Abstract
Secreted proteins play critical roles in physiological and pathological processes and can be used as biomarkers and therapies for aging and disease. Metrnl is a novel secreted protein homologous to the neurotrophin Metrn. But this protein, unlike Metrn that is mainly expressed in the brain, shows a relatively wider distribution in the body with high levels of expression in white adipose tissue and barrier tissues. This protein plays important roles in neural development, white adipose browning and insulin sensitization. Based on its expression and distinct functions, this protein is also called Cometin, Subfatin and Interleukin 39, which refer to its neurotrophic effect, adipokine function and the possible action as a cytokine, respectively. The spectrum of Metrnl functions remains to be determined, and the mechanisms of Metrnl action need to be elucidated. In this review, we focus on the discovery, structural characteristics, expression pattern and physiological functions of Metrnl, which will assist in developing this protein as a new therapeutic target or agent.
Keywords: secreted proteins, Metrnl, Cometin, Subfatin, adipokine, neurotrophin, insulin sensitization, white adipose browning
Introduction
Secreted proteins play critical roles in physiological and pathological processes and can be used as biomarkers and therapies for aging and disease1,2,3,4,5,6,7. Because adipose tissue is the largest endocrine organ, we have focused on adipose-derived secreted proteins (also known as adipokines) and explored their functions over the past decade8,9,10,11,12,13,14,15,16. In addition to clarifying the roles and molecular mechanisms as well as possible clinical applications of known adipokines (such as NAMPT/Visfatin)15,16,17,18,19,20,21,22,23,24,25,26,27,28, we have made efforts to discover new adipokines. Most recently, we identified a novel adipokine, Metrnl, also known as Meteorin(Metrn)-like, Cometin and Subfatin, and revealed its insulin sensitizing action, which may translate it into a promising therapeutic target for insulin resistance29,30.
Metrnl is a protein homologous to the neurotrophic factor Metrn. Although the expression and functions of Metrn have been explored extensively, studies on Metrnl are quite limited. In this review, we summarize the discovery, structural characteristics, expression pattern, functions and mechanisms of action of Metrnl that have been reported to date.
Discovery of Metrnl
The human genome contains approximately 20 687 protein-coding genes31, but most of their expression patterns and functions remain unknown. To discover novel functional proteins, advanced bioinformatic techniques show great potential. Using these techniques, a number of research groups noticed the Metrnl gene before the subsequent identification of the protein29,32,33,34.
The Metrnl gene is located on mouse chromosome 11qE2 and human chromosome 17q25.329,35. Its specific location on the q-arm terminal end of human chromosome 17 has recently attracted attention because a cyto-molecular analysis of a case of ring 17 syndrome showed that the breakpoints are very close to the telomeric ends, thus making Metrnl a candidate gene that is potentially involved in some of the phenotypic features related to the ring chromosome 1733.
The protein homologous to Metrnl, Metrn, was reported by Nishino et al in 200436. Considering the obvious role of Metrn in the central nervous system and following the demonstration of the Metrnl gene being a new, direct target of PAX2/5/8 for otic development32, Jorgensen et al first described Metrnl protein and demonstrated its function as a neurotrophic factor similar to Metrn in 201235. Our lab screened for new adipokines in a global gene expression profiling of different adipose depots with bioinformatic methods in 2007 and early 2008. We identified Metrnl as a novel adipokine29. Although Jorgensen et al and we discovered Metrnl protein in entirely independent ways, both labs verified Metrnl as a secreted protein29,35.
Metrnl as a novel secreted protein homologous to Metrn
Bioinformatic analysis predicts that the Metrnl proteins encoded in the mouse, rat, and human genomes contain 311 amino acids, with a NH2-terminal signal peptide of 45 amino acids and without any transmembrane region, suggesting a mature protein that contains 266 amino acids (∼30 kDa) when secreted. The secretion of Metrnl has been verified by both Jorgensen et al and us, independently. In brief, a C-terminally His-tagged version of mouse Metrnl was cloned into a eukaryotic expression vector, which was transfected into HEK293, COS-729 or HEK293F35 cells. Metrnl was detected in both cell lysate and serum-free medium of transfected cells by western blotting with a Metrnl antibody29,35.
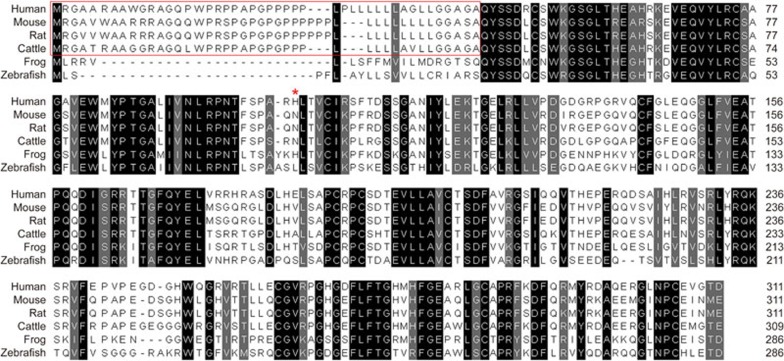
It is predicted that 239 amino acids (77%) are identical in the mouse and human Metrnl proteins (Figure 1). Moreover, orthologues for Metrnl are found in all vertebrates, including zebrafish and the frog Xenopus tropicalis (Figure 1), but not in invertebrates such as the fruit fly (Drosophila melanogaster) and the nematode (Caenorhabditis elegans)29,35,37.
Figure 1.
Amino acid sequences of Metrnl precursors in several vertebrates. Identical amino acids are marked in black, and similarity is marked in gray. The putative NH2-terminal signal sequences are indicated by the red frame, and the potential glycosylation site is indicated by a red star.
Metrnl shows approximately 40% amino acid identity with Metrn, with all ten cysteine residues in the mature sequence conserved. Structurally unrelated to other known proteins, Metrnl and Metrn constitute a new evolutionarily conserved two-member protein family29,35,37 (Figure 2).
Figure 2.
Amino acid sequences of human Metrnl and Metrn. Identical amino acids are marked in black, and similarity is marked in gray. The putative NH2-terminal signal sequences are indicated by the red frame.
The gene product encoded by Metrn was first described by Nishino and co-workers in 2004 and was shown to play an important role in the regulation of glial cell differentiation and in the induction of axonal extension. Thus, the initial name of this protein, Meteorin, was a vivid description of its function in transforming glial cells into cells with an elongated tail that look like meteors36. As the homologous protein, Metrnl protein was accordingly annotated as Meteorin-like in public databases at that time because neither its expression nor its functions had been reported29,35.
Unlike Metrn, the prediction of N-glycosylation sites in Metrnl indicates a single potential N-glycosylation site at amino acid 103 in mouse Metrnl. This was proven by both Jorgensen et al and us using recombinant mouse Metrnl-his6 protein. In both laboratories, the recombinant protein was purified by affinity chromatography and then separated by SDS-PAGE. Metrnl purified by this method appeared much heavier than the calculated molecular weight of Metrnl-his6 lacking the signal peptide29,35. In our case, we observed two bands between 34 and 43 kDa, which were recognized by anti-Metrnl and anti-His6 antibodies in SDS-PAGE analysis, and we identified both these bands as Metrnl by mass spectrometry29. In contrast, Jorgensen et al observed one band of approximately 34 kDa by SDS-PAGE analysis, which, when analyzed by MALDI-TOF MS, had a mass of 33.8 kDa with a shoulder at 33.4 kDa35. Because the bioinformatic prediction suggests that the mouse Metrnl contains one potential N-glycosylation site, the two labs then incubated the purified recombinant protein with glycopeptidase F29 or N-glycanase35, both obtaining a single band with a decreased molecular weight closer to the calculated size. These experimental results from both groups indicate that the larger molecular weight proteins represent glycosylated forms of Metrnl, thus proving that Metrnl is indeed post-translationally glycosylated, which may facilitate its secretion from the cells38.
However, the N-glycosylation site in mouse Metrnl is not conserved in Metrn or human Metrnl (Figure 1), suggesting that the glycosylation of mouse Metrnl may not play a central role in its function.
Metrnl expression in various tissues
Homologous to Metrnl, Metrn was discovered earlier and is mainly expressed in the nervous system both during development and in adult mice37. It has been reported that during embryonic mouse development, Metrn is widely expressed in undifferentiated neural progenitors and in the astrocyte lineage, including radial glia36. In the adult mouse brain, Metrn is highly expressed in Bergmann glia and in a few discrete neuronal populations, with low levels of Metrn in astrocytes distributed ubiquitously throughout the brain37,39,40.
In view of Metrn expression being closely associated with the brain, there is now great interest in the Metrnl expression pattern, especially concerning whether this novel protein is also expressed mainly in brain and whether it plays a similar role as a neurotrophic factor in neurogenesis like Metrn.
However, the structural similarity of Metrnl and Metrn do not seem to grant a similar expression pattern. Recent studies of Metrnl have demonstrated that it is widely expressed in adult mouse tissues at differing expression levels, but its highest expression is obviously not in the brain29.
In the nervous system
Assessed by in situ hybridization (ISH), Metrnl expression is found in very restricted sites within the brain during development. Metrnl mRNA expression appears weak in the otic vesicle of medaka embryos32, but during early mouse development, this transcript is found exclusively in the floor plate, and from E13.5, it is also found in dorsal root ganglions (DRG) and the inner ear but apparently not in the adult nervous system35.
Nevertheless, Metrnl is reported to be expressed in adult mouse brain, with a much lower expression level than that found in other tissues, such as white adipose tissue and skin29,41.
In adipose tissue
Examined by real-time PCR, the highest expression level of Metrnl is in subcutaneous white adipose tissue of both rodents and humans, with expression also detected in various tissues, including liver, spleen, muscle, heart, thymus, forebrain, midbrain, hindbrain, omental adipose tissue, subcutaneous adipose tissue, perivascular adipose tissue and interscapular adipose tissue29.
Metrnl protein is easily detected in the incubation medium of white adipose tissue by Western blot and ELISA29,30, which is consistent with its secretion. To further clarify the cell types in which Metrnl is mainly expressed, Metrnl expression was first compared between adipocytes and stromal cells separated by collagenase digestion of fat tissue, with no significant difference observed29. Upon comparing Metrnl expression between RAW264.7 macrophages and 3T3-L1 adipocytes, much lower expression was found in the macrophages29. These results indicate that both adipocytes and stromal cells but not unactivated macrophages are the main cell types expressing Metrnl. Immunohistochemical analysis of the fat tissue shows that Metrnl is distributed throughout the adipose tissue, except in the lipid droplets29.
In mucosal tissues, skin and activated macrophages
According to Metrnl expression data from the human BIGE database, the highest expression of Metrnl is in activated monocytes, followed by digestive and respiratory mucosal tissues and the skin41. Our unpublished results on the real-time PCR detection of Metrnl expression in various tissues also showed that Metrnl is highly expressed in digestive tract and lung (data not shown).
With strong expression in activated human monocytes according to the BIGE database, Metrnl was further found to be produced by alternatively activated macrophages and macrophage colony stimulating factors (M-CSF)-stimulated bone marrow macrophages41.
In addition, under resting conditions, Metrnl is expressed in fibroblasts but not in keratinocytes or peripheral blood mononuclear cells, whereas its expression is increased in IFNγ-treated keratinocytes41. Moreover, significant up-regulation of Metrnl expression was observed in familial primary localized cutaneous amyloidosis (FPLCA)42, psoriasis, prurigo nodularis, actinic keratosis and atopic dermatitis and in synovial membranes of human rheumatoid arthritis, thus suggesting a potential role of Metrnl in both innate and acquired immune responses41.
Function of Metrnl
Metrnl acts as a neurotrophic factor
Many secreted proteins, including NGF, GDNF and Metrn, are neurotrophic factors that nourish neurons and play central roles in neuronal development, maintenance and regeneration36,37,43,44,45,46,47. Recently, Jorgensen et al35 reported the neurotrophic activity of Metrnl in neurite outgrowth and neuroblast migration in vitro and in the survival of spiral ganglion neurons in vivo.
Using Metrn as a positive control, both proteins were tested on cultures of dissociated dorsal root ganglia. In the Metrnl treated group, neurite outgrowth was dose dependent and not significantly different from that induced by Metrn. Moreover, simultaneous treatment with Metrnl and Metrn showed an additive effect, with significantly more neurites compared to treatment with either factor alone35. Either the Jak inhibitor I (JAKiI) or the MEK inhibitor U0126 abrogates the Metrnl induced neurite outgrowth, indicating that both the Jak-STAT3 and MEK-ERK pathways are involved in the effect of Metrnl35. In rat subventricular zone explants, Metrnl induced a significant increase of neuroblast migration, with a similar effect to that induced by the positive control, stromal cell-derived factor 1a35. In a further investigation of the possible role of Metrnl in the adult inner ear, Jorgensen et al used a guinea pig model whose auditory sensory cells had been destroyed. The hearing-impaired animals treated with recombinant Metrnl exhibited a significant therapeutic effect in retaining electrical responsiveness of the auditory neurons, which was further supported by a stereological analysis indicating more spiral ganglion neurons in the treated animals than in the control group35.
Later, Watanabe et al48 reported that Metrnl is a latent process (LP) gene. The expression of these genes is upregulated during the latent process, a preparation step for cellular function, and this expression is required for subsequent neurite extension. Small interfering RNA targeting of Metrnl significantly inhibits NGF-induced neurite extension of PC12 cells, an adrenal chromaffin cell line that is a well-characterized model of nerve cells. This inhibition is partially prevented by Metrnl rescue constructs, which indicates an indispensable role of Metrnl in neurite extension. The effect of small interfering RNA knockdown of Metrnl expression on neurite extension is also observed in primary dissociated hippocampal neurons of rats, indicating that the role of Metrnl in neurite extension in primary neurons is consistent with that in the PC12 cell line48. Given that persistent activation of ERK is required for the NGF-induced differentiation of PC12 cells, Metrnl expression was shown to be dependent on ERK activity48. Further, the neurotrophic factors, ie, pituitary adenylate cyclase-activating peptide (PACAP) and forskolin, induce weaker ERK phosphorylation but greater Metrnl expression than NGF does, suggesting that Metrnl expression is regulated not only by ERK but also by other signaling pathways, at least in response to PACAP and forskolin48.
Metrnl induces white adipose browning
In mammals, there are two main types of adipose tissue: white adipose tissue (WAT), which stores energy, and brown adipose tissue (BAT), which dissipates energy49,50,51. WAT and BAT differ at the functional, morphological, and molecular levels51,52,53. However, upon thermogenic stimuli, WAT possesses the capacity to generate brown-like adipocytes (also called beige adipocytes), and this process is termed “browning” or “beiging”53,54. Due to its great therapeutic potential in developing new therapies for metabolic diseases, the browning of WAT has received much attention.
Recently, Rao et al55 showed a role for Metrnl in the browning of white adipose tissue. Metrnl, which Rao et al identified as a PGC-1α4-dependent myokine in skeletal muscle, can be induced in muscle after exercise, a physiological stimulus that also increases PGC-1α4 expression55. The authors demonstrated that muscle-specific expression of PGC-1α4 promotes browning of the subcutaneous and epididymal white adipose tissue55, a process that correlates with the ability to defend body temperature in cold environments53,56,57,58. These results suggest that Metrnl mediates muscle-fat crosstalk to promote the expression of genes that are associated with browning of the white adipose tissue55.
Rao et al also showed that increasing circulating Metrnl in mice, either by delivering a Metrnl-expressing adenoviral vectors to the liver or through the administration of recombinant Metrnl, produces remarkable increases in the expression of genes associated with beige fat thermogenesis and anti-inflammatory cytokines in white adipose tissue55. Metrnl was also shown to induce a thermogenic phenotype when expressed locally in adipose tissue in vivo. However, it should be noted that this phenotype can only be maintained for a short period (fewer than 10 days).
Examination of mRNA profiles from the subcutaneous white fat of mice with increased circulating Metrnl shows significant increases in several genes associated with alternative macrophage activation. Metrnl expression also increases the production of IL-4 and IL-13 as well as catecholamines in the adipose tissue. This suggests that Metrnl induces a phenotypic switch in adipose tissue macrophages in vivo, along with production of pro-thermogenic catecholamines, possibly via the induction of the M2-regulatory cytokines IL-4 and IL-1355. The browning response induced by Metrnl is IL-4/13 dependent because disruption of IL-4/13 signaling in STAT6 knockout mice causes no change in alternative macrophage activation but attenuates the effects of IL-4/13 on the regulation of thermogenic or β-oxidation genes and reduces the content of catecholamines55. The primary source of IL-4/13 upon Metrnl treatment is shown to be eosinophils, the number of which increases in the adipose tissue when circulating Metrnl is elevated55.
Finally, blocking Metrnl actions in vivo by anti-Metrnl antibody results in reduced mRNA expression of IL-4/13 along with a reduced number of eosinophils induced upon acute cold exposure for 24 h. The anti-Metml antibody also significantly inhibits the expression of genes that are characteristic of M2 macrophages and adipose thermogenesis induced by 72 h of cold exposure, thereby implicating the role of Metrnl in cold adaptation55.
Metrnl antagonizes insulin resistance
In addition to being the largest reservoir for energy storage, adipose tissue is a highly active endocrine organ that synthesizes and secretes proteins/peptides termed adipokines10,59. Adipokines participate in the regulation of multiple physiological functions, including metabolism, insulin sensitivity, cardiocerebrovascular function, immunity and inflammation10,60. Dysregulated production or secretion of adipokines is associated with the pathogenesis of obesity-linked disorders61,62. With potential clinical relevance, adipokines are promising candidates as new therapeutic compounds or targets in the treatment of obesity and its related diseases2,61.
Our lab has identified Metrnl as an adipokine that is abundantly expressed in rat, mouse and human subcutaneous white adipose tissue, with relatively lower expression levels found in brown adipose tissue and a much lower expression level in the brain29. In addition, Metrnl is downregulated in white adipose tissue of caloric restriction rats but is dramatically upregulated during white adipocyte differentiation and in the white adipose tissue of diet-induced obese mice29. These results suggested a role for Metrnl in white adipose biology and metabolic homeostasis, leading us to explore the function of Metrnl in white adipose tissue.
Adipogenesis, the differentiation of fibroblast-like mesenchymal stem cells into adipocytes, plays a central role in the regulation of whole body energy metabolism63. Metrnl is detectable both in the incubation medium of white adipose tissue and in the culture medium of either primary adipocytes or 3T3-L1 adipocytes29,30. To study the adipogenic potential of Metrnl, we employed gain-of-function and loss-of-function experiments using 3T3-L1 adipocytes as an in vitro model of adipogenesis to demonstrate that Metrnl promotes lipid accumulation and upregulates markers specific to mature adipocytes30. Importantly, the expression of PPARγ, which is the key regulator of adipocyte differentiation64,65,66, is induced by Metrnl protein in a concentration-dependent manner, indicating that secreted Metrnl promotes adipocyte differentiation30.
We further demonstrate an insulin sensitizing role of Metrnl using genetically engineered mouse models. Tested by various methods, insulin resistance induced by a high-fat diet (HFD) is exacerbated in adipocyte-specific Metrnl knockout mice, whereas transgenic mice overexpressing Metrnl specifically in adipocytes were protected from diet-induced insulin resistance. Moreover, the overexpression of Metrnl in adipose tissue also antagonizes insulin resistance in mice with leptin deficiency30.
We provide evidence that adipose Metrnl is most likely to ameliorate overall insulin resistance through its action on local adipose tissue in an autocrine or paracrine fashion30. First, though the phenotypes with regards to insulin resistance in these mouse models are obvious and unambiguous, their serum Metrnl concentrations remain unchanged compared to the corresponding control mice. Second, the insulin-stimulated phosphorylation of AKT is enhanced by adipocyte Metrnl in white adipose tissue, but not in other major metabolic tissues (brown adipose tissue, muscle and liver). Third, increasing the circulating Metrnl levels via the intravenous administration of recombinant Metrnl for 1 week is unable to rescue insulin resistance in adipose-specific Metrnl knockout mice fed a HFD. Moreover, acute intravenous injection with recombinant Metrnl has no hypoglycemic action in HFD-fed C57 obese mice or in leptin knockout obese mice30.
Both in vitro and in vivo, Metrnl promotes adipocyte differentiation, which is a key factor in forming functional fat for insulin sensitivity and lipid metabolism. We have detected gene markers related to adipocyte differentiation and lipid metabolism in white adipose tissue of both HFD-fed Metrnl knockout mice and Metrnl transgenic overexpression mice and found that Metrnl upregulates key transcription factors for adipocyte differentiation (PPARγ, C/EBPα) and lipid metabolism genes for lipid transport (FABP4, CD36), lipogenesis (ACC, FASN), lipolysis (Lipe, PNPLA), and lipid storage (Perilipin). In addition, the overexpression of Metrnl decreased the proportion of small adipose cells30, which is consistent with insulin sensitization67,68,69.
The transcription factor PPARγ is the key regulator of the fully differentiated and insulin-sensitive adipose cell phenotype, with consequences for the proper functioning of the adipose tissue and whole-body insulin sensitivity64,65,66. The expression of PPARγ is increased markedly in adipose tissue of Metrnl transgenic mice, in agreement with the in vitro results. We subsequently demonstrated that PPARγ plays a critical role in Metrnl-mediated beneficial effects by using long-term treatment with two different small-molecule inhibitors of PPARγ as well as the knockdown of PPARγ. The inhibition or knockdown of PPARγ completely abolished the insulin-sensitizing effect of Metrnl in HFD-fed Metrnl transgenic mice, indicating that Metrnl-mediated insulin sensitization occurs through the PPARγ pathway30.
We also investigated many other factors that are possibly related to the insulin sensitization of Metrnl30. For example, adipocyte Metrnl prevents an increase in TNF-α by chronic HFD but not acute LPS, indicating a role of Metrnl in the inhibition of HFD-induced adipose inflammation. Adipocyte Metrnl attenuates HFD-induced hypertriglyceridemia but not HFD-induced hypercholesterolemia or the accumulation of liver triglyceride. Metrnl enhances serum triglyceride clearance during acute lipid overload test and elevates the expression and activity of lipase in adipose tissue. These results all indicate a role of Metrnl in the activation of adipose lipid metabolism. However, no changes have been observed in body weight, food intake, lean/fat mass, distribution of adipose tissue, or energy expenditure in either Metrnl adipose-specific knockout or transgenic overexpression mice compared to control mice30. In particular, considering a physiological role for Metrnl in cold adaption as a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis, as reported by Rao et al55, we explored whether this mechanism is involved in our transgenic mouse models. We did not observe any significant changes in IL-4/13 expression, M2 macrophage activation, eosinophil accumulation, or thermogenic gene expression in the white adipose tissue of these transgenic mouse models, indicating that no browning of white adipose tissue occurs30. This discrepancy between these studies may be caused by the different animal models and intervention methods, for instance, the relatively acute models used by Rao et al versus the chronic models used in our study.
We have also noted a meeting abstract reporting that Metrnl is mainly expressed in undifferentiated osteoblasts and hypertrophic chondrocytes and reduces the terminal differentiation of human osteoblastic MG63, which may be associated with the inhibition of Metrnl on transcription activity of AP-170.
Concluding remarks
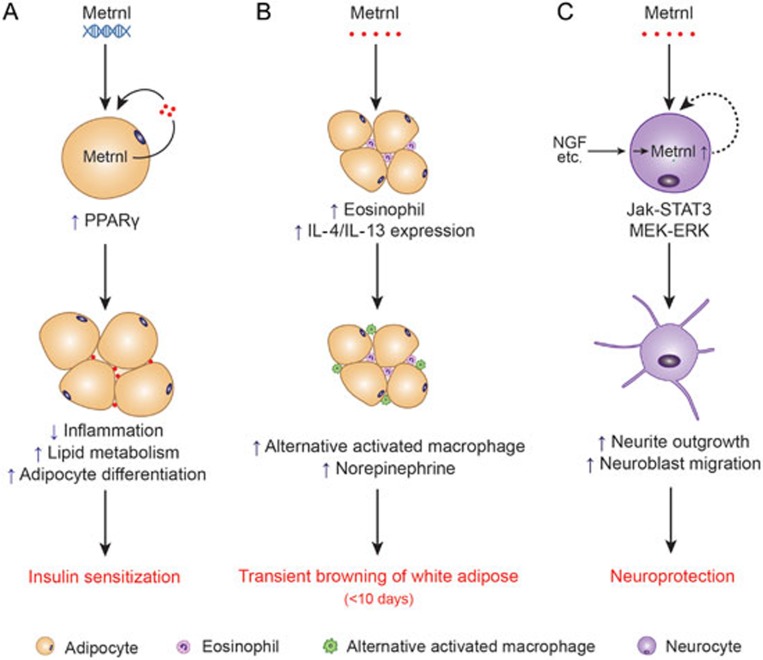
Metrnl is a novel secretory protein with three new emerging functions (Figure 3). First, Metrnl acts as a neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports the survival of spiral ganglion neurons in vivo. Second, Metrnl is involved in cold adaption by regulating immune-adipose interactions to increase beige fat thermogenesis. Third, Metrnl plays an important role in the biology of white adipose tissue, improving adipose function and antagonizing obesity-induced insulin resistance. With its neurotrophic activity and beneficial metabolic effect, this protein could be expected to provide novel therapeutic strategies for certain diseases. However, the utilization of this newly described protein as a therapeutic target or agent requires much work to be done to understand its functional spectrum in health and disease, especially the key mechanisms that initiate its actions. Important questions exist, such as what is the role of Metrnl in barrier tissues; how does Metrnl influence nerve system function; and what, if any, specific receptor exists for Metrnl?
Figure 3.
Known functions of Metrnl in white adipose tissue and neurocytes. (A) Overexpression of Metrnl upregulates PPARγ in adipocytes via an autocrine/paracrine mechanism, which inhibits adipose inflammation, enhances adipocyte differentiation, activates lipid metabolism, and ultimately reduces insulin resistance. (B) Administration of abundant Metrnl causes trafficking of eosinophils into white adipose tissue and increases local IL-4/13, which promotes alternative macrophage activation along with increased norepinephrine. Through this mechanism, Metrnl transiently induces white adipose browning for less than ten days. (C) Nerve growth factor (NGF), pituitary adenylate cyclase-activating peptide (PACAP) and forskolin induce Metrnl expression in PC12 cells. Metrnl protein promotes neurite outgrowth and neuroblast migration via the Jak-STAT3 and MEK-ERK pathways and plays a neuroprotective role in vivo.
Because research into this novel bioactive protein, Metrnl, has only just begun, its function and mechanisms of action remain largely unknown. As many as five names (Metrnl, Meteorin-like, Cometin, Subfatin, IL-39) have been proposed for this novel protein. Meteorin-like, simply highlighting Metrnl's homology with Meteorin (Metrn), was the initial name of the protein annotated in public databases at the time when neither its expression nor its functions had been reported. The most recent studies on Metrnl have revealed that the expression patterns and functions of Metrnl appear to be quite different from those of Meteorin (Metrn). Thus, the name “Meteorin-like” is not appropriate, which has led to new names being proposed, including Cometin, Subfatin and IL-39, based on the expression features and functions of this protein. However, one name for each function of the same protein is inappropriate, makes further work complicated for researchers, authors and readers, and is unfavorable for broad studies of this novel protein. To solve this issue, we suggest Metrnl, identical to the gene symbol, as the protein designation in the future literature. With the gene symbols being unique, Metrnl could uniquely represent the encoded protein, thus allowing for clear and unambiguous reference to Metrnl protein in scientific communications.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 81130061, 81202572, and 81373414).
References
- Kaiser J. Aging. 'Rejuvenation factor' in blood turns back the clock in old mice. Science 2014; 344: 570–1. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015; 36: 461–70. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Dodd GT, Tiganis T. Protein tyrosine phosphatases in hypothalamic insulin and leptin signaling. Trends Pharmacol Sci 2015; 36: 661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Miller D, Wanders D, Nanayakkara G, Amin R, Judd R, et al. Adiponectin downregulation is associated with volume overload-induced myocyte dysfunction in rats. Acta Pharmacol Sin 2016; 37: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueiros-Cendon T, Arevalo-Gallegos S, Iglesias-Figueroa BF, Garcia-Montoya IA, Salazar-Martinez J, Rascon-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 2014; 35: 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Chen YL, Li C, Ding XY, Xu GY, Hu LL, et al. The effect of inhibition of endoplasmic reticulum stress on lipolysis in white adipose tissue in a rat model of chronic kidney disease. Acta Pharmacol Sin 2014; 35: 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Li MX, Tang Z, Wang CH. Wogonin suppresses osteopontin expression in adipocytes by activating PPARalpha. Acta Pharmacol Sin 2015; 36: 987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 2009; 81: 370–80. [DOI] [PubMed] [Google Scholar]
- Wang P, Yang FJ, Du H, Guan YF, Xu TY, Xu XW, et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med 2011; 17: 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CY. Introduction: adipokines and cardiovascular disease. Clin Exp Pharmacol Physiol 2011; 38: 860–3. [DOI] [PubMed] [Google Scholar]
- Li ZY, Wang P, Miao CY. Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin Exp Pharmacol Physiol 2011; 38: 888–96. [DOI] [PubMed] [Google Scholar]
- Wang P, Vanhoutte PM, Miao CY. Visfatin and cardio-cerebro-vascular disease. J Cardiovasc Pharmacol 2012; 59: 1–9. [DOI] [PubMed] [Google Scholar]
- Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol 2012; 165: 643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Chen Y, Li ZY, Guan YF, Zou DJ, Miao CY. Protein tyrosine phosphatase 1B inhibits adipocyte differentiation and mediates TNFalpha action in obesity. Biochim Biophys Acta 2013; 1831: 1368–76. [DOI] [PubMed] [Google Scholar]
- Wang P, Miao CY. NAMPT as a Therapeutic Target against Stroke. Trends Pharmacol Sci 2015; 36:891–905. [DOI] [PubMed] [Google Scholar]
- Wang P, Li WL, Liu JM, Miao CY. NAMPT and NAMPT-controlled NAD metabolism in vascular repair. J Cardiovasc Pharmacol 2015 Oct 17. [Epub ahead of print] [DOI] [PubMed]
- Zhang RY, Qin Y, Lv XQ, Wang P, Xu TY, Zhang L, et al. A fluorometric assay for high-throughput screening targeting nicotinamide phosphoribosyltransferase. Anal Biochem 2011; 412: 18–25. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol 2011; 69: 360–74. [DOI] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 2012; 8: 77–87. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhang RY, Song J, Guan YF, Xu TY, Du H, et al. Loss of AMP-activated protein kinase-alpha2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes 2012; 61: 1051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Ke SF, Zhou CC, Zhang SL, Guan YF, Xu TY, et al. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci 2014; 69: 44–57. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu XZ, Tian WW, Guan YF, Wang P, Miao CY. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther 2014; 20: 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu TY, Wei K, Guan YF, Wang X, Xu H, et al. ARRB1/beta-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy 2014; 10: 1535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Du H, Zhou CC, Song J, Liu X, Cao X, et al. Intracellular NAMPT-NAD+-SIRT1 cascade improves post-ischaemic vascular repair by modulating Notch signalling in endothelial progenitors. Cardiovasc Res 2014; 104: 477–88. [DOI] [PubMed] [Google Scholar]
- Wang P, Guan YF, Li WL, Lu GC, Liu JM, Miao CY. Nicotinamide phosphoribosyltransferase facilitates post-stroke angiogenesis. CNS Neurosci Ther 2015; 21: 475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TY, Zhang SL, Dong GQ, Liu XZ, Wang X, Lv XQ, et al. Discovery and characterization of novel small-molecule inhibitors targeting nicotinamide phosphoribosyltransferase. Sci Rep 2015; 5: 10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu TY, Liu XZ, Zhang SL, Wang P, Li ZY, et al. Discovery of Novel Inhibitors and Fluorescent Probe Targeting NAMPT. Sci Rep 2015; 5: 12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Guan YF, Zhou XM, Li GQ, Li ZY, Zhou CC, et al. Regenerative neurogenesis after ischemic stroke promoted by nicotinamide phosphoribosyltransferase-nicotinamide adenine dinucleotide cascade. Stroke 2015; 46: 1966–74. [DOI] [PubMed] [Google Scholar]
- Li ZY, Zheng SL, Wang P, Xu TY, Guan YF, Zhang YJ, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther 2014; 20: 344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, et al. Adipocyte Metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes 2015; 64: 4011–22. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, et al. A draft map of the human proteome. Nature 2014; 509: 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramialison M, Bajoghli B, Aghaallaei N, Ettwiller L, Gaudan S, Wittbrodt B, et al. Rapid identification of PAX2/5/8 direct downstream targets in the otic vesicle by combinatorial use of bioinformatics tools. Genome Biol 2008; 9: R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace C, Piazzolla S, Sirleto P, Digilio MC, Roberti MC, Lombardo A, et al. Mild ring 17 syndrome shares common phenotypic features irrespective of the chromosomal breakpoints location. Clin Genet 2009; 76: 256–62. [DOI] [PubMed] [Google Scholar]
- Chung J, Kubota H, Ozaki Y, Uda S, Kuroda S. Timing-dependent actions of NGF required for cell differentiation. PLoS One 2010; 5: e9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JR, Fransson A, Fjord-Larsen L, Thompson LH, Houchins JP, Andrade N, et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp Neurol 2012; 233: 172–81. [DOI] [PubMed] [Google Scholar]
- Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, Hamada H. Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J 2004; 23: 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JR, Thompson L, Fjord-Larsen L, Krabbe C, Torp M, Kalkkinen N, et al. Characterization of Meteorin — an evolutionary conserved neurotrophic factor. J Mol Neurosci 2009; 39: 104–16. [DOI] [PubMed] [Google Scholar]
- Mitra N, Sinha S, Ramya TN, Surolia A. N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem Sci 2006; 31: 156–63. [DOI] [PubMed] [Google Scholar]
- Kim YY, Moon JS, Kwon MC, Shin J, Im SK, Kim HA, et al. Meteorin regulates mesendoderm development by enhancing nodal expression. PLoS One 2014; 9: e88811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JR, Emerich DF, Thanos C, Thompson LH, Torp M, Bintz B, et al. Lentiviral delivery of meteorin protects striatal neurons against excitotoxicity and reverses motor deficits in the quinolinic acid rat model. Neurobiol Dis 2011; 41: 160–8. [DOI] [PubMed] [Google Scholar]
- Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol 2015; 156: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Lai-Cheong JE, van den Akker PC, Nagy N, Millington G, Diercks GF, et al. The molecular skin pathology of familial primary localized cutaneous amyloidosis. Exp Dermatol 2010; 19: 416–23. [DOI] [PubMed] [Google Scholar]
- Lanni C, Stanga S, Racchi M, Govoni S. The expanding universe of neurotrophic factors: therapeutic potential in aging and age-associated disorders. Curr Pharm Des 2010; 16: 698–717. [DOI] [PubMed] [Google Scholar]
- Revilla S, Ursulet S, Alvarez-Lopez MJ, Castro-Freire M, Perpina U, Garcia-Mesa Y, et al. Lenti-GDNF gene therapy protects against Alzheimer's disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci Ther 2014; 20: 961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WK, Cheung AW, Yu SW, Sha O, Cho EY. Hepatocyte growth factor promotes long-term survival and axonal regeneration of retinal ganglion cells after optic nerve injury: comparison with CNTF and BDNF. CNS Neurosci Ther 2014; 20: 916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Pan J, Hua XM, Liu H, Shen S, Liu JF, et al. Endothelial nitric oxide synthase protects neurons against ischemic injury through regulation of brain-derived neurotrophic factor expression. CNS Neurosci Ther 2014; 20: 154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Wang NN, Zhang TY, Wang F, Wu CF, Yang JY. Neuroprotection by sildenafil: neuronal networks potentiation in acute experimental stroke. CNS Neurosci Ther 2014; 20: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Akimoto Y, Yugi K, Uda S, Chung J, Nakamuta S, et al. Latent process genes for cell differentiation are common decoders of neurite extension length. J Cell Sci 2012; 125: 2198–211. [DOI] [PubMed] [Google Scholar]
- Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell 2009; 5: 472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011; 12: 722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14: 324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis 2006; 16: 569–74. [DOI] [PubMed] [Google Scholar]
- Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig 2014; 19: 25–37. [DOI] [PubMed] [Google Scholar]
- Porter C, Chondronikola M, Sidossis LS. The therapeutic potential of brown adipocytes in humans. Front Endocrinol (Lausanne) 2015; 6: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157: 1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z, Wuren T, Liu S, Han S, Chen L, McClain D, et al. Intermittent cold exposure results in visceral adipose tissue “browning” in the plateau pika (Ochotona curzoniae). Comp Biochem Physiol A Mol Integr Physiol 2015; 184: 171–8. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10: 24–36. [DOI] [PubMed] [Google Scholar]
- Lee SD, Tontonoz P. Eosinophils in fat: pink is the new brown. Cell 2014; 157: 1249–50. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab 2014; 25: 348–55. [DOI] [PubMed] [Google Scholar]
- Xu TY, Lan XH, Guan YF, Zhang SL, Wang X, Miao CY. Chronic nicotine treatment enhances vascular smooth muscle relaxation in rats. Acta Pharmacol Sin 2015; 36: 429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M. Adipokines-removing road blocks to obesity and diabetes therapy. Mol Metab 2014; 3: 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lee P, Chisholm DJ, James DE. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol (Lausanne) 2015; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest 2006; 116: 581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett 2010; 584: 3242–9. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 2015; 241: 27–35. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007; 50: 1707–15. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Deng A, Yee G, Lamendola C, Reaven G, Tsao PS, et al. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia 2010; 53: 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014; 22: 673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong WY, Liu Y, Zheng RM, Oiu GX, Lin SO. Metrnl: A new secreted protein inhibit differentiation of MG-63. J Bone Miner Res 2007; 22: S142. [Google Scholar]