Abstract
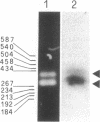
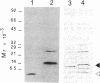
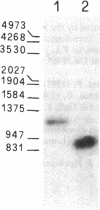
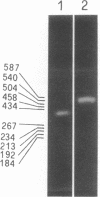
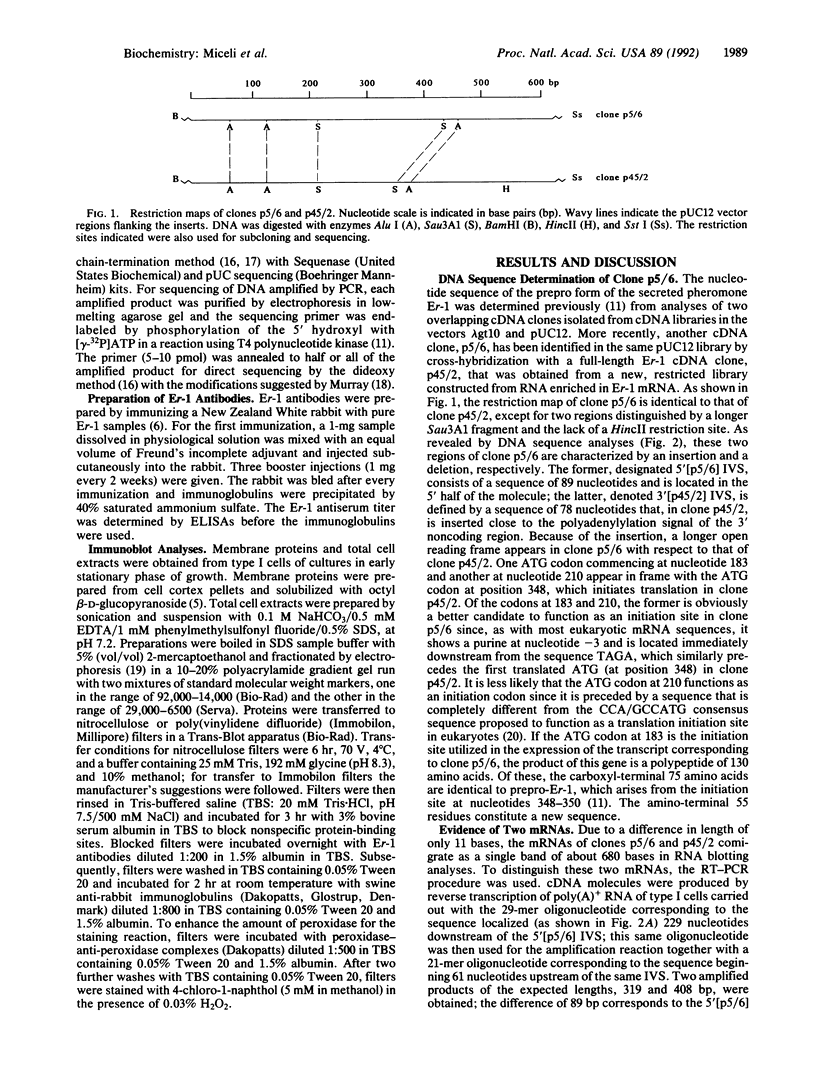
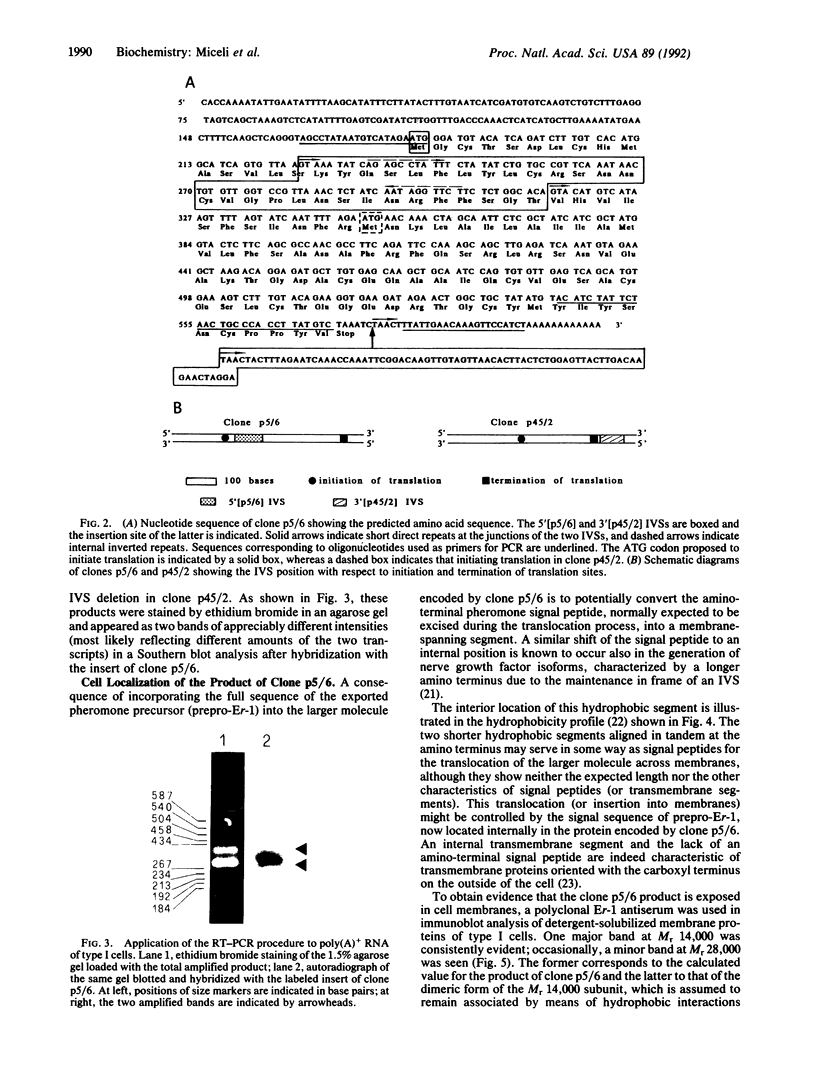
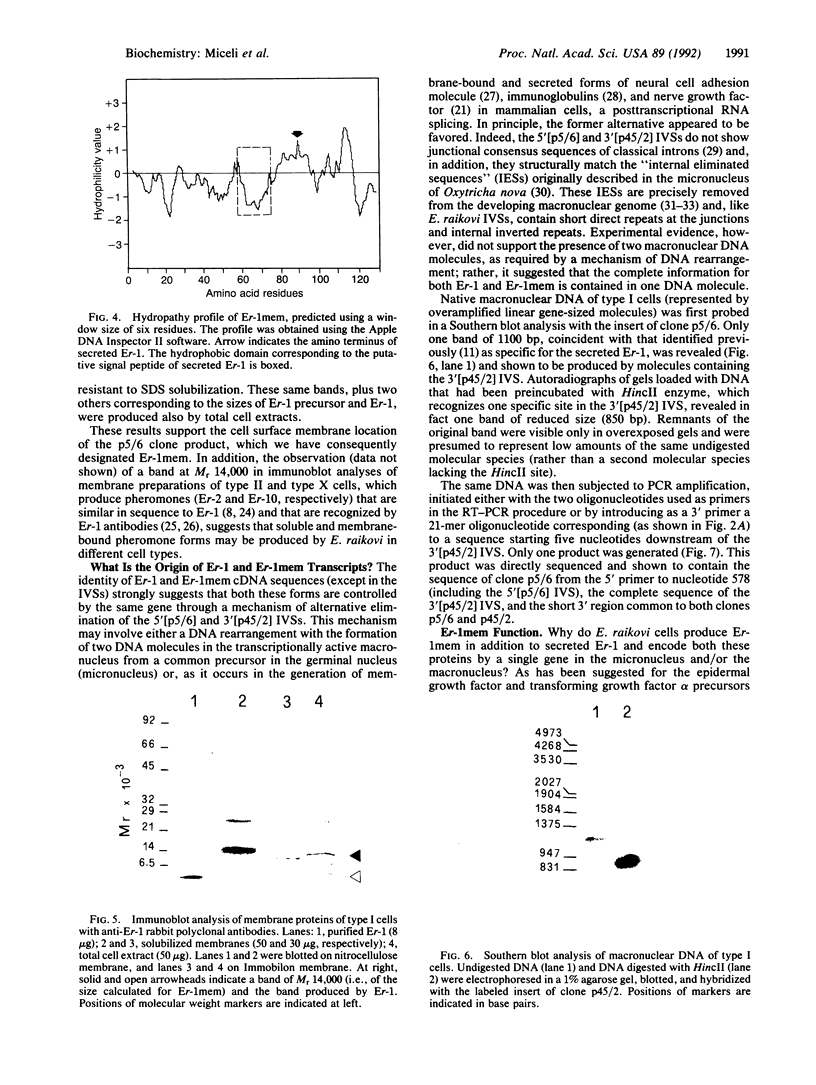
In the ciliate Euplotes raikovi, the same cell that secretes the pheromone Er-1, a polypeptide of 40 amino acids derived from a precursor (prepro-Er-1) of 75 amino acids, also produces a polypeptide of 130 amino acids, of which the 75 residues at the carboxyl terminus are identical to those of prepro-Er-1 and the 55 residues at the amino terminus form a new sequence. This larger Er-1 isoform is retained in membranes, where it may function as a binding site for soluble Er-1 in a mechanism of autocrine secretion. Membrane-bound and soluble Er-1 are translated from two mRNAs that apparently originate from a common micronuclear and/or macronuclear gene through alternative elimination of intervening sequences. This finding suggests that single genes responsible for the generation of isoform diversity in polypeptide hormones are present even in single-celled eukaryotes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Raffioni S., Luporini P., Bradshaw R. A., Eisenberg D. Crystallization of the Euplotes raikovi mating pheromone Er-1. J Mol Biol. 1990 Nov 5;216(1):1–2. doi: 10.1016/S0022-2836(05)80055-5. [DOI] [PubMed] [Google Scholar]
- Beale G. Self and nonself recognition in the ciliate protozoan Euplotes. Trends Genet. 1990 May;6(5):137–139. doi: 10.1016/0168-9525(90)90132-p. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Concetti A., Raffioni S., Miceli C., Barra D., Luporini P. Purification to apparent homogeneity of the mating pheromone of mat-1 homozygous Euplotes raikovi. J Biol Chem. 1986 Aug 15;261(23):10582–10586. [PubMed] [Google Scholar]
- Csank C., Taylor F. M., Martindale D. W. Nuclear pre-mRNA introns: analysis and comparison of intron sequences from Tetrahymena thermophila and other eukaryotes. Nucleic Acids Res. 1990 Sep 11;18(17):5133–5141. doi: 10.1093/nar/18.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Rutter W. J. Differential RNA splicing predicts two distinct nerve growth factor precursors. 1986 Feb 27-Mar 5Nature. 319(6056):784–787. doi: 10.1038/319784a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fiori P. L., Miceli C., Raffioni S., Vallesi A. Specific and common epitopes in mating pheromones of Euplotes raikovi revealed by monoclonal antibodies. J Protozool. 1990 May-Jun;37(3):187–190. doi: 10.1111/j.1550-7408.1990.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Gower H. J., Barton C. H., Elsom V. L., Thompson J., Moore S. E., Dickson G., Walsh F. S. Alternative splicing generates a secreted form of N-CAM in muscle and brain. Cell. 1988 Dec 23;55(6):955–964. doi: 10.1016/0092-8674(88)90241-3. [DOI] [PubMed] [Google Scholar]
- Greslin A. F., Prescott D. M., Oka Y., Loukin S. H., Chappell J. C. Reordering of nine exons is necessary to form a functional actin gene in Oxytricha nova. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6264–6268. doi: 10.1073/pnas.86.16.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luporini P., Raffioni S., Concetti A., Miceli C. The ciliate Euplotes raikovi heterozygous at the mat genetic locus coreleases two individual species of mating pheromone: Genetic and biochemical evidence. Proc Natl Acad Sci U S A. 1986 May;83(9):2889–2893. doi: 10.1073/pnas.83.9.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990 Dec 15;265(35):21393–21396. [PubMed] [Google Scholar]
- Miceli C., La Terza A., Bradshaw R. A., Luporini P. Structural characterization of mating pheromone precursors of the ciliate protozoan Euplotes raikovi. High conservation of pre and pro regions versus high variability of secreted regions. Eur J Biochem. 1991 Dec 18;202(3):759–764. doi: 10.1111/j.1432-1033.1991.tb16430.x. [DOI] [PubMed] [Google Scholar]
- Miceli C., La Terza A., Melli M. Isolation and structural characterization of cDNA clones encoding the mating pheromone Er-1 secreted by the ciliate Euplotes raikovi. Proc Natl Acad Sci U S A. 1989 May;86(9):3016–3020. doi: 10.1073/pnas.86.9.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortenzi C., Miceli C., Bradshaw R. A., Luporini P. Identification and initial characterization of an autocrine pheromone receptor in the protozoan ciliate Euplotes raikovi. J Cell Biol. 1990 Aug;111(2):607–614. doi: 10.1083/jcb.111.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffioni S., Luporini P., Bradshaw R. A. Purification, characterization, and amino acid sequence of the mating pheromone Er-10 of the ciliate Euplotes raikovi. Biochemistry. 1989 Jun 13;28(12):5250–5256. doi: 10.1021/bi00438a049. [DOI] [PubMed] [Google Scholar]
- Raffioni S., Luporini P., Chait B. T., Disper S. S., Bradshaw R. A. Primary structure of the mating pheromone Er-1 of the ciliate Euplotes raikovi. J Biol Chem. 1988 Dec 5;263(34):18152–18159. [PubMed] [Google Scholar]
- Raffioni S., Miceli C., Concetti A., Barra D., Luporini P. Purification and characterization of new mating pheromones of the ciliate Euplotes raikovi. Exp Cell Res. 1987 Oct;172(2):417–424. doi: 10.1016/0014-4827(87)90399-5. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Scott J., Bell G. I., Crawford R. J., Penschow J. D., Niall H. D., Coghlan J. P. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985 Jan 17;313(5999):228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- Ribas-Aparicio R. M., Sparkowski J. J., Proulx A. E., Mitchell J. D., Klobutcher L. A. Nucleic acid splicing events occur frequently during macronuclear development in the protozoan Oxytricha nova and involve the elimination of unique DNA. Genes Dev. 1987 Jun;1(4):323–336. doi: 10.1101/gad.1.4.323. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Kuehl M. Biosynthesis and regulation of immunoglobulins. Annu Rev Immunol. 1983;1:393–422. doi: 10.1146/annurev.iy.01.040183.002141. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]