Abstract
Background
Multiple hypersensitivities (MHS) have been described in humans, cats, and dogs, but not horses.
Hypotheses
Horses suffering from recurrent airway obstruction (RAO), insect bite hypersensitivity (IBH), or urticaria (URT) will have an increased risk of also being affected by another one of these hypersensitivities. This predisposition for MHS also will be associated with decreased shedding of strongylid eggs in feces and with a single nucleotide polymorphism (SNP BIEC2‐224511), previously shown to be associated with RAO.
Animals
The first population (P1) included 119 randomly sampled horses representative of the Swiss sporthorse population; the replication population (P2) included 210 RAO‐affected Warmblood horses and 264 RAO‐unaffected controls. All horses were Warmbloods, 14 years or older.
Methods
Associations between disease phenotypes (RAO, IBH, URT, MHS) fecal egg counts, the SNP BIEC2‐224511 as well as management and environmental factors were investigated.
Results
In P1, RAO‐affected horses had a 13.1 times higher odds ratio (OR) of also suffering from IBH (P = .004). In P2, the respective OR was 7.4 (P = .002) and IBH‐affected horses also showed a 7.1 times increased OR of concomitantly suffering from URT (P < .001). IBH, URT, and MHS phenotypes were significantly associated with the absence of nematode eggs in the feces.
Conclusions and Clinical Importance
This is the first report of MHS in horses. Specifically, an increased risk for IBH should be expected in RAO‐affected horses.
Keywords: Airway disease, Equine, Genetics, Horse Owner Assessed Respiratory Signs Index
Abbreviations
- CI
confidence interval
- EpG
eggs per gram of feces
- HOARSI
Horse Owner Assessed Respiratory Signs Index
- IBH+
IBH‐affected horses
- IBH−
IBH‐unaffected horses
- IBH
insect bite hypersensitivity
- IgE
serum immunoglobulin E
- IL4RA
interleukin‐4 receptor α chain
- MHS+
MHS‐affected horses
- MHS−
MHS‐unaffected horses
- MHS
multiple hypersensitivities
- OR
odds ratio
- P1
randomly sampled population 1
- P2
replication population 2
- RAO+
RAO‐affected horses
- RAO−
RAO‐unaffected horses
- RAO
recurrent airway obstruction
- SNP
single nucleotide polymorphism
- Th2
T‐helper‐2
- URT+
URT‐affected horses
- URT
urticaria
- URT−
URT‐unaffected horses
Hypersensitivity disorders are important in horses, with recurrent airway obstruction (RAO), insect bite hypersensitivity (IBH), and urticaria (URT) among the most frequently observed.1, 2, 3, 4, 5, 6 In other species, including humans, dogs, and cats, a combined occurrence of multiple hypersensitivities (MHS) affecting different organ systems has been described. Feline atopy, for instance, includes not only different syndromes of pruritic dermatoses, but also feline asthma.7 Furthermore, the combined occurrence of allergic dermatitis, conjunctivitis, rhinitis, and asthma‐like clinical signs has been reported both in naturally occurring and experimental hypersensitivities in dogs.8 The occurrence of MHS, such as asthma, rhinitis, and allergic skin diseases, in the same individual also has been described in humans.9, 10, 11, 12 A temporal sequence of hypersensitivities has been termed an “allergic march” with childhood atopic dermatitis being the precursor to allergic rhinitis and asthma years later.12
Investigating MHS may improve our understanding of the background of these hypersensitivity disorders. There is evidence that specific genetic determinants may predispose to an allergic phenotype in general.9 For instance, interleukin‐4 receptor α‐chain (IL4RA) has been identified as a candidate gene in atopic dermatitis, rhinitis, and asthma. In addition, IL4RA has been linked to an “allergic predisposition” and is associated with increased serum immunoglobulin E (IgE) concentrations in asthma‐affected humans.9, 10, 11 Polymorphisms in IL13, a gene involved in the same T‐helper‐2 (Th2)‐type response as IL4RA, are associated with both rhinitis and asthma, and rhinitis was shown to be a risk factor for the development of asthma.10 Furthermore, patients carrying a mutation in a profilaggrin/filaggrin gene which is associated with a severe form of atopic dermatitis also have an increased risk to develop allergic asthma.11
The pathogenesis of RAO has remarkable similarities to asthma in humans,1, 13, 14 one of these is a genetic association with IL4RA. Genetic linkage between equine RAO and a region on equine chromosome (ECA) 13 containing the gene for IL4RA was described in a high‐prevalence RAO family.15 An association of RAO with a single nucleotide polymorphism (SNP BIEC2‐224511) on ECA 13 near IL4RA recently was confirmed in unrelated cases and controls.16 Moreover, RAO has been shown to be associated with increased resistance against strongylid nematodes,17, 18 which is in agreement with the hygiene hypothesis first proposed by Strachnan.19 Except for a preliminary report describing an association of RAO with URT in a limited cohort of a RAO high‐prevalence family,20 MHS so far have not been reported in horses. Therefore, the objective of this study was to test the hypothesis that horses suffering from a hypersensitivity, specifically RAO, IBH, or URT, have an increased risk of also being affected by another one of these hypersensitivities. Furthermore, we wanted to investigate if environmental and management factors (eg, husbandry, bedding type, pasture access, deworming practice), nematode eggs per gram of feces (EpG), and SNP BIEC2‐224511 are linked to the occurrence of these hypersensitivities.
Materials and Methods
Study Design
First, a population consisting of a random sample representative of the Swiss sporthorse population (P1) was examined. To test whether results could be replicated, a second population of Warmblood horses consisting of RAO‐affected cases (RAO+) and RAO‐unaffected controls (RAO−) was investigated (P2). The study was approved by the ethical committee of the Canton of Berne (BE33/07, BE58/10, and BE10/13).
Horses
Warmbloods of at least 14 years of age were recruited into this study, to minimize false negative phenotyping. This is important especially with RAO, because clinical signs can develop relatively late in life.1, 14 Based on an error level of 5% and confidence level of 95%, a minimum sample size was calculated1 for P1, which then was randomly selected from the horse register of the Swiss Federation of the Equestrian Sports as described in detail in a previous publication.21 After selection based on age, P1 consisted of 119 horses.
P2 consisted of 474 horses recruited from the University clinics of Berne, Giessen, and Brno and living in Switzerland, Germany, the Czech Republic, Hungary, and Belgium. P2 represents a subset of a previously investigated population.16 Horses were selected based on age and breed (as defined above), and excluded if there was incomplete information or first degree parental relationships. The pedigree of the horse was investigated if possible with the aim to recruit a Warmblood population with minimal parental relationships to minimize genetic clustering bias and maximize genetic heterogeneity. First‐degree parental relationships were only accepted if either mare or stallion had >1 descendent in P2 and these showed different RAO phenotypes (ie, if an individual parent had >1 descendent that could be equally allocated in the category RAO+ and RAO−).
Questionnaire and Classification Criteria
The classification into RAO+ and RAO− was based on the respiratory signs reported by the owners (eg, coughing, nasal discharge, type of breathing, performance) as has been described before.2 The RAO− group consisted of Horse Owner Assessed Respiratory Signs Index (HOARSI) 1 (unaffected) and RAO+ of HOARSI 3 and HOARSI 4 (moderate to severe clinical signs). Horses belonging to the HOARSI 2 (mild signs) group were excluded from this study. The owners also were asked about the environment of their horses at the time when they had the most severe clinical signs, using a previously described standardized questionnaire,2 which included information about animal husbandry and management, such as bedding type, access to pasture, contact with donkeys, and frequency of deworming. All RAO+ horses had to have been exposed to hay at the time they showed signs of RAO. RAO− horses were included only if they had also been fed hay.
Owners were asked if their horses had been diagnosed with IBH, URT, or both. The horses were classified as having such if the diagnoses were confirmed by their private veterinarian based on clinical phenotypes. IBH was described as horses itching during the period of insect activity and presenting with loss of hair, mainly on the mane, tail, abdomen, or some combination of these. The clinical phenotype of URT was the sudden presence of wheals, occurring once or chronically. Owners were not asked if intradermal skin testing was performed. Occurrence of MHS was defined as ≥2 hypersensitivities (RAO, IBH, URT) manifesting in the same individual.
Fecal Sample Collection and Coprology
A majority of horses of P2 were available for collection of fecal samples by freshly voided or rectally obtained feces. If an anthelminthic agent was applied, the sample was collected after the expiration of the estimated egg reappearance period.22 Fecal samples were analyzed with a combined sedimentation and flotation method. If strongylid eggs were found, the individual was classified as positive for egg shedding.23 Results were expressed in EpG. Feces from horses that had contact with donkeys were further investigated using the Baermann funnel technique to detect evidence for infection with Dictyocaulus arnfieldi.23
Genotypic Association
Single nucleotide polymorphism BIEC2‐224511 genotypes were available for a subset of 471 P2 horses from a previously published study on the association of the T allele at this SNP with RAO.16 An association with the T allele at this SNP with RAO, IBH, URT, and MHS was investigated.
Statistics
Analyses were performed separately for the 2 populations with the statistical package NCSS 2007.2 After exploratory analysis, the variables of the coprologic and environmental factors were separated into 2 categories each:
Horses were classified as either positive or negative for egg shedding;
All horses were stabled. Stabling was classified either as indoor only without windows, or indoor with windows or free paddock access;
Type of bedding was classified as straw or other materials;
The access to pasture was either yes or no;
The frequency of deworming was 0–2 times per year or >2 times per year.
Cross tabulation tests (chi‐square or Fisher's exact test) then were performed to investigate associations of the above categorical egg shedding and management factors (P1 and P2) as well as the T allele at the SNP BIEC2‐224511 (P2) with RAO, IBH, and URT as well as with MHS. The alpha level of statistical significance was set at P < .05. When chi‐square or Fisher's exact test indicated a significant association, odds ratio (OR) and 95% confidence intervals (CI) were calculated by univariate or multivariate logistic regression analyses. Because age distributions were not normal in P1 and in P2, age differences between RAO+ and RAO− were tested by Kruskal‐Wallis one‐way ANOVA on ranks.
Results
Horses
The population of P1 comprised of 119 individuals aged 14–24 years, and 474 for P2, aged 14–31 years. The characteristics of both populations are shown in Table 1.
Table 1.
Descriptive statistics of horse populations P1 and P2 including the number affected by the various hypersensitivities (RAO, IBH, URT) and environmental factors
| Variable | Horse Population | |
|---|---|---|
| P1 (n = 119) | P2 (n = 474) | |
| Sex (%) | ||
| Male | 69 (58) | 283 (60) |
| Female | 50 (42) | 191 (40) |
| Age (years) | ||
| Mean age | 16.4, 95% CI = 16.1–16.9 | 18.3, 95% CI = 18–18.7 |
| Age RAO+ | 17.3, 95% CI = 15.5–19.1 | 18, 95% CI = 17.5–18.5 |
| Age RAO− | 16.4, 95% CI = 15.9–16.8 | 18.6, 95% CI = 18.1–19.1 |
| RAO (%) | ||
| RAO+ | 11 (9) | 210 (44) |
| RAO− | 108 (91) | 264 (56) |
| IBH (%) | ||
| IBH+ | 6 (5) | 22 (5) |
| IBH− | 113 (95) | 452 (95) |
| URT (%) | ||
| URT+ | 16 (13) | 34 (7) |
| URT− | 103 (87) | 440 (93) |
| Stabled inside without windows (%) | ||
| Yes | 23 (19) | 235 (49.5) |
| No | 96 (81) | 239 (50.5) |
| Straw bedding (%) | ||
| Yes | 95 (80) | 412 (87) |
| No | 24 (20) | 62 (13) |
| Pasture access (%) | ||
| Yes | 111 (93) | 458 (97) |
| No | 8 (7) | 16 (3) |
| Deworming frequency (%) | ||
| 0–2×/year | 49 (39) | 178 (38) |
| >2×/year | 70 (61) | 296 (62) |
RAO, recurrent airway obstruction; IBH, insect bite hypersensitivity; URT, urticaria.
Cross Tabulation
In P1, a significant association between RAO and IBH phenotypes was found, but not between RAO and URT or IBH and URT. In RAO+ horses, 3 of 11 (27%) suffered from IBH in contrast to 3 of 108 (3%) of RAO− horses (P = .01; Fig 1). No associations between RAO, IBH, URT, and environmental and management factors were observed.
Figure 1.
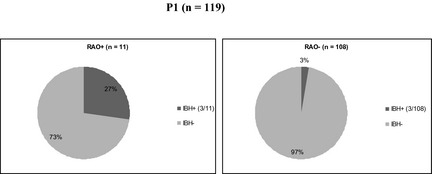
Pie chart illustrating the relative percentage (%) of recurrent airway obstruction (RAO+) (n = 11) and RAO− (n = 108) horses in P1 which were diagnosed with insect bite hypersensitivity (IBH) (27% and 3% respectively).
In P2, the occurrence of RAO, IBH, and URT were significantly associated. Nineteen of 210 (9%) of RAO+ also suffered from IBH, in contrast to 3 of 264 (1%) among RAO− horses (P < .001; Fig 2A). Among RAO+, 22 of 210 (11%) suffered from URT, in contrast to 12 of 264 (5%) of RAO− (P = .01; Fig 2B); among IBH+, 8 of 22 (36%) also were URT+, in contrast to 26 of 452 (6%) among IBH− (P < .001; Fig 2C).
Figure 2.
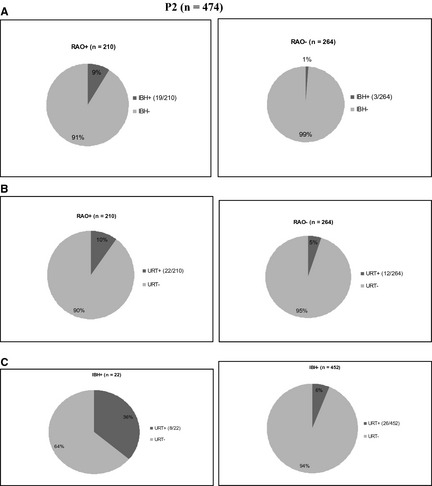
(A, B and C) Pie chart illustrating the relative percentage (%) of recurrent airway obstruction (RAO+) (n = 19) and RAO− (n = 3) horses in P2 which were diagnosed with insect bite hypersensitivity (IBH) (9% and 1% respectively; A) and RAO+ (n = 22) and RAO− (n = 12) horses with urticaria (URT) (10% and 5% respectively; B) and the relative percentage of (%) of IBH+ (n = 8) and IBH− (n = 26) horses in P2 which were diagnosed with URT (36% and 6% respectively; C).
Stabling and bedding type showed a significant association with RAO: 122 of 210 (58%) of RAO+ were stabled without windows and 192 of 210 (91%) were housed on straw bedding, in contrast to 117 of 264 (44%) and 220 of 264 (83%), respectively, of the RAO− (P = .002 and P = .009, respectively).
Deworming frequency showed a significant association with URT: 28 of 34 (82%) of URT+ were dewormed ≥3 times per year, in comparison to 268 of 440 (61%) of the URT− (P = .01). Furthermore, a significant association between IBH, URT, MHS, and the absence of nematode eggs in feces was found (see Table 2). In contrast, no significant association was found with RAO. In P2, a significant (P = .02) association of the T allele at the SNP BIEC2‐224511 with 125 of 209 (60%) of RAO+ in contrast to 136 of 262 (52%) of RAO− was detected, but not with IBH+, URT+ or MHS.
Table 2.
Association of the various hypersensitivities (RAO, IBH, URT, MHS) with egg shedding in a subset of P2 (n = 326)
| Variable | Positive | Negative | Total | P‐Value |
|---|---|---|---|---|
| RAO (%) | ||||
| RAO+ | 44 (30) | 103 (70) | 147 (100) | .71 |
| RAO− | 57 (32) | 122 (68) | 179 (100) | |
| Total | 101 (31) | 225 (69) | 326 (100) | |
| IBH (%) | ||||
| IBH+ | 1 (6) | 16 (94) | 17 (100) | .03 |
| IBH− | 100 (32) | 209 (68) | 309 (100) | |
| Total | 101 (31) | 225 (69) | 326 (100) | |
| URT (%) | ||||
| URT+ | 3 (11) | 25 (89) | 28 (100) | .02 |
| URT− | 98 (33) | 200 (67) | 298 (100) | |
| Total | 101 (31) | 225 (69) | 326 (100) | |
| MHS (%) | ||||
| MHS+ | 2 (7) | 27 (93) | 29 (100) | .003 |
| MHS− | 99 (33) | 198 (67) | 297 (100) | |
| Total | 101 (31) | 225 (69) | 326 (100) | |
RAO, recurrent airway obstruction; IBH, insect bite hypersensitivity; URT, urticaria; MHS, multiple hypersensitivities.
Logistic Regression Analyses
In P1, RAO+ had a 13.1 times higher OR to be affected with IBH compared to RAO− (univariate analysis; CI, 2.27–75.86; P = .004). In P2 (n = 474), the multivariate analysis including effects of URT, IBH, straw bedding, stabling with versus without windows on RAO showed no effect of URT+ on RAO+. However, IBH+ had a 7.8 times higher OR (CI, 2.20–27.38; P = .001) to be affected with RAO+ compared to IBH−. Horses kept on straw bedding had a 2.0 times increased OR (CI, 1.11–3.67; P = .02) to be RAO+ and horses stabled inside without windows had a 1.8 times higher OR (CI, 1.20–2.56; P = .004) to be RAO+.
In a multivariate analysis including effects of RAO+ and URT+ on IBH, URT+ had a 7.4 times higher OR (CI, 2.74–19.88; P < .001) and RAO+ a 7.4 increased OR (CI, 2.12–25.74; P = .002) of also being affected with IBH.
Horses dewormed ≥3 times per year had a 2.8 times higher OR (multivariate analysis; CI, 1.10–6.96; P = .03) to be URT+ than those that were dewormed less often. In addition, the OR of IBH+ was 7.1 (CI, 2.58–19.66; P < .001) to be URT+. RAO+ had no significant effect on URT.
In the subset of P2 (n = 326) for which EpGs were available, horses that were not shedding any eggs had a 7.7 times higher OR (univariate analysis; CI, 1.00–58.54; P = .05) to be IBH+, a 4.1 times higher OR (univariate analysis; CI, 1.20–13.85; P = .03) to be URT+ and a 6.7 times higher OR (univariate analysis; CI, 1.57–28.96; P = .01) to be MSH+ than those with positive egg shedding.
In the subset of P2 (n = 471) for which SNP BIEC 2‐224511 genotypes were available, the T allele was no longer significantly associated with RAO+ (univariate analysis), as found in the cross tabulation analysis.
Discussion
Except for a preliminary report in a limited cohort of a RAO high‐prevalence family,20 this study is, to our knowledge, the first report describing the occurrence of MHS in horses. Multiple hypersensitivity in horses was defined as suffering from at least 2 different hypersensitivities. Significant associations between RAO and IBH with OR of 13.1 and 7.4, respectively, was observed in a random sample representative of Swiss sporthorses and this could be replicated in a second larger population of RAO cases and controls. Furthermore, in the replication population, but not in the smaller random sample, an association between IBH and URT was observed, with IBH‐affected horses showing a 7.1 times increased OR of also suffering from URT. Cross tabulation also identified an association between RAO+ and URT+ in the RAO cases and controls, but this finding was not confirmed in the multivariate logistic regression analyses. This indicates that effects of stabling and bedding type, environmental factors that have previously been reported,1, 14 were stronger than that the presence of URT in RAO+ horses.
Frequent deworming also was associated with URT in P2. Although this was only observed in the larger population, and deworming frequency was not associated with the diagnosis of RAO or IBH, this finding in mature horses is interesting. In the present study, horses with positive egg counts in the feces also seemed to be protected against the development of IBH, URT, and MHS. An effect of deworming as well as the associations between decreased nematode egg shedding and both RAO+ in previous reports17, 18 and other hypersensitivities in the current study are in accordance with the “hygiene hypothesis”.19 Infection with strongylid nematodes may confer protection against the development of hypersensitivities. Alternatively, the genetic basis that predisposes to these hypersensitivities may award some protection against intestinal parasites. The Th2‐type pathway including IL4RA, IL13, and IL4 represents a promising candidate for the identification of such a common genetic predisposition. This pathway is not only involved in asthma, rhinitis, atopic dermatitis, psoriasis, increased serum IgE, and a common “allergic predisposition” in humans9, 10, 11, 24, 25, 26 as well as RAO in horses but also is involved in host defense against parasites.27
The SNP BIEC2‐224511 on ECA 13 near the IL4RA gene previously has been found to be associated with RAO both in a high‐prevalence family and in unrelated horses.16 As expected, because our P2 was a subset of the latter unrelated horses, cross tabulation confirmed these earlier results. However, regression analysis showed no significant effect of the T allele at the SNP BIEC2‐224511 on RAO. Also, no associations between IBH+, URT+, or MHS and the T allele at the SNP BIEC2‐224511 were observed. This can be interpreted as a lack of evidence for a common genetic predisposition for different and MHS in the horse. Alternatively, the lack of a significant association also may have been because of the bias of the larger study population (P2) toward selecting RAO+ cases and controls with a resulting low number of IBH+ and URT+ cases in both P1 and P2. Ideally, to achieve a comparable power to the study that demonstrated a link between SNP BIEC2‐224511 and RAO+,16 similar numbers (ie, several hundred) of IBH+ and URT+ would have to be tested.
A limitation of our study was the relatively low numbers of IBH+ and URT+ horses studied. Furthermore, the assessment of IBH and URT was less standardized than that of RAO, which was based on the HOARSI and a validated questionnaire. In contrast to RAO, there are no published standardized criteria for owner assessment of IBH and URT. The horse owners were simply interviewed and IBH as well as URT were described as detailed above in the Materials and Methods section. Also, diagnoses of IBH and URT were not confirmed by intradermal skin testing or other additional tests such as the Cellular Allergy Stimulation Test.28 Thus, due to of the lack of a gold standard for diagnosis of these conditions, a possible bias could have been introduced when classifying these cases. The random sampling procedure used to generate P1 conferred the advantage of a Warmblood horse population representative for sporthorses in Switzerland. However, after filtering for horses older than 13 years, the population was decreased to 119 individuals, resulting in a loss of power. We therefore also investigated P2, a prospectively chosen replication population, which provided higher statistical power with a total of 474 horses. The drawback of P2 (44% RAO+), however, obviously was a selection bias toward RAO and a relative scarcity of IBH and URT cases.
The prevalence of IBH+ in healthy (RAO−) horses (3% in P1 and 2% in P2) was similar to that observed in earlier studies investigating 3‐year‐old Freiberger (1%) and Warmblood (2%) horses in Switzerland.29, 30 Occurrence of IBH was thus markedly increased in RAO+ animals compared to healthy ones in this study as well as in these earlier reports.
Multiple hypersensitivities are best described in human medicine. For instance, patients suffering from allergic rhinitis and eczema are more susceptible to the development of drug hypersensitivity reactions such as itching or skin rash.9 The term “atopic disease” often is used to group clinically diverse hypersensitivity syndromes and symptoms. It is defined as an inherited predisposition to allergic disease via IgE‐mediated pathophysiology.11 Investigation of serum IgE in equine MHS would also be interesting, but was not possible in this study.
In domestic animals, similar syndromes of multiple manifestations of “atopic disease” are also unsubstantiated by measurements of IgE concentrations, but instead have been described based on clinical signs. The syndrome of feline atopy has been proposed to include miliary dermatitis, psychogenic alopecia, eosinophilic granuloma complex, and feline asthma.7, 30, 31, 32 Other investigators, however, found no association between allergic dermatitis and small airway disease in cats.33 Dogs suffering from atopic dermatitis appear to be predisposed to concurrently develop flea or food allergies as well as allergic rhinitis and asthma.8, 34, 35
Another limitation of our relatively large, noninterventional study was the lack of a direct comparison of egg shedding in the fecal samples of affected and unaffected horses in the same pasture and under the same deworming and management conditions. Additionally, fecal sampling was performed only once after the egg reappearance period, because this is when horses are expected to reach a maximum positive egg count after treatment. Nevertheless, the results must be interpreted with caution because one‐time sampling and measurement of EpG after expiration of the estimated ERP may not have been representative of the parasite status of the horse, specifically to the EpG before administration of the anthelmintic drug. This may explain why this study did not confirm the association of egg shedding with RAO observed in earlier investigations.17, 18
Furthermore, it would have been interesting to address the question whether or not a similar phenomenon described as the “allergic march” in humans12 occurs in horses with MHS. However, due to the lack of reliable questionnaire‐derived information concerning the age at which the individuals first developed IBH and URT, the chronology over which the different hypersensitivities were manifested could not be determined in the present study.
Future studies therefore should further investigate the intriguing phenomenon of equine MHS, ideally in prospective studies involving large numbers not only of RAO, but also of IBH and URT‐affected and control horses, for which information is available on management, fecal examinations (as in17, 18), on total and allergen‐specific IgE concentrations (to better define atopy as in humans26) as well as on the chronological appearance of the first clinical signs (to investigate a potential “allergic march” as described in humans12).
In conclusion, this study provides some of the first evidence of MHS in horses. In particular, an increased risk for IBH should be considered in horses affected with RAO.
Acknowledgments
We thank Selina Thomas for her help with the manuscript and Paivi Nussbaumer, Daniela Baleri, Caroline Ehrmann, Patricie Tumova, Caroline Frey, and the horse owners for their support.
Grant support: This work was supported by ISMEquine Research and the Swiss National Science Foundation grant nos 310030‐138295 and 310030‐129837/1.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
This work was performed at the Institut suisse de médecine équine, University of Berne and Agroscope, Switzerland.
This work was presented at the Swiss Equine Research Network Meeting 2012 in Avenches, Switzerland, in abstract form.
Footnotes
NCSS statistical software 2007; NCSS LLC, Kaysville, UT
References
- 1. Leclere M, Lavoie‐Lamoureux A, Lavoie JP. Heaves, an asthma‐like disease of horses. Respirology 2011;16:1027–1046. [DOI] [PubMed] [Google Scholar]
- 2. Ramseyer A, Gaillard C, Burger D, et al. Effects of genetic and environmental factors on chronic lower airway disease in horses. J Vet Intern Med 2007;21:149–156. [DOI] [PubMed] [Google Scholar]
- 3. Couëtil LL, Hoffman AM, Hodgson J, et al. Inflammatory airway disease of horses. J Vet Intern Med 2007;21:356–361. [DOI] [PubMed] [Google Scholar]
- 4. Marti E, Gerber V, Wilson AD, et al. Report of the 3rd Havemeyer workshop on allergic diseases of the Horse, Hòlar, Iceland, June 2007. Vet Immunol Immunopathol 2008;126:351–361. [DOI] [PubMed] [Google Scholar]
- 5. Rüfenacht S, Marti E, von Tscharner C, et al. Immunoglobulin E‐bearing cells and mast cells in skin biopsies of horses with urticaria. Vet Dermatol 2005;16:94–101. [DOI] [PubMed] [Google Scholar]
- 6. Lowell FC. Observations on heaves. An asthma‐like syndrome in the horse. J Allergy 1964;35:322–330. [DOI] [PubMed] [Google Scholar]
- 7. Carlotti D, Prost C. L'atopie féline. Point Vét 1988;20:777–784. [Google Scholar]
- 8. Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (IX): The controversy surrounding the route of allergen challenge in canine atopic dermatitis. Vet Immunol Immunopathol 2001;81:219–225. [DOI] [PubMed] [Google Scholar]
- 9. Kurt E, Demir AU, Cadirci O, et al. Immediate‐type drug hypersensitivity and associated factors in a general population. Allergol Immunopathol 2011;39:27–31. [DOI] [PubMed] [Google Scholar]
- 10. Bottema RWB, Nolte IM, Howard TD, et al. Interleukin 13 and interleukin 4 receptor‐α polymorphisms in rhinitis and asthma. Int Arch Allergy Immunol 2010;153:259–267. [DOI] [PubMed] [Google Scholar]
- 11. Bieber T. Atopic dermatitis. Ann Dermatol 2010;22:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003;112:118–127. [DOI] [PubMed] [Google Scholar]
- 13. Herszberg B, Ramos‐Barbón D, Tamaoka M, et al. Heaves, an asthma‐like equine disease, involves airway smooth muscle remodeling. J Allergy Clin Immunol 2006;118:382–388. [DOI] [PubMed] [Google Scholar]
- 14. Robinson NE. Chairperson's introduction: International Workshop on Equine Chronic Airway Disease, Michigan State University, 16–18 June 2000. Equine Vet J 2001;33:5–19. [DOI] [PubMed] [Google Scholar]
- 15. Jost U, Klukowska‐Rötzler J, Dolf G, et al. A region on equine chromosome 13 is linked to recurrent airway obstruction in horses. Equine Vet J 2007;39:236–241. [DOI] [PubMed] [Google Scholar]
- 16. Shakhsi‐Niaei M, Klukowska‐Rötzler J, Drögemüller C, et al. Replication and fine‐mapping of a QTL for recurrent airway obstruction in European Warmblood horses. Anim Genet 2012;43:627–631. [DOI] [PubMed] [Google Scholar]
- 17. Bründler P, Frey CF, Gottstein B, et al. Lower shedding of strongylid eggs by Warmblood horses with recurrent airway obstruction compared to unrelated healthy horses. Vet J 2011;190:12–15. [DOI] [PubMed] [Google Scholar]
- 18. Neuhaus S, Bründler P, Frey CF, et al. Increased parasite resistance and recurrent airway obstruction in horses of a high‐prevalence family. J Vet Intern Med 2010;24:407–413. [DOI] [PubMed] [Google Scholar]
- 19. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989;299:1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramseyer A, Gaillard C, Straub R, et al. Clinical manifestation of recurrent airway obstruction (RAO) in a Swiss Warmblood family. Proceedings of the Third World Equine Airways Symposium (WEAS). Cornell, NY, 2005. [Google Scholar]
- 21. Gerber V, Baleri D, Klukowska‐Rötzler J, et al. Mixed inheritance of equine recurrent airway obstruction. J Vet Intern Med 2009;23:626–630. [DOI] [PubMed] [Google Scholar]
- 22. Von Samson‐Himmelstjerna G. Helminthosen der Equiden In: Schneider T, ed. Veterinärmedizinische Parasitologie, 6th ed Stuttgart, PA: Parey; 2006:303–347. [Google Scholar]
- 23. Bauer C. Nachweis von Parasitenstadien im Kot In: Schneider T, ed. Veterinärmedizinische Parasitologie, 6th ed Stuttgart, PA: Parey; 2006:88–96. [Google Scholar]
- 24. Tamari M, Tanaka S, Hirota T. Genome‐wide association studies of allergic diseases. Allergol Int 2013;62:21–28. [DOI] [PubMed] [Google Scholar]
- 25. Moffatt MF, Gut IG, Demenais F, et al. A large‐scale, consortium‐based genomewide association study of asthma. N Engl J Med 2010;363:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair RP, Duffin KC, Helms C, et al. Genome‐wide scan reveals association of psoriasis with IL‐23 and NF‐κB pathways. Nat Genet 2009;41:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scales HE, Ierna MX, Lawrence CE. The role of IL‐4, IL‐13 and IL‐4Ra in the development of protective and pathological responses to Trichinella spiralis . Parasite Immunol 2007;29:81–91. [DOI] [PubMed] [Google Scholar]
- 28. Baselgia S, Doherr MG, Mellor P, et al. Evaluation of an in vitro sulphidoleukotriene release test for diagnosis of insect bite hypersensitivity in horses. Equine Vet J 2006;38:40–46. [DOI] [PubMed] [Google Scholar]
- 29. Mele M, Gerber V, Straub R, et al. Prevalence of hereditary disease in three‐year‐old horses of the Freiberger breed. Schweiz Arch Tierheilk 2007;149:151–159. [DOI] [PubMed] [Google Scholar]
- 30. Studer S, Gerber V, Straub R, et al. Prevalence of hereditary disease in three‐year‐old Swiss Warmblood horses. Schweiz Arch Tierheilk 2007;149:161–171. [DOI] [PubMed] [Google Scholar]
- 31. Halliwell REW. Efficacy of hyposensitization in feline allergic diseases based upon results of in vitro testing for allergen‐specific immunoglobulin E. J Am Anim Hosp Assoc 1997;33:282–288. [DOI] [PubMed] [Google Scholar]
- 32. Gilbert S, Prélaud P, Guaguère E. L'atopie féline. Prat Méd Chir Anim Comp 1999;34:15–31. [Google Scholar]
- 33. Hobi S, Linek M, Marignac G, et al. Clinical characteristics and causes of pruritus in cats: A multicentre study on feline hypersensitivity‐associated dermatoses. Vet Dermatol 2011;22:406–413. [DOI] [PubMed] [Google Scholar]
- 34. Moriello KA, Stepien RL, Henik RA, et al. Pilot study: Prevalence of positive aeroallergen reactions in 10 cats with small‐airway disease without concurrent skin disease. Vet Dermatol 2007;18:94–100. [DOI] [PubMed] [Google Scholar]
- 35. Marsella R, Sousa CA. The ACVD task force on canine atopic dermatitis (XIII): Threshold phenomenon and summation of effects. Vet Immunol Immunopathol 2001;81:251–253. [DOI] [PubMed] [Google Scholar]
- 36. Halliwell REW. The immunopathogenesis of allergic skin diseases in dogs and cats. EJCAP 2009;19:213–218. [Google Scholar]


