Abstract
Background
The indigenous Batwa of southwestern Uganda are among the most highly impoverished populations in Uganda, yet there is negligible research on the prevalence of malaria in this population. Plasmodium falciparum malaria parasitaemia prevalence was estimated in an indigenous Batwa and a non-indigenous neighbouring population, and an exploration of modifiable risk factors was carried out to identify potential entry points for intervention. Additionally, evidence of zooprophylaxis was assessed, hypothesizing that livestock ownership may play a role in malaria risk.
Methods
Two cross-sectional surveys of Batwa and non-Batwa communities were carried out in Kanungu District, Uganda in July 2013 and April 2014 based on a census of adult Batwa and a two-stage systematic random sample of adult non-Batwa in ten Local Councils where Batwa settlements are located. A community-based questionnaire and antigen rapid diagnostic test for P. falciparum were carried out in the cross-sectional health surveys. A multivariable logistic regression model was built to identify risk factors associated with positive malaria diagnostic test. A subset analysis of livestock owners tested for zooprophylaxis.
Results
Batwa experienced higher prevalence of malaria parasitaemia than non-Batwa (9.35 versus 4.45 %, respectively) with over twice the odds of infection (OR 2.21, 95 % CI 1.23–3.98). Extreme poverty (OR 1.96, 95 % CI 0.98–3.94) and having an iron sheet roof (OR 2.54, 95 % CI 0.96–6.72) increased the odds of infection in both Batwa and non-Batwa. Controlling for ethnicity, wealth, and bed net ownership, keeping animals inside the home at night decreased the odds of parasitaemia among livestock owners (OR 0.29, 95 % CI 0.09–0.94).
Conclusion
A health disparity exists between indigenous Batwa and non-indigenous community members with Batwa having higher prevalence of malaria relative to non-Batwa. Poverty was associated with increased odds of malaria infection for both groups. Findings suggest that open eaves and gaps in housing materials associated with iron sheet roofing represent a modifiable risk factor for malaria, and may facilitate mosquito house entry; larger sample sizes will be required to confirm this finding. Evidence for possible zooprophylaxis was observed among livestock owners in this population for those who sheltered animals inside the home at night.
Keywords: Indigenous health, Batwa, Malaria prevalence, Malaria risk factors, Livestock, Zooprophylaxis, Uganda
Background
There are renewed calls for malaria eradication with a focus on Africa [1, 2]. Malaria mortality rates are decreasing in many populations, with global incidence having fallen by approximately 37 % since 2000 to 214 million new cases in 2015 [3, 4]. Despite these gains, malaria remains a major global disease burden, with approximately 438,000 deaths annually [3, 5].
The elimination of malaria from many western nations has been attributed primarily to social and economic development allowing for screening of windows and doors, destruction of vector breeding sites, and rapid diagnosis and treatment [6]. The feasibility of Plasmodium falciparum malaria elimination in most of sub-Saharan Africa is low, with Uganda being among the countries with lowest feasibility [7]. Sub-Saharan African countries are disproportionately affected by malaria [4] due to the presence of highly competent mosquito vectors, widespread poverty, limited infrastructure, and overburdened health systems [8, 9]. Those living in extreme poverty are most vulnerable to infectious diseases, yet within-country disparities are often ignored [10, 11]. African indigenous populations in particular have consistently poorer health outcomes than their non-indigenous counterparts [12]. Social determinants of indigenous health include, but are not limited to, poverty, discrimination, limited access to health care, and loss of traditional lands [13]. Indigenous and ethnic minority populations outside of Uganda experience higher rates of malaria, which have been attributed to relative impoverishment, marginalization, and geographic remoteness [14–18]. In some cases, genetic variations have been identified as drivers of ethnic differences in malaria parasitaemia and immunological response [19].
Risk factors for malaria can be conceptualized as non-modifiable and modifiable. Non-modifiable factors such as age and sex have been inconsistently associated with higher malaria risk. In endemic areas, children under 5 years of age have high risk of malaria due to their immunological naïveté [10, 20, 21]. Beyond this, the relationship between age and malaria in adults is less clear [22]. It is known that regular exposure to malaria results in a functional immunity to the disease which quickly wanes in the absence of exposure. Excluding immunologically-suppressed pregnant women, sex-related variations in malaria risk are generally linked to gender-role and occupational exposures [23, 24]. Women in roles as household water-collectors may spend more time near mosquito breeding sites. Men with forest-related jobs may spend their time in mosquito-dense areas [10, 23].
Modifiable risk factors include environmental conditions and human behaviour. Local vector ecology, including the locations of swamps, forests, rice paddies where vectors breed, and the proximity of these sites to human habitations may bring humans into frequent contact with mosquitoes [25–27]. Housing conditions may also affect transmission. Open eaves and windows, for example, may permit mosquito entry into sleeping quarters [25, 28, 29]. Education and wealth are known to be protective against malaria. Understanding malaria transmission and prevention may result in behaviours such as staying indoors during peak vector activity, and having access to preventive strategies that may reduce exposure of humans to vectors [27, 30]. Many of these risk factors are mediated by poverty whereby access to building materials and bed nets may be dependent upon income [31–33]. Poverty has been described as a key modifiable determinant of malaria burden [6].
Livestock are routinely included in analyses of risk factors for malaria infection, yet their role in malaria transmission is not well understood. Livestock are dead-end hosts for human-infectious Plasmodium parasites and may reduce human malaria risk by drawing vectors away from humans (zooprophylaxis) [34–36]. In some cases, however, livestock act as additional blood meal sources, and they may alter vector longevity and population density to increase human malaria risk (zoopotentiation) [37, 38]. Empirical research exploring the association between livestock and malaria risk has been complicated by the confounding role of wealth [39]. Livestock symbolize social standing, provide food and services, and act as an asset to be sold in times of financial need [40–44]. Both livestock and wealth are generally associated with lower malaria prevalence. Given efforts to include zooprophylaxis in integrated vector control programs, there have been calls for further research to understand the impact of livestock on malaria transmission [39].
The Ugandan Batwa are an indigenous population with life expectancy and child mortality rates significantly worse than national averages [45]. There remain considerable gaps regarding our understanding of Batwa health; to our knowledge only two peer-reviewed studies currently report the prevalence and risk factors of health outcomes for Batwa in Uganda [23, 46]. While literature on Batwa livelihoods is currently limited, it is thought that Batwa engage in livestock livelihoods at a much lower rate than non-Batwa due to financial restrictions, in addition to their historical hunter-gatherer culture. Further, Namanya identified consistent differences across risk factors for malaria, including housing and education between Batwa and non-Batwa [47].
The aims of this paper were (1) to estimate malaria prevalence in indigenous Batwa of Kanungu District and compare this with their non-indigenous neighbours, (2) explore modifiable risk factors for malaria parasitaemia in order to identify potential entry points for intervention to reduce malaria prevalence among Batwa and non-Batwa and, (3) to test the hypothesis of zooprophylaxis in a sub-set of livestock owners from both Batwa and non-Batwa populations.
Methods
Study population
Kanungu District is located in southwestern Uganda bordering the Democratic Republic of Congo to the west. It contains part of the Bwindi Impenetrable National Park (Bwindi) [48] and has a population of approximately 250,000, 80 % of whom live in rural settlements [49]. The majority of the population are of Bakiga ethnicity [45, 50]. The non-Batwa (non-indigenous populations, including Bakiga) rely mainly on subsistence farming consisting of cash- and food-cropping, and small-scale livestock holdings [48, 50], but tourism centred around gorilla trekking in Bwindi also provides local employment [45]. The indigenous Batwa population of Kanungu District is approximately 900 [51]. When Bwindi received its national park designation in 1991, the Batwa were no longer permitted to enter the park. Thus, they lost their ancestral lands and livelihood as traditional hunter-gatherers. Since then, the Batwa have been transitioning to settled agriculture [45]. Kanungu District is remote and has limited infrastructure and service delivery [50]. It receives rainfall throughout the year, with two dryer seasons from December to February and from June to July. Malaria in Kanungu is characterized by low transmission intensity and low endemicity [8, 23, 52, 53].
Study design and sample
Two cross-sectional, in-person surveys were administered in the 10 Local Councils (LCs, the smallest government administration unit in Uganda) containing the 10 Batwa settlements in July 2013 and April 2014 (Fig. 1). These 10 LCs are located within the subcounties of Kayantorogo, Kayonza, Kirima, Mpungu and Butogota Town Council. Considering the small total population size of the Kanungu Batwa population, a census of all adult Batwa present (>18 years) was attempted. A two-step proportional systematic random-sample of adult non-Batwa was conducted, representing approximately 40 % of non-Batwa households in each LC. Sampling frames (a list of households in the LC) were provided by Batwa settlement and non-Batwa Local Council 1 (LC1) chairpersons. The sampling strategy consisted of the following: a number between 1 and 10 was randomly selected as the first household to be selected from the sampling frame. The total number of households was multiplied by 0.4 to obtain the sampling interval required to obtain a 40 % sample of the LC. Every nth household was then selected from the sampling frame. For the second stage, the LC1 chairperson conducted a census of all adults living in the selected households from stage 1. The LC1 chairperson selected the adult to be sampled out of a hat based on the census of adults in those households. The same individuals were interviewed in July and April.
Fig. 1.
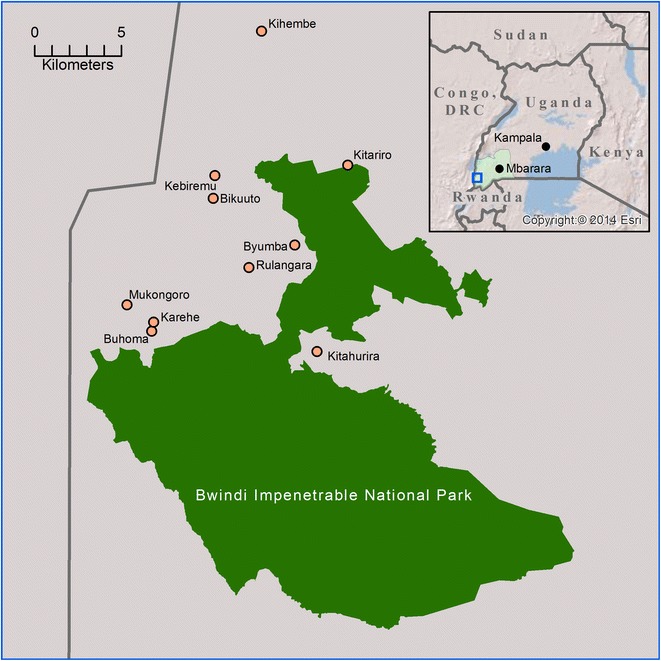
Kanungu District Batwa settlements surrounding Bwindi Impenetrable National Park, Uganda
Data collection
Plasmodium falciparum is the primary cause of malaria in this region [8]. Parasitaemia (outcome variable) was classified as a positive result on CareStart™ malaria rapid diagnostic test (RDT) detecting histidine-rich protein 2 (HRP2) of P. falciparum (Access Bio, Inc. NJ, USA) at either the July or April data collections [54]. The RDT has a sensitivity of 98 % and specificity of 97.5 % [55]. Having a positive test result at either collection period was defined as “RDT ever” while a negative outcome at both collection periods was defined as “RDT never”.
At each survey (July and April), participants were interviewed in Rukiga (the local language for Batwa and non-Batwa) by trained local survey administrators. The survey consisted of three parts: (1) an individual-level health questionnaire, (2) a household questionnaire and (3) a malaria RDT administered by a community health nurse. Part one (individual questionnaire) collected information pertaining to demographics and personal information, health and risk behaviour, and livelihoods. Part two (household questionnaire) collected information on household structure, housing materials, assets, water, and sanitation. For Batwa, each individual completed parts one and three, with the household head completing part two. The individual questionnaires were later matched to the household questionnaires. For non-Batwa, each individual completed all components. Data were collected in hard copy and entered into Microsoft Access (Microsoft Corp, USA).
Ethics
This study was designed using a community-based participatory research approach and was approved by the Research Ethics Boards at McGill University and the University of Guelph. The study design is consistent with the Canadian Tri-Council’s Policies and requirements of the Ethical Conduct of Research Involving Human Subjects. Informed consent was obtained verbally from all participants. This study is a part of the Indigenous Health Adaptation to Climate Change (IHACC) project, an international initiative with parallel field sites in the Canadian Arctic and Peruvian Amazon [56].
Statistical analysis
Malaria parasitaemia prevalence and risk factors
Among Batwa and non-Batwa a total of 649 questionnaires were carried out in July 2013 and 546 surveys were completed in April 2014. All questionnaires from July and April were pooled to create a single study population. This was done to: (1) increase the sample size, (2) achieve a prevalence reflective across seasons, and (3) account for the potential sporadic nature of infection and risk factors using only one point estimate in time. For individuals who were present for both the July and April surveys, and who were RDT negative at both times (n = 709), the risk factor data from the July surveys were used as baseline. If an individual was present in July and April and was RDT positive at one time (n = 49), data from the time at which they tested positive were used. If they were present at both July and April and positive at both times (n = 1), the July data were used. The period prevalence was calculated by dividing the total number of positive cases by the total number of participants tested.
To identify risk factors associated with parasitaemia, a series of univariable logistic regression models were constructed. Risk factors of interest were selected based on theorized relevance in the literature and data availability. Covariates included: age, sex, knowledge of malaria transmission (as reflected by knowing that one should avoid mosquito bites to prevent malaria), roofing material, bed net ownership, and wealth (Table 1). To measure wealth, a household-level asset-based wealth index was created using principal component analysis [46, 57, 58]. The variables included in the index were cell phone ownership, radio ownership, bicycle ownership, having a source of electricity, land ownership, receiving remittances, toilet type, and having access to hand washing facilities. The resultant component was categorized as a dichotomous variable representing the 50 % least poor relative to the 50 % poorest individuals. Livestock were not included in the wealth index to allow the evaluation of their specific role in malaria transmission. Collinearity between risk factor variables was assessed using a Spearman’s rank correlation coefficient with a cut-off of 60 %. Where collinearity was identified, the variable with the strongest association with the outcome variable was retained. A multivariable logistic regression model was built, and a final, reduced multivariable model based on variables significant in either the univariable or multivariable model. A mixed-effects logistic regression model with random intercept was tested, but there was no evidence of clustering at the LC-level. Data were analysed using Stata v.11 (Stata Corp., USA).
Table 1.
Variables relating to malaria risk factors for Batwa and non-Batwa communities in Kanungu District, Uganda
| Construct | Description | Variable | Justification |
|---|---|---|---|
| Dependent variable | |||
| Malaria parasitaemia (P. falciparum) | Positive RDT July 2013 Positive RDT April 2014 |
Dichotomous (ever positive/never positive) | RDTs are a widely accepted method of malaria detection and may outperform microscopy in some settings [4, 80–82] |
| Independent variables | |||
| Ethnicity | Survey date | Dichotomous dummy variable (Batwa/non-Batwa) | Indigenous populations and ethnic minorities have been identified in the literature as being more vulnerable to malaria [14–18] |
| Livestock ownership | Household ownership of animals (any) | Dichotomous (yes/no) | Livestock have been associated with increases in malaria prevalence through zoopotentiation [25, 38], and decreases in prevalence through zooprophylaxis [76, 77, 83] and wealth [26, 33] |
| Control variables | |||
| Individual-level | |||
| Sex | Observed sex of participant | Dichotomous (male/female) | Sex is associated with increased risk due to variations in exposure through livelihood or household activities [23, 24] |
| Age | Self-reported age of participant | Continuous (years) | Children, in areas of high transmission, are more vulnerable to infection than adults due to a lack of acquired immunity [10, 20, 21] |
| Malaria-related knowledge | Question: What is the best way to prevent malaria? | Dichotomous dummy variable based on correct identification of avoiding mosquito bites. | Understanding malaria transmission is associated with preventive behaviours [10, 84, 85] |
| Bed net use | Question: Does your household own a mosquito net? | Dichotomous (yes/no) | Insecticide treated and untreated bed nets are associated with significant reductions in malaria prevalence [21, 84, 85] |
| Household-level | |||
| Wealth | Question: Does your household have any of the following items? | Dichotomous dummy variable based on categorization of PCA of variables: cell phone, radio, bicycle, electricity, private latrine, hand washing facilities, remittance, land (lowest 50 % of scores/highest 50 % of scores) | Wealth is associated with access to malaria prevention and health care [10, 31, 84, 85] |
| House construction | Question: What is your roof made of? | Dichotomous (iron sheets/wood or thatch or banana fibre) | Wall materials, open eaves, and window coverings can facilitate vector entry into the home [24, 28, 29] |
| Animals kept inside at night | Question: If you own animals, do your animals come into the house during the night? | Dichotomous dummy variable (yes/no) | Zooprophylaxis/zoopotentiation may be determined by relative proximity of animals to human sleeping quarters [29, 86–88] |
Zooprophylaxis
A subset analysis of livestock owners assessed the impact of relative location of livestock to humans. The subset was achieved by selecting from the survey dataset all Batwa and non-Batwa individuals who had a positive outcome on the “any livestock” variable. Among those who owned any livestock, the risk factor of interest was whether livestock were housed inside or outside of the home at night. This analysis controlled for bed net use and non-livestock wealth, which have been proposed as potential confounding and effect modifying variables, respectively [39]. Sensitivity analyses were used to assess the effect of housing specific livestock species (goats and chickens) inside the house on parasitaemia. Although age and sex were not significant in the reduced multivariable model, sensitivity analysis also tested their potential effect on the final model.
Results
Batwa and non-Batwa demographics
A total of 759 individual questionnaires were completed in July 2013 and April 2014, of which 59.10 % (449) of participants were non-Batwa and 40.90 % (310) Batwa (Table 2). Females were overrepresented in the study population compared to males, slightly more so among non-Batwa than among Batwa. Men were more likely than women to be employed or away finding work and therefore unavailable to participate. The population had an average age of 36.6 years with the Batwa being slightly younger on average than non-Batwa (34.9 and 37.6 years, respectively, z 2.88, p < 0.01). Educational attainment differed significantly between indigenous and non-indigenous with 41.78 % of Batwa having any education compared to 73.21 % of non-Batwa (χ2 77.23, p < 0.01). About 40 % of adults were employed with no significant difference between groups (χ2 0.39, p 0.53). 70.55 % of Batwa were in the low wealth category compared to 38.07 % of non-Batwa (χ2 73.81, p < 0.01). While there was no difference in the average number of household members between the two ethnic groups (Batwa 5.16 people/household, non-Batwa 5.0 people/household, p 0.73), Batwa experienced a greater level of crowding than non-Batwa (2.81 individuals/sleeping room and 2.54 individuals/sleeping room, respectively, z −2.75, p < 0.01). Bed net ownership (50.49 % Batwa versus 60.09 % non-Batwa, χ2 6.84 p < 0.01) and use were lower among Batwa than non-Batwa (38.61 and 58.07 % slept under a bed net the previous night respectively, χ2 27.32, p < 0.01). Livestock ownership was consistently lower among Batwa than non-Batwa with 32.04 % of Batwa owning any livestock compared to over half of non-Batwa (χ2 35.68, p < 0.01). Goats were the most frequently owned livestock among non-Batwa (16.13 % of Batwa and 41.22 % of non-Batwa owned goats, χ2 53.81, p < 0.01) while Batwa most frequently owned chickens compared to other animals but still, at a lower frequency than non-Batwa (18.71 % Batwa owned chickens compared to 31.53 % non-Batwa, χ2 15.49, p < 0.01). Both Batwa and non-Batwa brought animals indoors at night, to protect against predation and theft (21.50 % Batwa, 22.90 % non-Batwa, χ2 0.19, p 0.66).
Table 2.
Demographics and parasitaemia status of Batwa and non-Batwa of Kanungu District, Uganda, July 2013 and April 2014
| Characteristic | Total surveyed participants | RDT never | RDT ever |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Individual characteristics | |||
| Sex | n = 749 | n = 701 | n = 48 |
| Female | 452 (60.35) | 423 (60.34) | 29 (60.42) |
| Male | 297 (39.65) | 278 (39.66) | 19 (39.58) |
| Age | n = 688 | n = 654 | n = 43 |
| Mean | 36.60 [95 % CI 35.44–37.8] | 36.40 [95 % CI 35.21–37.50] | 39.90 [95 % CI 33.50–46.20] |
| Ethnic group | n = 758 | n = 709 | n = 49 |
| Non-Batwa | 448 (59.10) | 428 (60.37) | 20 (40.82) |
| Batwa | 310 (40.90) | 281 (39.63) | 29 (59.18) |
| Malaria prevention knowledge (avoid bites) | n = 755 | n = 706 | n = 49 |
| No | 588 (77.88) | 545 (77.20) | 43 (87.76) |
| Yes | 167 (22.12) | 161 (22.80) | 6 (12.24) |
| Household characteristics | |||
| Roof type | n = 752 | n = 703 | n = 49 |
| Metal/iron sheets | 621 (82.58) | 577 (82.08) | 44 (89.80) |
| Grass/thatch/wood | 131 (17.42) | 126 (17.92) | 5 (10.20) |
| Own mosquito net | n = 755 | n = 706 | n = 49 |
| No | 331 (43.84) | 306 (43.34) | 25 (51.02) |
| Yes | 424 (56.16) | 400 (56.66) | 24 (48.98) |
| Relative wealth | n = 728 | n = 680 | n = 49 |
| Poorer 50 % | 372 (51.10) | 338 (49.71) | 34 (70.83) |
| Wealthier 50 % | 356 (48.90) | 342 (50.29) | 14 (29.17) |
| Livestock ownership | |||
| Any livestock | n = 753 | n = 704 | n = 49 |
| No | 414 (54.26) | 382 (54.26) | 32 (65.31) |
| Yes | 339 (45.02) | 322 (45.74) | 17 (34.69) |
| Chickens | n = 754 | n = 705 | n = 49 |
| No | 556 (73.74) | 515 (73.05) | 41 (83.67) |
| Yes | 198 (26.26) | 190 (26.95) | 8 (16.33) |
| Goats | n = 754 | n = 705 | n = 49 |
| No | 521 (69.10) | 479 (67.94) | 42 (85.71) |
| Yes | 233 (30.90) | 226 (32.06) | 7 (14.29) |
| Animals inside at night | n = 686 | n = 637 | n = 49 |
| Never/no animals | 533 (77.70) | 487 (76.45) | 46 (93.88) |
| Sometimes/often | 153 (22.30) | 150 (23.55) | 3 (6.12) |
Malaria parasitaemia prevalence and risk factors
Of the 759 questionnaires completed in July 2013 and April 2014, 50 participants tested positive for P. falciparum antigen on RDT. One participant tested positive at both data collection events. Only the July data were considered for this participant, resulting in a total of 49 positive cases. Of the positive cases, 29 (59.18 %) were Batwa and 20 (40.82 %) were non-Batwa. The parasite period prevalence was 9.35 % and 4.45 % for Batwa and non-Batwa, respectively, thus the odds of a positive RDT result were 2.21 times higher for Batwa than for non-Batwa (95 % CI 1.23–3.98).
Univariable analyses
Among the non-modifiable risk factors evaluated for Batwa and non-Batwa participants, individuals over 50 years of age appeared to be at increased odds (OR 1.49, 95 % CI 0.73–3.04) for malaria parasitaemia (relative to younger individuals), although this result was not precise, with wide confidence intervals (Table 3). Among modifiable risk factors for malaria, relative wealth had the strongest association with parasitaemia. Relative poverty was associated with higher odds of parasitaemia, having 2.48 times the odds compared to the wealthier individuals (95 % CI 1.30–4.66). Not owning a bed net, and iron sheet roofing compared to thatched roofs increased the odds of parasitaemia, although these estimates were imprecise. Not owning livestock (OR 1.59, 95 % CI 0.87–2.91), and not understanding the importance of avoiding mosquito bites (OR 2.11, 95 % CI 0.89–5.06) increased odds of positivity for malaria parasitaemia; again, however, estimates were imprecise with wide confidence intervals.
Table 3.
Univariable and multivariable explanatory logistic regression analyses of malaria parasitaemia for Batwa and non-Batwa of Kanungu District, Uganda, July 2013 and April 2014
| Risk factor | Univariable models Unadjusted odds (OR [CI])a |
Reduced multivariable model Adjusted odds (OR [CI])a |
|---|---|---|
| N = 717 | ||
| Non-modifiable | ||
| Age | N = 688 | |
| <50 years | Ref | |
| ≥50 years | 1.49 [0.73–3.04] | |
| Sex | N = 749 | |
| Female | Ref | |
| Male | 0.99 [0.55–1.81] | |
| Ethnicity | N = 758 | |
| Non-Batwa | Ref | Ref |
| Batwa | 2.21 [1.23–3.98] | 1.88 [1.00–3.58] |
| Modifiable | ||
| Relative wealth | N = 728 | |
| Wealthier 50 % | Ref | Ref |
| Poorer 50 % | 2.48 [1.30–4.66] | 1.96 [0.98–3.94] |
| Bed net ownership | N = 755 | |
| Yes | Ref | |
| No | 1.36 [0.76–2.43] | |
| Livestock ownership | N = 753 | |
| Yes | Ref | Ref |
| No | 1.59 [0.87–2.91] | 1.44 [0.75–2.79] |
| House construction (roof) | N = 752 | |
| Thatch | Ref | Ref |
| Iron sheets | 1.92 [0.75–4.94] | 2.54 [0.96–6.72] |
| Malaria knowledge (avoid mosquito bites) | N = 755 | |
| Yes | Ref | Ref |
| No | 2.11 [0.89–5.06] | 1.92 [0.79–4.63] |
aUnadjusted odds represents the logistic regression model for each variable alone and the outcome of interest (RDT positivity). Adjusted odds is the odds ratio when all listed variables are included in the logistic regression model. These results are consistent on sensitivity analysis for survey date
Reduced multivariable model
In reduced multivariable analyses Batwa ethnicity (OR 1.88, 95 % CI 1.00–3.58), poverty (OR 1.96, 95 % CI 0.98–3.94), and iron sheet roofing (OR 2.54, 95 % CI 0.96–6.72) had increased odds of positive RDT. There was no association between LC and parasitaemia, nor was there a significant difference between models that controlled for clustering at the LC-level and those that did not. There was minimal difference in the fit of the model with and without random intercepts. The model results were not sensitive to survey date, and showed consistent results on the removal of ethnicity and/or wealth.
Zooprophylaxis
Amongst the 339 Batwa and non-Batwa livestock owners within the total survey population of 759 respondents, 17 individuals had a positive RDT outcome. Of the 339 livestock owners, 99 (29.2 %) were Batwa and 240 (70.8 %) were non-Batwa. Keeping animals inside the house at night was significantly protective against malaria (OR 0.29, CI 0.09–0.94) in adjusted analyses (Table 4). The sample size was insufficient to disaggregate by livestock species (chickens and goats). Sensitivity analyses indicated consistency of results across species, as well as no effect on the results when adding age and/or sex in the model.
Table 4.
Multivariable logistic regression analysis of malaria risk for Batwa and non-Batwa livestock owners (zooprophylaxis) in Kanungu District Uganda, July 2013 and April 2014
| Variables | Adjusted odds (OR [CI]) |
|---|---|
| n = 325 | |
| Variable of interest | |
| Keep animals inside at night | |
| No | Ref |
| Yes | 0.29 [0.09–0.94] |
| Control variables | |
| Ethnicity | |
| Non-Batwa | Ref |
| Batwa | 1.79 [0.59–5.38] |
| Relative wealth | |
| Wealthier 50 % | Ref |
| Poorer 50 % | 3.39 [1.06–10.85] |
| Bed net ownership | |
| Yes | Ref |
| No | 0.88 [0.29–2.74] |
Adjusted odds is the odds ratio when all listed variables are included in the logistic regression model. These results are not sensitive to age and sex on sensitivity analysis
Discussion
The period prevalence of P. falciparum parasitaemia for July 2013 and April 2014 for adult Batwa was 9.35 %, which is higher than the 4.1 % rate previously reported for Batwa over the age of 5 years [23]. A bed net distribution was carried out in November 2012, which could explain the low prevalence rate found previously in this population in January 2013 [59]. These findings are consistent with parasite prevalence estimates for Kanungu District as being relatively low (10–40 % prevalence when including children) compared to other parts of Uganda [8, 60].
Ethnicity was associated with malaria parasitaemia, with Batwa at higher risk. This is an important result given that wealth and other theorized risk factors for parasitaemia were controlled for; there remains a residual effect of ethnicity beyond these factors. There are marked differences in livelihoods between the two populations that may be driving these results. Batwa are currently undergoing a transition as they adapt to life outside of the forest. This transition is reflected in the low levels of education and livestock ownership relative to non-Batwa. There may also be unmeasured genetic differences between these ethnic groups that influence malaria prevalence [61–64]. For instance, ethnic differences in immune responses [19, 65] and lower susceptibility to malaria infection among ethnic minority Fulani tribes relative to sympatric ethnic groups in West Africa have been attributed to genetic differences at various loci [66, 67]. It might be expected, given their higher prevalence of infection, that Batwa mount a weaker immune response relative to non-Batwa in the face of exposure. Immunological studies would be required to test this hypothesis, but it is unclear how the results of such studies would lead to intervention strategies [6].
These results provide tentative indication that there may be important modifiable risks for malaria infection among Batwa and non-Batwa. Iron sheet roofing may play a role in malaria risk. House construction is an important risk factor for malaria infection [68–70] and thatched houses have previously been related to higher malaria prevalence of inhabitants in other parts of sub-Saharan Africa [28] due to the propensity of mosquitoes to rest indoors [25]. However, a systematic review and meta-analysis on the effect of house construction on malaria found that modern roof materials (iron sheets, tiles) were not consistently associated with decreased odds of infection [71]. Thatched or wooden roofs may confer some protection to individuals relative to corrugated iron sheets. It is possible that the open eaves of iron sheet roofs facilitate mosquito house entry and thereby increased malaria risk. Open eaves and gaps in housing materials are frequently associated with higher rates of parasitaemia [24, 29, 71]. However, this result may be confounded by wealth. Among non-Batwa, iron sheet roofs are a reflection of wealth since they must be purchased at a high cost compared to thatch or banana fibre, which may be obtained at no cost from the surrounding environment. In contrast, iron sheet roofing is purchased for Batwa by a local NGO with priority given to the most impoverished families as identified by the community. A Ugandan study carried out in part in Kanungu District found that closed eaves and modern house construction were associated with significantly decreased human biting rates and incidence rates of malaria in children when controlling for age, sex, and household wealth [72]. Further evaluation of the impact of house construction on malaria risk among Batwa may help to inform and improve these community development strategies.
The analysis of livestock owners suggested that when controlling for wealth and bed net use, keeping animals inside at night reduced the odds of malaria infection. Previous studies suggest that keeping animals indoors at night increases malaria risk where mosquitoes are zoophilic [39]. Entomological studies in the region suggest that Anopheles funestus and Anopheles gambiae sensu lato are the predominant vector species [60]. Anopheles funestus tends towards anthropophily; however, An. gambiae s.l. consists of seven morphologically indistinguishable species [73] of which the most important vectors may be more zoophilic, as with Anopheles arabiensis, or anthropophilic, as with An. gambiae sensu stricto. This finding might suggest that the predominant local species are anthropophilic and, in turn, that keeping animals indoors at night results in reduced malaria risk among livestock owners, however further entomological evaluation of local vector populations is required. The mechanism through which livestock infer protection against malaria remains poorly understood and their role in malaria transmission has been much contested [32, 35, 37, 38, 74, 75]. Livestock may draw mosquitoes away from humans, reducing their exposure to malaria [76, 77], or may provide an abundance of blood-meals, increasing vector density and longevity [32, 38, 74]. The zooprophylatic effect is, however, complicated by the relationship between livestock and wealth [32, 39]. It is well recognized that livestock contribute significantly to household economies for the rural poor, including the most marginalized [40, 41, 43]. Wealthy community members in Kanungu are those with the greatest livestock and land holdings. The findings of this study are consistent with others showing that poverty is positively associated with malaria prevalence [31, 78, 79].
The cross-sectional nature of this study limits the ability to infer causal relationships between the risk factors and malaria infection. Unemployed people who were available to be surveyed during data collection events were overrepresented in the sample. This may have reduced variation within the population and perhaps led to an underestimation of the importance of employment for malaria infection. Similarly, men were more likely to be working away, leading to an overrepresentation of women. Given that the role of sex in malaria risk remains unclear, it is difficult to predict the direction of this effect. The small sample size and number of cases limited statistical power to detect effect sizes. Sample size also prevented the stratification of uni- and multi-variable analyses of parasitemia based on ethnicity, although sensitivity analysis suggested results were consistent between ethnic groups. Similarly, sample size prevented ethnicity-based stratification for the zooprophylaxis analysis. As a result, the identification of risk factors, and recommendations for malaria control, apply to the survey population as a whole. In highly vulnerable remote indigenous populations, sample sizes are typically small and demographics frequently differ from non-indigenous populations; lack of statistical power must be balanced with the importance of prioritizing research within vulnerable and at-risk sub-populations.
Conclusions
Indigenous Batwa in Kanungu District, Uganda experience a two-fold increase in malaria risk compared to their non-indigenous neighbours. This inequitable burden mirrors health disparities experienced by indigenous peoples worldwide. Tentative support for the role of housing construction and wealth in accounting for some of the risk differential was found. Investigation of roof construction and related vector entry may be a prudent follow-up measure given these results. High baseline poverty among both populations, but particularly among Batwa, will remain a major determinant of health inequity and transmission risk. The influence of livestock may be related to asset-based wealth and suggests that keeping livestock indoors at night may play a role in reducing human exposure to malaria in this setting. This evidence may support livestock-based interventions here as both a poverty reduction strategy as well as a component of malaria vector control.
Authors’ contributions
LBF, SL, DBN, SH and IHACC Research Team conceptualized, designed, and obtained funding for, the Indigenous longitudinal health survey tool Health. BD, LBF, NAR, and PM conceptualized and obtained funding for the non-indigenous sampling design and data collection. BD, ST, JL, SH, and MK carried out data collection. BD carried out statistical analysis and drafted the manuscript. LBF, NAR, and PM supervised statistical analysis. All authors contributed to manuscript editing. All authors read and approved the final manuscript.
Acknowledgements
We sincerely thank the ten Batwa communities in Kanungu District: Buhoma, Byumba, Bikuuto, Karehe, Kitahurira, Kihembe, Kebiremu, Mukongoro, Rurangara, and Kitariro, for their engagement with the project. We also thank the Local Council 1 chairpersons of Buhoma, Byumba, Bikuuto, Mukono, Mpungu, Kihembe, Kebiremu, Mukongoro, Kishanda, and Kitariro and the non-Batwa community members for their participation. We deeply appreciate the IHACC survey administrators Aine Tom, Ainembabazi Brian, Amanya Prince, Asaasira Grace, Asinath Kyomuhangi, City Tyson Magezi, Collins, Cranmar Magezi, Dan Mugume, Ivan Arineitwe, Kaheru John, Kenneth Kirihariwe, Kesande Annet, Kesande Charity, Kokunda Sylvia, Komuhangi Rovance, Matukunda Judith, Ninsiima Evas, Orikiriza Mathias, Orishaba Rabecca, Tom Kabategyire, Tumuhimbise Ishmael and Turyaeyira Bright. We also acknowledge the work of IHACC research team members Emmanuel Eloku, Hubert Nkabura, Jamen Kasumba, Fortunate Twembabaze, Christine Nantogo, Martin Kigozi, and Allan Gordon. We would like to thank Sierra Clark and Margot Charette for help with data entry. BD would like to thank Kaitlin Patterson for her assistance with statistical analysis. BD would like to acknowledge funding from an IDRC Doctoral Research Award.
This research is part of an international project entitled the “Indigenous Health Adaptation to Climate Change” (IHACC) project (http://www.ihacc.ca), with parallel field study sites in the Canadian Arctic and Peru. We thank our local partners; Ugandan Ministry of Health, Kanungu District Administration, Bwindi Community Hospital, Batwa Development Program. Funding was provided by CIHR/NSERC/SSHRC and IDRC Tri-Council Initiative on Adaptation to Climate Change, Indigenous Health Adaptation to Climate Change (IHACC), IDRC File N. 106372-003, 004, 005; CIHR Open Operating Grant, Adaptation to the health effects of climate change among Indigenous peoples in the global south (IP-ADAPT), Application N. 298312. We would also like to thank the anonymous reviewer for their thoughtful feedback.
IHACC Research Team: James Ford, Cesar Carcamo, Alejandro Llanos, and Victoria Edge.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- RDT
rapid diagnostic test
- LC
Local Council
Contributor Information
Blánaid Donnelly, Email: blanaid.donnelly@mail.mcgill.ca.
Lea Berrang-Ford, Email: lea.berrangford@mcgill.ca.
Jolène Labbé, Email: jolene.labbe@mcgill.ca.
Sabastian Twesigomwe, Email: twesigomwe.sabastian@yahoo.com.
Shuaib Lwasa, Email: shuaiblwasa@gmail.com.
Didacus B. Namanya, Email: didamanya@yahoo.com
Sherilee L. Harper, Email: harpers@uoguelph.ca
Manisha Kulkarni, Email: manisha.kulkarni@uottawa.ca.
Nancy A. Ross, Email: nancy.ross@mcgill.ca
Pascal Michel, Email: Pascal.Michel@phac-aspc.gc.ca.
References
- 1.Snow RW. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015;13:3. doi: 10.1186/s12916-014-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norheim OF, Jha P, Admasu K, Godal T, Hum RJ, Kruk ME, Gomez-Dantes O, Mathers CD, Pan H, Sepulveda J, et al. Avoiding 40 % of the premature deaths in each country, 2010–30: review of national mortality trends to help quantify the UN Sustainable Development Goal for health. Lancet. 2015;385:239–252. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Achieving the malaria MDG target: reversing the incidence of malaria 2000–2015. World Health Organization, United Nations Children’s Fund. 2015. http://www.apps.who.int/iris/bitstream/10665/184521/1/9789241509442_eng.pdf?ua=1. Accessed 13 Nov 2015.
- 4.Murray CJL, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton L, O’Neill MS, Kruk ME, Bell ML. The persistent problem of malaria: addressing the fundamental causes of a global killer. Soc Sci Med. 2008;67:854–862. doi: 10.1016/j.socscimed.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 7.Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Malaria elimination 2 ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010;376:1579–1591. doi: 10.1016/S0140-6736(10)61301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, et al. Malaria in Uganda: challenges to control on the long road to elimination I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wielgosz B, Kato E, Ringler C. Agro-ecology, household economics and malaria in Uganda: empirical correlations between agricultural and health outcomes. Malar J. 2014;13:11. doi: 10.1186/1475-2875-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates I, Fenton C, Gruber J, Lalloo D, Lara AM, Squire SB, Theobald S, Thomson R, Tolhurst R. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4:267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 11.Lawson DW, Mulder MB, Ghiselli ME, Ngadaya E, Ngowi B, Mfinanga SGM, Hartwig K, James S. Ethnicity and child health in northern Tanzania: Maasai pastoralists are disadvantaged compared to neighbouring ethnic groups. PLoS ONE. 2014;9:17. doi: 10.1371/journal.pone.0110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N Ohenjo, Willis R, Jackson D, Nettleton C, Good K, Mugarura B. Indigenous health 3—health of Indigenous people in Africa. Lancet. 2006;367:1937–1946. doi: 10.1016/S0140-6736(06)68849-1. [DOI] [PubMed] [Google Scholar]
- 13.Nettleton C, Stephens C, Bristow F, Claro S, Hart T, McCausland C, Mijlof I. Utz Wachil: findings from an international study of Indigenous perspectives on health and environment. EcoHealth. 2007;4:461–471. doi: 10.1007/s10393-007-0138-9. [DOI] [Google Scholar]
- 14.Hotez PJ, Woc-Colburn L, Bottazzi ME. Neglected tropical diseases in Central America and Panama: review of their prevalence, populations at risk and impact on regional development. Int J Parasitol. 2014;44:597–603. doi: 10.1016/j.ijpara.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Haque U, Magalhaes RJS, Mitra D, Kolivras KN, Schmidt WP, Haque R, Glass GE. The role of age, ethnicity and environmental factors in modulating malaria risk in Rajasthali, Bangladesh. Malar J. 2011;10:7. doi: 10.1186/1475-2875-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achidi EA, Apinjoh TO, Anchang-Kimbi JK, Mugri RN, Ngwai AN, Yafi CN. Severe and uncomplicated falciparum malaria in children from three regions and three ethnic groups in Cameroon: prospective study. Malar J. 2012;11:12. doi: 10.1186/1475-2875-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe T, Honda S, Nakazawa S, Tuong TD, Thieu NQ, Hung LX, Thuan LK, Moji K, Takagi M, Yamamoto T. Risk factors for malaria infection among ethnic minorities in Binh Phuoc, Vietnam. Southeast Asian J Trop Med Public Health. 2009;40:18–29. [PubMed] [Google Scholar]
- 18.Garnelo L, Brandao LC, Levino A. Dimensions and potentialities of the geographic information system on indigenous health. Rev Saude Publica. 2005;39:634–640. doi: 10.1590/S0034-89102005000400018. [DOI] [PubMed] [Google Scholar]
- 19.Bolad A, Farouk SE, Israelsson E, Dolo A, Doumbo OK, Nebie I, Maiga B, Kouriba B, Luoni G, Sirima BS, et al. Distinct interethnic differences in immunoglobulin G class/subclass and immunoglobulin M antibody responses to malaria antigens but not in immunoglobulin G responses to nonmalarial antigens in sympatric tribes living in West Africa. Scandi J Immunol. 2005;61:380–386. doi: 10.1111/j.1365-3083.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschmidt I, Sharp B. Patterns in age-specific malaria incidence in a population exposed to low levels of malaria transmission intensity. Trop Med Int Health. 2001;6:986–991. doi: 10.1046/j.1365-3156.2001.00817.x. [DOI] [PubMed] [Google Scholar]
- 21.Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:11. doi: 10.1186/1475-2875-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith T, Genton B, Betuela I, Rare L, Alpers MP. Mosquito nets for the elderly? Trans R Soc Trop Med Hyg. 2002;96:37–38. doi: 10.1016/S0035-9203(02)90232-4. [DOI] [PubMed] [Google Scholar]
- 23.Lewnard JA, Berrang-Ford L, Lwasa S, Namanya DB, Patterson KA, Donnelly B, Kulkarni MA, Harper SLL, Ogden NH, Carcamo CP, et al. Relative undernourishment and food insecurity associations with Plasmodium falciparum among Batwa Pygmies in Uganda: evidence from a cross-sectional survey. Am J Trop Med Hyg. 2014;91:39–49. doi: 10.4269/ajtmh.13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley J, Rehman AM, Schwabe C, Vargas D, Monti F, Ela C, Riloha M, Kleinschmidt I. Reduced prevalence of malaria iunfection in children living in houses with window screening or closed eaves on Bioko Island, Equatorial Guinea. PLOS ONE. 2013;8:7. doi: 10.1371/journal.pone.0080626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000;94:17–21. doi: 10.1016/S0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- 26.Mutero CM, Kabutha C, Kimani V, Kabuage L, Gitau G, Ssennyonga J, Githure J, Muthami L, Kaida A, Musyoka L, et al. A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop. 2004;89:171–186. doi: 10.1016/j.actatropica.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Clark TD, Greenhouse B, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Staedke SG, Seto E, Kamya MR, Rosenthal PJ, Dorsey G. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 28.Temu EA, Coleman M, Abilio AP, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;7:2. doi: 10.1371/journal.pone.0031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiscox A, Khammanithong P, Kaul S, Sananikhom P, Luthi R, Hill N, Brey PT, Lindsay SW. Risk factors for mosquito house entry in the Lao PDR. PLoS ONE. 2013;8:10. doi: 10.1371/journal.pone.0062769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickard DG, Dudovitz RN, Wong MD, Jen HC, Osborn RD, Fernandez HE, Donkor CI. Closing the gap between insecticide treated net ownership and use for the prevention of malaria. Prog Community Health Partnersh. 2011;5:123–131. doi: 10.1353/cpr.2011.0018. [DOI] [PubMed] [Google Scholar]
- 31.Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? A review of the literature. Trop Med Int Health. 2005;10:1047–1059. doi: 10.1111/j.1365-3156.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 32.Bogh C, Clarke SE, Walraven GEL, Lindsay SW. Zooprophylaxis, artefact or reality? A paired-cohort study of the effect of passive zooprophylaxis on malaria in The Gambia. Tran R Soc Trop Med Hyg. 2002;96:6. doi: 10.1016/S0035-9203(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 33.Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malar J. 2008;7:43. doi: 10.1186/1475-2875-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Environmental management for vector control. In: WHO Technical Report Series. 1980. http://www.apps.who.int/iris/bitstream/10665/41404/1/WHO_TRS_649.pdf. Accessed 19 Nov 2014.
- 35.WHO. Manual on environmental management for mosquito control. In: WHO Offset Publication, vol. 66. 1982. http://www.apps.who.int/iris/bitstream/10665/37329/1/9241700661_eng.pdf. Accessed 6 Mar 2015. [PubMed]
- 36.WHO. Action Plan for the Reduction of Reliance on DDT in Disease Vector Control. 2001. http://www.whqlibdoc.who.int/hq/2001/WHO_SDE_WSH_01.5.pdf. Accessed 5 Aug 2014.
- 37.Saul A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J. 2003;2:32. doi: 10.1186/1475-2875-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouma M, Rowland M. Failure of passive zooprophylaxis—cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans R Soc Trop Med Hyg. 1995;89:351–353. doi: 10.1016/0035-9203(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J. 2015;14:313. doi: 10.1186/s12936-015-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randolph TF, Schelling E, Grace D, Nicholson CF, Leroy JL, Cole DC, Dentment MW, Omore A, Zinsstag J, Ruel M. Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. J An Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- 41.Upton M. The role of livestock in economic development and poverty reduction. In: Pro-Poor Livestock Policy Initiative Working Papers. Rome: Food and Agriculture Organization; 2004;10. p. 66.
- 42.Kitalyi A, Mtenga L, Morton J, McLeod A, Thornton P, Dorward A, Sadullah M. Why keep livestock if you are poor? In: Owen EA, Kitalyi A, Jayasuriya N, Amith T, editors. Livestock and wealth creation: improving the husbandry of animals kept by resource-poor people in developing countries. Nottingham: Nottingham University Press; 2005. pp. 13–27. [Google Scholar]
- 43.Alary V, Corniaux C, Gautier D. Livestock’s contribution to poverty alleviation: how to measure it? World Dev. 2011;39:1638–1648. doi: 10.1016/j.worlddev.2011.02.008. [DOI] [Google Scholar]
- 44.McDermott JJ, Randolph TF, Staal SJ. The economics of optimal health and productivity in smallholder livestock systems in developing countries. Rev Sci Tech. 1999;18:399–424. doi: 10.20506/rst.18.2.1167. [DOI] [PubMed] [Google Scholar]
- 45.Berrang-Ford L, Dingle K, Ford JD, Lee C, Lwasa S, Namanya DB, Henderson J, Llanos A, Carcamo C, Edge V. Vulnerability of indigenous health to climate change: a case study of Uganda’s Batwa Pygmies. Soc Sci Med. 2012;75:1067–1077. doi: 10.1016/j.socscimed.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Clark S, Berrang-Ford L, Lwasa S, Namanya DB, Edge VL, Harper S. IHACC Team. The burden and determinants of self-reported acute gastrointestinal illness in an Indigenous Batwa Pygmy population in southwestern Uganda. Epidemiol Infect. 2015;143:2287–2298. doi: 10.1017/S0950268814003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Namanya DB. Comparative study of malaria risk factors and access to healthcare services by Batwa and non-Batwa communities in Kanungu District Southwestern Uganda. Master’s thesis. International Health Sciences University, Institute of Health Policy and Management; 2013.
- 48.Barasa B, Egeru A, Okello P, Mutuzo F. Dynamics of land use/cover trends in Kanungu District, south-western Uganda. J Appl Sci Environ Manag. 2010;14:67–70. [Google Scholar]
- 49.Ugandan Bureau of Statistics. National population and housing census 2014: provisional results. Revised Edition; 2014.
- 50.Tabuti JRS, Kukunda CB, Kaweesi D, Kasilo OMJ. Herbal medicine use in the districts of Nakapiripirit, Pallisa, Kanungu, and Mukono in Uganda. J Ethnobio Ethnomed. 2012;8:15. doi: 10.1186/1746-4269-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kayonza and Mpungu sub-counties household survey 2009. Buhoma: Bwindi Community Hospital; 2010.
- 52.Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna AO, Snow RW. Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC Med. 2011;9:37. doi: 10.1186/1741-7015-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bukirwa H, Yau V, Kigozi R, Filler S, Ouick L, Lugemwa M, Dissanayake G, Kamya M, Wabwire-Mangen F, Dorsey G. Short report: assessing the impact of indoor residual spraying on malaria morbidity using a sentinel site surveillance system in Western Uganda. Am J Trop Med Hyg. 2009;81:611–614. doi: 10.4269/ajtmh.2009.09-0126. [DOI] [PubMed] [Google Scholar]
- 54.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, Donegan S, Garner P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011;7:CD008122269. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A guide for the selection of malaria RDTs to be purchased with 3DF grants. http://www.3dfund.org/…Guidelines/Malaria-RDTs-Guideline-3DF-v1.0.pdf. Accessed 26 Jun 2015.
- 56.Indigenous Health Adaptation to Climate Change. http://www.ihacc.ca. Accessed 20 Mar 2015.
- 57.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 58.Booysen F, van der Berg S, Burger R, von Maltitz M, du Rand G. Using an asset index to assess trends in poverty in seven Sub-Saharan African countries. World Dev. 2008;36:1113–1130. doi: 10.1016/j.worlddev.2007.10.008. [DOI] [Google Scholar]
- 59.Clark S, Berrang-Ford L, Shuaib L, Namanya D, IHACC Research Team, Manisha K. Insecticide-treated net coverage and use in an Indigenous Batwa Pygmy population: a longitudinal analysis. Under review. 2015.
- 60.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D’Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 61.Frodsham AJ, Hill AVS. Genetics of infectious diseases. Hum Mol Gen. 2004;13:R187–R194. doi: 10.1093/hmg/ddh225. [DOI] [PubMed] [Google Scholar]
- 62.Mackinnon MJ, Gunawardena DM, Rajakaruna J, Weerasingha S, Mendis KN, Carter R. Quantifying genetic and nongenetic contributions to malarial infection in a Sri Lankan population. Proc Natl Acad Sci USA. 2000;97:12661–12666. doi: 10.1073/pnas.220267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackinnon MJ, Mwangi TW, Snow RW, Marsh K, Williams TN. Heritability of malaria in Africa. PLOS Med. 2005;2:1253–1259. doi: 10.1371/journal.pmed.0020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fortin A, Stevenson MM, Gros P. Susceptibility to malaria as a complex trait: big pressure from a tiny creature. Hum Mol Gen. 2002;11:2469–2478. doi: 10.1093/hmg/11.20.2469. [DOI] [PubMed] [Google Scholar]
- 65.Arama C, Giusti P, Bostrom S, Dara V, Traore B, Dolo A, Doumbo O, Varani S, Troye-Blomberg M. Interethnic differences in antigen-presenting cell activation and TLR responses in Malian children during Plasmodium falciparum malaria. PLoS ONE. 2011;6:e18319. doi: 10.1371/journal.pone.0018319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Israelsson E, Ekstrom M, Nasr A, Dolo A, Kearsley S, Arambepola G, Homann MV, Maiga B, Doumbo OK, ElGhazali G, et al. Marked differences in CRP genotype frequencies between the Fulani and sympatric ethnic groups in Africa. Malar J. 2009;8:136. doi: 10.1186/1475-2875-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiga B, Dolo A, Toure O, Dara V, Tapily A, Campino S, Sepulveda N, Corran P, Rockett K, Clark TG, et al. Fc gamma receptor IIa-H131R polymorphism and malaria susceptibility in sympatric ethnic groups, Fulani and Dogon of Mali. Scand J Immunol. 2014;79:43–50. doi: 10.1111/sji.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirby MJ, West P, Green C, Jasseh M, Lindsay SW. Risk factors for house-entry by culicine mosquitoes in a rural town and satellite villages in The Gambia. Parasit Vectors. 2008;1:41. doi: 10.1186/1756-3305-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana WR, Killeen GF, Moore SJ. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLOS NTD. 2010;4:e773. doi: 10.1371/journal.pntd.0000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu JX, Bousema T, Zelman B, Gesase S, Hashim R, Maxwell C, Chandramohan D, Gosling R. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLOS ONE. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, Lindsay SW. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:12. doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, Rek J, Bottomley C, Staedke SG, Kamya M, Dorsey G, Lindsay SW. Mind the gap: house structure and the risk of malaria in Uganda. PLoS ONE. 2015;10:15. doi: 10.1371/journal.pone.0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Besansky NJ, Powell JR, Caccone A, Hamm DM, Scott JA, Collins FH. Molecular phylogeny of the Anopheles gambiae complex suggests genetic introgression between principal malaria vectors. Proc Natl Acad Sci USA. 1994;91:6885–6888. doi: 10.1073/pnas.91.15.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bogh C, Clarke SE, Pinder M, Sanyang F, Lindsay SW. Effect of passive zooprophylaxis on malaria transmission in the Gambia. J Med Entomol. 2001;38:822–838. doi: 10.1603/0022-2585-38.6.822. [DOI] [PubMed] [Google Scholar]
- 75.Charlwood D. Zooprophylaxis: are we in Plato’s cave? Trends Parasitol. 2001;17:517. doi: 10.1016/S1471-4922(01)02090-6. [DOI] [PubMed] [Google Scholar]
- 76.Maia MF, Abonuusum A, Lorenz LM, Clausen P-H, Bauer B, Garms R, Kruppa T. The effect of deltamethrin-treated net fencing around cattle enclosures on outdoor-biting mosquitoes in Kumasi, Ghana. PLOS ONE. 2012;7:e45794. doi: 10.1371/journal.pone.0045794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tirados I, Gibson G, Young S, Torr SJ. Are herders protected by their herds? An experimental analysis of zooprophylaxis against the malaria vector Anopheles arabiensis. Malar J. 2011;10:68. doi: 10.1186/1475-2875-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 79.Somi MF, Butler JRG, Vahid F, Njau J, Kachur SP, Abdulla S. Is there evidence for dual causation between malaria and socioeconomic status? Findings from rural Tanzania. Am J Trop Med Hyg. 2007;77:1020–1027. [PubMed] [Google Scholar]
- 80.Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, Rapuoda B, Greenwood B, Cox J. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202. doi: 10.1186/1475-2875-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–518. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization. Guidelines for the treatment of malaria, 3rd edn. Geneva. 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1&ua=1. Accessed 25 Apr 2016.
- 83.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 84.De Beaudrap P, Nabasumba C, Grandesso F, Turyakira E, Schramm B, Boum Y, Etard JF. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar J. 2011;10:9. doi: 10.1186/1475-2875-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siri JG. Independent associations of maternal education and household wealth with malaria risk in children. Ecol Soc. 2014;19:33. doi: 10.5751/ES-06134-190133. [DOI] [Google Scholar]
- 86.Hadis M, Lulu M, Makonnen Y, Asfaw T. Host choice by indoor-resting Anopheles arabiensis in Ethiopia. Trans R Soc Trop Med Hyg. 1997;91:376–378. doi: 10.1016/S0035-9203(97)90245-5. [DOI] [PubMed] [Google Scholar]
- 87.Hewitt S, Kamal M, Muhammad N, Rowland M. An entomological investigation of the likely impact of cattle ownership on malaria in an Afghan refugee camp in the North-west Frontier Province of Pakistan. Med Vet Entomol. 1994;8:160–164. doi: 10.1111/j.1365-2915.1994.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 88.Iwashita H, Dida GO, Sonye GO, Sunahara T, Futami K, Njenga SM, Chaves LF, Minakawa N. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit Vectors. 2014;7:15. doi: 10.1186/1756-3305-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]


