Abstract
Difference in dispersal ability is a key driver of species coexistence in metacommunities. However, the available frameworks for interpreting species diversity patterns in natura often overlook trade-offs and evolutionary constraints associated with dispersal. Here, we build a metacommunity model accounting for dispersal evolution and a competition–dispersal trade-off. Depending on the distribution of carrying capacities among communities, species dispersal values are distributed either around a single strategy (evolutionarily stable strategy, ESS), or around distinct strategies (evolutionary branching, EB). We show that limited dispersal generates spatial aggregation of dispersal traits in ESS and EB scenarios, and that the competition–dispersal trade-off strengthens the pattern in the EB scenario. Importantly, individuals in larger (respectively (resp.) smaller) communities tend to harbour lower (resp. higher) dispersal, especially under the EB scenario. We explore how dispersal evolution affects species diversity patterns by comparing those from our model to the predictions of a neutral metacommunity model. The most marked difference is detected under EB, with distinctive values of both α- and β-diversity (e.g. the dissimilarity in species composition between small and large communities was significantly larger than neutral predictions). We conclude that, from an empirical perspective, jointly assessing community carrying capacity with species dispersal strategies should improve our understanding of diversity patterns in metacommunities.
Keywords: neutral theory, evolutionary branching, null models, coalescence, T-statistics
1. Introduction
Ecological differentiation is considered as a driver of stable coexistence among competing species since the origin of community ecology [1–3]. In this context, resource partitioning plays an important role, but numerous theoretical [4–6] and experimental [7,8] studies have emphasized that trade-offs between local competitive ability and dispersal of species in patchy environments (so-called competition–colonization or competition–dispersal trade-offs) also favour coexistence. Both empirical studies and field surveys suggest that these trade-offs are a distinctive characteristic of communities from many natural systems [9,10], and are sometimes associated with wide variation in dispersal among species belonging to the same community (e.g. [11] for the tree community of Barro Colorado Island). However, whether (and how) they affect patterns of species diversity in natural communities remains debatable [12,13]. One reason is that studies trying to explain such patterns using empirical data on dispersal variation and potential trade-offs with competitiveness remain scarce and focused on simple communities including a few species at best [12,13]. Gathering such data is, indeed, a highly demanding endeavour in complex natural communities.
A less demanding, though more indirect, approach to the role of trade-offs on species diversity patterns is the ‘pattern-to-process’ approach in which these patterns are used to infer the underlying processes, and therefore to distinguish among coexistence theories. However, the theory of species coexistence based on the competition–dispersal trade-off has mostly been developed in terms of ‘patch dynamics' [5,14,15], considering monospecific communities and focusing on species diversity at the ‘metacommunity’ scale only. Such assumptions are far from realistic for most natural systems and prevent studying diversity patterns within sites. The metacommunity framework provides more general models, giving more or less weight to basic processes such as dispersal, demographic stochasticity, or competition (see [15] for a review). In particular, neutral models [16–18] constitute an interesting entry into metacommunity dynamics: they assume ecological equivalence of species [6] and attribute a major role to the balance between demographic stochasticity and dispersal. Importantly, these models yield accurate quantitative descriptions of community structure [19]. An outstanding example is the fit of a neutral model to the rank–abundance diagram of tropical trees in Barro Colorado Island [20]. We suggest here that neutral models could be used for building predictions about the effects of interspecific competition–dispersal trade-offs on diversity patterns, providing a more sophisticated basis than patch-dynamics models for pattern-to-process approaches.
We know of only two studies that assessed the impact of interspecific heterogeneity of dispersal on species diversity patterns by comparing these patterns to neutral predictions. Liu et al. [21] introduced several dispersal levels in an otherwise neutral model. They showed that the rank–abundance diagram of a community could not be used to detect heterogeneous dispersal because this pattern is already very well predicted by a purely neutral model. Janzen et al. [22] showed that using seed-dispersal traits when analysing rank–abundance patterns of tree species in Barro Colorado Island could lead to the rejection of a purely neutral model in favour of an alternative hypothesis involving interspecific dispersal. Both [21] and [22] considered the effect of interspecific heterogeneity of dispersal on community structure through variation in propagule pressure: species with higher dispersal exert a stronger propagule pressure and are therefore better represented locally. However, in such models, dispersal does not trade-off with local competitive ability (assumed to be identical among species): species with higher dispersal always thrive in all communities. The conditions for regional coexistence are unfortunately not considered in these studies which focus on species diversity within communities. Introducing a metacommunity structure in which species can coexist within local communities and a competition–dispersal trade-off in models such as those of [21,22] should allow the study of conditions for the regional coexistence of dispersal strategies as a result of a trade-off with local competition; which is the focus of our study.
Dispersal has a genetic basis in many species [23–25] and can evolve quickly under selection [26,27], suggesting that interspecific variation along a competition–dispersal trade-off should not only be ecologically stable, but also evolutionarily stable, at the metacommunity scale. The evolution of dispersal has been considered in several theoretical studies [28,29], though mostly in single-species metapopulations, especially to explore whether dispersal is stabilized by selection around an evolutionarily stable strategy (ESS), or whether it can diversify towards several strategies (evolutionary branching, EB). We are particularly interested in the latter scenario in which the coexistence among strategies verifies both ecological and evolutionary stability. Several processes can generate EB on dispersal (e.g. spatial and temporal fluctuations of environmental conditions [30–32]). In particular, Massol et al. [33] showed that variation in the strength of kin selection owing to variation in carrying capacity among populations is sufficient to generate disruptive selection on dispersal and EB, without calling for fluctuations. Their model includes both an explicit metapopulation structure and a competition–dispersal trade-off. We upscaled it to metacommunity level which allowed us to explore whether dispersal polymorphism has an effect on diversity within communities and dissimilarity among communities compared with a neutral model without dispersal polymorphism. This extends the current framework for interpreting species distribution to the competition–dispersal coexistence theory.
2. Methods
(a). Model with dispersal evolution (model M1)
Model M1 simultaneously describes the evolutionary dynamics of dispersal and the temporal change in species diversity in a metacommunity. Individuals share several attributes: a species label, a dispersal parameter d, the cost of dispersal c, the probability (μ) and magnitude (σ) of mutations on dispersal, and the speciation probability ν. These attributes are real numbers between 0 and 1, except σ which lies between 0 and 0.1. Individuals belong to communities which are described by their carrying capacity K (i.e. the number of individuals they can harbour). The metacommunity is made of N communities which can have different carrying capacities.
Mortality occurs continuously, and dead individuals are immediately replaced through a lottery process. Each death–replacement cycle starts with a ‘death’ event: a randomly chosen individual is removed from the metacommunity. Each individual constantly produces a very large number of propagules normalized to 1 for the lottery. A fraction 1−d of propagules remains in the community and a proportion d is dispersed in the metacommunity, which induces a trade-off between local competition and dispersal. A proportion c of dispersed propagules are lost (dispersal cost). Dispersed propagules have equal chance of reaching all communities, meaning that an individual exports d(1 − c)/N propagules to each community. The model is therefore spatially implicit. A dead individual is replaced by either a local or an imported propagule in proportion to their occurrence (lottery). The new individual has the same dispersal probability as its parent (d) with probability 1 − μ. When mutation occurs (probability μ), the dispersal probability differs from that of the parent by ±σ min (10d, 10(1−d), 1), σ being the maximum amplitude of a mutation. This function was chosen to ensure both (i) a non-biased change in dispersal whatever the value of d and (ii) a mutant dispersal probability within the (0, 1) interval (σ = 0.01 in our simulations). In addition, with probability ν, the new individual belongs to a new species that never occurred before in the metacommunity (i.e. point speciation; see [18]). Speciation has no effect on dispersal and does not interact with the mutation dynamics. A new cycle then starts. Calling 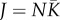 the total number of individuals in the metacommunity, the expected lifetime of an individual is J cycles, such that a ‘generation’ can be defined as J cycles.
the total number of individuals in the metacommunity, the expected lifetime of an individual is J cycles, such that a ‘generation’ can be defined as J cycles.
Following [33] (equations 4 and 5), the mean 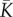 , squared coefficient of variation
, squared coefficient of variation 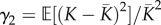 , and standardized skewness
, and standardized skewness 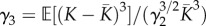 of carrying capacity distribution among communities determine whether EB or ESS of dispersal should occur. Here, we studied metacommunities in which communities can harbour only two carrying capacities K1 and K2 (K1 < K2). We note π1 the proportion of communities with carrying capacity K1. We selected two examples of metacommunities (‘symmetric’ and ‘asymmetric’) corresponding to the ESS and EB scenarios on dispersal (table 1 and electronic supplementary material, appendix S1). The symmetric metacommunity is comprised of an equal number of small and large communities (π1 = 0.5), whereas the asymmetric metacommunity is essentially made of small communities (π1 = 36/37 ≈ 0.97). These metacommunities display the same
of carrying capacity distribution among communities determine whether EB or ESS of dispersal should occur. Here, we studied metacommunities in which communities can harbour only two carrying capacities K1 and K2 (K1 < K2). We note π1 the proportion of communities with carrying capacity K1. We selected two examples of metacommunities (‘symmetric’ and ‘asymmetric’) corresponding to the ESS and EB scenarios on dispersal (table 1 and electronic supplementary material, appendix S1). The symmetric metacommunity is comprised of an equal number of small and large communities (π1 = 0.5), whereas the asymmetric metacommunity is essentially made of small communities (π1 = 36/37 ≈ 0.97). These metacommunities display the same 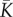 and γ2 values, as well as the same number of communities (N). Keeping
and γ2 values, as well as the same number of communities (N). Keeping 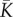 and N constant yields the same overall number of individuals in the metacommunity, which implies the same rate of occurrence of new species and the same singular value of dispersal
and N constant yields the same overall number of individuals in the metacommunity, which implies the same rate of occurrence of new species and the same singular value of dispersal 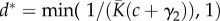 in the two metacommunities (i.e. the value at which branching or evolutionary stability may occur, see [33]). We set the mean carrying capacity of communities to
in the two metacommunities (i.e. the value at which branching or evolutionary stability may occur, see [33]). We set the mean carrying capacity of communities to 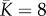 and the number of communities in the metacommunity to N = 1 110 (see electronic supplementary material, appendix S1), so that both metacommunities contained J = 8 880 individuals.
and the number of communities in the metacommunity to N = 1 110 (see electronic supplementary material, appendix S1), so that both metacommunities contained J = 8 880 individuals.
Table 1.
Parameters of symmetric and asymmetric metacommunities, corresponding to the evolutionary predictions and summary statistics of simulations. The two metacommunities verify: 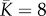 and γ2 = 9/16 ≈ 0.56, N = 1 110 patches. π1 represents the proportion of patches with carrying capacity K1. In both cases, other parameters are set to: μ = 0.01, σ = 0.01, ν = 10−4, c = 0.1. ESS, evolutionary stable strategy; EB, evolutionary branching.
and γ2 = 9/16 ≈ 0.56, N = 1 110 patches. π1 represents the proportion of patches with carrying capacity K1. In both cases, other parameters are set to: μ = 0.01, σ = 0.01, ν = 10−4, c = 0.1. ESS, evolutionary stable strategy; EB, evolutionary branching.
| metacommunity | symmetric | asymmetric | |
|---|---|---|---|
| distribution of carrying capacities | K1 | 2 | 7 |
| K2 | 14 | 44 | |
| π1 | 0.5 | 36/37≈0.97 | |
| prediction on dispersal evolution | ESS | EB |
At the beginning of the first cycle, all individuals harboured the same dispersal value set to the singular value d*. Species identities were attributed using the urn sampling scheme developed for the neutral model of species diversity with multiple communities [34]. In both cases, mutation and speciation parameters were set to: μ = 0.01, σ = 0.01, ν = 10−4. These parameters were chosen so as to (i) remain close to the assumption of adaptive dynamics (limiting the rate and size of mutations of dispersal), (ii) ensure evolutionary dynamics that are fast enough to exhibit the outcomes of interest with simulations of tractable length and size, and (iii) keep a separation of timescales between speciation and mutation processes. We explored three values of dispersal cost (c = 0, 0.1, and 0.5). As the results were qualitatively similar, we only reported results for c = 0.1 in the main text (full results are in the electronic supplementary material, appendix).
For both symmetric and asymmetric metacommunities, 100 independent simulations were run over 20 000 generations (ca 1.77 × 108 death–replacement cycles), a value large enough to reach a stationary dispersal distribution. Simulations were run using the Java programming language (source code doi:10.6084/m9.figshare.3145831).
(b). Neutral model (model M0)
Model M0 is a particular case of model M1 in which all individuals from the metacommunity display the same dispersal value and dispersal does not evolve (i.e. μ = 0). M0 is thus a neutral model in which individual dispersal is explicitly described. Expected patterns of species diversity can be derived analytically in this model using a coalescent approach (inspired by [35]; electronic supplementary material, appendix S4).
(c). Trait and species diversity patterns
We explored the spatial structure of the dispersal parameter in the final state of simulations produced by model M1 using T-statistics [36], a tool to decompose the metacommunity-wide variance of dispersal into the relative contributions of several levels of organization: individual (I), community (C), and metacommunity (R, for regional). We focused on TIC/IR, the proportion of dispersal variance at metacommunity level explained by the variation of individual dispersal within communities. Low TIC/IR means that individuals from a community tend to be similar, which we call dispersal clumpiness. We tested whether TIC/IR is lower than expected for two null models using a randomization procedure. First, we preserved the species label and dispersal value of individuals, but permuted their community identity among communities (keeping community size constant). Under the second model, we permuted dispersal values among species but preserved the occurrence and relative abundances of species in communities. In this second test, we overlooked intraspecific variation by first attributing to individuals the average metacommunity trait value of their species. Randomization proceeded such that individuals from species A were attributed the trait from species B, etc. The mean observed TIC/IR values in the 100 replicated simulations was compared with the mean of the null distribution, obtained by applying the randomization procedure to each simulation output and re-computing TIC/IR.
When comparing predictions from models M0 and M1 in terms of species diversity patterns, we focused on measures of (i) diversity within communities (α-diversity), (ii) differentiation among communities (β-diversity), and (iii) diversity at metacommunity scale (γ-diversity). We defined Dα(Ki) as the probability that two individuals sampled in the same community with carrying capacity Ki belong to different species. Dα(Ki) is a standard index of diversity usually called the Simpson index [37]. To evaluate the differentiation between community pairs, we used Dβ(Ki, Kj) defined as the probability that two individuals drawn from distinct communities with carrying capacities Ki and Kj belong to different species. Dγ, defined as the probability that two individuals randomly sampled in the metacommunity belong to different species, was used at the metacommunity scale.
3. Results
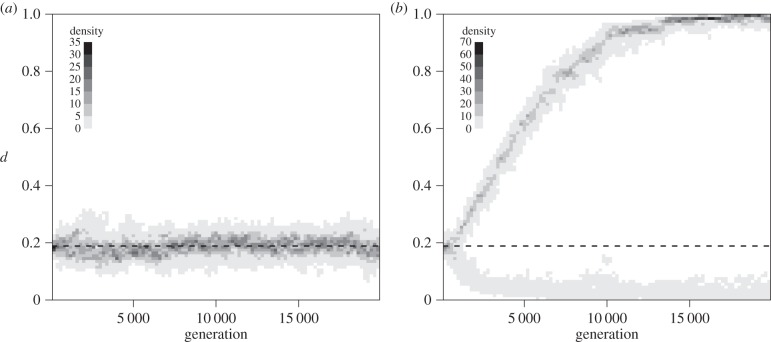
When allowing dispersal to evolve (model M1), our simulations corroborated the theoretical predictions of adaptive dynamics (figure 1). In the symmetric metacommunity (metacommunity 1), variation in dispersal remained concentrated around d*, in agreement with the ESS prediction. In the asymmetric metacommunity (metacommunity 2), two distinct values emerged owing to the evolutionary dynamics, in agreement with the EB prediction. The difference between these dispersal values decreased with the dispersal cost c (electronic supplementary material, appendix S2 and figure A2.1) and increased with the square coefficient of the variation in carrying capacity (γ2; electronic supplementary material, appendix S2 and figure A2.2). Following species composition of the asymmetric metacommunity over time during and after dispersal branching (figure 2) further showed that (i) species that appeared before branching could persist in both branches long after branching, but ultimately went extinct because of speciation and demographic stochasticity and (ii) species that appeared after branching stayed in their original branch. No species was present in both evolutionary branches at the end of 95 of our 100 replicates; it is therefore possible to characterize ‘dweller’ and ‘drifter’ species, harbouring low and high dispersal values, respectively. The situation is very different under the ESS scenario in which, by definition, all species had similar dispersal values at all time.
Figure 1.
Examples of dispersal distributions through time in model M1 for symmetric (a) and asymmetric metacommunity (b) respectively (see table 1 for metacommunity features). One simulation run is represented in each panel. The grey shades refer to individuals' density when all species are pooled together (null in the white areas). Time is given in generation numbers. Parameters describing dispersal evolution are μ = 0.01 and σ = 0.01, and the cost of dispersal is c = 0.1.
Figure 2.
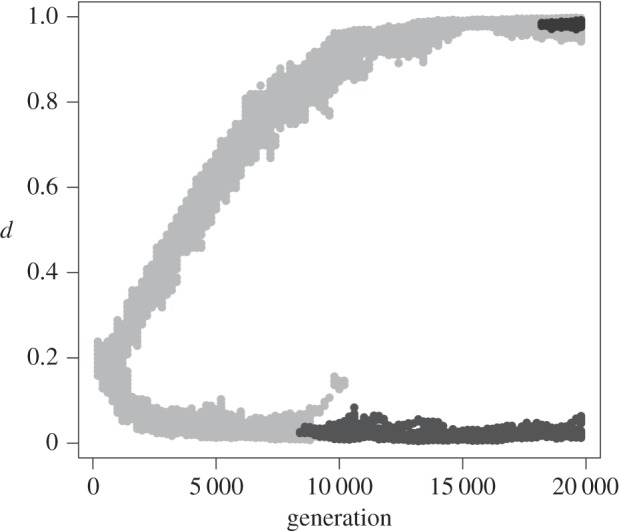
Monitoring of dispersal in three species from the asymmetric metacommunity during the simulation presented in figure 1b (out of 506 species observed over the whole simulation). Each species is represented by a shade of grey. A grey dot is drawn at (d, t) if an individual of the considered species with trait d occurs in the community at generation t. The species in light grey is initially present before branching and persists after branching, but goes extinct in the lower branch. The other two species appear after branching through speciation, in a single branch.
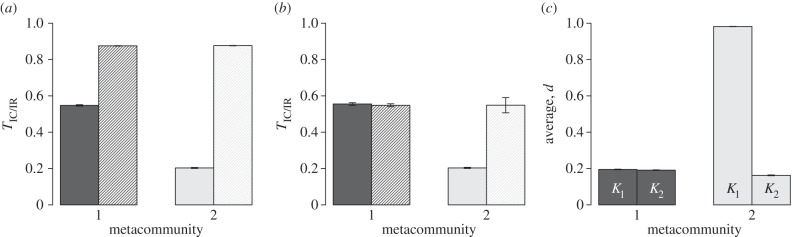
The proportion of variance of dispersal among individuals within communities was significantly lower than expected under a random permutation of individual position (i.e. TIC/IR was significantly lower than random expectation using the first randomization procedure; figure 3a), especially in the asymmetric metacommunity. When permuting species traits while preserving their abundance in each community (figure 3b), TIC/IR under the ESS scenario was not significantly different from the randomized value while a significantly lower value was observed under the EB scenario. We further observed that the average dispersal was significantly larger in communities with small carrying capacity than in those with large carrying capacity in the two metacommunities (figure 3c), a pattern that we call the ‘carrying capacity–dispersal relationship’ below. Electronic supplementary material, appendix S3 provides an analytical argument to explain the emergence of this pattern. The carrying capacity–dispersal relationship was much less pronounced in the symmetric metacommunity (ESS) than in the asymmetric one (EB). All these results are robust to variation in the dispersal cost (electronic supplementary material, appendix S3 and figures A3.1 and A3.2).
Figure 3.
Analysis of dispersal pattern in symmetric (1) and asymmetric (2) metacommunities. (a) Testing TIC/IR using full permutation of individual positions. Bars represent observed (plain bars) and randomized values (striped bars) of TIC/IR at generation 20 000 averaged over 100 independent simulations. Results for both metacommunity 1 (dark grey bar) and 2 (light grey bar) are shown. For each bar, a 95% CI based on central limit theorem is provided. (b) Similar to panel (a), but overlooking intraspecific variability and permuting only species dispersal values. (c) Average dispersal value in small (bars labelled ‘K1’) and large (bars labelled ‘K2’) communities for metacommunity 1 (dark grey) and 2 (light grey). 95% CI of these average value based on central limit theorem approximation are reported for each bar. Testing whether mean dispersal was higher in small communities yielded that the observed difference is statistically significant in both metacommunitites (test based on the central-limit theorem normal approximation; p = 0.0001 in metacommunity 1, p < 10−12 in metacommunity 2).
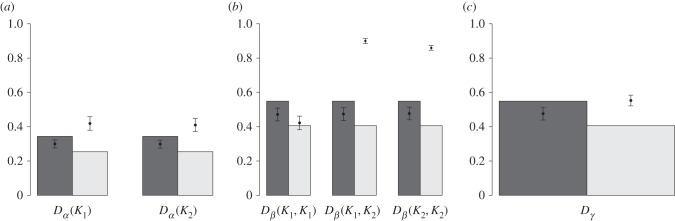
Under the ESS scenario, all species harboured similar dispersal strategies, and the carrying capacity–dispersal relationship was weak (but still significant). We therefore expected species diversity patterns (Dα, Dβ, and Dγ) to closely match the predictions of our neutral model M0. Under the EB scenario, dweller and drifter species emerged, as well as a strong carrying capacity–dispersal relationship. We therefore expected species diversity patterns to strongly deviate from model M0 predictions. In particular, we expected a higher Dβ(K1, K2) than in model M0, because small communities are occupied by drifter species, whereas large communities are occupied by dweller species. We derived analytical expressions of Dα(Ki), Dβ(Ki, Kj), and Dγ in model M0 (electronic supplementary material, appendix S4) which were validated using simulations (electronic supplementary material, figure A4.2). Dα(Ki) and Dβ(Ki, Kj) depend on the dispersal rate (d*), the dispersal cost (c), the speciation rate (ν) and the mean, variance and asymmetry of the distribution of the carrying capacities across the metacommunity, but not on the carrying capacities Ki and Kj. Higher asymmetry of the distribution always had a negative impact on Dα, Dβ, and Dγ (electronic supplementary material, figure A4.3). In the examples considered here, in which the coefficient of variation was kept constant but asymmetry varied, the neutral diversity at community (Dα) and metacommunity levels (Dγ) as well as the dissimilarity among communities (Dβ) in the symmetric metacommunity were thus predicted to be higher than in the asymmetric metacommunity (figure 4).
Figure 4.
Estimates and 95% CI of Dα (a), Dβ (b), and Dγ (c) in the symmetric and asymmetric metacommunities obtained from model M1 simulations compared with neutral predictions. Grey bars provide the analytical predictions of the neutral model M0 with d = d* (computed from the left column of electronic supplementary material, table A4.3) for symmetric (dark grey) and asymmetric (light grey) metacommunities. Simulation parameters are given in table 1. A point is associated with each grey bar, which represents the average estimate value obtained from 100 independent simulations of model M1. A 95% CI of the expected diversity/dissimilarity value under model M1 is reported around each point.
We computed the unbiased 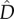 estimates (defined in electronic supplementary material, appendix S5) of Dα(K1), Dα(K2), Dβ(K1, K1), Dβ(K1, K2), Dβ(K2, K2), and Dγ from our simulations of model M1 and compared them with the analytical predictions of M0 (figure 4). In the symmetric metacommunity (dispersal ESS),
estimates (defined in electronic supplementary material, appendix S5) of Dα(K1), Dα(K2), Dβ(K1, K1), Dβ(K1, K2), Dβ(K2, K2), and Dγ from our simulations of model M1 and compared them with the analytical predictions of M0 (figure 4). In the symmetric metacommunity (dispersal ESS), 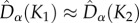 , and
, and 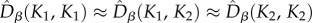 , in line with neutral predictions. However, all
, in line with neutral predictions. However, all 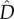 estimates were lower than the neutral predictions. In the asymmetric metacommunity (EB; figure 4), the
estimates were lower than the neutral predictions. In the asymmetric metacommunity (EB; figure 4), the 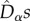 were also similar between small and large communities, in line with the predictions of model M0, but this time larger than the neutral expectations.
were also similar between small and large communities, in line with the predictions of model M0, but this time larger than the neutral expectations. 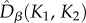 and
and 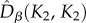 were, as expected, higher than the neutral expectations. These deviations from the neutral predictions of both
were, as expected, higher than the neutral expectations. These deviations from the neutral predictions of both 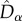 and
and 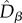 resulted in a larger
resulted in a larger 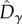 than predicted by the neutral model (figure 4). Note also that
than predicted by the neutral model (figure 4). Note also that 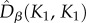 and
and 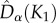 were very similar in the asymmetric metacommunity (figure 4), indicating that, when sampling two individuals in small communities, the probability of getting two different species did not depend on whether individuals were drawn from the same or from different communities. These results were robust to changing the dispersal cost (electronic supplementary material, appendix S5, figures A5.1 and A5.2).
were very similar in the asymmetric metacommunity (figure 4), indicating that, when sampling two individuals in small communities, the probability of getting two different species did not depend on whether individuals were drawn from the same or from different communities. These results were robust to changing the dispersal cost (electronic supplementary material, appendix S5, figures A5.1 and A5.2).
4. Discussion
(a). Dispersal in metacommunities
We built a metacommunity model including dispersal evolution which reproduces the central result obtained by Massol et al. [33] at the metapopulation scale: increasing asymmetry in the distribution of carrying capacities generates variance in the strength of kin competition among communities, which triggers evolutionary branching and the emergence of distinct dispersal strategies in the metacommunity. We could therefore compare an ESS scenario in which individual dispersal shows little variation around a single value (symmetric metacommunity) and an EB scenario in which dispersal is distributed around two distinct levels (asymmetric metacommunity).
Our analysis of dispersal distribution within and among communities showed that under both scenarios, a negative carrying capacity–dispersal relationship emerges. This pattern stems from the three following effects. First, all communities have similar immigrant pool compositions, because dispersal is not limited by distance in our model. Second, in small communities, the temporal dynamics of average species abundances in these small communities are mostly driven by immigration, and are similar to those of the immigrant pool. Third, the deterministic local competition effect dominates in large communities, which induces a shift of relative abundances towards the dominance of low dispersers and the extinction of high dispersers (i.e. competitive exclusion).
The carrying capacity–dispersal relationship echoes several observations on within-species dispersal polymorphism in natural metapopulations. For example, a majority of migrants originates from small ephemeral patches, rather than from large patches, in a rock pool metapopulation of the cladoceran Daphnia magna [38]. However, a competition–dispersal trade-off may not be the only driver of such patterns in natural metapopulations as variation in both age (when extinctions occur) and connectivity of populations play an important role in the distribution of dispersal phenotypes among populations [39]. Whether the carrying capacity–dispersal relationship occurs at the interspecific level in natural metacommunities is less documented to date. Our work predicts that species occurring preferentially in communities with large (resp. small) carrying capacities should be dwellers (resp. drifters). A comparison of traits assumed to be good proxies of dispersal, such as dispersal attributes of seeds in plant communities (see [40] for an example in grassland communities) might be a first approach to test this prediction. More refined statistical approaches based on dispersal kernel estimation [11,41] is a further possibility. A combination of genetic and demographic data collected in several species might also return relevant information on dispersal, as already done in metapopulations of single species [42].
A strong carrying capacity–dispersal relationship (like in the EB scenario) has potential to generate dispersal clumpiness, as small (resp. large) communities tend to harbour more drifters (resp. dwellers) than expected under random permutations of individuals in space. However, dispersal clumpiness can emerge even in the absence of strong carrying capacity–dispersal relationship as exemplified in the ESS scenario. This stems from the fact that when dispersal is limited, individuals which are in the same community tend to be more closely related than individuals randomly sampled at the metacommunity scale. This generates clumpiness of both species [43] and values of heritable traits, which in turn yields a lower TIC/IR than expected when permuting individuals across the metacommunity. Interestingly, this should caution against interpreting all significantly low TIC/IR as signals of some kind of ‘environmental filtering’ associated with the considered trait (as suggested by Violle et al. [36]) when using a null model randomizing individual position in space. By contrast, our second randomizing procedure (permuting average species trait only) exhibits two properties addressing the above-described limits: it preserves the variation of species abundance among communities and it removes the effects of increased relatedness within populations by considering average species traits. Under the ESS scenario, the variance within communities was not significantly lower than the one expected under the null model, which corroborates the idea that the significantly low TIC/IR observed when randomizing individual positions is mostly due to the effect of limited dispersal and not to dispersal polymorphism (which is limited in this case). Under the EB scenario, the dispersal strategies were still significantly more similar within communities than predicted by the null model. Permuting average species traits can therefore be used to disentangle the effects of limited dispersal on spatial patterns of dispersal (null model not rejected) from those of dispersal polymorphism (null model rejected), and should be preferred to permuting individual positions whenever limited dispersal is thought to play a role in diversity patterns.
(b). Neutral predictions on species diversity patterns
Our neutral model makes two important predictions about species diversity patterns. First, α- and β-diversity should not vary (in expectation) among communities with different carrying capacities. In several neutral metacommunity models, Simpson diversity (used here) can vary among patches [44,45]. However, this variation is mostly explained by differences in connectivity (i.e. the number of immigrants per generation) among patches induced by specific network structures. As we were primarily interested in the relationship between the evolutionary dynamics of dispersal and diversity patterns, we focused on a simple spatial structure in which connectivity is similar between all patches, irrespective of their carrying capacity, a situation for which we could derive evolutionary predictions. Our model might be extended to more complex community networks in which the neutral expectations have already been derived and that better describe the spatial structure of some real metacommunities, such as dendritic networks for rivers [46,47]. The limiting step here would be to derive eco-evolutionary predictions about dispersal polymorphism in complex networks with variable connectivity among patches, a point that has been little explored up to now [48].
Second, our neutral model predicts that species γ-diversity should decrease with the asymmetry of the carrying capacity distribution in the metacommunity. This can be intuitively explained by the fact that, in more asymmetric metacommunities, drift is stronger, because more individuals belong to small communities. Here, we focused on the prediction about asymmetry as it is the feature of carrying capacity distribution that discriminates dispersal evolution scenarios [33]. Yet, the analytical results derived from our neutral model may allow a more comprehensive exploration of the effects of carrying capacity distribution on species γ-diversity (electronic supplementary material, figure A4.3) and thus contribute to current research in conservation biology, in particular with respect to reserve design [49]. However, this is beyond the scope of this work.
In the two examples of metacommunities considered in our study, we derived neutral predictions about diversity patterns with a priori knowledge of the relevant parameters (carrying capacities and dispersal cost). However, it may not be straightforward to obtain these parameters in natural systems. One alternative would be to fit the neutral model to observed diversity patterns in order to estimate their most likely values. The sampling formulae that have been derived for classic neutral models considering spatially implicit metacommunities allow computing maximum-likelihood estimates [34,50,51]. Such a formula is not available for our model, but the carrying capacities of communities and the dispersal cost could be estimated by looking for values that yield predictions about expected diversity and dissimilarity that best fit the observed diversity and dissimilarity estimates (e.g. using a least-squares approach).
(c). Effects of dispersal polymorphism on species diversity
Our neutral predictions (model M0) incorporate the influence of speciation, drift, and limited dispersal. Comparing the neutral predictions of species diversity patterns with model M1, in which dispersal can evolve, reveals the additional effects (i) of mutational variance around the selective optima in the ESS scenario and (ii) of disruptive selection of dispersal in the EB scenario. To the best our knowledge, our study is the first to suggest studying the effects of a competition–dispersal trade-off as an evolutionarily driven deviation from the neutral theory of metacommunity assembly.
In the ESS scenario (symmetric metacommunity), both the diversity within communities and the dissimilarity among communities do not depend on carrying capacity, which is consistent with the neutral predictions. This was rather expected as species are distributed around the same dispersal value, with some variation owing to mutation, leading to metacommunity dynamics close to neutrality. However, lower diversity and lower dissimilarity than expected under a neutral model suggest that the mutational variance of dispersal generates fitness differences among individuals, which scale up to a slight fitness heterogeneity among species. Slightly maladapted species are more quickly eliminated than expected under the neutral model which yields a lower diversity at the metacommunity scale and consequently decreases both the diversity within communities and the dissimilarity among communities. More generally, our results suggest that when there is a unique selective optimum on a given trait (i.e. stabilizing selection), neutral models which overlook intra- and interspecific variance in this trait may overestimate species diversity.
Under EB, the following general picture of the metacommunity emerged: (i) each large community harbours a mostly endemic community of species that are good local competitors but nearly never disperse (dwellers) and (ii) drifter species freely disperse across the metacommunity, dominating small communities and maintaining themselves at low frequency through mass effect in large communities although they are locally outcompeted by dwellers. From point (i) derives the higher dissimilarity among large communities than expected under the neutral model. Point (ii) explains why the diversity of small communities equals the dissimilarity among small communities. The mass effect of drifters in large communities explains why diversity/dissimilarity in small communities (Dα(K1)) matches the diversity observed in a single large community (Dα(K2)) although the former includes many more individuals. We also emphasize that, when EB occurs, both α- and β-diversities are higher than predicted by the neutral model. Dispersal polymorphism increases the species coexistence potential at the metacommunity scale.
Our work suggests that the study of diversity patterns in metacommunities would greatly benefit from documenting dispersal traits of species, so as to identify dwellers and drifters. This point was previously made by Janzen et al. [22] in their study of the Barro Colorado tropical tree community: they concluded from combining local abundances of species with seed dispersal traits that immigration was stronger for biotically dispersed tree species (which would therefore be drifters) than for abiotically dispersed ones (which would therefore be dwellers). Importantly enough, biotically dispersed species are dominant in Barro Colorado Island. This point cannot be discussed in the Janzen et al. framework, as dispersal does not trade-off with any other features (it is a structural property of their model that drifters dominate in any community). By contrast, our model suggests a biological interpretation of drifters' dominance: the carrying capacity of Barro Colorado Island may be too small to let dwellers reach large enough population sizes to durably settle and counterbalance the immigration of drifters.
Beyond analysing a single community, testing whether small communities (i) have more similar species composition than they have with large communities and (ii) harbour species with higher dispersal abilities than large communities may reveal that a competition–dispersal trade-off affects species coexistence at the metacommunity scale. In addition, the EB scenario of our model illustrates how considering dispersal may contribute to avoid misinterpreting species diversity patterns. First, dweller species are abundant in one or a few large communities and absent elsewhere, but this is not due to local environmental filtering or adaptation. Dwellers should here show the same fitness in all communities, irrespective of size. Second, large communities simultaneously harbour dwellers and drifters the relative abundances of which fluctuate around stable values in time (results not shown), and this is not due to some local coexistence mechanisms but rather to mass effect from the metacommunity.
5. Conclusion
To the best of our knowledge, our study proposes the first theoretical model integrating dispersal evolution in a multi-species context and deriving predictions about species diversity patterns. Interestingly, it includes three of the four paradigms of metacommunity assembly identified by Leibold et al. [15]: it simultaneously borrows from (i) the ‘patch-dynamics' view by considering a competition–dispersal trade-off as a coexistence mechanism, (ii) the ‘mass-effect’ view as drifter species can persist in patches owing to immigration, although they are less fit locally, and (iii) the neutral view as species diversity in the metacommunity ultimately stems from an equilibrium between speciation and stochastic extinction. Our approach should now be extended to other traits contributing to the local adaptation of species in patches with heterogeneous environmental conditions (see [52]) but keeping the focus on the evolutionary and ecological significance of the considered polymorphisms, as we did here with dispersal. This would integrate the fourth metacommunity paradigm, species sorting [15], and might pave the way to a unified framework for pattern-based approaches of metacommunity assembly processes.
Supplementary Material
Acknowledgements
Many thanks are due to P. David N. Loeuille S. Maurice F. Munoz, and R. Vitalis for discussions and to F. Altermatt E. Klein S. Pavoine O. Ronce, and C. Vacher for their useful comments on the manuscript. We also thank associate editor O. Gaggiotti and two anonymous referees for significant improvements during the revision process. We thank the Center for Evolutionary and Functional Ecology and particularly M.-C. Quidoz (SIE platform) for access to computational resources.
Authors' contributions
F.L., P.J., and F.M. built the project. F.M. performed the analysis of dispersal evolution. T.P. ran the simulations. F.L. derived the neutral predictions and analysed the simulations. F.L., P.J., and F.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Funding
F.L. was supported by fellowships from AgroParistech, the Agropolis foundation and the Swedish University of Agricultural Science, and by money from IRSTEA. T.P. was supported by a fellowship from the Université de Montpellier. This work was supported by money from CNRS to P.J. and F.M., and from the French ANR (AFFAIRS project in the BIOADAPT programme–P.I.: Dr P. David—grant no. 12-ADAP-005; ARSENIC project—P.I.: Dr F. Massol—grant no. 14-CE02-0012).
References
- 1.Lotka AJ. 1926. Elements of physical biology. Am. Math. Mon. 33, 426–428. ( 10.2307/2298330) [DOI] [Google Scholar]
- 2.MacArthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385. ( 10.1086/282505) [DOI] [Google Scholar]
- 3.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.2307/221736) [DOI] [Google Scholar]
- 4.Hastings A. 1980. Disturbance, coexistence, history, and competition for space. Theor. Popul. Biol. 18, 363–373. ( 10.1016/0040-5809(80)90059-3) [DOI] [Google Scholar]
- 5.Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16. ( 10.2307/1939377) [DOI] [Google Scholar]
- 6.Calcagno V, Mouquet N, Jarne P, David P. 2006. Coexistence in a metacommunity: the competition–colonization trade-off is not dead. Ecol. Lett. 9, 897–907. ( 10.1111/j.1461-0248.2006.00930.x) [DOI] [PubMed] [Google Scholar]
- 7.Cadotte MW, Mai DV, Jantz S, Collins MD, Keele M, Drake JA. 2006. On testing the competition-colonization trade-off in a multispecies assemblage. Am. Nat. 168, 704–709. ( 10.1086/508296) [DOI] [PubMed] [Google Scholar]
- 8.Livingston G, Matias M, Calcagno V, Barbera C, Combe M, Leibold MA, Mouquet N. 2012. Competition-colonization dynamics in experimental bacterial metacommunities. Nat. Commun. 3, 1234 ( 10.1038/ncomms2239) [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson A, Eriksson O. 2000. A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos 88, 494–502. ( 10.1034/j.1600-0706.2000.880304.x) [DOI] [Google Scholar]
- 10.Nadell CD, Bassler BL. 2011. A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc. Natl Acad. Sci. USA 108, 14 181–14 185. ( 10.1073/pnas.1111147108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller-Landau HC, Wright SJ, Calderón O, Condit R, Hubbell SP. 2008. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 96, 653–667. ( 10.1111/j.1365-2745.2008.01399.x) [DOI] [Google Scholar]
- 12.Yu DW, Wilson HB, Pierce NE. 2001. An empirical model of species coexistence in a spatially structured environment. Ecology 82, 1761–1771. ( 10.1890/0012-9658(2001)082%5B1761:AEMOSC%5D2.0.CO;2) [DOI] [Google Scholar]
- 13.Lei G, Hanski I. 1998. Spatial dynamics of two competing specialist parasitoids in a host metapopulation. J. Anim. Ecol. 67, 422–433. ( 10.1046/j.1365-2656.1998.00204.x) [DOI] [Google Scholar]
- 14.Levins R, Culver D. 1971. Regional coexistence of species and competition between rare species. Proc. Natl Acad. Sci. USA 68, 1246–1248. ( 10.1073/pnas.68.6.1246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibold MA, et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. ( 10.1111/j.1461-0248.2004.00608.x) [DOI] [Google Scholar]
- 16.Caswell H. 1976. Community structure: a neutral model analysis. Ecol. Monogr, 327–354. ( 10.2307/1942257) [DOI] [Google Scholar]
- 17.Bell G. 2001. Neutral macroecology. Science 293, 2413–2418. ( 10.1126/science.293.5539.2413) [DOI] [PubMed] [Google Scholar]
- 18.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 19.Volkov I, Banavar JR, Hubbell SP, Maritan A. 2007. Patterns of relative species abundance in rainforests and coral reefs. Nature 450, 45–49. ( 10.1038/nature06197) [DOI] [PubMed] [Google Scholar]
- 20.Hubbell SP. 2006. Neutral theory and the evolution of ecological equivalence. Ecology 87, 1387–1398. ( 10.1890/0012-9658(2006)87%5B1387:NTATEO%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Zhou S. 2011. Asymmetry in species regional dispersal ability and the neutral theory. PLoS ONE 6, e0024128 ( 10.1371/journal.pone.0024128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janzen T, Haegeman B, Etienne RS. 2015. A sampling formula for ecological communities with multiple dispersal syndromes. J. Theor. Biol. 374, 94–106. ( 10.1016/j.jtbi.2015.03.018) [DOI] [PubMed] [Google Scholar]
- 23.Haag CR, Saastamoinen M, Marden JH, Hanski I. 2005. A candidate locus for variation in dispersal rate in a butterfly metapopulation. Proc. R. Soc. B 272, 2449–2456. ( 10.1098/rspb.2005.3235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saastamoinen M. 2007. Heritability of dispersal rate and other life history traits in the Glanville fritillary butterfly. Heredity 100, 39–46. ( 10.1038/sj.hdy.6801056) [DOI] [PubMed] [Google Scholar]
- 25.Charmantier A, Buoro M, Gimenez O, Weimerskirch H. 2011. Heritability of short-scale natal dispersal in a large-scale foraging bird, the wandering albatross. J. Evol. Biol. 24, 1487–1496. ( 10.1111/j.1420-9101.2011.02281.x) [DOI] [PubMed] [Google Scholar]
- 26.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fronhofer EA, Altermatt F. 2015. Eco-evolutionary feedbacks during experimental range expansions. Nat. Commun. 6, 6844 ( 10.1038/ncomms7844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253. ( 10.1146/annurev.ecolsys.38.091206.095611) [DOI] [Google Scholar]
- 29.Duputié A, Massol F. 2013. An empiricist's guide to theoretical predictions on the evolution of dispersal. Interface Focus 3, 20130028 ( 10.1098/rsfs.2013.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathias A, Kisdi E, Olivieri I. 2001. Divergent evolution of dispersal in a heterogeneous landscape. Evolution 55, 246–259. ( 10.1111/j.0014-3820.2001.tb01290.x) [DOI] [PubMed] [Google Scholar]
- 31.Massol F, Débarre F. 2015. Evolution of dispersal in spatially and temporally variable environments: the importance of life cycles. Evolution 69, 1925–1937. ( 10.1111/evo.12699) [DOI] [PubMed] [Google Scholar]
- 32.Johst K, Doebeli M, Brandl R. 1999. Evolution of complex dynamics in spatially structured populations. Proc. R. Soc. Lond. B 266, 1147–1154. ( 10.1098/rspb.1999.0756) [DOI] [Google Scholar]
- 33.Massol F, Duputié A, David P, Jarne P. 2011. Asymmetric patch size distribution leads to disruptive selection on dispersal. Evolution 65, 490–500. ( 10.1111/j.1558-5646.2010.01143.x) [DOI] [PubMed] [Google Scholar]
- 34.Etienne RS. 2007. A neutral sampling formula for multiple samples and an ‘exact’ test of neutrality. Ecol. Lett. 10, 608–618. ( 10.1111/j.1461-0248.2007.01052.x) [DOI] [PubMed] [Google Scholar]
- 35.Wakeley J. 2001. The coalescent in an island model of population subdivision with variation among demes. Theor. Popul. Biol. 59, 133–144. ( 10.1006/tpbi.2000.1495) [DOI] [PubMed] [Google Scholar]
- 36.Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. ( 10.1016/j.tree.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 37.Magurran AE. 2004. Measuring biological diversity. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 38.Altermatt F, Ebert D. 2010. Populations in small, ephemeral habitat patches may drive dynamics in a Daphnia magna metapopulation. Ecology 91, 2975–2982. ( 10.1890/09-2016.1) [DOI] [PubMed] [Google Scholar]
- 39.Hanski I, Erälahti C, Kankare M, Ovaskainen O, Sirén H. 2004. Variation in migration propensity among individuals maintained by landscape structure. Ecol. Lett. 7, 958–966. ( 10.1111/j.1461-0248.2004.00654.x) [DOI] [Google Scholar]
- 40.Lindborg R. 2007. Evaluating the distribution of plant life-history traits in relation to current and historical landscape configurations. J. Ecol. 95, 555–564. ( 10.1111/j.1365-2745.2007.01232.x) [DOI] [Google Scholar]
- 41.Clark CJ, Poulsen JR, Bolker BM, Connor EF, Parker VT. 2005. Comparative seed shadows of bird-, monkey-, and wind-dispersed trees. Ecology 86, 2684–2694. ( 10.1890/04-1325) [DOI] [Google Scholar]
- 42.Lamy T, Pointier JP, Jarne P, David P. 2012. Testing metapopulation dynamics using genetic, demographic and ecological data. Mol. Ecol. 21, 1394–1410. ( 10.1111/j.1365-294X.2012.05478.x) [DOI] [PubMed] [Google Scholar]
- 43.McGill BJ. 2010. Towards a unification of unified theories of biodiversity. Ecol. Lett. 13, 627–642. ( 10.1111/j.1461-0248.2010.01449.x) [DOI] [PubMed] [Google Scholar]
- 44.Economo EP, Keitt TH. 2008. Species diversity in neutral metacommunities: a network approach. Ecol. Lett. 11, 52–62. [DOI] [PubMed] [Google Scholar]
- 45.Economo EP, Keitt TH. 2010. Network isolation and local diversity in neutral metacommunities. Oikos 119, 1355–1363. ( 10.1111/j.1600-0706.2010.18272.x) [DOI] [Google Scholar]
- 46.Muneepeerakul R, Bertuzzo E, Lynch HJ, Fagan WF, Rinaldo A, Rodriguez-Iturbe I. 2008. Neutral metacommunity models predict fish diversity patterns in Mississippi–Missouri basin. Nature 453, 220–222. ( 10.1038/nature06813) [DOI] [PubMed] [Google Scholar]
- 47.Seymour M, Fronhofer EA, Altermatt F. 2015. Dendritic network structure and dispersal affect temporal dynamics of diversity and species persistence. Oikos 124, 908–916. ( 10.1111/oik.02354) [DOI] [Google Scholar]
- 48.Henriques-Silva R, Boivin F, Calcagno V, Urban MC, Peres-Neto PR. 2015. On the evolution of dispersal via heterogeneity in spatial connectivity. Proc. R. Soc. B 282, 20142879 ( 10.1098/rspb.2014.2879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Teeffelen AJA, Vos CC, Opdam P. 2012. Species in a dynamic world: consequences of habitat network dynamics on conservation planning. Biol. Conserv. 153, 239–253. ( 10.1016/j.biocon.2012.05.001) [DOI] [Google Scholar]
- 50.Etienne RS. 2005. A new sampling formula for neutral biodiversity. Ecol. Lett. 8, 253–260. ( 10.1111/j.1461-0248.2004.00717.x) [DOI] [Google Scholar]
- 51.Etienne RS, Olff H. 2004. A novel genealogical approach to neutral biodiversity theory. Ecol. Lett. 7, 170–175. ( 10.1111/j.1461-0248.2004.00572.x) [DOI] [Google Scholar]
- 52.Jabot F. 2010. A stochastic dispersal-limited trait-based model of community dynamics. J. Theor. Biol. 262, 650–661. ( 10.1016/j.jtbi.2009.11.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.