Abstract
DNMT3A mutations are observed in myeloid malignancies, including myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), and acute myeloid leukemia (AML). Transplantation studies have elucidated an important role for Dnmt3a in stem cell self-renewal and in myeloid differentiation. Here we investigated the impact of conditional hematopoietic Dnmt3a loss on disease phenotype in primary mice. Mx1-Cre-mediated Dnmt3a ablation led to the development of a lethal, fully penetrant myeloproliferative neoplasm with myelodysplasia (MDS/MPN) characterized by peripheral cytopenias and by marked, progressive hepatomegaly. We detected expanded stem/progenitor populations in the liver of Dnmt3a-ablated mice. The MDS/MPN induced by Dnmt3a ablation was transplantable, including the marked hepatomegaly. Homing studies showed that Dnmt3a-deleted bone marrow cells preferentially migrated to the liver. Gene expression and DNA methylation analyses of progenitor cell populations identified differential regulation of hematopoietic regulatory pathways, including fetal liver hematopoiesis transcriptional programs. These data demonstrate that Dnmt3a ablation in the hematopoietic system leads to myeloid transformation in vivo, with cell autonomous aberrant tissue tropism and marked extramedullary hematopoiesis (EMH) with liver involvement. Hence, in addition to the established role of Dnmt3a in regulating self-renewal, Dnmt3a regulates tissue tropism and limits myeloid progenitor expansion in vivo.
Keywords: Dnmt3a, myelodysplasia, hematopoietic stem cells, extramedullary hematopoiesis, HSPC homing
Introduction
Aberrant epigenetic patterning is a cornerstone of the molecular pathogenesis of cancers, including myeloid malignancies1. DNMT3A (DNA methyltransferase 3A) mutations are detected in 8–10% of myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS)2–4, and in 20–25% of acute myeloid leukemias (AML)5–8. These mutations occur as monoallelic or biallelic nonsense/frameshift alterations, or a dominant-negative R882 substitution9, 10. DNMT3A-mutant AMLs exhibit global and site-specific alterations in DNA methylation10, also observed in chronic myelomonocytic leukemia (CMML) patients with DNMT3A mutations11.
Although these data underscore the importance of DNMT3A mutations to myeloid transformation, the specific mechanisms by which DNMT3A functions as a tumor suppressor have not been fully elucidated. It is possible that mutations in epigenetic modifier genes alter the epigenetic state of normal hematopoietic stem/progenitor cells (HSPC), which allows malignant cells to re-access earlier developmental transcriptional programs. Notably, such features as enhanced hematopoietic stem cell self-renewal, increased proliferative capacity, myeloid bias, and extramedullary hematopoiesis (EMH), are shared between fetal liver hematopoiesis and MDS/MPN12, 13.
Previous studies on Dnmt3a loss in adult hematopoiesis used transplantation assays to document expansion of the stem/progenitor compartment, most prominently long-term HSCs, a gain in self-renewal, and a decline in the output of differentiated progeny14. Moreover, a subset of recipients developed different hematologic malignancies15, 16. However, these studies did not assess the tumor suppressor function of Dnmt3a in the absence of the selective pressure of serial transplantation, or whether Dnmt3a loss is sufficient to induce transformation in vivo. We therefore characterized the impact of conditional Dnmt3a loss in the hematopoietic compartment to assess impact on disease phenotype in vivo, and on steady-state adult hematopoiesis, coupled with studies of the effect of Dnmt3a loss on DNA methylation and transcriptional state.
Materials and Methods
Animal studies
were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center. Dnmt3af/f conditional knock-out (cKO) line17 was reconstituted from frozen embryos (The Jackson Laboratory, Bar Harbor, ME), backcrossed to C57BL/6 background, and crossed to Mx1-Cre-deletor line. See supplemental methods.
Colony-forming assay in semisolid media
was performed using previously published method18 with modifications. See supplemental methods.
Homing assay
was performed as described19 with modifications. See also supplemental methods.
Gene expression and DNA methylation analyses
were performed on FACS-sorted LSK and GMP cells. Enhanced Reduced Representation of Bisulfite Sequencing (ERRBS) was used to determine cytosine methylation patterns at base pair resolution20, 21. RNA was subjected to standard Illumina-based sequencing. For detailed description see supplemental methods.
Statistical analysis
Except where indicated, data are presented as mean ± standard deviation plotted using Prism version 6 (GraphPad Software). Statistical significance was determined using Student’s t-test with Holm-Sidak correction method, with α=5.0%.
Results
Hematopoietic-specific inactivation of Dnmt3a results in lethal hematologic disease
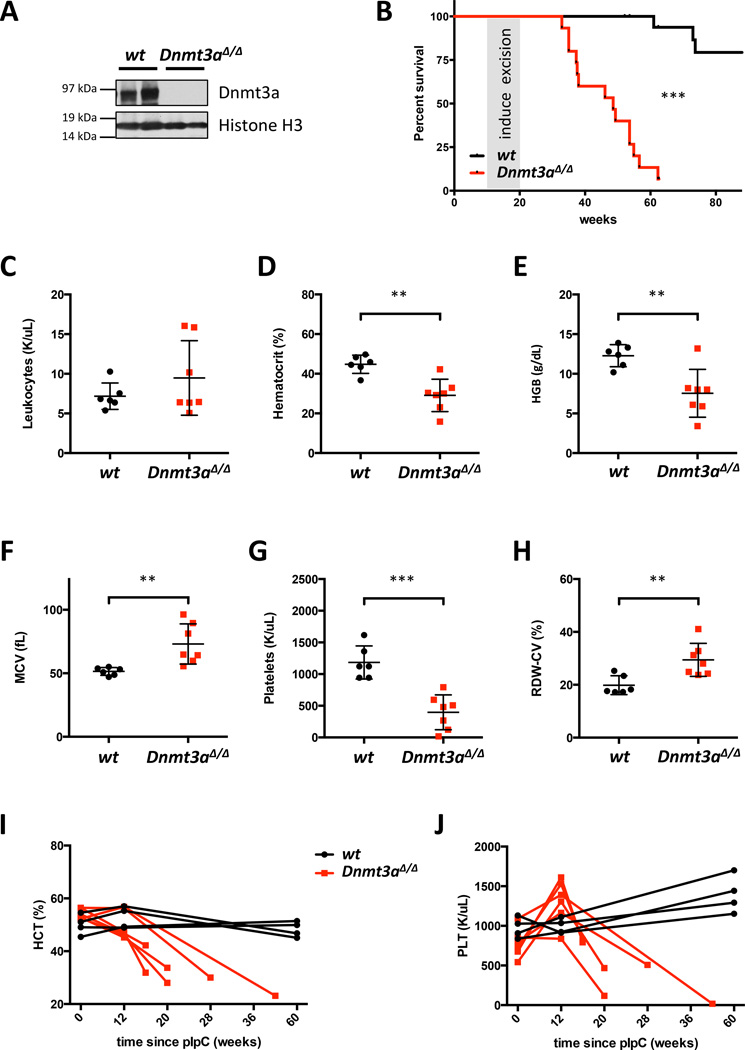
We first investigated the role of Dnmt3a in steady-state hematopoiesis. Mx1-Cre-driven recombination results in complete loss of Dnmt3a protein in the hematopoietic system (Figures 1A and S1A) and leads to a lethal, fully penetrant disease (median survival 48.6 weeks, p<0.0001, Figure 1B). None of the Dnmt3a+/+:Mx1-Cre+ or Dnmt3af/f:Mx1-Cre− animals developed hematologic abnormalities within a 90-week follow-up period. Dnmt3a-ablated mice developed progressive macrocytic anemia with anisocytosis, thrombocytopenia, and monocytosis (Figures 1C–H and S1B–G). Examination of the hematocrit and platelet counts over time revealed progressive anemia and thrombocytopenia (Figure 1I–J).
Figure 1. Primary Dnmt3a conditional knock-out mice have decreased survival and develop peripheral blood cytopenias.
A. Dnmt3a protein levels after excision in the spleens of Dnmt3af/f:Mx1-Cre+ and control mice.
B. Survival of Dnmt3a KO (n=15) and control (n=18) mice. Cre-mediated excision was induced by poly(I:C) injections (please see Supplementary Methods). Animals sacrificed as controls for diseased Dnmt3a KO mice were censored. Reasons for euthanasia in 2 control animals were rectal prolapse and severe bite wounds due to fighting; cause of death in 1 mouse was undetermined. None of the control mice exhibited signs of hematologic disease.
C. White blood cell counts at disease onset in Dnmt3a-ablated mice (n=6–7).
D–H. Dnmt3a-deleted mice develop macrocytic anemia (n=6–7) with decreased hematocrit (D) and hemoglobin levels (E), increased mean corpuscular volume (F), thrombocytopenia (G), and anisocytosis (H) (**, p<0.01; ***, p<0.001).
I–J. Hematocrit (I) and platelet counts (J) over time in 6 Dnmt3a KO and 2 representative control mice.
Dnmt3a loss induces mature myeloid and myeloid progenitor expansion in vivo
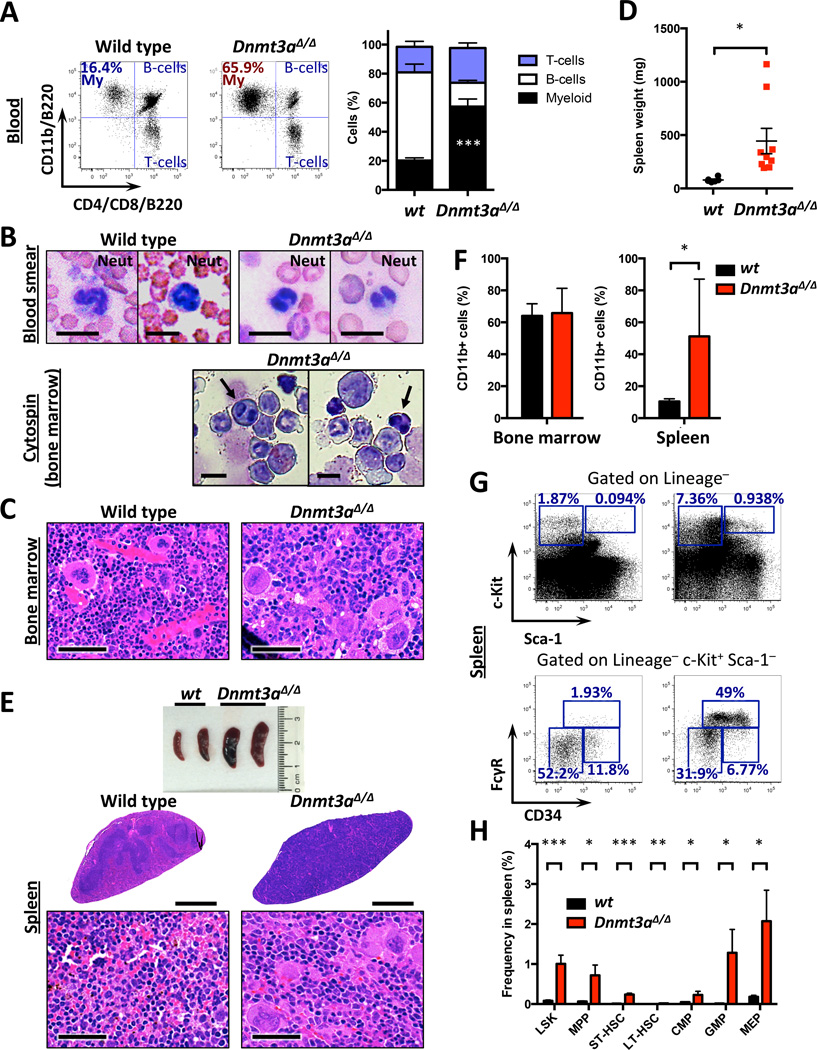
Detailed analysis of the hematopoietic system in Dnmt3a KO mice found marked myeloid bias and myeloid and erythroid dysplasia in peripheral blood (Figures 2A–B) accompanied by hypercellular bone marrow (Figure S2A) with megakaryocyte dysplasia (Figure 2C). We found increased spleen size (Figures 2D) and effacement of splenic architecture by myeloid infiltration, and scattered dysplastic megakaryocytes (Figure 2E), consistent with myeloproliferation, confirmed by flow cytometry (Figures 2F and S2B). We observed an increase in the stem-cell-enriched Lineage−Sca-1+c-Kit+ (LSK) and in Lineage−Sca-1−c-Kit+ (LK) myeloid progenitor cells, with significant expansion of GMPs (Figure 2G–H). The findings of hypercellular bone marrow with dysplasia, myeloid bias in the peripheral blood, and extramedullary hematopoiesis is consistent with a myeloproliferative/myelodysplastic disorder (MDS/MPN).
Figure 2. Dnmt3a-deleted mice develop myelodysplasia/myeloproliferation with extramedullary hematopoiesis.
A. Myeloid bias in peripheral blood of Dnmt3a-null mice (n=7–8; ***, p<0.001).
B. Peripheral blood smears and bone marrow cytospin preparations showing dysplasia of myeloid and erythroid lineages in Dnmt3a-null but not control mice, manifesting as nuclear hyposegmentation reminiscent of pseudo-Pelger-Huet neutrophils classically noted in human MDS and nucleated red blood cells (arrows) with dyserythropoiesis characterized by irregular nuclear contours. Bar – 10 µm.
C. Representative H/E-stained sections of control and Dnmt3a KO sterna. Arrows – megakaryocyte dysplasia. Bar – 50 µm.
D. Spleens weights in Dnmt3a KO and control mice at disease onset (n=6–9; *, p<0.05).
E. Gross pathology and H/E-stained sections of representative control and Dnmt3a-null spleens. Arrows – megakaryocytic infiltration. Bar – 1000 µm (low magnification), and 50 µm (high magnification).
F. Quantification of the CD11b+ cells shows elevated levels of myeloid cells in spleens of Dnmt3a-deleted mice at time of disease onset (n=5–6; *, p<0.05).
G. Representative FACS plots of immature stem and progenitor cells in the spleens of Dnmt3a-ablated and control mice.
H. Quantification of data presented in G (n=4–6; *, p<0.05; **, p<0.01; ***, p<0.001).
Impaired erythroid maturation after Dnmt3a loss
To gain insight into the mechanism of anemia in diseased Dnmt3a-null mice we examined erythroid maturation in bone marrow and spleens according to CD71 and Ter119 markers22, 23 and found a decrease in the mature stage IV erythroblasts and an increase in stage II CD71+Ter119+ cells, most pronounced in the spleens, without significant changes in apoptosis (Figure S2C–E), consistent with a previous report15. We also observed erythrophagocytosis in the spleens and livers of Dnmt3a-deleted animals (Figure S2F).
Perturbation of the stem/progenitor compartment and increased self-renewal of Dnmt3a deficient hematopoietic cells
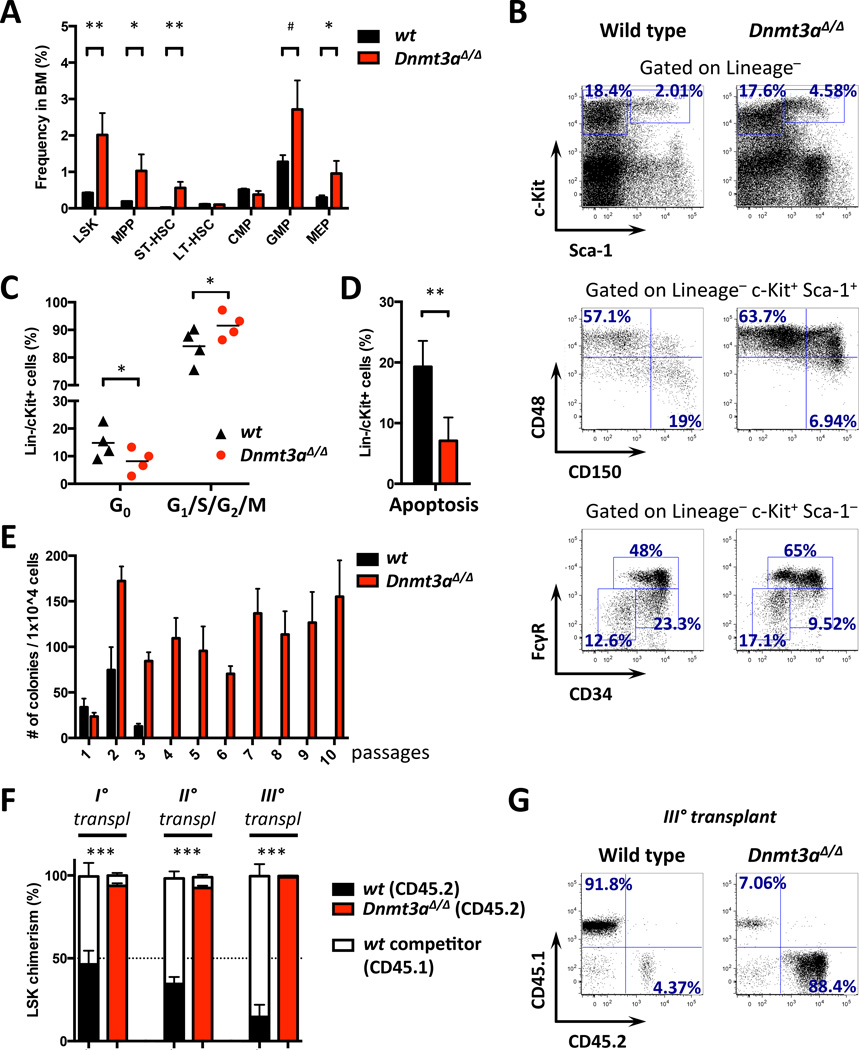
Previous studies found increased numbers of primitive HSCs, but not of immediate downstream progenitors, in recipient mice reconstituted with Dnmt3a-ablated HSCs14. In this setting the impact of stress from bone marrow transplantation could not be unequivocally separated from the cell-intrinsic HSC phenotype. Detailed analysis of HSPCs in diseased primary Dnmt3a KO animals showed a significant increase in the relative frequency of the immature LSK population. This expansion was due to elevated LSK CD48+ cells while the LSK CD48−CD150+ LT-HSC population remained unperturbed, and we observed an increase in committed myeloid progenitors (Figures 3A–B and S3A). Overall, Dnmt3a-deficient HSPCs were characterized by increased proliferation and decreased apoptosis (Figure 3C–D). The increased frequency of CD48+ LSK cells accompanied by predominant myelopoiesis relative to lymphopoiesis, and increased self-renewal and multi-lineage reconstitution potential are characteristic of fetal hematopoiesis13. We observed a prominent cKit+CD41+ population in the bone marrow of Dnmt3a-deleted mice, which is nearly absent in normal adult hematopoiesis (Figure S2G).
Figure 3. Conditional deletion of Dnmt3a results in perturbation of the hematopoietic stem and progenitor compartment and gain of self-renewal potential.
A. Relative stem and progenitor cell frequencies in Dnmt3a KO and control bone marrow at disease onset (n=4–6; #, p=0.065; *, p<0.05; **, p<0.01).
B. Representative FACS plots for data in A.
C–D. Increased proliferation and decreased quiescence (C) and decreased apoptosis (D) in Dnmt3a-deleted compared to wild-type HSPCs in animals competitively transplanted with bone marrow cells derived from Dnmt3a-null (CD45.2) and wild-type (CD45.1) mice. Analysis was performed in 4 transplant recipients 9 months after transplantation (*, p<0.05; **, p<0.01).
E. CFU assay on bone marrow cells from Dnmt3a-null or control littermates. Representative results of 3 independent experiments, each performed in triplicate.
F. CD45.1/CD45.2 chimerism in bone marrow LSK cells over three rounds of serial competitive transplantation. Test cells (CD45.2, red bars – Dnmt3a KO, black bars – WT) were competed against wild-type (CD45.1, white bars) and analyzed 16 weeks after transplantation (n=5; ***, p<0.001). Dotted line represents CD45.1/CD45.2 cell ratio at time of injection (50%).
G. CD45.1/CD45.2 peripheral blood chimerism in tertiary recipients 16 weeks after transplantation in D. Representative FACS plots.
To assess the self-renewal potential of bone marrow cells we performed colony-forming assays. Dnmt3a loss resulted in continuous serial replating, while control cells rapidly exhausted their colony-forming ability (Figure 3E). In serial competitive transplantation assays in vivo Dnmt3a KO cells showed robust repopulation advantage compared to wild-type control (Figure S3B–C), which was more pronounced in the bone marrow compartment, and continued to increase with each round of transplantation (Figure 3F–G). These observations suggest that loss of Dnmt3a augments stem cell function in vitro and in vivo.
Dnmt3a loss results in hepatomegaly due to liver-specific myeloproliferation and extramedullary hematopoiesis
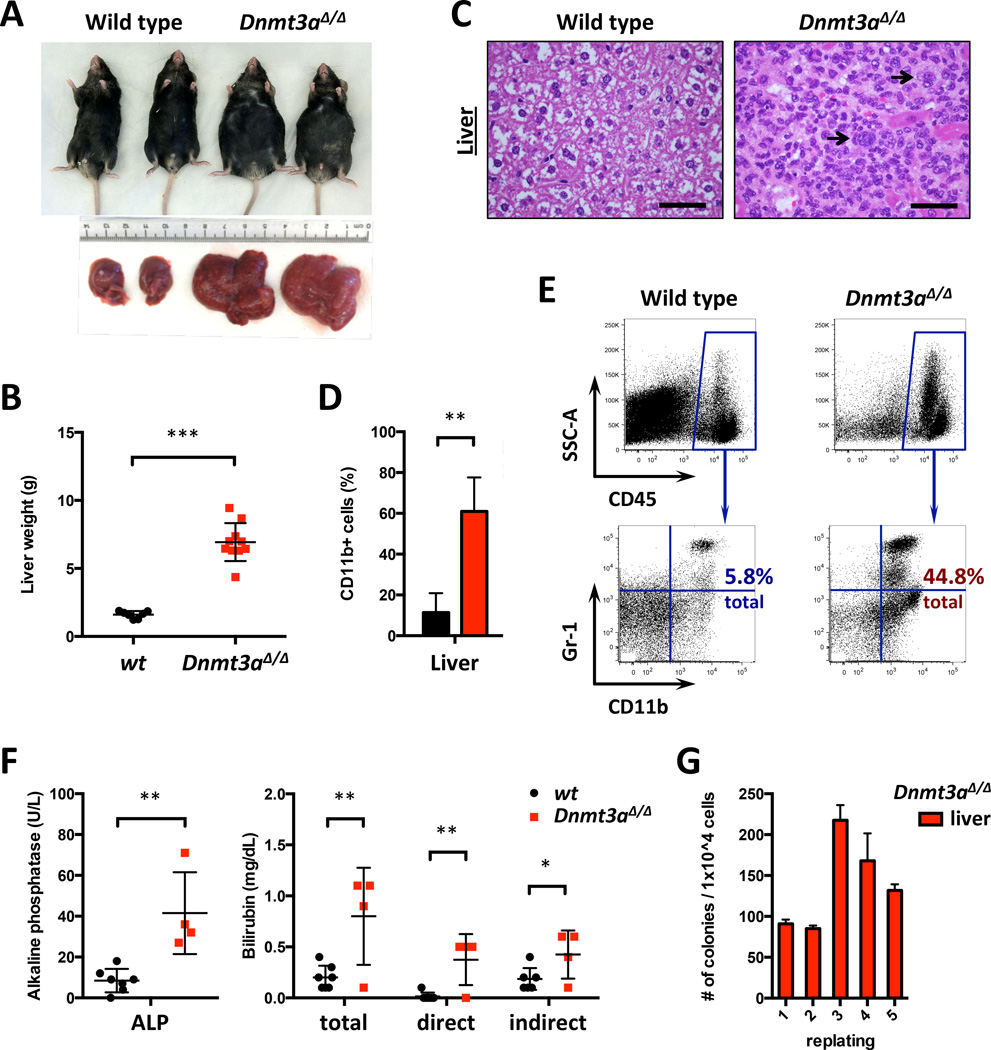
All moribund Dnmt3a-deleted animals presented with distended abdomens and marked hepatomegaly (Figure 4A–B). Histopathologically Dnmt3a KO livers showed portal, lobular, sinusoidal infiltration by immature myeloid cells with open chromatin and prominent nucleoli, scattered megakaryocytes, and occasional blasts (Figure 4C), confirmed by FACS analysis that also showed prominent monocytosis (Figure 4D–E and S4A). Alkaline phosphatase (ALP) and bilirubin levels were elevated and albumin levels decreased (Figures 4F and S4B), consistent with impaired liver function.
Figure 4. Hepatomegaly due to myeloproliferation and monocytic infiltration in conditional Dnmt3a knock-out mice.
A. Gross pathology of livers and overall appearance of control and diseased Dnmt3a-deleted mice, representative of all animals studied.
B. Liver weights in Dnmt3a-KO and WT mice at disease onset (n=7–10; *** p<0.001).
C. H/E-stained liver sections; arrows – scattered megakaryocytes in Dnmt3a-ablated but not control mice. Bar – 50 µm.
D. Quantification of CD11b+ cells in livers of control and diseased Dnmt3a KO mice (n=3–6; **, p<0.01).
E. Representative FACS plots for data presented in (D).
F. Serum alkaline phosphatase (ALP) and bilirubin levels in control and diseased Dnmt3a-deleted mice (n=4–7; *, p<0.05; **, p<0.01).
G. Colony-forming assay using liver cells from diseased Dnmt3a knock-out mouse, single experiment performed in triplicate.
Histological detection of megakaryocytes in infiltrated liver parenchyma after Dnmt3a inactivation suggested that liver was a site of hematopoiesis. Analysis of HSPCs residing in the liver detected myeloid progenitor LK and stem-cell-enriched LSK cells in Dnmt3a-null mice, but not in wild-type animals (Figure S4C). Plating of Dnmt3a KO liver cells yielded colonies with characteristic granulocyte-erythroid-macrophage-megakaryocyte (GEMM) and granulocyte-macrophage (GM) morphology that serially replated (Figure 4G) similar to continuously self-renewing Dnmt3a-ablated bone marrow cells. By contrast, cells derived from wild-type control livers were unable to give rise to hematopoietic colonies in vitro.
Dnmt3a-deleted bone marrow cells exhibit cell-intrinsic liver tropism
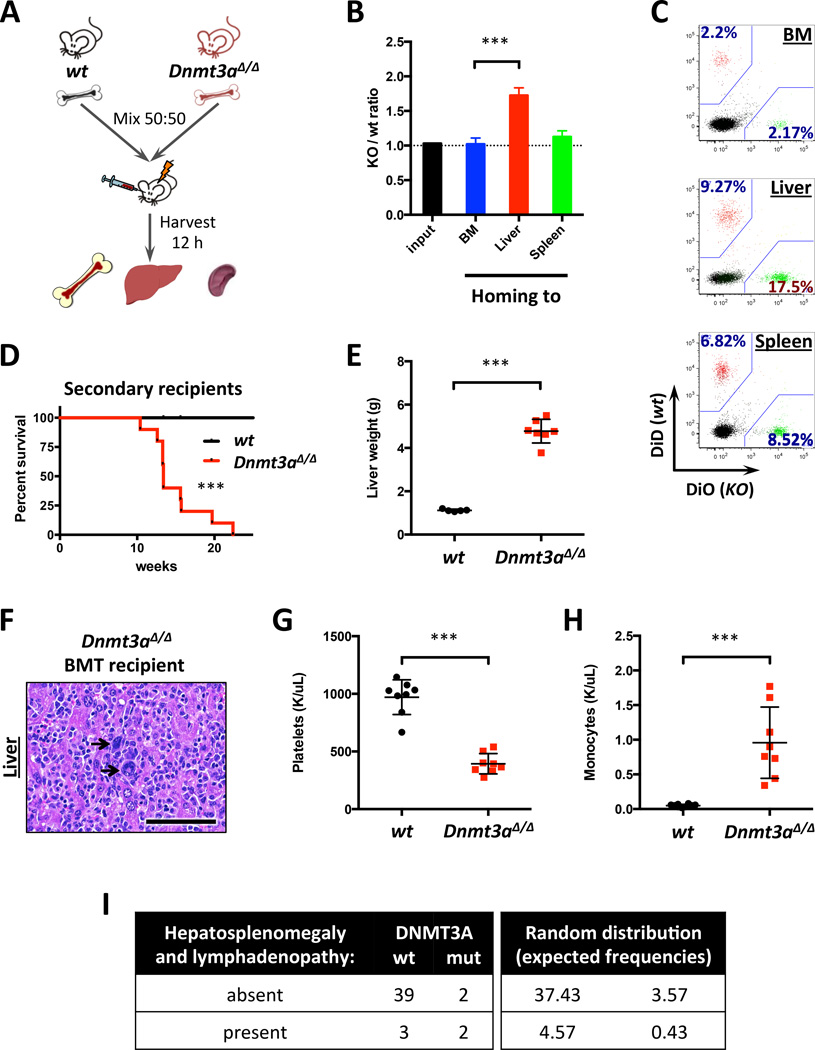
As Mx1-Cre is able to mediate excision, in addition to HSCs, in hepatic and perivascular cells24, 25, we examined if extramedullary tropism of Dnmt3a KO hematopoietic cells was due to cell-intrinsic or niche-dependent mechanism by performing short-term homing studies (Figure 5A). While the ability of Dntm3a-deficient cells to home to the bone marrow was unaltered, they showed preferential liver tropism (Figure 5B–C). Interestingly, CD45+ Dnmt3a KO liver cells exhibited even stronger hepatotropism than bone marrow cells from the same animals (Figure S5A). These findings demonstrate that preferential liver homing of Dnmt3a-null hematopoietic cells is mediated by a cell-intrinsic mechanism.
Figure 5. Conditional loss of Dnmt3a results in cell-autonomous liver tropism.
A. Schematic depiction of the homing assay workflow.
B. Relative homing tropism calculated as Dnmt3a KO/wt ratio in the bone marrow, livers and spleens of recipient mice (n=4; ***, p<0.001). Dotted line represents ratio at time of injection (1.0).
C. Representative FACS plots showing distribution of fluorescently labeled WT and Dnmt3a KO cells homed to the indicated sites, gated on CD45+.
D. Survival of lethally-irradiated recipient mice transplanted with total bone marrow from diseased Dnmt3a-null or control animals (n=10; ***, p=0.0003 by Gehan-Breslow-Wilcoxon test).
E–F. Liver weights (E; n=5–7; ***, p<0.001) and H/E-stained sections (F) in primary recipients transplanted with Dnmt3a-ablated bone marrow cells at time of disease onset and in control wild-type mice. Arrows – megakaryocytes indicative of EMH.
G–H. Platelet (G) and monocyte (H) counts in the peripheral blood of mice transplanted with Dnmt3a-null bone marrow (n=8; ***, p<0.001).
I. Increased frequency of extramedullary hematopoiesis in CMML patients with DNMT3A mutations. Left panel represents observed frequencies of hepatosplenomegaly and lymphadenopathy in patients with different DNMT3A (wt or mutant) status (n=46); right panel shows expected frequencies for random distribution (p=0.053, Phi=+0.39, 2-tailed Fisher’s exact test).
Hematopoietic-specific Dnmt3a loss results in cell-autonomous myeloproliferation and liver-specific myeloid expansion in secondary recipients
We tested the ability of Dnmt3a-deficient hematopoietic cells to re-initiate MDS/MPN by transplantation into sublethally-irradiated congenic hosts. No engraftment was observed 12 weeks post-transplant confirming that the hematologic disease represents MDS/MPN, and not AML. Conversely, transplantation into lethally-irradiated recipients resulted in a fully penetrant, rapidly fatal disease with a median latency of 13.4 weeks (Figure 5D, p<0.0001) that recapitulated the MDS/MPN seen in primary mice with hepatosplenomegaly (Figure 5E and S5B). Similar results were obtained when recipient mice were transplanted with CD45+ cells isolated from Dnmt3a-deleted livers, suggesting that disease-initiating cells are present in the liver (data not shown). Dnmt3a KO-transplanted livers showed myeloid infiltration and histiocytic hyperplasia (Figure 5F). Recipient mice developed thrombocytopenia, decreased hematocrit and peripheral blood leukocytosis with monocytosis (Figures 5G–H and S5C–D). In contrast to primary Dnmt3a KO mice, transplant recipients showed both myeloid and T-lymphoid bias, and bi-lineage expansion (Figure S5E–F).
Extramedullary hematopoiesis in MDS/MPN patients with DNMT3A mutations
To extend our studies to the human context we examined whether DNMT3A mutations are associated with EMH in a well-annotated cohort of 46 patients with the MDS/MPN overlap syndrome CMML11. DNMT3A-mutant patients had a higher rate of clinically evident EMH (Figure 5I): 2 of 4 DNMT3A-mutant patients (50%) presented with hepatosplenomegaly and lymphadenopathy due to extramedullary CMML, while only 7.14% (3/42) DNMT3A-WT patients showed similar extramedullary involvement (p=0.053 and Phi=+0.39, 2-tailed Fisher’s exact test). These data underscore the tumor suppressor role of Dnmt3a and association of DNMT3A loss with EMH in the clinical context.
DNA methylation profiling reveals decreased methylation upon Dnmt3a loss at loci with regulatory potential
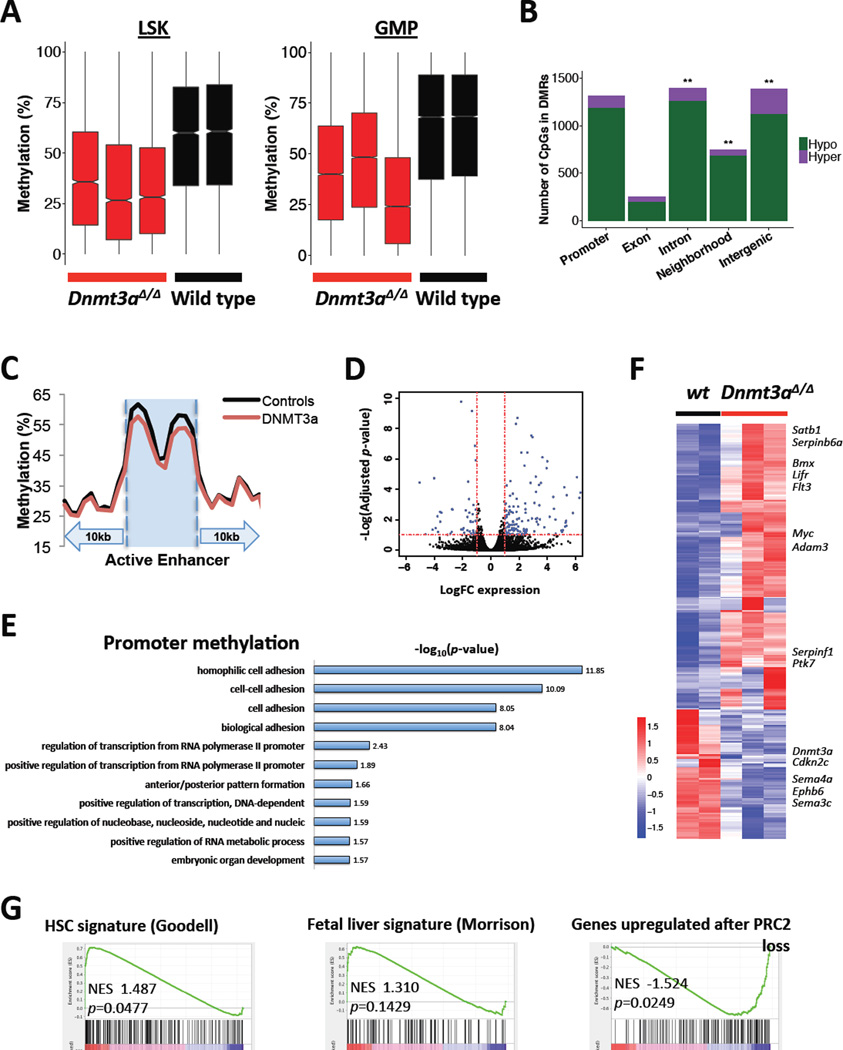
To assess whether the Dnmt3a KO phenotype was linked to aberrant epigenetic programming we analyzed DNA methylation patterns in LSK and GMP cells. Enhanced reduced-representation bisulfite sequencing (ERRBS) followed by unsupervised hierarchical clustering of genome-wide cytosine methylation profiles showed segregation of Dnmt3a wild-type and mutant samples (Figure S6A). Genome-wide CpG methylation values revealed regions of marked DNA demethylation upon Dnmt3a loss (Figure 6A; p<0.01, Wilcoxon two-tailed rank sum test). Of the 822 differentially methylated regions in GMPs (DMRs - methylKit: q<0.01 and ≥20% methylation difference; methylSig: beta-binomial p≤0.25 and methylation difference ≥10%; Table S1) 797 (97%) were hypomethylated in Dnmt3a-ablated mice (Figure S6B) with particular enrichment for hypomethylated CpGs within introns, gene neighborhoods, and intergenic regions (p<0.01, 1-tailed Fisher’s exact test; Figure 6B). We next focused our analyses on GMPs as we observed a marked increase in the proportion of GMPs in vivo. A trend toward hypomethylation was seen at gene promoters (Figures 6B and S6C). Overall, GMP-stage active enhancers showed a greater degree of methylation loss than promoters, exons, introns, gene neighborhoods, or intergenic regions (p<0.01, Wilcoxon one-tailed rank-sum test with continuity correction; Figure 6C). A subset of genes associated with hypomethylated DMRs had increased expression by the RNA-sequencing analysis (Figure S6E). Furthermore, in GMPs significantly hypomethylated regions were enriched in binding sites with homology to Ets family transcription factor Elk4, zinc finger and BTB domain containing transcription factor Zbtb3, and Forkhead class transcription factor FoxP1, implicated as tumor suppressors, while HoxA9 was among notable findings in LSKs (Figure S6D)26–28. Gene ontology analysis by biological process applied to DMCs within gene promoters revealed significant enrichment of genes involved in cell adhesion (CD97, Icam2, Ppard, Cldn4, Eng, Ninj2, protocadherins and cadherin-related protein genes), regulation of RNA polymerase II-driven transcription (Srebf1, FoxH1, HoxB1, Cebpb, Hand1, Nr4a2, Tead2, Jak3), and embryonic development (HoxB1, Cebpb, Hand1, Hlx, HoxA6, HoxB6; Figure 6E). These data suggest that the hypomethylation observed with Dnmt3a loss may have a functional role in regulating transcriptional gene states.
Figure 6. ERRBS DNA methylation and gene expression profiling identifies genomic features dysregulated by Dnmt3a loss.
A. Dnmt3a-null LSK and GMP cells show overall methylation loss relative to wild-type in differentially methylated regions (DMRs).
B. CpGs contained in DMRs are enriched within introns, gene neighborhoods, and intergenic regions. CpGs with lower %methylation in Dnmt3a-null GMP cells compared to wild-type are defined as hypomethylated, and those with greater methylation in Dnmt3a-null GMP cells as hypermethylated (**, genomic compartments enriched for DMR-contained CpGs relative to other regions of the genome, Fisher’s exact test, one-tailed, p<0.01).
C. Dnmt3a-null GMPs show methylation loss at GMP active enhancers relative to wild-type. GMP active enhancers ± 10kb were divided into 30 bins and the average methylation value in each was plotted.
D. Differential expression of genes covered by RNA-sequencing shows preferential upregulation of a subset of genes in Dnmt3a-null GMPs compared to wild-type controls. Dotted lines represent significance cutoffs set at ±1 Log(FC expression) (vertical) or −1 Log(adjusted p-value) (horizontal).
E. List of gene ontology terms by DAVID functional annotation tool derived from DMCs mapping to gene promoters in GMP cells from Dnmt3a-null and control animals.
F. Heatmap of differentially expressed genes (adjusted p<0.1) based on RNA-sequencing of GMPs derived from Dnmt3a-null and wild-type control animals, z-score scale.
G. GSEA plots showing enrichment of the HSC gene signature36, modified fetal liver HSC signature37, and PRC2_EED_UP.V1_UP51, in Dnmt3a-null GMPs compared to wild-type controls.
Gene expression profiling identifies reprogramming towards a more primitive developmental state with Dnmt3a loss
RNA-sequencing identified 218 upregulated and 98 downregulated genes in Dnmt3a-null GMPs (Figure 6D; adjusted p<0.1), including genes implicated in embryonic and cancer stem cell phenotypes cMyc29, Lifr30, Bmx31, Satb132, positive and negative regulators of tumor invasion Adam3, Ptk733, serpin34 and semaphorin35 family proteins and ephrin receptor ligands, and networks associated with hematopoietic cell lineage, cell surface receptors, and regulation of immune systems (Figures 6F and S6F, Table S2). Among hypomethylated genes that showed corresponding expression changes, in addition to Adam3 and Ptk7, were Svep1, Hivep2, Lrp1, Cds1, Padi1, Zfp618 that regulate cell attachment and amplify cellular signaling responses from cytokines. Gene set enrichment analysis (GSEA) found enrichment of HSC signature genes36 in Dnmt3a-null GMPs consistent with enhanced self-renewal. Comparison with the fetal hematopoiesis gene expression signature37 (Figure 6G) along with positive enrichment for PRC2-regulated genes and negative enrichment for the ES cell differentiation genes confirmed transcriptional reactivation of early developmental programs after Dnmt3a loss. In line with previous reports6, 38 we found increased expression of Hox genes and of their targets in Dnmt3a-null GMPs (Figure S6G–H). Increased expression of the cell adhesion molecules is consistent with hypomethylation within their promoters and altered cell tropism of the Dnmt3a-ablated bone marrow cells (Figures 6E and S6H). These data indicate that key regulator genes and gene networks are transcriptionally modified by Dnmt3a loss.
Discussion
Our findings emphasize the crucial role of Dnmt3a in the pathogenesis of hematologic malignancies, by showing Dnmt3a in primary mice gives rise to a fully penetrant, lethal myeloid disease. Although studies of Dnmt3a-deficient hematopoiesis in adoptive transfer experiments has produced invaluable insights14–16, the variable impact on disease phenotype may be due to the selective pressure of transplantation, which has been suggested to be necessary to unmask the malignant transformation induced in Dnmt3a-deficient HSCs15. Our data provide an unequivocal role for Dnmt3a in inducing myeloid transformation in primary mice in the absence of transplantation. Our studies and previous studies relied on interferon-activated Mx1-Cre excision14–16 which may force HSCs to enter cell cycle39, 40. Hence an inflammatory component may be an important contributing factor into the pathogenesis of hematopoietic failure syndromes, consistent with clinical observations41. Dnmt3a loss resulted in expansion of the immature compartment in the bone marrow, including LSK and GMP cells, concomitant with pronounced monocytosis. This pattern of quantitative changes reveals compromised differentiation at several stages, paralleling high-risk subtypes of MDS and a disproportionate association of DNMT3A-mutations with monocytic disease observed in the clinic42, 43. These findings reinforce the clinical relevance of Dnmt3a-deficient hematopoiesis as a faithful animal model of human disease, including MDS/MPN.
Dnmt3a-deficient hematopoiesis shares similarities with HSC expansion within the fetal liver, including expansion of CD48+ LSK ST-HSCs and MPPs and lower relative frequencies of CD150+CD48− LSK LT-HSCs, preferential myelopoiesis with lower Gr-1 expression, and changes in erythroid maturation, including a prominent CD41+ cKit+ population not found in adult hematopoiesis. Most importantly, deletion of functional Dnmt3a leads to robust gain in self-renewal and long-term multilineage reconstituting potential, which is a hallmark of fetal hematopoiesis13, 37, 44, 45. We observed enrichment of the HSC and fetal liver gene expression signatures in Dnmt3a-null GMPs, along with upregulation of embryonic and cancer stem cell-associated genes. It is tempting to speculate that these phenotypic changes arise due to partial reprogramming towards developmentally more primitive state by impaired epigenetic control following Dnmt3a loss. Further studies examining expression of fetal stem/progenitor markers and transcriptomic switches will be necessary to address the potential role of Dnmt3a function in hematopoietic development.
The most striking finding in Dnmt3a-deleted myeloproliferation/myelodysplasia is the hepatomegaly due to EMH and liver-specific myeloproliferation. The ability of adult hematopoietic cells to home, colonize, and mature in the liver reflects primitive developmental patterns of hematopoiesis and re-establishment of a stem cell niche46. Reminiscent of fetal hematopoiesis, hepatic EMH in Dnmt3a-null animals was predominantly localized within sinusoids. The mechanism of homing to the liver is distinct from other organs, as the lower blood flow and fenestrated endothelium in the sinusoids do not require selectin-mediated rolling for leukocyte recruitment, suggesting other adhesion mechanisms play a prominent role47. Gene expression analysis of Dnmt3a-deleted GMPs identified enrichment of a cell adhesion signature that may account for the enhanced liver tropism. At the same time, elevated expression of the extracellular matrix degradation and tissue invasion genes may contribute to cell mobilization from the bone marrow to the liver48, 49, facilitated through epigenetic changes after Dnmt3a loss targeting genomic loci with regulatory potential, including Ets binding sites50. These observations may have important implications for the treatment of DNMT3A-mutated MDS in humans as EMH in the liver may serve as a safe heaven environment and a reservoir for the disease-initiating clone. Future interrogation of the specific cell adhesion determinants will help clarify molecular mechanisms of hepatotropism of Dnmt3a-ablated hematopoietic cells.
Taken together, Dnmt3a inactivation in primary animals leads to the development of a myeloid malignancy with features of human MDS/MPN, highlighting the relevance of this model to chronic myeloid neoplasms. We speculate that increased epigenetic plasticity allows reactivation of developmental gene expression programs. Subsequent studies are needed to explore the role for concomitant disease alleles, and to determine if therapies can be developed to prevent and/or reverse the myeloid transformation induced by Dnmt3a loss.
Supplementary Material
Acknowledgements
This work was supported by NCI K99 grant CA178191 and by Lauri Strauss Leukemia Foundation discovery grant to OAG, by a Gabrielle’s Angel Fund grant to RLL and AMM, by grant CA172636 to RLL and AMM, and by the Samuel Waxman Cancer Research Center. AMM is a Burroughs Wellcome Clinical Translational Scholar, and is supported by the Sackler Center for Biomedical and Physical Sciences. RLL is a LLS Scholar. MSKCC cores are supported by grant P30 CA008748. We thank Caroline Sheridan, Jorge Gandara and the WCMC Epigenomics Core for preparation and sequencing of Next generation sequencing assays.
Footnotes
Conflict of interest: the authors declare no competing financial interests.
Supplementary information is available at Leukemia’s website.
References
- 1.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nature reviews Cancer. 2012 Aug 17; doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011 Jul;25(7):1219–1220. doi: 10.1038/leu.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stegelmann F, Bullinger L, Schlenk RF, Paschka P, Griesshammer M, Blersch C, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011 Jul;25(7):1217–1219. doi: 10.1038/leu.2011.77. [DOI] [PubMed] [Google Scholar]
- 4.Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011 Jul;25(7):1153–1158. doi: 10.1038/leu.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. The New England journal of medicine. 2010 Dec 16;363(25):2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nature genetics. 2011 Apr;43(4):309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 7.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. The New England journal of medicine. 2012 Mar 22;366(12):1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013 May 30;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013 Dec 12;122(25):4086–4089. doi: 10.1182/blood-2013-02-483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R, et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer cell. 2014 Apr 14;25(4):442–454. doi: 10.1016/j.ccr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meldi K, Qin T, Buchi F, Droin N, Sotzen J, Micol JB, et al. Specific molecular signatures predict decitabine response in chronic myelomonocytic leukemia. The Journal of clinical investigation. 2015 May;125(5):1857–1872. doi: 10.1172/JCI78752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bejar R, Levine R, Ebert BL. Unraveling the molecular pathophysiology of myelodysplastic syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Feb 10;29(5):504–515. doi: 10.1200/JCO.2010.31.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1995 Oct 24;92(22):10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nature genetics. 2011;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celik H, Mallaney C, Kothari A, Ostrander EL, Eultgen E, Martens A, et al. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood. 2015 Jan 22;125(4):619–628. doi: 10.1182/blood-2014-08-594564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2015 Jan 22;125(4):629–638. doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R. Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Developmental dynamics : an official publication of the American Association of Anatomists. 2007 Jun;236(6):1663–1676. doi: 10.1002/dvdy.21176. [DOI] [PubMed] [Google Scholar]
- 18.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011 Jul 12;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf RZ, Scadden DT. Homing of hematopoietic cells to the bone marrow. Journal of visualized experiments : JoVE. 2009;(25) doi: 10.3791/1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett-Bakelman FE, Sheridan CK, Kacmarczyk TJ, Ishii J, Betel D, Alonso A, et al. Enhanced reduced representation bisulfite sequencing for assessment of DNA methylation at base pair resolution. Journal of visualized experiments : JoVE. 2015;(96) doi: 10.3791/52246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I, et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS genetics. 2012;8(6):e1002781. doi: 10.1371/journal.pgen.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2009 Oct 13;106(41):17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001 Dec 1;98(12):3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 24.Joseph C, Quach JM, Walkley CR, Lane SW, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell stem cell. 2013 Nov 7;13(5):520–533. doi: 10.1016/j.stem.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995 Sep 8;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 26.Day BW, Stringer BW, Spanevello MD, Charmsaz S, Jamieson PR, Ensbey KS, et al. ELK4 neutralization sensitizes glioblastoma to apoptosis through downregulation of the anti-apoptotic protein Mcl-1. Neuro-oncology. 2011 Nov;13(11):1202–1212. doi: 10.1093/neuonc/nor119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer letters. 2013 Jan 28;328(2):198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Lim JH. Zinc finger and BTB domain-containing protein 3 is essential for the growth of cancer cells. BMB reports. 2014 Jul;47(7):405–410. doi: 10.5483/BMBRep.2014.47.7.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurenti E, Wilson A, Trumpp A. Myc's other life: stem cells and beyond. Current opinion in cell biology. 2009 Dec;21(6):844–854. doi: 10.1016/j.ceb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Okita K, Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Current stem cell research & therapy. 2006 Jan;1(1):103–111. doi: 10.2174/157488806775269061. [DOI] [PubMed] [Google Scholar]
- 31.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer cell. 2011 Apr 12;19(4):498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Will B, Vogler TO, Bartholdy B, Garrett-Bakelman F, Mayer J, Barreyro L, et al. Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment. Nature immunology. 2013 May;14(5):437–445. doi: 10.1038/ni.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golubkov VS, Prigozhina NL, Zhang Y, Stoletov K, Lewis JD, Schwartz PE, et al. Protein-tyrosine pseudokinase 7 (PTK7) directs cancer cell motility and metastasis. The Journal of biological chemistry. 2014 Aug 29;289(35):24238–24249. doi: 10.1074/jbc.M114.574459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, et al. Update of the human and mouse SERPIN gene superfamily. Human genomics. 2013;7:22. doi: 10.1186/1479-7364-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nature reviews Drug discovery. 2014 Aug;13(8):603–621. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- 36.Chambers SM, Boles NC, Lin KY, Tierney MP, Bowman TV, Bradfute SB, et al. Hematopoietic fingerprints: an expression database of stem cells and their progeny. Cell stem cell. 2007 Nov;1(5):578–591. doi: 10.1016/j.stem.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He S, Kim I, Lim MS, Morrison SJ. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes & development. 2011 Aug 1;25(15):1613–1627. doi: 10.1101/gad.2052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro AF, Pratcorona M, Erpelinck-Verschueren C, Rockova V, Sanders M, Abbas S, et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood. 2012 Jun 14;119(24):5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- 39.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010 Jun 10;465(7299):793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietras EM, Lakshminarasimhan R, Techner JM, Fong S, Flach J, Binnewies M, et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. The Journal of experimental medicine. 2014 Feb 10;211(2):245–262. doi: 10.1084/jem.20131043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirantes C, Passegue E, Pietras EM. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Experimental cell research. 2014 Dec 10;329(2):248–254. doi: 10.1016/j.yexcr.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Will B, Zhou L, Vogler TO, Ben-Neriah S, Schinke C, Tamari R, et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood. 2012 Sep 6;120(10):2076–2086. doi: 10.1182/blood-2011-12-399683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias HK, Schinke C, Bhattacharyya S, Will B, Verma A, Steidl U. Stem cell origin of myelodysplastic syndromes. Oncogene. 2014 Oct 30;33(44):5139–5150. doi: 10.1038/onc.2013.520. [DOI] [PubMed] [Google Scholar]
- 44.Babovic S, Eaves CJ. Hierarchical organization of fetal and adult hematopoietic stem cells. Experimental cell research. 2014 Dec 10;329(2):185–191. doi: 10.1016/j.yexcr.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006 Oct;133(19):3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 46.Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Veterinary pathology. 2012 May;49(3):508–523. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- 47.Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. Journal of hepatology. 2008 Mar;48(3):504–512. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 48.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. The Journal of biological chemistry. 2001 Nov 23;276(47):43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 49.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002 May 31;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogart A, Lichtenberg J, Ajay SS, Anderson S, Center NIHIS. Margulies EH, et al. Genome-wide DNA methylation profiles in hematopoietic stem and progenitor cells reveal overrepresentation of ETS transcription factor binding sites. Genome research. 2012 Aug;22(8):1407–1418. doi: 10.1101/gr.132878.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes & development. 2006 May 1;20(9):1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.