Abstract
Learning, defined as a change in behaviour evoked by experience, has hitherto been investigated almost exclusively in multicellular neural organisms. Evidence for learning in non-neural multicellular organisms is scant, and only a few unequivocal reports of learning have been described in single-celled organisms. Here we demonstrate habituation, an unmistakable form of learning, in the non-neural organism Physarum polycephalum. In our experiment, using chemotaxis as the behavioural output and quinine or caffeine as the stimulus, we showed that P. polycephalum learnt to ignore quinine or caffeine when the stimuli were repeated, but responded again when the stimulus was withheld for a certain time. Our results meet the principle criteria that have been used to demonstrate habituation: responsiveness decline and spontaneous recovery. To distinguish habituation from sensory adaptation or motor fatigue, we also show stimulus specificity. Our results point to the diversity of organisms lacking neurons, which likely display a hitherto unrecognized capacity for learning, and suggest that slime moulds may be an ideal model system in which to investigate fundamental mechanisms underlying learning processes. Besides, documenting learning in non-neural organisms such as slime moulds is centrally important to a comprehensive, phylogenetic understanding of when and where in the tree of life the earliest manifestations of learning evolved.
Keywords: learning, slime moulds, habituation, Physarum polycephalum
1. Introduction
Learning and memory are indisputably two of the key features of animal success [1]. Using information about past experiences is often critical for optimal decision-making in a fluctuating environment and is involved in every aspect of an animal's life, including foraging and interacting with other individuals [1]. We usually think of learning as a trait that is limited to organisms with brains and nervous systems. Indeed, learning is often equated with neuronal changes such as synaptic plasticity, implicitly precluding its existence in non-neural organisms [1,2]. While the evolutionary benefits of learning are clear, very little is known about its origins. Even the ‘simplest’ organisms adapt to changing environments, raising the exciting possibility that mechanisms for learning might pre-date the evolution of nervous systems, possibly existing in a breadth of as yet unstudied organisms. Tantalizing results suggest that the hallmarks for learning can occur at the level of single cells [3–6]. If true, then studies of unicellular model species displaying learning abilities promise exciting insights to the earliest manifestations of learning, a key innovation in the history of life. However, evidence for learning in single-celled organisms remains scant and, to date, very few unequivocal reports of such processes have been described [3,4]. For example, the fact that bacteria respond faster to a signal already encountered in the past or the fact that they are able to predict environmental changes has been recognized as ‘learning’ by some researchers [6–8]. However, for others, it should be called adaptation [9,10] or plasticity [11], because in most experiments bacteria did not learn to respond to the stimulus within a lifetime but rather they evolved to respond over multiple generations. In this paper, we attempt to demonstrate learning in a single-celled organism Physarum polycephalum within a single lifetime.
Physarum polycephalum, also known as the true slime mould, is a unicellular but multi-nucleated eukaryote. Slime moulds have been known for decades to display complex behaviours, including finding their way through a maze [12], solving nutritional challenges [13], avoiding traps [14] and anticipating periodic events [15]. However, despite their increasing recognition as a model for studies of complex behaviours, very little is known about their learning abilities. In this paper, using a habituation paradigm, we explore the learning capacity of these non-neural organisms.
Habituation, one of the simplest forms of learning, is defined as a decline in a behavioural response in response to a repeated, irrelevant stimulus. The processes do not involve sensory adaptation or sensory fatigue or motor fatigue. If the stimulus is withheld, then the behavioural response recovers to its original state over time [16,17]. Habituation of the gill and siphon withdrawal reflex in Aplysia is a textbook example of such a process [18]. Habituation phenomena have been observed across many phyla of the animal kingdom from invertebrates to vertebrates [16,17]. Here we designed a protocol to investigate habituation phenomena in slime moulds using chemotaxis as the behavioural response under scrutiny. Chemotaxis is the directed motion of an organism towards favourable chemicals (attractants) and away from unfavourable ones (repellents) [19,20]. To test whether slime moulds are capable of habituation, individuals were repeatedly exposed to quinine or caffeine, known repellents for P. Polycephalum [21,22] and their behavioural responses were recorded.
2. Material and methods
(a). Species
We used plasmodia of the slime mould P. polycephalum (Hakodate University) exhibiting a typical yellow colour. They are large amoeba-like cells, scaling up to several square metres, usually inhabiting shady, cool and humid environments such as forest litter. They can crawl over their support in search for food by extending tubular structures called pseudopods. For this experiments, plasmodia were cultured in large Petri dishes (Ø 145 mm) on a 1% agar gel containing 10% of blended oat flakes (Quaker Oats Company®) at 25°C in the dark.
Before starting the experiment, to accustom the slime moulds to the set-up, we introduced 416 slime moulds resting on their rearing substrate (H = 2 mm, Ø = 18 mm) in experimental arenas (Petri dish Ø = 90 mm). The slime moulds were taken directly from the culture using a template. Then, we connected each slime mould with a food patch (1% agar gel containing 10% of blended oat flakes, H = 2 mm, Ø = 18 mm) using an agar gel bridge (1% agar gel, H = 2 mm, L = 13 mm, W = 15 mm). The slime mould explored the bridge by expanding pseudopods. When it found the food patch it left both the bridge and its initial position to exploit the food patch. After 24 h, the slime mould resting on the food patch was transferred to a new arena and the experiment started. Six individuals failed in crossing the bridge and were excluded from the experiment.
(b). Habituation experiment
The experiment consisted of three treatments: quinine treatment (Q, n = 140), caffeine treatment (CAF, n = 70) and control treatment (C, n = 200), which are described below. The experiment lasted 9 days and was organized in four different phases.
(i). Habituation phase
The habituation phase lasted for 5 days. In treatment C, slime moulds had to reach a new food patch crossing an agar gel bridge (1% agar gel, H = 2 mm, L = 13 mm, W = 15 mm) every day for 5 days. Typically, every day, the food patch covered by the slime mould after crossing the bridge was transferred to a new arena together with a new bridge and a new food patch, and so on. Every daily transfers were done at the same time of the day. In Q and CAF treatment, we followed the same procedure; except that slime moulds were required to cross an agar bridge (1% agar) containing quinine (4 mM) or caffeine (1 mM) to reach the food patch every day for 5 days. Accordingly, the Q and CAF slime moulds were in contact with quinine or caffeine once a day while crossing the bridge. We chose a quinine concentration of 4 mM as, when encountering such a concentration, slime moulds typically stop moving for several hours before migrating through this repellent region. Higher quinine concentrations would induce the slime mould to turn back [21]. Caffeine was used as an alternative bitter repellent. As caffeine, contrary to quinine, has never been tested before in a comparable chemotaxis experiment, we had to test various concentrations (1, 2, 3 and 4 mM). Slime moulds would only cross the bridge if the concentrations were less than 2 mM. High concentrations of caffeine could turn out to be harmful for slime moulds causing extrusions of cytoplasm at concentrations 5–15 mM [22,23].
(ii). Test phase 1
On the sixth day, we tested the slime moulds for habituation and stimulus generalization abilities. Q, CAF and half of the C slime moulds (n = 100) were offered a bridge containing either quinine or caffeine, whereas the other half of the C slime moulds (n = 100) were once more offered a plain agar bridge. First, by offering the same repellent for the sixth time to Q and CAF slime moulds, we determined if there was a difference in responsiveness between habituated slime moulds (Q or CAF) and unhabituated ones (C). Second, by offering another repellent on day 6 to Q and CAF slime moulds, we tested whether the slime moulds recovered an aversive response that would show stimulus specificity and rule out any form of sensory adaptation or motor fatigue.
(iii). Recovery phase
In the third phase of the experiment, the recovery phase, Q, CAF and C slime moulds were all required to cross a plain agar bridge to reach the food once a day for 2 days.
(iv). Test phase 2
On the ninth day, we tested the slime moulds for recovery. Q, CAF and half of the C slime moulds were offered a bridge containing quinine or caffeine, whereas the other half of the C slime moulds were once again offered a plain agar bridge.
(c). Response variables
First, we recorded the time needed for each individual to make the first contact with the bridge and to cross the bridge (i.e. to make the first contact with the new food patch). Second, we measured the area (pseudopod size) and the perimeter of the pseudopod just as it contacted the food patch. We quantified the pseudopod shape using a circularity index (circularity = 4π × area/perimeter2). High values of time to contact the bridge and time to cross the bridge as well as low values of pseudopod size and pseudopod shape would indicate that the slime mould is avoiding contact with the bridge surface by extending a slow and elongated pseudopod. All this variables were recorded every day for each individual.
(d). Experimental procedure
We used different temperature-controlled chambers set to 25°C. In each chamber, a high definition camera (EOS 60D, Canon®) took a picture every 5 min of the set-ups. All pictures were analysed using ImageJ software. Statistical analyses were performed using IBM® SPSS® software (22.0). All statistical analyses are presented in the electronic supplementary material.
3. Results
The behavioural variables measured throughout the whole experiment are presented in figure 1 (time to contact the bridge), figure 2 (time to cross the bridge), figure 3 (pseudopod shape) and figure 4 (pseudopod size). The results are also illustrated with a detailed example comparing the C and Q slime moulds in figure 5.
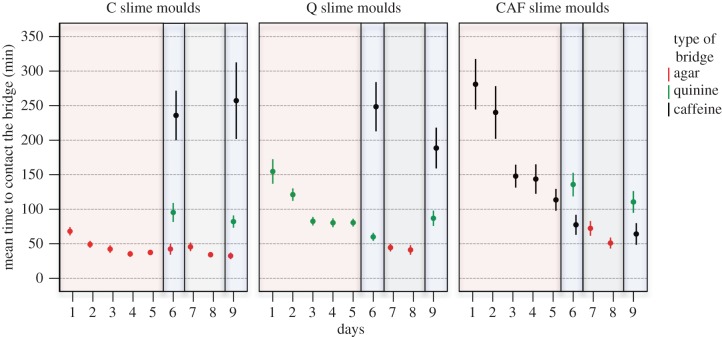
Figure 1.
Time to make first contact with the bridge from day 1 to 9 for each treatment. High values of time to contact the bridge indicate an aversive response. The colours of the frames indicate the various phases of the experiment: habituation phase (pale red), test phases 1 and 2 (pale blue) and recovery phase (pale grey). n = 200, n = 140 and n = 70 for C, Q and CAF treatment, respectively. Error bars indicate ±95% CI.
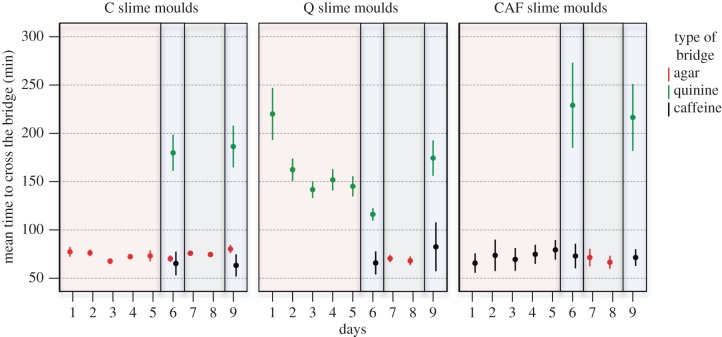
Figure 2.
Time to cross the bridge from day 1 to 9 for each treatment. High values of time to cross the bridge indicate an aversive response. The colours of the frames indicate the various phases of the experiment: habituation phase (pale red), test phases 1 and 2 (pale blue) and recovery phase (pale grey). n = 200, n = 140 and n = 70 for C, Q and CAF treatment, respectively. Error bars indicate ±95% CI.
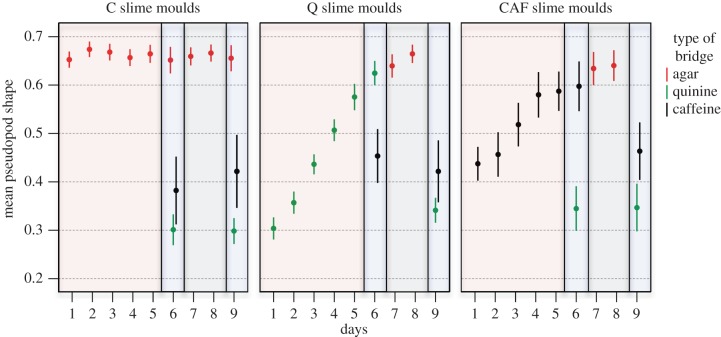
Figure 3.
Shape of the pseudopod just as it contacted the food patch from day 1 to 9 for each treatment. Shape is measured as circularity, 4π × (area)/(perimeter)2 with a value of 1 indicating a circle and values approaching 0 indicating an increasingly elongated shape. Low values of pseudopod shape indicate an aversive response. The colours of the frames indicate the various phases of the experiment: habituation phase (pale red), test phases 1 and 2 (pale blue) and recovery phase (pale grey). n = 200, n = 140 and n = 70 for C, Q and CAF treatment, respectively. Error bars indicate ± 95% CI.
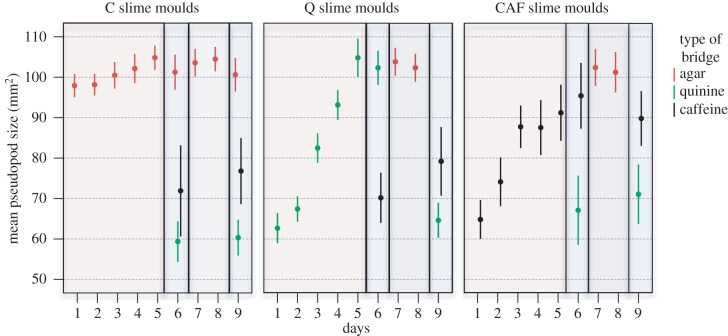
Figure 4.
Size of the pseudopod just as it contacted the food patch from day 1 to 9 for each treatment. Low values of pseudopod size indicate an aversive response. The colours of the frames indicate the various phases of the experiment: habituation phase (pale red), test phases 1 and 2 (pale blue) and recovery phase (pale grey). n = 200, n = 140 and n = 70 for C, Q and CAF treatment, respectively. Error bars indicate ± 95% CI.
Figure 5.
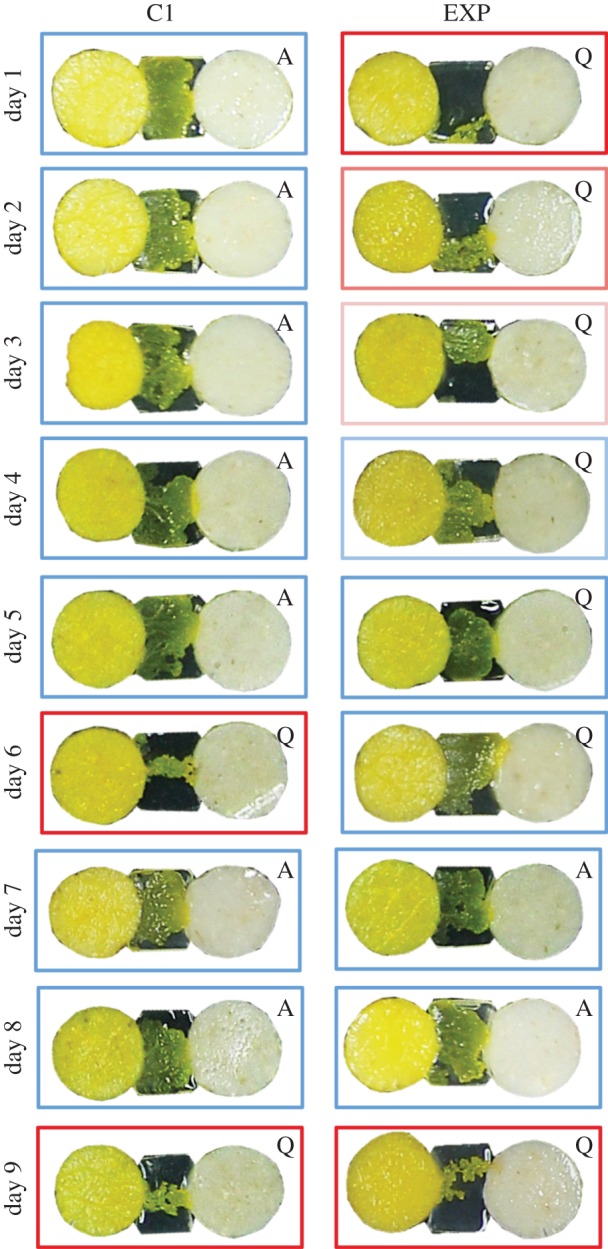
Photographs of a control slime mould (C) and a quinine slime mould (Q) from day 1 to 9. The slime moulds are crossing a bridge of agar gel containing quinine (Q) or not (A) to reach a food patch. The colours of the frames indicate the pseudopod size, ranging from small pseudopods (red) to large ones (blue).
(a). Habituation phase
On day 1, C slime moulds encountering agar entered the bridge after an hour, spread widely and crossed the bridge rapidly in about an hour (figures 1–4). In contrast, slime moulds encountering quinine (Q) or caffeine (CAF) for the first time showed a clear aversive behaviour. Q slime moulds entered the bridge only after 2.5 h and it took them 4 h to cross the bridge by way of a very narrow pseudopod (figures 1–4 and electronic supplementary material, table S1). CAF slime moulds were the longest to enter the bridge (almost 5 h) and also built thin pseudopods (figures 1–4 and electronic supplementary material, table S1). However, contrary to Q slime moulds, they crossed the bridge as quickly as a slime mould crossing an agar bridge (figure 2 and electronic supplementary material, table S1).
The following days of the habituation phase, Q and CAF slime moulds started to contact the bridge sooner and sooner and to form larger and larger pseudopods (figures 1–4 and electronic supplementary material, table S2). Q slime moulds also crossed the bridge twice as fast (figure 2 and electronic supplementary material, table S2). For both CAF and Q slime moulds, the decrease in responsiveness is shown as an exponential function of the number of repellent presentations (figures 1–4 and electronic supplementary material, table S3 and figure S1). In contrast, the behaviour of the C slime moulds encountering agar day after day remained unchanged except for a slight decrease in the time to contact the bridge (figures 1–4 and electronic supplementary material, table S3).
(b). Test phase 1
The Q and CAF slime moulds crossing a bridge containing quinine or caffeine for the sixth time showed reduced aversive behaviour. They behaved like the C slime moulds offered a plain agar bridge for 6 days in terms of shape and pseudopods size (figures 3 and 4; electronic supplementary material, table S4). However, Q slime moulds remained somewhat slower than the C slime moulds (figure 2 and electronic supplementary material, table S4) and CAF slime moulds still entered the bridge later than the C slime moulds (figure 1 and electronic supplementary material, table S4). These results indicate that the CAF and Q slime moulds habituated to their repellent, but still exhibited the main trait of their aversive behaviour, respectively time to contact the bridge and time to cross it, though in a diminished way. In addition, Q and CAF slime moulds offered the same repellent for 6 days behaved very differently from the C slime moulds, which were offered the repellents for the first time and showed aversive behaviour (figures 1–4 and electronic supplementary material, table S5), confirming habituation. In contrast, Q and CAF slime moulds facing a different repellent from the one they habitually encountered behaved like the C slime moulds facing the repellents for the first time. They showed a strong aversive behaviour, avoiding contact with the bridge and building slow and narrow pseudopods (figures 1–4 and electronic supplementary material, table S5). The results indicate that the response decrement shows some stimulus specificity, ruling out motor or sensory fatigue.
(c). Recovery phase
All slime moulds behaved almost similarly, crossing the agar bridge quickly with large pseudopods throughout the recovery phase (figures 1–4 and electronic supplementary material, table S6). Only, CAF slime moulds took longer to enter the bridge on the first day of the recovery phase, but this difference vanished the second day of the recovery phase (figure 1 and electronic supplementary material, table S6).
(d). Test phase 2
Contrary to what we observed during test 1, after recovery, Q and CAF slime moulds encountering their respective repellent behaved differently to C slime moulds encountering agar in terms of pseudopod shape and size (electronic supplementary material, table S7).
Q and C slime moulds encountering quinine or caffeine showed a similar aversive behaviour, i.e. narrow and slow pseudopods (electronic supplementary material, table S8 and figures 1–4). The behavioural response of the Q slime moulds to quinine after recovery was similar to their behaviour when they encountered the quinine for the first time on day 1 indicating that Q slime moulds had recovered from the habituation phase (electronic supplementary material, table S9).
CAF and C slime moulds also showed a similar aversive behaviour towards quinine (electronic supplementary material, table S8 and figures 1–4). However, when facing caffeine, CAF and C slime moulds behaved differently regarding the time to enter the bridge, which is the strongest manifestation of aversive behaviour towards caffeine (electronic supplementary material, table S8). CAF slime moulds facing caffeine entered the bridge quicker than the C slime moulds and also quicker than when they faced caffeine for the first time on day 1 (figure 1 and electronic supplementary material, table S9). This indicates that the CAF slime moulds did not recover completely after 2 days.
4. Discussion
In this paper, we demonstrate that a single-celled organism fulfilled the two major criteria of habituation learning. P. polycephalum learns to ignore a stimulus when the stimulus is repeated and spontaneously recovers when it is withheld for 2 days. Moreover, we were able to show stimulus specificity: the aversive response still occurred to another stimulus. This characteristic can be used to distinguish habituation from more general sensory adaptation or motor fatigue [17]. As stimulus generalization within the same sensory modality could not be shown, this suggests that habituation does not happen at a central level, rather at the level of primary sensors [17].
While the specific mechanisms of quinine and caffeine chemoreception in P. polycephalum are still unknown, the process is likely similar to that of chemoreception across other single-celled organisms [24–27]. Quinine and caffeine likely bind to chemoreceptors on the cell membrane, which in turn trigger a signalling cascade that leads to a new migration direction [28]. While high levels of such stimulants could cause such a severe reaction that the organism changes its direction of migration, here, we chose sufficiently low concentrations of repellent to enable the plasmodium to cross the bridge while not harming it [21,23]. In our experiment, quinine and caffeine affects slime mould motile behaviour, causing a significant reduction in velocity and spreading.
We show that when exposed to quinine and caffeine on several successive days, this aversive response progressively decreased. As indicated by Ginsburg & Jablonka [3], new insights into the evolution of eukaryotic signal transduction and gene regulation offer a theoretical framework for understanding learning in single-celled organisms. Epigenetic regulatory mechanisms such as DNA methylation, histone modifications or changes in transcription factors encode past experiences of a single cell, and are often considered one of the most fundamental types of memory. Such mechanisms can strengthen adaptive response patterns that depend on prior exposure to various stimuli in a single cell [3]. Thus, we can imagine that the signalling pathway behind quinine and caffeine chemoreception may lead to transient inhibitory epigenetic markings that reduce the level of expression of key receptor genes [3]. After each stimulation, the number of quinine and caffeine receptors would decrease eventually leading to a lack of aversive response to the repellent. We can also imagine that the three-dimensional conformation of the chemoreceptors may experience small, semi-persistent modifications after each stimulation, which may raise their activation threshold, leading to a decrease in the aversive response of the organism following several exposures to quinine and caffeine [3].
Many of the processes we might consider fundamental features of the brain, such as sensory integration, decision-making and now, learning, have all been displayed in these non-neural organisms. The survival of slime moulds depends on their ability to respond and adapt to changing environmental conditions. In all organisms, habituation allows animals to ignore irrelevant stimuli and focus selectively on important stimuli that are key to their survival. Stimulus specificity, as we showed in this study, seems relevant to the evolution of habituation. Habituation reduces the response to innocuous stimuli while still enabling the organism to respond to novel and potentially harmful stimuli that would fall in the same sensory modality. Challenges for the future include the elucidation of how slime moulds integrate environmental cues in order to produce an adapted response. Whether capacities for simple learning in slime moulds extend beyond habituation and constitute a prerequisite for other forms of learning such as associative learning remains an open question. Previous attempts at showing associative learning processes in slime moulds had significant shortcomings [29]. In living organisms with nervous systems, behavioural habituation is the result of an alteration in neurons and synapses [16]. Slime moulds clearly have no neurons making them an excellent model in which to understand and elucidate the molecular components of cell-mediated habituation and learning.
Supplementary Material
Acknowledgements
We thank Doug Emlen, Devin O'Brien and Erin McCullough for their comments on earlier versions of the manuscript.
Data accessibility
Data are deposited in the Dryad repository at http://datadryad.org/review?doi=doi:10.5061/dryad.51j89.
Authors' contributions
A.D. designed the experiment. A.D., R.B. and D.V. conducted the experiments. A.D. and R.B. analysed the data and wrote the paper. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
A.D. was supported by the CNRS and D.V. by the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture.
References
- 1.Pearce JM. 2013. Animal learning cognition: an introduction. Hove, UK: Psychology Press. [Google Scholar]
- 2.Dukas R. 2009. Learning: mechanisms, ecology and evolution. In Cognitive ecology II (eds Dukas R, Ratcliffe JM). Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Ginsburg S, Jablonka E. 2009. Epigenetic learning in non-neural organisms. J. Biosci. 34, 633–646. ( 10.1007/s12038-009-0081-8) [DOI] [PubMed] [Google Scholar]
- 4.Wood DC. 1992. Learning and adaptive plasticity in unicellular organisms. In Encyclopedia of learning and memory (ed. Squire LR.), pp. 623–624. New York, NY: Macmillan. [Google Scholar]
- 5.Armus HL, Montgomery AR, Jellison JL. 2006. Discrimination learning in paramecia (P. caudatum). Psychol. Rec. 56, 489–498. [DOI] [PubMed] [Google Scholar]
- 6.Hoffer SM, Westerhoff HV, Hellingwerf KJ, Postma PW, Tommassen J.. 2001. Autoamplification of a two-component regulatory system results in ‘learning’ behavior. J. Bacteriol. 183, 4914–4917. ( 10.1128/jb.183.16.4914-4917.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyon P. 2015. The cognitive cell: bacterial behavior reconsidered. Front. Microbiol. 6, 1–18. ( 10.3389/fmicb.2015.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagkopoulos I, Liu YC, Tavazoie S. 2008. Predictive behavior within microbial genetic networks. Science 320, 1313–1317. ( 10.1126/science.1154456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellingwerf KJ. 2005. Bacterial observations: a rudimentary form of intelligence? Trends Microbiol. 13, 152–158. ( 10.1016/j.tim.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 10.Yi TM, Huang Y, Simon MI, Doyle J. 2000. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc. Natl Acad. Sci. USA 97, 4649–4653. ( 10.1073/pnas.97.9.4649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukas R. 2013. Effects of learning on evolution: robustness, innovation and speciation. Anim. Behav. 85, 1023–1030. ( 10.1016/j.anbehav.2012.12.030) [DOI] [Google Scholar]
- 12.Nakagaki T, Yamada H, Tóth Á. 2000. Maze-solving by an amoeboid organism. Nature 407, 470 ( 10.1038/35035159) [DOI] [PubMed] [Google Scholar]
- 13.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid CR, Latty T, Dussutour A, Beekman M. 2012. Slime mold uses an externalized spatial ‘memory’ to navigate in complex environments. Proc. Natl Acad. Sci. USA 109, 17 490–17 494. ( 10.1073/pnas.1215037109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, 018101 ( 10.1103/PhysRevLett.100.018101) [DOI] [PubMed] [Google Scholar]
- 16.Thompson RF. 2009. Habituation: a history. Neurobiol. Learn. Mem. 92, 127–134. ( 10.1016/j.nlm.2008.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin CH, et al. 2009. Habituation revisited: an updated revised description of the behavioural characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138. ( 10.1016/j.nlm.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsker H, Kupfermann I, Castellucci V, Kandel E. 1970. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167, 1740–1742. ( 10.1126/science.167.3926.1740) [DOI] [PubMed] [Google Scholar]
- 19.Ueda T, Terayama K, Kurihara K, Kobatake Y. 1975. Threshold phenomena in chemoreception and taxis in slime mold Physarum polycephalum. J. Gen. Physiol. 65, 223–234. ( 10.1085/jgp.65.2.223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles DJ, Carlile MJ. 1978. The chemotactic response of plasmodia of the myxomycete Physarum polycephalum to sugars and related compounds. J. Gen. Microbiol. 108, 17–25. ( 10.1099/00221287-108-1-17) [DOI] [PubMed] [Google Scholar]
- 21.Ueda KI, Takagi S, Nishiura Y, Nakagaki T. 2011. Mathematical model for contemplative amoeboid locomotion. Phys. Rev. E. 83, 021916 ( 10.1103/PhysRevE.83.021916) [DOI] [PubMed] [Google Scholar]
- 22.Kincaid RL, Mansour TE. 1979. Cyclic 3′, 5′-AMP phosphodiesterase in Physarum polycephalum I. Chemotaxis toward inhibitors and cyclic nucleotides. Biochim. Biophys. Acta 588, 332–341. ( 10.1016/0304-4165(79)90341-6) [DOI] [PubMed] [Google Scholar]
- 23.Kukulies J, Stockem W, Wohlfarth-Bottermann KE. 1983. Caffeine-induced surface blebbing and budding in the acellular slime mold Physarum polycephalum. Z. Naturforsch. C 38, 589–599. [Google Scholar]
- 24.Walden J, Speckmann EJ. 1981. Effects of quinine on membrane potential and membrane currents in identified neurons of Helix pomatia. Neurosci. Lett. 27, 139–143. ( 10.1016/0304-3940(81)90258-5) [DOI] [PubMed] [Google Scholar]
- 25.Misra UK, Gawdi G, Pizzo SV. 1997. Chloroquine, quinine and quinidine inhibit calcium release from macrophage intracellular stores by blocking inositol 1,4,5-trisphosphate binding to its receptor. J. Cell. Biochem. 64, 225–232. ( 10.1002/(SICI)1097-4644(199702)64:2%3C225::AID-JCB6%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 26.Fishman MC, Spector I. 1981. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc. Natl Acad. Sci. USA 78, 5245–5249. ( 10.1073/pnas.78.8.5245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook NS, Haylett DG. 1985. Effects of apamin, quinine, and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J. Physiol. 358, 373–394. ( 10.1113/jphysiol.1985.sp015556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natsume K, Miyake Y, Yano M, Shimizu H. 1993. Information propagation by spatio-temporal pattern change of Ca2+ concentration throughout Physarum polycephalum with repulsive stimulation. Cell Struct. Funct. 18, 111–115. ( 10.1247/csf.18.111) [DOI] [PubMed] [Google Scholar]
- 29.Shirakawa T, Gunji YP, Miyake Y. 2011. An associative learning experiment using the plasmodium of Physarum polycephalum. Nano Commun. Netw. 2, 99–105. ( 10.1016/j.nancom.2011.05.002) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in the Dryad repository at http://datadryad.org/review?doi=doi:10.5061/dryad.51j89.