Significance
Lysergic acid diethylamide (LSD), the prototypical “psychedelic,” may be unique among psychoactive substances. In the decades that followed its discovery, the magnitude of its effect on science, the arts, and society was unprecedented. LSD produces profound, sometimes life-changing experiences in microgram doses, making it a particularly powerful scientific tool. Here we sought to examine its effects on brain activity, using cutting-edge and complementary neuroimaging techniques in the first modern neuroimaging study of LSD. Results revealed marked changes in brain blood flow, electrical activity, and network communication patterns that correlated strongly with the drug’s hallucinatory and other consciousness-altering properties. These results have implications for the neurobiology of consciousness and for potential applications of LSD in psychological research.
Keywords: LSD, serotonin, consciousness, brain, psychedelic
Abstract
Lysergic acid diethylamide (LSD) is the prototypical psychedelic drug, but its effects on the human brain have never been studied before with modern neuroimaging. Here, three complementary neuroimaging techniques: arterial spin labeling (ASL), blood oxygen level-dependent (BOLD) measures, and magnetoencephalography (MEG), implemented during resting state conditions, revealed marked changes in brain activity after LSD that correlated strongly with its characteristic psychological effects. Increased visual cortex cerebral blood flow (CBF), decreased visual cortex alpha power, and a greatly expanded primary visual cortex (V1) functional connectivity profile correlated strongly with ratings of visual hallucinations, implying that intrinsic brain activity exerts greater influence on visual processing in the psychedelic state, thereby defining its hallucinatory quality. LSD’s marked effects on the visual cortex did not significantly correlate with the drug’s other characteristic effects on consciousness, however. Rather, decreased connectivity between the parahippocampus and retrosplenial cortex (RSC) correlated strongly with ratings of “ego-dissolution” and “altered meaning,” implying the importance of this particular circuit for the maintenance of “self” or “ego” and its processing of “meaning.” Strong relationships were also found between the different imaging metrics, enabling firmer inferences to be made about their functional significance. This uniquely comprehensive examination of the LSD state represents an important advance in scientific research with psychedelic drugs at a time of growing interest in their scientific and therapeutic value. The present results contribute important new insights into the characteristic hallucinatory and consciousness-altering properties of psychedelics that inform on how they can model certain pathological states and potentially treat others.
Lysergic acid diethylamide (LSD) is a potent serotonergic hallucinogen or “psychedelic” that alters consciousness in a profound and characteristic way. First synthesized in 1938, its extraordinary psychological properties were not discovered until 1943 (1). LSD would go on to have a major effect on psychology and psychiatry in the 1950s and 1960s; however, increasing recreational use and its influence on youth culture provoked the drug’s being made illegal in the late 1960s. As a consequence, human research with LSD has been on pause for half a century. However, inspired by a revival of research with other psychedelics, such as psilocybin and ayahuasca, a small number of new reports on the psychological effects of LSD have recently been published (2–6).
LSD has a high affinity for a range of different neurotransmitter receptors, but its characteristic psychological effects are thought to be mediated by serotonin 2A receptor (5-HT2AR) agonism (7). Previous neurophysiological research with LSD is limited to electroencephalography (EEG) studies in the 1950s and 1960s. These reported reductions in oscillatory power, predominantly in the lower-frequency bands, and an increase in the frequency of alpha rhythms (8). Broadband decreases in cortical oscillatory power have been observed in modern EEG and magnetoencephalography (MEG) studies with psilocybin (9, 10), with EEG and the dimethyltryptamine-containing brew “ayahuasca” (11), and with rodent brain local-field potential recordings and a range of different 5-HT2AR agonists (12–14).
The effects of psychedelics (other than LSD) on human brain activity have also previously been investigated with positron emission tomography (PET) (15) and functional magnetic resonance imaging (fMRI) (16). fMRI studies with psilocybin revealed decreased cerebral blood flow (CBF) and blood oxygen level-dependent (BOLD) signal in connector hubs (16), decreased resting state functional connectivity (RSFC) in major resting state networks (RSNs) such as the default-mode network (DMN) (17), and the emergence of novel patterns of communication (18, 19), whereas increased cortical glucose metabolism was found with PET (15). Notably, the spatial locations of the PET-, fMRI-, EEG-, and MEG-measured effects of psychedelics are relatively consistent; for example, high-level cortical regions, such as the posterior cingulate cortex (PCC), and some of the principal effects of psilocybin revealed by fMRI (e.g., decreased DMN RSFC) were recently replicated by a separate team working with ayahuasca (20).
Consistent with a prior hypothesis (17), these studies suggest that an “entropic” effect on cortical activity is a key characteristic of the psychedelic state. However, a putative excitation of hippocampal/parahippocampal gyri activity has also been observed with fMRI and psychedelics in humans (19) and animals (14). Moreover, depth EEG studies in the 1950s reported activations in medial temporal lobe regions during psychosis-like states under LSD and other psychedelics (21, 22). Further, patients with epilepsy with resection of the medial temporal lobes showed attenuated LSD effects postsurgery (23), and electrical stimulation of medial temporal lobe circuitry produces visual hallucinations of somewhat similar nature to those produced by psychedelics [e.g., distorted visual perception (24) and dreamlike “visions” (25)].
The present study sought to investigate the acute brain effects of LSD in healthy volunteers, using a comprehensive placebo-controlled neuroimaging design incorporating ASL, BOLD signal measures, and MEG resting state scans. It was predicted that major RSNs (e.g., the DMN) and hippocampal/parahippocampal gyri circuitry would be implicated in the drug’s mechanism of action.
Twenty healthy participants attended two scanning days (LSD and placebo) at least 2 wk apart in a balanced-order, within-subjects design. Sessions included an fMRI followed by a MEG scan, each lasting 75 min. Data were acquired during eye-closed, task-free, “resting state” conditions. Drug/placebo were administered in solution and injected i.v. over the course of 2 min. Two resting state ASL scans totaling 16 min were completed 100 min after i.v. administration of LSD (75 µg in 10 mL saline) or placebo (10 mL saline), corresponding to the initial phase of the peak subjective effects of LSD (peak effects were reached ∼120–150 min postinfusion). Two resting state BOLD scans totaling 14 min were completed 135 min postinfusion, and two resting state MEG scans totaling 14 min were completed 225 min postinfusion. All analyses applied multiple comparison correction (SI Appendix) and two-tailed hypothesis testing unless particularly strong prior hypotheses were held.
Results
The intensity of LSD’s subjective effects was relatively stable for the ASL and BOLD scans but attenuated somewhat for the MEG (SI Appendix, Table S1). Participants carried out VAS-style ratings via button-press and a digital display screen presented after each scan (SI Appendix), and the 11-factor altered states of consciousness (ASC) questionnaire (26) was completed at the end of each dosing day (SI Appendix, Fig. S1). All participants reported eyes-closed visual hallucinations and other marked changes in consciousness under LSD.
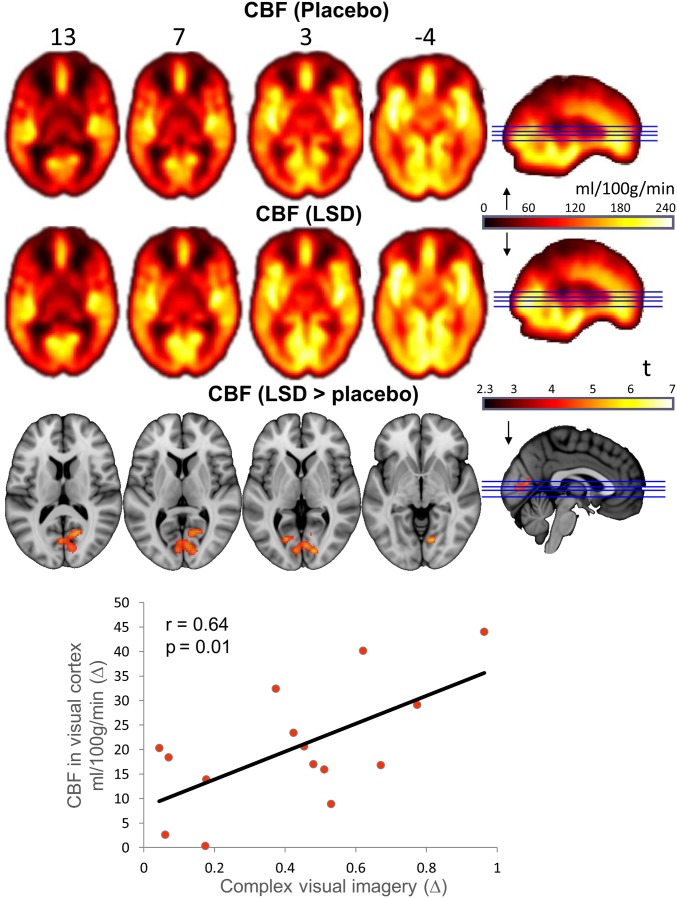
Data from 15 volunteers were suitable for the ASL and BOLD analyses (four females; mean age, 30.5 ± 8.0 y; SI Appendix). Differences in CBF in the two conditions were calculated using a whole-brain analysis (cluster-correction, P < 0.05). Greater CBF under LSD was observed in the visual cortex (Fig. 1), and the magnitude of these increases correlated positively with ratings of complex imagery on the ASC (r = 0.64; P = 0.01; Bonferroni corrected P = 0.04; SI Appendix, Fig. S9A). The unthresholded difference in CBF can be viewed in Neurovault (27) (neurovault.org/collections/FBVSAVDQ/).
Fig. 1.
Whole-brain cerebral blood flow maps for the placebo and LSD conditions, plus the difference map (cluster-corrected, P < 0.05; n = 15).
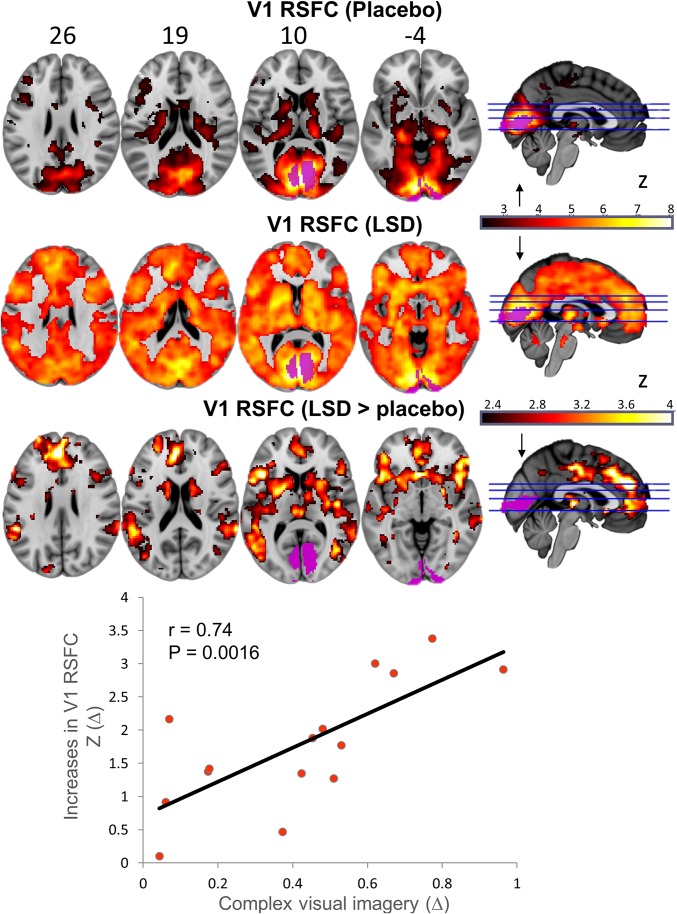
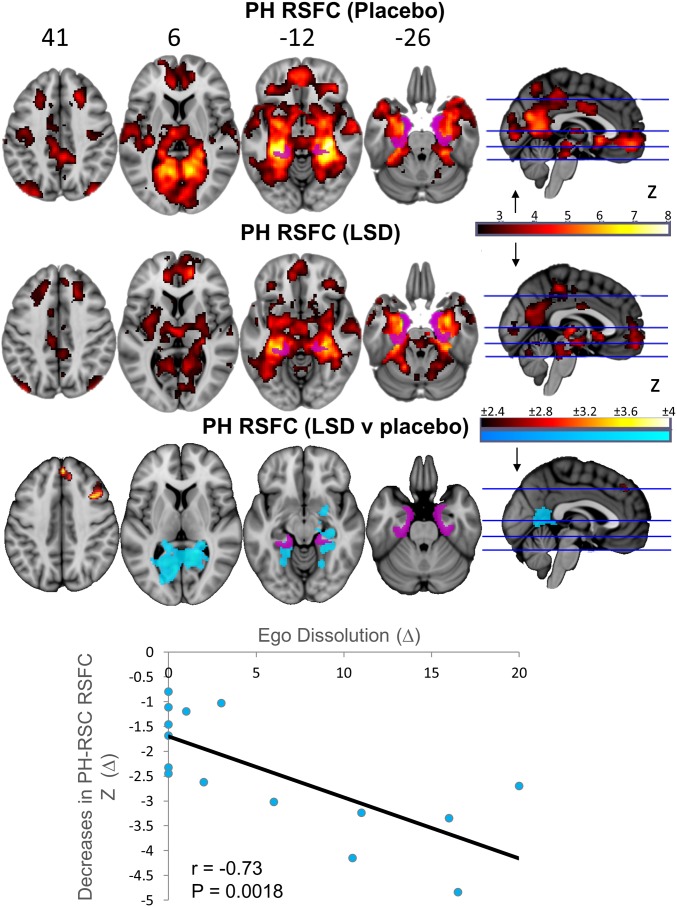
Seed-based RSFC analyses were also performed. A bilateral parahippocampal (PH) seed was chosen based on previous findings with psilocybin (19) and a primary visual cortex (V1) seed was chosen based on the characteristic visual perceptual effects of psychedelics. V1 was identified using a retinotopic-localizer paradigm (SI Appendix). Finally, the ventromedial PFC (vmPFC) was chosen because of our previous focus on this region in studies with psilocybin and MDMA (16, 28). Analyses revealed increased RSFC between V1 and a large number of cortical and subcortical brain regions (Fig. 2 and SI Appendix, Table S3), decreased RSFC between the PH and the retrosplenial cortex (RSC) and PCC, and increased RSFC between the PH and dorsal mPFC and right dorsolateral PFC (Fig. 3 and SI Appendix, Table S4). Increased RSFC between the vmPFC and the bilateral caudate and inferior frontal gyrus was also observed, as was decreased vmPFC-PCC RSFC (SI Appendix, Fig. S4 and SI Appendix, Table S5). All the relevant unthresholded maps can be viewed in Neurovault (27) (neurovault.org/collections/FBVSAVDQ/).
Fig. 2.
Significant between-condition differences (orange = increases) in RSFC between the V1 seed region (purple) and the rest of the brain. Unthresholded maps can be viewed here: neurovault.org/collections/FBVSAVDQ/ (n = 15).
Fig. 3.
Significant between-condition differences in RSFC between the PH seed and the rest of the brain (orange = increases; blue = decreases). Unthresholded maps can be viewed here: neurovault.org/collections/FBVSAVDQ/ (n = 15).
Increased V1 RSFC (to the most significant regions shown in Fig. 2: P < 0.01; 5,000 permutations; SI Appendix, Fig. S8) correlated with VAS ratings of simple hallucinations (r = 0.62; P = 0.012; Bonferroni corrected P = 0.048; SI Appendix, Fig. S9B), as well as ASC ratings of elementary (r = 0.63; P = 0.012; Bonferroni corrected P = 0.048; SI Appendix, Fig. S9C) and complex (r = 0.74; P = 0.0016; Bonferroni corrected P = 0.006; SI Appendix, Fig. S9D) imagery. Decreased PH RSFC (to the significant regions shown in Fig. 3) correlated with VAS ratings of ego-dissolution (r = 0.73; P = 0.0018; SI Appendix, Fig. S9E) and “altered meaning” on the ASC (r = 0.82; P = 0.0002; Bonferroni corrected P = 0.002; SI Appendix, Fig. S9F). Importantly, some of these (hypothesized) correlations were phenomenology selective (SI Appendix, Table S7): increased visual cortex CBF and V1 RSFC correlated more strongly with the visual hallucinatory aspect of the drug experience than the altered meaning/ego-dissolution aspect, whereas the opposite was true for decreased PH RSFC. Changes in vmPFC RSFC did not correlate with any of the ratings.
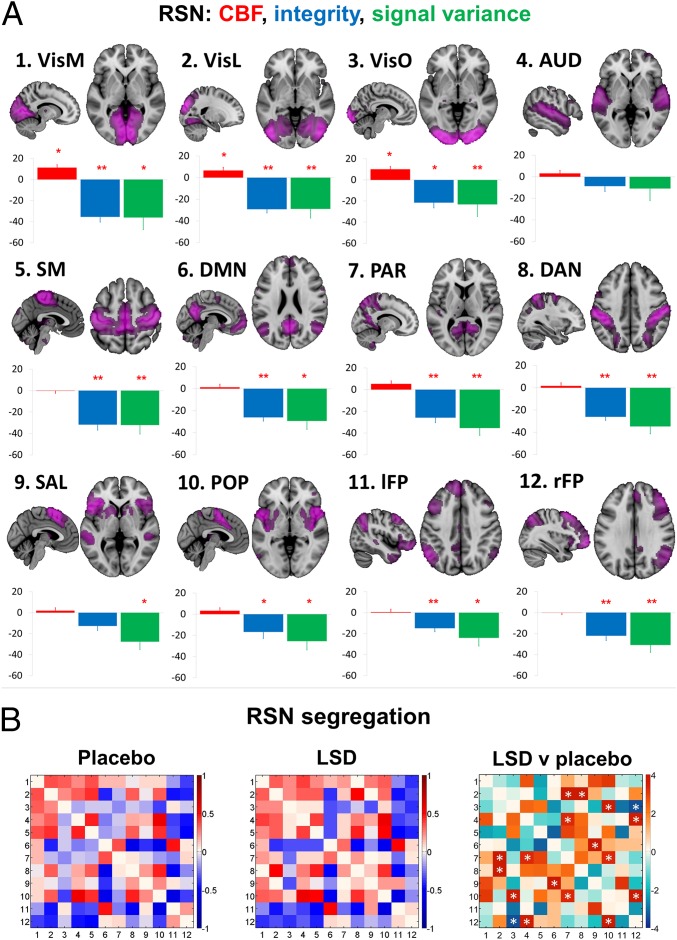
Next, the effect of LSD on brain network properties was investigated. Twelve functionally familiar RSNs were identified in a set of 20 spatially independent components derived from independent data (human connectome project; SI Appendix). These RSNs are as follows: a medial visual network, a lateral visual network (VisL), an occipital pole network (VisO), an auditory network (AUD), a sensorimotor network, the DMN, a parietal cortex network (PAR), the dorsal attention network, the salience network, a posterior opercular network (POP), the left frontoparietal network, and the right frontoparietal network (rFP).
Four metrics were calculated for each RSN: within-RSN CBF, within-RSN RSFC or “integrity,” within-RSN BOLD signal variance, and between-RSN RSFC or “segregation.” Between-condition differences in the first three metrics are shown in Fig. 4A, and the between-RSN RSFC results are shown in Fig. 4B. Differences (increases) in CBF were restricted to the visual RSNs, whereas differences in variance and integrity (decreases) were much more pronounced and universal. According to previous research with psilocybin (17), it was predicted that decreased DMN integrity (or DMN “disintegration”) would correlate with ratings of ego-dissolution, and this hypothesis was supported (r = 0.49; P = 0.03; SI Appendix, Fig. S9G). Given the large number of possible permutations, additional correlational analyses were not performed; however, to test the selectivity of the relationship between DMN disintegration and ego-dissolution, correlations were calculated for ego-dissolution and the integrity of the other 11 RSNs, and none were significant (SI Appendix, Table S2). Disintegration of the visual RSNs did not correlate with ratings of visual hallucinations. See SI Appendix, Fig. S5, for brain images of the RSN integrity results.
Fig. 4.
(A) Mean percentage differences (+SEM) in CBF (red), integrity (blue), and signal variance (green) in 12 different RSNs under LSD relative to placebo (red asterisks indicate statistical significance, *P < 0.05; **P < 0.01, Bonferroni corrected). (B) Differences in between-RSN RSFC or RSN “segregation” under LSD vs placebo. Each square in the matrix represents the strength of functional connectivity (positive = red, negative = blue) between a pair of different RSNs (parameter estimate values). The matrix on the far right displays the between-condition differences in covariance (t values): red = reduced segregation and blue = increased segregation under LSD. White asterisks represent significant differences (P < 0.05, FDR corrected; n = 15).
Between-RSN RSFC or RSN segregation was also markedly modulated by LSD. Decreased segregation (red squares with white asterisks in Fig. 4B, right matrix) was observed between eight RSN pairs (VisL–PAR, VisL–dorsal attention network, VisO–POP, AUD–PAR, AUD–rFP, DMN–salience network, PAR–POP, POP–rFP), with only one pair (VisO–rFP) showing increased segregation (blue square with white asterisk in Fig. 4B, right matrix). Contrary to a prior hypothesis, decreased RSN segregation (in the eight networks that showed this effect) did not correlate with ratings of ego-dissolution (r = 0.12; P > 0.05).
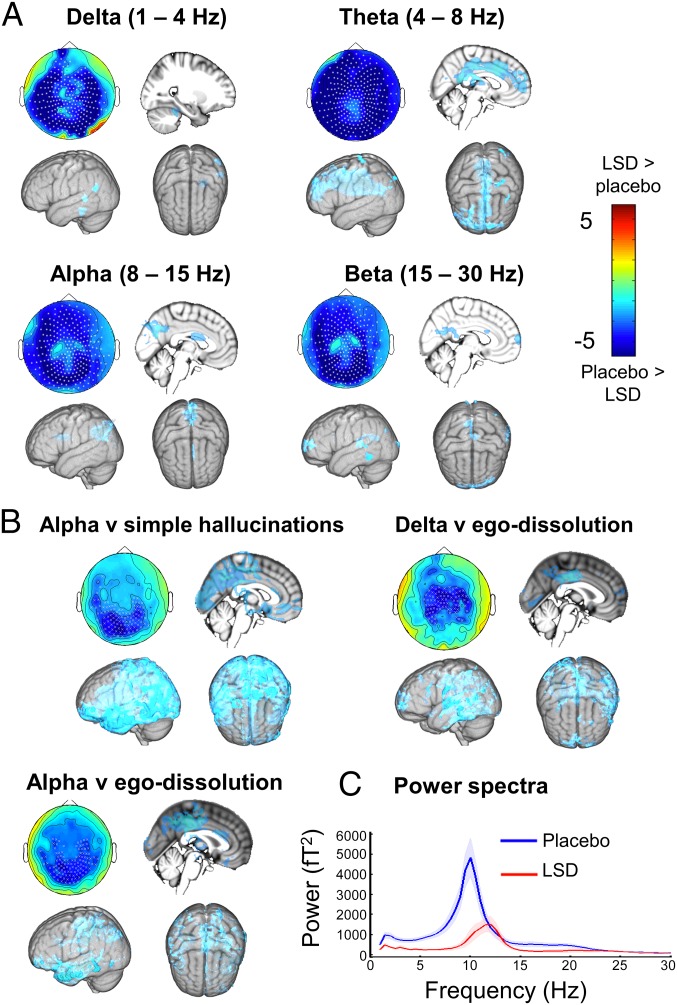
Data from 14 volunteers were suitable for the MEG analyses (three females; mean age, 32.1 ± 8.3 y). Primary analyses focused on between-condition differences in frequency-specific oscillatory power, measured during eyes-closed rest. The relevant data (14 min of rest) were acquired ∼50 min after completion of the MRI protocol and filtered into the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–15 Hz), beta (15–30 Hz), low gamma (31–49 Hz), and high gamma (51–99 Hz). Results revealed decreased oscillatory power under LSD in four frequency bands (Fig. 5A), with some suspected residual muscle artifact confounding the gamma results. For the lower-frequency bands (i.e., 1–30 Hz), the decreases reached significance in most of the sensors. To explore relationships between these outcomes and subjective measures, VAS ratings of ego-dissolution and visual hallucinations (simple and complex) were entered into regression analyses, using cluster permutation testing. Significant relationships were found between ego-dissolution and decreased delta (mean cluster, r = −0.54; P < 0.05) and alpha power (mean cluster, r = −0.29; P < 0.05) and between simple hallucinations and decreased alpha power (mean cluster, r = −0.61; P < 0.05) (Fig. 4B). Plotting the power spectrum independently for each condition for the significant alpha cluster (Fig. 5C), it is evident that the distribution of power is decreased across a broad frequency range under LSD, and the peak alpha rhythm is reduced in amplitude and of higher frequency (i.e., 10 Hz under placebo, 12 Hz under LSD; t = 4.21; P = 0.0009). Source modeling revealed that sources of the power decreases were relatively distributed throughout the brain (SI Appendix, Table S8), with significant effects in the PCC/precuneus (theta, alpha, and beta) and other high-level cortical regions (delta-beta).
Fig. 5.
MEG results. (A) Statistical analysis of planar gradiometer-configured MEG data comparing LSD with placebo in the eyes-closed condition. Blue indicates less power under LSD. Units are t-statistics. Significant sensor clusters are marked such that stars correspond to P < 0.01 and crosses to P < 0.05 (corrected). Source localization results are also displayed. (B) Significant correlations between changes (decreases) in oscillatory power and subjective phenomena. (C) Power spectra for the significant sensor cluster in B (simple hallucinations), with placebo data plotted in blue and LSD in red (n = 14).
This study’s multimodal design enabled correlational analyses to be performed between the various (significant) imaging outcomes. This was done in a hypothesis-driven manner, and because the outcomes’ directions were already known, one-tailed tests were performed. Relationships were observed between the increases in CBF (localized to the visual cortex) and decreases in alpha power in posterior (occipital cortex) sensors (r = −0.59; P = 0.029; SI Appendix, Fig. S6) and between increases in V1 RSFC (to the most significant regions: P < 0.01; 5,000 permutations; SI Appendix, Fig. S8) and decreased posterior-sensor alpha power (r = −0.81; P = 0.0015; SI Appendix, Fig. S6), but there was only a trend-level relationship between increases in visual cortex CBF and increases in V1 RSFC (r = 0.43; P = 0.055). The mean change (decreases) in the integrity of the 12 RSNs correlated very strongly with the mean change (decreases) in their variance (r = 0.89; P = 4 × 10−6; SI Appendix, Fig. S6). Neither metric correlated with the mean change in CBF, however [r = 0.1 (P > 0.05) and r = 0.33 (P > 0.05) for integrity and variance, respectively], nor head motion (SI Appendix), but they did correlate with the mean decrease in power (significant sensors) for the four displayed frequency bands [r = 0.79 (P = 0.001; SI Appendix, Fig. S6) and r = 0.76 (P = 0.002) for integrity and variance, respectively]. Mean decreases in RSN segregation (for the eight pairs that showed this effect) correlated with mean decreases in RSN integrity (mean of all 12 RSNs, r = 0.53; P = 0.02; SI Appendix, Fig. S6) and reduced oscillatory power (delta-beta combined, r = 0.67; P = 0.017; SI Appendix, Fig. S6), but not decreased RSN variance (r = 0.33, P > 0.05) nor increased CBF (r = 0.18; P > 0.05). Given the number of possible permutations, we chose not to explore beyond these relationships.
Discussion
The present findings offer a comprehensive new perspective on the changes in brain activity characterizing the LSD state, enabling us to make confident new inferences about its functional neuroanatomy. Principal findings include increased visual cortex CBF, RSFC, and decreased alpha power, predicting the magnitude of visual hallucinations; and decreased DMN integrity, PH-RSC RSFC, and delta and alpha power (e.g., in the PCC), correlating with profound changes in consciousness, typified by ego-dissolution. More broadly, the results reinforce the view that resting state ASL, BOLD FC, and MEG measures can be used to inform on the neural correlates of the psychedelic state (9, 16). Importantly, strong relationships were found between the different imaging measures, particularly between changes in BOLD RSFC (e.g., network “disintegration” and “desegregation”) and decreases in oscillatory power, enabling us to make firmer inferences about their functional meaning.
The present study sheds new light on the relationship between changes in spontaneous brain activity and psychedelic-induced visual hallucinations. Strong relationships were observed between increased V1 RSFC and decreased alpha power, as well as ratings of both simple and complex visual hallucinations. The latter result is consistent with previous findings with psilocybin (29). Importantly, a very strong relationship was also observed between increased V1 RSFC and decreased alpha power in occipital sensors, suggesting that as well as being commonly related to visual hallucinations, these physiological effects are closely interrelated. The increase in V1 RSFC under LSD is a particularly novel and striking finding and suggests that a far greater proportion of the brain contributes to visual processing in the LSD state than under normal conditions. This expansion of V1 RSFC may explain how normally discreet psychological functions (e.g., emotion, cognition, and indeed the other primary senses) can more readily “color” visual experience in the psychedelic state.
Biologically informed modeling has suggested that instability within the primary visual cortex may facilitate the emergence of geometric hallucinations via self-organized patterns of neural excitation (30), and eyes-closed fMRI recordings during ayahuasca hallucinations suggest the visual cortex behaves “as if” there is external input when there is none (31) (see also ref. 29). The present findings of increased visual cortex CBF, expanded V1 RSFC, and decreased alpha power may be seen as consistent with the notion of “seeing with eyes-shut” under psychedelics, because they are all properties normally associated with visual stimulation (32, 33). Cortical alpha has been hypothesized to serve a general inhibitory function, filtering out “stimulus-irrelevant” information (34). Thus, reduced alpha power (9, 29, 35) could have disinhibitory consequences, facilitating the release of anarchic patterns of excitation that manifest spontaneously and experientially as visual hallucinations. This hypothesis is leant (indirect) support by two prior studies that found reduced spontaneous visual cortex alpha power under psilocybin alongside reduced evoked visual responses (9, 29). Further work, using higher-resolution brain imaging, machine learning techniques, dynamic measures of functional and effective connectivity, and improved “capture” of visual hallucinations (e.g., via button press or experience sampling), may help to develop this appealing model (e.g., see ref. 36).
The present data also inform on another fundamental question; namely, how do psychedelics alter brain function to (so profoundly) alter consciousness? Interestingly, although the effects of LSD on the visual system were pronounced, they did not significantly correlate with its more fundamental effects on consciousness. Instead, a specific relationship was found between DMN disintegration and ego-dissolution, supporting prior findings with psilocybin (17). Also consistent with previous psilocybin research (9), a significant relationship was found between decreased PCC alpha power and ego-dissolution. Moreover, an especially strong relationship was found between PH-RSC decoupling and ego-dissolution (see also ref. 10). Thus, in the same way the neurobiology of psychedelic-induced visual hallucinations can inform on the neurobiology of visual processing, so the neurobiology of psychedelic-induced ego-dissolution can inform on the neurobiology of the “self” or “ego” (37), and the present results extend our understanding in this regard, implying that the preservation of DMN integrity, PH-RSC communication, and regular oscillatory rhythms within the PCC may be important for the maintenance of one’s sense of self or ego.
Linking these results to pathology, an especially strong relationship was found between PH-RSC decoupling and the “altered meaning” factor on the ASC. Interestingly, altered activity within the PH-RSC circuit under psilocybin has previously been found to correlate with the spiritual experience and insightfulness dimensions of the 11-factor ASC (10), and altered RSC/PCC activity has been found to correlate with ego-dissolution (9), suggesting modulation of this particular circuit may be an important feature of especially profound psychedelic experiences. The altered meaning factor of the ASC is composed of items such as “some unimportant things acquired a special meaning” and “things in my surroundings had a new or alien meaning” that are phenomenologically resonant with the notion of “aberrant salience” in schizophrenia research (38). Impaired reality testing as a corollary of impaired ego functioning may explain an association between ego-dissolution and altered meaning. Similarities between aspects of psychosis and the psychedelic state have long been debated, and one of the most influential hypotheses on the neurobiology of schizophrenia proposes a functional disconnect between certain brain structures in the disorder (39). In this context, it is intriguing to consider whether the PH-RSC circuit is involved in certain psychosis-related experiences (e.g., refs. 40 and 41). More specifically, it would be interesting to examine the integrity of the PH-RSC connection in cases of endogenous psychoses in which phenomena such as altered meaning, ego-dissolution, and/or impaired reality-testing are observed. To our knowledge, these specific phenomena have never been formally investigated in imaging studies involving patients exhibiting endogenous psychoses, but studies on early psychosis and the at-risk mental state may be informative in this regard (e.g., ref. 40).
When the present results are considered in relation to previous human neuroimaging studies with psychedelics, some general principles emerge. It seems increasingly evident that psychedelics reduce the stability and integrity of well-established brain networks (e.g., ref. 16) and simultaneously reduce the degree of separateness or segregation between them (e.g., ref. 42); that is, they induce network disintegration and desegregation. Importantly, these effects are consistent with the more general principle that cortical brain activity becomes more “entropic” under psychedelics (17). Furthermore, with the benefit of the present study’s multimodal imaging design, we can extend on these generic insights to postulate some more specific physiological properties of the psychedelic state and how these relate to some of its key psychological properties; namely, expanded V1 RSFC relates to the magnitude of visual hallucinations and decoupling of the PH-RSC circuit relates to the level of ego-dissolution, and perhaps also the profundity of a psychedelic experience more generally (also see refs. 9 and 10 in this regard).
Before concluding, we should highlight some general limitations of the present study and address a discrepant finding in the field. Regarding limitations, a fully randomized, double-blind design is often considered the gold standard; however, experimental blinding is known to be ineffective in studies with conspicuous interventions. Thus, a single-blind, balanced-order design with an inert placebo (offering the simplest and “cleanest” possible control condition) was considered an effective compromise. Also, although the multimodal design of this study was an advantage, the experimental protocol was demanding for participants, and the different scan types (ASL, BOLD, and MEG) occurred separately in time. Simultaneous EEG-fMRI may therefore offer some advantageous in this regard. Another general limitation of imaging studies involving potent psychoactive drugs, is the issue of between-condition differences in head motion and related artifacts. In this study, we opted to use the most rigorous motion-correction strategies available (SI Appendix), despite motion levels being no higher than those seen in previous studies by our group (16). Regarding the discrepant finding, a previous psilocybin ASL study of ours revealed decreased CBF postpsilocybin (i.v.) during eyes-open rest (16), whereas the present i.v. LSD study found increased CBF localized to the visual cortex with eyes-closed rest. One must be cautious of proxy measures of neural activity (that lack temporal resolution), such as CBF or glucose metabolism, lest the relationship between these measures, and the underlying neural activity they are assumed to index, be confounded by extraneous factors, such as a direct vascular action of the drug (43). For this reason, more direct measures of neural activity (e.g., EEG and MEG) and/or more dynamic fMRI measures (e.g., RSFC) should be considered more reliable indices of the functional brain effects of psychedelics, and it is notable in this regard that our previous MEG (9) and RSFC (16, 19, 42) findings with psilocybin are highly consistent with those observed here with LSD. Thus, rather than speculate on the above-mentioned discrepancy, it may be more progressive to highlight the advantages of EEG/MEG and dynamic fMRI and conclude that further work would be required to resolve discrepancies in the literature regarding the effects of psychedelics on metabolically related metrics that lack temporal resolution.
Finally, as evidence supporting the therapeutic potential of psychedelics mounts (6, 44–46), so does our need to better understand how these drugs work on the brain. In many psychiatric disorders, the brain may be viewed as having become entrenched in pathology, such that core behaviors become automated and rigid. Consistent with their “entropic” effect on cortical activity (17), psychedelics may work to break down such disorders by dismantling the patterns of activity on which they rest. Future work is required to test this hypothesis and the others that have been presented here as part of a broader initiative to properly utilize these valuable scientific tools.
Methods
This study was approved by the National Research Ethics Service committee London-West London and was conducted in accordance with the revised declaration of Helsinki (2000), the International Committee on Harmonization Good Clinical Practice guidelines, and National Health Service Research Governance Framework. Imperial College London sponsored the research, which was conducted under a Home Office license for research with schedule 1 drugs. For more methods see SI Appendix, Methods.
Supplementary Material
Acknowledgments
We thank supporters of the Walacea.com crowdfunding campaign for helping secure the funds required to complete this study. This report presents independent research carried out at the National Institute of Health Research/Wellcome Trust Imperial Clinical Research Facility. This research received financial support from the Safra Foundation (which funds D.J.N. as the Edmond J. Safra Professor of Neuropsychopharmacology) and the Beckley Foundation (the study was conducted as part of the Beckley-Imperial research programme). R.L.C.-H. is supported by an Medical Research Council clinical development scheme grant. S.M. is supported by a Royal Society of New Zealand Rutherford Discovery Fellowship. K.M. is supported by a Wellcome Trust Fellowship (WT090199).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518377113/-/DCSupplemental.
References
- 1.Hofmann A. LSD: My Problem Child. McGraw-Hill; New York: 1980. [Google Scholar]
- 2.Carhart-Harris RL, et al. LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl) 2015;232(4):785–794. doi: 10.1007/s00213-014-3714-z. [DOI] [PubMed] [Google Scholar]
- 3.Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME. Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans. Int J Neuropsychopharmacol. 2015;19(1):pyv072. doi: 10.1093/ijnp/pyv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaelen M, et al. LSD enhances the emotional response to music. Psychopharmacology (Berl) 2015;232(19):3607–3614. doi: 10.1007/s00213-015-4014-y. [DOI] [PubMed] [Google Scholar]
- 5.Schmid Y, et al. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry. 2015;78(8):544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Gasser P, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):513–520. doi: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink M. EEG and human psychopharmacology. Annu Rev Pharmacol. 1969;9:241–258. doi: 10.1146/annurev.pa.09.040169.001325. [DOI] [PubMed] [Google Scholar]
- 9.Muthukumaraswamy SD, et al. Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci. 2013;33(38):15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kometer M, Pokorny T, Seifritz E, Volleinweider FX. Psilocybin-induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berl) 2015;232(19):3663–3676. doi: 10.1007/s00213-015-4026-7. [DOI] [PubMed] [Google Scholar]
- 11.Riba J, et al. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol. 2002;53(6):613–628. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32(9):3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celada P, Puig MV, Díaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: Reversal by antipsychotic drugs. Biol Psychiatry. 2008;64(5):392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Riga MS, Soria G, Tudela R, Artigas F, Celada P. The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: Reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2014;17(8):1269–1282. doi: 10.1017/S1461145714000261. [DOI] [PubMed] [Google Scholar]
- 15.Vollenweider FX, et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16(5):357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 16.Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carhart-Harris RL, et al. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20. doi: 10.3389/fnhum.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri G, et al. Homological scaffolds of brain functional networks. J R Soc Interface. 2014;11(101):20140873. doi: 10.1098/rsif.2014.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR. Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp. 2014;35:5442–5456. doi: 10.1002/hbm.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palhano-Fontes F, et al. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One. 2015;10(2):e0118143. doi: 10.1371/journal.pone.0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe RR, Heath RG. Effects of lysergic acid and various derivatives on depth and cortical electrograms. J Neuropsychiatry. 1961;3:75–82. [PubMed] [Google Scholar]
- 22.Schwarz BE, Sem-Jacobsen CW, Petersen MC. Effects of mescaline, LSD-25, and adrenochrome on depth electrograms in man. AMA Arch Neurol Psychiatry. 1956;75(6):579–587. doi: 10.1001/archneurpsyc.1956.02330240017002. [DOI] [PubMed] [Google Scholar]
- 23.Serafetinides EA. The EEG effects of LSD-25 in epileptic patients before and after temporal lobectomy. Psychopharmacology (Berl) 1965;7(6):453–460. doi: 10.1007/BF00402367. [DOI] [PubMed] [Google Scholar]
- 24.Mégevand P, et al. Seeing scenes: Topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area. J Neurosci. 2014;34(16):5399–5405. doi: 10.1523/JNEUROSCI.5202-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignal JP, Maillard L, McGonigal A, Chauvel P. The dreamy state: Hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain. 2007;130(Pt 1):88–99. doi: 10.1093/brain/awl329. [DOI] [PubMed] [Google Scholar]
- 26.Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS One. 2010;5(8):e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgolewski KJ, et al. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carhart-Harris RL, et al. The effects of acutely administered 3,4-methylenedioxymethamphetamine on spontaneous brain function in healthy volunteers measured with arterial spin labeling and blood oxygen level-dependent resting state functional connectivity. Biol Psychiatry. 2015;78(8):554–562. doi: 10.1016/j.biopsych.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kometer M, Schmidt A, Jäncke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33(25):10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler TC, et al. Evolutionary constraints on visual cortex architecture from the dynamics of hallucinations. Proc Natl Acad Sci USA. 2012;109(2):606–609. doi: 10.1073/pnas.1118672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Araujo DB, et al. Seeing with the eyes shut: Neural basis of enhanced imagery following Ayahuasca ingestion. Hum Brain Mapp. 2012;33(11):2550–2560. doi: 10.1002/hbm.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolias AS, et al. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48(6):901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Cavonius CR, Estévez-Uscanga O. Local suppression of alpha activity by pattern in half the visual field. Nature. 1974;251(5474):412–414. doi: 10.1038/251412a0. [DOI] [PubMed] [Google Scholar]
- 34.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirahashi K. Electroencephalographic study of mental disturbances experimentally induced by LSD25. Psychiatry Clin Neurosci. 1960;14(2):140–155. [Google Scholar]
- 36.Horikawa T, Tamaki M, Miyawaki Y, Kamitani Y. Neural decoding of visual imagery during sleep. Science. 2013;340(6132):639–642. doi: 10.1126/science.1234330. [DOI] [PubMed] [Google Scholar]
- 37.Lebedev AV, et al. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. 2015;36(8):3137–3153. doi: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 39.Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- 40.Seidman LJ, et al. Medial temporal lobe default mode functioning and hippocampal structure as vulnerability indicators for schizophrenia: A MRI study of non-psychotic adolescent first-degree relatives. Schizophr Res. 2014;159(2-3):426–434. doi: 10.1016/j.schres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Lui S, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45(1):97–108. doi: 10.1017/S003329171400110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci. 2014;8:204. doi: 10.3389/fnhum.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyer DC, Gant DW. Vasoconstriction produced by hallucinogens on isolated human and sheep umbilical vasculature. J Pharmacol Exp Ther. 1973;184(2):366–375. [PubMed] [Google Scholar]
- 44.Grob CS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 45.Bogenschutz MP, et al. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol. 2015;29(3):289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983–992. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.