Abstract
The epidemic of overweight and obesity presents a major challenge to chronic disease prevention and health across the life course around the world. Fueled by economic growth, industrialization, mechanized transport, urbanization, an increasingly sedentary lifestyle, and a nutritional transition to processed foods and high calorie diets over the last 30 years, many countries have witnessed the prevalence of obesity in its citizens double, and even quadruple. Rising prevalence of childhood obesity, in particular, forebodes a staggering burden of disease in individuals and healthcare systems in the decades to come. A complex, multifactorial disease, with genetic, behavioral, socioeconomic, and environmental origins, obesity raises risk of debilitating morbidity and mortality. Relying primarily on epidemiologic evidence published within the last decade, this non-exhaustive review discusses the extent of the obesity epidemic, its risk factors—known and novel—, sequelae, and economic impact across the globe.
1. Introduction
Obesity is a complex, multifactorial, and largely preventable disease (1), affecting, along with overweight, over a third of the world’s population today (2,3). If secular trends continue, by 2030 an estimated 38% of the world’s adult population will be overweight and another 20% will be obese (4). In the USA, the most dire projections based on earlier secular trends point to over 85% of adults being overweight or obese by 2030 (5). While growth trends in overall obesity in most developed countries seem to have leveled off (2), morbid obesity in many of these countries continues to climb, including among children. In addition, obesity prevalence in developing countries continues to trend upwards toward US levels.
Obesity is typically defined quite simply as excess body weight for height, but this simple definition belies an etiologically complex phenotype primarily associated with excess adiposity, or body fatness, that can manifest metabolically and not just in terms of body size (6). Obesity greatly increases risk of chronic disease morbidity—namely disability, depression, type 2 diabetes, cardiovascular disease, certain cancers—and mortality. Childhood obesity results in the same conditions, with premature onset, or with greater likelihood in adulthood (6). Thus, the economic and psychosocial costs of obesity alone, as well as when coupled with these comorbidities and sequealae, are striking.
In this article, we outline the prevalence and trends of obesity, then review the myriad risk factors to which a preventive eye must be turned, and finally present the costs of obesity in terms of its morbidity, mortality, and economic burden.
2. Classification of Body Weight in Adults
The current most widely used criteria for classifying obesity is the body mass index (BMI; body weight in kilograms, divided by height in meters squared, Table 1), which ranges from underweight or wasting (<18.5 kg/m2) to severe or morbid obesity (≥40 kg/m2). In both clinical and research settings, waist circumference, a measure of abdominal adiposity, has become an increasingly important and discriminating measure of overweight/obesity (7). Abdominal adiposity is thought to be primarily visceral, metabolically active fat surrounding the organs, and is associated with metabolic dysregulation, predisposing individuals to cardiovascular disease and related conditions (8). Per internationally used guidelines of metabolic syndrome—a cluster of dysmetabolic conditions that predispose individuals to cardiovascular disease of which abdominal adiposity is one component—a waist circumference resulting in increased cardiovascular risk is defined as ≥94 cm in European men, and ≥80 cm in European women, with different cut points recommended in other races and ethnicities (e.g., ≥90 and ≥80 cm in men and women, respectively, in South Asians, Chinese, and Japanese) (8,9).
Table 1.
Common Classifications of Body Weight in Adults and Children
| Age | Indicator | Normal Weight | Overweight | Obese | |
|---|---|---|---|---|---|
| Adultsb | ≥20 years | BMI (kg/m2) | 18.50 to 24.99 | ≥25.00 Preobesec: 25.00 to 29.99 |
≥30.00a
Class 1: 30.00 to 34.99 Class 2: 35.00 to 39.99 Class 3: ≥40.00 |
| Children | |||||
| International | |||||
| WHO 2006d | 0-60 months | BMI Z or WH Z | >−2 to ≤2 SD At risk of overweight: >1 to ≤2 SD |
>2 to ≤3 SD | >3 SD |
| WHO 2007e | 5-19 years | BMI Z | >−2 to ≤1 SD | >1 to ≤2 SD | >2 SD |
| IOTFf | 2-18 years | Growth curve for BMI at age 18 |
BMI = 25 | BMI = 30 | |
| USA g | 2-19 years | BMI percentile | ≥5th to <85th | ≥85th to <95th | ≥95th |
Abbreviations used: BMI, body mass index; IOTF, International Obesity Task Force; SD, standard deviation; WHO, World Health Organization; WH weight-for-height; Z, z score.
In the USA, typically “Class” is referred to as “Grade”. Obesity has an unofficial cut point of BMI ≥27 kg/m2 in Asian populations.
Per WHO 2000 classifications, in BMI as kg/m2 (139). These categories, if not the exact terminology, of adult weight status have been adopted by other major health organizations, including the US National Heart, Lung, and Blood Institute and National Institute of Diabetes and Digestive and Kidney Diseases (135).
Preobesity has an unofficial cut point of 23-<27 kg/m2 in Asian populations.
Per WHO 2006 classifications, BMIZ are BMI z scores, and WHZ are WH z scores, based on age- and sex-specific growth standards for children 0-60 months old. In children aged <2 years, weight-for-length is used (10).
Per WHO 2007 classifications, BMIZ are BMI z scores are based on age- and sex-specific growth standards and references for children aged 5-19 years (11).
Per Cole et al. (140), for the IOTF based on age- and sex-specific curves defined to pass through BMIs of 25 or 30 kg/m2 at age 18, for children aged 2-18 years.
Per CDC 2000 classifications, BMI percentiles are based on age- and sex-specific growth references for children aged 2-19 years (12).
3. Classification of Body Weight in Children
In children, body weight classifications (Table 1) differ from those of adults because body composition varies greatly as a child develops, and further varies between boys and girls primarily owing to differences in sexual development and maturation. The World Health Organization (WHO) Child Growth Standards are the most widely currently used classification system of weight and height status for children from birth to 5 years old, based on data from children in six regions across the globe born and raised in optimal conditions (10). In 2007, the WHO published updated growth references combining the 1977 National Center for Health Statistics (NCHS)/WHO growth reference and the 2006 WHO Child Growth Standards to create the most recent BMI-for-age references for individuals aged 5–19 years (11). Thus, the latest WHO guidelines are designed to represent relatively seamless standards and references from birth all the way into late adolescence/early adulthood.
In the USA, the Centers for Disease Control and Prevention (CDC) currently use the 2000 CDC growth references based on 1963–1994 US children’s data, to determine age- and sex-specific BMI percentiles for children aged 2–19 years (12). Overweight is defined in US children as age- and sex-specific BMI ≥85th and <95th percentile, while obesity is ≥95th percentile (13). Cut points for severe obesity in childhood have been proposed in recognition of the alarming growing prevalence of this extreme condition, defined as the 99th BMI percentile (13) or 120% of the 95th percentile (14). For US children <2 years old, the CDC currently uses the 2006 WHO Child Growth Standards, described above (15).
4. Prevalence and Trends
4.1. Adult Obesity—US and Europe
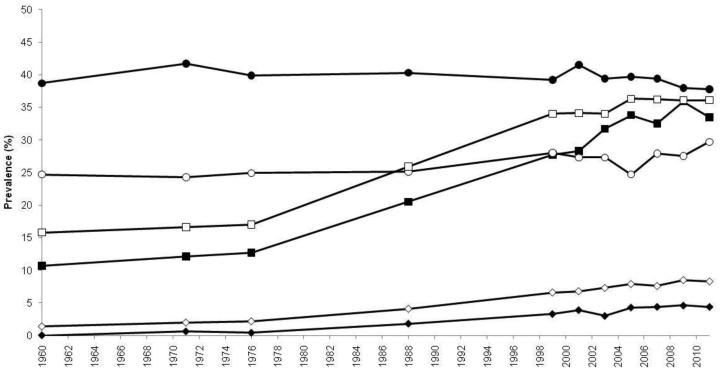
The first indications that obesity was taking on epidemic proportions originated in the USA and Europe. With few restrictions on access to or availability of food, the prevalence of overweight and obesity in the USA climbed virtually unmitigated over the last 50 years. Today, those who are overweight (BMI 25–<30 kg/m2) or obese (BMI ≥30 kg/m2) in the USA eclipse two-fold the numbers of those who are normal weight (16). In US adults, 1960–1994 trends showed that while levels of overweight hovered at approximately 31% over the time period, in contrast, age-adjusted obesity jumped from 13 to 23%, bringing the crude prevalence of overweight or obesity to 55% of the American population (17). Unfortunately, 1994 did not represent the endpoint of the upward trend, as the following decade saw adult obesity rise from 23 to 32% by 2003–2004 (16). In the last 10 years, national estimates of obesity seem to indicate that the steady upward trend of obesity in Americans has leveled off at a prevalence of about 35% (16) (Figure 1), perhaps having reached some “Malthusian” obesity limit. However, certain subpopulations are faring worse than others, as 2011–2012 obesity rates in Hispanics and non-Hispanic blacks were 43 and 48%, respectively, pointing to a disproportionate burden in differing racial/ethnic and/or socioeconomic status (SES) groups. Gender also plays a role, with women being disproportionately affected by extreme obesity (classes 2–3, BMI ≥35 kg/m2) than men, regardless of age or race/ethnicity (16).
Fig. 1.
Trends in age-adjusted prevalence of overweight, obesity, and extreme obesity in US adults, aged 20–74 years, 1960–2012. Trends in prevalence of overweight as BMI 25–<30 kg/m2 (circles), and upward trends in obesity as BMI ≥30 kg/m2 (squares), and extreme obesity as BMI ≥40 kg/m2 (diamonds) in adult males (closed points) and females (open points). The figure is based on data from NHES I (1960–1962), NHANES I (1971–1974), NHANES II (1976–1980), NHANES III (1988–1994), and NHANES (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012). Data derived are derived from Ogden, et al., and Fryar, et al. (16,141). BMI, body mass index; NHANES, National Health and Nutrition Examination Survey; NHES, National Health Examination Survey.
Meanwhile, in Europe, longitudinal data (1992–1998 to 1998–2005) from participants in five countries involved in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (Italy, the United Kingdom, the Netherlands, Germany, and Denmark), indicate that adult obesity increased modestly from 13 to 17% in that time period (18). However, were such linear trends were to continue, the overall obesity prevalence in these populations could reach 30% by 2015, paralleling US rates. A more conservative projection suggests a prevalence of just 20% obesity in these populations by 2015, if public awareness and public health measures take hold (18).
European studies including populations beyond EPIC indicate there is considerable disparity in overweight/obesity between European countries. A systematic review of national and regional surveys conducted between 1990 and 2008 points to obesity rates as low as 4.0 and 6.2% in French men and women, respectively (regional survey, 1994–1996), and as high as 30.0 and 32.0% in Czech men and women, respectively (national survey, 2002–2005) (19). Regional trends within Europe are apparent, with southern Italy and southern Spain, and Eastern European countries showing higher prevalence of obesity than countries in Western and Northern Europe (19). As in the USA, these data suggest that socioeconomic disparities and relatively recent/ongoing economic transitions are playing a considerable role in apparent differences across and within countries with respect to obesity risk.
4.2. Child Obesity—USA and Europe
US children may be faring better than their adult counterparts in some ways (16), potentially offsetting earlier dire predictions of rampant obesity by 2030 (5). In national surveys, levels of overweight in children, as in adults, seem to have leveled off (or even declined) at approximately 30% of US children aged 2–19 years (16,20). However, this belies a potentially disturbing long-term trend in the rising prevalence of extreme obesity (equivalent to adult class 2 obesity and higher, BMI ≥35 kg/m2). Since 1999–2000, the prevalence of class 2 obesity in children (BMI ≥120% of the 95th percentile) has risen from 3.8 to 5.9% and class 3 obesity (BMI ≥140% of the 95th percentile) has doubled from 0.9 to 2.1%, the latter category jumping 30% since 2009–2010 alone (20). Again, as in their adult counterparts, certain sub-populations appear to be faring worse than others, notably Hispanic girls and Black boys, in whom overweight, obesity, and class 2 obesity have increased significantly (20).
Childhood obesity prevalence varies considerably between and within countries as well. Relatively recent estimates based on 2007–2008 data of children aged 6–9-years collected in 12 European countries as a part of the WHO European Childhood Obesity Surveillance Initiative observed overweight/obesity (BMI z score >+1 standard deviation [SD]) prevalence of 19.3–49.0% of boys and 18.4–42.5% of girls, while obesity (BMI z score >+2 SD) affected 6.0–26.6% of boys and 4.6–17.3% of girls. Researchers continued to observe the trend of north-south and west-east gradients evident in adults, with the highest levels of overweight in southern European countries (21).
4.3. Obesity Beyond North America and Europe
The data discussed above focus on the USA and European countries, many with robust national health surveillance programs. While historical data tends to be scarcer outside of these regions, an alarming picture has emerged over the last decades in low- and middle-income countries around the globe, complicated by rapidly changing socioeconomic environments. While country-specific trends are not discussed in this article, regional and national estimates of long-term changes in child (<20 years old) and adult (>20 years old) overweight and obesity have increased in nearly all countries and regions since 1980 (Figure 2) (2,3). While the USA still may boast the largest absolute numbers of overweight and obese individuals, several other nations exceed the USA in terms of overall prevalence and, moreover, the rate of growth in certain countries is disheartening. For example, the prevalence of overweight and obesity in nationally representative Mexican adults was estimated to be 71.3% overweight/obese, with overweight at 38.8% and obesity at 32.4% (22). This prevalence represents an increase of 15% since 2000, placing this population among the most rapidly accelerating in terms of obesity prevalence over the last decade. Further, while rates of overweight remained relatively stable since 2000 at approximately 38% overall, extreme obesity (class 3, BMI ≥40 kg/m2) increased by an estimated 76.5% from 2000 to 2012. These trends are also evident in countries outside of the Americas. In China, for example, between 1993 and 2009, overweight (BMI 25 to <27.5 kg/m2) doubled in men (8 to 17%) and increased from 11 to 14% in women. Meanwhile, obesity (BMI ≥27.5 kg/m2) nearly quadrupled in men, from 3 to 11%, and doubled in women, from 5 to 10%. Chinese children are faring as badly as their adult counterparts: overweight/obesity doubled from 6 to 13% in children aged 6–17 years over the same time period, suggesting that the obesity epidemic will continue to deepen in this country (23).
Fig. 2.
Prevalence of overweight and obesity in adults aged ≥20 years by global region, 1980–2008. From left to right, each column represents the estimated regional prevalence of overweight and obesity for 1980, 1985, 1990, 1995, 2000, 2005, and 2008. For a given region, a dark gray column indicates the lowest estimated prevalence in the trend, while the highest estimated prevalence is indicated by a black column. As is evident, the vast majority of regions demonstrate the lowest estimated prevalence of overweight and obesity in 1980, and the highest in 2008, demonstrating the global reach of obesity. The scale shows 25, 50, and 100% prevalence columns, for reference. Asterisks denotes high income. Data are sourced from Stevens, et al. (3).
5. Risk Factors for Obesity
Currently, our greatest gap in knowledge is not regarding the numbers of risk factors, nor in their independent impact on risk, but rather in how they interact with one another—their confluence—to produce today’s aptly if unfortunately named “globesity” epidemic. Obesity arises as the result of an energy imbalance between calories consumed and the calories expended, creating an energy surplus and a state of positive energy balance resulting in excess body weight. This energy imbalance is partially a result of profound social and economic changes at levels well beyond the control of any single individual. These “obesogenic” changes—economic growth, growing availability of abundant, inexpensive, and often nutrient-poor food, industrialization, mechanized transportation, urbanization—have been occurring in high-income countries since the early 20th century, and today these forces are accelerating in low- and middle-income countries. And yet, not all of us living in obesogenic environments experience the same growth in our waistlines. Hereditary factors—genetics, family history, racial/ethnic differences—and our particular socioeconomic and sociocultural milieus have been shown to affect risk of obesity (Table 2) even in ostensibly similar obesogenic environments. So while body weight regulation is and should be viewed as a complex interaction between environmental, socioeconomic, and genetic factors, ultimately, personal behaviors in response to these conditions continue to play a dominant role in preventing obesity. Importantly, apart from genetics, every risk factor discussed below is modifiable.
Table 2.
Risk Factors, Comorbidities, and Sequelae of Obesity
| Risk Factors (non-exhaustive) |
|---|
|
| Comorbidities and Sequelae (non-exhaustive) |
|
5.1. Genetics of Obesity
To date, over 60 relatively common genetic markers1 have been implicated in elevated susceptibility to obesity (24,25); however, the 32 most common genetic variants are thought to account for <1.5% of the overall inter-individual variation in BMI (24). When these 32 “top” genetic hits are combined into a genetic risk of obesity score, those with the highest genetic risk (i.e., carriers of over 38 risk alleles), have just a 2.7 kg/m2 higher BMI on average than those with a low genetic risk. This translates into about a 15-lb (7-kg) weight difference between two 5’3” (160 cm) individuals with high versus low genetic risk (24). Although genetics undoubtedly play a role, this relatively small difference in BMI, coupled with the dramatic rise in obesity over the last half century in developed and developing nations alike point to obesity risk factors beyond genetics. A concomitant and rich area of research has therefore evolved investigating gene-environment interaction based on the idea that underlying genetic risk predisposes individuals to particularly adverse (or beneficial) effects of behavioral or environmental exposures such as diet and exercise, a concept scientifically popularized in, for example, the “thrifty gene” hypothesis (26). In many ways, these types of gene-environment interactions are playing out in population research: for example, a variant in FTO (rs9939609)—the strongest obesity susceptibility locus—increases odds of obesity in risk allele carriers by an estimated 23% per allele; however, this risk is modified by physical activity in adults (27,28) and children (29), among other factors. Nevertheless, these types of interactions have so far been investigated in relatively few genetic risk loci out of millions, and with just a handful of environmental factors, raising important questions of how to aggregate this complexity for public health and ultimately personalized medicine.
In addition, parental diet, lifestyle, and other exposures have been implicated in subsequent offspring obesity risk, including famine exposure (30), parental obesity (31–33), smoking (34), endocrine-disrupting and other chemicals (35,36), and weight gain during gestation and gestational diabetes (33,37). These and other studies point to lasting effects of fetal programming that via differing mechanisms, likely epigenetic, result in substantial repercussions in life course health, with implications across the socioeconomic/food availability spectrum. Careful management of diet and lifestyle in pre- and perinatal periods could exert a considerable impact on the obesity epidemic for generations to come (37).
5.2. Individual Behaviors
5.2.1. Diet
In the decades preceding the 21st century, the vast majority of research on obesity risk factors focused on individual-level, largely modifiable behaviors. The role of diet and physical activity in mitigating obesity risk and reducing prevalent obesity have received the most attention, and with good reason: 15% of deaths in 2000 in the USA were attributable to excess weight, owing to poor diet and physical inactivity (38). Caloric intake and expenditure needed for weight maintenance or healthy growth has historically taken center stage (39), and caloric restriction remains today a primary focus of most popular and clinical weight-management and weight-loss approaches.
Beyond overall caloric intake to regulate body weight, a tremendous amount of research has attempted to resolve the roles of diet quality and dietary patterns, including those specifying combinations of macronutrients (40). Evidence from clinical trials have almost universally shown that caloric restriction, regardless of dietary pattern, is associated with better weight outcomes (40). Although the metabolic nuances and relative merits of the differing dietary patterns for various comorbid conditions are still being investigated, the evidence seems to suggest that merely adhering to a diet—nearly irrespective of what type of healthy diet it is—has an impact on weight loss/control (41–43).
For long-term maintenance of healthy weight, evidence from observational cohorts indicate that diets that are considered “healthier” lead to better long-term weight maintenance, or at least mitigate weight gain typically associated with aging through middle age. For example, research in US health professionals pointed to averaged 4-year weight gain throughout middle age as being strongly associated with increasing intake of potato chips and potatoes, sugar-sweetened beverages, and processed and unprocessed red meats, but inversely associated with the intake of vegetables, fruits, whole grains, nuts, and yogurt (44). Specific food groups, such as sugar-sweetened beverages, have received considerable attention largely because added sugar consumption (primarily as sugar-sweetened beverages) has been rising concomitantly with prevalent obesity (45). Indeed, the weight of the evidence about the role of sugar-sweetened beverages in obesity (46,47) is a strong impetus for public health interventions and policies, such as limiting advertising on these beverages as in Mexico (48), attempts to limit beverage sizes permitted for sale as in New York City (49), taxation, eliminating sale in schools, etc.
5.2.2. Physical Activity, Sedentary Behaviors, and Sleep
Personal behaviors beyond diet (physical activity, sleep, sedentary and screen time, and stress) have also been independently associated with weight change and maintenance in adulthood. Combined with diet, these elements have synergistic and likely cumulative effects on an individual’s ability to maintain or obtain a healthy body weight over the life course. Recently reviewed evidence from randomized trials and observational studies support 2008 US recommendations for weight management (50), consistently showing that in general, 150–250 minutes per week of moderate intensity activity is required to prevent weight gain, or aid in weight loss when accompanied by dietary restriction (51). Activity (>250 minutes per week) is associated with weight loss and weight maintenance after weight loss (51). Leisure-time activities involving sitting, but which are not truly restful behaviors, such as getting <6 or >8 hours of sleep in adults and adolescents (44,52–55) or <10–11 hours of sleep in children (52), television viewing or screen time (44,56,57), and other leisure-time sitting (58) are also associated with weight gain.
5.3. Socioeconomic Risk Factors: Income and Education
Income has had a shifting role in obesity risk over the last century. As late as the mid-20th century, the USA and Europe could link wealth directly with obesity—the wealthier an individual, the more likely to be overweight. Over the last few decades, however, perhaps owing to the abundance of cheap and highly available food, coupled with changing sociocultural norms, this link has flipped. Today, wealth in the USA tends to be inversely correlated with obesity, and it is those who are at or below the level of poverty who appear to have the highest rates of obesity (59). Indeed, in US cities where the homeless are surveyed, the prevalence of overweight and obesity parallels that of non-homeless populations, contrary to our typical beliefs about thinness accompanying food insecurity or homelessness (60,61).
More broadly, across 11 Organisation for Economic Co-Operation and Development (OECD) countries, SES, whether defined by household income or occupation-based social class, showed an inverse relationship with obesity: women, in particular, had consistently higher prevalence of overweight/obesity the less affluent they were (62). In men, too, those in low income strata tended to have higher prevalence of obesity, but the gradient for overweight reversed in about half of the countries surveyed. That is, in some countries, poverty was associated with more prevalent overweight than wealth, but in others, lower income was associated with more favorable weight status. The differences between sexes in terms of income status and obesity, in particular the trend reversal in men, may be in part due to low-paying jobs typically involving more physically demanding work performed by men more than by women (62). Adding complexity to this picture is the role of education: in the 11 OECD countries discussed above, education showed a strong inverse relationship with overweight/obesity, particularly in women, who had consistently higher prevalence of overweight/obesity the less educated they were (62).
As wealth rises in low- and middle-income countries, it is expected for poverty-obesity patterns to begin more closely mimicking those of high-income countries. Evidence of this transition is already accumulating. In explorations of the role of education and wealth in women and weight status in four middle-income countries (Colombia, Peru, Jordan, and Egypt), authors observed a significant interaction between education and wealth: in women with little or no education, higher income was associated with 9–40% higher odds of obesity, while in those with higher levels of education, the association with income was either not present (Egypt, Peru) or associated with 14–16% lower odds of obesity (Jordan, Colombia) (63). This suggests that in currently transitioning economies, education may offset the apparently negative effects of increasing purchasing power in emerging obesogenic environments. However, the protective effect of education has yet to be seen in the poorer countries, such as India, Nigeria, and Benin, where both education and wealth were directly associated with increased odds of obesity (63).This is perhaps unsurprising, as obesity was relatively rare at <6.0% of women in these countries, and >50% of women had little or no education.
The glimmer of hope, then, is that in the context of a paradigm of diseases of affluence, in which the transition to wealth seem to invariably lead to higher obesity and thus greater chronic disease burden, higher education levels may yet offset some of the frightening challenges that lay before us.
5.4. Environmental2 Risk Factors
5.4.1. The Built Environment
Research on the built environment tends to focus on a few measurable characteristics of neighborhoods as they relate to weight status, while holding sociodemographic and other person-level characteristics constant. Such neighborhood characteristics range from more concrete factors (e.g., fast food restaurants, supermarkets, parks, transportation, etc.) to more variably scored factors (e.g., walkability, neighborhood healthiness). Most studies of the built environment have been cross-sectional, tending to focus on one or two characteristics; thus, findings on the relative importance or effects of given characteristics on obesity have been inconsistent (66–72), revealing the fundamental challenge of teasing out whether neighborhood characteristics play a causal role in weight status, or whether health-minded folks inhabit health-friendly areas to begin with (residential selection bias) (73). However, the emerging picture points to the primacy of diet-related built environments over those associated with physical activity. While presence of neighborhood physical activity or recreational spaces has been associated with increased physical activity levels or energy expenditure (71,72), healthy food environments, characterized by availability of produce or presence of supermarkets over convenience stores or fast food restaurants, play a potentially more important role (68,70,74,75).
Research on the causality of the built environment as obesity-inducing or health-promoting is critical for municipalities and public health authorities to justify potentially costly improvements to public spaces and/or zoning regulations. There is an unmet need for standardized measures, definitions, and criteria, established residential and occupational geographic radii relevant to health, and research methodologies that can take into account the complexity of something as seemingly simple as a neighborhood.
5.4.2. Environmental “Pathogens”: Viruses, Microbiomes, and Social Networks
Growing evidence from animal and human studies indicates that obesity may be attributable to infection, or that obesity itself may be a contagion. Infectious agents include viruses, the trillions of microbiota inhabiting the human gut, and, of course, obese humans as infectious agents themselves.
Although several viruses have been identified as potentially having a causal role in obesity (76), Ad-36 is among the most studied, being causally associated with adiposity in animals. Studies in diverse human populations generally support greater Ad-36 viral loads as probably causal of obesity in both children and adults (76–79), with links to other metabolic traits (77,79).
Ground-breaking research in the last decade has emerged on the role of trillions of gut bacteria—the human microbiome—in relation to obesity, energy metabolism, and carbohydrate and lipid digestion, opening promising therapeutic avenues for obesity and disease (80). Two primary phyla of bacteria differ in their proportions in lean vs. obese populations; these proportions change as obese individuals lose weight and correlate highly with the percentage of body weight lost (81). Broad and sometimes dramatic changes in microbiome populations have been catalogued following gastric bypass surgery (80), and in both the short- (82,83) and long-term (81,83) in response to changes in dietary composition (80). Research in mice indicates that increased adiposity is a transmissible trait via microbiome transplantation (84), and has prompted similar experimental fecal transplantation research in humans for the promotion of weight loss (85). In addition, other research has examined the feeding of pre- and probiotics as therapeutic modalities designed to manipulate the gut microbiome; these strategies also show promise for a range of conditions (85).
Finally, the importance of social networks—real and virtual—in obesity is a fascinating, relatively new area of research that capitalizes on known characteristics of infectious disease transmission. In a landmark 2007 study examining the spread of obesity due to social ties using 32-year prospective data from the Framingham Heart Study, Christakis and Fowler (86) showed that an individual’s chances of becoming obese increased by 57% if he or she had a friend who became obese in a given 4-year interval. This was a stronger risk ratio than that observed between pairs of adult siblings or even between spouses. Conversely, it may be possible to capitalize on the social contagion of obesity in the reverse direction, that is, in the promotion of healthy weight and behavior. Intervention studies of weight loss often include a social-relational component, although the evidence supporting any single approach or its efficacy is relatively scarce (87). In theory, a supportive network, community, or coaching relationship is supposed to improve weight loss; despite a lack of strong evidence, it is a key component of many popular commercial (e.g., Weight Watchers), trial/intervention, and online approaches.
6. Costs of Obesity: Co-Morbidities, Mortality, and Economic Burden
Obesity is associated with concomitant or increased risk of nearly every chronic condition, from diabetes, to dyslipidemia, to poor mental health. Its impacts on risk of stroke and cardiovascular disease, certain cancers, and osteoarthritis are significant.
6.1. Overall Mortality
In the year 2000 in the USA, 15% of deaths were attributable to excess weight, owing to poor diet and physical inactivity (38). Overweight/obesity in middle age shortens life expectancy by an estimated 4–7 years (88). Many long-term cohort studies, as well as three recent major syntheses of pooled data from established cohorts (89–91), which adequately accounted for history of smoking and chronic disease status, unequivocally show that overweight and obesity over the life course is associated with excess risk of total mortality, death from cardiovascular disease, diabetes, cancer, or accidental death (89–97).
Some studies suggest that excess body weight may be protective against mortality from certain chronic conditions—resulting in a so-called “obesity paradox.” However, most studies that have shown an obesity paradox, or no association between obesity and mortality, have been conducted in groups of older (>65) or elderly patients or in those with chronic conditions, or have inadequately accounted for smoking. Indeed, the role of excess adiposity in old age is unclear. While the protective effects of overweight in specific instances of diseased older populations may be real, these observations are fraught with methodological problems, especially reverse causation, and belie the limitations of generalizing excess adiposity’s supposed benefits to younger populations over the life course, not least because excess body weight leads to higher disease incidence to begin with (7).
6.2. Diabetes
Excess weight and diabetes are so tightly linked that the American Diabetes Association recommends physicians test for type 2 diabetes and assess risk of future diabetes in asymptomatic people ≥45 years old simply if they are overweight/obese, and regardless of age if they are severely obese (98). Overweight raises risk of developing type 2 diabetes by a factor of three, and obesity by a factor of seven, compared to normal weight (99). Excess weight in childhood and in young adulthood, and weight gain through early to mid-adulthood are strong risk factors for diabetes (100–102). While not every overweight/obese individual has diabetes, some 80% of those with diabetes are overweight/obese (103). Obesity itself raises diabetes risk even in the absence of other metabolic dysregulation (insulin resistance, poor glycemic control, hypertension, dyslipidemia). While metabolically healthy obese individuals are estimated to have half the risk of their metabolically unhealthy counterparts, they still have four times the risk of those who are normal weight and metabolically healthy (104).
6.3. Heart and Vascular Diseases
Ischemic heart disease and stroke are the leading causes of death in the USA and globally (105). Excess body weight is a well-known risk factor for heart disease and ischemic stroke, including their typical antecedents—dyslipidemia and hypertension. Recent studies have consistently shown that benign obesity appears to be a myth (106–108); overweight clearly adds to risk of heart disease and stroke beyond its implications for hypertension, dyslipidemia, and dysglycemia.
Given childhood obesity rates, research has lately focused on the role of obesity in early life and subsequent adulthood disease. Obesity in childhood or adolescence has been associated with twofold or higher risk of adult hypertension, coronary heart disease, and stroke (100). A recent study pooling data from four child cohorts (aged 11 years at baseline with average 23-year follow-up), observed that, compared with individuals who were normal weight in childhood and non-obese as adults, those who were normal weight or overweight but became obese as adults, or who were obese and stayed obese into adulthood, had considerably higher risk of high-risk dyslipidemia, hypertension, and higher carotid intima-media thickness. Notably, those individuals who were overweight/obese as children, but non-obese as adults, had similar risk profiles to those individuals who were never obese, indicating that the potential health effects of childhood obesity can be offset by weight loss prior to or while entering into adulthood (109).
6.4. Cancer
An estimated 6% of all cancers (4% in men, 7% in women) diagnosed in 2007 were attributable to obesity (110). Beyond being a major risk factor for diabetes, which itself is a risk factor for most cancers, obesity has long been understood to be associated with increased risk of esophageal, colon, pancreatic, postmenopausal breast, endometrial, and renal cancers (111). More recently, evidence has accumulated that overweight and/or obesity raise risk of cancers of the gallbladder (112), liver (113), ovaries (epithelial) (114), and advanced cancer of the prostate (115), as well as leukemia (116).
6.5. Trauma and Infection
A study in Pennsylvania (USA) trauma centers (2000–2009) showed that in-hospital mortality and risk of major complications of surgery were increased in obese patients as compared to non-obese patients. Severely obese patients had upwards of 30% increased risk of mortality from their trauma than non-obese patients, and double the risk of major complications. Severely obese females also had more than double the risk of developing wound complications, and quadruple the risk of developing decubitus ulcers (117). A recent meta-analysis of obesity in trauma care concluded that obesity was associated with 45% increased odds of mortality, longer stays in the intensive care unit, and higher rates of complications, and tended to associate with longer durations of mechanical ventilation and longer stays in the hospital overall, compared to non-obese patients, despite equivalent injury severity (118).
While elevated risk of chronic disease is a seemingly obvious consequence of obesity, increasing attention is being given to increased risk of infection and infectious disease in obesity, including surgical-site, intensive care unit (ICU)-acquired catheter, blood, nosocomial, urinary tract, and cellulitis and other skin infections (119), community-acquired infections, and poorer recovery outcomes owing to higher risk of influenza, pneumonia, bacteremia, and sepsis (119). Impaired immunological response may be an underlying mechanism; recent research has demonstrated lower vaccine efficacy and serological response to vaccination in the obese. For example, a recent study estimated an eightfold increase in the odds of non-responsiveness to hepatitis-B vaccination in obese versus normal-weight women (120).
The consequences of a global obesity epidemic may not merely be greater chronic and infectious disease burden for the obese, but also a greater global burden of infectious disease owing to obesity. Greater infectious disease vigilance may be required in populations with high levels of overweight/obesity, and there is a clear need for better clinical practice guidelines (e.g., use and dosage of antimicrobials, vaccines, other pharmaceuticals) for obese individuals.
6.6. Mental Health
The role of weight in mental health faces causal challenges, but what is clear is that obesity and adiposity are associated with anatomical as well as functional changes in the human brain. Studies in older populations have shown that BMI is inversely correlated with brain volume, and that obese older adults, compared to normal weight counterparts, show atrophy in the frontal lobes, anterior cingulate gyrus, hippocampus, and thalamus (121). In addition, obesity in children and adolescents (aged >9 years) has been associated with smaller orbitofrontal cortex gray matter volume, along with poorer performance in certain domains of executive function (e.g., inhibitory control) (122). Being overweight in midlife increases risk of Alzheimer's disease, vascular dementia, or any type of dementia by 35, 33, and 26%, respectively; even higher risk is observed for obesity (123). Importantly, physical activity, even among overweight individuals, may stave off poor mental functioning: moderately active or highly active adult overweight Finns did not have significantly increased risk of poor mental functioning at a 7-year follow-up compared to those who were normal weight and highly active, but inactive and overweight patients presented a nearly 40% increased risk of poor mental functioning (124). Thus, exercise may play an important mediating role in the relationship between excess body weight and age-related cognitive decline.
6.7. Economic Burden of Obesity
In the USA, recent estimates indicate that obese men are thought to incur an additional US$1,152 per year in medical spending, particularly due to hospitalizations and prescription drugs, compared to their non-obese counterparts, while obese women incur over double that of obese men, an additional US$3,613 per year in medical spending (year 2005 values). Extrapolating these costs to the national level, authors estimate some US$190 billion per year of healthcare spending, approximately 21% of US healthcare expenditures, is due to treating obesity and obesity-related conditions (125).
Total hospital costs account for a part of this: another author group studied non-bariatric, non-obstetric hospital procedures for obese patients, finding they were US$648 higher (year 2009 values) per capita than for non-obese patients. The estimated national hospital expenditures for the largest volume surgical procedures was US$160 million higher per year for obese than for their non-obese counterparts (126).
Employers bear a substantial brunt of obesity-related costs in the USA. Data from the Human Capital Management Services Research Reference Database (2001–2012) on employees and their dependents was used to compare medical, drug, sick leave, short-term disability, and workers’ compensation costs as well as absent days across three BMI strata: <27, ≥27–<30, and ≥30 kg/m2. Each of the costs was incrementally higher in ascending BMI categories. For example, total annual costs and total days absent in the highest vs. lowest BMI strata were US$6,313 versus US$4,258 (year 2012 values), and 7.5 versus 4.5 days. In addition, productivity was lowest in the obese group (127).
Finally, lifetime direct incremental medical costs of obesity in childhood in the USA were estimated to range from US$12,660 to US$19,630 (year 2012 values) for an obese 10-year old compared to a normal-weight 10-year old, if expected weight gain through adulthood among the normal weight child occurs (128). If normal weight children were to not continue on the typical weight gain trajectory into overweight/obesity, estimated incremental medical costs for today’s 10-year old obese child ranges between US$16,310 and US$39,080. Putting these figures into perspective, multiplying the lifetime medical cost estimate of US$19,000 by the number of obese 10-year-olds today generates a total direct medical cost of obesity of roughly US$14 billion for this 10-year old age group alone. In terms of big picture savings, the upper estimate of US$39,000 per case represents two years of public college tuition for that child (128).
In Europe, a 2008 review of 13 studies in ten Western European countries estimated the obesity-related healthcare burden had a relatively conservative upper limit of €10.4 billion annually (in Germany, in 1995 € equivalent), and ranging between <0.1 to 0.61% of each country’s gross domestic product (GDP). The review relied on study data from as early as the 1980s in the Netherlands, through 2002 in most of the remaining countries surveyed (129). A more recent review focused on 19 studies published in 2007–2010 in eight Western European countries (predominantly Germany, Denmark, and the United Kingdom). Excess health care costs of obesity or derivations of excess health care costs by comparisons of mean costs between normal weight and obese individuals in seven of the reviewed studies were between €117 and €1,873 per person (based on the € valuation given in each study year). Excess costs increased particularly due to severe obesity. Approximately 23% of medication costs and 6.9% of out-of-pocket costs were attributable to overweight or obesity. Health economic models estimated that 2.1–4.7% of total health care costs and 2.8% of total hospital costs were due to overweight and obesity. Total (direct and indirect) costs were generally unchanged from the 2008 estimate of the earlier review, accounting for 0.47–0.61% of GDP in these countries (130).
In the context of the Brazilian Unified Health System (i.e., public hospitals), estimated direct costs of diseases related to overweight/obesity in outpatient and inpatient care based on 2008–2010 data were US$2.1 billion annually (year 2010 values), 68.4% of which was attributable to hospitalizations, and the remainder due to ambulatory procedures (131). The largest costs of outpatient and inpatient care in both sexes were due to cardiovascular disease (US$747 million) followed by overweight- and obesity-related neoplasms (US$299.8 million), asthma (US$34 million), type 2 diabetes (US$3.7 million), and osteoarthritis (US$3.9 million). Authors estimated that these direct costs were a considerable underestimate of the true burden of overweight/obesity in Brazil, which would include private health care expenditures, as well as indirect costs due to lost productivity, premature death, and home care (131).
Given the predicted rise in obesity in Brazil, coronary heart disease, stroke, hypertension, cancers, osteoarthritis, and diabetes are projected to at least double by 2050, with concomitant doubling in health care costs, from US$5.8 billion in 2010 to US$10.1 billion per year—totaling US$330 billion over 40 years (year 2010 values). It is estimated that a 5% reduction in mean BMI across the population could save Brazil some US$57 billion over that time frame (132). A similar analytic approach that substituted Mexican prevalence and trends for the Brazilian ones estimated 2010 costs of obesity at US$806 million (year 2000 values), which were projected to increase to US$1.7 billion by 2050, at which point a mere 1% reduction in BMI prevalence in Mexico could save an estimated US$85 million per year (133).
Of course, none of these estimates include dollars spent on the weight-loss industry, which is estimated to be over US$60 billion dollars in 2014 in the USA alone (134), and includes non-prescription drugs and supplements, diet plans, gym memberships, workout videos, and an endless stream of money-making schemes.
7. Touching on Solutions, and Some Conclusions
Obesity is a major contributor to preventable disease and death across the globe, and poses a nearly unprecedented challenge not just to those tasked with addressing it at the public health level, or at the healthcare provider level, but to each of us as individuals, for none of us are immune. Increasing ease of life, owing to reduced physical labor and automated transportation, an increasingly sedentary lifestyle, and liberal access to calorie-dense food, driven by dramatic economic growth in many parts of the world in the last century, have turned a once rare disease of the affluent into one of the most common diseases—increasingly of the poor. That barely one in three people in the USA today are normal weight portends, quite simply, an astounding and frightening future. Significant reductions in public health and healthcare expenditures could occur around the world if we were able to stem the tide of childhood obesity trends, and if young and middle-aged overweight and obese adults lost approximately10% of their body weight, as recommended for a considerably reduced risk of debilitating chronic conditions (135).
Obesity is complex. Although its risk factors are myriad and compounding, there is an urgent need for deeper understanding of the way risk factors interact with each other, and the potential solutions to the epidemic are as multi-leveled and complex as its causes. There are calls for applying systems-level (136) and systems epidemiology (137) approaches to this and related nutrition and metabolic diseases, approaches which attempt to comprehensively address biological, behavioral, and environmental contributors to disease as well as their intricate feedback loops. Additional research on solutions to this epidemic would include, for example, examining the relative cost/benefit to individuals and populations of individual versus systemic policies and/or interventions, concurrently or independently, particularly when individuals and communities must decide between approaches given limited resources, and moreover, with the currently limited evidence in the case of broad industry, agricultural, or public health policies. For example, we could attempt to limit national production and import of sugar-sweetened beverages, tax sugar-sweetened beverages, or restrict fast food restaurant zoning. These largely political acts seem relatively inexpensive, but may have economic impacts in communities and regions beyond what we currently understand. We may push for the increasing medicalization of obesity, including developing an obesity vaccine. While such a “cure” may someday arise, the medicalization of a condition typically improves its treatment rather than its prevention, and prevention is key in the case of obesity. However, preventing and remediating obesity in children and adults—e.g., via health and wellness incorporation into curricula at every educational level from kindergarten through medical school—requires vast resources allocated to educators, as well as earlier diagnosis and treatment of overweight (education, counseling, drug treatment, etc.). Given these resource costs, perhaps greater attention should be given to pregnancy, a condition which is already highly medicalized and which may be an ideal preventive avenue for the provision of nutrition education and intensive monitoring of weight gain, to ensure that children have the most optimal start with respect to their future obesity risk. Clearly, no single approach is optimal, but with limited resources, an evidence base supporting one or more approaches or their combination is needed, as is tenacity and perhaps some audacity by local government and public health authorities in testing some of these approaches within their populations. However, an epidemic of this magnitude needs, quite simply, more resources. One of the reasons why the American Medical Association opted to declare obesity a “disease” was to give obesity the label it needs for greater allocation of resources for research, prevention, and treatment (1).
Despite the many unknowns, we can be cautiously optimistic about our ability to address the obesity epidemic. Indeed, we have relatively successfully faced similarly daunting public health challenges before: smoking, to name just one. While tobacco can loosely be thought of as a single product, and our food culture is infinitely more complex, as a case study in how to approach obesity, it provides numerous lessons in multi-level solutions to a major health threat in terms of both mitigation and prevention. We began by developing an understanding of smoking’s epidemiological impact and the healthcare costs borne by society, uncovered its biological basis, learned about and applied behavior change, and initiated and carried out vast public health, public policy, political, and economic strategies that ultimately affected whole environments as well as sociocultural norms.
It took over half a century to achieve the immense success we have with regard to smoking in the USA and still we are not yet tobacco-free (138); other parts of the world continue to wrestle with it to a greater degree. It has only been a couple decades since we first deeply appreciated that obesity was epidemic. We clearly still have a long way to go.
Key Points for Decision Makers.
In 2013, an estimated one in three adults worldwide was overweight or obese, and adult obesity exceeded 50% in several countries around the globe. While the prevalence of adult obesity in the developed world seems to have stabilized, the prevalence of obesity in children and adolescents globally, as well as adults obesity in developing countries, is still increasing. In addition, some developed countries continue to observe increasing prevalence of extreme classes of obesity.
Overweight and obesity—defined as excess body weight for height—have genetic, behavioral, socioeconomic, and environmental origins.
Obesity increases risk of major chronic diseases, including heart disease, diabetes, depression, and many cancers, as well as premature death.
Estimates of annual healthcare costs attributable to obesity are US$190 billion per year in the USA, approximately 21% of US healthcare expenditures.
Given its complexity, the obesity epidemic requires multilevel and integrated solutions, from individual intervention, to broad food policy, industry, and agriculture initiatives.
Acknowledgements
The authors declare no conflict of interest. AH is supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship award. FH is supported by NIH grants DK51158, HL60712, P30 DK46200, and U54CA155626. The authors broadly thank the researchers in this field for their consistent and tireless work in illuminating the etiology, sequelae, and solutions to this complex condition.
Footnotes
See also http://www.genome.gov/gwastudies/
We do not review the impact of food, agriculture, trade, and nutrition policy on obesity in the present paper, but refer interested readers to a recent review (64). Further, we do not address the body of growing evidence on the role of environmental pollutants–“obesogens”–in obesity, specifically those known as endocrine-disrupting chemicals. We refer readers to recent reviews on the topic (35,36,65).
Author Contributions
AH wrote the first draft of the paper. AH and FH contributed to writing, revised, and edited the paper. AH is the final guarantor of this work and takes full responsibility for its contents. Both authors read and approved the final manuscript.
References
- 1.American Medical Association AMA Adopts New Policies on Second Day of Voting at Annual Meeting [Internet] 2013 [cited 2014 Apr 7]. Available from: http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page.
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet [Internet] doi: 10.1016/S0140-6736(14)60460-8. (0). Available from: http://www.sciencedirect.com/science/article/pii/S0140673614604608. [DOI] [PMC free article] [PubMed]
- 3.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes 2005. 2008 Sep;32(9):1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obes Silver Spring Md. 2008 Oct;16(10):2323–30. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB. Obesity epidemiology. Oxford University Press; Oxford; New York: 2008. p. 498. [Google Scholar]
- 7.Hu FB. Obesity and Mortality: Watch Your Waist, Not Just Your Weight. Arch Intern Med. 2007 May 14;167(9):875. doi: 10.1001/archinte.167.9.875. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 10.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Oslo Nor 1992 Suppl. 2006 Apr;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 11.De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007 Sep;85(9):660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 13.Barlow SE, the Expert Committee Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007 Dec 1;120(Supplement 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009 Nov;90(5):1314–20. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 15.Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease Control and Prevention (CDC) Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2010 Sep 10;59(RR-9):1–15. [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014 Feb 26;311(8):806. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1998 Jan;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 18.Von Ruesten A, Steffen A, Floegel A, van der ADL, Masala G, Tjønneland A, et al. Trend in obesity prevalence in European adult cohort populations during follow-up since 1996 and their predictions to 2015. PloS One. 2011;6(11):e27455. doi: 10.1371/journal.pone.0027455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8(1):200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999-2012. JAMA Pediatr [Internet] 2014 Apr 7; doi: 10.1001/jamapediatrics.2014.21. [cited 2014 Apr 8]; Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2014.21. [DOI] [PubMed]
- 21.Wijnhoven TMA, van Raaij JMA, Spinelli A, Rito AI, Hovengen R, Kunesova M, et al. WHO European Childhood Obesity Surveillance Initiative 2008: weight, height and body mass index in 6–9-year-old children. Pediatr Obes. 2013;8(2):79–97. doi: 10.1111/j.2047-6310.2012.00090.x. [DOI] [PubMed] [Google Scholar]
- 22.Barquera S, Campos-Nonato I, Hernández-Barrera L, Pedroza A, Rivera-Dommarco JA. [Prevalence of obesity in Mexican adults 2000-2012] Salud Pública México. 2013;55(Suppl 2):S151–60. [PubMed] [Google Scholar]
- 23.Liang Y-J, Xi B, Song A-Q, Liu J-X, Mi J. Trends in general and abdominal obesity among Chinese children and adolescents 1993-2009: General and abdominal obesity in Chinese children. Pediatr Obes. 2012 Oct;7(5):355–64. doi: 10.1111/j.2047-6310.2012.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010 Nov;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci. 2009 Jun 9;106(23):9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962 Dec;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 27.Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical Activity Attenuates the Influence of FTO Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children. In: Lewis C, editor. PLoS Med. 11. Vol. 8. Nov 1, 2011. p. e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankinen T, Rice T, Teran-Garcia M, Rao DC, Bouchard C. FTO genotype is associated with exercise training-induced changes in body composition. Obes Silver Spring Md. 2010 Feb;18(2):322–6. doi: 10.1038/oby.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott RA, Bailey MES, Moran CN, Wilson RH, Fuku N, Tanaka M, et al. FTO genotype and adiposity in children: physical activity levels influence the effect of the risk genotype in adolescent males. Eur J Hum Genet EJHG. 2010 Dec;18(12):1339–43. doi: 10.1038/ejhg.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999 Nov 1;70(5):811–6. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 31.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010 Sep 1;140(3):387–98. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 32.Gaillard R, Steegers EAP, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood Cardiometabolic Outcomes of Maternal Obesity During Pregnancy: The Generation R Study. Hypertension. 2014 Apr 1;63(4):683–91. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 33.Bammann K, Peplies J, De Henauw S, Hunsberger M, Molnar D, Moreno LA, et al. Early Life Course Risk Factors for Childhood Obesity: The IDEFICS Case-Control Study. In: Bruce A, editor. PLoS ONE. 2. Vol. 9. Feb 13, 2014. p. e86914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M. Prepubertal start of father’s smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur J Hum Genet EJHG. 2014 Apr 2; doi: 10.1038/ejhg.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janesick AS, Shioda T, Blumberg B. Transgenerational inheritance of prenatal obesogen exposure. Mol Cell Endocrinol. 2014 Sep 15; doi: 10.1016/j.mce.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Cock M, van de Bor M. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies? Environ Int. 2014 Sep;70:15–24. doi: 10.1016/j.envint.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev. 2013 Oct;71:S62–7. doi: 10.1111/nure.12061. [DOI] [PubMed] [Google Scholar]
- 38.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA J Am Med Assoc. 2004 Mar. 10;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 39.Hill JO, Wyatt HR, Peters JC. Energy Balance and Obesity. Circulation. 2012 Jul 3;126(1):126–32. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle Modification for Obesity: New Developments in Diet, Physical Activity, and Behavior Therapy. Circulation. 2012 Mar 6;125(9):1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N Engl J Med. 2008 Jul 17;359(3):229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 42.Gardner CD. Comparison of the Atkins, Zone, Ornish, and LEARN Diets for Change in Weight and Related Risk Factors Among Overweight Premenopausal Women<SUBTITLE>The A TO Z Weight Loss Study: A Randomized Trial</SUBTITLE>. JAMA. 2007 Mar 7;297(9):969. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 43.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 Feb 26;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N Engl J Med. 2011 Jun 22;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik VS, Popkin BM, Bray GA, Despres J-P, Hu FB. Sugar-Sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation. 2010 Mar 23;121(11):1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013 Oct 1;98(4):1084–102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013 Aug 1;14(8):606–19. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallagher J. Mexico restricts soft drink TV ads to fight obesity [Internet] BBC News. 2014 [cited 2014 Jul 16]. Available from: http://www.bbc.com/news/world-latin-america-28325105.
- 49.New York City bans supersize sodas [Internet] BBC News. 2012 [cited 2014 Jul 21]. Available from: http://www.bbc.com/news/world-us-canada-19593012.
- 50.2008 Physical Activity Guidelines for Americans [Internet] [cited 2014 Apr 21]. Available from: http://www.health.gov/paguidelines/guidelines/
- 51.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009 Feb;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 52.Patel SR, Hu FB. Short Sleep Duration and Weight Gain: A Systematic Review. Obesity. 2008 Mar;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010 Feb;33(2):161–7. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath Schlaf Atm. 2012 Sep;16(3):829–33. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- 55.Lyytikäinen P, Rahkonen O, Lahelma E, Lallukka T. Association of sleep duration with weight and weight gain: a prospective follow-up study. J Sleep Res. 2011 Jun;20(2):298–302. doi: 10.1111/j.1365-2869.2010.00903.x. [DOI] [PubMed] [Google Scholar]
- 56.Hu FB. Television Watching and Other Sedentary Behaviors in Relation to Risk of Obesity and Type 2 Diabetes Mellitus in Women. JAMA. 2003 Apr 9;289(14):1785. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 57.Boone JE, Gordon-Larsen P, Adair LS, Popkin BM. Screen time and physical activity during adolescence: longitudinal effects on obesity in young adulthood. Int J Behav Nutr Phys Act. 2007;4(1):26. doi: 10.1186/1479-5868-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martínez-González MA, Martínez JA, Hu FB, Gibney MJ, Kearney J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1999 Nov;23(11):1192–201. doi: 10.1038/sj.ijo.0801049. [DOI] [PubMed] [Google Scholar]
- 59.Levine JA. Poverty and Obesity in the U.S. Diabetes. 2011 Nov 1;60(11):2667–8. doi: 10.2337/db11-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh KA, Hoy JS, O’Connell JJ, Montgomery P. The hunger-obesity paradox: obesity in the homeless. J Urban Health Bull N Y Acad Med. 2012 Dec;89(6):952–64. doi: 10.1007/s11524-012-9708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai J, Rosenheck RA. Obesity among chronically homeless adults: is it a problem? Public Health Rep Wash DC 1974. 2013 Feb;128(1):29–36. doi: 10.1177/003335491312800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devaux M, Sassi F. Social inequalities in obesity and overweight in 11 OECD countries. Eur J Public Health. 2013 Jun 1;23(3):464–9. doi: 10.1093/eurpub/ckr058. [DOI] [PubMed] [Google Scholar]
- 63.Aitsi-Selmi A, Bell R, Shipley MJ, Marmot MG. Education modifies the association of wealth with obesity in women in middle-income but not low-income countries: an interaction study using seven national datasets, 2005-2010. PloS One. 2014;9(3):e90403. doi: 10.1371/journal.pone.0090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013 Jan;9(1):13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 65.Regnier SM, Sargis RM. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta. 2014 Mar;1842(3):520–33. doi: 10.1016/j.bbadis.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29:129–43. doi: 10.1093/epirev/mxm009. [DOI] [PubMed] [Google Scholar]
- 67.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009 Jan;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Holsten JE. Obesity and the community food environment: a systematic review. Public Health Nutr. 2009 Mar;12(3):397–405. doi: 10.1017/S1368980008002267. [DOI] [PubMed] [Google Scholar]
- 69.McCormack GR, Shiell A. In search of causality: a systematic review of the relationship between the built environment and physical activity among adults. Int J Behav Nutr Phys Act. 2011;8:125. doi: 10.1186/1479-5868-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morland KB, Evenson KR. Obesity prevalence and the local food environment. Health Place. 2009 Jun;15(2):491–5. doi: 10.1016/j.healthplace.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sallis JF, Floyd MF, Rodríguez DA, Saelens BE. Role of built environments in physical activity, obesity, and cardiovascular disease. Circulation. 2012 Feb 7;125(5):729–37. doi: 10.1161/CIRCULATIONAHA.110.969022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durand CP, Andalib M, Dunton GF, Wolch J, Pentz MA. A systematic review of built environment factors related to physical activity and obesity risk: implications for smart growth urban planning. Obes Rev Off J Int Assoc Study Obes. 2011 May;12(5):e173–82. doi: 10.1111/j.1467-789X.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berry TR, Spence JC, Blanchard CM, Cutumisu N, Edwards J, Selfridge G. A longitudinal and cross-sectional examination of the relationship between reasons for choosing a neighbourhood, physical activity and body mass index. Int J Behav Nutr Phys Act. 2010;7:57. doi: 10.1186/1479-5868-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boone-Heinonen J, Diez-Roux AV, Goff DC, Loria CM, Kiefe CI, Popkin BM, et al. The neighborhood energy balance equation: does neighborhood food retail environment + physical activity environment = obesity? The CARDIA study. PloS One. 2013;8(12):e85141. doi: 10.1371/journal.pone.0085141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Auchincloss AH, Mujahid MS, Shen M, Michos ED, Whitt-Glover MC, Diez Roux AV. Neighborhood health-promoting resources and obesity risk (the multi-ethnic study of atherosclerosis) Obes Silver Spring Md. 2013 Mar;21(3):621–8. doi: 10.1038/oby.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, et al. Ten Putative Contributors to the Obesity Epidemic. Crit Rev Food Sci Nutr. 2009 Dec 10;49(10):868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parra-Rojas I, Del Moral-Hernández O, Salgado-Bernabé AB, Guzmán-Guzmán IP, Salgado-Goytia L, Muñoz-Valle JF. Adenovirus-36 seropositivity and its relation with obesity and metabolic profile in children. Int J Endocrinol. 2013;2013:463194. doi: 10.1155/2013/463194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes 2005. 2005 Mar;29(3):281–6. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- 79.Almgren M, Atkinson R, He J, Hilding A, Hagman E, Wolk A, et al. Adenovirus-36 is associated with obesity in children and adults in Sweden as determined by rapid ELISA. PloS One. 2012;7(7):e41652. doi: 10.1371/journal.pone.0041652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O’Toole PW, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes. 2012 Jun;3(3):186–202. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 82.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013 Dec 11;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 2011 Oct 7;334(6052):105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 85.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Obesity. 2013 Feb;34(1):39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Christakis NA, Fowler JH. The Spread of Obesity in a Large Social Network over 32 Years. N Engl J Med. 2007 Jul 26;357(4):370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 87.Leroux JS, Moore S, Dubé L. Beyond the “I” in the Obesity Epidemic: A Review of Social Relational and Network Interventions on Obesity. J Obes. 2013;2013:1–10. doi: 10.1155/2013/348249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003 Jan 7;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 89.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N Engl J Med. 2010 Dec 1;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. 2013 Oct 1;347:f5446–f5446. doi: 10.1136/bmj.f5446. oct01 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai SP, Donnelly RP, Wendt JK. Obesity and mortality in a prospective study of a middle-aged industrial population. J Occup Environ Med Am Coll Occup Environ Med. 2006 Jan;48(1):22–7. doi: 10.1097/01.jom.0000184866.49000.e5. [DOI] [PubMed] [Google Scholar]
- 93.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006 Aug 24;355(8):763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 94.Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A, et al. Body Mass and Weight Change in Adults in Relation to Mortality Risk. Am J Epidemiol. 2014 Jan 15;179(2):135–44. doi: 10.1093/aje/kwt254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-Mass Index and Mortality in a Prospective Cohort of U.S. Adults. N Engl J Med. 1999 Oct 7;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 96.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 97.Park Y, Wang S, Kitahara CM, Moore SC, Berrington de Gonzalez A, Bernstein L, et al. Body Mass Index and Risk of Death in Asian Americans. Am J Public Health. 2014 Mar;104(3):520–5. doi: 10.2105/AJPH.2013.301573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care. 2012 Jan 1;35(Supplement 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010 Sep;89(3):309–19. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 100.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011 Jul;35(7):891–8. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 101.Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta-analysis: Body weight gain and type 2 diabetes. Obes Rev. 2014 Mar;15(3):202–14. doi: 10.1111/obr.12129. [DOI] [PubMed] [Google Scholar]
- 102.Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care. 2007 Jun 1;30(6):1562–6. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 103.National Diabetes Information Clearinghouse, US Department of Health and Human Services Diabetes Fact Sheet [Internet] [cited 2014 Apr 24]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/overview/
- 104.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014 doi: 10.1111/obr.12157. n/a – n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012 Dec 15;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flint AJ, Hu FB, Glynn RJ, Caspard H, Manson JE, Willett WC, et al. Excess Weight and the Risk of Incident Coronary Heart Disease Among Men and Women. Obesity. 2010 Feb;18(2):377–83. doi: 10.1038/oby.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramer CK, Zinman B, Retnakaran R. Are Metabolically Healthy Overweight and Obesity Benign Conditions?: A Systematic Review and Meta-analysis. Ann Intern Med. 2013 Dec 3;159(11):758. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 108.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014 Mar 15;383(9921):970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N Engl J Med. 2011 Nov 16;365(20):1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 110.Polednak AP. Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev. 2008 Jan;32(3):190–9. doi: 10.1016/j.cdp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 111.Vainio H, Bianchini F. Weight control and physical activity. IARC Press; Lyon: 2002. [DOI] [PubMed] [Google Scholar]
- 112.Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer [Internet] 2007 Mar 20; doi: 10.1038/sj.bjc.6603703. [cited 2014 Apr 24]; Available from: http://www.nature.com/doifinder/10.1038/sj.bjc.6603703. [DOI] [PMC free article] [PubMed]
- 113.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007 Oct 8;97(7):1005–8. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer Oxf Engl 1990. 2007 Mar;43(4):690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012 Jul;23(7):1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 116.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer J Int Cancer. 2008 Mar 15;122(6):1418–21. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 117.Glance LG, Li Y, Osler TM, Mukamel DB, Dick AW. Impact of Obesity on Mortality and Complications in Trauma Patients. Ann Surg. 2014 Mar;259(3):576–81. doi: 10.1097/SLA.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 118.Liu T, Chen J, Bai X, Zheng G, Gao W. The effect of obesity on outcomes in trauma patients: A meta-analysis. Injury. 2013 Sep;44(9):1145–52. doi: 10.1016/j.injury.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 119.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013 Mar;37(3):333–40. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 120.Young KM, Gray CM, Bekker L-G. Is Obesity a Risk Factor for Vaccine Non-Responsiveness? Ahuja SK, editor. PLoS ONE. 2013 Dec 11;8(12):e82779. doi: 10.1371/journal.pone.0082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20870. NA – NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reinert KRS, Po’e EK, Barkin SL. The Relationship between Executive Function and Obesity in Children and Adolescents: A Systematic Literature Review. J Obes. 2013;2013:1–10. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev Off J Int Assoc Study Obes. 2011 May 12;(5):e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 124.Lindholm V, Lahti J, Rahkonen O, Lahelma E, Lallukka T. Joint association of physical activity and body weight with subsequent physical and mental functioning: a follow-up study. BMC Public Health. 2013;13(1):197. doi: 10.1186/1471-2458-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cawley J, Meyerhoefer C. The medical care costs of obesity: An instrumental variables approach. J Health Econ. 2012 Jan;31(1):219–30. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Mason RJ, Moroney JR, Berne TV. The cost of obesity for nonbariatric inpatient operative procedures in the United States: national cost estimates obese versus nonobese patients. Ann Surg. 2013 Oct;258(4):541–51. doi: 10.1097/SLA.0b013e3182a500ce. discussion 551–3. [DOI] [PubMed] [Google Scholar]
- 127.Kleinman N, Abouzaid S, Andersen L, Wang Z, Powers A. Cohort Analysis Assessing Medical and Nonmedical Cost Associated With Obesity in the Workplace: J Occup Environ Med. 2014 Feb;56(2):161–70. doi: 10.1097/JOM.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 128.Finkelstein EA, Graham WCK, Malhotra R. Lifetime Direct Medical Costs of Childhood Obesity. PEDIATRICS [Internet] 2014 Apr 7; doi: 10.1542/peds.2014-0063. [cited 2014 Apr 22]; Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2014-0063. [DOI] [PubMed]
- 129.Müller-Riemenschneider F, Reinhold T, Berghöfer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol. 2008 Aug;23(8):499–509. doi: 10.1007/s10654-008-9239-1. [DOI] [PubMed] [Google Scholar]
- 130.Von Lengerke T, Krauth C. Economic costs of adult obesity: A review of recent European studies with a focus on subgroup-specific costs. Maturitas. 2011 Jul;69(3):220–9. doi: 10.1016/j.maturitas.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 131.Bahia L, Coutinho ES, Barufaldi L, de Azevedo Abreu G, Malhão T, Ribeiro de Souza C, et al. The costs of overweight and obesity-related diseases in the Brazilian public health system: cross-sectional study. BMC Public Health. 2012;12(1):440. doi: 10.1186/1471-2458-12-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rtveladze K, Marsh T, Webber L, Kilpi F, Levy D, Conde W, et al. Health and economic burden of obesity in Brazil. PloS One. 2013;8(7):e68785. doi: 10.1371/journal.pone.0068785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rtveladze K, Marsh T, Barquera S, Sanchez Romero LM, Levy D, Melendez G, et al. Obesity prevalence in Mexico: impact on health and economic burden. Public Health Nutr. 2014 Jan;17(01):233–9. doi: 10.1017/S1368980013000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marketdata Enterprises, Inc. The U.S. Weight Loss Market: 2014 Status Report & Market Research [Internet] 2014 [cited 2014 Apr 23]. Available from: http://www.marketresearch.com/Marketdata-Enterprises-Inc-v416/Weight-Loss-Status-Forecast-8016030/
- 135.National Institutes of Health, National Heart, Lung, and Blood Institute . Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. [Internet] U.S. Department of Health and Human Services; Bethesda, MD: Oct, 1998. Obesity Education Initiative: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. [cited 2014 Apr 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK2003/ [Google Scholar]
- 136.Huang TT, Drewnosksi A, Kumanyika S, Glass TA. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009 Jul;6(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 137.Cornelis MC, Hu FB. Systems Epidemiology: A New Direction in Nutrition and Metabolic Disease Research. Curr Nutr Rep. 2013 Dec;2(4) doi: 10.1007/s13668-013-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health . The Health Consequences of Smoking —50 Years of Progress: A Report of the Surgeon General. [Internet] U.S. Department of Health and Human Services; Atlanta, GA: 2014. [cited 2014 Apr 1]. Available from: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/ [Google Scholar]
- 139.World Health Organization . Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organization; Geneva: 2000. p. 253. [PubMed] [Google Scholar]
- 140.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000 May 6;320(7244):1240. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fryar CD, Carroll MD, Ogden CL. NCHS Health E Stat - Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960-1962 Through 2009-2010 [Internet] [cited 2014 Feb 26]. Available from: http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm.