Abstract
Marrow cells from male mice pretreated with 5-fluorouracil were infected with helper-free neomycin-resistant (neor) recombinant retrovirus and then used to initiate long-term cultures (LTC) on irradiated adherent marrow feeder layers. Four weeks later LTC cells were harvested and injected into lethally irradiated female recipients either alone or together with 2 x 10(5) female marrow cells with selectively compromised long-term repopulating potential to assay for totipotent and competitive repopulating units (CRU), respectively. A total of 46 unique clones were detected in recipients 5 wk to 7 mo after transplant. Half of these clones (22 of 46) included both lymphoid and myeloid progeny. Eight of the 22 lympho-myeloid clones were represented in multiple recipients, in some cases after injection of limiting numbers of CRU, thus indicating repopulation from sibling totipotent stem cells generated during the initial 4-wk period in LTC. Serial analysis of cells released into the nonadherent fraction of LTC for up to 7 wk provided additional evidence of the continuing proliferation in LTC of totipotent stem cells with long-term repopulating potential. The frequency of CRU determined from limiting-dilution analyses of LTC-derived cells was the same for recipients analyzed at 5 wk or 7 mo after transplantation and was also the same whether marrow or thymus repopulation was assessed. These assays showed that concurrent with the expansion of some totipotent cells revealed by retroviral marking, there was a slow but net 6.5-fold decrease in total CRU numbers after 4 wk in LTC. These results show the capacity of some totipotent hematopoietic stem cells to be maintained and amplified over extensive time periods in vitro without diminution of their long-term in vivo repopulating potential. These results also set the stage for analogous studies of human stem cell selection and expansion in vitro, which may be important for future gene therapy protocols.
Full text
PDF

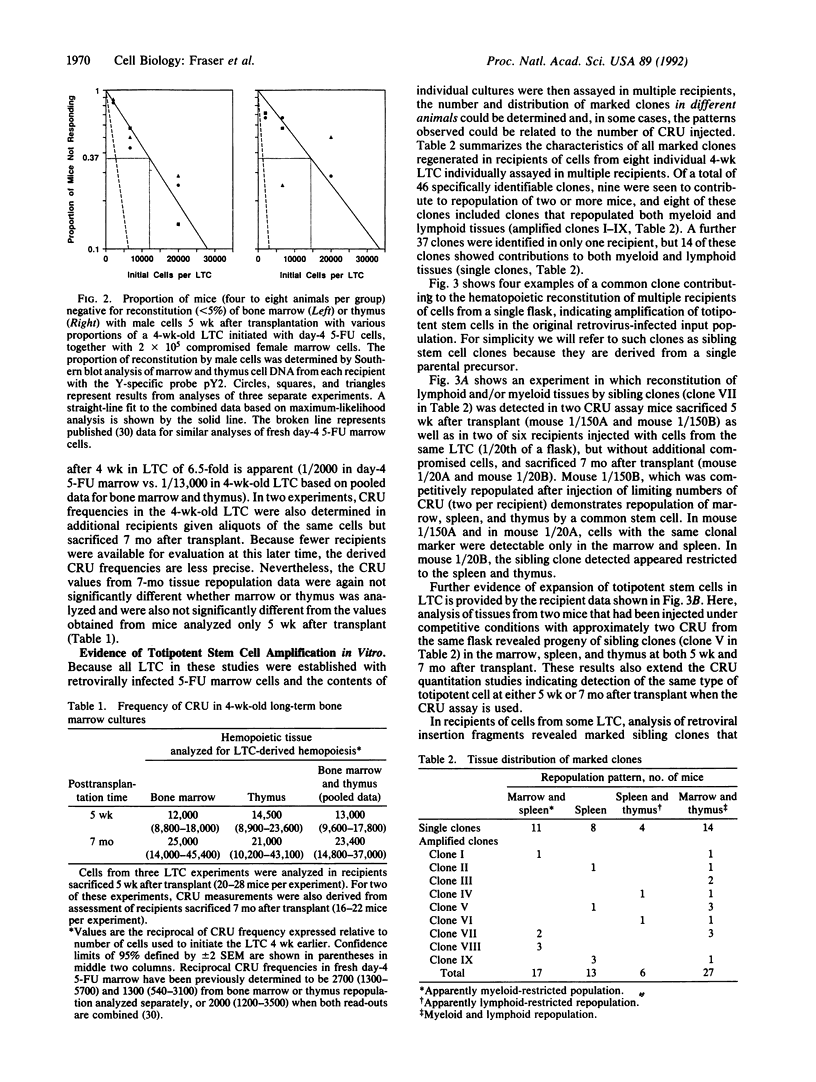
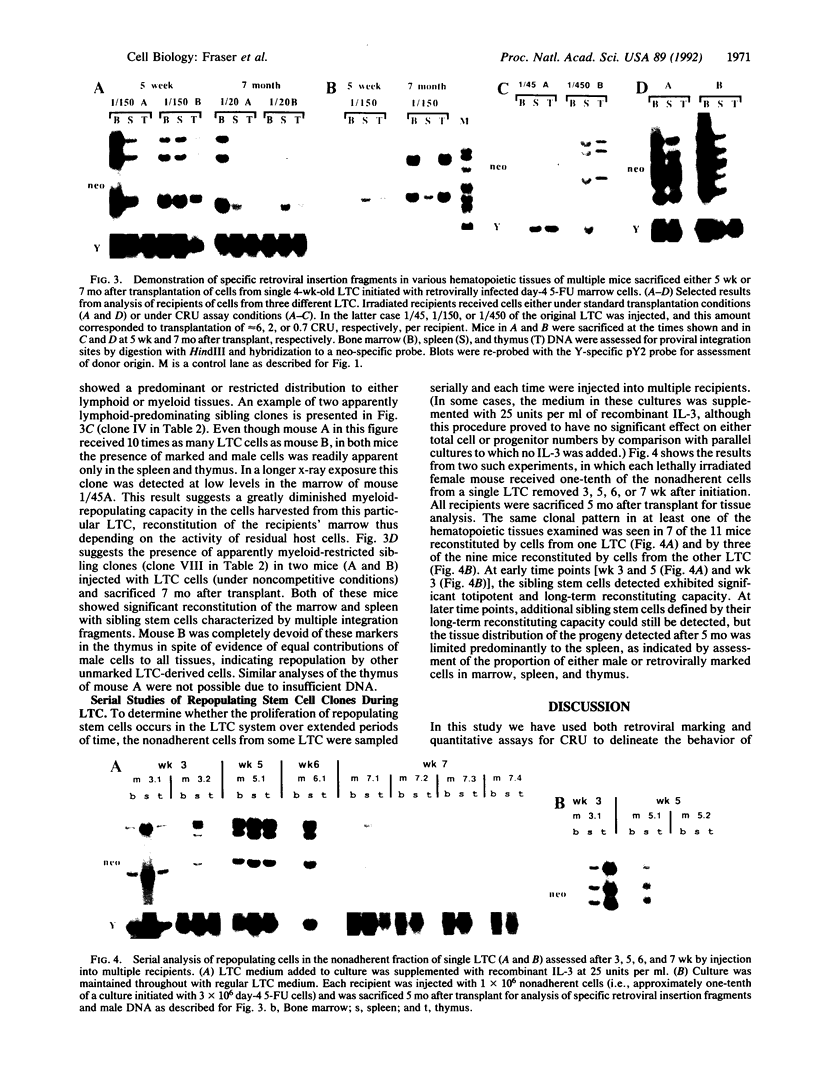

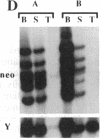
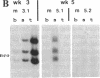
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. E., Braun J., McFarland-Starr E. C. Erythrocyte replacement precedes leukocyte replacement during repopulation of W/Wv mice with limiting dilutions of +/+ donor marrow cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7332–7335. doi: 10.1073/pnas.85.19.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUDKOWICZ G., UPTON A. C., SHEARER G. M., HUGHES W. L. LYMPHOCYTE CONTENT AND PROLIFERATIVE CAPACITY OF SERIALLY TRANSPLANTED MOUSE BONE MARROW. Nature. 1964 Jan 11;201:165–167. doi: 10.1038/201165a0. [DOI] [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Capel B., Hawley R., Covarrubias L., Hawley T., Mintz B. Clonal contributions of small numbers of retrovirally marked hematopoietic stem cells engrafted in unirradiated neonatal W/Wv mice. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4564–4568. doi: 10.1073/pnas.86.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. Y., Takei F. Molecular cloning and characterization of a novel murine T cell surface antigen, YE1/48. J Immunol. 1989 Mar 1;142(5):1727–1736. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Fraser C. C., Eaves C. J., Szilvassy S. J., Humphries R. K. Expansion in vitro of retrovirally marked totipotent hematopoietic stem cells. Blood. 1990 Sep 15;76(6):1071–1076. [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. Use of scid mice to identify and quantitate lymphoid-restricted stem cells in long-term bone marrow cultures. Blood. 1989 Oct;74(5):1537–1544. [PubMed] [Google Scholar]
- Harrison D. E., Lerner C. P., Spooncer E. Erythropoietic repopulating ability of stem cells from long-term marrow culture. Blood. 1987 Apr;69(4):1021–1025. [PubMed] [Google Scholar]
- Harrison D. E., Stone M., Astle C. M. Effects of transplantation on the primitive immunohematopoietic stem cell. J Exp Med. 1990 Aug 1;172(2):431–437. doi: 10.1084/jem.172.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S., Botnick L. E., Hannon E. C., Vigneulle R. M. Proliferative capacity of murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Jones R. J., Wagner J. E., Celano P., Zicha M. S., Sharkis S. J. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990 Sep 13;347(6289):188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar E. E., Palmer E. Y-encoded, species-specific DNA in mice: evidence that the Y chromosome exists in two polymorphic forms in inbred strains. Cell. 1984 May;37(1):171–177. doi: 10.1016/0092-8674(84)90312-x. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Mauch P., Greenberger J. S., Botnick L., Hannon E., Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci U S A. 1980 May;77(5):2927–2930. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch P., Hellman S. Loss of hematopoietic stem cell self-renewal after bone marrow transplantation. Blood. 1989 Aug 1;74(2):872–875. [PubMed] [Google Scholar]
- Mintz B., Anthony K., Litwin S. Monoclonal derivation of mouse myeloid and lymphoid lineages from totipotent hematopoietic stem cells experimentally engrafted in fetal hosts. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7835–7839. doi: 10.1073/pnas.81.24.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons R. H. Separation of CFU-S from primitive cells responsible for reconstitution of the bone marrow hemopoietic stem cell compartment following irradiation: evidence for a pre-CFU-S cell. Exp Hematol. 1989 Mar;17(3):263–266. [PubMed] [Google Scholar]
- Ross E. A., Anderson N., Micklem H. S. Serial depletion and regeneration of the murine hematopoietic system. Implications for hematopoietic organization and the study of cellular aging. J Exp Med. 1982 Feb 1;155(2):432–444. doi: 10.1084/jem.155.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Lansdorp P. M., Thacker J. D., Hogge D. E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991 Aug 1;78(3):666–672. [PubMed] [Google Scholar]
- Szilvassy S. J., Fraser C. C., Eaves C. J., Lansdorp P. M., Eaves A. C., Humphries R. K. Retrovirus-mediated gene transfer to purified hemopoietic stem cells with long-term lympho-myelopoietic repopulating ability. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8798–8802. doi: 10.1073/pnas.86.22.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Humphries R. K., Lansdorp P. M., Eaves A. C., Eaves C. J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Lansdorp P. M., Humphries R. K., Eaves A. C., Eaves C. J. Isolation in a single step of a highly enriched murine hematopoietic stem cell population with competitive long-term repopulating ability. Blood. 1989 Aug 15;74(3):930–939. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]
- Van Zant G., Chen J. J., Scott-Micus K. Developmental potential of hematopoietic stem cells determined using retrovirally marked allophenic marrow. Blood. 1991 Feb 15;77(4):756–763. [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]