Abstract
Purpose
We previously demonstrated that tenascin-C was highly expressed in the fibrovascular membranes (FVMs) of patients with proliferative diabetic retinopathy (PDR). However, its role in the pathogenesis of FVMs has not been determined. The purpose of this study was to investigate what role tenascin-C plays in the formation and angiogenesis of FVMs.
Methods
The level of tenascin-C was determined by sandwich enzyme-linked immunosorbent assay in the vitreous samples collected from patients with PDR and with a macular hole as control. The locations of tenascin-C, α- smooth muscle actin (SMA), CD34, glial fibrillary acidic protein (GFAP), and integrin αV in the FVMs from PDR patients were determined by immunohistochemistry. We also measured the in vitro expression of the mRNA and protein of tenascin-C in vascular smooth muscle cells (VSMCs) stimulated by interleukin (IL)-13. The effects of tenascin-C on cell proliferation, migration, and tube formation were determined in human retinal endothelial cells (HRECs) in culture.
Results
The mean vitreous levels of tenascin-C were significantly higher in patients with PDR than in patients with a macular hole (p<0.001). Double immunofluorescence analyses of FVMs from PDR patients showed that tenascin-C co-stained FVMs with α-SMA, CD34, and integrin αV but not with GFAP. In addition, IL-13 treatment increased both the expression and secretion of tenascin-C by VSMCs in a dose-dependent manner. Tenascin-C exposure promoted proliferation, migration, and tube formation in HRECs. Tenascin-C neutralizing antibody significantly blocked the tube formation by HRECs exposed to VSMC-IL-13-conditioned medium.
Conclusions
Our findings suggest that tenascin-C is secreted from VSMCs and promotes angiogenesis in the FVMs associated with PDR.
Introduction
Proliferative diabetic retinopathy (PDR) is a common complication of diabetes mellitus and is the leading cause of blindness among working-age adults worldwide [1]. PDR is characterized by preretinal neovascularization and development of epiretinal fibrovascular membranes (FVMs) [2]. The formation of FVMs is a wound-healing process in which newly formed blood vessels progress to form FVMs, which can then cause traction on the retina resulting in retinal detachment and blindness [3]. Despite recent advances in the treatment of PDR, such as vitreoretinal surgery with the use of anti-vascular endothelial growth factor (VEGF) drugs, PDR still remains an important vision-threatening disease. Therefore, it is important to develop new therapies based on comprehensive understandings of the molecular mechanisms involved in the development of FVMs.
To achieve this, we recently performed a comprehensive gene expression-profiling study of FVMs using DNA microarray analysis. The results revealed significant upregulations of matricellular proteins, e.g., periostin and tenascin-C [4]. Subsequently, we demonstrated that periostin plays a pivotal role in the development of periretinal fibro(vascular) membranes [5-7].
Tenascin-C is a large, hexameric, extracellular glycoprotein. Monomers of tenascin-C contain a globular N-terminal assembly domain, a 14.5 epidermal growth factor (EGF)-like repeat domain, a fibronectin type III–like repeat domain, and a fibrinogen-like sequence. It has been shown that the fibronectin type III–like repeats of tenascin-C affect cell adhesion through the integrin receptors or annexin II [8]. Tenascin-C is expressed at lower levels in most adult tissues but is transiently upregulated during acute inflammation and is continuously expressed during chronic inflammation and tissue repair [9]. Other studies have demonstrated that tenascin-C plays a vital role in cardiac ischemia [10-12], tumor angiogenesis, and metastatic tissues [13-16].
Relevant to this study, it has been reported that there is a higher level of tenascin-C in the vitreous of patients with PDR and at the active stage of PDR [17]. However, the roles played by tenascin-C in the development and angiogenesis of FVMs in PDR patients have not been definitively determined. Thus, the purpose of this study was to determine whether tenascin-C is expressed in the vitreous of PDR patients and to determine what its role is in the development and angiogenesis of FVMs.
Methods
Human specimens
This study was approved by the Ethics Committees of the Kyushu University Hospital, and the surgical specimens were handled in accordance with the Declaration of Helsinki. All patients included in this study signed an informed consent form before undergoing surgery.
FVM samples were collected from six eyes of six patients (age 54.2±8.8 years; 3 men, 3 women) with PDR. Vitreous samples were collected from 133 eyes of 112 patients (age 59.0±10.2 years; 66 men, 46 women) with PDR during the initial pars plana vitrectomy. For the control, vitreous samples were collected from 41 eyes of 39 patients (age 65.0±1.3 years; 19 men, 20 women) who were undergoing vitrectomy for a macular hole (MH). Undiluted vitreous samples (0.5–1.0 ml) were aspirated at the beginning of vitrectomy under standardized conditions and were immediately transferred to sterile tubes. The samples were centrifuged for 10 min at 1630 ×g at 4 °C, and the supernatants were divided into aliquots and stored at −70 °C until laboratory analyses.
Enzyme-linked immunosorbent assay
Sandwich enzyme-linked immunosorbent assay (ELISA) was performed to measure the concentration of tenascin-C using an ELISA kit (27767; IBL, Fujioka, Gunma, Japan) in accordance with the protocol of the manufacturer. The samples were added to a buffer solution in 96-well plates and incubated 1 h at room temperature. The solution was aspirated and washed. Mouse monoclonal antibody against tenascin-C (4F10TT) conjugated to horseradish peroxidase was added to each well and incubated for 30 min at 4 °C, after which the solution was collected and washed again. The substrate solution was added to each well and incubated for 30 min at room temperature. The stop solution was then added to each well, and the level of tenascin-C was determined with a microplate reader at 450 nm (ImmunoMini NJ-2300; NJ InterMed, Tokyo, Japan).
Immunofluorescence staining
FVMs were embedded in paraffin and cut into 3-μm sections. After removing the paraffin, the sections were rehydrated, blocked, and incubated with primary antibodies overnight. The next morning, the sections were incubated with the secondary antibodies for 30 min at room temperature. Nuclei were counterstained with Hoechst 33342 (H3570, 1:400 dilution; Life Technologies, Gaithersburg, MD). The primary antibodies were tenascin-C supplied by Juntendo University (20 μg/ml), α-smooth muscle actin (α-SMA, ab21027, 1:50 dilution; Abcam, Cambridge, MA), CD34 (NCL-L-END, 1:2000 dilution; Leica Biosystems, Newcastle, UK), glial fibrillary acidic protein (GFAP, Z0334, 1:500 dilution; Dako, Glostrup, Denmark), integrin αV (AB1930, 1:1000 dilution; Millipore, Bedford, MA), smooth muscle myosin heavy chain SM1 (7599, 1:3000; YAMASA, Tokyo, Japan), and SM2 (7601, 1:400; YAMASA). The secondary antibodies were anti-mouse Alexa Fluor 488 (A11001, 1:800 dilution; Life Technologies), anti-rat Alexa Fluor 488 (A11006, 1:800 dilution; Life Technologies), anti-mouse Alexa Fluor 647 (A21247, 1:800 dilution; Life Technologies), anti-goat Alexa Fluor 647 (A21469, 1:800 dilution; Life Technologies), and anti-rabbit Alexa Fluor 647 (A21244: 1:200 dilution; Life Technologies). Sections were examined and photographed with a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan).
Cell cultures
Human vascular smooth muscle cells (VSMCs, CC-2571; Lonza, Visp, Switzerland) were grown in endothelial cell growth medium (EGM)-2 plus bullet kit media (CC-3162; Lonza). Human retinal endothelial cells (HRECs, ACBRI181; Cell Systems, Kirkland, WA) were grown in CSC complete recombinant media (SF-4Z0; Cell Systems). To study the role of VSMCs, they were exposed to or not exposed to 2, 10, and 50 ng/ml of recombinant interleukin (IL)-13 (213-ILB; R&D Systems, Minneapolis, MN). After incubating for 24 h, the total RNA was extracted using TRIzol reagent (Life Technologies). After a 72-h incubation, the supernatant was collected and the lysated protein was extracted with lysis buffer.
Real-time quantitative transcriptase PCR
Total RNA was isolated and reverse-transcribed with a first-strand cDNA synthesis kit for real-time (RT) PCR (Roche, Mannheim, Germany) and the GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). The level of gene expression in the cDNA was determined by RT-PCR using a LightCycler 96 (Roche). The quantification was performed with either TaqMan-probe-based or SYBR Green–based chemistry. Probe-based PCR was performed with FastStart Essential DNA Probes Master (Roche) and TaqMan Gene Expression Assays targeting tenascin-C (Hs01115665_m1, Roche). Thermal cycling was done at 95 °C for 10 min, followed by 45 cycles of 95 °C for 10 s and 60 °C for 30 s. The fluorescent signal was acquired using the FAM channel. The SYBR Green–based PCR was performed with SYBR Premix Ex Taq (Takara, Otsu, Shiga, Japan). Thermal cycling was done at 95 °C for 10 s followed by 50 cycles of 95 °C for 5 s and 60 °C for 20 s, and one cycle of final extension at 60 °C for 1 min. The fluorescent signal was acquired using the SYBR Green channel. Melting curve analysis was performed between 65 °C and 95 °C to evaluate the specificity of the PCR amplification. Primer nucleotide sequences for GAPDH were: forward; 5′-GAG TCA ACG GAT TTG GTC GT-3′, reverse: 5′-CTT GAT TTT GGA GGG ATC TCG C-3′.
Western blot analysis
Western blot analysis was performed as described in detail [6]. The cell lysates of VSMCs stimulated by IL-13 were subjected to NuPAGE 3%–8% Tris-Acetate Gel (EA0375; Life Technologies), and the blots were incubated with a monoclonal antibody of tenascin-C (4F10TT, 10,337:1:3000; IBL). Differences in the loading were normalized by blotting the membranes with an antibody against β-actin (#4970, 1:5000; Cell Signaling, Beverly, MA).
Cell proliferation assay
The degree of proliferation of the HRECs was determined by bromodeoxyuridine (BrdU)-ELISA (Roche) incorporation performed according to the manufacturer's protocol. Briefly, HRECs were plated at 1.0 × 104 cells on 96-well plates. After starvation in CSC medium with 0.5% fetal bovine serum (FBS), the cells were treated with or without tenascin-C at 30, 100, and 300 ng/ml (CC065; Millipore). Subsequently, the cells were incubated for 48 h at 37 °C. The cells were labeled with 10 μM BrdU for 2 h and fixed. After 1.5 h of incubation with a peroxidase-coupled anti-BrdU-antibody, cells were washed three times with phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4). Thereafter, peroxidase substrate tetramethyl-benzidine was added for 10 min, and measurements were performed on a microplate reader at 450 nm (ImmunoMini NJ-2300; NJ InterMed).
Cell migration assay
To examine the effect of tenascin-C on the migration of HRECs, trans-well assays were performed using 8-µm pore-size cell culture inserts (Corning, Corning, NY). The lower surface of the inserts was coated with tenascin-C or fibronectin (F2006; Sigma-Aldrich, St. Louis, MO) or bovine serum albumin (BSA; 2 µg/insert each). HRECs were suspended at 8 × 105 cells/ml in CSC with 0.5% FBS on the upper surface of the inserts. The cells were allowed to migrate to the lower surface of the membrane for 5 h at 37 °C. The inserts were fixed with 4% paraformaldehyde in PBS and stained with Hoechst 33342 (H3570, 1:1000 dilution; Life Technologies) for 15 min. The stained cells in four random fields were counted under a 20X objective lens, and the data were analyzed with ImageJ software (National Institutes of Health, Bethesda, MD).
Preparation of vascular smooth muscle cell-conditioned media
To produce VSMC-conditioned media, VSMCs were cultured for 2 days on type 1 collagen at 37 °C in Dulbecco's Modified Eagle's Medium (DMEM, D6046; Sigma-Aldrich) with 10% FBS. After 2 days, the cells were removed and cultured in DMEM with 0.5% FBS for 24 h. On day 3, the cells were rinsed twice with PBS, and the medium was replaced with serum-free CSC medium with or without recombinant IL-13 (213-ILB, 2 ng/ml; R&D Systems). After 24 h, the conditioned media was collected from the VSMC culture dishes. The conditioned media was filtered through 0.2 μM filters (Corning) and frozen at −70 °C until use.
Cellular tube formation assay
HRECs were plated in 96-well plates that had been precoated with Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA) at 1.8 × 104 cells/well. The CSC medium or VSMC-controlled media with or without tenascin-C neutralizing antibody (MAB2138, 50 μg/ml; R&D Systems) was supplemented with 0.5% FBS. The cells were subsequently incubated for 20 h at 37 °C. The data were analyzed by ImageJ software (National Institutes of Health).
Statistical analyses
All results are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed with a commercial statistical software package (JMP, version 11.0; SAS Institute, Cary, NC). The significance of the differences among groups was determined by the Mann–Whitney U tests or two-tailed Student t tests. To determine whether a significant correlation existed between vitreous concentrations of tenascin-C, periostin, and VEGF, Spearman rank correlation test was used. A value of p<0.05 was considered statistically significant.
Results
Higher concentrations of tenascin-C in vitreous of eyes with PDR than with an MH
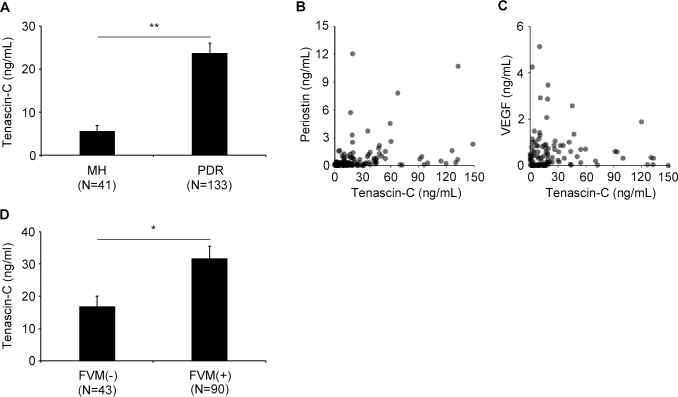
The vitreous concentration of tenascin-C was significantly higher at 26.91±2.80 ng/ml in eyes with PDR than in eyes with an MH at 5.15±1.35 ng/ml (Figure 1A; p<0.0001).
Figure 1.
Concentrations of tenascin-C in the vitreous collected from eyes with a macular hole (MH) or eyes with proliferative diabetic retinopathy (PDR). A: Tenascin-C concentrations in vitreous samples collected from eyes with a macular hole (MH; n=41) and eyes with proliferative diabetic retinopathy (PDR; n = 133). B and C: Correlations between vitreous concentrations of tenascin-C and periostin (B), or tenascin-C and VEGF (C) in eyes with PDR (n = 133). B: There is a significant correlation between the vitreous concentration of tenascin-C and periostin (r = 0.470, P <0.001; Spearman correlation coefficient). C: There is a weak correlation between tenascin-C and VEGF in the same patients with PDR (r = 0.198, P = 0.040; Spearman correlation coefficient). D: Vitreous concentrations of tenascin-C in eyes with fibrovascular membranes (FVMs; n=90) compared to those eyes without (n=43). *p <0.05, **p <0.001. Bars are mean ± standard error of the mean (SEM). N is number of patients.
Significant correlation between vitreous concentrations of tenascin-C and periostin in eyes with PDR
We have reported that the vitreous concentration of periostin in eyes with PDR was significantly higher than that in eyes of nondiabetic controls (MH and epiretinal membrane) [18]. To determine whether there were significant associations among the concentrations of tenascin-C, periostin, and VEGF, we calculated the correlations among the vitreous concentrations of these three molecules. There was a highly significant correlation between the vitreous concentration of tenascin-C and periostin in the 133 eyes with PDR (r = 0.470, p<0.001; Spearman correlation; Figure 1B). The correlation between tenascin-C and periostin in the 41 eyes with MH was not significant (p=0.111). In addition, only a weak but significant correlation was found between tenascin-C and VEGF in the same eyes with PDR (r = 0.198, p = 0.040; Spearman correlation coefficient: Figure 1C).
High vitreous concentration of tenascin-C in PDR eyes with FVMs
We demonstrated previously by microarray analysis that the expression of the tenascin-C gene was significantly higher in FVMs than in normal human retinas [4]. To determine the relationship between tenascin-C and FVMs, we subdivided the PDR patients into those with an FVM and those without an FVM (epicenter only). The mean vitreous level of tenascin-C was 31.70±3.76 ng/ml in the 90 eyes with an FVM and 16.90±3.10 ng/ml in the 43 eyes without an FVM. This difference was statistically significant (p = 0.030, Figure 1D).
Localization of tenascin-C in FVMs
To determine the location of the cellular expression of tenascin-C and integrin αV, a tenascin-C receptor, we stained the FVM sections with antibodies against tenascin-C, integrin αV, α-SMA, CD34, and GFAP. α-SMA is a marker for VSMCs and mesenchymal cells, CD34 for vascular endothelial cells, and GFAP for glial cells. Double immunofluorescence analyses showed that tenascin-C was co-expressed with α-SMA and CD34 in FVMs (Figure 2). Most α-SMA-positive cells were co-stained with SM1 and SM2, specific markers of smooth muscle cells. This indicates that the α-SMA-positive cells in the FVMs were not mesenchymal cells transdifferentiated from retinal endothelial cells but VSMCs (Appendix 1). In contrast, the glial cells rarely co-expressed tenascin-C with any of the other antibodies. In addition, the vascular endothelial cells in the FVMs expressed both tenascin-C and integrin αV (Figure 2). These findings indicate that tenascin-C can be produced from or bound to VSMCs or vascular endothelial cells in the FVMs of eyes with PDR.
Figure 2.
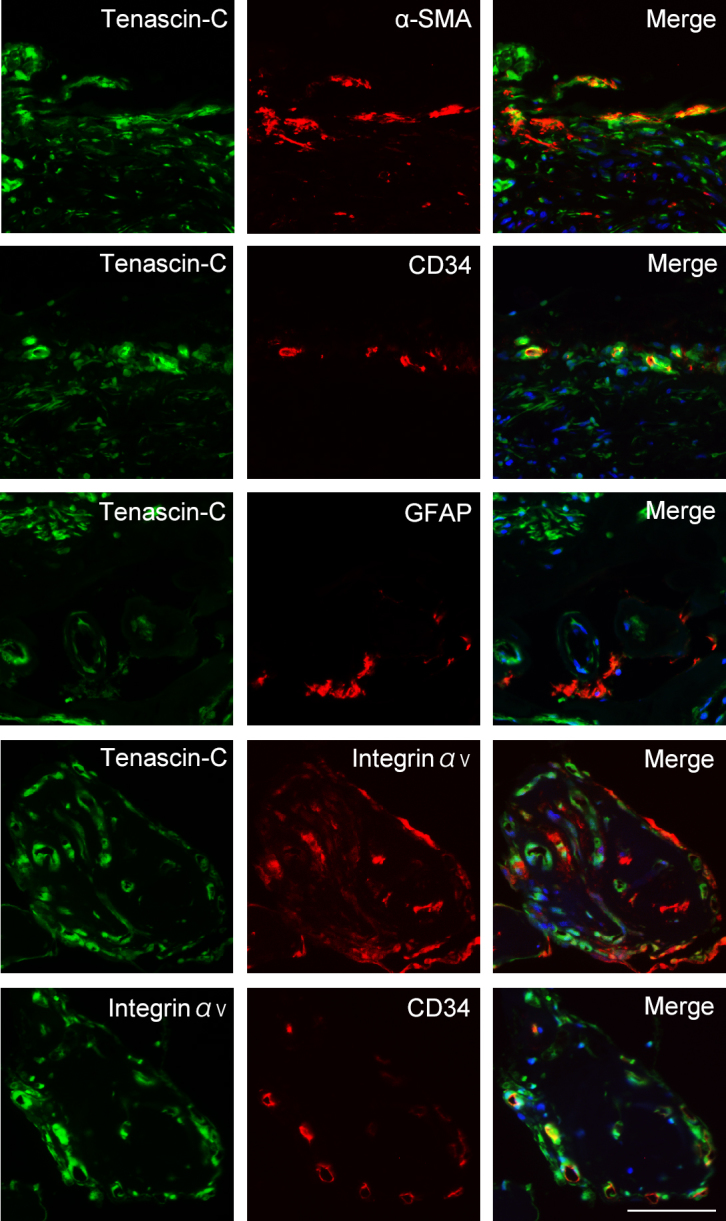
Triple immunofluorescence staining for tenascin-C, α- smooth muscle actin (SMA; marker for vascular smooth muscle cells), CD34 (marker for vascular endothelial cells), glial fibrillary acidic protein (GFAP; marker for glial cells), and integrin αV in fibrovascular membranes (FVMs) from eyes with proliferative diabetic retinopathy (PDR) patients. Nuclei are stained blue. Scale bar, 100 μm.
Tenascin-C expression in VSMCs stimulated by IL-13
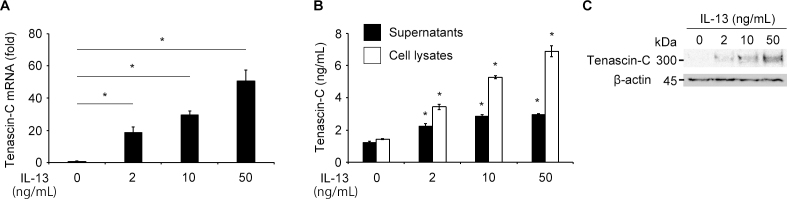
Next, we tested the possible synthesis of tenascin-C by VSMCs and HRECs stimulated by inflammatory cytokines. We determined the level of the mRNAs of tenascin-C in VSMCs and HRECs after exposure to cytokines that are present in the vitreous of eyes with PDR [19-22], viz., IL-6, IL-8, IL-13, monocyte chemoattractant protein (MCP)-1, insulin-like growth factor (IGF)-1, transforming growth factor (TGF)-β2, or VEGF. We have reported that the concentration of IL-13 in the vitreous of patients with PDR is significantly higher than that in the vitreous of patients with nondiabetic ocular diseases [22]. Only the VSMCs stimulated by IL-13 expressed the mRNA (Figure 3A) and protein (Figure 3B) of tenascin-C. VSMCs or HRECs stimulated by other cytokines did not induce tenascin-C expression (data not shown). Western blot analysis of the antibodies in the cell lysates from VSMCs treated with IL-13 using an anti-tenascin-C antibody had a band of tenascin-C at approximately 300 kDa (Figure 3C). These results indicate that the IL-13-induced tenascin-C production and secretion by the VSMCs in the FVMs of eyes with PDR.
Figure 3.
Western blot showing expression of the mRNA of tenascin-C in vascular smooth muscle cells (VSMCs). A: Expression of the mRNA of tenascin-C normalized to GAPDH in VSMCs stimulated with the indicated concentrations of IL-13. B: ELISA analysis of tenascin-C in cell lysates and supernatants in VSMCs stimulated with the indicated concentrations of IL-13. VSMCs stimulated by IL-13 produced and secreted tenascin-C in a dose-dependent manner. C: Western blot analysis of tenascin-C in cell lysates in VSMCs stimulated with the indicated concentrations of IL-13. Mouse monoclonal anti-tenascin-C clone 4F10TT antibody shows a bond at approximately 300 kDa. β-actin was used as a loading control. *p <0.001, compared to control. Bars are mean ± standard error of the mean (SEM, n = 4 per group).
Proliferation, migration, and tube formation of HRECs induced by tenascin-C
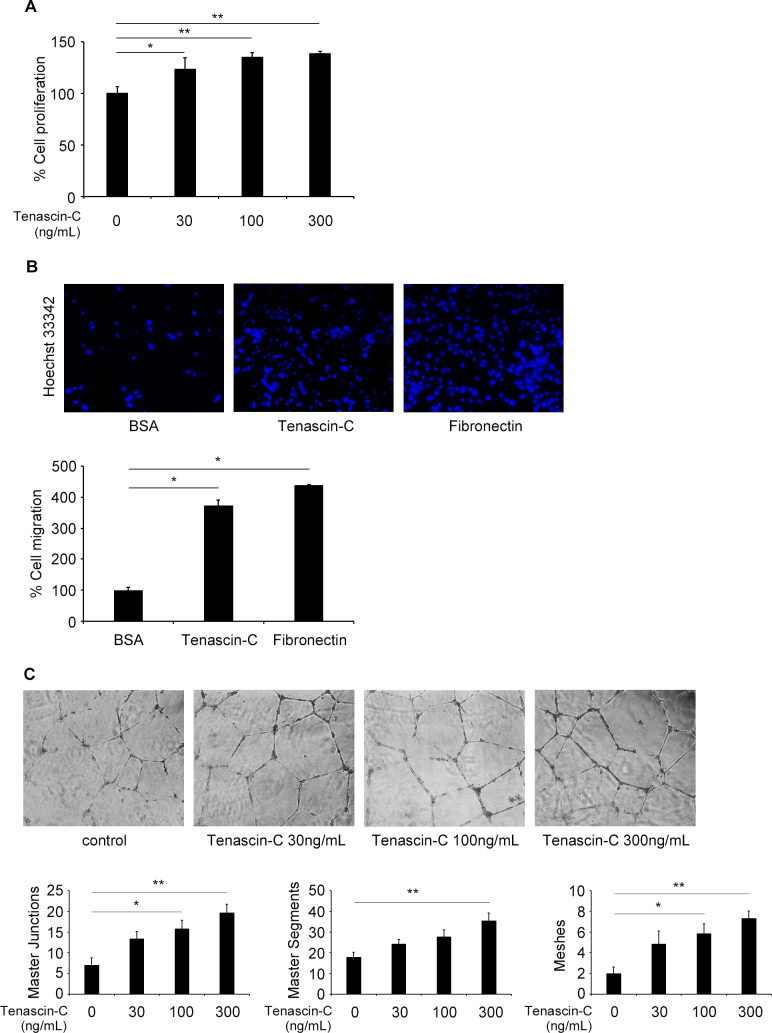
To examine whether tenascin-C affects the different angiogenic processes, we examined the proliferation, migration, and tube formation of HRECs. We first studied the effects of tenascin-C on the proliferation in HRECs using the BrdU assay. Tenascin-C exposure of 0, 30, 100, and 300 ng/ml significantly increased the proliferation of cells in a dose-dependent manner (Figure 4A).
Figure 4.
Effect of tenascin-C on human retinal endothelial cells (HRECs) in culture. A: Cell proliferation assay of HRECs. Bromodeoxyuridine (BrdU) incorporation (n = 4 per group) was measured in the presence of varying concentrations of tenascin-C (30, 100, and 300 ng/ml). *p <0.05, **p <0.01, compared to control. B: Cellular migration assay of HRECs. HRECs were placed in the upper chamber and allowed to migrate toward the lower side of the insert coated with tenascin-C, fibronectin, or BSA (25 μg/ml each). The photographs were taken (20× objective) after Hoechst 33342 staining. *p<0.001, compared to control. C: Matrigel tube formation assay of HRECs. The network formation with HRECs was quantified by the total numbers of master junctions, master segments, or meshes in the presence of varying concentrations of tenascin-C (30, 100, and 300 ng/ml). *p <0.05, **p <0.01, compared to control. Bars are mean ± standard error of the mean (SEM, n = 4-6 per group).
We next investigated the effect of tenascin-C on the migration of HRECs using trans-well with inserts coated with tenascin-C. Fibronectin-coated trans-well inserts were used as a positive control to induce cell migration [23]. Tenascin-C and fibronectin significantly promoted cell migration (Figure 4B). In addition, we measured the ability of HRECs to form master junctions, master segments, or meshes in the presence and absence of 0, 30, 100, 300 ng/ml of tenascin-C to quantify tube formation in a manner similar to a previous report [24]. In the tube formation assay, tenascin-C promoted all three aspects of tube formation on a Matrigel BM matrix in a dose-dependent manner (Figure 4C).
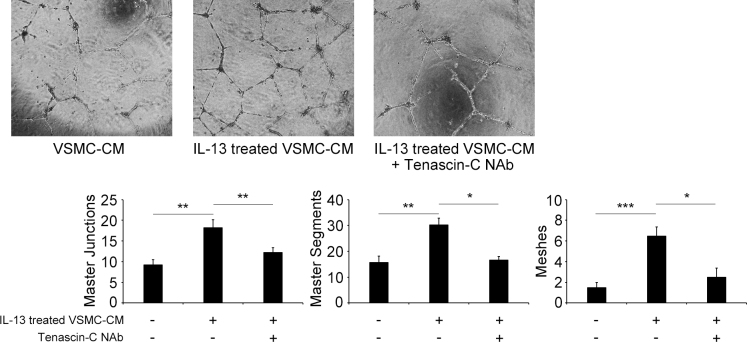
To examine if tenascin-C secreted from VSCMs can promote tube formation by HRECs, a VSMC-conditioned medium stimulated by IL-13 (VSMC-IL-13-conditioned medium) was prepared. VSMC-IL-13-conditioned medium significantly increased all three aspects of tube formation by HRECs on the Matrigel BM matrix (Figure 5). We confirmed that the VSMC-IL-13-conditioned medium with or without cryopreservation at −70 °C had the same effect (Appendix 2). In addition, tenascin-C neutralizing antibody significantly blocked the tube formation by HRECs exposed to VSMC-IL-13-conditioned medium (Figure 5). These results indicate that tenascin-C, which is secreted by VSMCs stimulated by IL-13, facilitates retinal neovascularization.
Figure 5.
Tube formation assay on matrigel by human retinal endothelial cells (HRECs) in vascular smooth muscle cell-conditioned medium (VSMC-CM). HRECs were cultured for 20 h in 96-well plates coated with a matrigel. The cells were cultured in VSMC-CM stimulated with or without IL-13 (2 ng/ml) and treated with or without tenascin-C neutralizing antibody (NAb; 50 μg/ml). The network formation with HRECs was quantified by the total numbers of master junctions, master segments, or meshes. *p <0.05, **p <0.01, ***p <0.001. Bars are mean ± standard error of the mean (SEM, n = 4 per group).
Discussion
Our results showed that tenascin-C was present at significantly higher concentrations in the vitreous and FVMs of patients with PDR than in the vitreous of eyes with an MH. In addition, our results showed that tenascin-C was produced by VSMCs stimulated by IL-13 and that it promoted cellular processes associated with angiogenesis, such as proliferation, migration, and tube formation, in HRECs.
We identified the VSMCs as the synthesizers of tenascin-C in the FVMs of eyes with PDR. This is consistent with the results of an earlier study that showed that the VSMCs of hypertensive rats expressed tenascin-C [25]. Our results showed that IL-13 stimulated VSMCs to express tenascin-C. IL-13 is a pro-angiogenic cytokine [26] produced by helper T (Th2) cells, one of the cellular components present in the FVMs of patients with PDR [27]. Moreover, our earlier study showed an increase in the IL-13 level in the vitreous of patients with PDR [22]. These findings suggest that IL-13 is secreted from Th2 cells and induces tenascin-C production by the VSMCs in the FVMs associated with PDR.
Our results also showed that the conditioned media from VSMC-IL13 promoted cell proliferation, migration, and tube formation and that tenascin-C neutralizing antibody blocked the tube formation of HRECs stimulated by the conditioned media from VSMC-IL-13. These findings indicate that tenascin-C secreted from VSMCs by IL-13 facilitated angiogenic processes of retinal endothelial cells. This demonstrated a pro-angiogenic role of tenascin-C.
In immunohistochemical studies, we found a co-staining of the vascular endothelial cells by the antibodies of tenascin-C and integrin αVβ3. Integrin αVβ3, a well-known receptor involved in retinal angiogenesis [28], is a tenascin-C-binding receptor on vascular endothelial cells [29]. The results of earlier studies have demonstrated that tenascin-C binds to the integrin αVβ3 of HRECs during tube formation [30]. Our observations of the co-localization of integrin αV and tenascin-C in FVMs support the idea that tenascin-C binds to integrin αVβ3, which results in retinal angiogenesis during the process of FVM formation.
We found that the concentration of vitreous tenascin-C was significantly correlated with the vitreous periostin concentration. Tenascin-C and periostin are both matricellular proteins and share a common facilitatory role in angiogenesis and fibrosis during tumor formation [7,31,32]. An earlier study proposed that periostin acts as a scaffold for tenascin-C to form a hexamer and mediates the incorporation of tenascin-C into the architecture of extracellular matrixes, which helps maintain structural homeostasis [7]. The results of our recent studies showed a pro-fibrotic and pro-angiogenic role of periostin in fibrous membrane formation in proliferative vitreoretinal diseases [5,6]. The present results showed the pro-angiogenic role of tenascin-C in retinal neovascularization. Overall, the interaction of tenascin-C and periostin may play an important role in angiogenesis of FVMs.
Anti-VEGF therapy has recently been used on patients with intraocular neovascular diseases. Many patients have had good initial responses, but some of the patients became resistant to additional treatment. The possible mechanisms for resistance are tolerance or tachyphylaxis, which is a phenomenon causing reduced drug efficacy by repeated administration [33]. Some eyes need shorter treatment intervals or a switch to another anti-VEGF antibody. Because the vitreous concentration of tenascin-C was only weakly correlated with those of VEGF in patients with PDR, this suggests that tenascin-C and VEGF act independently in the development of FVMs. Therefore, anti-tenascin-C but not anti-VEGF drugs has a potential for being an effective therapeutic treatment that would inhibit angiogenesis.
In conclusion, we found a higher concentration of tenascin-C in the vitreous and VSMCs of FVMs in patients with PDR. Tenascin-C exposure promoted proliferation, migration, and tube formation in HRECs, and tenascin-C neutralizing antibody significantly blocked the tube formation by HRECs exposed to VSMC-IL-13-conditioned medium. These results indicate that tenascin-C is secreted from VSMCs and promotes angiogenesis in the FVMs associated with PDR.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (B; No. 15H04995 and 26293374), Grant-in-Aid for Challenging Exploratory Research (No. 26670757), and Grant-in-Aid for JSPS Fellows from Japan Society for the Promotion of Science (No.15J03433).
Appendix 1.
To access the data, click or select the words “Appendix 1.” Triple immunofluorescence staining for tenascin-C, α-SMA, M1, and SM2 in FVMs from eyes with PDR. SM1 and SM2 are specific markers for smooth muscle cells. Most of α-SMA-positive cells are co-stained with SM1 and SM2, specific markers of smooth muscle cells. Nuclei are stained blue. Scale bar, 100 μm.
Appendix 2.
To access the data, click or select the words “Appendix 2.” Tube formation assay on matrigel by human retinal endothelial cells (HRECs) in vascular smooth muscle cell-conditioned medium (VSMC-CM) with or without cyopreservation at -70° C. HRECs were cultured for 20 h in 96-well plates coated with a matrigel. The cells were cultured in VSMC-CM stimulated with or without IL-13 (2 ng/ml). The network formation with HRECs was quantified by the total numbers of master junctions, master segments, or meshes. VSMC-IL-13-conditioned medium with or without cryopreservation at -70° C has same effect. *p <0.05, **P <0.01. Bars are mean ± standard error of the mean (SEM, n = 4 per group).
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–83. doi: 10.1080/09286580701396720. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17896294&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 2.Garner A. Histopathology of diabetic retinopathy in man. Eye (Lond) 1993;7:250–3. doi: 10.1038/eye.1993.58. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7607344&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18628999&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa K, Yoshida S, Kobayashi Y, Zhou Y, Nakama T, Nakao S, Sassa Y, Oshima Y, Niiro H, Akashi K, Kono T, Ishibashi T. Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:932–46. doi: 10.1167/iovs.14-15589. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25604687&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T. Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Ther. 2015;22:127–37. doi: 10.1038/gt.2014.112. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25503692&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, Oshima Y, Murakami N, Niiro H, Ono J, Matsuda A, Goto Y, Akashi K, Izuhara K, Kudo A, Kono T, Hafezi-Moghadam A, Ishibashi T. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB. 2014;28:131–42. doi: 10.1096/fj.13-229740. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24022401&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cellular and molecular life sciences. Cell Mol Life Sci. 2011;68:3201–7. doi: 10.1007/s00018-011-0784-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21833583&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244:143–63. doi: 10.1016/j.canlet.2006.02.017. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16632194&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–99. doi: 10.1002/path.1415. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12845616&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Taki J, Inaki A, Wakabayashi H, Imanaka-Yoshida K, Ogawa K, Hiroe M, Shiba K, Yoshida T, Kinuya S. Dynamic expression of tenascin-C after myocardial ischemia and reperfusion: assessment by 125I-anti-tenascin-C antibody imaging. J Nucl Med. 2010;51:1116–22. doi: 10.2967/jnumed.109.071340. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20554738&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Wallner K, Sharifi BG, Shah PK, Noguchi S, DeLeon H, Wilcox JN. Adventitial remodeling after angioplasty is associated with expression of tenascin mRNA by adventitial myofibroblasts. J Am Coll Cardiol. 2001;37:655–61. doi: 10.1016/s0735-1097(00)01117-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11216993&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol. 1996;179:321–5. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8774490&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Pezzolo A, Parodi F, Marimpietri D, Raffaghello L, Cocco C, Pistorio A, Mosconi M, Gambini C, Cilli M, Deaglio S, Malavasi F, Pistoia V. Oct-4+/Tenascin C+ neuroblastoma cells serve as progenitors of tumor-derived endothelial cells. Cell Res. 2011;21:1470–86. doi: 10.1038/cr.2011.38. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21403679&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvo A, Catena R, Noble MS, Carbott D, Gil-Bazo I, Gonzalez-Moreno O, Huh JI, Sharp R, Qiu TH, Anver MR, Merlino G, Dickson RB, Johnson MD, Green JE. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene. 2008;27:5373–84. doi: 10.1038/onc.2008.155. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18504437&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka K, Hiraiwa N, Hashimoto H, Yamazaki Y, Kusakabe M. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer. 2004;108:31–40. doi: 10.1002/ijc.11509. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14618612&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Garcion E, Faissner A. ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development. 2001;128:2485–96. doi: 10.1242/dev.128.13.2485. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11493565&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Mitamura Y, Takeuchi S, Ohtsuka K, Matsuda A, Hiraiwa N, Kusakabe M. Tenascin-C levels in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2002;25:1899. doi: 10.2337/diacare.25.10.1899. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12351514&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Yoshida S, Nakama T, Zhou Y, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol. 2015;99:451–6. doi: 10.1136/bjophthalmol-2014-305321. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25281471&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, Ueno A, Hata Y, Yoshida H, Ishibashi T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19997642&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirase K, Ikeda T, Sotozono C, Nishida K, Sawa H, Kinoshita S. Transforming growth factor beta2 in the vitreous in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116:738–41. doi: 10.1001/archopht.116.6.738. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9639441&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Burgos R, Mateo C, Canton A, Hernandez C, Mesa J, Simo R. Vitreous levels of IGF-I, IGF binding protein 1, and IGF binding protein 3 in proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2000;23:80–3. doi: 10.2337/diacare.23.1.80. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10857973&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Yoshida S, Kobayashi Y, Nakama T, Zhou Y, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol. 2015;99:629–34. doi: 10.1136/bjophthalmol-2014-305860. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25355804&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.McIntosh LC, Muckersie L, Forrester JV. Retinal capillary endothelial cells prefer different substrates for growth and migration. Tissue Cell. 1988;20:193–209. doi: 10.1016/0040-8166(88)90041-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3406938&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Lipo E, Cashman SM, Kumar-Singh R. Aurintricarboxylic acid inhibits complement activation, membrane attack complex, and choroidal neovascularization in a model of macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7107–14. doi: 10.1167/iovs.13-12923. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24106121&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackie EJ, Scott-Burden T, Hahn AW, Kern F, Bernhardt J, Regenass S, Weller A, Buhler FR. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol. 1992;141:377–88. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1379781&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushi J, Ono M, Morikawa W, Iwamoto Y, Kuwano M. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J Immunol. 2000;165:2818–23. doi: 10.4049/jimmunol.165.5.2818. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10946314&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Tang S, Le-Ruppert KC. Activated T lymphocytes in epiretinal membranes from eyes of patients with proliferative diabetic retinopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1995;233:21–5. doi: 10.1007/BF00177781. [DOI] [PubMed] [Google Scholar]
- 28.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci USA. 1996;93:9764–9. doi: 10.1073/pnas.93.18.9764. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8790405&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci. 1993;105:1001–12. doi: 10.1242/jcs.105.4.1001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7693733&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 30.Castellon R, Caballero S, Hamdi HK, Atilano SR, Aoki AM, Tarnuzzer RW, Kenney MC, Grant MB, Ljubimov AV. Effects of tenascin-C on normal and diabetic retinal endothelial cells in culture. Invest Ophthalmol Vis Sci. 2002;43:2758–66. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12147613&dopt=Abstract [PubMed] [Google Scholar]
- 31.Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cellular and molecular life sciences. Cell Mol Life Sci. 2011;68:3175–99. doi: 10.1007/s00018-011-0783-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21818551&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15082792&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96:1–2. doi: 10.1136/bjophthalmol-2011-301236. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22157632&dopt=Abstract [DOI] [PubMed] [Google Scholar]