the mice were dissected and their intestinal polyps were counted.
Figure 1. Anti-tumor effect of 5-Aza-CdR on intestinal tumor formation in Min mice.
(a) Experimental design of the animal study and growth curves of male and female Min mice. From 6 weeks of age, 1 μg per body weight (g) 5-Aza-CdR or PBS was injected subcutaneously into Min mice once a week for 100 days (15 injections). The mice were then dissected at 21 weeks of age, and the number of polyps was counted. (b) Examples of the macroscopic and microscopic (HE staining) features of the intestine in Min mice treated with either 5-Aza-CdR or PBS. Scale bars: 1000 μm. (c) Average numbers of polyps in the intestine of male and female Min mice treated with either 5-Aza-CdR or PBS (upper). Average numbers of large polyps (>3 mm) and small polyps (<3 mm) in the intestine of Min mice treated with either 5-Aza-CdR or PBS (lower). *p < 0.05.
Polyp analysis
The gastrointestinal tract (duodenum to rectum) of Min mice was removed immediately after sacrifice, washed with PBS, cut longitudinally and rinsed with PBS, and then fixed with Davidson’s fixative. The number and size of the polyps along the entire length of the intestine were analyzed by investigators who were blinded to the treatment regime used for the mice. Tissues were fixed in buffered formalin, embedded in paraffin blocks, and then processed for histologic analyses using standard protocols with HE staining.
Establishment of intestinal tumor organoids and 5-Aza-CdR treatment
Isolation and dissociation of stem cells from intestinal tumors of Min mice and normal epithelia from wild-type C57BL/6 mice were performed as described previously8. Isolated tumor cells and epithelial cells were embedded in Matrigel on ice (growth factor-reduced, phenol red-free; BD Biosciences) and seeded in 48-well plates. The Matrigel was polymerized for 10 minutes at 37 °C, and overlaid with 250 μL/well basal culture medium (advanced Dulbecco’s modified Eagle medium/F12 supplemented with penicillin/streptomycin, 10 mmol/L HEPES, Glutamax, 1 × N2, 1 × B27 [all from Thermo Fisher Scientific], and 1 mmol/L N-acetylcysteine [Sigma-Aldrich]) containing the following optimized growth factor combinations: epidermal growth factor (EGF) and noggin for intestinal tumors; EGF, noggin and Rspo1 for intestinal epithelia. Intestinal tumor organoids were treated with 1 or 3 μM 5-Aza-CdR. Twenty-four hours after treatment, 5-Aza-CdR was removed from the culture medium, and regular culture medium was employed thereafter.
Lentivirus-mediated knockdown of Dnmt1 in tumor organoids
Lentivirus-mediated shRNA against the mouse Dnmt1 gene (GIPZ lentiviral shRNA) and a shRNA control (GIPZ lentiviral empty vector shRNA) were purchased from Thermo Fisher Scientific. Preparation of organoids and lentiviral infection were performed as described previously14. Twenty-four hours after transduction, the infected organoids were selected with puromycin (1 μg/ml).
Cell proliferation assay
Cell proliferation activity of tumor organoids was examined by WST assay using the Cell Counting Kit-8 (Dojindo, Japan) in accordance with the manufacturer’s instructions. Absorbance at 450 nm was measured using an Infinite M1000 PRO microplate reader (TECAN).
RNA extraction and microarray analyses
Total RNAs from cultured tumor organoids were extracted using a mirVana isolation kit (Thermo Fisher Scientific). Microarray analyses were conducted by Toray Industries (www.toray.com: Tokyo, Japan). All data were submitted to the GEO database, under the accession number GSE72768. Gene Ontology (GO) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses of the microarray data were performed using Genecoids (http://genecoids.dacya.ucm.es/analysis/). Hypergenometric p-values corrected by the false discovery rate (FDR) were calculated. The threshold for the significant GO and pathway was set at p < 0.05.
Quantitative RT-PCR
Levels of Dnmt1 expression were analyzed by quantitative RT-PCR using the TaqMan gene expression assay (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions. Expression levels of interferon-responsive genes (Oas1, Irf7, Isg15, Rig1, Mda5) and murine ERVs (IAP-1, MLV, LV30-2, MuRRS) were analyzed by quantitative RT-PCR using the Universal SYBR Select Master Mix (Thermo Fisher Scientific) as described previously15,16. The primer sequences are summarized in Supplementary Table S1.
Statistical analysis
Data obtained from polyp counting, WST assay and quantitative RT-PCR were analyzed by independent t-test using the SPSS 23.0 statistical software package. Differences at p < 0.05 were considered significant.
Results
Anti-tumor effect of 5-Aza-CdR on intestinal tumor formation in Min mice
To study the effects of DNA methylation inhibitor on intestinal tumor formation in vivo, we treated Min mice with 5-Aza-CdR. The experimental design of this study is shown in Fig. 1a. From 6 weeks of age, 1 μg per body weight (g) 5-Aza-CdR was injected subcutaneously into Min mice once a week for 100 days (15 injections). Control Min mice received PBS instead of 5-Aza-CdR. The mice were then dissected at 21 weeks of age, and the polyps were counted to investigate the suppressive effects of 5-Aza-CdR on tumor progression. Growth curves of male and female Min mice treated with either 5-Aza-CdR or PBS are shown in Fig. 1a. As can be seen, treatment with 5-Aza-CdR did not have any significant effect on body weight.
The number of intestinal polyps at 21 weeks of age was determined by examining the mucosa over the entire length of the intestine. Figure 1b shows examples of the macroscopic and microscopic (HE staining) features of the intestine in Min mice treated with either 5-Aza-CdR or PBS. Numerous adenomas were found throughout the entire intestine of control Min mice treated with PBS, whereas Min mice treated with 5-Aza-CdR showed a decrease of adenoma formation. The graphs shown in Fig. 1c demonstrate the number of polyps in male and female Min mice treated with either 5-Aza-CdR or PBS. Treatment of male and female Min mice with 5-Aza-CdR significantly reduced the average number of intestinal adenomas from 66 to 44, and from 65 to 47, respectively. In addition, the average number of large adenomas (>3 mm) in Min mice treated with 5-Aza-CdR was significantly decreased from 24 to11, whereas there was no significant difference in the average number of small adenomas (<3 mm). These results indicate that treatment of Min mice with the DNA methylation inhibitor 5-Aza-CdR suppresses the development of intestinal tumors.
Anti-tumor effect of 5-Aza-CdR on growth of intestinal tumor organoids
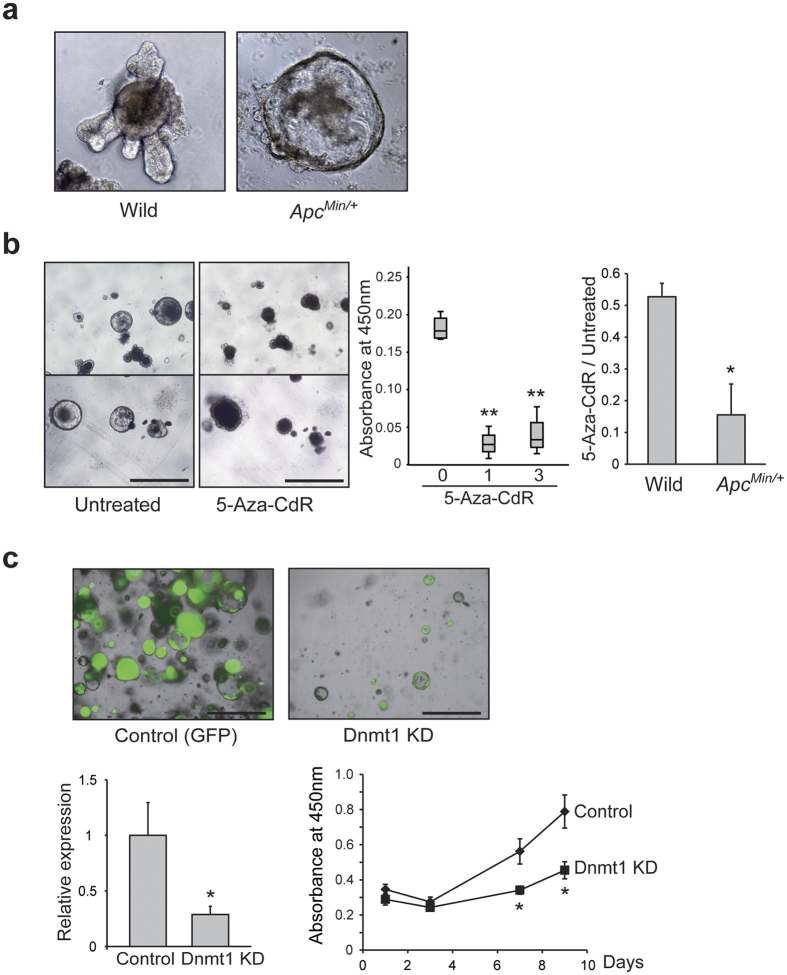
To investigate the effects of the DNA methylation inhibitor on intestinal tumor cells in vitro, we established cultured organoids from intestinal tumors of Min mice as well as intestinal epithelia of wild-type C57BL/6 mice. As described previously8, organoids derived from Apc-deficient tumors formed cystic organoid structures without the “budding” that is observed in intestinal epithelial organoids derived from wild-type C57BL/6 mice (Fig. 2a). Since Apc loss constitutively activates the Wnt pathway, Rspo1 was dispensable for the culture of Apc-deficient tumor organoids. We treated Apc-deficient intestinal tumor organoids with 5-Aza-CdR and analyzed the WST cell proliferation assay of the organoids at 4 days after 5-Aza-CdR treatment. As shown in Fig. 2b, the organoids were unable to proliferate after treatment with 5-Aza-CdR. The results of the WST assay clearly indicated that cell proliferation activity of the tumor organoids was significantly reduced after treatment with both 1 μM and 3 μM 5-Aza-CdR. In addition, the cell proliferation activity after treatment with 1 μM 5-Aza-CdR was markedly lower in organoids derived from intestinal tumors of Min mice than in organoids derived from normal intestinal epithelia of wild-type mice (Fig. 2b). These results indicate that 5-Aza-CdR exerts an anti-tumor effect on tumor organoids but is much less toxic to normal intestinal organoids.
Figure 2. Anti-tumor effect of 5-Aza-CdR treatment and Dnmt1 knockdown in intestinal tumor organoids.
(a) Bright-field images of organoids derived from intestinal epithelia of wild-type C57BL/6 mice (Wild) and intestinal tumors of Min mice (ApcMin/+). (b) Bright-field images of intestinal tumor organoids of Min mice after treatment with 5-Aza-CdR. Scale bars: 1000 μm (left). WST cell proliferation assay of intestinal tumor organoids of Min mice after treatment with 1 μM or 3 μM 5-Aza-CdR. **p < 0.001 compared to untreated control (middle). WST cell proliferation assay of organoids derived from intestinal epithelia of wild-type C57BL/6 mice (Wild) and intestinal tumors of Min mice (ApcMin/+) after treatment with 1 μM 5-Aza-CdR. Values were normalized to untreated controls. *p < 0.005 compared to Wild (right). We analyzed the WST cell proliferation assay of the organoids at 4 days after 5-Aza-CdR treatment. (c) Images of intestinal tumor organoids transfected with lentiviral shRNA against Dnmt1 and control shRNA. Transfection of lentivirus vectors was confirmed by GFP expression in the organoids (upper). Relative expression of Dnmt1 normalized to Gapdh and WST cell proliferation assay in intestinal tumor organoids transfected with lentiviral shRNA against Dnmt1 and control shRNA. We seeded the cells obtained from the organoids of Dnmt1 knockdown and control (GFP) at 2 × 104 cells per well, and then examined cell proliferation until 9 days after cell plating. *p < 0.05 compared to control (lower).
Anti-tumor effect of Dnmt1 knockdown on growth of intestinal tumor organoids
The cellular toxicity of 5-Aza-CdR may affect gene expression and proliferation of intestinal tumor organoids. Therefore, to evaluate the effect of DNA demethylation in the absence of cellular toxicity, we next performed lentivirus-mediated knockdown of the major DNA methyltransferase, Dnmt1, in intestinal tumor organoids. Successful transfection of lentiviral shRNA against Dnmt1 and control shRNA was confirmed by expression of GFP in the organoids (Fig. 2c). WST assay was performed to investigate the cell proliferation activity of intestinal tumor organoids. We seeded the cells obtained from the organoids of Dnmt1 knockdown and control (GFP) at 2 × 104 cells per well, and then examined cell proliferation until 9 days after cell plating. As shown in Fig. 2c, cell proliferation activity of Dnmt1 knockdown organoids was significantly suppressed in comparison with the control after 7 and 9 days of culture.
Treatment with 5-Aza-CdR and knockdown of Dnmt1 induce an anti-viral response in intestinal tumor organoids
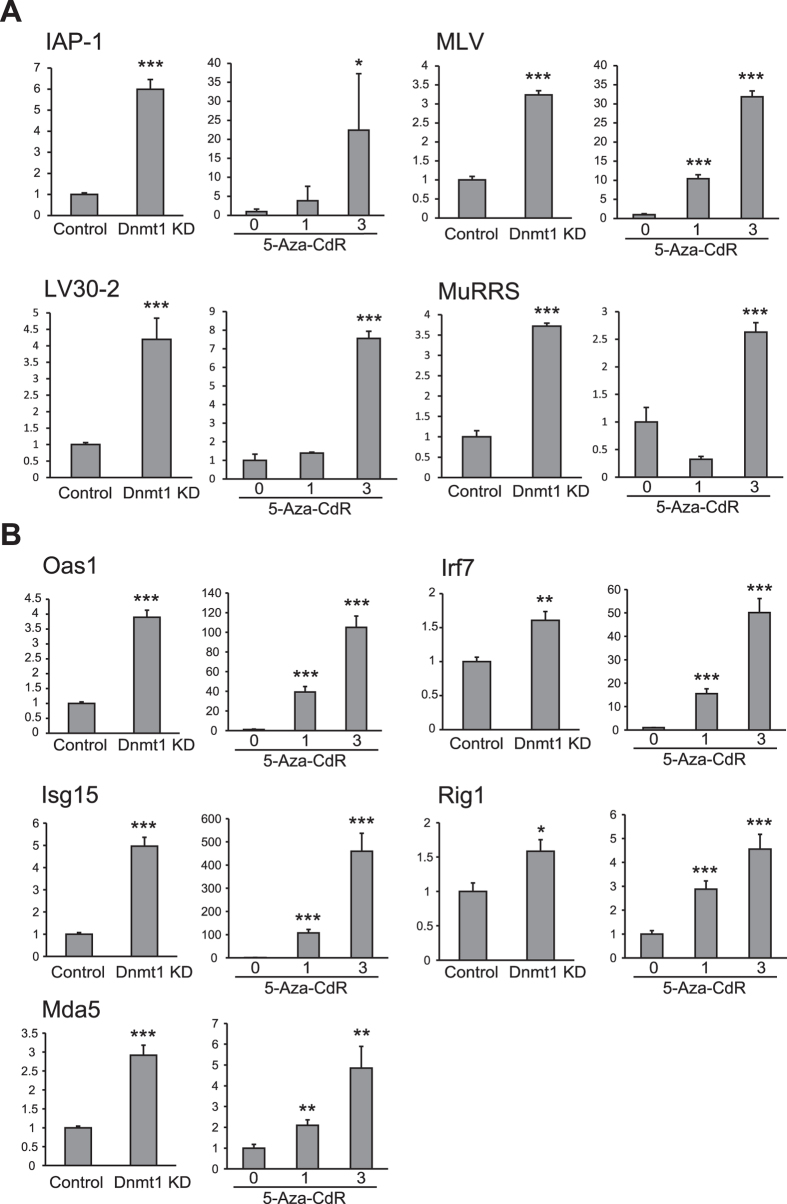
To identify the genes that were differentially expressed upon inhibition of DNA methylation, we conducted microarray analyses of Apc-deficient intestinal tumor organoids after treatment with 1 μM 5-Aza-CdR and knockdown of Dnmt1. Total RNAs were extracted at 4 days after 1 μM 5-Aza-CdR treatment and at 9 days after the lentivirus infection, respectively. At these points, we confirmed that the growth of the organoids and Dnmt1 expression were significantly decreased. A number of genes were significantly up-regulated in the organoids by pharmacologic and genetic inhibition of DNA methylation. Table 1 shows the top 25 genes that were commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1 knockdown in the tumor organoids. All data for the genes activated by DNA demethylation are available in Supplementary Table S2. As shown in Table 1, interferon-responsive genes including 2′-5′ oligoadenylate synthetase (Oas) and interferon regulatory factor 7 (Irf7) were markedly activated in the tumor organoids after treatment with 5-Aza-CdR and knockdown of Dnmt1. Fifteen out of the top 25 up-regulated genes (60%) included the term “immune” or “interferon” in the gene name descriptor and GO annotation, indicating that these genes activated by DNA demethylation are involved in the pathway of the interferon and immune response. To determine whether the genes activated by DNA demethylation play a role in biological process, GO analysis was performed. The top 10 terms demonstrated by GO analysis of the genes commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1 knockdown in the tumor organoids are shown in Table 2. The GO analysis revealed that the genes that were activated by DNA demethylation in the tumor organoids are involved in the anti-viral response, immune response and cellular response to interferon and exogenous dsRNA. Moreover, pathway enrichment analysis also revealed that these genes up-regulated by DNA demethylation were associated with the KEGG pathways of the anti-viral responses to measles and hepatitis C (Table 2). Finally, to validate activation of the RIG-1/MDA5 RNA response pathway, we examined expression levels of interferon-responsive genes and murine ERVs in intestinal tumor organoids after 5-Aza-CdR treatment and Dnmt1 knockdown. Expression levels of murine ERVs such as IAP-1, MLV, LV30-2 and MuRRS were significantly increased in the organoids after 5-Aza-CdR treatment and Dnmt1 knockdown (Fig. 3A). Moreover, expression levels of interferon-responsive genes such as Oas1, Irf7, Isg15, Rig1 and Mda5 were also increased by 5-Aza-CdR treatment in a dose-dependent manner and Dnmt1 knockdown (Fig. 3B).
Table 1. The top 25 genes that were commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1-knockdown.
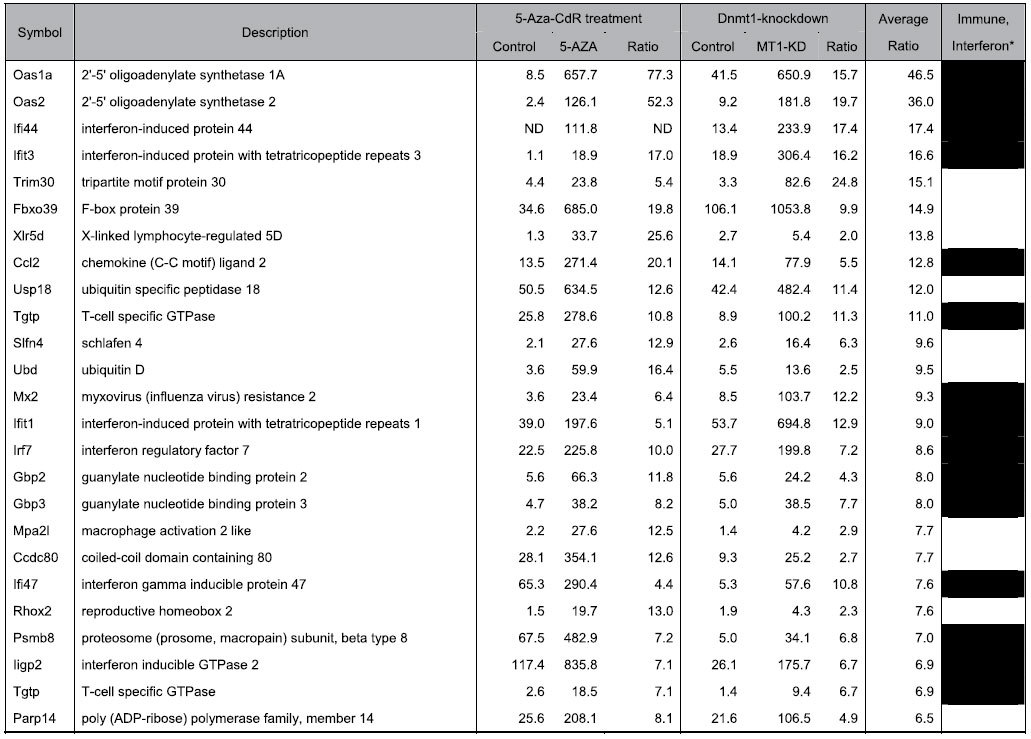
ND, not detected. *Genes that include the term “immune” or “interferon” in their description and gene ontology annotation are denoted by black.
Table 2. The GO and KEGG pathway analyses of the genes commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1-knockdown.
| Term | Description | Genes | NGR | NG | p-value |
|---|---|---|---|---|---|
| GO:0009615 | Response to virus | Klra8,Zc3hav1,Ifih1,Irf7,Oas1b,Rsad2,Ifit1,Mx2 | 79 (36814) | 8 (105) | 2.36441E-08 |
| GO:0035458 | Cellular response to interferon-beta | Gbp3,Gbp2,Ifit3,Ifit1 | 17 (36814) | 4 (105) | 2.31915E-05 |
| GO:0006955 | Immune response | B2m,Oasl2,Oas1b,Ccl2,Oas1a,Ccl5 | 151 (36814) | 6 (105) | 0.00053118 |
| GO:0071346 | Cellular response to interferon-gamma | Gbp3,Gbp2,Ccl2 | 16 (36814) | 3 (105) | 0.000986171 |
| GO:0019060 | Intracellular transport of viral proteins in host cell | Tap1,Ifit1 | 4 (36814) | 2 (105) | 0.00309224 |
| GO:0043123 | Positive regulation of I-kappaB kinase/NF-kappaB cascade | Bst2,Rnf31,Zc3hav1,Ubd | 98 (36814) | 4 (105) | 0.00535993 |
| GO:0010759 | Positive regulation of macrophage chemotaxis | Ccl2,Ccl5 | 7 (36814) | 2 (105) | 0.00538121 |
| GO:0045071 | Negative regulation of viral genome replication | Ifit1,Ccl5 | 7 (36814) | 2 (105) | 0.00538121 |
| GO:0071360 | Cellular response to exogenous dsRNA | Zc3hav1,Ifit1 | 7 (36814) | 2 (105) | 0.00538121 |
| GO:0032020 | ISG15-protein conjugation | Ube2l6,Usp18 | 6 (36814) | 2 (105) | 0.00550129 |
| Pathway | |||||
| KEGG 05162 | Measles | Mx2,Adar,Irf7,Stat2,Oas1a,Ifih1,Oas2,Oas1b | 135 (36814) | 8 (105) | 2.85178E-07 |
| KEGG 05160 | Hepatitis C | Irf7,Stat2,Ifit1,Oas1a,Oas2,Oas1b | 137 (36814) | 6 (105) | 7.35785E-05 |
| KEGG 04623 | Cytosolic DNA-sensing pathway | Ccl5,Adar,Irf7 | 62 (36814) | 3 (105) | 0.0130779 |
| KEGG 04620 | Toll-like receptor signaling pathway | Ccl5,Irf7,Cd80 | 100 (36814) | 3 (105) | 0.0387771 |
The top 10 terms of GO analysis of the genes commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1-knockdown.
The KEGG Pathway analysis of the genes commonly up-regulated by both 5-Aza-CdR treatment and Dnmt1-knockdown.
GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; NGR, Number of annotated genes in the reference list (Total number of genes in the reference list); NG, Number of annotated genes in the input list (Total number of genes in the input list).
Figure 3. Expression levels of murine ERVs and interferon-responsive genes in intestinal tumor organoids after 5-Aza-CdR treatment and Dnmt1 knockdown.
(A) Relative expression levels of IAP-1, MLV, LV30-2 and MuRRS normalized to TBP in intestinal organoids after 5-Aza-CdR treatment (1 μM and 3 μM) and Dnmt1 knockdown. *p < 0.05 and ***p < 0.0001 compared to control. (B) Relative expression levels of Oas1, Irf7, Isg15, Rig1 and Mda5 normalized to β-actin in intestinal organoids after 5-Aza-CdR treatment (1 μM and 3 μM) and Dnmt1 knockdown. *p < 0.05, **p < 0.001 and ***p < 0.0001 compared to control.
Discussion
Several studies have demonstrated that DNA methylation inhibitors such as 5-Aza-CdR and zebularine suppress intestinal tumors in Min mice17,18. However, the molecular mechanisms underlying this anti-tumor effect remain to be clarified. So far, the major mechanism responsible for the anti-tumor effect of DNA methylation inhibitors has been considered to be re-activation of silenced tumor suppressor genes by DNA demethylation of their CpG island promoters. Recently, however, two groups, Roulois et al. and Chiappinelli et al., have demonstrated that DNA demethylating agents can induce a cell-autonomous immune activation response by stimulating the expression of dsRNA-containing ERVs3,4. This may represent a paradigm shift in our understanding of the anti-tumor mechanism of DNA demethylating agents.
In Rspo1-based 3D culture system, Lgr5-positive stem cells form ever-expanding organoids that retain their tissue identity. The tumor organoids derived from Apc-deficient tumors can be stably maintained in culture medium without Rspo1 and form cystic organoid structures, because Apc loss constitutively activates the Wnt signaling pathway in tumor cells. Our present results show that pharmacologic and genetic DNA demethylation by 5-Aza-CdR treatment and Dnmt1 knockdown suppresses the proliferation of Apc-deficient intestinal tumor organoids. In addition, the data from microarray analyses clearly demonstrate that the majority of genes commonly up-regulated by 5-Aza-CdR treatment and Dnmt1 knockdown are associated with the immune response to virus and dsRNAs and the interferon response. Indeed, expression levels of murine ERVs and interferon-responsive genes including the Rig1/Mda5 RNA response pathway were significantly increased after 5-Aza-CdR treatment and Dnmt1 knockdown. We investigated the levels of DNA methylation around the promoter regions of these genes, including Irf7, by pyrosequencing in intestinal tumor organoids, but found no significant difference after 5-Aza-CdR treatment and Dnmt1 knockdown (data not shown). Therefore, our present results strongly support the hypothesis that the main anti-tumor effect of DNA demethylating agents is induction of an anti-viral response via activation of dsRNA-containing ERVs3,4.
Taken together, these findings indicate that inhibition of DNA methylation suppresses intestinal tumor organoids by inducing a growth-inhibitory immune response, including activation of interferon-responsive genes, in response to ERVs. Treatment with DNA methylation inhibitors to activate a growth-inhibiting immune response could be an effective therapeutic approach for colon cancers. In the future, patient-derived tumor organoids may allow evaluation of sensitivity to DNA methylation inhibitors and personalized epigenetic therapy for cancer.
Additional Information
How to cite this article: Saito, Y. et al. Inhibition of DNA Methylation Suppresses Intestinal Tumor Organoids by Inducing an Anti-Viral Response. Sci. Rep. 6, 25311; doi: 10.1038/srep25311 (2016).
Supplementary Material
Acknowledgments
This work was supported by a Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects (to Y.S.), a Grant-in-Aid for Scientific Research B (26290049) from the Japan Society for Promotion of Science (to Y.S.) and the Takeda Science Foundation (to Y.S.).
Footnotes
Author Contributions Y.S., T.K. and H.S. designed and performed experiments, analyzed data and wrote the manuscript. T.N., K.S., K.T. and K.M. performed experiments. T.M. and T.S. performed organoid culture.
References
- Gal-Yam E. N., Saito Y., Egger G. & Jones P. A. Cancer epigenetics: modifications, screening, and therapy. Annu. Rev. Med. 59, 267–280 (2008). [DOI] [PubMed] [Google Scholar]
- Yoo C. B. & Jones P. A. Epigenetic therapy of cancer: past, present and future. Nat. Rev. Drug Discov. 5, 37–50 (2006). [DOI] [PubMed] [Google Scholar]
- Roulois D. et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 162, 961–973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli K. B. et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 162, 974–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). [DOI] [PubMed] [Google Scholar]
- Barker N. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010). [DOI] [PubMed] [Google Scholar]
- Sato T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011). [DOI] [PubMed] [Google Scholar]
- Huch M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon K. S., Gong X., Lin Q., Thomas A. & Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011). [DOI] [PubMed] [Google Scholar]
- van de Wetering M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B. K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2012). [DOI] [PubMed] [Google Scholar]
- Stengel A. et al. Prion infection influences murine endogenous retrovirus expression in neuronal cells. Biochem. Biophys. Res. Commun. 343, 825–831 (2006). [DOI] [PubMed] [Google Scholar]
- Yan K. et al. Toll-like receptor 3 and RIG-I-like receptor activation induces innate antiviral responses in mouse ovarian granulosa cells. Mol. Cell. Endocrinol. 372, 73–85 (2013). [DOI] [PubMed] [Google Scholar]
- Laird P. W. et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell 81, 197–205 (1995). [DOI] [PubMed] [Google Scholar]
- Yoo C. B. et al. Long-term epigenetic therapy with oral zebularine has minimal side effects and prevents intestinal tumors in mice. Cancer Prev Res 1, 233–240 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.