Abstract
AIM: To determine the efficacy of calcium supplementation in reducing the recurrence of colorectal adenomas.
METHODS: We conducted a systematic review and meta-analysis of published studies. We searched PubMed, Scopus, the Cochrane Library, the WHO International Clinical Trials Registry Platform, and the ClinicalTrials.gov website, through December 2015. Randomized, placebo-controlled trials assessing supplemental calcium intake for the prevention of recurrence of adenomas were eligible for inclusion. Two reviewers independently selected studies based on predefined criteria, extracted data and outcomes (recurrence of colorectal adenomas, and advanced or “high-risk” adenomas), and rated each trial’s risk-of-bias. Between-study heterogeneity was assessed, and pooled risk ratio (RR) estimates with their 95% confidence intervals (95%CI) were calculated using fixed- and random-effects models. To express the treatment effect in clinical terms, we calculated the number needed to treat (NNT) to prevent one adenoma recurrence. We also assessed the quality of evidence using GRADE.
RESULTS: Four randomized, placebo-controlled trials met the eligibility criteria and were included. Daily doses of elemental calcium ranged from 1200 to 2000 mg, while the duration of treatment and follow-up of participants ranged from 36 to 60 mo. Synthesis of intention-to-treat data, for participants who had undergone follow-up colonoscopies, indicated a modest protective effect of calcium in prevention of adenomas (fixed-effects, RR = 0.89, 95%CI: 0.82-0.96; random-effects, RR = 0.87, 95%CI: 0.77-0.98; high quality of evidence). The NNT was 20 (95%CI: 12-61) to prevent one colorectal adenoma recurrence within a period of 3 to 5 years. On the other hand, the association between calcium treatment and advanced adenomas did not reach statistical significance (fixed-effects, RR = 0.92, 95%CI: 0.75-1.13; random-effects, RR = 0.92, 95%CI: 0.71-1.18; moderate quality of evidence).
CONCLUSION: Our results suggest a modest chemopreventive effect of calcium supplements against recurrent colorectal adenomas over a period of 36 to 60 mo. Further research is warranted.
Keywords: Calcium, Colorectal adenoma, Recurrence, Cancer chemoprevention, Colorectal cancer, Systematic review, Meta-analysis, Polyp
Core tip: To assess the efficacy of calcium supplementation in reducing the recurrence of colorectal adenomas, we conducted a systematic review and meta-analysis of randomized, placebo-controlled trials. We found a modest protective effect of calcium in prevention of adenomas (fixed-effects, RR = 0.89, 95%CI: 0.82-0.96; random-effects, RR = 0.87, 95%CI: 0.77-0.98; high quality of evidence). On the other hand, the association between calcium treatment and advanced (“high-risk”) colorectal adenomas was not statistically significant (fixed-effects, RR = 0.92, 95%CI: 0.75-1.13; random-effects, RR = 0.92, 95%CI: 0.71-1.18; moderate quality of evidence). Further targeted research is warranted.
INTRODUCTION
Colorectal cancer is a tumour resulting from a complex interaction between inherited susceptibility and environmental factors, as demonstrated by genetic, experimental, and epidemiological studies[1-3]. It represents the third most common malignancy, and the fourth most common cause of cancer deaths globally, accounting for 1.35 million new cases and 0.7 million deaths annually[4].
The magnitude of the colorectal cancer problem, and the failure of advanced disease chemotherapy to effect significant reductions in the respective mortality rates, indicate that an intensive approach to the prevention of this disease is necessary. Accordingly, research on chemopreventive agents for colorectal cancer has received much attention over the last 30 years. Among several promising compounds (including vitamins A, C, and E, folate and other B vitamins, aspirin, sulindac and other non-aspirin non-steroidal anti-inflammatory drugs, statins, bisphosphonates, selenium, and fiber)[5-16], calcium has also been studied. It was proposed by Newmark et al[17] that calcium binds bile acids in the bowel lumen, inhibiting their well-known proliferative and carcinogenic effects. In addition, calcium has demonstrated a direct antiproliferative effect on cells, as well as promoting cellular differentiation and death (apoptosis)[18]. Evidence from epidemiologic studies also suggests that higher calcium intake may reduce the risk of colorectal cancer[19].
Most colorectal tumors develop from adenomas arising from the lining of the intestine. Progression - described as the adenoma-cancer (or polyp-cancer) sequence - is characterized by morphological and histological changes[20]. For instance, a small tubular adenoma acquires villoglandular characteristics as it grows. On the molecular level, the adenoma-cancer sequence reflects an accumulation of genomic defects. Generally, a single adenoma has a risk of progressing into neoplasia of 0.25% per year[21], depending on its size, location, histological type, and the presence of dysplasia.
The standard treatment for colorectal adenomas is endoscopic resection that interrupts the progression to invasive disease[22]. However, even after polypectomy, rates of adenoma recurrence may be up to 50% within 3 years of follow-up[23,24]. That is why research on colorectal cancer prevention has often focused on prevention of recurrent adenomas. Assuming that the effects of chemopreventive agents on adenomas reflect those on cancer, this endpoint provides a convenient surrogate for the study of colorectal cancer prevention[25,26].
Contrary to expectations, the recent randomized placebo-controlled trial published by Baron et al[27] showed that daily supplementation with 1200 mg of calcium did not significantly reduce the risk of colorectal adenomas over a time period of 3 to 5 years. In view of earlier promising clinical trial data[28,29], we sought to obtain a comprehensive snapshot of the existing evidence on the clinical efficacy of calcium supplementation for the prevention of colorectal adenomas. Therefore, we carried out an updated systematic review and meta-analysis of randomized controlled trials (RCTs) published in the peer-reviewed literature.
MATERIALS AND METHODS
Data sources and search strategy
To identify the studies of interest, we systematically searched the PubMed and Scopus bibliographic databases from their inception to 15 December 2015 (date of final search). Search terms included: “calcium” combined with “adenoma” or “polyp”. The search was limited to RCTs and human studies. No language restrictions were applied.
We also searched the Cochrane Library for any recently published systematic review on the subject, the WHO International Clinical Trials Registry Platform, and the ClinicalTrials.gov website, for completed but unpublished studies.
Two authors (Bonovas S and Lytras T) independently reviewed titles and abstracts to identify studies for inclusion. The full texts of the selected articles were carefully examined for eligibility, and their reference lists (as well as those of relevant systematic reviews[30-33]) were also investigated to identify any studies missed by the electronic database search.
Selection criteria
Studies were eligible for inclusion if they were randomized, placebo-controlled trials assessing supplemental calcium intake for the prevention of recurrent colorectal adenomas. All studies had to include follow-up evaluation (i.e., endoscopy) to confirm the presence or absence of adenomas. If the results of a study were reported in multiple publications and/or at multiple time-points, we selected the most updated publication and extracted the data for the maximum follow-up time reported, as long as it remained a randomized trial and fully reported the outcomes of interest.
Studies were excluded if they were observational; did not report (or provided insufficient data to calculate) the outcomes of interest; or evaluated multi-interventional therapies, in which the effect of calcium treatment could not be separated out. We did not apply restrictions on eligibility according to dosage, or duration of calcium supplementation.
Types of outcomes and data extraction
We analyzed the following two outcomes: (1) recurrence of colorectal adenomas (at least one adenoma detected during follow-up colonoscopies); and (2) recurrence of advanced or “high-risk” adenomas (defined as those that have a diameter ≥ 10 mm, villous or tubulovillous features, or severe dysplasia).
Data extraction was independently undertaken by two authors (Bonovas S and Lytras T) using a predesigned form. The following information was extracted from each study: first author, journal and year of publication, study design and duration, number and characteristics of participants, intervention parameters, and number of subjects with the outcomes of interest reported for the intervention and control groups.
Disagreements were resolved via consensus, referring back to the original articles.
Assessment of risk of bias
We assessed the risk of bias (RoB) in included studies using the Cochrane Collaboration’s tool[34], which addresses the following key-domains: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other sources of bias, such as extreme baseline imbalances in prognostic factors, etc. These items were considered for RoB assessment and were classified as “adequate” (low RoB), “inadequate” (high RoB), or “unclear” (uncertain RoB).
Studies reporting adequate procedures in all domains were classified as “low RoB”, studies with inadequate procedures in at least one domain were classified as “high RoB”, and those with unclear procedures in one or more domains were classified as “uncertain RoB”. Discrepancies among reviewers were discussed and agreement was reached by consensus.
Data synthesis and analysis
The risk ratio (RR) was used to measure treatment effects. Study-level RRs along with their 95%CI were calculated using intention-to-treat data for study participants who completed the follow-up evaluation (i.e., follow-up colonoscopies).
Meta-analyses were performed twice, assuming a fixed-effects model (Mantel-Haenszel approach[35]) and a random-effects model (DerSimonian-Laird approach[36]). Under a fixed-effects model, we assume that the included studies share a common true effect, and the pooled effect is an estimate of the common effect size. Under a random-effects model, we assume that the true effects vary between the studies, and the pooled effect is a weighted average of the effects reported in the different studies. The random-effects model often leads to broader confidence intervals (i.e., it is a more conservative approach)[37].
The between-study heterogeneity was evaluated using the Cochran’s Q test[38], with a 0.10 level of significance, and the I-squared metric[39], which describes the percentage of variation across studies that is due to heterogeneity rather than chance. I-squared values of less than 25%, 25%-50%, or higher than 50% indicate low, moderate, or high heterogeneity, respectively[40].
Publication bias was not assessed, because the relevant statistical tests lack power when the number of included studies is limited[41].
To express the treatment effect in clinical terms, we calculated the number needed to treat (NNT) to prevent one adenoma recurrence using the Mantel-Haenszel fixed-effects risk difference (risk in the placebo group minus risk in the calcium group), in cases in which a statistically significant RR was detected. The NNT is the inverse of this risk difference.
The quality of evidence (confidence in the synthesized effect estimates) was assessed using GRADE (Grading of Recommendations Assessment, Development and Evaluation)[42].
For all statistical analyses, we used the R software[43], version 3.2.2, and the “meta” package for R[44], version 4.3-0. All P-values are two-tailed. For all tests (except for heterogeneity), a P-value less than 0.05 indicates statistical significance.
Our study was performed in accordance with the Cochrane Handbook for intervention reviews[41], and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement[45].
The study did not involve any experiment on humans or animals, thus an ethical approval was not required.
RESULTS
Search results
A summary of the literature search and selection process is shown in Figure 1 (Flow diagram). Four randomized studies of calcium supplementation met the eligibility criteria and were included: (1) the Vitamin D/Calcium Polyp Prevention Study[27,46]; (2) the Southwest Oncology Group (SWOG) Calcium Chemoprevention Pilot Study[47,48]; (3) the European Cancer Prevention Organisation (ECP) Calcium Fibre Polyp Prevention Study[28,49,50]; and (4) the Calcium Polyp Prevention Study[29,51,52].
Figure 1.
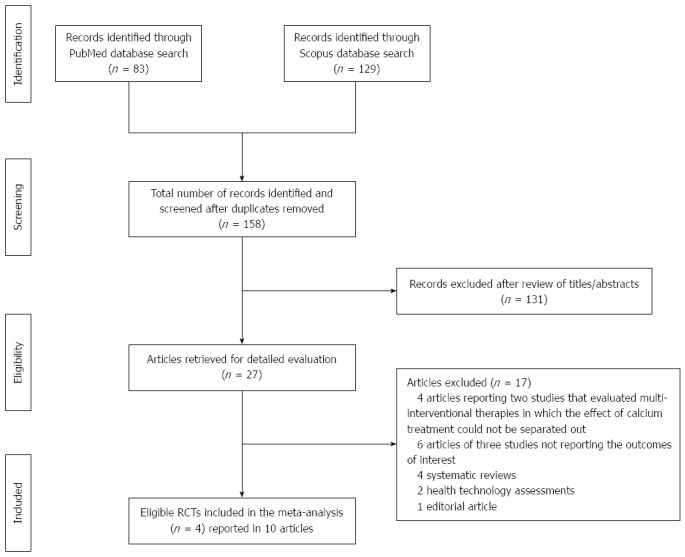
Summary of the evidence search and selection process (flow diagram). RCTs: Randomized controlled trials.
They were multicenter, randomized, placebo-controlled trials of supplementation with calcium for the prevention of colorectal adenomas. Daily doses of elemental calcium ranged from 1200 to 2000 mg, while the duration of treatment and follow-up of patients ranged from 36 to 60 mo. A summary of the trials’ characteristics is given in Table 1.
Table 1.
Randomized, double-blind, placebo-controlled trials of calcium supplementation for prevention of colorectal adenomas
| Study or subgroup1 | Participants randomized | Mean age (yr) | Women | Follow-up (mo) | Amount of elemental calcium supplemented (mg/d) |
| Vitamin D/Calcium Polyp Prevention Study[27] | 1675 | 59 | 15% | 36 or 60 | 1200 |
| SWOG Calcium Chemoprevention Pilot Study[47] | 220 | 682 | 37% | 60 | 1800 |
| ECP Calcium Fibre Polyp Prevention Study[28] | 439 | 59 | 37% | 36 | 2000 |
| Calcium Polyp Prevention Study[51,52] | 930 | 61 | 28% | 48 | 1200 |
For the Vitamin D/Calcium Polyp Prevention Study, only a subgroup was taken into account in the analysis;
Median value. ECP: European Cancer Prevention Organisation; SWOG: Southwest Oncology Group.
Patients underwent endoscopy at baseline; subsequent colonoscopies were then undertaken to assess adenoma recurrence during the follow-up. The studies differ in that the SWOG Calcium Chemoprevention Pilot Study[47,48] recruited patients with completely resected colorectal cancer, while all the other studies included participants with colorectal adenomas removed before enrollment. All studies reported the number of subjects with adenomas (and advanced adenomas) identified during the follow-up endoscopies; thus, we were able to conduct a post hoc analysis of these clinical trials, calculate RRs for the outcomes of interest, and incorporate them in the meta-analyses.
Another two clinical trials were identified, but did not meet the eligibility criteria, and were excluded. The first study - included in two previous systematic reviews[31,32] - examined a mixed intervention consisting of calcium, β-carotene, vitamin C, vitamin E, and selenium, compared with placebo[53,54]. The second clinical trial, which was evaluating a combination treatment of aspirin, calcitriol, and calcium, compared with placebo, was terminated early because no positive tendency was shown in a preplanned interim analysis[55,56]. In both studies, the effect of calcium could not be separated out; thus, they were not included in the evidence synthesis.
Risk of bias in included studies
The Vitamin D/Calcium Polyp Prevention Study[27,46], the ECP Calcium Fibre Polyp Prevention Study[28,49,50], and the Calcium Polyp Prevention Study[29,51,52], were judged to be at low RoB: their allocation sequences appeared to be adequately generated and concealed; patients and staff were masked; participants excluded from the analyses (those who had not undergone follow-up colonoscopy) were balanced in numbers and reasons across intervention groups; and the outcomes of interest for this review were fully reported.
The SWOG Calcium Chemoprevention Pilot Study[47,48] was considered to have uncertain RoB, because information was insufficient to permit judgement about the sequence generation process, the method used to conceal allocation, and attritions/exclusions.
Quality assessment items are presented in Figure 2.
Figure 2.
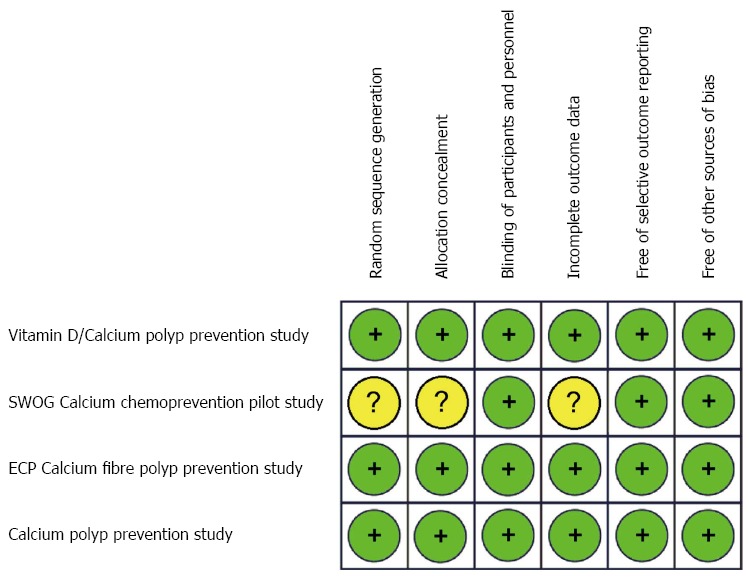
Risk-of-bias assessment for the studies included in the meta-analysis. Green (+): Low risk-of-bias; Yellow (?): Unclear risk-of-bias. ECP: European Cancer Prevention Organisation; SWOG: Southwest Oncology Group.
Results of quantitative synthesis
Recurrence of colorectal adenomas: Intention-to-treat data for 2984 participants, who underwent follow-up colonoscopies, were analyzed. Each one of the four included trials reported a lower recurrence rate of colorectal adenomas in the calcium group, as compared to the placebo group; however, the results only from two studies (the Calcium Polyp Prevention Study[29,51,52] and the SWOG Calcium Chemoprevention Pilot Study[47,48]) were statistically significant. The overall recurrence rate, on all four RCTs, was 41.1% in calcium groups and 46.2% in placebo groups, over a treatment and follow-up period of 3 to 5 years.
We found a statistically significant modest protective effect (about 10%-15% risk reduction) of calcium supplements in the prevention of colorectal adenomas, both under the assumption of a fixed-effects model (RR = 0.89, 95%CI: 0.82-0.96) and a random-effects model (RR = 0.87, 95%CI: 0.77-0.98). The RRs with their 95%CIs for the individual studies, and the pooled results, are shown in Figure 3. The Cochran’s Q test had a P-value of 0.18 and the corresponding I-squared value was 39%, indicating moderate heterogeneity between the studies.
Figure 3.
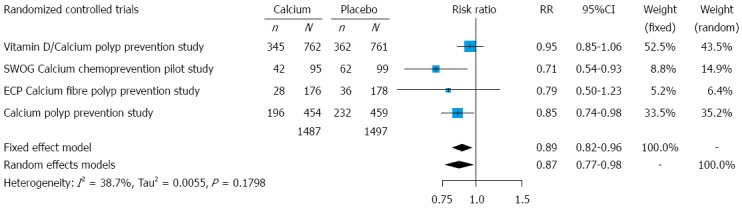
Forest plot for adenomas: results from individual studies and meta-analysis. For the analysis we used intention-to-treat data for patients who underwent follow-up evaluation (follow-up colonoscopies). ECP: European Cancer Prevention Organisation; SWOG: Southwest Oncology Group; n: Number of subjects with at least one adenoma detected during the follow-up evaluation; N: Number of subjects who underwent follow-up evaluation; RR: Risk ratio.
For patients treated with calcium supplements in the included trials, the NNT was 20 (95%CI: 12-61) to prevent one colorectal adenoma recurrence within a period of 3 to 5 years.
Recurrence of advanced (high-risk) adenomas: We analyzed data for 2998 participants, who completed their follow-up evaluations (colonoscopies). None of the studies reported statistically significant results for advanced adenomas. Their overall occurrence, on all four RCTs, was 10.4% in calcium groups and 11.3% in placebo groups.
In meta-analysis, the association between calcium treatment and advanced adenomas did not reach statistical significance, either assuming a fixed-effects model (RR = 0.92, 95%CI: 0.75-1.13) or a random-effects model (RR = 0.92, 95%CI: 0.71-1.18). The RRs with their 95%CIs for the individual studies, and the pooled results, are presented in Figure 4. The Cochran’s Q test had a P-value of 0.30 and the corresponding I-squared value was 17%, indicating low heterogeneity between the studies.
Figure 4.
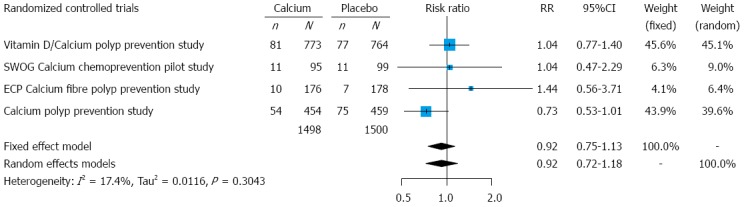
Forest plot for advanced adenomas: results from individual studies and meta-analysis. For the analysis we used intention-to-treat data for patients who underwent follow-up evaluation (follow-up colonoscopies). ECP: European Cancer Prevention Organisation; SWOG: Southwest Oncology Group; n: Number of subjects with at least one adenoma detected during the follow-up evaluation; N: Number of subjects who underwent follow-up evaluation; RR: Risk ratio.
Quality of the evidence
Using the GRADE approach[42], our confidence in the synthesized evidence is “high” for the first outcome (adenomas), but “moderate” for the second one (advanced adenomas), for the following reasons: (1) the data were derived from RCTs, which are considered as the gold standard for assessing drugs[57]; (2) the synthesized effect estimates are precise for adenomas, but imprecise for advanced adenomas; (3) heterogeneity is low-to-moderate across studies; and (4) publication bias is not likely.
A high quality of evidence means that “we are very confident that the true effect lies close to that of the estimate of the effect”, while a moderate quality of evidence means that “we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different”[58].
A summary of findings and strength of evidence is shown in Table 2.
Table 2.
Summary of findings
|
Illustrative comparative risks (95%CI) |
|||
| Assumed risk | Corresponding risk | ||
| Placebo | Calcium | ||
| Recurrence of adenomas (follow-up: 3 to 5 yr) | 462 per 1000 | 411 per 1000 | Relative effect (95%CI): RR = 0.89 (0.82-0.96) |
| (379 to 444) | No. of patients with follow-up evaluation: 2984 | ||
| No. of RCTs: 4 | |||
| Quality of evidence (GRADE): ++++ (high) | |||
| Advanced adenomas (follow-up: 3 to 5 yr) | 113 per 1000 | 104 per 1000 | Relative effect (95%CI): RR = 0.92 (0.75-1.13) |
| (85 to 128) | No. of patients with follow-up evaluation: 2998 | ||
| No. of RCTs: 4 | |||
| Quality of evidence (GRADE): +++- (moderate) | |||
(1) the basis for calculating the assumed risk is the overall event rate across the trial groups receiving placebo; (2) the corresponding risk (calcium group) is based on the assumed risk and the relative effect estimate (risk ratio); (3) the relative effect estimate and its 95%CI come from a Mantel-Haenszel fixed-effects meta-analytic model; and (4) the overall quality of evidence is judged as “high” for recurrence of adenomas, and “moderate” for advanced adenomas. A high quality of evidence means that “we are very confident that the true effect lies close to that of the estimate of the effect”, while a moderate quality of evidence means that “we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different”[58]. Population: Patients with colorectal adenomas removed before enrollment. Intervention: Calcium supplementation (1200-2000 mg/d) to prevent recurrence of adenomas. Comparison: Placebo. RR: Risk ratio; GRADE; Grading of Recommendations Assessment, Development and Evaluation; RCTs: Randomized controlled trials.
DISCUSSION
Chemoprevention is a promising area of cancer research focusing on prevention of malignancies through pharmacological, biological, and nutritional interventions[59]. As first defined by Sporn[60], cancer chemoprevention uses natural, synthetic, or biologic agents to reverse, suppress, or prevent either the initial phase of carcinogenesis or the progression of malignant cells to cancer[61-65]. Regarding chemoprevention of colorectal cancer, several studies suggest that calcium may have chemopreventive potential[66-72]. Our knowledge on the underlying mechanism is incomplete. It has been proposed that calcium may protect against neoplasia in the large bowel by binding bile and fatty acids, thus decreasing their proliferative and carcinogenic effects on colonic epithelial cells[17].
Meta-analysis is a statistical methodology for combining the findings from independent studies[73]. We undertook this systematic review and meta-analysis to assess and synthesize the existing evidence on the efficacy of calcium supplements in prevention of colorectal adenomas.
In the recent literature, we have identified three systematic reviews with meta-analyses of RCTs examining the efficacy of calcium supplementation for the prevention of colorectal adenomas[31-33]. Carroll et al[31] and Shaukat et al[32] performed similar three-trial meta-analyses including the ECP Calcium Fibre Polyp Prevention Study[28,49,50], the Calcium Polyp Prevention Study[29,51,52], as well as the Hofstad et al[53,54] study that examined a mixed intervention consisting of calcium, β-carotene, vitamin C, vitamin E, and selenium, compared with placebo. Both meta-analyses found a significant 20% risk reduction associated with calcium. On the other hand, the third meta-analysis by Weingarten et al[33] reported a larger protective effect for calcium (OR = 0.74, 95%CI: 0.58-0.95) including only the ECP Calcium Fibre Polyp Prevention Study[28,49,50] and the Calcium Polyp Prevention Study[29,51,52], and excluding the Hofstad et al[53,54] study because of the use of antioxidants as a co-intervention. However, Weingarten et al[33] used the numbers of randomized subjects, rather than the numbers of subjects who completed the follow-up evaluation (colonoscopy), as the denominator in the analysis. This approach assumes that none of the subjects lost to follow-up experienced the outcomes[74,75]; however, this assumption does not appear to be valid.
In our study, a rigorous and extensive literature search was conducted; four eligible randomized trials were identified (the Vitamin D/Calcium Polyp Prevention Study[27,46], the SWOG Calcium Chemoprevention Pilot Study[47,48], the ECP Calcium Fibre Polyp Prevention Study[28,49,50], and the Calcium Polyp Prevention Study[29,51,52]); two further trials were excluded (the Hofstad et al[53,54] study and the Pommergaard et al[55] study[56]) because they evaluated multi-interventional treatments where the effect of calcium could not be separated out; data extraction was carefully undertaken by two independent investigators; and the evidence was synthesized using appropriate statistical techniques. Our results indicate a modest chemopreventive effect of calcium supplements against colorectal adenomas (approximately 10%-15% risk reduction; high quality of evidence). However, this effect was not statistically significant for the advanced (high-risk) adenomas (imprecise pooled effect estimates; moderate quality of evidence). These findings extend the results of the primary trials and have important implications for future research.
The strengths of this systematic review should be weighed against a number of limitations. Firstly, the number of available studies was limited. Secondly, a colorectal adenoma typically requires 10-15 years to evolve into clinically invasive cancer[76]. Therefore, we did not examine whether calcium supplementation affects the progression of adenomas into invasive cancer. To address this question, studies with longer durations of treatment and follow-up are necessary. Thirdly, we could not analyze whether the dose of calcium affected the results; however, the dose range was relatively narrow in the included trials (range: 1200-2000 mg of elemental calcium daily).
Despite these limitations, our study is the most up-to-date meta-analysis on the topic and adheres to the recommended PRISMA reporting standards. Calcium does not appear to strongly reduce the risk of adenomas; however, there is high quality evidence suggesting a modest overall risk reduction, which might be a composite of an effect of calcium supplements in some populations (e.g., the non-obese[27]) and some adenoma types (e.g., the right-colon adenomas[28]), and lack of effect in others. Therefore, we consider that the recent negative results published by Baron et al[27] is not the end of the road for calcium as a potential chemopreventive agent against colorectal carcinoma; rather a new research approach is warranted. There is good reason to focus again on basic research, and perform clinical and epidemiologic studies to answer questions related to dosing and duration of treatment, and identify populations for whom calcium might be particularly beneficial for prevention of adenomas and colorectal cancer.
ACKNOWLEDGMENTS
We would like to thank Dr. Petros Kopterides, MD, PhD, University of Pittsburgh, for language revision.
COMMENTS
Background
Colorectal cancer is the 3rd most common malignancy and the 4th most common cause of cancer deaths globally, with 1.35 million new cases and 0.7 million deaths annually. Most colorectal malignancies develop from adenomas arising from the lining of the intestine. That is why research on colorectal cancer prevention has often focused on prevention of recurrent adenomas.
Research frontiers
It has been suggested that calcium binds bile acids in the bowel lumen, inhibiting their well-known proliferative and carcinogenic effects. Calcium has also demonstrated a direct antiproliferative effect on cells, as well as promoting cellular differentiation and apoptosis.
Innovations and breakthroughs
Contrary to expectations, the recent randomized controlled trial published by Baron et al showed that daily supplementation with 1200 mg of calcium did not significantly reduce the risk of colorectal adenomas over a period of 3 to 5 years. In view of earlier promising clinical data, the authors sought to obtain a comprehensive picture of the evidence by conducting a systematic review and meta-analysis of randomized controlled trials.
Applications
The results show a modest chemopreventive effect of calcium supplements against recurrent colorectal adenomas. Further clinical and epidemiological research is warranted to answer questions related to dosing and duration of treatment, and identify populations for whom calcium might be particularly beneficial.
Terminology
Meta-analysis is a statistical methodology for combining the findings from independent studies.
Peer-review
This study presented the effect of calcium supplementation on colorectal adenoma recurrence through a meta-analysis of clinical trials. This is a valuable paper on secondary adenoma prevention in the colon. The authors give a comprehensive view of an important clinical topic.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest exist.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 15, 2016
First decision: March 21, 2016
Article in press: April 15, 2016
P- Reviewer: Hoensch HP, Lee JE S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
References
- 1.Willett W. The search for the causes of breast and colon cancer. Nature. 1989;338:389–394. doi: 10.1038/338389a0. [DOI] [PubMed] [Google Scholar]
- 2.Reddy B, Engle A, Katsifis S, Simi B, Bartram HP, Perrino P, Mahan C. Biochemical epidemiology of colon cancer: effect of types of dietary fiber on fecal mutagens, acid, and neutral sterols in healthy subjects. Cancer Res. 1989;49:4629–4635. [PubMed] [Google Scholar]
- 3.Potter JD. Reconciling the epidemiology, physiology, and molecular biology of colon cancer. JAMA. 1992;268:1573–1577. [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–147. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 6.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Manson JE, Lee IM, Cook NR, Paul L, Selhub J, Giovannucci E, Zhang SM. Effect of combined folic acid, vitamin B(6), and vitamin B(12) on colorectal adenoma. J Natl Cancer Inst. 2012;104:1562–1575. doi: 10.1093/jnci/djs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 9.Hull MA, Sandell AC, Montgomery AA, Logan RF, Clifford GM, Rees CJ, Loadman PM, Whitham D. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized controlled trial. Trials. 2013;14:237. doi: 10.1186/1745-6215-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut. 1997;40:344–349. doi: 10.1136/gut.40.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson P, Roe DJ, Fales L, Buckmeier J, Wang F, Hamilton SR, Bhattacharyya A, Green S, Hsu CH, Chow HH, et al. Design and baseline characteristics of participants in a phase III randomized trial of celecoxib and selenium for colorectal adenoma prevention. Cancer Prev Res (Phila) 2012;5:1381–1393. doi: 10.1158/1940-6207.CAPR-12-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol. 2014;20:1858–1870. doi: 10.3748/wjg.v20.i7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonovas S, Nikolopoulos G, Bagos P. Bisphosphonate use and risk of colorectal cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76:329–337. doi: 10.1111/bcp.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007;25:3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 16.Bonovas S. Statins: do they have a potential role in cancer prevention and modifying cancer-related outcomes? Drugs. 2014;74:1841–1848. doi: 10.1007/s40265-014-0309-2. [DOI] [PubMed] [Google Scholar]
- 17.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72:1323–1325. [PubMed] [Google Scholar]
- 18.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 19.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 20.Hoff G, Vatn MH. Colonic adenoma: natural history. Dig Dis. 1991;9:61–69. doi: 10.1159/000171293. [DOI] [PubMed] [Google Scholar]
- 21.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 22.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 23.Neugut AI, Jacobson JS, Ahsan H, Santos J, Garbowski GC, Forde KA, Treat MR, Waye J. Incidence and recurrence rates of colorectal adenomas: a prospective study. Gastroenterology. 1995;108:402–408. doi: 10.1016/0016-5085(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 24.Brand L, Munding J, Pox CP, Ziebarth W, Reiser M, Hüppe D, Schmiegel W, Reinacher-Schick A, Tannapfel A. ß-Catenin, Cox-2 and p53 immunostaining in colorectal adenomas to predict recurrence after endoscopic polypectomy. Int J Colorectal Dis. 2013;28:1091–1098. doi: 10.1007/s00384-013-1667-z. [DOI] [PubMed] [Google Scholar]
- 25.Bonovas S, Tsantes A, Drosos T, Sitaras NM. Cancer chemoprevention: a summary of the current evidence. Anticancer Res. 2008;28:1857–1866. [PubMed] [Google Scholar]
- 26.Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994;16:273–297. doi: 10.1093/oxfordjournals.epirev.a036154. [DOI] [PubMed] [Google Scholar]
- 27.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonithon-Kopp C, Kronborg O, Giacosa A, Räth U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356:1300–1306. doi: 10.1016/s0140-6736(00)02813-0. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 30.Keum N, Lee DH, Greenwood DC, Zhang X, Giovannucci EL. Calcium intake and colorectal adenoma risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2015;136:1680–1687. doi: 10.1002/ijc.29164. [DOI] [PubMed] [Google Scholar]
- 31.Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32:789–803. doi: 10.1016/j.clinthera.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Shaukat A, Scouras N, Schünemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol. 2005;100:390–394. doi: 10.1111/j.1572-0241.2005.41220.x. [DOI] [PubMed] [Google Scholar]
- 33.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;(1):CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Petitti DB. Statistical methods in meta-analysis. In: Petitti DB, editor. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis. New York: Oxford University Press; 1999. [Google Scholar]
- 38.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;810:101–129. [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins JPT, Green S, editors . Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration. New York: Oxford University Press; 2011. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 42.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 44.Schwarzer G. Meta: An R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 45.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitamin D/Calcium Polyp Prevention Study. Available from: https://clinicaltrials.gov/ct2/show/NCT00153816.
- 47.Chu DZ, Hussey MA, Alberts DS, Meyskens FL, Fenoglio-Preiser CM, Rivkin SE, Mills GM, Giguere JK, Blanke CD, Goodman GE. Colorectal Chemoprevention Pilot Study (SWOG-9041), randomized and placebo controlled: the importance of multiple luminal lesions. Clin Colorectal Cancer. 2011;10:310–316. doi: 10.1016/j.clcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu DZ, Chansky K, Alberts DS, Meyskens FL, Fenoglio-Preiser CM, Rivkin SE, Mills GM, Giguere JK, Goodman GE, Abbruzzese JL, et al. Adenoma recurrences after resection of colorectal carcinoma: results from the Southwest Oncology Group 9041 calcium chemoprevention pilot study. Ann Surg Oncol. 2003;10:870–875. doi: 10.1245/aso.2003.03.037. [DOI] [PubMed] [Google Scholar]
- 49.Faivre J, Couillault C, Kronborg O, Rath U, Giacosa A, De Oliveira H, Obrador T, O’Morain O. Chemoprevention of metachronous adenomas of the large bowel: design and interim results of a randomized trial of calcium and fibre. ECP Colon Group. Eur J Cancer Prev. 1997;6:132–138. [PubMed] [Google Scholar]
- 50.Faivre J, Doyon F, Boutron MC. The ECP calcium fibre polyp prevention study. The ECP Colon Group. Eur J Cancer Prev. 1991;1 Suppl 2:83–89. doi: 10.1097/00008469-199110002-00015. [DOI] [PubMed] [Google Scholar]
- 51.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, et al. Calcium supplements and colorectal adenomas. Polyp Prevention Study Group. Ann N Y Acad Sci. 1999;889:138–145. doi: 10.1111/j.1749-6632.1999.tb08731.x. [DOI] [PubMed] [Google Scholar]
- 52.Wallace K, Baron JA, Cole BF, Sandler RS, Karagas MR, Beach MA, Haile RW, Burke CA, Pearson LH, Mandel JS, et al. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96:921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 53.Hofstad B, Almendingen K, Vatn M, Andersen SN, Owen RW, Larsen S, Osnes M. Growth and recurrence of colorectal polyps: a double-blind 3-year intervention with calcium and antioxidants. Digestion. 1998;59:148–156. doi: 10.1159/000007480. [DOI] [PubMed] [Google Scholar]
- 54.Hofstad B, Vatn M, Hoff G, Larsen S, Osnes M. Growth of colorectal polyps: design of a prospective, randomized, placebo-controlled intervention study in patients with colorectal polyps. Eur J Cancer Prev. 1992;1:415–422. [PubMed] [Google Scholar]
- 55.Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Aspirin, Calcitriol, and Calcium Do Not Prevent Adenoma Recurrence in a Randomized Controlled Trial. Gastroenterology. 2016;150:114–122.e4. doi: 10.1053/j.gastro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 56.A clinical trial evaluating the efficacy and safety of a combination treatment administered over three years in patients at risk of experiencing recurrence of colorectal adenomas. Available from: https://clinicaltrials.gov/ct2/show/NCT00486512.
- 57.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 58.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Bonovas S. Cancer chemoprevention: progress and perspectives. Curr Drug Targets. 2011;12:1871–1873. doi: 10.2174/138945011798184137. [DOI] [PubMed] [Google Scholar]
- 60.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 61.Greenwald P. Cancer chemoprevention. BMJ. 2002;324:714–718. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 63.Lippman SM, Benner SE, Hong WK. Cancer chemoprevention. J Clin Oncol. 1994;12:851–873. doi: 10.1200/JCO.1994.12.4.851. [DOI] [PubMed] [Google Scholar]
- 64.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–530. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 65.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–543. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 66.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 67.Lipkin M. Early development of cancer chemoprevention clinical trials: studies of dietary calcium as a chemopreventive agent for human subjects. Eur J Cancer Prev. 2002;11 Suppl 2:S65–S70. [PubMed] [Google Scholar]
- 68.Slattery ML. Diet, lifestyle, and colon cancer. Semin Gastrointest Dis. 2000;11:142–146. [PubMed] [Google Scholar]
- 69.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 70.Martínez ME, Willett WC. Calcium, vitamin D, and colorectal cancer: a review of the epidemiologic evidence. Cancer Epidemiol Biomarkers Prev. 1998;7:163–168. [PubMed] [Google Scholar]
- 71.Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl. 1995;22:65–73. doi: 10.1002/jcb.240590810. [DOI] [PubMed] [Google Scholar]
- 72.Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res. 1993;290:87–95. doi: 10.1016/0027-5107(93)90036-f. [DOI] [PubMed] [Google Scholar]
- 73.Nikolopoulos GK, Bagos PG, Bonovas S. Developing the evidence base for cancer chemoprevention: use of meta-analysis. Curr Drug Targets. 2011;12:1989–1997. doi: 10.2174/138945011798184191. [DOI] [PubMed] [Google Scholar]
- 74.Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165:1339–1341. [PMC free article] [PubMed] [Google Scholar]
- 75.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]


