Abstract
Stomata, formed by pairs of guard cells in the epidermis of terrestrial plants, regulate gas exchange, thus playing a critical role in plant growth and stress responses. As natural openings, stomata are exploited by microbes as an entry route. Recent studies reveal that plants close stomata upon guard cell perception of molecular signatures from microbes, microbe associated molecular patterns (MAMPs), to prevent microbe invasion. The perception of MAMPs induces signal transduction including recruitment of second messengers, such as Ca2+ and H2O2, phosphorylation events, and change of transporter activity, leading to stomatal movement. In the present review, we summarize recent findings in signaling underlying MAMP-induced stomatal movement by comparing with other signalings.
Keywords: guard cell, microbe-associated molecular patterns, reactive oxygen species, ion channels, Ca2+ signaling, mitogen-activated protein kinase, open stomata 1, Ca2+-dependent protein kinase
Introduction
Stomata are microscopic pores formed by pairs of guard cells in the shoot epidermis of plants and regulate gas exchange, notably CO2 uptake for photosynthesis and water loss during transpiration, thus playing a critical role in plant growth and stress responses such as drought stress. As natural openings, stomata are exploited as a major entry route by a wide range of microbes including bacteria, oomycetes, and fungi (Grimmer et al., 2012; Sawinski et al., 2013). On the other hand, plants proactively induce stomatal closure and inhibit stomatal opening to prevent microbe invasion, which is later termed as stomatal immunity (Melotto et al., 2008; Sawinski et al., 2013). Stomatal immunity is closely related to susceptibility to a variety of pathogenic microbe infections. For example, defection in stomatal closure in response to pathogenic bacteria increased infection in Arabidopsis (Melotto et al., 2006; Singh et al., 2012), while pre-closing stomata by abscisic acid (ABA) reduced infection in grapevine by oomycete, Plasmopara viticola (Allègre et al., 2009).
Microbe-associated molecular patterns (MAMPs) are molecular signatures that are highly conserved in whole classes of microbes but are absent from the host, such as chitin for fungi and flagellin for bacteria (Boller and Felix, 2009). Recognition of each MAMP is performed by specific surface-localized receptors containing various ligand-binding ectodomains in plants, which are termed as pattern-recognition receptors (PRRs). The perception of MAMPs by PRRs triggers signaling converging to common responses, such as ion fluxes including Ca2+ influx, K+ efflux, and anion efflux, production of reactive oxygen species (ROS) and phosphorylation events, which are critical for plant innate immunity (Boller and Felix, 2009; Zipfel, 2014). Recent studies revealed that several MAMPs induce stomatal closure and inhibit stomatal opening, including flg22 (a conserved 22-amino-acid peptide near the N terminus of bacterial flagellin (Melotto et al., 2006; Zhang et al., 2008). Further results showed that plants with loss-of-function of the PRR for flg22, FLAGELLIN-SENSITIVE 2 (FLS2), did not close stomata in response to flg22 and coronatine-deficient Pseudomonas syringae pv tomato (Pst) strain DC3000, Pst DC3118 (Melotto et al., 2006; Zeng and He, 2010). As a result, the mutant is more susceptible to Pst DC3118 than wild type. These results indicate that plants mainly sense MAMPs to induce stomatal closure to prevent microbe invasion. The past decade has seen increasing efforts in elucidating MAMP signaling in guard cells and exciting findings every year. Several excellent reviews have been published covering this topic (Melotto et al., 2008; Zeng et al., 2010; Sawinski et al., 2013; McLachlan et al., 2014; Arnaud and Hwang, 2015). In the present review, we concentrate on the current knowledge of MAMP signaling in guard cells and discuss the latest findings by comparing with other signalings.
Abscisic Acid Signaling in Guard Cells
Phytohormones play critical roles in regulating stomatal movement. Almost all the phytohormones are reported to be involved in stomatal movement, among which ABA, methyl jasmonic (MeJA), and salicylic acid (SA) are believed to induce stomatal closure in various plants (Acharya and Assmann, 2009; Murata et al., 2015). Particularly, mechanism of stomatal movement has been well characterized in the context of ABA signaling in guard cells. In this section, we briefly overview ABA signaling in Arabidopsis guard cells. For details on this topic, we refer readers to excellent reviews (Hubbard et al., 2010; Kim et al., 2010; Joshi-Saha et al., 2011).
Abscisic acid is mainly produced in response to drought stress and the ABA synthesized in guard cells plays a critical role in regulation of stomatal movement (Bauer et al., 2013). An Snf1-related protein kinase 2 (SnRK2), SnRK2.6 also known as Open stomata 1 (OST1), is a Ca2+-independent protein kinase and an essential positive regulator in ABA signaling in Arabidopsis guard cells. In resting condition, OST1 kinase activity is inhibited by clade A Type 2C protein phosphatases (PP2Cs). Upon ABA perception, the interaction of ABA receptors, PYR/PYL/RCAR, and PP2Cs releases the inhibition of OST1, resulting in increment of OST1 kinase activity (Cutler et al., 2010; Hubbard et al., 2010; Joshi-Saha et al., 2011). In guard cell ABA signaling, OST1 is essential for recruitment of second messengers, such as H2O2, NO, and Ca2+, which are important for regulation of transporters in the plasma membrane including S-type anion channels and H+-ATPases (Mustilli et al., 2002; Bright et al., 2006; Acharya et al., 2013; Yin et al., 2013). OST1 has been reported to directly regulate ion channels including S-type anion channel SLAC1 (Geiger et al., 2009; Lee et al., 2009; Brandt et al., 2012), R-type anion channel ALMT12 (Meyer et al., 2010; Sasaki et al., 2010; Imes et al., 2013), inward-rectifying K+ channels (Kin channels) KAT1 (Sato et al., 2009; Siegel et al., 2009; Uraji et al., 2012), K+ uptake transporter 6 (KUP6; Osakabe et al., 2013), vacuolar anion exchanger CLCa (Wege et al., 2014) and Plasma membrane Intrinsic Protein 2;1 (PIP2;1; Grondin et al., 2015). Downstream components are also involved in ABA regulation of transporters including several Ca2+-dependent protein kinases (CDPK), CPK3, CPK4, CPK5, CPK6, CPK10, CPK11, and CPK23 (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010; Brandt et al., 2015), and mitogen-activated kinases (MAPKs), MPK9 and MPK12 (Jammes et al., 2009). The regulation of transporters by ABA, especially the activation of anion channels and suppression of H+-ATPases, causes depolarization of plasma membrane. For open stomata, this leads to efflux of anion, K+ and water, resulting in stomatal closure. For closed stomata, this keeps stomata closed in response to light.
MAMPs Known to Induce Stomatal Closure
A wide range of MAMPs from bacteria, fungi and oomycetes have been identified and characterized (Zipfel, 2014). New types of MAMPs are emerging such as peptides from Necrosis and ethylene-inducing peptide 1 (Nep1)-like proteins (Oome et al., 2014) and glycoside hydrolase family 12 (GH12) protein (Ma et al., 2015). The well-known MAMPs that induce stomatal movement are bacterium-derived MAMPs including flg22, elf18/elf26 (the first 18/26-amino-acid peptide of the N terminus of bacterial elongation factor Tu) and bacterial lipopolysaccharide (LPS), and fungus-derived MAMPs including chitin, chitosan and YEL. β-1→3-linked glucans are major components in oomycete cell wall and have been used to study stomatal movement (Allègre et al., 2009; Fu et al., 2011; Robinson and Bostock, 2014). In this section, we briefly introduce the molecular nature and perception of these MAMPs. For detail, we refer readers to excellent reviews (Boller and Felix, 2009; Zipfel, 2014; Sánchez-Vallet et al., 2015; Shinya et al., 2015).
MAMPs Derived from Bacteria
The widely used flg22, QRLSTGSRINSAKDDAAGLQIA, is an epitope in the flagellin of P. syringae pv. tabaci 6605, and induces stomatal closure and inhibits light-trigged stomatal opening in Arabidopsis (Melotto et al., 2006; Zhang et al., 2008). Though this epitope is widely conserved, studies have shown that variations of this epitope that are not sensed by Arabidopsis exist in several pathogenic bacteria (Pfund et al., 2004; Robatzek et al., 2006). The flg22 used here only refers to the one from P. syringae pv. tabaci 6605, which has been used in most of studies on stomatal movement. The perception of flg22 is mediated by a plasma membrane-localized leucine-rich repeat type receptor-like kinase (LRR-RLK), Flagellin-sensitive 2 (FLS2; Gomez-Gomez and Boller, 2000), which is expressed in guard cells (Melotto et al., 2006; Beck et al., 2014). Loss-of-function mutation of FLS2 abolishes flg22-induced stomatal movement (Melotto et al., 2006; Zhang et al., 2008). Recent studies identified another epitope in the flagellin from various P. syringae pv. tomato, 28 amino acid long peptides (flgII-28), which induces stomatal closure in tomato (Cai et al., 2011). However, the PRR for flgII-28 remains to be identified.
Elongation factor is one of the most abundant proteins in bacteria. The widely used elf18, N-acetylated SKEKFERTKPHVNVGTIG, and elf26, N-acetylated SKEKFERTKPHVNVGTIGHVDHGKTT, are the bioactive N terminals of elongation factor with the first 18 and 26 amino acid residues from Escherichia coli and both induce stomatal closure in Arabidopsis (Zeng and He, 2010). The PRR for these two peptides in Arabidopsis is Elongation factor Tu receptor (EFR), which is also an LRR-RLK expressed in guard cells (Zipfel et al., 2006; Liu et al., 2009). Plants outside of the Brassicaceae are believed not to respond to this epitope of EF-Tu due to lack of EFR (Zipfel et al., 2006). Recently, a new epitope from the middle region comprising Lys176 to Gly225 of the Acidovorax avenae EF-Tu, termed as EFa50, is identified as a MAMP sensed by rice (Furukawa et al., 2014). It remains to be clarified whether EFa50 regulates stomatal movement in rice.
Upon binding of flg22 or elf18/elf26, FLS2 or EFR forms receptor complexes with LRR-RLKs belonging to Somatic-embryogenesis receptor-like kinase (SERK) family, among which Brassinosteroid insensitive1-associated kinase1/SERK3 (BAK1/SERK3) plays a dominant role (Chinchilla et al., 2007; Roux et al., 2011). Structure data revealed that the C-terminal region of flg22 functions as a molecular glue between ectodomains of FLS2 and BAK1 (Sun et al., 2013). Recent studies revealed that receptor-like cytoplasmic kinases (RLCKs) are direct substrates of the receptors complexes and transduce the signal of MAMPs to downstream events (Macho and Zipfel, 2014). A RLCKs, Botrytisinduced kinase1 (BIK1), is essential for stomatal closure induced by flg22 but not ABA (Li et al., 2014).
Lipopolysaccharides are characteristic components of Gram-negative bacteria and composed of conserved lipid A, core oligosaccharide regions and a long-chain poly saccharide (the O antigen) that can have variable composition, length, and branching of its carbohydrate subunits. Due to variety in structure and easy contamination during preparation, LPS used in many of the plant researches can be consider a mixture of MAMPs and is derived from human pathogen P. aeruginosa. The LPS induces stomatal closure in Arabidopsis and tomato (Melotto et al., 2006; Liu et al., 2009; Desclos-Theveniau et al., 2012). Lipopolysaccharides from E. coli O55:B5 induced stomatal closure in Arabidopsis (Melotto et al., 2006). The PRRs for LPS are still under investigation. It has been shown that plants sense the O antigen, core oligosaccharide and lipid A (Bedini et al., 2005; Silipo et al., 2005). Recent studies further revealed that a bulb-type (B-type) lectin S-domain (SD)-1 RLK, Lipooligosaccharide-specific reduced elicitation (LORE), functions as a putative PRR for LPS from Pseudomonas and Xanthomonas species including P. aeruginosa by sensing lipid A moiety, and is restricted to the Brassicaceae family of plants (Ranf et al., 2015). Interestingly, Arabidopsis responds to LPS from E. coli K12 and E. coli O111:B4 in an LORE-independent manner (Ranf et al., 2015). These results suggest that multiple PRRs including LORE are involved in LPS-induced stomatal closure.
MAMPs Derived from Fungi and Oomycetes
Chitin is an insoluble polymer of β-1,4-linked N-acetylglucosamineone and one of the major components of fungal cell wall. Chitosan is the deacetylated form of chitin. Caution has to be paid that commercially available chitosan can be only partially deacetylated. For example, some chitosan from Sigma is 75 to 85% deacetylated. Chitin induces stomatal closure in Arabidopsis (Lozano-Duran et al., 2014; Bourdais et al., 2015). Chitosan induces stomatal closure and inhibits light-induced stomatal opening in various plant species, such as Arabidopsis (Klüsener et al., 2002), tomato (Lee et al., 1999), pea (Srivastava et al., 2009), rapeseed (Li et al., 2009), tobacco (Fu et al., 2011), and barley (Koers et al., 2011). In these studies, both chitin and chitosan are a mixture of polymers with different degree of polymerization (DP). Actually, the degree of polymerization is critical for the plant responses to chitin and chitosan (Kauss et al., 1989; Yamada et al., 1993; Vander et al., 1998; Liu et al., 2012). A current model shows that perception of chitin in Arabidopsis involves three lysin motif type RLKs (LysM-RLKs), Lysin motif receptor kinase 5 (LYK5), LYK4 and chitin elicitor receptor kinase 1 (CERK1) (Miya et al., 2007; Cao et al., 2014). It has been reported that LYK5 has much higher affinity to chitin than CERK1 does, interacts with CERK1, and is indispensable for chitin-triggered CERK1 phosphorylation (Cao et al., 2014). These results suggest that LYK5 and CERK1 form a receptor complex for chitin. Recent studies also identified several RLCKs as downstream components of the receptor complex (Zhang et al., 2010; Shinya et al., 2014). For the perception of chitosan, high DP of chitosan seems to be critical (Kauss et al., 1989; Vander et al., 1998; Iriti and Faoro, 2009). Chitosan oligomers only weakly bind to CERK1 and is unlikely to induce several plant responses including ROS production (Kauss et al., 1989; Vander et al., 1998; Petutschnig et al., 2010). It is further suggested that surface charge of fully deacetylated chitosan polymers is responsible for their effects on plants (Kauss et al., 1989).
Mixtures of β-1→3-linked glucans with different DP have been shown to induce stomatal closure and inhibits stomatal opening in grapevine and tobacco (Allègre et al., 2009; Fu et al., 2011). The strength of stomatal closure induced by the glucans showed dependency on DP, which may reflect the different perceptions of these glucans. However, the molecular mechanism of β-1→3-linked glucan perception is poorly understood.
Elicitors from baker’s yeast (YEL) extracted by ethanol precipitation mainly contains fungal cell wall fraction including mannan, β-1→3-linked glucans, chitin, and glycopeptides, and has been widely used as a fungal MAMP to induce plant immune response including stomatal responses (Hahn and Albersheim, 1978; Schumacher et al., 1987; Gundlach et al., 1992; Blechert et al., 1995; Kollar et al., 1997; Klüsener et al., 2002; Zhao et al., 2004; Ge and Wu, 2005; Khokon et al., 2010a; Salam et al., 2013; Ye et al., 2013b, 2015; Moon et al., 2015; Narusaka et al., 2015). YEL induces stomatal closure and inhibits light-induced stomatal opening in Arabidopsis (Klüsener et al., 2002; Khokon et al., 2010a; Salam et al., 2013; Ye et al., 2013b, 2015).
Core Signaling Events Downstream of Mamp Perception in Guard Cells
Guard cell signaling induced by MAMPs involves in recruitment of second messengers, such as H2O2, NO, and Ca2+, phosphorylation events mediated by CDPKs, OST1, and MAPKs, and changes of transporter activity. In this section, we review the main signaling events upon perception of MAMPs with focus on findings from Arabidopsis guard cells.
Production of Reactive Oxygen Species in Guard Cell MAMP Signaling
Reactive oxygen species, particularly H2O2, are important second messengers in guard cell signaling induced by abiotic and biotic factors (Pei et al., 2000; Zhang et al., 2001; Khokon et al., 2010a; Hoque et al., 2012; Hossain et al., 2013; Ye et al., 2013a; Kadota et al., 2014; Sobahan et al., 2015). Researches using leaf disks have provided most of the current knowledge of MAMP-induced H2O2 production. Recent studies further revealed that MAMPs including flg22, elf26, LPS, chitosan, β-1→3-linked glucans and YEL induce accumulation of H2O2 in guard cells (Desikan et al., 2008; Allègre et al., 2009; Ma W. et al., 2009; Desclos-Theveniau et al., 2012; Ma et al., 2013; Salam et al., 2013; Ye et al., 2015).
One of the main mechanisms involved in H2O2 production is mediated by plasma membrane-localized NAD(P)H oxidases, RBOHD and RBOHF. NAD(P)H oxidases transfer electrons from cytosolic NAD(P)H to apoplastic oxygen, leading to superoxide production. The superoxide can be converted to H2O2 through dismutation by unknown superoxide dismutase (Jannat et al., 2011; Suzuki et al., 2011). The produced H2O2 can accumulate in guard cells through diffusion and water channels (Henzler and Steudle, 2000; Bienert et al., 2007; Grondin et al., 2015). In ABA and MeJA signaling, both RBOHD and RBOHF redundantly regulate H2O2 production and stomatal closure (Kwak et al., 2003; Suhita et al., 2004; Munemasa et al., 2007). On the other hand, it seems that RBOHD plays a prominent role in flg22- and elf18-induced stomatal closure and H2O2 production in leaf (Mersmann et al., 2010; Macho et al., 2012; Kadota et al., 2014; Figure 1). Regarding the activation of NAD(P)H oxidases, studies have shown that elevation of free cytosolic Ca2+ concentration ([Ca2+]cyt) and phosphorylation by CDPKs are important for flg22- and elf18-induced H2O2 production in leaf disks (Boudsocq et al., 2010; Dubiella et al., 2013; Kadota et al., 2014), but not for ABA- and MeJA-induced H2O2 production in guard cells (Suhita et al., 2004; Munemasa et al., 2011; Brandt et al., 2015). Ca2+-independent phosphorylation is essential to the activation of NAD(P)H oxidases. In ABA signaling, the Ca2+-independent protein kinase, OST1, phosphorylates RBOHF at Ser13 and Ser174 and interacts with RBOHD (Sirichandra et al., 2009; Acharya et al., 2013) and is essential for H2O2 production in guard cells (Mustilli et al., 2002; Yin et al., 2013). Recent studies revealed that phosphorylation of several phosphorylation sites at the N-terminal part of RBOHD including Ser39 and Ser343 is increased in response to flg22 and elf18, which is independent on the elevation of [Ca2+]cyt but dependent on the Ca2+-independent activation of RLCKs, particularly BIK1 (Kadota et al., 2014; Li et al., 2014). Further results showed that phosphor-dead mutations of these phosphorylation sites suppress H2O2 production and stomatal closure induced by flg22 and elf18 and phosphor-mimetic mutations of the sites do not induce H2O2 production and stomatal closure but complement the flg22- and elf18-induced H2O2 production and stomatal closure in plants of loss-of-function mutations of BIK1 and another RLCK, PBL1. These results indicate that phosphorylation of these sites by RLCKs is essential but not sufficient to induce H2O2 production in leaf disks. To integrate the Ca2+-dependent and -independent regulation of RBOHD, the authors proposed that the BIK1-mediated phosphorylation primes RBOHD activation by increasing the sensitivity to the Ca2+-based regulation (Kadota et al., 2014, 2015; Li et al., 2014). Future work is needed to elucidate this hypothesis.
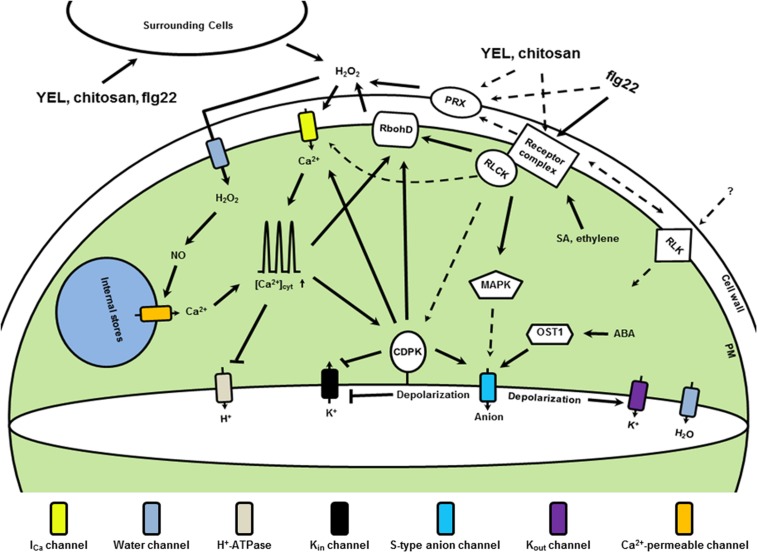
FIGURE 1.
Simplified microbe associated molecular patterns (MAMP) signaling in Arabidopsis guard cells. Upon perception of MAMPs, such as YEL, chitosan and flg22, receptor complexes are formed in the plasma membrane, which activate receptor-like cytoplasmic kinases (RLCKs). Downstream events are H2O2 production mediated by RbohD and PRXs, and activation of MAPKs and unidentified ICa channels. H2O2 accumulates in the cytosol and induces production of NO, which regulates Ca2+ release from internal stores. Elevation of [Ca2+]cyt negatively regulates the activity of H+-ATPases and activates CDPKs. Regulation by upstream components is also essential for CDPK activation. CDPKs negatively regulate Kin channel activity but positively regulate ICa channels and S-type anion channels. MAPK and resting OST1 activity are essential for S-type anion channel activation. Ethylene and SA at resting level regulate receptor levels. ABA at resting level may contribute to the resting OST1 activity. Increasing RLKs, such as GHR1, CRKs, and LecRKs, are found to be involved in MAMP signaling, but their activation is largely unknown. Arrowheads designate positive regulation and bars show negative regulation. Dashed lines indicate possible pathways need to be clarified, and question marks unconfirmed components.
Another important mechanism involved in H2O2 production in guard cell is mediated by class III peroxidases (PRXs; Figure 1). Both internal factors, such as SA (Mori et al., 2001; Khokon et al., 2011), isothiocyanates (ITCs; Hossain et al., 2013; Sobahan et al., 2015) and methylglyoxal (Hoque et al., 2012), and external factors, such as YEL (Khokon et al., 2010a), chitosan (Khokon et al., 2010b), flg22 (O’Brien et al., 2012), and elf26 (O’Brien et al., 2012), induce H2O2 production mediated by PRXs. Pharmacological and genetic studies further showed that salicylhydroxamic acid (SHAM)-sensitive PRXs but not RBOHD or RBOHF are essential for stomatal closure and H2O2 production in guard cells induced by SA, ITCs, methylglyoxal, chitosan and YEL (Khokon et al., 2010a,b, 2011; Hoque et al., 2012; Hossain et al., 2013). The chemistry of H2O2 production by PRXs can be affected by factors including pH and reductants. For example, under an acidic reaction condition, SA itself functions as electron donor to produce superoxide catalyzed by PRXs, which then can be converted to H2O2 by dismutases (Mori et al., 2001). Under an alkali condition, PRXs can directly utilize electron donors to produce H2O2, in which it seems that thiol compounds are preferred as the electron donors (Bolwell et al., 1995; Bolwell et al., 2002). While the identification of reductants in a physiological context remains challenging, apoplast alkalization is widely observed in plants treated by MAMPs such as flg22, elf18, and chitin (Felix et al., 1999; Felle et al., 2004; Zipfel et al., 2006; Boller and Felix, 2009). Recent studies have tried to identify the PRXs involved in the H2O2 production induced by MAMPs. Two PRXs, PRX33, and PRX34, were shown to be involved in H2O2 production induced by flg22, elf26, and fungal derived MAMPs (Daudi et al., 2012; O’Brien et al., 2012). However, it remains unknown whether these PRXs are involved in stomatal movement.
Production of Nitric Oxide Induced by MAMPs in Guard Cells
Nitric oxide has been widely shown to be involved in stomatal movement induced by various biotic and abiotic stimuli such as ABA, MeJA, SA, allyl isothiocyanate (AITC), and flg22 (Neill et al., 2002; Melotto et al., 2006; Garcia-Mata and Lamattina, 2007; Munemasa et al., 2007; Khokon et al., 2010a, 2011). Flg22, LPS, YEL and chitosan induce NO production in guard cells, which is required for stomatal closure (Melotto et al., 2006; Srivastava et al., 2009; Khokon et al., 2010a,b). Application of H2O2 has been shown to induce NO production in Arabidopsis guard cells (Bright et al., 2006). It is likely that H2O2 contributes to NO production induced by MAMPs. Genetic and pharmacological studies have shown that H2O2 is involved in ABA-induced NO production (Bright et al., 2006). Pharmacological studies have also shown that H2O2 is involved in NO production induced by YEL and chitosan (Srivastava et al., 2009; Khokon et al., 2010a,b). However, the mechanism how H2O2 is involved in NO production remains unknown. Cyclic AMP has been shown to be involved in LPS-induced NO production (Ma W. et al., 2009). Enzymatic mechanism mediated by nitrate reductases and NO synthase-like enzymes and non-enzymatic mechanism are involved in generation of NO in plant cells (Desikan et al., 2002; Moreau et al., 2008; Neill et al., 2008; Gayatri et al., 2013). Pharmacological studies have suggested that NO synthase-like enzymes were involved in flg22-, LPS-, and chitosan-induced stomatal closure (Melotto et al., 2006; Srivastava et al., 2009). However, the genes of NO synthase-like enzymes remain to be identified. It has been suggested that nitrate reductases were involved in chitosan-induced NO production (Srivastava et al., 2009). Two Arabidopsis, NIA1 and NIA2, were reported to be involved in ABA- and SA-induced NO production and stomatal closure (Desikan et al., 2002; Hao et al., 2010). Further investigation of the roles of NIA1 and NIA2 in MAMP-induced NO production and stomatal closure may move the field forward.
Elevation of [Ca2+]cyt Induced by MAMPs in Guard Cells
Cytosolic Ca2+ has long been considered as an important second messenger in guard cell signaling induced by various internal and external stimuli (Shimazaki et al., 2007; Kim et al., 2010; Roelfsema and Hedrich, 2010). A lot of methods have been introduced to monitor the [Ca2+]cyt in guard cells including fluorescent dyes such as fura-2 and fluo-3, and genetically encoded indicators such as aequorin, yellow cameleon and R-GECO1 (Irving et al., 1992; Allen et al., 1999a,b; Harada and Shimazaki, 2009; Keinath et al., 2015). Among these methods, real-time imaging of live guard cells expressing the fluorescence resonance energy transfer-based indicator, yellow cameleon, has greatly advanced our understanding of Ca2+ signaling (Allen et al., 1999b; Mori et al., 2006). Based on this technique, we learn that spontaneous repetitive elevation of [Ca2+]cyt occurs in guard cells, that stomata respond differently to different patterns of elevation of [Ca2+]cyt (Allen et al., 2000, 2001), and that Ca2+ influx from the apoplast and Ca2+ releasing from internal stores are essential for the elevation of [Ca2+]cyt induced by stimuli such as ABA and MeJA (Allen et al., 2000; Hamilton et al., 2000; Pei et al., 2000; Klüsener et al., 2002; Garcia-Mata et al., 2003; Munemasa et al., 2007; Akter et al., 2012; Hossain et al., 2014). Several MAMPs including chitosan, YEL, flg22 and chitin have been demonstrated to induce elevation of [Ca2+]cyt in guard cells (Klüsener et al., 2002; Khokon et al., 2010a; Salam et al., 2013; Ye et al., 2013b; Thor and Peiter, 2014; Keinath et al., 2015). Pharmacological and genetic studies reveal that Ca2+ influx and Ca2+ release mechanisms are essential for elevation of [Ca2+]cyt induced by chitosan, YEL and flg22 (Klüsener et al., 2002; Ye et al., 2013b; Thor and Peiter, 2014). Influx of Ca2+ is mediated by plasma membrane non-selective Ca2+-permeable cation channels (ICa channels), which are activated by hyperpolarization (Hamilton et al., 2000; Pei et al., 2000). It has been shown that LPS, chitosan and YEL activate ICa channels in guard cells (Klüsener et al., 2002; Ali et al., 2007; Ye et al., 2013b). The identification of ICa channels involved in MAMP signaling remains challenging. Studies have suggested that glutamate receptor-like channels (GLRs) are involved in elevation of [Ca2+]cyt in seedlings induced by flg22, elf18, and chitin (Kwaaitaal et al., 2011) and that cyclic nucleotide-gated channels (CNGCs) are involved in elevation of [Ca2+]cyt induced by LPS (Ali et al., 2007). On the other hand, recent studies suggest that neither GLRs nor CNGCs are essential for flg22-induced elevation of [Ca2+]cyt (Thor and Peiter, 2014). Recently, OSCA1 and its homologs have been identified as an ICa channel involved in hyperosmolality response (Hou et al., 2014; Yuan et al., 2014). It remains unknown whether OSCA1 is involved in MAMP signaling. Pharmacological studies suggested that many factors involved in Ca2+ releasing in guard cells, such as NO, cGMP, cADPR, and IP3 (Klüsener et al., 2002; Garcia-Mata et al., 2003; Hossain et al., 2014). Recent studies showed that Ca2+ releasing is essential for flg22-induced elevation of [Ca2+]cyt (Thor and Peiter, 2014). Application of NO does not activate ICa channels in the plasma membrane, but induces Ca2+ releasing, probably through cGMP-cADPR pathway (Garcia-Mata et al., 2003; Joudoi et al., 2013; Hossain et al., 2014). Therefore, it is likely that NO produced by MAMPs contributes to MAMP-induced elevation of [Ca2+]cyt in guard cells. The identification of the transporters involved in Ca2+ releasing remains challenging.
Sensing of Ca2+ in MAMP-induced Stomatal Movement
In Arabidopsis, there are a maximum of 250 proteins possibly having the Ca2+-binding EF-hand motif (Day et al., 2002), which play important roles in transducing the signal of Ca2+ to downstream events. In addition to the ubiquitous eukaryotic Ca2+ sensor calmodulin (CaM), other proteins containing EF-hand are CDPKs, CaM-like proteins (CML) and calcineurin B-like protein (CBL). CBLs can form complexes with CBL-interacting protein kinases (CIPKs) to convert the Ca2+ signal to phosphorylation events (Steinhorst and Kudla, 2013).
Ca2+-dependent protein kinases comprise a gene family of 34 members in Arabidopsis. The roles of the 34 CDPKs in plant growth and stress response can differ from each other due to their specificities in tissue expression, subcellular localization, [Ca2+]cyt dependency, substrates and regulation mechanisms (Boudsocq and Sheen, 2013; Liese and Romeis, 2013). So far, several members of CDPKs including CPK3, CPK4, CPK5, CPK6, CPK8, CPK10, CPK11, and CPK23 are identified as positive regulators in stomatal movement induced by ABA, MeJA, CO2 and exogenous H2O2 (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010, 2015; Munemasa et al., 2011; Hubbard et al., 2012; Merilo et al., 2013; Brandt et al., 2015). Recent works showed that CPK6 positively functions in YEL-induced stomatal closure and inhibition of light-induced stomatal opening (Ye et al., 2013b). On the other hand, CPK6 shows a negative effect on H2O2 scavenging mechanism induced by YEL. Since the CPK6 also positively functions in stomatal closure induced by ABA and MeJA (Mori et al., 2006; Munemasa et al., 2011; Brandt et al., 2015), the results suggest that CPK6 is a convergent point of signaling for stomatal closure induced by abiotic and biotic stimuli. The important role of CPK6 is related to its regulation of S-type anion channels (see Activation of S-type Anion Channels in Response to MAMPs in Guard Cells). CDPKs including CPK6 are activated by flg22 and involved in flg22-induced H2O2 production in leaf disks and mesophyll cells (Boudsocq et al., 2010; Dubiella et al., 2013; Guzel et al., 2015). Recent studies showed that cpk3 cpk6 cpk5 cpk11 loss-of-function mutation shows a trend to partly impair flg22-induced stomatal closure in a nanoinfusion experiment (Guzel et al., 2015). Nevertheless, the quadruple mutation did not strongly impair flg22-induced stomatal closure, which may attribute to the compensatory mechanism by other Ca2+ sensing mechanisms. A recent study showed that CPK28 negatively regulates flg22-induced stomatal closure and that BIK1 is a target of CPK28 (Monaghan et al., 2014). Results from in vitro experiments have shown that activation of CDPKs is dependent on [Ca2+]cyt (Boudsocq et al., 2012). In ABA signaling, the activation of CDPKs including CPK6 seems to be solely dependent on the elevation of [Ca2+]cyt induced by ABA (Laanemets et al., 2013; Brandt et al., 2015). On the other hand, the activation of CDPKs including CPK6 in response to flg22 is dependent on both elevation of [Ca2+]cyt and FLS2-dependent signaling (Boudsocq et al., 2010; Dubiella et al., 2013). These results suggest the activation of CDPK differs in different signalings.
Research on involvement of CMLs and CBL-CIPK pairs in MAMP signaling in guard cells is scarce. In Arabidopsis, CML24 has been shown to be involved in LPS-induced stomatal closure, probably by regulating NO production (Ma et al., 2008; Walker, 2011). Recent studies have suggested that CBL-CIPK pairs are involved in stomatal response (Maierhofer et al., 2014). Future research is needed to identify CMLs and CBL-CIPKs involved in guard cell MAMP signaling.
Ca2+-sensing receptor (CAS) represents a Ca2+ sensor of low Ca2+ affinity/high-capacity that does not contain EF-hand motif and is associated with thylakoid membranes (Han et al., 2003; Nomura et al., 2008; Weinl et al., 2008). Studies have revealed that CAS is involved in stomatal closure induced by high extracellular Ca2+ and flg22, but not ABA (Han et al., 2003; Weinl et al., 2008; Nomura et al., 2012). In these studies, CAS has been shown to be important for elevation of [Ca2+]cyt induced by high extracellular Ca2+ in guard cells and is required for flg22-induced elevation of [Ca2+]cyt in Arabidopsis plants. These results suggest that Ca2+ releasing from chloroplast involving CAS is essential for flg22-induced stomatal closure.
Open Stomata 1 Involvement in MAMP-induced Stomatal Closure
There are increasing results showing that OST1 plays a central role in guard cell signaling induced by various stimuli such as high CO2, low humidity, and ozone (Xie et al., 2006; Ache et al., 2010; Vahisalu et al., 2010; Xue et al., 2011; Merilo et al., 2013). Recent studies have revealed that OST1 kinase is also involved in stomatal closure induced by MAMPs including flg22, LPS, and YEL (Melotto et al., 2006; Montillet et al., 2013; Guzel et al., 2015; Ye et al., 2015). The importance of OST1 seems to be related to its essential role in activation of S-type anion channels (see Activation of S-type Anion Channels in Response to MAMPs in Guard Cells), because OST1 is not involved in elevation of [Ca2+]cyt and H2O2 accumulation induced by YEL but is involved in S-type anion channel activation induced by ABA, CO2, YEL, and high Ca2+ (Xue et al., 2011; Hua et al., 2012; Brandt et al., 2015; Ye et al., 2015).
To our knowledge, there is no evidence that kinase activity of OST1 is increased by stimuli other than ABA in guard cells. On the other hand, recent studies have shown that flg22 does not increase OST1 kinase in Arabidopsis suspension cells and YEL does not increase OST1 kinase in Arabidopsis guard cells (Montillet et al., 2013; Ye et al., 2015). OST1 is mainly activated upon inhibition of PP2Cs by ABA perception in plants (Mustilli et al., 2002; Ma Y. et al., 2009; Park et al., 2009). Flg22 does not increase ABA content in plant and YEL does not induce transcription of ABA responsive gene, RD29B, in guard cells (Nomura et al., 2012; Ye et al., 2015), suggesting that flg22 and YEL do not increase ABA content in guard cells. These results taken together raise the possibility that resting activity of OST1 kinase, which itself is very weak, is involved in stomatal closure induced by stimuli including flg22 and YEL (Figure 1). Further research is needed to validate this possibility.
Mitogen-activated Protein Kinase Involvement in MAMP-induced Stomatal Movement
Activation of MAPKs including MPK3, MPK4, and MPK6, is one of the early response induced by MAMPs such as flg22, elf18, LPS, and chitin (Zipfel et al., 2006; Boller and Felix, 2009; Ranf et al., 2015). It has been shown that MPK3 and MPK6 are essential for stomatal closure induced by flg22 and LPS (Gudesblat et al., 2009; Montillet et al., 2013). On the other hand, MPK3 and MPK6 seem not to be activated by ABA or involved in ABA-induced stomatal closure (Gudesblat et al., 2007; Montillet et al., 2013). Interestingly, MPK3 is involved in ABA inhibition of light-induced stomatal opening (Gudesblat et al., 2007), which is reminiscent of the involvement of OST1 in stomatal closure induced by flg22 and YEL. MPK4 is reported to be negatively involved in stomatal closure induced by Pst DC3000, in which flg22 is the dominant MAMP sensed by guard cells (Zeng and He, 2010; Hettenhausen et al., 2012). MPK9 and MPK12 function redundantly in stomatal closure induced by ABA, MeJA, YEL, and chitosan (Jammes et al., 2009; Salam et al., 2012, 2013). Further results reveal that mutation of mpk9 mpk12 increased the susceptibility of Arabidopsis to spray-inoculated Pst DC3000 (Zeng and He, 2010; Jammes et al., 2011). On the other hand, it has been reported that MPK9 and MPK12 were not involved in stomatal closure induced by flg22 at 5 μM (Montillet et al., 2013). These results suggest that different MAMPs recruit different MAPKs to induce stomatal closure. MPK9 and MPK12 function redundantly in inhibition of light-induced stomatal opening induced by YEL while only mutation in MPK12 impaired ABA inhibition of light-induced stomatal opening (Salam et al., 2013; Des Marais et al., 2014). These results suggest that the regulation of MPK9 and MPK12 are differently regulated by ABA and YEL.
It has been reported that MPK3 and MPK6 are not involved in elevation of [Ca2+]cyt induced by flg22 and elf18 and that MPK9 and MPK12 are not involved in elevation of [Ca2+]cyt induced by YEL and chitosan (Salam et al., 2012, 2013). The activation of MAPKs can be induced by flg22 in a Ca2+-independent manner (Boudsocq et al., 2010; Ranf et al., 2011). These results suggest that MAPKs can function in parallel with Ca2+-dependent pathways to regulate stomatal movement induced by MAMPs. Note that activation of MAPKs can differs in mechanism among different MAMPs, as seen in recent studies that a RLCK, PBL27, is specifically required for activation of MAPKs by chitin but not flg22 (Shinya et al., 2014). MPK9 and MPK12 have been shown to be essential for activation of S-type anion channels induced by ABA, MeJA, and high extracellular Ca2+ (Jammes et al., 2009; Brandt et al., 2015; Khokon et al., 2015). It is likely that the function of MAPKs is related to regulation of S-type anion channels in MAMP-induced stomata movement.
Regulation of Plasma Membrane Transporters in MAMP-induced Stomatal Movement
Guard cell volume is tightly regulated by transporters, especially the ones in plasma membrane. Recent studies have revealed that MAMPs regulate plasma membrane transporters, including ICa channels, Kin channels, S-type anion channels and H+-ATPases in guard cells (Klüsener et al., 2002; Zhang et al., 2008; Liu et al., 2009; Koers et al., 2011; Ye et al., 2013b, 2015; Guzel et al., 2015). In this section, we review these findings.
Activation of ICa Channels in Response to MAMPs in Guard Cells
ICa channels in the plasma membrane function as a pathway for Ca2+ influx, which is activated at hyperpolarization condition (Hamilton et al., 2000; Pei et al., 2000). Patch clamp results have shown that ICa channels are activated by ABA, MeJA, exogenous H2O2, and MAMPs including YEL, chitosan and LPS in Arabidopsis guard cells (Pei et al., 2000; Murata et al., 2001; Klüsener et al., 2002; Ali et al., 2007; Munemasa et al., 2011; Ye et al., 2013b, 2015). Intriguingly, activation of ICa channels is observed with cytosol dialyzed with ATP-free solution. These results suggest that MAMPs including YEL, chitosan and LPS can activate ICa channels in a phosphorylation-independent manner. Since application of H2O2 can activate ICa channels, it is likely that MAMP-induced H2O2 contributes to activation of ICa channels. YEL and chitosan activate ICa channels in guard cells that have most of the cell wall-bind peroxidases removed and LPS activate ICa channels in guard cells with cytosol dialyzed with NAD(P)H-free solution (Klüsener et al., 2002; Ali et al., 2007; Ye et al., 2013b). These results suggest that MAMPs can activate ICa channels in a H2O2-independent manner and/or that ICa channels activated by exogenous H2O2 are different from the ones by MAMPs in these experimental conditions (Figure 1). Further studies reveal that CPK6 is required for activation of ICa channels induced by ABA, MeJA, and YEL (Mori et al., 2006; Munemasa et al., 2011; Ye et al., 2013b). On the other hand, it has been shown that suppression of EF-hand-containing proteins by an inhibitor, W7, is required for LPS activation of ICa channels (Ali et al., 2007). These results suggest that Ca2+-dependent mechanism can play both negative and positive roles in activation of ICa channels induced by different MAMPs.
Activation of S-type Anion Channels in Response to MAMPs in Guard Cells
Early studies have identified two types of anion channels in guard cells, S-type and R-type anion channels (Schroeder and Hagiwara, 1989; Hedrich et al., 1990; Schroeder and Keller, 1992). While R-type anion channel is activated transiently and shows strong voltage dependency, S-type is weakly voltage-dependent and lack of time-dependent inactivation. Therefore, activation of S-type anion channel is likely to induce long-term anion efflux and sustained depolarization, representing a hallmark of stomatal closure. In Arabidopsis guard cells, several genes have been identified as S-type anion channels including SLAC1 and SLAC1 homolog 3 (SLAH3), and R-type channels, ALMT12. However, SLAC1 shows the most prominent role in regulation of stomatal closure induced by various stimuli, such as ABA, CO2, darkness and high extracellular Ca2+ (Negi et al., 2008; Vahisalu et al., 2008; Meyer et al., 2010; Sasaki et al., 2010). Electrophysiological studies have shown that S-type anion channels are activated by chitosan, YEL and flg22 in guard cells (Koers et al., 2011; Ye et al., 2013b, 2015; Guzel et al., 2015). Further results have shown that both SLAC1 and SLAH3 were required for flg22-induced stomatal closure and activation of S-type anion channel (Montillet et al., 2013; Guzel et al., 2015). It is unknown whether ALMT12 is involved in MAMP-induced stomatal movement. While the regulation R-type anion channels remains largely unknown, the regulation of S-type anion channels has been extensively studied. Here, we briefly review the regulation of SLAC1.
In Arabidopsis guard cells, elevation of [Ca2+]cyt is essential for S-type anion channel activation induced by ABA, high extracellular Ca2+ and CO2, but itself is not sufficient for activation of S-type anion channels (Allen et al., 2002; Siegel et al., 2009; Xue et al., 2011). These results raised the hypothesis that external and internal stimuli enhance/prime guard cells to respond to increased [Ca2+]cyt levels and to activate S-type anion channels (Young et al., 2006; Siegel et al., 2009; Kim et al., 2010; Hubbard et al., 2012). Since the identification of SLAC1 as the main S-type anion channel in guard cells, many regulators have been revealed by in vitro experiments including CDPKs, CBL-CIPK complexes, OST1 and GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1) as positive regulators and PP2Cs as negative regulators (Geiger et al., 2009, 2010, 2011; Lee et al., 2009; Brandt et al., 2012, 2015; Hua et al., 2012; Scherzer et al., 2012; Maierhofer et al., 2014). However, only a few of the regulators have been shown to positively function in stomatal closure, including CPK3, CPK5, CPK6, CPK23, OST1, and GHR1 (Mustilli et al., 2002; Mori et al., 2006; Hua et al., 2012; Merilo et al., 2013; Brandt et al., 2015). In case of CPK23 there is also evidence that CPK23 negatively regulates salt and drought response (Ma and Wu, 2007). These results point out that the regulation of S-type anion channels can be complicated in guard cells in response to different stimuli. CPK6 and OST1 are required for stomatal closure induced by YEL and flg22 and activation by YEL of S-type anion channel (Melotto et al., 2006; Hua et al., 2012; Montillet et al., 2013; Ye et al., 2013b, 2015; Guzel et al., 2015). CPK6 does not seem to be engaged in activation of OST1 kinase by ABA and OST1 is not involved in [Ca2+]cyt elevation in guard cells induced by YEL (Brandt et al., 2015; Ye et al., 2015). These results raise the possibility that CPK6 and OST1 directly regulate S-type anion channels in flg22 and YEL signaling as they do in vitro. Note that OST1 may function at resting activity. Further research on identification of phosphorylation sites of SLAC1 and their regulation by components such as CDPKs and OST1 is needed in order to further elucidate the regulation of S-type anion channels by MAMPs (Figure 2).
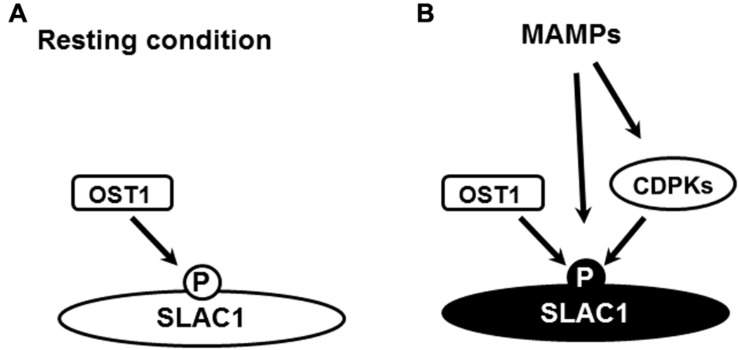
FIGURE 2.
Hypothetical regulation of SLAC1 by phosphorylation in guard cell MAMP signaling. (A) Resting OST1 activity contributes to resting level of SLAC1 phosphorylation, particularly to the level of S120 phosphorylation, which is essential for the activation of SLAC1 by MAMPs. (B) In response to MAMPs, CDPK-dependent and –independent mechanisms change the phosphorylation status of SLAC1 including the phosphorylation of S59, resulting in SLAC1 activation. The change of color of letter “P” and “SLAC1” from black in (A) to white in (B) indicates a changed phosphorylation status and activation status, respectively.
Studies have demonstrated that CPK6 and OST1 both can phosphorylate SLAC1 at Ser59 and Ser120 in vitro, which are probably dephosphorylated by PP2Cs (Geiger et al., 2009; Vahisalu et al., 2010; Brandt et al., 2012, 2015; Maierhofer et al., 2014). Recent studies showed that phosphorylation of Ser59 and S120 redundantly function in S-type anion channel activation by ABA in guard cells (Maierhofer et al., 2014; Brandt et al., 2015). On the other hand, mutation of Ser120 impaired stomatal closure induced by various stimuli other than ABA (Vahisalu et al., 2010; Merilo et al., 2013). These results suggest that different stimuli induce different phosphorylation pattern of S-type anion channels leading to stomatal closure. Further results showed that phosphorylation of Thr531 in SLAC1 results in constitutive activation of anion currents and phosphorylation of Ser59 and Ser120 are not sufficient for SLAC1 activation in oocytes (Maierhofer et al., 2014). These phosphorylation sites are strong candidates for the regulation by kinases and phosphatases in guard cells. The exist of different sites with different functions provides the molecular basis for Ca2+ priming model mentioned above.
Regulation of Potassium Channels and H+-ATPases by MAMPs in Guard Cells
Inward-rectifying K+ channels function as the main gate for K+ influx to the cytosol, while outward-rectifying K+ channels (Kout channels) function as the main gate for K+ efflux to the apoplast. Both Kin channels and Kout channels are voltage-dependent and do not inactivate with time (Schroeder, 1988; Blatt, 1990). The non-inactivation property of potassium channels allows long-term efflux of K+ needed during stomatal closure and influx of K+ needed during stomatal opening. Kout channels are activated but Kin channels are deactivated by depolarization of plasma membrane. In addition to the plasma membrane potential, flg22 suppressed both Kin channels, which was mediated by FLS2 and G-protein (Zhang et al., 2008). Recent studies also showed that YEL suppresses Kin channels, which is mediated by CPK6 (Ye et al., 2013b). Intriguingly, flg22 seems not to affect the voltage-dependency of Kin channels (Zhang et al., 2008), while ABA shifts the voltage dependency of Kin channels to more hyperpolarization (Armstrong et al., 1995). These results indicate that the suppression of Kin channel activity by ABA and MAMPs can be different in mechanism and suggest that decrease in number of active Kin channels contributes to the inhibition by MAMPs. It has been shown that KAT1, the dominant Kin channel in guard cells, undergoes internalization during stomatal closure (Sutter et al., 2007; Eisenach et al., 2012), and traffic system is important for flg22-induced stomatal closure (Spallek et al., 2013). Further studies suggested that phosphorylation of CDPK recognition sites in KAT1 by protein kinase C activator suppresses KAT1-mediated currents with voltage dependency unchanged (Sato et al., 2010). These results raise the possibility that phosphorylation by CPK6 of KAT1 contributes to YEL suppression of Kin channel activity. OST1 has been shown to phosphorylate KAT1 at Ter306 in vitro, which is critical for KAT1 activation in oocytes (Sato et al., 2009). It is likely that OST1 is involved in suppression of Kin channel activity by MAMPs. Flg22 also suppresses Kout channels in Arabidopsis guard cell (Zhang et al., 2008). It remains unknown whether this is common response for other MAMPs. Though activity of Kout channels is decreased, K+ efflux is sufficient for stomatal closure induced by flg22.
Plasma membrane H+-ATPases transport H+ into the apoplast at the expense of ATP, leading to hyperpolarization of plasma membrane, the driving force for stomatal opening in the light. It is known that ABA inhibits H+-ATPases in guard cells, which is essential for stomatal closure and inhibition of light-induced stomatal opening (Goh et al., 1996; Merlot et al., 2007). It has been shown that H2O2, NO, Ca2+, and phosphatidic acid contribute to ABA inhibition of H+-ATPases (Kinoshita et al., 1995; Zhang et al., 2004, 2007; Takemiya and Shimazaki, 2010; Uraji et al., 2012; Yin et al., 2013). Various MAMPs including flg22, chitosan, YEL and β-1→3-linked glucan inhibit light-induced stomatal opening. Studies have also shown that constitutive activation of AHA1 by ost2 mutation, the dominant H+-ATPase in guard cells, impairs stomatal closure induced by flg22 and LPS (Liu et al., 2009). These results suggest that H+-ATPases are suppressed by MAMPs in guard cells.
Involvement of Phytohormones in MAMP-induced Stomatal Response
It has been reported that stomatal closure induced by flg22 and LPS was impaired in ABA-deficient mutant (Melotto et al., 2006; Montillet et al., 2013; Du et al., 2014), suggesting endogenous ABA is involved in flg22 and LPS signaling in guard cells. This idea is supported by the fact that the master regulator of ABA signaling, OST1, is required for stomatal closure induced by flg22 and LPS. On the other hand, flg22 was shown not to induce ABA synthesis in Arabidopsis leaves and activation of OST1 in Arabidopsis suspension cells (Nomura et al., 2012; Montillet et al., 2013). These results suggest that resting level of ABA but not the elevating level of ABA induced by flg22 is required for signaling in guard cells. A possible function of the resting level of ABA is to produce the resting activity of OST1 (Figure 1). On the other hand, YEL and chitosan induced stomatal closure in ABA-deficient mutant (Issak et al., 2013). However, we may not be able to exclude the possibility that endogenous ABA is not required for stomatal closure induced by YEL and chitosan, since there is considerable ABA in these ABA-deficient mutants. For example the content of ABA in aba2-2 is around 23% of those in wild type (Nambara et al., 1998). These results raise the possibility that the remained ABA is enough for stomatal closure induced by YEL and chitosan. Future work is needed to elucidate the role of endogenous ABA in stomatal closure induced by MAMPs.
Stomatal closure induced by LPS and Pst DC3000 is impaired in SA-deficient mutant (Melotto et al., 2006; Zeng and He, 2010), suggesting that endogenous SA is involved in stomatal closure induced by LPS and flg22. It seems that SA functions through NPR1, a master regulator of SA signaling, in flg22 signaling, since stomatal closure induced by Pst DC3000 and Pst DC3118 was impaired in npr1-1 mutant (Zeng and He, 2010). Flg22 and LPS also induces SA production and expression of SA-responsive gene, PR1, in Arabidopsis leaves (Denoux et al., 2008; Tsuda et al., 2008; Nomura et al., 2012). A role of SA may be related to the regulation of PRR levels in guard cells, since recent works have shown that SA finely regulates levels of PRRs including FLS2 in Arabidopsis (Tateda et al., 2014). The endogenous SA may be recruited as a substrate for PRXs that are involved in flg22-indued stomatal closure (Mori et al., 2001). Future work is needed to elucidate how endogenous SA functions in guard cell MAMP signaling.
Studies have shown that ethylene signaling components, ETR1 and EIN2, are involved in flg22-induced stomatal closure probably by regulating FLS2 transcription and protein level and H2O2 production, but only in an unwounded condition (Mersmann et al., 2010). These results suggest that ethylene signaling is required for guard cell flg22 signaling in a stimulus-dependent manner. Ethylene production has been widely observed to be induced by various MAMPs including flg22, elf18, elf26, chitin, and YEL (Felix et al., 1991; Kunze et al., 2004; Zipfel et al., 2006). It is likely that ethylene signaling is also involved in stomatal closure induced by other MAMPs.
Other Components Involved in MAMP Signaling in Guard Cells
Increasing evidences are emerging that reactive carbonyl species (RCS), such as 4-hydroxy-2-nonenal and acrolein, is produced by both enzymatic and non-enzymatic mechanisms and regulates guard cell signalings (Montillet et al., 2013; Islam et al., 2015). Recent studies have shown that RCS production mediated by a lipoxygenase (LOX), LOX1, is required for stomatal closure induced by flg22 but not ABA (Montillet et al., 2013). It remains unknown how the RCS is involved in flg22 signaling.
In addition to GHR1, recent studies identified several RLKs, including L-type lectin receptor kinase-VI.2 (LecRK-VI.2), LecRK-V.5, and Cysteine-rich receptor-like kinases (CRKs), which are involved in guard cell MAMP signaling. LecRK-V.5 has been reported to negatively regulate stomatal closure and H2O2 accumulation in guard cells induced by flg22, LPS, elf26 and ABA (Desclos-Theveniau et al., 2012). LecRK-VI.2 positively regulates stomatal closure induced by flg22 and elf26 but not ABA (Singh et al., 2012). Further results show LecRK-VI.2 is not involved in H2O2 production but activation of MAPKs induced by flg22 in leaves. Recent phenotypic studies of the T-DNA insertion mutants of CRK family have identified many of its members are involved in stomatal closure induced by stimuli including flg22, chitin and ABA, and shown that CRKs provide signaling specificity (Bourdais et al., 2015). For these RLKs, the future challenge is to elucidate their regulation and substrates in MAMP signaling.
Redundancy in Guard Cell Mamp Signaling
Great advance in understanding guard cell signaling including the one induced by MAMPs has been made based on genetic methods, particularly using mutant plants. In these studies, functionally redundant mechanisms are suggested. Typical examples can be found in H2O2 production, sensing of Ca2+, MAPK function and regulation of anion channels. However, these mechanisms do have their own specificity. For example, CPK3 and CPK6 have different Ca2+ sensitivity, with CPK6 activated at lower Ca2+ concentration, but both can phosphorylate SLAC1 (Boudsocq et al., 2012; Scherzer et al., 2012; Laanemets et al., 2013). Theoretically, CPK6 functions at lower Ca2+ concentration, while CPK6 and CPK3 both function at higher concentration, which are therefore considered functionally redundant at higher concentration. It can be expected that functional redundancy appears depending on conditions and the strength of stimuli is an important factor to determine the occurrence of redundancy. A challenge for future dissection of signaling is to define the biological conditions and mimic them in the labs. It is also needed to mention that the functional redundancy in signaling should not be confused with compensatory mechanisms that have been widely observed in extreme experimental conditions for plants, such as constitutive loss-of-function mutations. It is likely that to change the properties of functionally redundant components is a common mechanism for compensation. For example, the gene expression level of SLAH3 doubles in slac1 mutant, which may account for the partial flg22-induced stomatal closure in slac1 mutant (Geiger et al., 2011; Guzel et al., 2015). On the other hand, flg22-induced stomatal closure was abolished in ost1 mutant in the same study but OST1 seems not to activate SLAH3. It is therefore possible that SLAC1 plays a dominant role but not function redundantly with SLAH3 in flg22-induced stomatal closure in wild-type plants. In the future, the challenge is to validate the contribution of these suggested redundant components to MAMP signaling in wild-type plants. Elucidation of the compensatory mechanisms in these mutants may also contribute to our understanding and is of particular importance in practical aspect.
Comparison of Mamp Signalings in Leaf Epidermal Cells, Mesophyll Cells and Guard Cells
Unlike epidermal and mesophyll cells, guard cells do not have plasmodesmata but function autonomously. FLS2 and co-receptor, BAK1, are expressed in epidermal cells, mesophyll cells and guard cells, suggesting that the similar perception mechanism of flg22 exist in these three cell types (Robatzek et al., 2006; Shang et al., 2015). Future biochemical studies in a cell-specific context are needed to validate this suggestion. While many downstream components such as RbohD, CPK6, MPK3, and MPK6 seem to be expressed ubiquitously in the leaf, components, such as MPK9, MPK12, OST1, SLAC1, and ALMT12 are mainly expressed in guard cells (Mustilli et al., 2002; Mori et al., 2006; Negi et al., 2008; Vahisalu et al., 2008; Jammes et al., 2009; Meyer et al., 2010; Sasaki et al., 2010). Endogenous hormone ABA concentration is much higher in guard cells than epidermal and mesophyll cells (Waadt et al., 2014). These differences in signaling component levels determine the output of MAMP responses in different cell types. For example, flg22 induces H2O2 production and [Ca2+]cyt elevation in guard cells, epidermal cells and mesophyll cells (Ranf et al., 2008; Jeworutzki et al., 2010; Desclos-Theveniau et al., 2012; Macho et al., 2012; Thor and Peiter, 2014; Guzel et al., 2015; Keinath et al., 2015). Flg22-induced depolarization of plasma membrane, the driven force of stomatal closure, is not impaired in rbohD, slah3 and fusicoccin-treated mesophyll protoplasts, but flg22-induced stomatal closure is impaired in rbohD, slah3, and ost2 plants, suggesting that flg22-induced depolarization of plasma membrane is different in mechanism in the two cell types (Jeworutzki et al., 2010; Macho et al., 2012; Kadota et al., 2014; Li et al., 2014; Guzel et al., 2015).
Concluding Remarks and Outlooks
Since 2006, stomatal immunity has emerged as an important part of plant immunity. The output is stomatal closure and inhibition of stomatal opening to prevent microbe invasion. Though it is known that MAMPs are important signals to trigger stomatal immunity, we know little about how and how much MAMPs are exposed to the surveillance of guard cells. For example, stomatal closure by Pst DC3118 but not E. coli is abolished in fls2 mutant (Melotto et al., 2006; Zeng and He, 2010), indicating that guard cells differently sense different pathogens even though it seems that they have the same set of MAMPs. It also remains unclear whether MAMPs including elf18/26, normally considered to exist inside the cells, are exposed to cell surface. A recent study suggests that Arabidopsis plants sense elongation factor Tu in the outer membrane vesicles secreted by Gram-negative bacteria (Bahar et al., 2016).
Though plasma membrane-localized PRRs in guard cells are widely accepted to function to perceive MAMPs, MAMP signaling also happens in the cell wall. It remains unknown whether the apoplast signaling is regulated by the plasm membrane-localized PRRs or there are PRRs in the apoplasts. Upon binding of PRRs and ligands, co-receptors are immediately recruited to form receptor complexes, which determine the specificity of signalings. The core signaling downstream to induce stomatal closure is that MAMP perception induces Ca2+-dependent mechanisms to activate S-type anion channels, which is dependent on Ca2+-independent mechanisms (Figures 1 and 2). Future research is needed to unravel new Ca2+-dependent mechanisms. Emergent challenge is to elucidate how Ca2+-independent mechanisms, such as the ones mediated by OST1 and MAPKs, are involved in MAMP signaling in guard cells. Signaling specificity provided by RLCKs and CRKs has been observed in MAMP-induced stomatal response. Further elucidation of these specificities is essential for dissecting MAMP signaling in guard cells.
The roles of phytohormones in guard cell MAMP signaling are still unclear. Though increase of phytohormone by methods such as direct application can induce stomatal movement, it is less likely that MAMPs increase the level of phytohormone to trigger stomatal movement, as seen in the case that flg22 does not increase ABA content. Therefore, it is important to note that the function of elevating level of phytohormone can be different from that of phytohormone in unstressed plants. An emerging role is that phytohormones, such as ethylene and SA, in unstressed plants regulate the level of PRRs. A possible role is that phytohormones, such as ABA, are important for providing basal activity of important signaling components. The future challenge is to elucidate the roles of endogenous phytohormones in guard cell MAMP signaling.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to colleagues whose relevant studies were not cited owing to space constraints.
Footnotes
Funding. This work was supported by the Japan Society for the Promotion of Science (JSPS) [Grants-in-Aid for Fellows (to WY)].
References
- Acharya B., Assmann S. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69 451–462. 10.1007/s11103-008-9427-0 [DOI] [PubMed] [Google Scholar]
- Acharya B. R., Jeon B. W., Zhang W., Assmann S. M. (2013). Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 200 1049–1063. 10.1111/nph.12469 [DOI] [PubMed] [Google Scholar]
- Ache P., Bauer H., Kollist H., Al-Rasheid K. A. S., Lautner S., Hartung W., et al. (2010). Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 62 1072–1082. 10.1111/j.1365-313X.2010.04213.x [DOI] [PubMed] [Google Scholar]
- Akter N., Sobahan M. A., Uraji M., Ye W., Hossain M. A., Mori I. C., et al. (2012). Effects of depletion of glutathione on abscisic acid- and methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 76 2032–2037. 10.1271/bbb.120384 [DOI] [PubMed] [Google Scholar]
- Ali R., Ma W., Lemtiri-Chlieh F., Tsaltas D., Leng Q., von Bodman S., et al. (2007). Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and Innate Immunity. Plant Cell 19 1081–1095. 10.1105/tpc.106.045096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allègre M., Héloir M., Trouvelot S., Daire X., Pugin A., Wendehenne D., et al. (2009). Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol. Plant Microbe Interact. 22 977–986. 10.1094/MPMI-22-8-0977 [DOI] [PubMed] [Google Scholar]
- Allen G. J., Chu S. P., Harrington C. L., Schumacher K., Hoffmann T., Tang Y. Y., et al. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057. 10.1038/35082575 [DOI] [PubMed] [Google Scholar]
- Allen G. J., Chu S. P., Schumacher K., Shimazaki C. T., Vafeados D., Kemper A., et al. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342. 10.1126/science.289.5488.2338 [DOI] [PubMed] [Google Scholar]
- Allen G. J., Kuchitsu K., Chu S. P., Murata Y., Schroeder J. I. (1999a). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11 1785–1798. 10.2307/3871054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. J., Kwak J. M., Chu S. P., Llopis J., Tsien R. Y., Harper J. F., et al. (1999b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19 735–747. 10.1046/j.1365-313x.1999.00574.x [DOI] [PubMed] [Google Scholar]
- Allen G. J., Murata Y., Chu S. P., Nafisi M., Schroeder J. I. (2002). Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14 1649–1662. 10.1105/tpc.010448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F., Leung J., Grabov A., Brearley J., Giraudat J., Blatt M. R. (1995). Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1 a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc. Natl. Acad. Sci. U.S.A. 92 9520–9524. 10.1073/pnas.92.21.9520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud D., Hwang I. A. (2015). sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant 8 566–581. [DOI] [PubMed] [Google Scholar]
- Bahar O., Mordukhovic G., Luu D. D., Schwessinger B., Daudi A., Jehle A. K., et al. (2016). Bacterial outer membrane vesicles induce plant immune responses. Mol. Plant Microbe Interact. 25 846–848. 10.1094/MPMI-12-15-0270-R [DOI] [PubMed] [Google Scholar]
- Bauer H., Ache P., Lautner S., Fromm J., Hartung W., Al-Rasheid K. A. S., et al. (2013). The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23 53–57. 10.1016/j.cub.2012.11.022 [DOI] [PubMed] [Google Scholar]
- Beck M., Wyrsch I., Strutt J., Wimalasekera R., Webb A., Boller T., et al. (2014). Expression patterns of FLAGELLIN SENSING 2 map to bacterial entry sites in plant shoots and roots. J. Exp. Bot. 65 6487–6498. 10.1093/jxb/eru366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedini E., De Castro C., Erbs G., Mangoni L., Dow J. M., Newman M., et al. (2005). Structure-dependent modulation of a pathogen response in plants by synthetic O-antigen polysaccharides. J. Am. Chem. Soc. 127 2414–2416. 10.1021/ja0452166 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Møller A. L. B., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., et al. (2007). Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 282 1183–1192. 10.1074/jbc.M603761200 [DOI] [PubMed] [Google Scholar]
- Blatt M. R. (1990). Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta 180 445–455. 10.1007/BF00198799 [DOI] [PubMed] [Google Scholar]
- Blechert S., Brodschelm W., Hölder S., Kammerer L., Kutchan T. M., Mueller M. J., et al. (1995). The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. U.S.A. 92 4099–4105. 10.1073/pnas.92.10.4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. A. (2009). Renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60 379–406. 10.1146/annurev.arplant.57.032905.105346 [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Bindschedler L. V., Blee K. A., Butt V. S., Davies D. R., Gardner S. L., et al. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53 1367–1376. 10.1093/jexbot/53.372.1367 [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Buti V. S., Davies D. R., Zimmerlin A. (1995). The origin of the oxidative burst in plants. Free Radic. Res. 23 517–532. 10.3109/10715769509065273 [DOI] [PubMed] [Google Scholar]
- Boudsocq M., Droillard M. J., Regad L., Lauriere C. (2012). Characterization of Arabidopsis calcium-dependent protein kinases: activated or not by calcium? Biochem. J. 447 291–299. 10.1042/BJ20112072 [DOI] [PubMed] [Google Scholar]
- Boudsocq M., Sheen J. (2013). CDPKs in immune and stress signaling. Trends Plant Sci. 18 30–40. 10.1016/j.tplants.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M., Willmann M. R., McCormack M., Lee H., Shan L., He P., et al. (2010). Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464 418–422. 10.1038/nature08794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdais G., Burdiak P., Gauthier A., Nitsch L., Salojärvi J., Rayapuram C., et al. (2015). Large-scale phenomics identifies primary and fine-tuning roles for CRKs in responses related to oxidative stress. PLoS Genet. 11:e1005373 10.1371/journal.pgen.1005373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Brodsky D. E., Xue S., Negi J., Iba K., Kangasjarvi J., et al. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. U.S.A. 109 10593–10598. 10.1073/pnas.1116590109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Munemasa S., Wang C., Nguyen D., Yong T., Yang P. G., et al. (2015). Calcium specificity signaling mechanisms in abscisic acid signal transduction in guard cells. Elife 4:e3599 10.7554/eLife.03599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J., Desikan R., Hancock J. T., Weir I. S., Neill S. J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45 113–122. 10.1111/j.1365-313X.2005.02615.x [DOI] [PubMed] [Google Scholar]
- Cai R., Lewis J., Yan S., Liu H., Clarke C. R., Campanile F., et al. (2011). The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 7:e1002130 10.1371/journal.ppat.1002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Liang Y., Tanaka K., Nguyen C. T., Jedrzejczak R. P., Joachimiak A., et al. (2014). The kinase LYK5 is a major chitin receptor in and forms a chitin-induced complex with related kinase CERK1. Elife 3:e3766 10.7554/eLife.03766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nurnberger T., Jones J. D. G., et al. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448 497–500. 10.1038/nature05999 [DOI] [PubMed] [Google Scholar]
- Cutler S. R., Rodriguez P. L., Finkelstein R. R., Abrams S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61 651–679. 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- Daudi A., Cheng Z., O’Brien J. A., Mammarella N., Khan S., Ausubel F. M., et al. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24 275–287. 10.1105/tpc.111.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day I. S., Reddy V. S., Shad A. G., Reddy A. S. (2002). Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol. 3:Research0056 10.1186/gb-2002-3-10-research0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoux C., Galletti R., Mammarella N., Gopalan S., Werck D., De Lorenzo G., et al. (2008). Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 1 423–445. 10.1093/mp/ssn019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D. L., Auchinclossb L. C., Sukamtoha E., McKayc J. K., Logana T., Richardsb J. H., et al. (2014). Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc. Natl. Acad. Sci. U.S.A. 111 2836–2841. 10.1073/pnas.1321429111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M., Arnaud D., Huang T. Y., Lin G. J., Chen W. Y., Lin Y. C., et al. (2012). The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 8:e1002513 10.1371/journal.ppat.1002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R., Griffiths R., Hancock J., Neill S. A. (2002). A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 99 16314–16318. 10.1073/pnas.252461999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R., Horák J., Chaban C., Mira-Rodado V., Witthöft J., Elgass K., et al. (2008). The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS ONE 3:e2491 10.1371/journal.pone.0002491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Zhai Q., Deng L., Li S., Li H., Yan L., et al. (2014). Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26 3167–3184. 10.1105/tpc.114.128272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiella U., Seybold H., Durian G., Komander E., Lassig R., Witte C. P., et al. (2013). Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. U.S.A. 110 8744–8749. 10.1073/pnas.1221294110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C., Chen Z. H., Grefen C., Blatt M. R. (2012). The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J. 69 241–251. 10.1111/j.1365-313X.2011.04786.x [DOI] [PubMed] [Google Scholar]
- Felix G., Duran J. D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18 265–276. 10.1046/j.1365-313X.1999.00265.x [DOI] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Basse C. W., Boller T. (1991). Elicitor-induced ethylene biosynthesis in tomato cells: characterization and use as a bioassay for elicitor action. Plant Physiol. 97 19–25. 10.1104/pp.97.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. H., Herrmann A., Hanstein S., Huckelhoven R., Kogel K. H., Apoplastic P. H. (2004). Signaling in barley leaves attacked by the powdery mildew fungus Blumeria graminis f. sp. hordei. Mol. Plant Microbe Interact. 17 118–123. 10.1094/MPMI.2004.17.1.118 [DOI] [PubMed] [Google Scholar]
- Fu Y., Yin H., Wang W., Wang M., Zhang H., Zhao X., et al. (2011). β-13-Glucan with different degree of polymerization induced different defense responses in tobacco. Carbohydr. Polym. 86 774–782. 10.1016/j.carbpol.2011.05.022 [DOI] [Google Scholar]
- Furukawa T., Inagaki H., Takai R., Hirai H., Che F. S. (2014). Two distinct EF-Tu epitopes induce immune responses in rice and Arabidopsis. Mol. Plant Microbe Interact. 27 113–124. 10.1094/MPMI-10-13-0304-R [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C., Gay R., Sokolovski S., Hills A., Lamattina L., Blatt M. R. (2003). Nitric oxide regulates K+ and Cl- channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 100 11116–11121. 10.1073/pnas.1434381100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C., Lamattina L. (2007). Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium-and nitric oxide-mediated signaling pathways. Nitric Oxide 17 143–151. 10.1016/j.niox.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Gayatri G., Agurla S., Raghavendra A. S. (2013). Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 4:425 10.3389/fpls.2013.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Wu J. (2005). Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci. 168 487–491. 10.1016/j.plantsci.2004.09.012 [DOI] [Google Scholar]
- Geiger D., Maierhofer T., Al-Rasheid K. A. S., Scherzer S., Mumm P., Liese A., et al. (2011). Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 4:a32 10.1126/scisignal.2001346 [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., et al. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. U.S.A. 107 8023–8028. 10.1073/pnas.0912030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., et al. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. U.S.A. 106 21425–21430. 10.1073/pnas.0912021106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C. H., Kinoshita T., Oku T., Shimazaki K. I. (1996). Inhibition of blue light-dependent H+ pumping by abscisic acid in Vicia guard-cell protoplasts. Plant Physiol. 111 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L., Boller T. (2000). FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5 1003–1011. 10.1016/S1097-2765(00)80265-8 [DOI] [PubMed] [Google Scholar]
- Grimmer M. K., John Foulkes M., Paveley N. D. (2012). Foliar pathogenesis and plant water relations: a review. J. Exp. Bot. 63 4321–4331. 10.1093/jxb/ers143 [DOI] [PubMed] [Google Scholar]
- Grondin A., Rodrigues O., Verdoucq L., Merlot S., Leonhardt N., Maurel C. (2015). Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27 1945–1954. 10.1105/tpc.15.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat G. E., Iusem N. D., Morris P. C. (2007). Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 173 713–721. 10.1111/j.1469-8137.2006.01953.x [DOI] [PubMed] [Google Scholar]
- Gudesblat G. E., Torres P. S., Vojnov A. A. (2009). Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 149 1017–1027. 10.1104/pp.108.126870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. (1992). Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. U.S.A. 89 2389–2393. 10.1073/pnas.89.6.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel D. A., Scherzer S., Nuhkat M., Kedzierska J., Kollist H., Brosche M., et al. (2015). Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 208 162–173. 10.1111/nph.13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Albersheim P. (1978). Host-pathogen interactions: XIV. Isolation and partial characterization of an elicitor from yeast extract. Plant Physiol. 62 107–111. 10.1104/pp.62.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. W. A., Hills A., Köhler B., Blatt M. R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. U.S.A. 97 4967–4972. 10.1073/pnas.080068897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Tang R., Anderson L. K., Woerner T. E., Pei Z. M. A. (2003). cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425 196–200. 10.1038/nature01932 [DOI] [PubMed] [Google Scholar]
- Hao F., Zhao S., Dong H., Zhang H., Sun L., Miao C. (2010). Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 52 298–307. 10.1111/j.1744-7909.2010.00920.x [DOI] [PubMed] [Google Scholar]
- Harada A., Shimazaki K. (2009). Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant Cell Physiol. 50 360–373. 10.1093/pcp/pcn203 [DOI] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. (1990). Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 9 3889–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler T., Steudle E. (2000). Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 51 2053–2066. 10.1093/jexbot/51.353.2053 [DOI] [PubMed] [Google Scholar]
- Hettenhausen C., Baldwin I. T., Wu J. (2012). Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol. 158 759–776. 10.1104/pp.111.190074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque T. S., Uraji M., Ye W., Hossain M. A., Nakamura Y., Murata Y. (2012). Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J. Plant Physiol. 169 979–986. 10.1016/j.jplph.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Ye W., Munemasa S., Nakamura Y., Mori I. C., Murata Y. (2014). Cyclic adenosine 5’-diphosphoribose (cADPR) cyclic guanosine 3′,5′-monophosphate positively function in Ca2+ elevation in methyl jasmonate-induced stomatal closure, cADPR is required for methyl jasmonate-induced ROS accumulation NO production in guard cells. Plant Biol. (Stuttg.) 16 1140–1144. [DOI] [PubMed] [Google Scholar]
- Hossain M. S., Ye W., Hossain M. A., Okuma E., Uraji M., Nakamura Y., et al. (2013). Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 77 977–983. 10.1271/bbb.120928 [DOI] [PubMed] [Google Scholar]
- Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., et al. (2014). DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24 632–635. 10.1038/cr.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., et al. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24 2546–2561. 10.1105/tpc.112.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard K. E., Nishimura N., Hitomi K., Getzoff E. D., Schroeder J. I. (2010). Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 24 1695–1708. 10.1101/gad.1953910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard K. E., Siegel R. S., Valerio G., Brandt B., Schroeder J. I. (2012). Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann. Bot. 109 5–17. 10.1093/aob/mcr252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D., Mumm P., Bohm J., Al-Rasheid K. A., Marten I., Geiger D., et al. (2013). Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 74 372–382. 10.1111/tpj.12133 [DOI] [PubMed] [Google Scholar]
- Iriti M., Faoro F. (2009). Chitosan as a MAMP, searching for a PRR. Plant Signal. Behav. 4 66–68. 10.4161/psb.4.1.7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving H. R., Gehring C. A., Parish R. W. (1992). Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc. Natl. Acad. Sci. U.S.A. 89 1790–1794. 10.1073/pnas.89.5.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M., Ye W., Matsushima D., Khokon M. A., Munemasa S., Nakamura Y., et al. (2015). Inhibition by acrolein of light-induced stomatal opening through inhibition of inward-rectifying potassium channels in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 79 59–62. 10.1080/09168451.2014.951028 [DOI] [PubMed] [Google Scholar]
- Issak M., Okuma E., Munemasa S., Nakamura Y., Mori I. C., Murata Y. (2013). Neither endogenous abscisic acid nor endogenous jasmonate is involved in salicylic acid-, yeast elicitor-, or chitosan-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 77 1111–1113. 10.1271/bbb.120980 [DOI] [PubMed] [Google Scholar]
- Jammes F., Song C., Shin D., Munemasa S., Takeda K., Gu D., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. U.S.A. 106 20520–20525. 10.1073/pnas.0907205106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F., Yang X., Xiao S., Kwak J. M. (2011). Two Arabidopsis guard cell-preferential MAPK genes, MPK9 and MPK12, function in biotic stress response. Plant Signal. Behav. 6 1875–1877. 10.4161/psb.6.11.17933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannat R., Uraji M., Morofuji M., Islam M. M., Bloom R. E., Nakamura Y., et al. (2011). Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J. Plant Physiol. 168 1919–1926. 10.1016/j.jplph.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeworutzki E., Roelfsema M. R. G., Anschutz U., Krol E., Elzenga J. T. M., Felix G., et al. (2010). Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. Plant J. 62 367–378. 10.1111/j.1365-313X.2010.04155.x [DOI] [PubMed] [Google Scholar]
- Joshi-Saha A., Valon C., Leung J. A. (2011). Brand New START: abscisic acid perception and transduction in the guard cell. Sci. Signal. 4:re4 10.1126/scisignal.2002164 [DOI] [PubMed] [Google Scholar]
- Joudoi T., Shichiri Y., Kamizono N., Akaike T., Sawa T., Yoshitake J., et al. (2013). Nitrated cyclic GMP modulates guard cell signaling in Arabidopsis. Plant Cell 25 558–571. 10.1105/tpc.112.105049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y., Shirasu K., Zipfel C. (2015). Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 56 1472–1480. 10.1093/pcp/pcv063 [DOI] [PubMed] [Google Scholar]
- Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., et al. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54 43–55. 10.1016/j.molcel.2014.02.021 [DOI] [PubMed] [Google Scholar]
- Kauss H., Jeblick W., Domard A. (1989). The degrees of polymerization and N-acetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus roseus. Planta 178 385–392. 10.1007/BF00391866 [DOI] [PubMed] [Google Scholar]
- Keinath N., Waadt R., Brugman R., Schroeder J. I., Grossmann G., Schumacher K., et al. (2015). Live cell imaging with R-GECO1 sheds light on flg 22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Mol. Plant 8 1188–1200. 10.1016/j.molp.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon M., Okuma E., Hossain M. A., Munemasa S., Uraji M., Nakamura Y., et al. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34 434–443. 10.1111/j.1365-3040.2010.02253.x [DOI] [PubMed] [Google Scholar]
- Khokon M. A. R., Hossain M. A., Munemasa S., Uraji M., Nakamura Y., Mori I. C., et al. (2010a). Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol. 51 1915–1921. 10.1093/pcp/pcq145 [DOI] [PubMed] [Google Scholar]
- Khokon M. A. R., Salam M. A., Jammes F., Ye W., Hossain M. A., Uraji M., et al. (2015). Two guard cell mitogen-activated protein kinases, MPK9 and MPK12, function in methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Plant Biol. (Stuttg.) 17 946–952. 10.1111/plb.12321 [DOI] [PubMed] [Google Scholar]
- Khokon M. A. R., Uraji M., Munemasa S., Okuma E., Nakamura Y., Mori I. C., et al. (2010b). Chitosan-induced stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis. Biosci. Biotechnol. Biochem. 74 2313–2315. 10.1271/bbb.100340 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Bohmer M., Hu H., Nishimura N., Schroeder J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid. CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61 561–591. 10.1146/annurev-arplant-042809-112226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Nishimura M., Shimazaki K. I. (1995). Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B., Young J. J., Murata Y., Allen G. J., Mori I. C., Hugouvieux V., et al. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 130 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koers S., Guzel Deger A., Marten I., Roelfsema M. R. G. (2011). Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard-cell S-type anion channels. Plant J. 68 670–680. [DOI] [PubMed] [Google Scholar]
- Kollar R., Reinhold B. B., Petrakova E., Yeh H. J., Ashwell G., Drgonova J., et al. (1997). Architecture of the yeast cell wall. Beta(1– > 6)-glucan interconnects mannoprotein, beta(1– >) 3-glucan, and chitin. J. Biol. Chem. 272 17762–17775. [DOI] [PubMed] [Google Scholar]
- Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaaitaal M., Huisman R., Maintz J., Reinstadler A., Panstruga R. (2011). Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem. J. 440 355–365. [DOI] [PubMed] [Google Scholar]
- Kwak J. M., Mori I. C., Pei Z. M., Leonhardt N., Torres M. A., Dangl J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K., Brandt B., Li J., Merilo E., Wang Y. F., Keshwani M. M., et al. (2013). Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol. 163 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Choi H., Suh S., Doo I., Oh K., Jeong Choi E., et al. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Lan W., Buchanan B. B., Luan S. A. (2009). protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. U.S.A. 106 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li M., Yu L., Zhou Z., Liang X., Liu Z., et al. (2014). The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15 329–338. [DOI] [PubMed] [Google Scholar]
- Li Y., Yin H., Wang Q., Zhao X., Du Y., Li F. (2009). Oligochitosan induced Brassica napus L. production of NO and H2O2 and their physiological function. Carbohydr. Polym. 75 612–617. [Google Scholar]
- Liese A., Romeis T. (2013). Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta 1833 1582–1589. [DOI] [PubMed] [Google Scholar]
- Liu J., Elmore J. M., Fuglsang A. T., Palmgren M. G., Staskawicz B. J., Coaker G., et al. (2009). RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 7:e1000139 10.1371/journal.pbio.1000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Liu Z., Song C., Hu Y., Han Z., She J., et al. (2012). Chitin-induced dimerization activates a plant immune receptor. Science 336 1160–1164. [DOI] [PubMed] [Google Scholar]
- Lozano-Duran R., Bourdais G., He S. Y., Robatzek S. (2014). The bacterial effector HopM1 suppresses PAMP-triggered oxidative burst and stomatal immunity. New Phytol. 202 259–269. [DOI] [PubMed] [Google Scholar]
- Ma S., Wu W. (2007). AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 65 511–518. [DOI] [PubMed] [Google Scholar]
- Ma W., Qi Z., Smigel A., Walker R. K., Verma R., Berkowitz G. A. (2009). Ca2+, cAMP, and transduction of non-self perception during plant immune responses. Proc. Natl. Acad. Sci. U.S.A. 106 20995–21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Smigel A., Tsai Y. C., Braam J., Berkowitz G. A. (2008). Innate immunity signaling: cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 148 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., et al. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324 1064–1068. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Walker R. K., Berkowitz G. A. (2013). Molecular steps in the immune signaling pathway evoked by plant elicitor peptides (Peps): CPKs, NO and ROS are downstream from the early Ca2+ signal. Plant Physiol. 163 1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Song T., Zhu L., Ye W., Wang Y., Shao Y., et al. (2015). A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27 2057–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A. P., Boutrot F., Rathjen J. P., Zipfel C. (2012). Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol. 159 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A. P., Zipfel C. (2014). Plant PRRs and the activation of innate immune signaling. Mol. Cell 54 263–272. [DOI] [PubMed] [Google Scholar]
- Maierhofer T., Diekmann M., Offenborn J. N., Lind C., Bauer H., Hashimoto K., et al. (2014). Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 7:ra86 10.1126/scisignal.2005703 [DOI] [PubMed] [Google Scholar]
- McLachlan D. H., Kopischke M., Robatzek S. (2014). Gate control: guard cell regulation by microbial stress. New Phytol. 203 1049–1063. [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., He S. Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126 969–980. [DOI] [PubMed] [Google Scholar]
- Merilo E., Laanemets K., Hu H., Xue S., Jakobson L., Tulva I., et al. (2013). PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness- and CO2-induced stomatal regulation. Plant Physiol. 162 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S., Leonhardt N., Fenzi F., Valon C., Costa M., Piette L., et al. (2007). Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 26 3216–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann S., Bourdais G., Rietz S., Robatzek S. (2010). Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K. A. S., et al. (2010). AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63 1054–1062. [DOI] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., et al. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 104 19613–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J., Matschi S., Shorinola O., Rovenich H., Matei A., Segonzac C., et al. (2014). The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16 605–615. [DOI] [PubMed] [Google Scholar]
- Montillet J. L., Leonhardt N., Mondy S., Tranchimand S., Rumeau D., Boudsocq M., et al. (2013). An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 11:e1001513 10.1371/journal.pbio.1001513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S., Han S., Kim D., Yoon I. S., Shin D., Byun M., et al. (2015). Ectopic expression of a hot pepper bZIP-like transcription factor in potato enhances drought tolerance without decreasing tuber yield. Plant Mol. Biol. 89 421–431. [DOI] [PubMed] [Google Scholar]
- Moreau M., Lee G. I., Wang Y., Crane B. R., Klessig D. F. (2008). AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 283 32957–32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I. C., Murata Y., Yang Y. Z., Munemasa S., Wang Y. F., Andreoli S., et al. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion-and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4:e327 10.1371/journal.pbio.0040327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I. C., Pinontoan R., Kawano T., Muto S. (2001). Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 42 1383–1388. [DOI] [PubMed] [Google Scholar]
- Munemasa S., Hossain M. A., Nakamura Y., Mori I. C., Murata Y. (2011). The Arabidopsis calcium-dependent protein kinase, CPK6 functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 155 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S., Oda K., Watanabe-Sugimoto M., Nakamura Y., Shimoishi Y., Murata Y. (2007). The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 143 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Mori I. C., Munemasa S. (2015). Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66 369–392. [DOI] [PubMed] [Google Scholar]
- Murata Y., Pei Z. M., Mori I. C., Schroeder J. I. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A. C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E., Kawaide H., Kamiya Y., Naito S. (1998). Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol. 39 853–858. [DOI] [PubMed] [Google Scholar]
- Narusaka M., Minami T., Iwabuchi C., Hamasaki T., Takasaki S., Kawamura K., et al. (2015). Yeast cell wall extract induces disease resistance against bacterial and fungal pathogens in Arabidopsis thaliana and Brassica Crop. PLoS ONE 10:e115864 10.1371/journal.pone.0115864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., et al. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452 483–486. [DOI] [PubMed] [Google Scholar]
- Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59 165–176. [DOI] [PubMed] [Google Scholar]
- Neill S. J., Desikan R., Clarke A., Hancock J. T. (2002). Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 128 13–16. [PMC free article] [PubMed] [Google Scholar]
- Nomura H., Komori T., Kobori M., Nakahira Y., Shiina T. (2008). Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 53 988–998. [DOI] [PubMed] [Google Scholar]
- Nomura H., Komori T., Uemura S., Kanda Y., Shimotani K., Nakai K., et al. (2012). Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 3 926. [DOI] [PubMed] [Google Scholar]
- O’Brien J. A., Daudi A., Finch P., Butt V. S., Whitelegge J. P., Souda P., et al. (2012). A Peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 158 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oome S., Raaymakers T. M., Cabral A., Samwel S., Bohm H., Albert I., et al. (2014). Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111 16955–16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Arinaga N., Umezawa T., Katsura S., Nagamachi K., Tanaka H., et al. (2013). Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Fung P., Nishimura N., Jensen D. R., Fujii H., Zhao Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z. M., Murata Y., Benning G., Thomine S., Klusener B., Allen G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. [DOI] [PubMed] [Google Scholar]
- Petutschnig E. K., Jones A. M. E., Serazetdinova L., Lipka U., Lipka V. (2010). The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J. Biol. Chem. 285 28902–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfund C., Tans-Kersten J., Dunning F. M., Alonso J. M., Ecker J. R., Allen C., et al. (2004). Flagellin is not a major defense elicitor in Ralstonia solanacearum cells or extracts applied to Arabidopsis thaliana. Mol. Plant Microbe Interact. 17 696–706. [DOI] [PubMed] [Google Scholar]
- Ranf S., Eschen-Lippold L., Pecher P., Lee J., Scheel D. (2011). Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 68 100–113. [DOI] [PubMed] [Google Scholar]
- Ranf S., Gisch N., Schaffer M., Illig T., Westphal L., Knirel Y. A., et al. (2015). A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 16 426–433. [DOI] [PubMed] [Google Scholar]
- Ranf S., Wunnenberg P., Lee J., Becker D., Dunkel M., Hedrich R., et al. (2008). Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 53 287–299. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Chinchilla D., Boller T. (2006). Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 20 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. M., Bostock R. M. (2014). β-glucans and eicosapolyenoic acids as MAMPs in plant-oomycete interactions: past and present. Front. Plant Sci. 5:797 10.3389/fpls.2014.00797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema M. R. G., Hedrich R. (2010). Making sense out of Ca2+ signals: their role in regulating stomatal movements. Plant Cell Environ. 33 305–321. [DOI] [PubMed] [Google Scholar]
- Roux M., Schwessinger B., Albrecht C., Chinchilla D., Jones A., Holton N., et al. (2011). The Arabidopsis leucine-rich repeat receptor–like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23 2440–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam M. A., Jammes F., Hossain M. A., Ye W., Nakamura Y., Mori I. C., et al. (2012). MAP kinases, MPK9 and MPK12, regulate chitosan-induced stomatal closure. Biosci. Biotechnol. Biochem. 76 1785–1787. [DOI] [PubMed] [Google Scholar]
- Salam M. A., Jammes F., Hossain M. A., Ye W., Nakamura Y., Mori I. C., et al. (2013). Two guard cell-preferential MAPKs, MPK9 and MPK12, regulate YEL signalling in Arabidopsis guard cells. Plant Biol. (Stuttg.) 15 436–442. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vallet A., Mesters J. R., Thomma B. P. H. J., de Wit P. (2015). The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol. Rev. 39 171–183. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Mori I. C., Furuichi T., Munemasa S., Toyooka K., Matsuoka K., et al. (2010). Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 51 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Gambale F., Dreyer I., Uozumi N. (2010). Modulation of the Arabidopsis KAT1 channel by an activator of protein kinase C in Xenopus laevis oocytes. FEBS J. 277 2318–2328. [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., et al. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2. 6 protein kinase. Biochem. J. 424 439–448. [DOI] [PubMed] [Google Scholar]
- Sawinski K., Mersmann S., Robatzek S., Bohmer M. (2013). Guarding the green: pathways to stomatal immunity. Mol. Plant Microbe Interact. 26 626–632. [DOI] [PubMed] [Google Scholar]
- Scherzer S., Maierhofer T., Al-Rasheid K. A. S., Geiger D., Hedrich R. (2012). Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant 5 1409–1412. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I. (1988). K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J. Gen. Physiol. 92 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430. [Google Scholar]
- Schroeder J. I., Keller B. U. (1992). 2 types of anion channel currents in guard-cells with distinct voltage regulation. Proc. Natl. Acad. Sci. U.S.A. 89 5025–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H. M., Gundlach H., Fiedler F., Zenk M. H. (1987). Elicitation of benzophenanthridine alkaloid synthesis in Eschscholzia cell cultures. Plant Cell Rep. 6 410–413. [DOI] [PubMed] [Google Scholar]
- Shang Y., Dai C., Lee M. M., Kwak J. M., Nam K. H. (2015). BRI1-Associated Receptor Kinase 1 regulates guard cell ABA signaling mediated by Open Stomata 1 in Arabidopsis. Mol. Plant 9 447–460. [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Doi M., Assmann S. M., Kinoshita T. (2007). Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58 219–247. [DOI] [PubMed] [Google Scholar]
- Shinya T., Nakagawa T., Kaku H., Shibuya N. (2015). Chitin-mediated plant–fungal interactions: catching, hiding and handshaking. Curr. Opin. Plant Biol. 26 64–71. [DOI] [PubMed] [Google Scholar]
- Shinya T., Yamaguchi K., Desaki Y., Yamada K., Narisawa T., Kobayashi Y., et al. (2014). Selective regulation of the chitin-induced defense response by the Arabidopsis receptor-like cytoplasmic kinase PBL27. Plant J. 79 56–66. [DOI] [PubMed] [Google Scholar]
- Siegel R. S., Xue S., Murata Y., Yang Y., Nishimura N., Wang A., et al. (2009). Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. Plant J. 59 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silipo A., Molinaro A., Sturiale L., Dow J. M., Erbs G., Lanzetta R., et al. (2005). The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 280 33660–33668. [DOI] [PubMed] [Google Scholar]
- Singh P., Kuo Y., Mishra S., Tsai C., Chien C., Chen C., et al. (2012). The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H. C., Davanture M., Lee S., Djaoui M., et al. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583 2982–2986. [DOI] [PubMed] [Google Scholar]
- Sobahan M. A., Akter N., Okuma E., Uraji M., Ye W., Mori I. C., et al. (2015). Allyl isothiocyanate induces stomatal closure in Vicia faba. Biosci. Biotechnol. Biochem. 79 1737–1742. [DOI] [PubMed] [Google Scholar]
- Spallek T., Beck M., Ben K. S., Salomon S., Bourdais G., Schellmann S., et al. (2013). ESCRT-I mediates FLS2 endosomal sorting and plant immunity. PLoS Genet. 9:e1004035 10.1371/journal.pgen.1004035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N., Gonugunta V., Puli M., Raghavendra A. (2009). Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229 757–765. [DOI] [PubMed] [Google Scholar]
- Steinhorst L., Kudla J. (2013). Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol. 163 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D., Raghavendra A. S., Kwak J. M., Vavasseur A. (2004). Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol. 134 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li L., Macho A. P., Han Z., Hu Z., Zipfel C., et al. (2013). Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342 624–628. [DOI] [PubMed] [Google Scholar]
- Sutter J. U., Sieben C., Hartel A., Eisenach C., Thiel G., Blatt M. R. (2007). Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17 1396–1402. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Morales J., Shulaev V., Torres M. A., Mittler R. (2011). Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 14 691–699. [DOI] [PubMed] [Google Scholar]
- Takemiya A., Shimazaki K. (2010). Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase. Plant Physiol. 153 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda C., Zhang Z., Shrestha J., Jelenska J., Chinchilla D., Greenberg J. T. (2014). Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signaling. Plant Cell 26 4171–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K., Peiter E. (2014). Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 204 873–881. [DOI] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Glazebrook J., Cohen J. D., Katagiri F. (2008). Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 53 763–775. [DOI] [PubMed] [Google Scholar]
- Uraji M., Katagiri T., Okuma E., Ye W., Hossain M. A., Masuda C., et al. (2012). Cooperative function of PLDδ and PLDα1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 159 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y. F., Nishimura N., Chan W. Y., Valerio G., et al. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., Puzorjova I., Brosche M., Valk E., Lepiku M., Moldau H., et al. (2010). Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 62 442–453. [DOI] [PubMed] [Google Scholar]
- Vander P., Vrum K. M., Domard A., Eddine E. G. N., Moerschbacher B. M. (1998). Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol. 118 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Hitomi K., Nishimura N., Hitomi C., Adams S. R., Getzoff E. D. (2014). FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. Elife 3:e01739 10.7554/eLife.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. K. (2011). Delineation of Molecular Signal Transduction Events Facilitating Pathogen Defense Responses in Plants. Ph.D. dissertation, University of Connecticut, Mansfield, CT. [Google Scholar]
- Wege S., De Angeli A., Droillard M. J., Kroniewicz L., Merlot S., Cornu D., et al. (2014). Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 7:ra65. [DOI] [PubMed] [Google Scholar]
- Weinl S., Held K., Schlucking K., Steinhorst L., Kuhlgert S., Hippler M., et al. (2008). A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol 179 675–686. [DOI] [PubMed] [Google Scholar]
- Xie X., Wang Y., Williamson L., Holroyd G. H., Tagliavia C., Murchie E., et al. (2006). The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr. Biol. 16 882–887. [DOI] [PubMed] [Google Scholar]
- Xue S., Hu H., Ries A., Merilo E., Kollist H., Schroeder J. I. (2011). Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 30 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A., Shibuya N., Kodama O., Akatsuka T. (1993). Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci. Biotechnol. Biochem. 57 405–409. [Google Scholar]
- Ye W., Adachi Y., Munemasa S., Nakamura Y., Mori I. C., Murata Y. (2015). Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 56 1239–1248. [DOI] [PubMed] [Google Scholar]
- Ye W., Hossain M. A., Munemasa S., Nakamura Y., Mori I. C., Murata Y. (2013a). Endogenous abscisic acid is involved in methyl jasmonate-induced reactive oxygen species and nitric oxide production but not in cytosolic alkalization in Arabidopsis guard cells. J. Plant Physiol. 170 1212–1215. [DOI] [PubMed] [Google Scholar]
- Ye W., Muroyama D., Munemasa S., Nakamura Y., Mori I. C., Murata Y. (2013b). Calcium-dependent protein kinase, CPK6 positively functions in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in Arabidopsis. Plant Physiol. 163 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Adachi Y., Ye W., Hayashi M., Nakamura Y., Kinoshita T., et al. (2013). Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol. 163 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. J., Mehta S., Israelsson M., Godoski J., Grill E., Schroeder J. I. (2006). CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. U.S.A. 103 7506–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F., Yang H., Xue Y., Kong D., Ye R., Li C., et al. (2014). OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514 367–371. [DOI] [PubMed] [Google Scholar]
- Zeng W., He S. Y. (2010). A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 153 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Melotto M., He S. Y. (2010). Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 21 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li W., Xiang T., Liu Z., Laluk K., Ding X., et al. (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7 290–301. [DOI] [PubMed] [Google Scholar]
- Zhang W., He S. Y., Assmann S. M. (2008). The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 56 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Takemiya A., Kinoshita T., Shimazaki K. (2007). Nitric oxide inhibits blue light-specific stomatal opening via abscisic acid signaling pathways in Vicia guard cells. Plant Cell Physiol. 48 715. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Takemiya A., Song C., Kinoshita T., Shimazaki K. (2004). Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 136 4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang L., Dong F., Gao J., Galbraith D. W., Song C. P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Guo Y., Kosaihira A., Sakai K. (2004). Rapid accumulation and metabolism of polyphosphoinositol and its possible role in phytoalexin biosynthesis in yeast elicitor-treated Cupressus lusitanica cell cultures. Planta 219 121–131. [DOI] [PubMed] [Google Scholar]
- Zhu S., Yu X., Wang X., Zhao R., Li Y., Fan R., et al. (2007). Two calcium-dependent protein kinases, CPK4 and CPK11 regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19 3019–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. (2014). Plant pattern-recognition receptors. Trends Immunol. 35 345–351. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J. D., Boller T., et al. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediatv ed transformation. Cell 125 749–760. [DOI] [PubMed] [Google Scholar]
- Zou J., Li X., Ratnasekera D., Wang C., Liu W., Song L., et al. (2015). Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H. Plant Cell 27 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Wei F., Wang C., Wu J., Ratnasekera D., Liu W., et al. (2010). Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 154 1232–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]