Abstract
Background
Back pain has many causes. In Germany, about 70% of adults have at least one episode of back pain per year.
Methods
This review is based on a selective literature search and on the German National Disease Management Guideline for Low Back Pain.
Results
The physician taking the history from a patient with back pain should ask about the nature, onset, course, localization, and radiation of the pain and its dependence on physical activity and/or emotional stress. In the differential diagnosis, neurologic deficits and any “red flags” suggesting dangerous conditions such as spinal fracture, bacterial infection, and tumors must be ruled out. If no specific cause of the pain can be identified, no imaging studies are indicated on initial presentation. The treatment of acute, nonspecific low back pain focuses on pain relief and functional improvement. Adequate patient education and counseling are essential. Exercise therapy is no more effective than the continuation of normal daily activities. Restriction of activity, including bed rest, is of no benefit and merely prolongs recovery and the resumption of normal activity. Further diagnostic testing is indicated if there is any suspicion of a fracture, infection, or tumor.
Conclusion
After dangerous conditions have been ruled out, low back pain can be pragmatically classified as either nonspecific or specific. More research is needed so that the diagnostic assessment and individualized treatment of acute lower back pain can be further refined.
Low back pain is not a disease in itself, but rather a symptom with many causes. The term “low back pain” refers to pain felt near the midline in the lumbar or sacral region. Its cause need not lie in the spine, as it can also be due to abdominal or pelvic disease. Physicians and patients are confronted by a bewildering variety of treatment options for low back pain. According to the German Health Ministry’s Expert Council for the Assessment of Developments in Health Care (Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen), the management of low back pain in Germany is currently characterized by overtreatment, undertreatment, and mistreatment (1).
Learning objectives
Readers of this article should become able to
understand that low back pain is a symptom with many causes, and undertake a practical differential diagnostic assessment;
know and apply the appropriate methods of history-taking, diagnostic evaluation, and treatment;
recognize and avoid early risk factors for the chronification of low back pain.
Epidemiology
The high prevalence of low back pain in Germany has been documented in primary epidemiologic data from the Federal Health Survey, the Lübeck Back Pain Study, and a multicenter study of the German Back Pain Research Association (Deutscher Forschungsverbund Rückenschmerz, DFRS), among other sources. It ranges from 30% to 70% among German adults (aged 18–74) depending on the period over which it is determined (point prevalence vs. seven-day, three-month, and one-year prevalence) (e1).
Prevalence.
In Germany, about 70% of adults have at least one episode of back pain per year.
The prognosis of acute back pain is uncertain. It is generally presumed that the pain resolves within six weeks in about half of all cases (2) and that 68–86% of the affected persons resume work within a month (e2), but it has also been reported that 62% of the affected persons still have pain 12 months later, and that 16% do not resume work within six months. Recurrent low back pain is common (47–54%) (3), as is recurrent inability to work (33%) (e2). The interpretation of the available data is further complicated by the fact that only one-third of patients tell their primary caregiver that they rarely or never had back pain before (4). In any case, it clearly cannot assumed that a patient’s first episode of back pain will also be his or her last.
Definition and causes
Low back pain (lumbar back pain) is defined as pain in the back from the level of the lowest rib down to the gluteal fold, with or without radiation into the legs (5). An episode of low back pain is called acute if it has arisen for the first time in a patient’s life, or after a pain-free interval of at least six months, and lasts no longer than six weeks (6).
Definition.
Low back pain (lumbar back pain) is defined as pain in the back from the level of the lowest rib down to the gluteal fold, with or without radiation into the legs.
Low back pain due to a specific, serious disease is rare. Moreover, pathophysiologically oriented diagnostic categories for low back pain are often not reproducible, and they generally have no clear-cut implications for treatment. Therefore, in the German National Disease Management Guideline for Low Back Pain (6), low back pain is pragmatically classified as either nonspecific or specific. Treatment-based or functional-cognitive classifications, though they may seem promising, are still in need of validation by an adequate evidence base (7–9). Back pain is called nonspecific when there is no clear causal relationship between the symptoms, physical findings, and imaging findings. Physicians should accordingly exercise caution before ordering further diagnostic tests and treatments.
In specific low back pain, by definition, a patho-anatomical relationship can be demonstrated between the pain and one or more pathological processes, including compression of neural structures, joint inflammation, and/or instability of one or more spinal motion segments. Specific diagnostic investigations and cause-directed treatments should be initiated.
Among all patients whose low back pain had a specific, clinically relevant cause, 4% were diagnosed with disk herniation, 3% with spinal stenosis, and 2% with spondylolisthesis. Roughly 1–4% of patients were found to have a vertebral body fracture on their primary investigation; 0.7% had a tumor (primary or metastatic), 0.2% had ankylosing spondylitis, and 0.01% had spondylodiscitis (10).
Overall, 15% of all instances of low back pain showed pathological findings. It follows that some 80–90% of cases of low back pain are nonspecific, i.e., have no clear patho-anatomical correlate (11).
Nonspecific back pain.
Back pain is called nonspecific when there is no clear causal relationship between the symptoms, physical findings, and imaging findings.
Low back pain is often caused by non-pathological functional disturbances that are best detected by physical examination and cannot be adequately demonstrated by imaging studies, especially the following:
segmental dysfunction (e.g., “blockages” [12]),
sacroiliac joint syndrome,
altered spinal statics (e.g., hyperlordosis or straightening of the normal lumbar lordosis),
muscle dysfunction (e.g., Janda’s crossed syndromes, shortened muscles, trigger points),
connective-tissue changes (e.g., swelling, fascial hypomobility), and
systemic conditions (e.g., incoordination, inadequate deep stabilization, or constant hypermobility).
Causes.
Low back pain is often caused by non-pathological functional disturbances that are best detected by physical examination and cannot be adequately demonstrated by imaging studies.
The current differential diagnostic methods generally do not enable a clear diagnosis to be made when low back pain is of muscular origin; this situation is very common. Pain of this type is perceived differently from patient to patient and is associated with variable symptoms and signs. More research is needed in this area (6). It is also hard to classify the spinal degenerative changes of various kinds that are now revealed by advanced neuroimaging techniques in 15–45% of patients with low back pain (10, 13). Degenerative changes are a part of normal aging, but they should be considered pathological if they involve inflammation, e.g., activated spondylarthrosis. Lumbar facet syndrome, a familiar clinical condition, is not an entity that can be definitively diagnosed, although an evidence base does exist for its diagnosis and satisfactory treatment by local anesthetic infiltration (e3). The same holds for spinal canal stenosis, an anatomical condition commonly revealed by MRI in elderly persons, which only needs treatment if there are typical symptoms and signs of neurogenic intermittent claudication and if other important entities in the differential diagnosis (peripheral vascular disease, polyneuropathy) have been ruled out. eTable 1 contains a list of physical findings without pathological significance that are commonly seen in patients with low back pain.
eTable 1. Types of low back pain associated with physical findings of no clear pathoanatomical significance.
| Syndrome | Findings | Assessment/Plan |
|---|---|---|
| Facet syndrome |
History and physical examination:
Radiological findings (not indicated on intial evaluation):
|
Differential diagnosis:
analgesics (1–3 days), muscle stabilization, manual medicine, facet injection if indicated |
| Sacro-iliac joint syndrome |
History and physical examination:
|
Functional disturbance: muscular imbalance Treatment: stabilizing exercises, analgesics (1–3 days) if needed, manual medicine, sacro-iliac joint injection if indicated |
| Myofascial pain syndrome |
History and physical examination:
Radiological and histological findings:
|
Local treatment: active physiotherapy, manual therapy, infiltration, acupuncture |
| Functional instability |
History and physical examination:
|
|
MRI, magnetic resonance imaging
Low back pain typically takes a chronic relapsing and remitting course, and its character often varies over time. It is traditionally classified as acute (lasting up to 6 weeks), subacute (6–12 weeks), or chronic (more than 12 weeks) (6). This purely temporal classification, however, often does not adequately reflect the prognostically highly important process of chronification, i.e., the transition from acute to chronic pain. The typical feature of chronification is the increasing multidimensionality of pain, involving a loss of mobility, restriction of function, abnormal perception and mood, unfavorable cognitive patterns, pain-related behavior, and, on the social level, disturbances of social interaction and occupational difficulties (14).
Either the Numerical Rating Scale (NRS) or the Visual Analog Scale (VAS) is recommended as a means of rating the subjective intensity of pain, along a scale ranging from “none” to “unbearable” (6).
Typical features of the increasing multidimenstionality of pain include.
loss of mobility and restriction of function
abnormal perception and mood
pain-related behavior, disturbances of social interaction, occupational difficulties
History and diagnostic evaluation
A meticulously obtained history generally yields important information for the assessment of the back pain experience. The physician should ask about the onset and course of the pain, earlier pain episodes (if any), the site and radiation (if present) of the pain, its quality and intensity, and its dependence on rest and/or exercise, as well as about sleep disturbances, impairment in the activities of everyday living, and any other stress factors in the patient’s personal life or at work. The overriding goal in the primary treatment of low back pain is symptomatic relief, i.e., acute reduction of the pain, with simultaneous attention to the following:
the exclusion of serious disease (“red flags”),
the detection of clues that might suggest a specific diagnosis, and
the early detection of psychosocial factors that promote chronification (“yellow flags”) (e3).
“Red flags” are the current clinical features and prior illnesses that warn of a possible specific cause which may lead to serious problems unless it is treated immediately (6) (Table 1).
Table 1. Warning signs (“red flags”) for specific spinal causes of low back pain requiring urgent treatment*1, 2.
| Suspicion oftraumatic lesion | Suspicion oftumor | Suspicion ofinfection | Suspicion of radiculopathy, cauda equina syndrome |
|---|---|---|---|
|
|
|
|
Recent studies demand that the physician searching for red flags should have a narrowly focused and specific list of red flags in mind, as it has been found that some 80% of patients will be found to have at least one red flag that might prompt further diagnostic investigation (15) (eTable 2). Decisions about further diagnostic and therapeutic measures should depend on multiple features in combination, rather than on one feature alone, and always in the light of the physical findings (e5).
eTable 2. Sensitivity and specificity of red flags (6).
| Sensitivity | Specificity | |
|---|---|---|
| Malignancy | ||
| Age ≥ 50 years History of cancer Unintentional weight loss No relief after 4 weeks of treatment |
0.77 0.31 0.15 0.31 |
0.71 0.98 0.94 0.90 |
| No relief with bed rest Persistence for more than one month Age ≥ 50 years or history of cancer or unintentional weight loss or no relief after one month of treatment |
> 0.90 0.50 1.00 |
0.46 0.81 0.60 |
| Spinal osteomyelitis | ||
| Intravenous drug abuse, urinary tract infection, or skin infection | 0.40 | – |
| Compression fracture | ||
| Age ≥ 50 years Age ≥ 70 years |
0.84 0.22 |
0.61 0.96 |
| Trauma | 0.30 | 0.85 |
| Corticosteroid use | 0.06 | 0.99 |
| Ankylosing spondylitis | ||
| Age ≤ 35 years Morning stiffness |
0.90 0.64–0.95 |
0.30 0.29–0.59 |
| No improvement of pain in the supine position Improvement of pain and stiffness on movement |
0.80 0.69–0.75 |
0.49 0.45–0.90 |
| Insidious onset | 0.53–0.88 | 0.51–0.76 |
| Duration of symptoms >3 months Four of the above five signs positive |
0.71–0.86 0.95 |
0.09–0.54 0.85 |
| Disc herniation | ||
| Sciatica (assumed prevalence* 5%) | 0.95 | 0.88 |
| Cauda equina syndrome | ||
| Urinary retention Saddle anesthesia |
0.90 0.75 |
– – |
| Sphincter dysfunction | 0.60–0.80 | – |
*artifact
Pain documentation.
Either the Numerical Rating Scale (NRS) or the Visual Analog Scale (VAS) is recommended as a means of rating the subjective intensity of pain, along a scale ranging from “none” to “unbearable.”
The history should also include any psychosocial risk factors for the chronification of low back pain (“yellow flags”) (Table 2). Cognitive-psychoemotional and behavioral traits favoring the transition from acute to chronic pain (16) should be recognized as early as possible and addressed in the treatment plan. Further important elements of the history are:
Table 2. Psychosocial risk factors (yellow flags) for the chronification of nonspecific back pain.
| Strong evidence | Moderately strong evidence | Limited evidence | No evidence |
|---|---|---|---|
|
|
|
|
lifting and poor posture as possible causes of pain (17),
iatrogenic factors, e.g., faulty diagnosis,
preference for passive and pain-avoidant behavior,
excessive preoccupation with somatic and radiological findings.
Several screening instruments for assessing the risk of chronification are now available, including the Heidelberger Kurzfragebogen (Short Heidelberg Questionnaire) HKF-R10 (18), the Örebro Musculoskeletal Pain Screening Questionnaire (ÖMPSQ) (19, e7), the Risk-R (20), and the Start Back-Screening Tool (SBST) (e9) (17, e9). No particular one can be recommended above the others, both because evaluations of individual instruments have yielded varying results (18, e8, e9) and because the utility of early psychosocial intervention has not been clearly shown (21).
As only a few patients with low back pain have red flags, while far more have functional disturbances (eTable 1), physical examination plays an important role as well (especially tests of muscle and joint function) (6) (Box 1).
Box 1. Basic clinical examination*.
Inspection: general condition, gait, asymmetry (muscle atrophy), deformities, skin changes
Palpation of the local musculature (tone, tenderness)
Pain on palpation and percussion of spinal structures, esp. spinous processes (fracture), and kidneys
Range of motion of the lumbar spine (esp. for follow-up) and hip joints (hip arthritis and other joint diseases as part of the differential diagnosis)
Nerve-stretching tests, esp. Lasègue and femoral nerve stretch test
General testing of sensation, motor function, and reflexes (hypesthesia, hyperesthesia, allodynia; strength grading; reflexes)
*modified from (6)
The utility of physical examination is limited by the inability to test all relevant structures and by the poor discriminatory ability of many of the tests. Systematic statistical evaluations of the physical examination have shown that even common tests like the straight-leg-raising test, though they may be highly sensitive (87–95%), are often not very specific (22–35%); the figures depend on the reference method used for statistical purposes (e.g., MRI findings, surgery) (22). Provocative tests, e.g., compression and mobilization tests of the sacro-iliac joint, are more reliable than tests of mobility (e10). Combinations of tests are more informative than single ones (6, e8, e11, e12).
The therapeutic consequences of nonspecific acute low back pain
The treatment of the patient with nonspecific low back pain begins with thorough patient information and counselling (Box 2) (e13).
Box 2. What to tell the patient after specific causes of low back pain have been ruled out*.
Everyday activities should be continued or resumed as soon as possible
Bed rest should be avoided
The patient’s low back pain is benign and reversible
The pain may recur, but the patient can have an influence on his/her symptoms and their consequences
Imaging studies are of little use in this situation, and therefore not indicated
*modified from (e13)
History.
A meticulously obtained history generally yields important information for the assessment of the back pain experience. The patient’s description of the pain should be thoroughly documented.
Treatment should be given sparingly and oriented to the patient’s pain and current functional status.
With regard to non-pharmacological treatments for acute low back pain, exercise therapy is no more effective than the continuation of normal activity (e14). Conversely, reduced activity and bed rest have been shown to have no effect or to lead to worsening of the pain and delayed resumption of daily activities (6). Patients suffering from subacute (> 6 weeks) nonspecific low back pain who have psychosocial risk factors for chronification should be offered cognitive behavioral therapy (CBT) tailored to their individual risk profile (6). It is best for CBT and progressive muscle relaxation to be introduced after the patient has been assessed in an interdisciplinary, multimodal treatment program. Preventive back exercises, techniques of manual medicine, and relaxation techniques can be used (grade B recommendation) if the first-line treatments mentioned above are ineffective.
The goal of pharmacotherapy for low back pain is to enable patients to continue or recommence their normal daily activities (Table 3).
Table 3. Recommendations for the oral drug treatment of nonspecific low back pain, with evidence-based doses*1.
| Drug recommendation | Dosage | Recommendation*2 | Recommendation grade |
|---|---|---|---|
|
Nonsteroidal anti-inflammatory drugs Ibuprofen Diclofenac Naproxen |
1.2 g/d, at most 2.4 g 100 mg/d, at most 150 mg 750 mg/d, at most 1.25 g |
Positive (“should”) (“should”) (“should”) |
B B B |
| COX-2 inhibitors (off-label use for acute low back pain) |
Celecoxib 200 mg/d Etoricoxib 60–90 mg/d |
Open (“can”) | 0 |
| Paracetamol (acetaminophen) | 500–1000 mg/d, at most 3 g | Open (“can”) | 0 |
|
Low-potency opioids Tramadol Tilidin N |
Depending on the preparation 50–100 mg 50–100 mg |
Open (“can”) | 0 |
*1modified from (6)
*2The recommendations and grades listed here (positive [“should”] and open [“can”]) are derived from the German National Disease Management Guideline for Low Back Pain (6),which employs the evidence classification of the Centre for Evidence Based Medicine (CEBM) at the University of Oxford.
Initial treatment.
The overriding goal in the primary treatment of low back pain is symptomatic relief, i.e., acute reduction of the pain.
Paracetamol (acetaminophen) is considered an optional drug in view of its questionable efficacy and insufficiently recognized side effects (e15, e16, 23). Rather, the traditional nonsteroidal anti-inflammatory drugs (t-NSAIDs) are recommended, with adherence to the recommended doses and monitoring for side effects (Table 3). In general, any analgesic drug for low back pain should be given at the lowest effective dose for the shortest possible time (6). The parenteral administration of NSAIDs or COX-2 inhibitors is not recommended because of their adverse effects and unproven efficacy (6). Metamizole is considered a reserve analgesic in the light of current data, particularly concerning side effects (6). COX-2 inhibitors can be used to treat acute, non-specific back pain (as long as the relevant warnings are heeded) if the traditional NSAIDs are contraindicated or poorly tolerated (6). Flupirtine has additional muscle-relaxing properties, but, in the light of current evidence, particularly concerning side effects, it should only be given to treat acute pain for a maximum of two weeks, with weekly checking of the liver function (e17). Insufficient evidence is available to judge other muscle relaxants, e.g., methocarbamol, for the systemic treatment of painful muscle tension (6). If the recommended analgesic drugs (and NSAIDs in particular) are ineffective or poorly tolerated, patients with nonspecific low back pain can be given low-potency opioids such as tramadol or tilidine, with close clinical follow-up (6). Invasive treatments and surgery are not recommended (6).
Acute, specific low back pain
Patients with neurologic findings such as muscle weakness, impaired sensation in the lower limbs, and bladder or bowel disturbances should undergo a neurological examination including testing of sensation, muscle strength (on the 5-point MRC scale), intrinsic muscle reflexes, and nerve-stretching tests.
Electrophysiologic testing is indicated if the patient’s pain is unclear or difficult to classify or if it is apparently of peripheral origin. Electromyography (EMG) is unnecessary if the clinical and radiological findings are entirely concordant.
An overview of the differential diagnosis and treatment of specific low back pain for patients who need immediate medical attention is given in eTable 3, and a comparable table for patients with non-urgent problems is given in eTable 4.
eTable 3. Specific causes of low back pain that need immediate treatment (red flags).
| Disease | Findings | Further evaluation | Treatment |
|---|---|---|---|
Fracture
|
|
Imaging studies:
|
Conservative:
Prevention:
|
| Massive disc herniation |
|
|
Surgical:
|
| Bacterial infection(spondylitis/ spondylodiscitis, epidural or paravertebral abscess) |
|
|
The indication for conservative vs. operative treatment (debridement, filling of defects, instrumentation) depends on:
|
| Tumor |
|
Imaging studies - local at first, then staging studies to rule out instability (SINS):
|
Neurologic deficit present:
Neurologic deficit absent: discuss plan in interdisciplinary tumor board Conservative:
|
CBC, complete blood count; CRP, C-reactive protein; CT, computerized tomography; EMG, electromyography; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; SINS, spinal instability in neoplastic disease; SSEP, somatosensory evoked potentials
eTable 4. Specific types of low back pain that require further diagnostic evaluation.
| Disease | Findings | Further evaluation | Treatment |
|---|---|---|---|
| Disk herniation |
|
Imaging studies: (DD herniation vs. stenosis vs. tumor)
Neurological/electrophysiological testing:
|
Depending on the clinical findings: conservative/interventional:
|
| Spinal canal stenosis / degenerative instability |
|
Abnormally flexed posture of trunk imaging studies:
neurological/electrophysiological testing:
|
Depending on the clinical findings: conservative:
interventional: PDA, sacral block surgical:
|
| Axial spondylitis andseronegative spondyloarthropathy |
Inflammatory back pain syndrome
|
Imaging studies:
rheumatologic consultation |
|
Deformities
|
Clinical features:
|
Early detection in children! Imaging studies:
|
Depending on the patient’s age and on the cause and severity of the deformity:
|
| Herpes zoster |
|
Lumbar puncture and CSF examination:
|
|
| Diabetic radiculopathy |
|
|
Pharmacotherapy:
|
| Neuroborreliosis |
|
Lumbar puncture and CSF examination:
|
|
| Spinal ischemia |
|
|
|
AB, antibodies; CT, computerized tomography; CSF, cerebrospinal fluid; DD, differential diagnosis; ENG, electroneurography; EMG, electromyography; MRI, magnetic resonance imaging; NCS, nerve conduction study; NSAID, nonsteroidal anti-inflammatory drug; PDA, peridural anesthesia; SSEP, somatosensory evoked potentials; SSNRI, selective serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant
Acute, nonspecific low back pain.
As only a few patients with low back pain have red flags, while far more have functional disturbances, physical examination plays an important role.
The vertebral bodies are generally overrated as a source of low back pain. Pain of extravertebral origin, arising from neighboring organs rather than from the bony spine or its associated muscles, discs, and ligaments (Box 3), is estimated to account for at least 2% of the cases of low back pain that are seen in primary care (10) and should therefore always be kept in mind (6).
Box 3. Extraspinal causes of low back pain*.
abdominal and visceral processes, e.g., cholecystitis, pancreatitis, tumors
vascular processes, e.g., aortic aneurysm
gynecological/urological processes, e.g., urolithiasis, renal tumors, perirenal abscess, endometriosis, pelvic tumors
neurological diseases, e.g., polyneuropathy, herpes zoster
psychosomatic and psychiatric diseases
*modified from (6)
Imaging studies
These should only be ordered for strict indications because of their possible side effects and the danger of overdiagnosis leading to chronification. Imaging is necessary if any red flags are present (5). The clinical suspicion of a fracture, infection, or radiculopathy is an indication for MRI in preference to CT, as MRI is more sensitive than CT for these conditions and, unlike CT, does not expose the patient to ionizing radiation (5). This also holds for fractures whose precise locality, type, and age (osteoporotic fracture) are of clinical importance. Moreover, dynamic plain films obtained after acute traumatic changes have been ruled out permit assessment of the spine in motion. The choice of imaging study can also be influenced by local availability and cost (6). No imaging is needed in the initial evaluation of acute low back pain if there are no features in the history or physical examination that suggest a specific cause (24). If the pain acutely worsens, or persists and remains intractable for six weeks or more, an imaging study is indicated (6).
Non-pharmacological treatment.
With regard to non-pharmacological treatments for acute low back pain, exercise therapy is no more effective than the continuation of normal activity.
All imaging studies should be read by a radiologist, and the ordering physician should discuss the findings with the patient. These findings should be rationally correlated with the findings of the history and physical examination.
Laboratory testing
No laboratory tests should be obtained except to evaluate specific disease entities that are suspected on the basis of the history and physical examination. Ancillary laboratory testing is needed if there is clinical evidence that the pain has a specific cause.
Special aspects of a few important specific conditions will be discussed in what follows.
Lumbar disc herniation
The clinical recognition of neurologic deficits (if any are present) is the cornerstone of the diagnosis and treatment of lumbar disc herniation (Box 1).
Pharmacotherapy.
COX-2 inhibitors can be used to treat acute, non-specific back pain (as long as the relevant warnings are heeded) if the traditional NSAIDs are contraindicated or poorly tolerated.
In most cases of disc herniation, the pain abates spontaneously within six weeks. Further diagnostic studies are indicated if the pain persists or if neurologic deficits arise (eTable 3). The L5 and S1 nerve roots are the ones most commonly affected (in more than 80% of cases), owing to herniations of the L4/5 and L5/S1 intervertebral discs (25).
Radicular pain with no more than mild weakness is generally treated in the same way as pain of non-radicular origin, mainly with anti-inflammatory drugs, but sometimes also with drugs specifically directed against neuropathic pain, such as tricyclic antidepressants; the evidence base is inconsistent (26, 27). Patients should be mobilized as soon as possible with active physiotherapy, and they should return to work as soon as possible while being given adequate analgesic medication, generally NSAIDs, but sometimes also opioids over the short term. There is no evidence to support the use of oral steroid tapers (27).
If the pain persists despite treatment, and neurologic deficits arise, periradicular injections can relieve pain and promote physical activity (28, 30). Epidural steroid injections bring short- to intermediate-term relief (e18). Transforaminal epidural techniques are superior to periradicular injections (29).
If severe radicular symptoms persist despite appropriate, intensive conservative management for six weeks or more, with concordant clinical and radiological findings, surgery can be considered. Surgery is unequivocally indicated in cases of cauda equina syndrome with acute paraparesis and in cases of acute or progressive severe motor deficits due to nerve root compression (strength 3 or less on the MRC scale) (25). The main manifestations of cauda equina syndrome are urinary retention and a sensory deficit of variable extent in the lower lumbar and sacral dermatomes (“saddle anaesthesia”), which may be accompanied by severe radicular pain and mild weakness of the legs.
There is no significant difference between the long-term outcomes of patients treated conservatively and surgically in terms of symptoms and disability (29), but surgery brings more rapid recovery (e19, 30).
Tumors
Spinal tumors usually manifest themselves initially with nonspecific pain, and later with general functional deficits (e20). An actual swelling is seen in only 16% of cases (e21). The vast majority of spinal tumors (96%) are metastases (e22). The remaining 4% consist of primary benign and malignant tumors and so-called “tumor-like lesions” (e22, 31).
Imaging studies.
Imaging studies should only be ordered for strict indications because of their possible side effects and the danger of overdiagnosis leading to chronification. Imaging is necessary if any red flags are present.
Any clinical suspicion of a spinal tumor should prompt further diagnostic studies (e23, e24). Plain films, although they are a part of the standard diagnostic work-up, only reveal osteolytic processes when at least 30–50% of the bone substance is lost (e25). MRI is the current gold standard of diagnostic screening for spinal tumors (31) (eTable 3). The diagnosis and treatment of patients with spinal tumors should be discussed in an interdisciplinary tumor board.
Infections
Bacterial infections of the axial skeleton can arise by continuity, by hematogenous spread from an extraspinal infection, or iatrogenically by contamination during an invasive procedure (e26). They typically cause nonspecific pain that persists when the patient is at rest (e.g., in bed at night).
The acute phase of discitis/spondylodiscitis has nonspecific manifestations and is thus easily misinterpreted. This entity is rare, with an incidence of only 0.4-2.4 cases per 100,000 persons per year. The radiologically visible changes arise late in its course, and the rate of false-negative cultures can be as high as 30% (32). Nonspecific spondylodiscitis accounts for 2–7% of all cases of osteomyelitis and is the most common infectious entity; most cases of nonspecific spondylodiscitis are in the lumbar region (e27). This condition has two incidence peaks, one in early childhood and another between the ages of 50 and 60.
Plain films do not reveal destruction of the upper and lower vertebral body end plates until several weeks after the onset of spondylodiscitis.
Lumbar disc herniation.
The clinical recognition of neurologic deficits (if any are present) is the cornerstone of the diagnosis and treatment of lumbar disc herniation.
MRI can be used to diagnose this entity with high sensitivity (96–100%) and specificity (92%); as it reveals soft-tissue processes, it can detect discitis as well as the early stages of spondylodiscitis (33). CT is an alternative (e28). Scintigraphy can be used to search for the primary source of infection.
The most common pathogen is Staphylococcus aureus, accounting for 42–84% cases, followed by Gram-negative bacteria (4–30%) and streptococci/enterococci (5–30%) (33). There is no single, uniform treatment concept for spondylodiscitis. Successful conservative treatment is based on antibiotic administration and bed rest until the inflammatory parameters return to the normal range, followed by external immobilization in a corset. High-level evidence for this form of treatment is lacking (33).
Surgical treatment involves thorough debridement of the infected area, internal immobilization of the infected spinal segments with dorsal and, sometimes, ventral instrumentation, and prolonged antibiotic administration (34, 35).
Spinal tumors.
Spinal tumors usually manifest themselves initially with nonspecific pain, and later with general functional deficits.
Fractures
The spine can be injured in a traumatic event involving massive force, with resulting low back pain, but spinal fractures often arise spontaneously or after relatively mild trauma, generally because of osteoporosis. The incidence of radiologically detectable fractures in 55- to 79-year-old women is 1% per year; in men in the same age group, it is 0.6% per year (36). A woman over age 50 has a more than 60% chance of sustaining an osteoporotic fracture (e29).
Plain films still play an important role in diagnosis and follow-up observation. MRI (STIR sequence) is the method of choice for assessing the age of a fracture, which is an important consideration in the indications for treatment (eTable 3).
According to the current guidelines, osteoporotic fractures of the spine that do not cause spinal instability or neurologic deficits should be treated conservatively at first (36). Progressive vertebral body collapse and/or severe, intractable pain can be an indication for surgical measures such as cement augmentation (vertebroplasty, kyphoplasty) and spinal realignment with intravertebral weight-bearing prosthetic material (e30). 10–30% of patients with a first osteoporotic fracture will have a second one (37); thus, proper management involves not only the treatment of the fracture, but also the appropriate diagnosis and treatment of osteoporosis (a systemic disease) in line with current guidelines, to prevent further fractures.
Infections.
Bacterial infections of the axial skeleton can arise by continuity, hematogenous spread from an extraspinal infection, or iatrogenic contamination. They typically cause nonspecific pain when the patient is at rest (e.g., in bed at night).
An algorithm for the management of acute low back pain
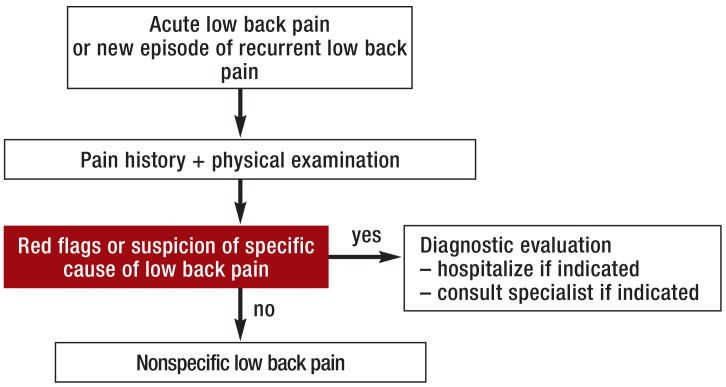
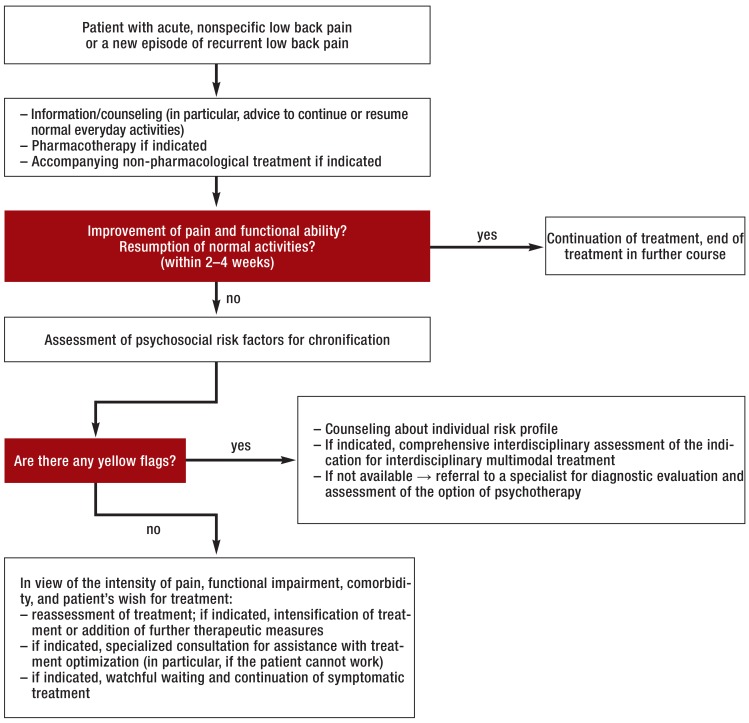
Red flags (Figure 1) should immediately prompt further diagnostic investigation and, if necessary, transfer to a center where spinal surgery can be performed. Patients with back pain of any specific type should be referred to the appropriate specialist(s). If a meticulously taken history and a thorough physical examination do not reveal any red flags or clear-cut patho-anatomical findings, there is no immediate indication for further ancillary diagnostic testing or invasive treatment (Figure 2). If there are psychosocial risk factors for the chronification of low back pain (yellow flags), and especially if the pain is persistent, the patient should undergo interdisciplinary assessment four to six weeks after the onset of pain to evaluate the indication for a multimodal treatment program; this is because payors in Germany now generally request a statement from the treating physician as soon as the patient has been unable to work for four weeks because of back pain. The remaining patients without any red or yellow flags should be extensively informed and counselled, in line with current guidelines, and should be given analgesic medication as needed (Figure 2). If low back pain persists despite six weeks of treatment in conformity with the guidelines, the patient should undergo comprehensive interdisciplinary evaluation (38) to determine whether treatment should be continued in the current setting or whether the patient should instead undergo an interdisciplinary multimodal pain treatment program, on either an inpatient or an outpatient basis, followed by an end assessment and an official statement on the prognosis, further treatment, and ability to work (39).
Figure 1.
The initial managment of acute low back pain
Figure 2.
Further diagnostic evaluation and treatment in acute, nonspecific low back pain (modified from [6])
Fractures.
Spinal fractures often arise spontaneously or after relatively mild trauma, generally because of osteoporosis.
The treatment of acute low back pain.
Red flags should immediately prompt further diagnostic investigation and, if necessary, transfer to a center where spinal surgery can be performed.
Further information on CME. This article has been certified by the North Rhine Academy for Postgraduate and.
Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing
medical education (CME) in accordance with the requirements of the Medical
Associations of the German federal states (Länder). CME points of the Medical
Associations can be acquired only through the Internet, not by mail or fax, by the
use of the German version of the CME questionnaire. See the following website:
cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit
“uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must
be entered in the appropriate field in the cme.aerzteblatt.de website under
“meine Daten” (“my data”), or upon registration. The EFN appears on each
participant’s CME certificate. This CME unit can be accessed until 26 June 2016, and earlier CME units until the dates indicated:
“The Presentation, Diagnosis, and Treatment of Sexually Transmitted Infections” (Issue 1–2/2016) until 4 April 2016;
“Inflammatory Bowel Disease” (Issue 5/2016) until 2 May 2016;
“Evidence-Based Hernia Treatment in Adults” (Issue 9/2016) until 29 May 2016.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the most appropriate answer.
Question 1
Which of the following is an indication for further diagnostic studies?
Weakness of hip flexion (strength 3 on the Janda scale)
Trigger points for the pain
Sacro-iliac joint syndrome
Lumbar hyperlordosis
Lumbago in the absence of trauma
Question 2
Which of the following is an indication for imaging (plain films and MRI)?
Lumbago of one week’s duration
A fall on the back with pain afterward
Lumbago of three weeks’ duration
Myogelosis in the lumbar paravertebral muscles
A functional disturbance of muscles
Question 3
What is the most important component of the initial treament of nonspecific acute low back pain?
Invasive treatment
Cortisone taper
Opioids
Bed rest
Thorough patient information and counseling
Question 4
What is the most important part of initial history-taking in a patient with acute low back pain?
The exclusion of red flags
The documentation of yellow flags
Monitoring of sleep behavior
Occupational stress situations
The documentation of earlier episodes of back pain
Question 5
What is the main clinical manifestation of cauda equina syndrome?
Marked lordosis
Stabbing pelvic pain
Multisegmental sensory deficit in the pelvic and crural area
Polyneuropathy
Circulatory disturbance in the legs
Question 6
What is the most common initial symptom of a spinal tumor?
Local swelling
Nonspecific pain
Papular rash
Saddle anesthesia
Lumbar myelogelosis
Question 7
What should the patient be told when specific causes of low back pain have been ruled out?
That he or she has an irreversible disturbance of spinal function
That he or she should continue all normal daily activities
That surgery is necessary, with various available options
That he or she should change jobs as soon as possible
That further diagnostic testing will soon follow
Question 8
What should be done if yellow flags are found?
Assessment of the indication for an interdisciplinary, multimodal treatment program
Further imaging studies
Intensified invasive treatment
Prescription of higher opioid doses
Avoidance of communication with other persons involved in treatment
Question 9
What is the most important diagnostic test for patients presenting with acute low back pain?
Electromyography
Plain films of the lumbar spine
Physical examination of sensation and motor function
MRI of the lumbar spine
Quantitative sensory testing (QST)
Question 10
What pathogen is the most common cause of discitis/spondylodiscitis?
Clostridium difficile
Influenza virus A/H2N2
Neisseria meningitidis
Staphylococcus aureus
Streptococcus pneumoniae
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Casser has served as a paid consultant for TEVA, Mucos Pharma, Grünenthal, and Janssen and has been paid for preparing continuing medical education events by Pfizer, TEVA, Grünenthal, Recordati, and Mundipharma.
Dr. Seddigh has received reimbursement of meeting participation fees from Grünenthal and has been paid for preparing scientific meetings by Lilly.
Prof. Rauschmann has been paid for preparing continuing medical education events by Aesculap, biomet depuy, Medacta, AAP, Spontec, and Paradigmen Spike.
References
- 1.Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen. Band III - 3. Bedarf, bedarfsgerechte Versorgung, Über-, Unter- und Fehlversorgung. www.svr-Gesundheit.de/index.php?id=160. (last accessed on 5 February 2016)
- 2.da C Menezes CL, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184:613–624. doi: 10.1503/cmaj.111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehling WE, Gopisetty V, Bartmess E, et al. The prognosis of acute low back paen in primary care in the United States. A 2-year prospective cohort study. Spine. 2012;37:678–684. doi: 10.1097/BRS.0b013e318230ab20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker A, Kögel K, Donner-Banzhoff N, et al. Kreuzschmerzpatienten in der hausärztlichen Praxis: Beschwerden, Behandlungserwartungen und Versorgungsdaten. Z Allg Med. 2003;79:126–131. [Google Scholar]
- 5.Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM) Kreuzschmerzen. Düsseldorf: DEGAM. (DEGAM-Leitlinie; 3). www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/LL-03_Kreuz_mod-007.pdf. 2003. (last accessed on 15 March 2016)
- 6.Nationale Versorgungs. Leitlinie Kreuzschmerz. www.leitlinien.de/mdb/downloads/nvl/kreuzschmerz/kreuzschmerz-1aufl-vers5-lang.pdf. (last accessed on 15 March 2016)
- 7.Brennan GP, Fritz JM, Hunter SJ, et al. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623–631. doi: 10.1097/01.brs.0000202807.72292.a8. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer A, Gärtner-Tschacher N, Schöttker-Königer T. Subgruppenspezifische Therapie lumbaler Rückenschmerzen. Darstellung und Gütekriterien zweier Klassifikationssysteme. Orthopäde. 2013;42:90–99. doi: 10.1007/s00132-012-2041-5. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan P. Diagnosis and classification of chronic low back disorder maladaptive movement and motor control impairments a underlying mechanism. Man Ther. 2005;10:242–255. doi: 10.1016/j.math.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 11.Koes BW, van Tulder MW, Thomcaas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–1434. doi: 10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymann W. Differenzialdiagnostik und Therapie des akuten Kreuzschmerzes. Manuelle Medizin. 2013;51:77–88. [Google Scholar]
- 13.Fanuele JC, Birkmeyer NJ, Abdu WA, Tostison TD, Weinstein JN. The impact of spinal problems on the health status of patients: have we underestimated the affect? Spine. 2000;25:1509–1514. doi: 10.1097/00007632-200006150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Diener HC. Schmerzbegriffe. In: Diener HC, Meier CH, editors. Schmerztherapie - medikamentös - interventionell - psychologisch. München: Urbach & Schwarzenberg; 1997. pp. 3–5. [Google Scholar]
- 15.Downie A, Williams CM, Henschke N, et al. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ. 2013;347 doi: 10.1136/bmj.f7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;(Suppl 2):192–300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt CO, Raspe H, Pfingsten M. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine. 2007;32:2005–2011. doi: 10.1097/BRS.0b013e318133fad8. [DOI] [PubMed] [Google Scholar]
- 18.Neubauer E, Junge A, Pirron P. HKF-R 10—screening for predicting chronicity in acute low back pain (LBP): a prospective clinical trial. Eur J Pain. 2006;10:559–566. doi: 10.1016/j.ejpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive-behavior intervention and two forms of information for patients with spinal pain. Spine. 2000;25:2825–2831. doi: 10.1097/00007632-200011010-00017. [DOI] [PubMed] [Google Scholar]
- 20.Hallner D, Hasenbring M. Classification of psychosocial risk factors (yellow flags) for the development of chronic low back and leg pain using artificial neural network. Neurosci Lett. 2004;361:151–154. doi: 10.1016/j.neulet.2003.12.107. [DOI] [PubMed] [Google Scholar]
- 21.van der Windt D, Hay E, Jellema P, Maen C. Psychosocial interventions for low back pain in primary care. Spine. 2008;33:81–89. doi: 10.1097/BRS.0b013e31815e39f9. [DOI] [PubMed] [Google Scholar]
- 22.van der Windt DA, Simons E, Riphagen II, et al. Physical examination for lumbar radiculopathy due two disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD007431.pub2. (2:CD007431) [DOI] [PubMed] [Google Scholar]
- 23.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: A double-blind randomized controlled trial. Lancet. 2014;384:1586–1596. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 24.Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463–472. doi: 10.1016/S0140-6736(09)60172-0. [DOI] [PubMed] [Google Scholar]
- 25.Deutsche Gesellschaft für Neurologie. Leitlinie lumbale Radikulopathie. www.awmf.org/uploads/tx_szleitlinien/030-058l_S2k_Lumbale_Radikulopathie_2013_1.pdf. (last accessed on 5. February 2016)
- 26.Moskowitz MH. Pharmacotherapy neuropathic low back pain. Current Pain and Headache Reports. 2003;7:178–187. doi: 10.1007/s11916-003-0071-8. [DOI] [PubMed] [Google Scholar]
- 27.Balagoe F, Piguet V, Dudler J. Steroids for LBP-from rationale two inconvenient truth. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13566. [DOI] [PubMed] [Google Scholar]
- 28.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the maine lumbar spine study. Spine. 2005;30:927–935. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 29.Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: Guidance and recommendations. Pain Physician. 2013;16:49–283. [PubMed] [Google Scholar]
- 30.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CM, Henschke N, Maher CG, et al. Red flags to screen for vertebral fracture in patients presenting with low back pain. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD008643.pub2. CD008643. [DOI] [PubMed] [Google Scholar]
- 32.Frangen TM, Kalicke T, Gottwald M. Surgical management of spondylodiscitis. An analysis of 78 cases. Unfallchirurg. 2006;109:743–753. doi: 10.1007/s00113-006-1084-7. [DOI] [PubMed] [Google Scholar]
- 33.Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181–187. doi: 10.3238/arztebl.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiedenhöfer B, Hemmer S, Akbar M, Lehner B, Schmidmaier G, Klöckner C. Goldstandard bei der Implantatwahl zur operativen Therapie der Spondylitis/Spondylodiszitis. Orthopäde. 2012;41:721–726. doi: 10.1007/s00132-012-1916-9. [DOI] [PubMed] [Google Scholar]
- 35.Akbar M, Lehner B, Doustdar S, et al. Pyogene Spondylodiszitis der Brust- und Lendenwirbelsäule. Eine neue Klassifikation zur Entscheidungsfindung bei der Wahl der operativen Therapie. Der Orthopäde. 2011;40:614–623. doi: 10.1007/s00132-011-1742-5. [DOI] [PubMed] [Google Scholar]
- 36.Dachverband deutschsprachiger wissenschaftlicher Fachgesellschaften für Osteologie. DVO-Leitlinie 2009 zur Prophylaxe, Diagnostik und Therapie der Osteoporose bei Erwachsenen. Osteologie. 2009;18:304–328. [Google Scholar]
- 37.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture: a randomized controlled trial. Lancet. 2009;373:1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 38.Casser HR, Arnold B, Brinkschmidt T, et al. Interdisziplinäres Assessment zur multimodalen Schmerztherapie. Indikation und Leistungsumfang. Schmerz. 2013;27:363–370. doi: 10.1007/s00482-013-1337-7. [DOI] [PubMed] [Google Scholar]
- 39.Arnold B, Brinkschmidt T, Casser HR, et al. Multimodale Schmerztherapie. Konzepte und Indikation. Schmerz. 2009;23:112–120. doi: 10.1007/s00482-008-0741-x. [DOI] [PubMed] [Google Scholar]
- e1.Lieb K. Chronifizierung von Rückenschmerzen in der Lübecker Bevölkerung. Eine Analyse unter besonderer Berücksichtigung des Amplifikationsmodells. Inauguraldissertation. www.zhb.uni-luebeck.de/epubs/ediss322.pa (last accessed on 14 March 2016) [Google Scholar]
- e2.Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327 doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12:437–460. [PubMed] [Google Scholar]
- e4.Arzneimittelkommission der deutschen Ärzteschaft. Empfehlungen zur Therapie von Kreuzschmerzen. www.akdae.de/Arzneimitteltherapie/TE/A-Z/PDF/Kreuzschmerz.pdf. 3rd edition. (last accessed on 14 March 2016)
- e5.Downie A, Williams CHM, Henschke N, et al. Red flags to scream for malignancy and fracture in patients with low back pain: Systematic review. BMJ. 2013;347 doi: 10.1136/bmj.f7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Grotle M, Vollestad NK, Brox JI. Screening for yellow flags in first-time acute low back pain: reliability and validity of a Norwegian version of the Acute Low Back Pain Screening Questionnaire. Clin J Pain. 2006;22:458–467. doi: 10.1097/01.ajp.0000208243.33498.cb. [DOI] [PubMed] [Google Scholar]
- e7.Hurley DA, Dusoir TE, McDonough SM, Moore AP, Baxter GD. How effective is the acute low back pain screening questionnaire for predicting 1-year follow-up in patients with low back pain? Clin J Pain. 2001;17:256–263. doi: 10.1097/00002508-200109000-00012. [DOI] [PubMed] [Google Scholar]
- e8.Riddle DL, Freburger JK. Evaluation of the presence of sacroiliac joint region dysfunction using a combination of tests: a multicenter intertester reliability study. Phys Ther. 2002;82:772–781. [PubMed] [Google Scholar]
- e9.Hill JC, Whitehurst DGT, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;37:1560–1571. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10:207–218. doi: 10.1016/j.math.2005.01.003. [DOI] [PubMed] [Google Scholar]
- e12.Kokmeyer DJ, van der Wurff P, Aufdemkampe G, Fickenscher TC. The reliability of multitest regimens with sacroiliac pain provocation tests. J Manipulative Physiol Ther. 2002;25:42–48. doi: 10.1067/mmt.2002.120418. [DOI] [PubMed] [Google Scholar]
- e13.Bertelsmann-Stiftung (eds.) Gesundheitspfad Rücken - Innovative Konzepte zur Verbesserung der Versorgung von Patienten mit Rückenschmerzen. www.bertelsmann-stiftung.de/de/publikationen/publikation/did/gesundheitspfad-ruecken. (last accessd on 5 February 2016)
- e14.Hayden JA, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev 2005. doi: 10.1002/14651858.CD000335.pub2. CD000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Wehling M. Paracetamol. Wirksam und sicher bis ins hohe Alter. Schmerz. 2013;27:20–25. doi: 10.1007/s00482-012-1270-1. [DOI] [PubMed] [Google Scholar]
- e16.Davies RA, Maher CG, Hancock MJ. A systematic review of paracetamol for non-specific low back pain. Eur Spine. 2008;17:1423–1430. doi: 10.1007/s00586-008-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Rote-Hand-Brief Flupirtin. www.akdae.de/Arzneimittelsicherheit/RHB/Archiv/2013/20130716.pdf. (last accessed on 14 March 2016) [Google Scholar]
- e18.Kang SS, Hwang BM, Son HJ, et al. The dosages of corticosteroid in transforaminal epidural steroid injections for lumbar radicular pain due to a herniated disc. Pain Physician. 2011;14:361–370. [PubMed] [Google Scholar]
- e19.Peul WC, van Houwelingen HC, van den Hout WB. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- e20.Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level - listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol) 2002;14:472–480. doi: 10.1053/clon.2002.0098. [DOI] [PubMed] [Google Scholar]
- e21.Landreneau FE, Landreneau RJ, Keenan RJ, Ferson PF. Diagnosis and management of spinal metastases from breast cancer. J Neurooncol. 1995;23:121–134. doi: 10.1007/BF01053417. [DOI] [PubMed] [Google Scholar]
- e22.Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;3:141–151. doi: 10.1016/j.suronc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- e23.Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control. 2012;19:122–128. doi: 10.1177/107327481201900206. [DOI] [PubMed] [Google Scholar]
- e24.Quraishi NA, Kokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal dord. J Bone Joint Surg Br. 2010;92:1054–1060. doi: 10.1302/0301-620X.92B8.22296. [DOI] [PubMed] [Google Scholar]
- e25.Schirrmeister H. Detection of bone metastases in breast cancer by positron emission tomography. Radio Clin North Am. 2007;45:669–676. doi: 10.1016/j.rcl.2007.05.007. [DOI] [PubMed] [Google Scholar]
- e26.Wirth CJ, Mutschler W. Stuttgart: Thieme Verlag; 2007. Praxis der Orthopädie und Unfallchirurgie. [Google Scholar]
- e27.Bhagat S, Mathieson C, Jandhyala R, Hohnston R. Spondylodiscitis (disc space infection) associated with negative microbiological tests: comparison of outcome of suspected disc space infections to documented non-tuberculous pyogenic discitis. Br J Neurosurg. 2007;21:473–477. doi: 10.1080/02688690701546155. [DOI] [PubMed] [Google Scholar]
- e28.Eichler M, Weber MA, Hähnel S, Rehnitz CH. Radiologische Diagnostik entzündlicher Wirbelkörpererkrankungen - Was ist „state of art“? Orthopäde. 2012;41:711–720. doi: 10.1007/s00132-012-1915-x. [DOI] [PubMed] [Google Scholar]
- e29.Bergmann P, Body JJ, Boonen S, et al. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2002;1:19–26. doi: 10.1111/j.1742-1241.2008.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e30.van Meirhaeghe J, Bastian L, Boonen S, et al. A randomized trial of balloon kyphoplasty and non-surgical management for treating acute vertebral compression fractures: Vertebral body kyphosis correction and surgical parameters. Spine. 2013;38:971–983. doi: 10.1097/BRS.0b013e31828e8e22. [DOI] [PMC free article] [PubMed] [Google Scholar]