Abstract
Aim:
The substrate cocktail is frequently used to evaluate cytochrome P450 (CYP) enzyme-mediated drug interactions and potential interactions among the probe substrates. Here, we re-optimized the substrate cocktail method to increase the reliability and accuracy of screening for candidate compounds and expanded the method from a direct CYP inhibition assay to a time-dependent inhibition (TDI) assay.
Methods:
In the reaction mixtures containing human liver microsome (0.1 mg/mL), both the concentrations of a substrate cocktail (phenacetin for 1A2, coumarin for 2A6, bupropion for 2B6, diclofenac for 2C9, dextromethorphan for 2D6, and testosterone for 3A4) and the incubation time were optimized. Metabolites of the substrate probes were simultaneously analyzed by multiple-reaction monitoring (MRM) using a routine LC/MS/MS. Direct CYP inhibition was validated using 7 inhibitors (α-naphthoflavone, tranylcypromine, ticlopidine, fluconazole, quinidine, ketoconazole and 1-ABT). The time-dependent inhibition was partially validated with 5 inhibitors (ketoconazole, verapamil, quinidine, paroxetine and 1-ABT).
Results:
The inhibition curve profiles and IC50 values of 7 CYP inhibitors were approximate when a single substrate and the substrate cocktail were tested, and were consistent with the previously reported values. Similar results were obtained in the IC50 shifts of 5 inhibitors when a single substrate and the substrate cocktail were tested in the TDI assay.
Conclusion:
The 6-in-1 substrate cocktail (for 1A2, 2A6, 2B6, 2C9, 2D6 and 3A) is reliable for assessing CYP inhibition and time-dependent inhibition of drug candidates.
Keywords: CYP inhibition, time-dependent inhibition, substrate cocktail, drug interaction
Introduction
Drug-Drug Interactions (DDIs) may restrict prescribing and significantly change the way a drug interacts with the body. For example, co-administration of itraconazole and tacrolimus or of leflunomide and warfarin can be dangerous, although individually, these drugs are safe1,2. Even worse, many drugs have been refused approval or have been withdrawn from the market by regulatory agencies, including mibefradil, terfenadine and cisapride3,4,5.
One major category of DDIs is the mechanism-based DDIs. Cytochrome P450 (CYP) enzymes are responsible for over 75% of the biotransformation of the top 200 drugs used in the US6. A change in the metabolic clearance of these drugs due to changes in the CYP activities can produce severe adverse reactions or a loss of efficacy when two or more drugs are co-administered. To avoid failures in the later stages of drug development or post-marketing, an evaluation of the effects of NMEs (new molecular entities) on CYP activities during the early stages of drug discovery is crucial. The FDA recommends an in vitro CYP inhibition assay for the 7 major human hepatic CYP isoforms: CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A7. Assessing the inhibition of other CYP enzymes (CYP2A6 and 2E1) involved in the metabolism of certain drugs and performing assays to examine significant ethnic differences are also recommended, especially for herbal medicines8,9,10.
Currently, substrate cocktails, which are mixtures of two or more probe substrates, have been popularized as in vitro screening assays to evaluate the inhibitory potency of NMEs in pharmaceutical industries in an attempt to reduce the costs and increase the efficiency of screening strategies. The challenges of using substrate cocktails are 1) the potential interactions among the probe substrates in the mixture; 2) the maintenance of substrate specificity and sensitivity to the enzymes; and 3) the limitations of liquid chromatography mass spectrometry. Significant research has focused on the CYP inhibition assay using substrate cocktails, and the number of probe substrates has increased from 5 (5 CYP isoforms) to 10 (9 CYP isoforms)11,12,13,14. However, the reported methods still have some flaws. For example, to recognize more probe metabolites from the mixtures, UPLC must be performed in combination with stable-labeled metabolites as the internal standard11 or the running time for concentration determination must be prolonged to 8 min, with a polarity switch for the positive and negative ion modes13; see table 1. The high expense of reagents and instruments and significant time investment that are needed greatly limit the throughput of these methods for industrial applications. In addition, the interference and transformation of probe substrates in metabolism can create complications. Amodiaquine, substrate of CYP2C8, produces a non-specific inhibition on other CYP isoforms11,14, and Otten et al tried to optimize this by lowering its concentration to 0.1 μmol/L, less than 10% of its Km value11,14. However, its biotransformation was higher than 50%, even at 5 min of incubation, which did not conform to the rule of “no more than 10%–30% substrate depletion,” as delineated in the FDA guidelines15. Therefore, the method of optimizing the substrate cocktail remains the best strategy for optimization.
Table 1. Comparison of the different assay conditions in reported methods.
| 5-in-112 | 7-in-114 | 9-in-111 | 10-in-113 | 6-in-1 (in our lab) | |
|---|---|---|---|---|---|
| Conc. of HLM | 0.1 mg/mL | 0.25 mg/mL | 0.2 mg/mL | 0.5 mg/mL | 0.1 mg/mL |
| Incubation Time | 10 min | 10 min | 5 min | 20 min | 10 min |
| Conc. of Substrate (μmol/L) 1A2 | 2 (Tacrine) | 50 (Phenacetin) | 20 (Phenacetin) | 4 (Melatonin) | 15 (Phenacetin) |
| 2A6 | / | / | 2 (Coumarin) | 2 (Coumarin) | 2.5 (Coumarin) |
| 2B6 | / | 50 (Bupropion) | 5 (Bupropion) | 1 (Bupropion) | 5 (Bupropion) |
| 2C8 | / | 0.1 (Amodiaquine) | 0.1 (Amodiaquine) | 2 (Amodiaquine) | / |
| 2C9 | 5 (Diclofenac) | 100 (Tolbutamide) | 1 (Diclofenac) | 4 (Tolbutamide) | 5 (Diclofenac) |
| 2C19 | 40 (S-Mephenytoin) | 120 (S-Mephenytoin) | 40 (S-Mephenytoin) | 2 (Omeprazole) | / |
| 2D6 | 5 (Dextromethorphan) | 5 (Dextromethorphan) | 5 (Bufuralol) | 0.2 (Dextromethorphan) | 5 (Dextromethorphan) |
| 2E1 | / | / | / | 6 (Chlorzoxazone) | / |
| 3A | 2 (Midazolam) | 5 (Midazolam) | 2 (Midazolam) | 0.4 (Midazolam) | / |
| / | / | 10 (Testosterone) | 1 (Testosterone) | 10 (Testosterone) | |
| LC | / | Binary Agilent 1100 | Waters ACQUITY | Waters 2695 Alliance | Shimadzu LC-20AD |
| HPLC | UPLC | HPLC | HPLC | ||
| MS/MS | Sciex API 4000 QTRAP | Sciex API 4000 | Waters Wevo TQ | Waters Micromass Quattro Micro API | Sciex API 4000 |
| Run Time | 1 min | 3 min | 1.5 min | 8 min | 5 min |
The time-dependent inhibition (TDI) assay can be used to determine whether the inhibition of an enzyme by a test article is time-dependent. Time-dependent inhibitors more frequently cause DDIs. The procedure of the TDI assay is more complex than that of the inhibition assay and always uses a single probe substrate, which limits the use of the TDI assay for massive candidate compounds in the early stages of drug discovery. Only a few reports have addressed the application of the substrate cocktail method for TDI, although it will likely be useful for lowering the expense and increasing the throughput of this method.
In this study, a new substrate cocktail approach was optimized and validated to increase the reliability and accuracy when screening candidate compounds and to expand the method to the TDI assay.
Materials and methods
Chemicals and reagents
Phenacetin, acetaminophen, testosterone, coumarin, 7-hydroxycoumarin, hydroxybupropion, amodiaquine, paclitaxel, dextromethorphan, chlorzoxazone, 6β-hydroxytestosterone, α-naphthoflavone, tranylcypromine, quinidine, 1-aminobenzotriazole(1-ABT) and tolbutamide were obtained from Sigma-Aldrich (St Louis, MO, USA). Bupropion, diclofenac and fluconazole were obtained from Tokyo Chemical Industry (Tokyo, Japan). Paclitaxel, 6α-hydroxypaclitaxel, N-desethylamodiaquine, 4′-hydroxydiclofenac, (S)-mephenytoin, hydroxymephenytoin, dextrophan, hydroxychlorzoxazone and (S)-(+)-N-3-benzylnirvanol were purchased from Toronto Research Chemicals (North York, ON, Canada). Midazolam was obtained from the National Institutes for Food and Drug Control (Shanghai, China). 1-Hydroxymidazolam was from the Cerilliant Corporation (Round Rock, TX, USA). Ketoconazole was obtained from CiviChem & Applications (Shanghai, China).
The pooled human liver microsomes (HLM, 20 mg/mL) of 200 donors were obtained from Xenotech (Lenexa, KS, USA). D-glucose 6-phosphate (G6P) and β-nicotinamide adenine dinucleotide phosphate (NADP) were obtained from Chem-Impex International (Wood Dale, IL, USA). Glucose-6-phosphate dehydrogenase (G6PDH) was purchased from Sigma-Aldrich (St Louis, MO, USA). Potassium phosphate buffer (pH 7.4) and magnesium chloride (MgCl2) were analytical reagents. HPLC-grade methanol, acetonitrile (ACN) and formic acid were provided by Merck KGaA (Darmstadt, Germany). Purified water was made in-house using a Millipore Ultrapure water system with a resistivity of 18.2 MΩ·cm.
CYP enzyme reaction system
All experiments were performed in 100 μL reaction mixtures, containing 0.1 mg protein/mL of the HLM, NADPH regeneration system (1.2 mmol/L NADP, 2.4 mmol/L G6P and 1.2 U/mL G6PDH), 2.88 mmol/L MgCl2, 0.1 mol/L potassium phosphate buffer (pH 7.4), and probe substrates for each CYP (15 μmol/L phenacetin for 1A2, 2.5 μmol/L coumarin for 2A6, 5 μmol/L bupropion for 2B6, 2 μmol/L amodiaquine/10 μmol/L paclitaxel for 2C8, 5 μmol/L diclofenac for 2C9, 40 μmol/L S-mephenytoin for 2C19, 5 μmol/L dextromethorphan for 2D6, 40 μmol/L chlorzoxazone for 2E1, and 10 μmol/L testosterone/3 μmol/L midazolam for 3A). The concentrations of DMSO and methanol in the reaction system were lower than 0.1%. A single probe substrate or a cocktail of probe substrates, whose concentrations were determined based on their Km values (70 μmol/L phenacetin, 1 μmol/L coumarin, 100 μmol/L bupropion, 3 μmol/L amodiaquine, 10 μmol/L diclofenac, 30 μmol/L S-mephenytoin, 10 μmol/L dextromethorphan, 100 μmol/L chlorzoxazone, 1.5 μmol/L midazolam and 40 μmol/L testosterone) in preliminary experiments, and the substrate cocktail concentrations in the literature were studied (Table 1).
Time course assays were performed at 5, 10, 15 and 20 min at 37 °C. The incubation was terminated with acetonitrile using 100 ng/mL tolbutamide as the internal standard. The samples were determined using LC/MS/MS.
Microsomal protein linearity was determined by plotting data points at 10-min intervals with incubation at 4 protein concentrations (0.05, 0.1, 0.2 and 0.3 mg/mL).
Validation of direct CYP inhibition
Six selective CYP inhibitors (α-naphthoflavone for 1A2, tranylcypromine for 2A6, ticlopidine for 2B6, fluconazole for 2C9 and 3A4, quinidine for 2D6, ketoconazole for 3A4 and a non-specific CYP inhibitor for 1-ABT) at 7 concentrations were used as positive controls for incubation with a single substrate or a substrate cocktail at 37 °C for 10 min with an optimized concentration (15 μmol/L phenacetin, 2.5 μmol/L coumarin, 5 μmol/L bupropion, 5 μmol/L diclofenac, 5 μmol/L dextromethorphan, 10 μmol/L testosterone).
Validation of time-dependent inhibition
1-ABT was selected to evaluate the effectiveness of the cocktail system because 1-ABT is also a time-dependent inhibitor for the 6 CYP isoforms16,17. Two known reversible CYP2D6 and 3A inhibitors (quinidine & ketoconazole) and 2 irreversible CYP2D6 and 3A inhibitors (paroxetine & verapamil) were selected for the comparison between the 6-in-1 substrate cocktail and the single probe substrate. All inhibitors were pre-incubated with HLM (0.2 mg/mL) in the presence and absence of the NADPH-regeneration system (1.2 mmol/L NADP, 2.4 mmol/L G6P and 1.2 U/mL G6PDH) for 30 min18. An aliquot (2-fold dilution) was transferred to a secondary incubation containing the 6-in-1 substrate cocktail (15 μmol/L phenacetin, 2.5 μmol/L coumarin, 5 μmol/L bupropion, 5 μmol/L diclofenac, 5 μmol/L dextromethorphan, 10 μmol/L testosterone) or a single probe substrate, which was then incubated for 10 min.
LC-MS/MS conditions
The sample concentrations were determined using an API 4000 mass spectrometer (Applied Biosystems, Concord, Ontario, Canada) in the positive electro-spray ionization (ESI) mode, linked to a LC-20AD HPLC (Shimadzu, Kyoto, Japan). Briefly, the separation was achieved using a 4 μm, 30×2 mm Synergi Hydro-RP C18, 80A column (Phenomenex, Torrance, CA, USA). The mobile phase was water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The gradient eluted program consisted of the following: 0 to 0.3 min, 5% (B); 0.3 to 3 min, 5%–40% (B); 3 to 4 min, 40%–90% (B); 4 to 4.5 min, 90% (B); 4.5 to 5 min, 90%–5% (B). The flow rate was set at 0.4 mL/min. The injection volume was 10 μL. Data were collected and processed using the Analyst Software (version 1.6.1, Applied Biosystems/MDS SCIEX). The metabolites of the probe CYP substrates and IS were analyzed in the multiple-reaction monitoring (MRM) mode. The details of the MRM transitions and mass spectrometry parameters are shown in Table 2.
Table 2. MRM transitions and mass spectrometry parameters for metabolites of CYP probe substrates.
| Compound | MRM Transition (m/z) |
DP | Collision energy (eV) | |
|---|---|---|---|---|
| Q1 Ion | Q3 Ion | |||
| Acetaminophen | 151.9 | 110.1 | 64 | 21 |
| 7-Hydroxycoumarin | 162.9 | 106.9 | 86 | 29 |
| Hydroxybupropion | 256.1 | 238 | 61 | 18 |
| 4-Hydroxydiclofenac | 312 | 231 | 32 | 29 |
| Dextrophan | 258.1 | 157 | 103 | 60 |
| 1-Hydroxymidazolam | 342.2 | 203 | 52 | 36 |
| 6β-Hydroxytestosterone | 305.2 | 269.3 | 80 | 19 |
| Tolbutamide (IS) | 271.3 | 155 | 70 | 26 |
Data analysis
The negative control was prepared by replacing the inhibitor with solvent. The amount of metabolite was transformed to the percent (%) of the negative control and plotted versus the inhibition concentration. The IC50 values were analyzed by nonlinear regression against four-parameter logistic equations using SigmaPlot v11.0 (Systat Software Inc, San Jose, CA, USA).
The IC50 determination was accomplished using the following formula:
where Emin is the minimum % inhibition, Emax is the maximum % inhibition, and I is the concentration of the inhibitor. The Hillslope value should be in the range of 0.5–1.5.
For the TDI assays, the IC50 fold-shift is the shift ratio of the IC50 values obtained pre-incubation with and without NADPH.
Results
LC-MS/MS method
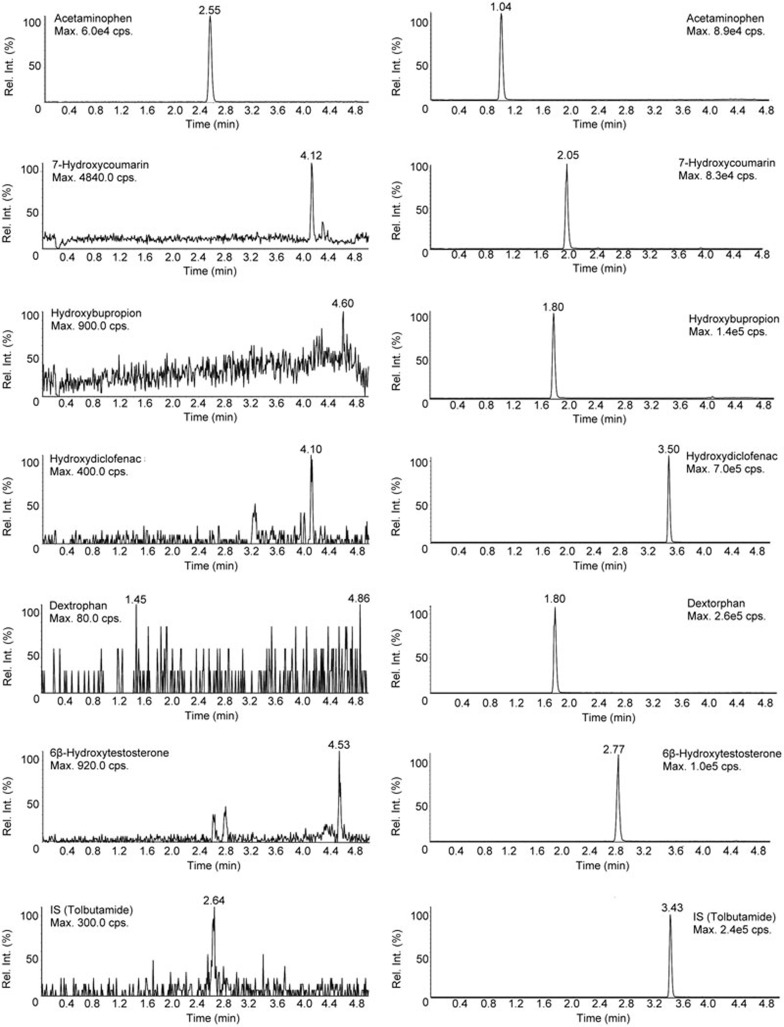
Usually, the cocktail method is recommended for a screening assay, which can then be semi-quantitative. IC50 values can be calculated by comparing the peak area of the metabolite with the different inhibitor concentrations (including a condition with no inhibitor). To evaluate the sensitivity, reliability and specificity of the LC/MS/MS method, the limit of detection, linearity of standard curve, accuracy and precision for each probe metabolite were validated. An eight-point calibration curve was plotted with the ratio of the metabolite and the IS peak area versus the metabolite concentration by weighted (1/x2) linear regression analysis. The values of the coefficient correlation (R) are shown in Table 3. The results of a typical chromatograph are shown in Figure 1 and indicate that the response of endogenous compounds co-eluted with the analyte had no effect on determining the metabolites in the current conditions. Hence, the current LC-MS/MS method was found to be suitable for determining the 6 CYP probe metabolites in the cocktail system.
Table 3. Calibration ranges and types of regression for substrate metabolites.
| Enzyme | Metabolite | R2 value | Weighting | Range (nmol/L) |
|---|---|---|---|---|
| CYP1A2 | Acetaminophen | 0.998 | 1/X2 | 4.69–600 |
| CYP2A6 | 7-Hydroxycoumarin | 0.996 | 1/X2 | 15.6–2000 |
| CYP2B6 | Hydroxybupropion | 0.997 | 1/X2 | 0.78–100 |
| CYP2C9 | 4′-Hydroxydiclofenac | 0.996 | 1/X2 | 15.6–2000 |
| CYP2D6 | Dextrophan | 0.998 | 1/X2 | 3.13–400 |
| CYP3A4 | 6β-Hydroxytestosterone | 0.992 | 1/X2 | 7.81–1000 |
Figure 1.
The integration of MRM trace of each CYP-specific metabolite and internal standard (IS). The left is the blank matrix with cocktail substrates, and the right a representative HLM incubation sample.
Optimization of reaction conditions
To optimize the reaction conditions, the highest throughput method with a 9-in-1 substrate cocktail was first performed. The probe substrates and initial concentrations were selected based on the FDA draft guidelines15, literature values11,14 and Km values, which were generated in-house.
S-Mephenytoin (2C19 probe substrate) and paclitaxel (2C8 probe substrate) were excluded due to their low response in the LC/MS/MS detection. Chlorzoxazone (2E1 probe substrate) was excluded from the substrate cocktail because the response of its metabolite, 6-hydroxychlorzoxazone, was much lower under a positive mode of ionization than under a negative mode. Bupropion (2B6 probe substrate) showed a highly potent inhibition of other CYP isoforms at its Km value, so its concentration in the substrate cocktail was reduced to 1/30 of its Km value (5 μmol/L) to avoid the drug interaction among substrates.
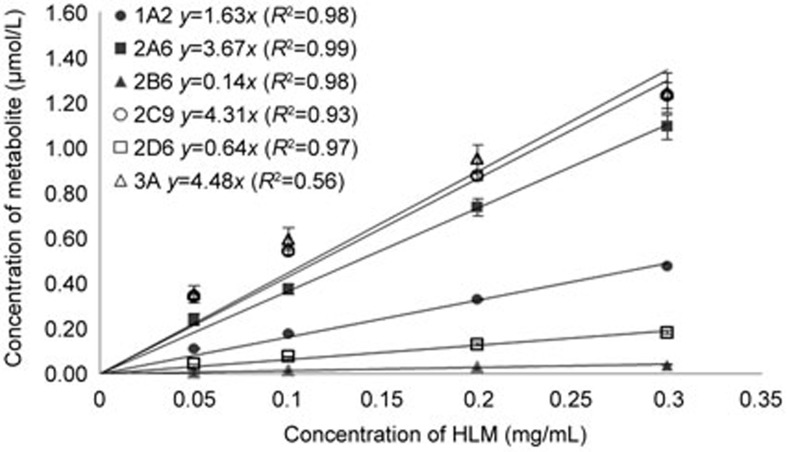
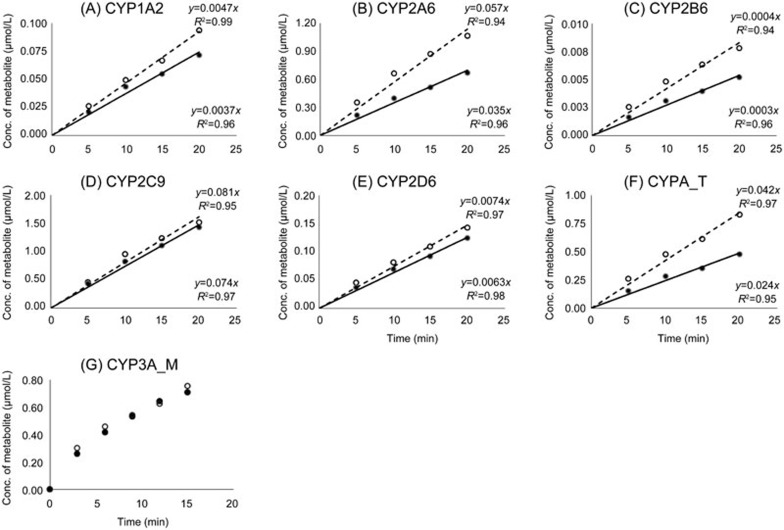
The plot of the protein concentration (mg/mL) vs the formed metabolite (μmol/L) is presented in Figure 2. Aside from CYP3A, the other 5 probe metabolites of the CYPs showed a good linearity relationship from 0.05 to 0.3 mg/mL HLM at 10 min of incubation. The relationship between the metabolite formation and the use of either a single substrate or the substrate cocktail is shown in Figure 3, and the depletion of each substrate was found to be less than 20% after a 10-min incubation.
Figure 2.
HLM concentration vs metabolite formed after 10 min incubation (1A2: acetaminophen; 2A6: 7-hydroxycoumarin; 2B6: hydroxybupropion; 2C9: 4-hydroxydiclofenac; 2D6: dextrophan; 3A: 6β-hydroxytestosterone). Metabolite formation is represented as mean±SD of a single experiment run in triplicate.
Figure 3.
Time course experiment, incubation time vs concentration of metabolite formed for single substrate (○, ...) or 9-in-1 cocktail (•, ─). (A) acetaminophen, (B) 7-hydroxycoumarin, (C) hydroxybupropion, (D) 4-hydroxydiclofenac, (E) Dextrophan, (F) 6β-hydroxytestosterone, (G) 1-hydroxymidazolam. Metabolite formation is represented as mean of a single experiment run in triplicate.
Finally, a cocktail of six probe substrates (15 μmol/L phenacetin, 2.5 μmol/L coumarin, 5 μmol/L bupropion, 5 μmol/L diclofenac, 5 μmol/L dextromethorphan and 10 μmol/L testosterone) was selected for a 10-min incubation with 0.1 mg protein/mL of HLM as the optimized condition. Comparison of the CYP activities obtained from the 2 approaches as performed on 3 different days showed that all 6 CYP activities in the 6-in-1 cocktail were in the range of 83%–104% of those in the single probe substrate reactions (Table 4).
Table 4. Comparison of CYP activities between single substrate and 6-in-1 cocktail. Mean±SD.
| CYP activity (pmol·min−1·mg−1) |
Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 (n=3) | Day 2 (n=3) | Day 3 (n=3) | |||||
| 6-in-1 | Single | 6-in-1 | Single | 6-in-1 | Single | ||
| 1A2 | 186.1±14.2 | 165.5±14.8 | 201±7.8 | 191±4.2 | 189.7±16.1 | 201±12.7 | 104% |
| 2A6 | 488.1±32.2 | 561.5±50.2 | 532.6±22.5 | 584.5±6.4 | 487.9±27.4 | 515±18.4 | 91% |
| 2B6 | 10.1±0.7 | 14.8±0.4 | 10.4±0.6 | 13.6±0.1 | 12.3±1.4 | 11.7±0.6 | 83% |
| 2C9 | 836±38.9 | 807±33.9 | 829.6±51.5 | 916.5±108.2 | 583.3±29.8 | 623.5±50.2 | 96% |
| 2D6 | 87.8±4.1 | 109±0 | 75.8±2.9 | 84.3±4.3 | 82.2±7.2 | 88.1±4.5 | 88% |
| 3A4_T | 705.6±42.2 | 665.3±52.2 | 617±18.6 | 653±44.9 | 643.9±39.8 | 663.3±20.4 | 99% |
Validation of the experimental system
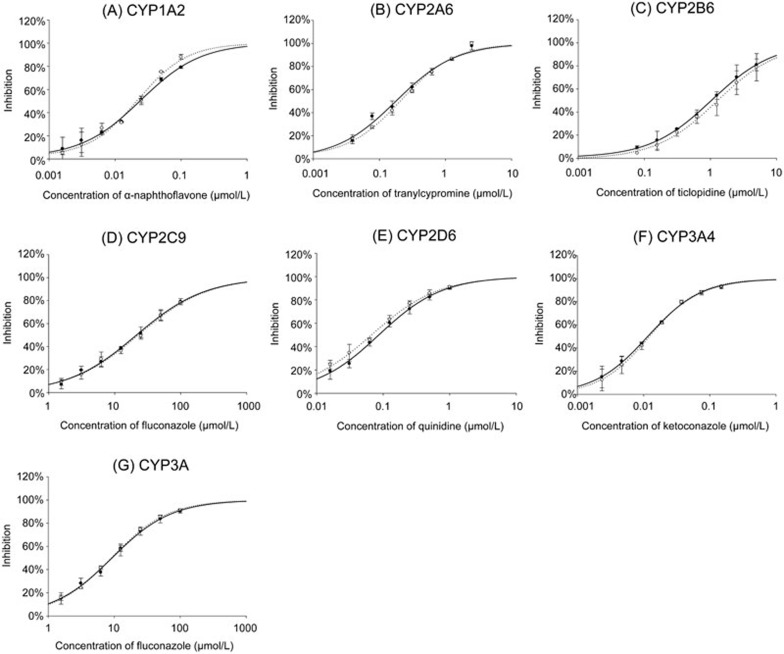
To validate the above method, six known specific inhibitors listed in the FDA guidelines in 201215, ie, α-naphthoflavone, tranylcypromine, ticlopidine, fluconazole, quinidine, and ketoconazole, and 1-aminobenzotriazole (1-ABT), as a non-specific CYP inhibitor, were used to compare the IC50 values between the single substrates and the substrate cocktail17. The inhibition curves obtained from these 2 approaches are shown in Figure 4, and the IC50 values are shown in Table 5. The results of single substrates were found to be consistent with the published values14,17,19,20,21,22,23 and correlated well with the results from the 6-in-1 substrate cocktail.
Figure 4.
Inhibition curves of CYP-specific inhibitors using single substrate (○, ...) or 6-in-1 cocktail substrate (•, ─). The inhibition is expressed as percentage of decline of enzyme activity (no inhibitor control). The results are the means of triplicate experiments in different days. (A) Inhibition of phenacetin O-deethylation by α-naphthoflavone. (B) Inhibition of coumarin hydroxylation by tranylcypromine. (C) Inhibition of bupropion hydroxylation by ticlopidine. (D) Inhibition of diclofenac 4′-hydroxylation by fluconazole. (E) Inhibition of dextromethorphan O-demethylation by quinidine. (F) and (G) Inhibition of testosterone 6β-hydroxylation by ketoconazole and fluconazole. Percentage inhibition is represented as mean±SD of a single experiment run in triplicate.
Table 5. Comparison of IC50 values between single substrate and 6-in-1 cocktail.
| Inhibitor | CYP enzyme | IC50 value (μmol/L) |
Ratio | IC50 value (μmol/L) Literature15,18,20,21,22,23,24 | |
|---|---|---|---|---|---|
| 6-in-1 | Single | ||||
| α-Naphthoflavone | 1A2 | 0.025±0.0001 | 0.022±0.0021 | 114% | 0.04–0.12 |
| Tranylcypromine | 2A6 | 0.180±0.027 | 0.21±0.013 | 86% | 0.2–0.6 |
| Ticlopidine | 2B6 | 1.03±0.14 | 0.67±0.25 | 153% | 0.33–0.78 |
| Fluconazole | 2C9 | 21.7±4.6 | 22.8±2.1 | 95% | 30.3 |
| 3A4 | 9.2±1.6 | 9.0±0.6 | 102% | 13.1 | |
| Quinidine | 2D6 | 0.087±0.017 | 0.068±0.013 | 128% | 0.09–0.27 |
| Ketoconazole | 3A4 | 0.011±0.0014 | 0.011±0.0021 | 94% | 0.01–0.03 |
| 1-ABT | 1A2 | 444.7±69.4 | 673.4±19.7 | 66% | 340, >1000 |
| 2A6 | 58.0±8.3 | 57.1±2.3 | 102% | 282 | |
| 2B6 | 1178.5±41.5 | 921.6±108.0 | 128% | >1000 | |
| 2C9 | 1418.9±194.0 | 1498.5±97.7 | 95% | >1000 | |
| 2D6 | 215.1±13.4 | 655.3±137.3 | 33% | 120, >1000 | |
| 3A4 | 6.5±1.2 | 7.6±1.7 | 85% | 0.58 | |
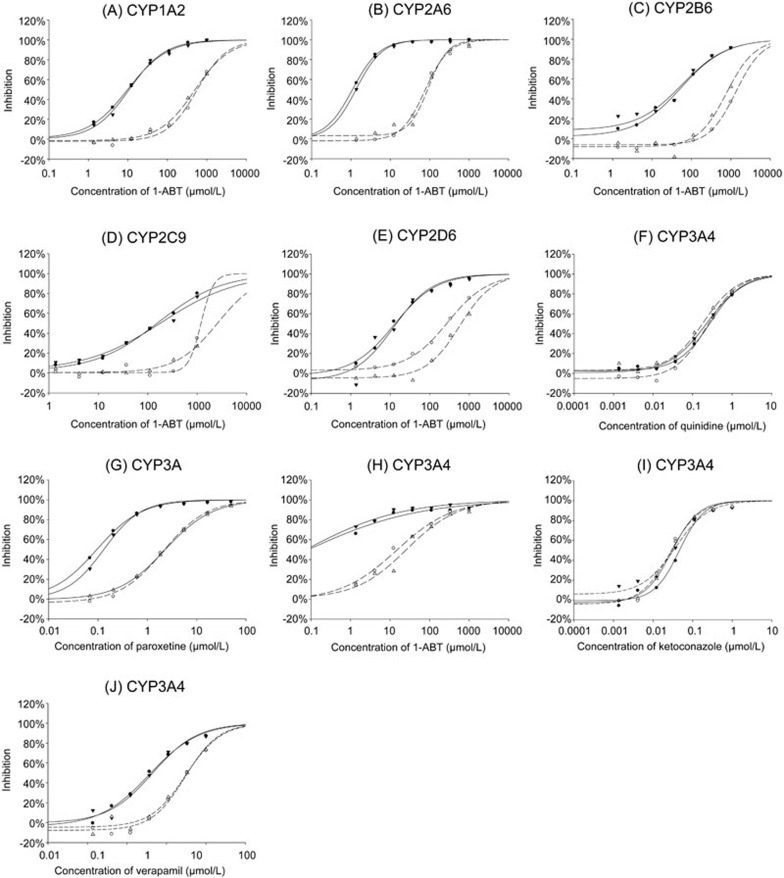
A comparison of the results of the reversible and irreversible inhibitors using the 6-in-1 cocktail and single probe substrate in the TDI assay are shown in Figure 5 and Table 6. The fold shift of IC50 for the reversible inhibitors (quinidine and ketoconazole) was less than 1, and there was a strong correlation between the results of the 6-in-1 substrate cocktail and the single substrates. However, the pre-incubation IC50 curve of the irreversible inhibitors (paroxetine and verapamil) with NADPH shifted towards the left side. The ratio of the verapamil IC50 values without NADPH compared to those with NADPH were 8.2- and 6.9-fold higher relative to the 6-in-1 substrate cocktail and testosterone, respectively. The ratio of the paroxetine IC50 values without NADPH against those with NADPH was 24- and 15.9-fold higher in the 6-in-1 substrate cocktail and dextromethorphan, respectively. The non-selective CYP inhibitor 1-ABT also showed a significant IC50 fold-shift (the IC50 fold-shifts of all 6 P450s were more than 5), indicating that 1-ABT is a potential time-dependent inhibitor24. Furthermore, the IC50 fold-shift between the single substrate and cocktail approach showed good correlation.
Figure 5.
The comparison of the shifted IC50 curves of time-dependent inhibition (TDI) using 6-in-1 cocktail and single probe substrate. •: 6-in-1 cocktail substrate pre-incubation with NADPH, ○: 6-in-1 cocktail substrate pre-incubation without NADPH, ▾: Single substrate pre-incubation with NADPH; Δ: Single substrate pre-incubation without NADPH. (A) TDI of phenacetin O-deethylation by 1-ABT; (B) TDI of coumarin hydroxylation by 1-ABT; (C) TDI of bupropion hydroxylation by 1-ABT; (D) TDI of diclofenac 4′-hydroxylation by 1-ABT; (E) TDI of dextromethorphan O-demethylation by 1-ABT; (F) TDI of dextromethorphan O-demethylation by quinidine; (G) TDI of dextromethorphan O-demethylation by paroxetine; (H) TDI of testosterone 6β-hydroxylation by 1-ABT; (I) TDI of testosterone 6β-hydroxylation by ketoconazole; (J) TDI of testosterone 6β-hydroxylation by verapamil. Percentage inhibition is represented as mean of a single experiment run in duplicate.
Table 6. Comparison of IC50 shift of reversible and irreversible inhibitors using 6-in-1 cocktail and single substrate.
| Inhibitor | Substrate | Pre-incubation with NADPH |
Pre-incubation w/o NADPH |
IC50 fold-shift | ||
|---|---|---|---|---|---|---|
| IC50 | Hill slope | IC50 | Hill slope | |||
| Ketoconazole | 6-in-1 cocktail | 0.0458±0.0092 | −1.39 | 0.0271±0.0057 | −1.16 | 0.59 |
| Testosterone | 0.0367±0.0099 | −1.04 | 0.0293±0.0041 | −1.29 | 0.80 | |
| Verapamil | 6-in-1 cocktail | 3.66±1.32 | −0.70 | 29.8±2.15 | −1.02 | 8.2 |
| testosterone | 4.45±2.10 | −0.77 | 30.8±3.06 | −0.98 | 6.9 | |
| Quinidine | 6-in-1 cocktail | 0.283±0.085 | −1.06 | 0.227±0.046 | −0.921 | 0.80 |
| Dextromethorphen | 0.230±0.105 | −1.09 | 0.189±0.080 | −1.02 | 0.82 | |
| Paroxetine | 6-in-1 cocktail | 0.095±0.0060 | −0.961 | 2.30±0.068 | −0.908 | 24.2 |
| Dextromethorphen | 0.132±0.0093 | −1.15 | 2.10±0.304 | −0.976 | 15.9 | |
| 1-ABT | 6-in-1 cocktail | 10.0±0.73 | −0.869 | 551.9±47.0 | −1.14 | 55.0 |
| Phenacetin | 10.8±1.69 | −0.956 | 492.9±34.2 | −0.977 | 45.6 | |
| 6-in-1 cocktail | 1.10±0.063 | −1.26 | 76.3±7.91 | −1.45 | 69.1 | |
| Coumarin | 1.35±0.082 | −1.31 | 92.6±16.0 | −1.76 | 68.6 | |
| 6-in-1 cocktail | 54.3±19.3 | −0.762 | >1000 | NA | >10 | |
| Bupropion | 57.7±4.26 | −0.758 | 779.9±66.1 | −1.21 | >10 | |
| 6-in-1 cocktail | 162.7±12.5 | −0.676 | >1000 | NA | >5 | |
| Diclofenac | 191.4±23.4 | −0.563 | >1000 | NA | >5 | |
| 6-in-1 cocktail | 12.6±2.20 | −0.816 | 285.0±13.1 | −0.854 | 22.6 | |
| Dextromethorphen | 12.2±6.77 | −0.884 | 568.2±75.3 | −1.03 | 46.8 | |
| 6-in-1 cocktail | <1 | NA | 14.7±4.16 | −0.613 | >10 | |
| Testosterone | <1 | NA | 26.5±14.2 | −0.670 | >10 | |
Discussion
An easy and reliable substrate cocktail system was optimized to evaluate the effects of NCEs on 6 CYPs (through direct or time-dependent inhibition): CYP1A2, 2A6, 2B6, 2C9, 2D6 and 3A in one reaction. The objective of this substrate cocktail assay was to establish a faster, higher capacity and lower cost way to assess CYP inhibition potential at the early stages of drug discovery and development and to provide a medical chemistry tool to researchers that provides information on the structure-activity relationship (SAR) and re-designs and synthesizes preferable NCEs.
In Table 1, the different assay conditions of the published literature and those of our lab were compared. The protein concentration of HLM used in our lab was lowest (0.1 mg/mL) to minimize the non-special protein binding of HLM and probe substrates. The concentration of probe substrates was also reduced to avoid compound-compound interactions. In the optimized assay conditions, all 6 metabolite concentrations were more than 10-fold the amount of LLOQ (Tables 3 and 4) to provide sufficient sensitivity for the measurement of the inhibition of the enzyme activity. Without the use of UPLC11 or QTRAP12, we utilized a highly selective and sensitive 6-in-1 method for the reversible and irreversible CYP inhibition assay. At the same time, the injection time was limited to 5 min to save time and money.
Due to the presence of multiple substrate-binding sites on the CYP3A enzyme, two or more specific CYP3A substrates were recommended for the evaluation of CYP3A inhibition because of the multiple substrate-binding sites in the CYP3A enzyme15. Based on the published literature, two CYP3A probe substrates (midazolam and testosterone) were added to the substrate cocktail11,18. However, comparing the CYP activities of the single substrate to those of the 7-in-1 and 6-in-1 cocktails (7-in-1 cocktail contained 2 μmol/L midazolam, whereas the 6-in-1 cocktail did not), the addition of midazolam had a significant effect on the activity of 2B6 (7.9±1.4 pmol·min−1·mg−1 of 7-in-1, 10.9±1.2 pmol·min−1·mg−1 of 6-in-1 and 13.4±1.6 pmol·min−1·mg−1 of single) and on the metabolism of the other 3A4 probe substrate testosterone (478.0±28.8 pmol·min−1·mg−1 of 7-in-1, 655.5±45.4 pmol·min−1·mg−1 of 6-in-1 and 660.5±40.2 pmol·min−1·mg−1 of single). At the same time, the activity of 3A4 with midazolam as a substrate was 424.0±19.3 pmol·min−1·mg−1 in the 7-in-1 cocktail but 708.8±8.8 pmol·min−1·mg−1 in the single substrate condition. This is likely because there is a mutual site for all CYP3A4 probe substrates, thus allowing the partial cross-inhibition of the hydroxylation pathways of the other substrates25. Therefore, only testosterone was added into the cocktail in this research, unlike the conditions used in previous reports11,18.
A good correlation between the 6-in-1 cocktail and the single probe was shown both in the CYP activity (Table 4) and the IC50 values of the known specific and non-specific CYP inhibitors, containing time-dependent inhibitors (Tables 5 and 6, with a linear regression equation y=1.03x1.02, R2=0.99). The difference in the IC50 values in the cocktail and single substrate approaches was within a 2-fold range. The IC50 ratio of 1-ABT on CYP2D6 between 2 approaches was equal and more than 2 in the multiple assays (Table 5, 215.1 vs 655.3; Table 6, 285.0 vs 568.2). However, all of the IC50 values from the 2 approaches were more than 200 μmol/L, which had little effect on the judgment of the compound's characters.
The IC50 values obtained in the single substrate and 6-in-1 substrate cocktail approaches were in a wide range, from 0.01–1000 μmol/L, showing that the new experimental system may be used to evaluate the potential CYP inhibition of test articles across 6 CYP isoforms reliably.
Usually, selective inhibitors for each CYP isoform are used to validate the cocktail approach14, but these compounds or drugs always inhibit at least two enzymes simultaneously. Thus, the non-specific inhibitor 1-ABT was used to examine our substrate cocktail for the direct inhibition assay and time-dependent inhibition assay. Further, 1-ABT can also irreversibly deactivate almost all major CYP enzymes that are involved in metabolism of xenobiotics16,17. In addition to 1-ABT, several known specific reversible and irreversible inhibitors were selected to test these two approaches.
In the TDI assay, a 6-in-1 cocktail system may also distinguish the known reversible and irreversible inhibitors accurately. Inhibition curves plotted with data generated from the 6-in-1 cocktail system nearly coincided with those from the single probe system (Figure 5). Measuring the TDI parameters kinact/KI is a time-consuming and labor-intensive process. Usually, Kobs, kinact and KI need not be determined unless the test compounds are proven to cause a IC50-shift in the time-dependent inhibition assay. Considering the potential risks of the complex inhibition between unknown drugs with a substrate cocktail, the 6-in-1 approach is more suitable for initial screening than the determination of inhibition parameters. Thus, the most reliable single-substrate approach should be applied to calculate inhibition constants.
In conclusion, an improved cocktail approach was validated to measure the activity of 6 CYP isoforms to establish a rapid and low-cost method for direct CYP inhibition and TDI assays, thus providing an easier and more reliable evaluation system for assessing the drug-drug interactions of 6 major CYPs.
Author contribution
Zhong-hua CHEN and Su-xing ZHANG designed the research; Na LONG, Xue-qin LV and Pei-zhen YE performed the experiments; Ning LI contributed some reagents and analytic tools; Li-shan LIN, Tao CHEN and Fei-peng ZHANG analyzed the data; Ke-zhi ZHANG and Su-xing ZHANG wrote the paper.
Acknowledgments
The Industry-Academia-Research Foundation of Chancheng, Foshan (No 2013B1009), and the Foundation of Foshan Innovation Team (No 2014IT100031) provided the financial support for this study.
References
- Ideura T, Muramatsu T, Higuchi M, Tachibana N, Hora K, Kiyosawa K. Tacrolimus/itraconazole interactions: a case report of ABO-incompatible living-related renal transplantation. Nephrol Dial Transplant 2000; 15: 1721–3 [DOI] [PubMed] [Google Scholar]
- Chonlahan J, Halloran MA, Hammonds A. Leflunomide and warfarin interaction: case report and review of the literature. Pharmacotherapy 2006; 26: 868–71 [DOI] [PubMed] [Google Scholar]
- Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR. Terfenadine-ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences. JAMA 1993; 269: 1513–8 [PubMed] [Google Scholar]
- Krayenbuhl JC, Vozeh S, Kondo-Oestreicher M, Dayer P. Drug-drug interactions of new active substances: mibefradil example. Eur J Clin Pharmacol 1999; 55: 559–65 [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Bacsanyi J. Cisapride and fatal arrhythmia. N Engl J Med 1996; 335: 290–1 [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos 2004; 32: 1201–8 [DOI] [PubMed] [Google Scholar]
- Food U, Administration D. Guidance for industry drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations. Food Drug Administration: Rockville, MD 2012.
- Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 1992; 13: 1789–94. [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Guengerich FP. Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 1996; 26: 395–403. [DOI] [PubMed] [Google Scholar]
- Ueng YF, Chen CC, Chung YT, Liu TY, Chang YP, Lo WS, et al. Mechanism-based inhibition of cytochrome P450 (CYP)2A6 by chalepensin in recombinant systems, in human liver microsomes and in mice in vivo. Br J Pharmacol 2011; 163: 1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakai K, Yamada Y, Oshikata M, Kawase T, Suzuki E, Haramaki Y, et al. Reliable high-throughput method for inhibition assay of 8 cytochrome P450 isoforms using cocktail of probe substrates and stable isotope-labeled internal standards. Drug Metab Pharmacokinet 2012; 27: 520–9. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Lyons R, Payne L, Jones BC, Saunders K. An automated, high-throughput, 384 well cytochrome P450 cocktail IC50 assay using a rapid resolution LC-MS/MS end-point. J Pharm Biomed Anal 2008; 48: 92–9. [DOI] [PubMed] [Google Scholar]
- Tolonen A, Petsalo A, Turpeinen M, Uusitalo J, Pelkonen O. In vitro interaction cocktail assay for nine major cytochrome P450 enzymes with 13 probe reactions and a single LC/MSMS run: analytical validation and testing with monoclonal anti-CYP antibodies. J Mass Spectrom 2007; 42: 960–6. [DOI] [PubMed] [Google Scholar]
- Otten JN, Hingorani GP, Hartley DP, Kragerud SD, Franklin RB. An in vitro, high throughput, seven CYP cocktail inhibition assay for the evaluation of new chemical entities using LC-MS/MS. Drug Metab Lett 2011; 5: 17–24. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry-Drug Interaction Studies-Study Design. Data Analysis, and Implications for Dosing and Labelling 2006. [DOI] [PubMed]
- Ortiz de Montellano PR, Mathews JM. Autocatalytic alkylation of the cytochrome P-450 prosthetic haem group by 1-aminobenzotriazole. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem J 1981; 195: 761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder CD, Renaud NA, Hutzler JM. Is 1-aminobenzotriazole an appropriate in vitro tool as a nonspecific cytochrome P450 inactivator? Drug Metab Dispos 2009; 37: 10–3. [DOI] [PubMed] [Google Scholar]
- Kozakai K, Yamada Y, Oshikata M, Kawase T, Suzuki E, Haramaki Y, et al. Cocktail-substrate approach-based high-throughput assay for evaluation of direct and time-dependent inhibition of multiple cytochrome P450 isoforms. Drug Metab Pharmacokinet 2014; 29: 198–207. [DOI] [PubMed] [Google Scholar]
- Emoto C, Murase S, Sawada Y, Iwasaki K. In vitro inhibitory effect of 1-aminobenzotriazole on drug oxidations in human liver microsomes: a comparison with SKF-525A. Drug Metab Pharmacokinet 2005; 20: 351–7. [DOI] [PubMed] [Google Scholar]
- Dierks EA, Stams KR, Lim HK, Cornelius G, Zhang H, Ball SE. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab Dispos 2001; 29: 23–9. [PubMed] [Google Scholar]
- Bu HZ, Knuth K, Magis L, Teitelbaum P. High-throughput cytochrome P450 (CYP) inhibition screening via a cassette probe-dosing strategy. V. Validation of a direct injection/on-line guard cartridge extraction—tandem mass spectrometry method for CYP1A2 inhibition assessment. Eur J Pharm Sci 2001; 12: 447–52. [DOI] [PubMed] [Google Scholar]
- Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull 2005; 28: 1805–8. [DOI] [PubMed] [Google Scholar]
- Dinger J, Meyer MR, Maurer HH. Development of an in vitro cytochrome P450 cocktail inhibition assay for assessing the inhibition risk of drugs of abuse. Toxicol Lett 2014; 230: 28–35. [DOI] [PubMed] [Google Scholar]
- Grimm SW, Einolf HJ, Hall SD, He K, Lim HK, Ling KH, et al. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug Metab Dispos 2009; 37: 1355–70. [DOI] [PubMed] [Google Scholar]
- Galetin A, Clarke SE, Houston JB. Multisite kinetic analysis of interactions between prototypical CYP3A4 subgroup substrates: midazolam, testosterone, and nifedipine. Drug Metab Dispos 2003; 31: 1108–16. [DOI] [PubMed] [Google Scholar]