Abstract
When chemerin was discovered in 1997, it was relegated to being a protein associated with the normal skin function contrasting the setting of psoriasis. However, with the discovery of multiple receptors for the chemerin protein and a vast collection of associations with various pathologies, chemerin has global influence capable of regulating chemotactic, adipokine, autocrine/paracrine, adipogenic, angiogenic, and reproductive functions. These individual abilities of chemerin are important for understanding its basic pharmacology and physiology, but application of these principles to human pathology relies on the ability of scientists and physicians to view this protein from a much wider, all-encompassing angle. A global participant in the action of chemerin is the cardiovascular system (CVS). Although the CVS may not have as many direct interactions (e.g. smooth muscle in endothelium) with chemerin as it does indirect (e.g. chemerin activation in the lumen by proteases), our basic understanding of the CVS and its relation to chemerin is necessary to form a proper grasp of its individual actions and make the applications to pathology. This review provides a fundamental, yet comprehensive review of chemerin that inherently identifies the CVS as a necessary link between chemerin and its associated pathologies, but also calls for basic cardiovascular research as the solution to this chasm between knowledge and application.
Keywords: Chemerin, Cardiovascular System, Pharmacology, Physiology, Pathology
1 - Introduction
Although the fields of medical research tend to be divided into basic, translational, and epidemiology, the communication and interplay between these three fields is of greatest importance in the unearthing and analysis of new drugs and their functions. Chemerin is a protein that emerged in 1997 [1] but due to a lack of these essential exchanges, has largely failed to produce useful medical applications. The epidemiology and associations between the protein and certain disorders is being investigated in great depth, but without knowledge of its mechanisms the epidemiology argues correlative conclusions without finding causative ones.
The discovery of chemerin (as tazarotene-induced gene 2, TIG2; also known as retinoic acid receptor responder gene 2, RARRES2) was in the context of psoriasis and hypothesized to be involved in cell-cell or cell-extracellular matrix interactions [1]. However, our knowledge of its receptors is just as important as the investigation of chemerin itself. G protein-coupled receptor 1 (GPR1) was first described in 1994 in the human hippocampus [2] but was not linked to chemerin until 2007 [3].
As mentioned above, CMKLR1 is also a receptor associated with chemerin and was next to be discovered in 1996 [4] followed by the Chemerin Receptor 23 (ChemR23) in 1998 [5]. Coincidentally, these separately described receptors are one in the same. Although it is unclear when the scientific community came to this realization, Zabel, Silverio, and Butcher seemed to be acutely aware of this situation when they pointed this out in 2004 [6]. ChemR23 was linked to chemerin in 2003 [7,8]. The mouse ortholog of ChemR23 is also known as DEZ (named in 1997) [9] and a rat ortholog was once named CMKRL3 [10].
The last receptor to be associated with chemerin is chemokine (CC motif) receptor-like 2 (CCRL2) which was first discovered in the human in 1998 (then named human chemokine receptor, HCR) [11]. The link to chemerin was not made until 2008 when Zabel et al. investigated the mechanisms of the receptor [12].
Chemerin and these receptors can be found throughout the human body and the evidence seems to point towards it playing a multifunctional role as a chemokine, adipokine, and possibly a growth factor. When thinking about inflammation, it seems to have connections to all three of these areas but centers both passively (using the system for transport) and actively (having an effect on the endothelium or smooth muscle) around the cardiovascular system. The field of basic research currently has strong support for the connections between chemerin and its respective specific functions (relating chemerin as a chemokine to immune cells or as an adipokine to adipose cells), but lacks the next step in its connection to the cardiovascular system.
2 - Biochemistry
2.1 - Sequencing: chemerin protein and receptors
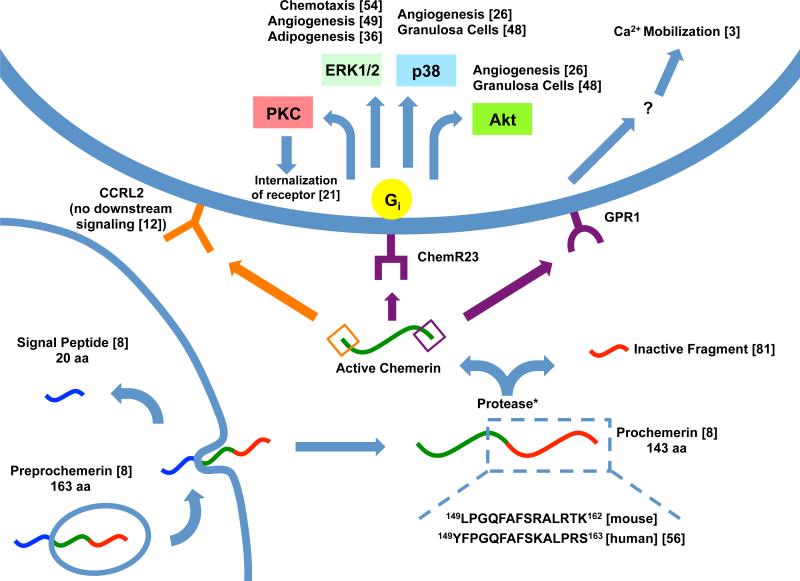
When chemerin and many of its receptors were discovered, amino acid sequencing was quickly performed on chemerin because the biochemistry of the protein offered an identification of the new receptor or ligand as well as insights to its possible actions. Upon the cloning of the cDNA analog of chemerin (TIG2), the 830 bp unit was found to code for a 164 amino acid sequence. At the N-terminus of the sequence was a hydrophobic residue that led researchers to believe chemerin was membrane-associated [1]. Wittamer revealed a 20 amino acid signal peptide and a resulting 143 amino acid sequence which is released from the cell. More importantly, she found that in active chemerin, the carboxyl-terminal end of the eighth peptide was missing six of its predicted amino acids. These missing amino acids led to the proposal that they were lost in proteolytic processing and created the active protein (about 137 amino acids) known as chemerin (see Figure 1) [8].
Figure 1. Processing and pathways of chemerin.
Chemerin is depicted with the N-terminus on the left and C-terminus on the right. When preprochemerin is released, the membrane-bound signal peptide (blue) is released and the protein becomes prochemerin [8]. This is then cleaved at various locations by a protease (*see Table 1), creating an inactive prochemerin fragment (red) and active chemerin (green) [81]. The N-terminus (orange) can bind CCRL2 [12] or the C-terminus (purple) can bind ChemR23 or GPR1. CHO-K1 cultured cells demonstrate Gi signaling to various pathways [8] which differ depending on the process being carried out. GPR1 has been shown to bind chemerin and induce Ca2+ mobilization but the specific mechanisms are still unknown [3].
Initial processing of the ChemR23 gene revealed it to be closer related to chemoattractant receptors (e.g. anaphylatoxin C3a and C5a receptors) rather than CC or CXC chemokine receptors. It was also discovered that the receptor did not contain the extra cysteine residues that normally link the N-terminus with the extracellular loop, a common characteristic of chemokine receptors, therefore setting it apart. The mouse receptor DEZ was also found to contain 80.3% homology and determined to be the mouse counterpart of human ChemR23 [5] with similar methods of regulation [13].
Discovery and sequencing of the GPR1 revealed a close relationship with the G protein-coupled receptor family. There was also a high level of homology between the rat and human analogs (80%) [2]. Thanks to the sequencing and publishing of these data, the GPR1 was shown to have significant homology with the ChemR23 receptor and was linked to chemerin [3].
CCRL2 contains a reading frame with a predicted 345 amino acids. Because of its similarity to other human chemokine receptors it was first named human chemokine receptor (HCR) [11]. Just before its association with chemerin, scientists discovered that CCRL2 had an unusual DRYLAIV motif and postulated it may cause CCRL2 to be a silent receptor capable of binding ligands but not transducing a signal. The function of this receptor is to present the carboxy-terminal domain to ChemR23 without transduction, essentially concentrating extracellular chemerin and colocalizing it to another receptor [12].
2.2 - Synthesis of Chemerin
Central to the synthesis of chemerin is its extracellular proteolytic cleavage (Figure 1). Not only was it shown that chemerin needed to be cleaved from prochemerin to become active [8], but it was also shown that the cleavage must be extremely specific in order to maintain the active chemerin S157 peptide as an agonist to ChemR23 [14]. Several proteases have been implicated in the activation of this protein including inflammatory serine proteases tryptase, elastase and plasmin (Table 1). Interestingly, these proteases do not each cut the peptide in the same location [15] and this variation in cleavage points gives rise to one of the most unrecognized aspects of chemerin in the epidemiology world, isoforms.
Table 1.
Proteases of chemerin
| Proteases | Chemerin Substrate | Chemerin Product | Source | Notes | Model | Ref |
|---|---|---|---|---|---|---|
| Calpains | Prochemerin | Not Determined | Macrophage | Anti-inflammatory | Mouse | 18 |
| Carboxy-peptidase B and N | S157 | Can be used sequentially with plasmin | Human | 19 | ||
| Cathepsin G | Prochemerin | F148, F156 | Neutrophil | Human | 15,16 | |
| Cathepsin K | Prochemerin | S157, R125 | Antibacterial | Human | 81 | |
| Cathepsin L | Prochemerin | S157, R125 | Antibacterial | Human | 81 | |
| Cathepsin S | Prochemerin | Not Determined | Macrophage | Anti-inflammatory | Mouse | 18 |
| Elastase | Prochemerin | S157, A155, G152 | Neutrophil | Human | 15 | |
| Factor XIIa | Prochemerin | Not Determined | Coagulation Cascade | Human | 15 | |
| Kallikrein 7 | Prochemerin | F156 | Skin Psoriasis | Human | 56 | |
| Mast Cell Chymase | S157 and F156 | F154 | Mast Cell | F154 is inert | Human | 20 |
| Plasmin | Prochemerin | K158 | Fibrinolytic Cascade | Human | 15 | |
| Proteinase-3 | Prochemerin | A155 | Neutrophil | A155 is inert | Human | 20 |
| Staphopain B | Prochemerin | S157 | S. aureus | Human | 82 | |
| Tryptase | Prochemerin | K158, A155 | Mast Cell | Human | 15 |
Proteases are listed alphabetically with their substrate and product, both of which are named for their C-terminal amino acid (prochemerin in humans has a C-terminus of S163, see Table 2a). In cases where the product is not determined, controlled trials revealed that the protease could be fed prochemerin (inert) and turn it into a form that elicits a response by ChemR23.
Isoforms of chemerin are named for their carboxy-terminal amino acid. The isoform cleaved by plasmin (named above) would be chemerin K158. The defined serum chemerin reported by Zabel et al. is chemerin A155 [15]. However, the most active form in the serum that stimulated calcium mobilization and chemotaxis in murine pre-B lymphoma cell line L1.2 is chemerin S157 (calcium mobilization EC50 = 1.17 nM and % migration EC50 = 3.15 nM) [16]. In addition, chemerin K158 predominates in CSF and synovial fluid [17]. But most astoundingly, in 2008, Cash et al. demonstrated that specific proteolytic cleavage and creation of chemerin-15 (mouse, A140-A154) demonstrated anti-inflammatory qualities on the ChemR23 receptor [18] (for a full list of currently known isoforms of chemerin, see Table 2). Already, the great variation in distribution and effectiveness of isoforms is staggering and the fact that the medical community has not specified these isotypes in their various pathologies is extremely limiting to advancement of this research, but is also an opportunity to provide clarity for many epidemiological questions.
Table 2.
a – Synthetic peptides of chemerin
| Isoform | Sequence | Function of Isoform | Notes | Model | Ref |
|---|---|---|---|---|---|
| Chemerin-15 | AGEDPHGYFLPGQFA | Mimics chemerin A154 | Inhibit Macrophage | Mouse | 18 |
| FA Peptide | HSFYFPGQFA | Mimics chemerin A155 | No Cell Chemotaxis | Human | 16 |
| 11-mer | HSFYFPGQFAF | Mimics chemerin F156 | Slight Cell Chemotaxis | Human | 16 |
| Chemerin-9 | YFPGQFAFS | Mimics chemerin S157 | Highest Biological Activity | Human Rat | 14, 25 |
| Chemerin-9 | FLPGQFAFS | Mimics chemerin S158 | Same potency as human | Mouse | 22 |
| 10-mer | YFPGQFAFSK | Mimics chemerin K158 | Inert | Human | 19 |
| 15-mer | YFPGQFAFSKALPRS | Mimics chemerin S163 | Inert | Human | 16 |
| Table 2b – Biologically derived peptides of chemerin | ||||
|---|---|---|---|---|
| Isoform | ChemR23 Activity | Other | Model | Ref |
| Chemerin R125 | Inert | Human | 81 | |
| Chemerin F154 | Inert | Human | 20 | |
| Chemerin A155 | Inert, antagonist of Chemerin-9 | Most Abundant in Serum | Human | 15, 16 |
| Chemerin F156 | Psoriasis | Human | 56 | |
| Chemerin S157 | Significant Ca2+ mobilization, chemotaxis | Most Active in Serum | Human | 16, 80 |
| Chemerin K158 | Abundant in CSF and Synovial, Glioblastoma, RA | Human | 16, 17 | |
| Chemerin S163 | Prochemerin, inert | Human | 15 | |
A list of synthetic peptides are given with their sequence and are sorted based on the C-terminal amino acid of chemerin they were created to emulate. Chemerin-9 continues to be the primary agonist of the ChemR23 receptor (a). A list of biologically active isoforms is sorted according to their C-terminal amino acid. All are assumed to begin with amino acid E21. Although no full mouse isoforms are listed, full-length mouse peptides begin with E23 [18] (b).
A full list of proteases for chemerin is given in Table 1 but proteases of particular interest are carboxypeptidase B (CPB), carboxypeptidase N (CPN), and mast cell chymase. CPB and CPN have the ability to cleave chemerin K158 (inert) to the active chemerin-9. These proteases can be paired with plasmin (which creates the inert chemerin K158) to create an active product [19]. Mast cell chymase is also of note because it can take the active chemerin S157 and turn it into the inert chemerin F154 form [20].
3 - Pharmacology
3.1 - ChemR23
Chemerin was the first ligand associated with ChemR23, before which it was considered an orphan G protein-coupled receptor. Short peptides are often used to explore the actions of the receptor. Use of the 13 amino acid “YHSFFFPGQFAFS” peptide on CHO-K1 cells caused release of intracellular calcium, inhibition of cAMP, and phosphorylation of p44 and p42 MAP kinases (extracellular signal-regulated kinase 1 and 2, ERK1/2). When pretreated with pertussis toxin, all of the previously described effects were inhibited indicating an association with the Gi family [8]. There are many other peptides and derivatives of chemerin that can agonize the receptor but of them, human chemerin-9 (YFPGQFAFS) appears to simulate the same pharmacology as recombinant chemerin. Furthermore, Wittamer et al. also found that Gly152 and the aromatic amino acids of Tyr149, Phe150, Phe154, and Phe156 are critical in the binding of chemerin to ChemR23 [14]. Binding of chemerin-9 to ChemR23 caused concentration-dependent internalization of the receptor. This does not occur through clathrin but may be compartmentalized with caveolae [21]. The chemerin-15 peptide (141AGEDPHSFYFPGQFA155) is not normally an agonist for ChemR23 and is able to block this internalization [21]. Chemerin-9 is metabolized in both the mouse and human at a high rate which makes in vivo experiments difficult. Substitutions of the following peptides in chemerin-9 resulted in a near 100% potency for the entire length of the four hour in vivo experiment: d-Tyr147, d-Ser151, d-Ala154, Tic155 [22]. Although chemerin-15 has been shown in some studies to be inactive with respect to ChemR23 (does not cause downstream signaling), in mouse macrophages it has induced anti-inflammatory effects through unknown downstream signaling mechanisms [18].
Another anti-inflammatory agonist for ChemR23 is the lipid Resolvin E1 (RvE1). Both chemerin and RvE1 share the same binding sites but the transmission of signals is believed to be different [23]. This is not the first time peptide and lipid ligands have exhibited different effects on the same receptor. The ALX receptor on neutrophils can be triggered by peptides or LXA4 and elicit separate responses [24].
An antagonist of ChemR23 was recently described. CCX832 reduced chemerin/ChemR23-stimulated contraction of isolated arteries but had no affinity for the GPR1 or CCRL2 receptors, indicating a preferential use of the ChemR23 receptor in chemerin induced smooth muscle signaling [25].
As previously mentioned, ChemR23 is widely known to act through Gi and ERK1/2 [8] but others have reported separate post-receptor signaling events specific to a particular action of the receptor. Angiogenesis through ChemR23 tends to activate Akt (protein kinase B) and p38 along with ERK1/2 [26]. The protein kinase C (PKC) pathway is important for internalization of the receptor but seems to be separate from ERK signaling (Figure 1). When receptor internalization is halted, ERK phosphorylation is maximized whereas if internalization is allowed to occur via PKC, ERK phosphorylation is halted before it reaches maximal rates [21]. This suggests that the internalization of the receptor itself is what limits receptor functioning.
3.2 - GPR1
Recombinant chemerin is also an agonist for GPR1 with an EC50 of 240 pM compared to 3 nM of ChemR23 in HEK293T cell lines. Although this may suggest that chemerin is a more potent agonist for GPR1 than for ChemR23, subsequent calcium mobilization assays for GPR1 displayed only one-third of the mobilization seen in ChemR23 [3]. In mice, GPR1 was found in highest concentrations in the stromal vascular fraction of white adipose tissue. Functionally, GPR1-knockout mice experienced worsened glucose intolerance, elevated blood glucose, and reduced insulin compared to wild type [27]. Although the pharmacologic mechanisms of the chemerin/GPR1 interaction are still relatively unknown, it seems to take on similar characteristics as ChemR23 which is predictable considering their close homology [3]. Due to lack of evidence, all that is currently known about GPR1 post-receptor signaling with chemerin is that it carries out its effects through calcium mobilization (Figure 1) [3].
3.3 - CCRL2
As previously mentioned, CCRL2 does not internalize chemerin but can bind the N-terminus with high affinity allowing for chaperoning and concentrating of the chemerin ligand to ChemR23 (Figure 1). Binding with chemerin in L1.2 cell cultures occurs at an EC50 of 0.2 nM suggesting that CCRL2 binds chemerin with higher affinity than ChemR23 (3.1 nM). Binding with chemerin-9, however, only produces an EC50 of 26.2 nM [12]. Although the concentrating effect of CCRL2 on chemerin is a very probable explanation of the data, CCRL2 transduces signals stimulated by other ligands: CCL5 agonizes CCRL2 via ERK1/2 [28] and CCL19 displays internalization with CCRL2 in a clathrin-dependent manner [29]. Although CCRL2 seems to have a definitive singular action with chemerin, its capabilities are varied and diverse so it should not be assumed that CCRL2 responds to all chemerin isoforms the same in all tissues.
4 - Physiology
4.1 - A Chemotactic Agent
Although chemerin's association with inflammation and role as a chemokine is clear, our knowledge about its direct influence on inflammation is still hazy and compounded by the lack of data on the previously mentioned isotypes. At its discovery, scientists found that chemerin is present in normal skin but significantly reduced in psoriatic lesions. This led them to hypothesize that chemerin was involved in maintaining the normal physiology of the human skin [1]. Since then, both scientists and physicians have provided evidence to the contrary.
When the mechanism of extracellular proteolytic cleavage was uncovered to be the activator of chemerin in the human body, these proteases were associated with coagulation, fibrolytic cascades, elastase from activated neutrophils, and tryptase from mast cells [15]. All of these processes point towards active chemerin being a result of inflammation and not a cause of normal physiologic states. The situations under which active chemerin isoforms are generated can give us insight as to its chemotactic purpose, but so will the location and activity of its receptors.
Chemokine receptor CCRL2 was originally found to be in high concentrations in the spleen, fetal liver, lymph nodes, and bone marrow [11]. In addition, it is constitutively expressed on mast cells. Seeing as CCRL2 only binds chemerin and does not internalize it, supposedly to concentrate it for the ChemR23 receptors, this suggests that mast cells are holding chemerin in preparation for activation and such an event that would require an inflammatory response. Macrophage exposure to lipopolysaccharide (LPS) will up-regulate CCRL2 concentration on its surface but interestingly, also down-regulate ChemR23 levels. The functional significance of this relationship is still unclear but there is a period after activation where both ChemR23 and CCRL2 are present on the cell [12].
ChemR23 also has its own unique distribution on cells of the immune system. At its discovery, ChemR23 was found on the antigen presenting monocyte-derived dendritic cell and macrophages (which can also release chemerin at physiologic levels [8]). Low concentrations were also found on T lymphocytes [5].
Dendritic cells are often divided into myeloid (mDC) and plasmacytoid (pDC). mDCs population is greater in the blood and responds to inflammatory chemokines. On the contrary, pDCs are relatively rare in the blood and produce interferons to activate adaptive immunity. Although pDCs do not react to typical inflammatory chemokines, they still retain the ability of chemotaxis. Investigation narrowed down the receptor's presence from any DCs to immature pDCs which are further down-regulated upon maturation. Because of these findings, it is believed that chemerin is activated during clotting and inflammatory cascades, allowing for the chemotaxis of pDCs to the site of injury [6].
Along with macrophages and dendritic cells, natural killer cells have been added to the list of immune cells that express ChemR23. Like dendritic cells, natural killers (NK) come in two varieties: CD56lowCD16+ and CD56highCD16−. The CD56low population tends to be the predominant type to migrate into inflamed tissue. NK cells with the CD56high phenotype tend to be scarce and migrate into secondary lymphoid tissue [30]. Along the common theme of inflammation, ChemR23 was only present in the CD56low population. More importantly, the cooperation of dendritic cells and NK cells in the adaptive immune response led researchers to believe chemerin's involvement in these two cell types is somehow linked. Experiments confirmed that these two cells are colocalized in the presence of active chemerin [31]. On the surface, chemerin seems to offer an easy explanation for the relationship between NK cells and dendritic cells. However, the previously mentioned research on dendritic cells cited that only pDCs expressed ChemR23 and mDCs do not, while the recently mentioned research on NK cells demonstrates that both pDCs and mDCs express ChemR23 and both subtypes colocalize the NK cells in the presence of chemerin. This is a discrepancy that has not yet been explained, but does not detract from the chemotactic nature of chemerin.
A large topic of research in the 1990's that brought about the initial discovery of the chemerin receptors was Human (also Simian) Immunodeficiency Virus (HIV/SIV). While investigating the mechanisms of viral adhesion and invasion of cells, researchers found that chemokine receptors were being used as cofactors of fusion, and mutations in these chemokine receptors could confer a certain level of resistance to the host [32]. Ultimately, ChemR23 was only used by a very small population of HIV strains and a slightly larger SIV population. Although this receptor is not considerably active in HIV/SIV fusion, Samson et al. suggested it might be a path for the virus to escape therapeutic interventions [5].
As mentioned previously, RvE1 is a lipid that can competitively bind to ChemR23 and cause a response (often anti-inflammatory) different to that of chemerin. Radioligand binding assays using tritium-labeled RvE1 displayed binding to ChemR23 and competition with chemerin-9. In the inflammatory response, dendritic cells in the spleen (containing ChemR23) migrate towards T lymphocytes and express IL-12. With application of RvE1, IL-12 production is inhibited and when small interference RNA is applied to the same cells for ChemR23, essentially eliminating ChemR23 expression, IL-12 production is restored (experiments were performed in murine tissue with its version of ChemR23) [23]. This tells us that RvE1 may compete with chemerin for the ChemR23 receptor and act to inhibit inflammation by affecting dendritic cell cytokine production.
In addition to RvE1, chemerin may also have its own anti-inflammatory effects by inhibiting phosphorylation of NF-κB and p38 MAPK and inhibiting TNFα-induced VCAM-1 expression through ChemR23 [33]. These elements play an important role in cell adhesion of the inflammatory response. Contrary to these results, other researchers have reported chemerin and ChemR23 to activate MAPK pathways and up-regulate TNFα, IL-1β, IL-6, and MMPs [26]. Yamawaki et al. using human umbilical vein endothelial cells and Kaur et al. utilizing human microvascular endothelial cells suggest that different endothelial sites could react differently to chemerin in their post-receptor signaling and TNFα production.
As previously stated, chemerin can influence TNFα production, but TNFα can also influence chemerin production, as demonstrated by Parlee et al. using mice and mouse adipocyte cell lines (3T3-L1). Their first finding was that TNFα induced chemerin mRNA synthesis in differentiated 3T3-L1 adipocytes. These findings appear to be tissue specific because TNFα did not induce the same effect in hepatocytes (a major producer of chemerin in the body [34]). Second, they investigated the depth of regulation on chemerin synthesis. Because actinomycin D (an inhibitor of mRNA synthesis) was cytotoxic to the cells, they were unable to determine if TNFα regulated transcription. However, using cycloheximide and brefeldin A, they were successful in determining that TNFα regulated chemerin synthesis on the levels of protein synthesis and secretion (respectively) [35]. Although TNFα is typically involved in inflammatory responses and the production of chemerin could then take on a chemotactic function, the presence of chemerin in adipocytes points towards a new and completely different role in human physiology: autocrine and paracrine signaling.
4.2 - An Adipokine, Autocrine, and Paracrine Agent
The second major role of chemerin concerns adipose tissue, both in regulating lipid metabolism and adipocyte growth. This function of chemerin surfaced in 2007 (almost 10 years after discovering its function in the immune system) after physicians started to note the associations between chemerin and obesity. One of the major sites of chemerin production is the liver [34] which has been confirmed by in vitro hepatocyte cultures [35]. Both chemerin and ChemR23 are found in high concentrations in white adipose tissue (WAT). Using 3T3-L1 cultures, differentiated adipose tissue produces prochemerin and processes it to an active form. It was not investigated how this process happens in adipose tissue but it is predicted to be intracellularly cleaved (which would be a novel process for chemerin) or to be secreted along with serine proteases [36]. The autocrine/paracrine functions of chemerin were also suggested at this early time because active chemerin was produced at levels well above physiologic minimums for the also expressed ChemR23 receptor. This action seemed to be based on the state of differentiation in the adipocyte. Therefore, it was hypothesized chemerin played a role in adipogenesis and development. To support this, chemerin and ChemR23 expression were knocked down before, during, and after adipocyte differentiation. If done before, the adipocytes did not develop. If done during the maturation cycle, lipid regulators like GLUT4 were reduced [36].
The same article maintained that chemerin and ChemR23 are expressed at their highest levels in mature adipocytes. If chemerin and its receptor are knocked down at this stage, the morphology of the cells change indicating a continuing role of chemerin in the mature adipocyte [36]. This role is supported by others who have found mRNA levels of both chemerin and its receptor at its highest in the mature state and even higher if fed a high fat diet [37]. Human studies also found a positive correlation between chemerin and LDL cholesterol and a negative correlation between chemerin and HDL cholesterol in obese patients [38].
Although there is some agreement that both chemerin and its receptor are increased during differentiation [39], there are some who have found ChemR23 levels to decrease during differentiation and reach their lowest levels in the fully differentiated form [34]. Most experiments were done using the 3T3-L1 cell line but regardless, we know there are vast differences in chemerin and adipocyte expression when it comes to location within the body: within different levels of fat, WAT vs. brown adipose tissue [36] or subscapular vs. visceral, and also between different visceral organs [34]. The vast majority of data appears point towards abdominal visceral WAT as being the largest determinant for the variance in serum chemerin levels [34,40] while the liver continues to be the largest overall producer [34]. ChemR23 expression also appears to play a vital role in the perivascular adipose tissue as agonism with active chemerin can cause contraction in the smooth muscle of blood vessels [25].
Additional evidence suggests chemerin levels may also vary with the time of day. Studies in mice in which serum chemerin levels were continuously monitored showed peaks during the day and troughs at night that may correspond with eating habits and levels of starvation. It was specifically noted that researchers working with mice and chemerin should consider this variable when planning their sample collection [35]. Further studies in the human, however, have refuted this pattern and cited the discrepancy as a fundamental difference between species [41]. Although the mouse and human ChemR23 receptor retain over 80% homology [5], this discrepancy between animal models elucidates an important caution when designing chemerin experiments.
Central to the large variety of circumstances where chemerin is expressed or down regulated as an adipokine, is how this action of the adipokine relates to adipocyte pathology. In addition to chemerin's role in adipocyte differentiation, incubation of 3T3-L1 cells with chemerin increased insulin-dependent uptake of glucose uptake by 41% [39]. Furthermore, chemerin and its receptor have been identified in β-islet cells of the pancreas. Chemerin deficiency in these cells down-regulate expression of human musculoaponeurotic fibrosarcoma oncogene homolog A (MafA, a transcription factor needed for normal functioning and deficiency will cause glucose intolerance) [42]. There has been one report citing that the presence of chemerin down-regulates glucose uptake [43], however, the preponderance of evidence, including that derived from ChemR23 and chemerin knockouts [36,39,44], suggest chemerin's role as supporting normal glucose uptake. Either way, the regulation of lipid and glucose metabolism exists on more than one level.
Adipokines in general have the ability to act on cells other than adipocytes as seen with leptin [45] and adiponectin [46] and their activity in the reproductive axis. Chemerin was discovered in the ovary in 2003 [7,8] but later expanded to the placenta [36] and almost the entire ovary including follicles, follicular fluid, granulosa, theca, corpus luteum, and cortex [47,48]. Human ChemR23 [47] and bovine GPR1 and CCRL2 [48] have been found on granulosa and theca cells and suggests an autocrine/paracrine function for chemerin. Bovine ChemR23 was also identified on ovarian cumulus cells [48]. In the human, chemerin decreases Insulin-like Growth Factor 1 (IGF1)-induced production of progesterone and estradiol. Chemerin was also found in higher concentrations in the follicular fluid just before oocyte release. However, the researchers measured total chemerin, including active and the inactive precursors so the exact effects of active chemerin are unclear [47]. In the bovine ovary, insulin, IGF1, and insulin sensitizers increase chemerin mRNA production but decreased mRNA production of all chemerin's receptors while TNFα and adiponectin increased both chemerin and ChemR23. More importantly, chemerin displayed the ability to decrease follicle-stimulating hormone (FSH)-induced steroidogenesis through ChemR23 and arrested nuclear development of the oocytes [48]. These results need to be replicated in the human but could have a large potential impact on our understanding of reproductive pharmacology.
4.3 - A Growth Factor
With the knowledge of chemerin's association to obesity and metabolic syndrome, scientists investigated possible genetic factors involved in chemerin expression and regulation. Their first finding was that 25% of variation in serum chemerin levels was hereditary or genetic (n = 1354 individuals) [49]. This led to an investigation of the specific single nucleotide polymorphisms involved. The strongest association between plasma chemerin levels and a genetic factor was found in epithelial growth factor-like repeats and discoidin I-like domains 3 (EDIL3) which plays a role in angiogenesis. These findings were supported with subsequent coculture studies in which chemerin successfully induced the growth of capillary-like structures in a concentration dependent manner using ERK1/2 [49]. Another report published at the same time (also identifying chemerin and its role in angiogenesis) supports the angiogenic nature of chemerin through MAPKs but adds that there was also induction of MMPs [26]. This role in angiogenesis along with its known role in adipocytes fosters the belief that chemerin may support adipogenesis through the expansion of capillary blood flow.
Chemerin's role as a growth factor in osteogenesis was discovered when the protein became associated with osteoporosis. In this disease, the balance between adipogenesis and osteoblastogenesis is shifted towards adipogenesis. Because of this dysregulation, it is believed that adipogenic compounds may influence osteoblast function. In knockdowns of chemerin and ChemR23 (created by small hairpin RNA targeting), there was increased osteoblast expression and mineralization in bone marrow stromal cells [44].
Dysregulation of chemerin has been noted in certain tumor progressions [50-52], but with reports of chemerin's involvement in angiogenesis emerging rather recently, researchers have only started to investigate possible connections between the two. In squamous cell carcinoma of the oral tongue, chemerin has been associated with poor prognosis, poor differentiation, metastasis, and increased microvessel density [53]. Although the mechanisms underlying all these findings need to be substantiated and investigated, these associations with cancer automatically heighten the threat level of chemerin from just a slow working adipokine to a cancer-enabling angiogenic growth factor.
5 - Pathophysiology
When describing the roles of chemerin with respect to physiology, it is convenient to separate them into chemokine, adipokine, and growth factor roles. But when evaluating the number of different pathologies chemerin has been associated with, these divisions often merge and chemerin plays multiple roles at once.
5.1 - Psoriasis
The relationship between psoriasis and chemerin has been a controversial one. As previously noted, when chemerin was first discovered it was associated with maintaining normal skin physiology [1]. However, more was discovered about the mechanisms of chemerin and the characteristics of psoriasis, the scene began to change. Psoriasis is known to be T-cell mediated and driven by interferons produced by pDC recruitment. With this information, chemerin was hypothesized to be linked to psoriasis through pDCs, which was later supported by data showing increased expression in early lesions, especially in fibroblasts [54]. The association of high chemerin levels with psoriatic lesions has been further supported [55] with some expanding upon the method with which chemerin becomes active in the human skin [56]. This trend of elevated chemerin in epithelial lesions holds true for both Crohn's disease and ulcerative colitis [57] so it is possible that at the discovery of chemerin, scientists mistook prochemerin for the active form. Conversely, there have been some studies in lung epithelium that chemerin S157 may be anti-inflammatory [58] (this contrasts the report by Cash et al. where he described the synthetic analog of chemerin A154 as being anti-inflammatory [18]). Because many of the studies were only epidemiological, we do not know if the anti-inflammatory forms of chemerin (as previously described) are present in certain types of epithelium, but clearly, there is need for research into the pharmacology of chemerin and its receptors in different epithelial regions. As for psoriasis, the current research points towards a positive correlation with circulating chemerin levels and an inflammatory component of this protein.
5.2 - Rheumatoid Arthritis
Similar to psoriasis, the relationship of rheumatoid arthritis (RA) and chemerin to dendritic cells prompted investigation into a possible relationship between the chemokine and pathology. Compared to osteoarthritis, chemerin and ChemR23 are highly expressed in RA synovial fluid with high production by local fibroblasts [59] and could be used as a biomarker of RA [60]. Because of the wide variety of other pathologies that may increase serum chemerin, its use as a biomarker for RA is not practical, but the chemerin expansion beyond the synovial fluid indicates chemerin may be involved in the global recruitment of the immune cells. These global effects, however, may play a role in the adipokine function of chemerin. Dessein et al. reports that RA can be linked to an increased risk of cardiovascular disease, particularly atherosclerosis. They also report that serum chemerin is a positive predictive measure of this increased risk [61].
5.3 - Non-Alcoholic Fatty Liver Disease
In the case of non-alcoholic fatty liver disease (NAFLD), both inflammation and metabolic homeostasis are known to play vital roles in the progression to steatosis, hepatitis and cirrhosis. In patients with NAFLD, chemerin is positively associated with the disease itself as well as the progression through the stages. The homeostasis model assessment for insulin resistance (HOMA-IR) was similarly associated. Based on these findings, Kukla et al. hypothesized that chemerin may play a role in the pathogenesis (both inflammation and insulin resistance) of NAFLD leading to lipid deposition [62]. These findings have been supported in the setting of obese children [63] and adults [64] and chemerin has been proposed as an effective biomarker for predicting advanced steatosis. However, these results require caution because chemerin is also produced in large quantities in the liver [34]. Further research is needed to determine if chemerin is actually leading to the deposition of fat in the liver or if it is simply a product of the fat and inflammation.
5.4 - Obesity
Although obesity can present with NAFLD, obesity itself and the subsequent physiologic parameters associated with obesity are often positively associated with serum chemerin levels. Articles often site body mass index (BMI) and C-reactive protein (CRP) to be positively correlated to chemerin and high-density lipoproteins (HDLs) to be negatively correlated [64]. Increased chemerin presents in childhood obesity [65] but the parameters of BMI, CRP, and triglycerides were also confirmed in children along with increased endothelial activation of ICAM-1 and E-Selectins [66]. Additional evidence for the link between chemerin and obesity can be seen when obesity is largely eliminated (mostly through bariatric surgery) and chemerin levels decrease [67] along with triglycerides, HOMA-IR, and blood glucose levels [64]. Some measures of inflammation and pathology, like adiponectin, can be difficult to interpret because of the discrepancies between reports [64,68] but on average, chemerin maintains its association with obesity. One implication from these data is that the high circulating levels of chemerin may derive from the excess adipose tissue found in obesity which then may cause certain other pathologies like insulin sensitivity. To quote Chakaroun et al., “reduced adipose tissue chemerin expression may contribute to improved insulin sensitivity and subclinical inflammation beyond significant weight loss” [67]. This is a good example of physicians in an epidemiological setting trying to make correlative statements without the support of basic research. This is not to discourage hypotheses, but rather to point out the significant lack of basic research that could be used to match correlational findings to causative theories.
5.5 - Obesity-related Comorbidities
Often paired with obesity is type II diabetes. Normal weight type II diabetics also exhibit elevated chemerin and CRP levels indicating a more inflammatory role for chemerin rather than that of the adipokine [57]. But the data in type II diabetes seem to be more conflicting compared to other pathologies, with reports failing to observe differences between serum chemerin in normal and type II diabetic patients [34] and one study observing that chemerin is only elevated in those with macroalbuminuria [69].
Metabolic syndrome presents with raised blood pressure, dyslipidemia, raised fasting glucose, and central obesity. A diagnosis is made by the presence of three of the following: elevated waist circumference, elevated triglycerides, reduced HDL-C, elevated blood pressure, or elevated fasting glucose [70]. Many of the parameters associated with NAFLD and obesity still apply but new to metabolic syndrome are the positive correlations between blood pressure (both systolic and diastolic) [71,72], dyslipidemia [38,68], and coronary artery disease [72]. Common to the hypertension, atherosclerotic, and other cardiovascular components of metabolic syndrome is smooth muscle. A recent report remarks that the ChemR23 receptor is found both on the endothelium of blood vessels and also on their underlying smooth muscle layers [25]. This has two implications: first, agonism of ChemR23 may cause alterations in vascular tone, potentially leading to pathologies like hypertension; and second, pathologies with damaged endothelium may expose ChemR23 receptors on smooth muscle cells leading to the progression seen in atherosclerosis. A recent report has confirmed via immunohistochemistry that ChemR23 is present on the smooth muscle and foam cells in human atherosclerotic lesions [73]. An additional article adds that chemerin reduces NO-induced relaxation and cGMP signaling in the vasculature [74], adding weight to the argument that chemerin is involved in atherosclerosis, hypertension, and overall metabolic syndrome.
Related to hypertension, pregnant women with preeclampsia have increased serum chemerin but also have increased levels of chemerin six months after pregnancy [75]. Possible explanations for the chemerin increase after pregnancy could be metabolic patterns independent of the pregnancy (some women had a pre-pregnancy BMI of 43 kg/m2) but women with diabetes and renal problems were excluded from the study. This relationship to preeclampsia and continually elevated chemerin levels is reminiscent of the previously mentioned hereditability of chemerin [49]. If, in fact, chemerin can agonize ChemR23 and cause hypertension, than persistently elevated levels caused by an inherited trait could lead to the preeclampsia seen in these cases with elevated levels well after delivery.
5.6 - Cancer
The mechanism associating chemerin with cancer, specifically tumor metastasis, is angiogenesis. In cases of gastric cancer, patients were observed with elevated serum chemerin levels but these concentrations also positively correlated to the stage of the cancer progression. It was delineated that chemerin promotes tumor invasion through phosphorylation of p38 and ERK1/2 which increases levels of VEGF, MMP-7, and IL-6 [76]. Squamous cell carcinoma of the tongue (SCCOT) has also been associated with chemerin but more specifically to microvessel density [53]. It is of no doubt that with chemerin's involvement in the basic metastasizing mechanisms of tumors, it will be associated with a number of cancers and possibly cancer treatments. Although we can confidently associate chemerin with angiogenesis, its association with neoplasms in general is more difficult to understand because numerous reports find chemerin down-regulated in melanomas,[50] skin squamous cell carcinoma [51], and hepatocellular carcinoma [52]. Given the diversity of neoplasms and their development, these contradictory findings are hardly surprising.
6 - Evaluating Chemerin in the Cardiovascular System
The cardiovascular system is inherently difficult to understand because it has functions of its own (e.g. endothelial cells) but is also intertwined in every other body system. Over the past two centuries, chemerin has been described as a chemokine, adipokine, paracrine/autocrine agent, and growth factor. Every time chemerin is described in one of these roles, there are relations made to the cardiovascular system but always in a secondary manner: as a chemokine chemerin allows for chemoattraction through the vasculature [8], changes endothelial adhesion levels [33], and is extracellularly activated in the lumen [8]; as an adipokine chemerin adjusts lipid [36] and glucose levels (through glucose intolerance) [42] possibly altering their infiltration into endothelium; and as a growth factor it promotes microvessel growth to support adipocytes [49] and effects osteoblastogenesis [44]. Understanding chemerin as a more global protein with diverse actions both within the cardiovascular system and without will allow us to better understand the pathologies and apply proper treatments.
6.1 - What Do We Know About Chemerin in the Cardiovascular System?
Chemerin is activated by proteases involved with the coagulation, fibrinolytic, and other inflammatory cascades [15]. With one of the roles of chemerin being a chemoattractant, this would allow chemerin to radiate from a point of injury and interact with macrophages, dendritic cells, and natural killers [5,6,8,12,31]. Similarly, the adhesion molecules of ICAM-1 and E-selectin, which act on endothelium, are also induced by chemerin [66]. The same article that cites ICAM-1 expression notes the lack of VCAM-1 expression but others have gone as far to say that VCAM-1 expression may be inhibited leading to an anti-inflammatory role [33].
There is also small molecule regulation on the level of MMPs. Chemerin stimulation has lead to the induction of MMP-2, MMP-9,[26] and MMP-7 [76]. This suggests chemerin can influence the growth and remodeling of blood vessels [26,49] which has been supported with in vitro experiments [76]. The application of this remodeling is in both adipogenesis[36] and tumor metastasis [53,76]. Although the mechanisms of most pathologies like hypertension [71,72], preeclampsia [75], and coronary artery disease [72] have not been thoroughly investigated in their relation to chemerin, they still have known positive associations with chemerin levels. The perivascular adipose tissue and chemerin receptors on vascular smooth muscle are proving to play important roles in hypertension because of its ability to induce tone and smooth muscle contraction.
6.2 - What Chemerin Mechanisms of Known Associated Pathologies are Uncertain?
Chemerin is predicted to be highly involved in the pathology of hypertension but there is still quite a bit we have yet to learn about its mechanisms. In their review of hypertension and immunity Anders et al. conclude, “the contribution of immunity to hypertension is mostly likely rather a secondary phenomenon upon vascular injury.” They also state, “hypertension activates dendritic cells ... that in turn aggravate hypertension-related adaptive immunity, which further aggravates hypertension” [77]. These two conclusions are important because it suggests that chemerin has the ability to act through dendritic cells (as previously supported) but only when secondary to vascular injury. This conclusion can be rationalized by evidence that chemerin is activated by proteases of damage and inflammation. So in the presence of inflammation, activated chemerin can activate dendritic cells and influence hypertension. The missing link in this scenario, however, is that between the immunity and the hypertension. There is evidence that chemerin is interacting with smooth muscle of the vasculature [25,73], but an immune axis of hypertension may also be responsible and should be investigated as we learn more about the general associations between hypertension and immunity.
Preeclampsia is another cardiovascular pathology that could greatly benefit from basic research investigating the role of chemerin. Already there are correlations between preeclampsia and serum chemerin levels [75]. Similar to hypertension, preeclampsia involves high blood pressure and vascular tone. However, as Duhig and Shennan pointed out in their recent review, an emerging and significant factor in this disease is angiogenesis. Abnormal spiral artery remodeling in placentation causes the hypertensive vascular response, which is then used in the diagnosis [78]. Seeing as how chemerin is involved in both angiogenesis and the hypertensive response, it is a prime target for study of the specific internal mechanisms.
Atherosclerosis, and all its associated pathologies, provides another instance where chemerin has been epidemiologically associated [61,72] but could be participating on more than one level. Because of the receptors presence on macrophages [5,8] and chemerin's activity in inflammatory cascades [15], it is likely to be potentiating the macrophage activity in the damaged tissue and assisting immune cell migration to the site of injury. ChemR23s presence on smooth muscle cells [25,73] could help establish the injury in the first place through abnormal contraction or progress the disease by acting on the cells once the fatty streak is established. This hypothesis has been partially supported by evidence of chemerin-induced NO dysregulation [74] but its relation to vascular pathology needs to be proven in vivo and this signaling disruption needs to be replicated in human tissue. Chemerin's role in adipogenesis and lipid metabolism [36] is also a candidate for the progression of this disease. Additionally, as an atherosclerotic plaque develops and creates a necrotic core, it requires a blood supply. Its regulation of MMPs and other growth factors [26,76] could allow for the dangerous late stage progression to a thrombus or embolus. Like preeclampsia, chemerin in atherosclerosis has the ability to be involved in every stage of the pathology making it a prime suspect for basic research.
6.3 - What New Roles Could Chemerin Have in the Cardiovascular System?
The three previous pathologies have already been associated with elevated serum chemerin levels but one possibly new cell type where chemerin has yet to be investigated is pericytes. Pericytes are the mural cells of the endothelium and are involved in a number of important regulatory functions: they are contractile, stabilize vessels in angiogenesis, regulate permeability, and can act as a stem cell for white adipose tissue [79]. All of these actions have strong connections to the actions of chemerin. Because of the importance of the pericyte to angiogenesis and chemerin's association as a growth factor, it is plausible that they would display chemerin receptors. The fact that these pericytes can give rise to perivascular adipocytes [79] also indicates a possible adipokine role for chemerin if the proper receptors are present on the pericyte. Regulation of permeability during inflammation is less likely to be influenced by chemerin because chemerin acts as more of chemotactic agent to immune cells. To date, there are no articles the authors are aware of that even consider chemerin as being associated with pericytes. Considering chemerin as a possible ligand for pericyte interaction could enhance our knowledge of the workings of the protein in the microvasculature.
Another possible role of chemerin in the cardiovascular system involves the chemerin receptor GPR1. Chemerin has high affinity for this receptor and depending on the isotype, chemerin will bind with more affinity to GPR1 than it will to ChemR23 [3]. But before GPR1 and chemerin were ever associated, GPR1 was related to opioid receptors and found in both the rat and human hippocampus [2]. Active chemerin peptides have also been found in cerebral spinal fluid [17]. Although there may be other ligands for GPR1 in the brain, chemerin's high affinity for this receptor raises the question of its interaction with the blood/brain barrier and/or its production in the brain itself. Certainly this barricade must be very selective against chemokines and proteins that interact with the immune system, but the other proven functions of chemerin could certainly have implications on the physiology of the brain. Thus, chemerin's presence and function in the vasculature of the brain is a promising new field.
One last possible role for chemerin is related to its angiogenic potential. This function has been noted in tumor metastasis but it should not be limited to just one pathology, especially if there are other compounding factors, like insulin resistance or obesity where chemerin might also be involved. Cases like diabetic impaired wound healing or diabetic retinopathy where chemerin is already active in altered sugar metabolism may then go on to alter angiogenesis leading to these disease states.
In conclusion, chemerin is a global player in human physiology and pathology. Until there is more basic research on this protein, it has potential for involvement in a myriad of diseases. A particular target of research should be the cardiovascular system because elucidation of mechanisms in this area can directly lead to clinical applications of known pathologies and help integrate the current knowledge of chemerin's diverse roles.
Acknowledgements
Thank you to Dr. John Castellot of Tufts University for his guidance and review of this manuscript.
Abbreviations
- AP-4
Adaptor protein-4 complex
- BMI
Body mass index
- CRP
C-reactive protein
- CPB
Carboxypeptidase B
- CPN
Carboxypeptidase N
- CVS
Cardiovascular system
- C/EBP
CCAAT/enhancer binding protein
- CCL
CC chemokine ligands
- CSF
Cerebrospinal fluid
- ChemR23
Chemerin Receptor 23
- CMKRL3
Chemoattractant receptor-like 3
- CCRL2
Chemokine (CC motif) receptor-like 2
- CMKLR1
Chemokine-like receptor 1
- DC
Dendritic cell
- DEZ
Actual name
- EDIL3
Discoidin I-like domains 3
- ERK1/2
Extracellular signal-regulated kinase 1 and 2
- GPR1
G protein-coupled receptor 1
- GATA
Nucleotide sequence
- GLUT4
Glucose transporter type 4
- HDL
High-density lipoprotein
- HOMA-IR
Homeostasis model assessment for insulin resistance
- HCR
Human chemokine receptor
- HIV
Human immunodeficiency virus
- IGF1
Insulin-like Growth Factor 1
- ICAM-1
Intercellular adhesion molecule 1
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- IL-12
Interleukin 12
- KLK7
Kallikrein 7
- LPS
Lipopolysaccharide
- LDL
Low-density lipoprotein
- MMP
Matrix Metalloproteinase
- MAPK
Mitogen-activated protein kinase
- MCP
Monocyte chemotactic protein
- MafA
Musculoaponeurotic fibrosarcoma oncogene homolog A
- mDC
Myeloid dendritic cell
- NK
Natural killer cell
- NAFLD
Non-alcoholic fatty liver disease
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OM
Omental fat
- pDC
Plasmacytoid dendritic cell
- Akt
Protein kinase B
- PKC
Protein kinase C
- RvE1
Resolvin E1
- RARRES2
Retinoic acid receptor responder gene 2
- RA
Rheumatoid arthritis
- SIV
Simian immunodeficiency virus
- SP-1
Specificity protein-1
- SCCOT
Squamous cell carcinoma of the tongue
- SspB
Staphopain B
- Subcutaneous fat
SC *subscapular fat is also referenced in the text but is not referred to by “SC”
- TIG2
Tazarotene-induced gene 2
- TNFα
Tumor Necrosis Factor α
- VCAM
Vascular cell adhesion molecule
- VEGF
Vascular endothelial growth factor
- WAT
White adipose tissue
References
- 1.Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, Teng M, Duvic M, Chandraratna RAS. Tazarotene-induced gene 2 (tig2), a novel retinoid-responsive gene in skin. Journal of Investigative Dermatology. 1997;109:91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 2.Marchese A, Docherty JM, Nguyen T, Heiber M, Cheng R, Heng HH, Tsui LC, Shi X, George SR, O'Dowd BF. Cloning of human genes encoding novel g protein- coupled receptors. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- 3.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantz I, Konda Y, Yang YK, Miller DE, Dierick HA, Yamada T. Molecular cloning of a novel receptor (cmklr1) with homology to the chemotactic factor receptors. Cytogenet Cell Genet. 1996;74:286–290. doi: 10.1159/000134436. [DOI] [PubMed] [Google Scholar]
- 5.Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms RW, Parmentier M. Chemr23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for siv and some primary hiv-1 strains. European Journal of Immunology. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. The Journal of Immunology. 2004;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 7.Meder W, Wendland M, Busmann A, Kutzleb C, Spodsberg N, John H, Richter R, Schleuder D, Meyer M, Forssmann WG. Characterization of human circulating tig2 as a ligand for the orphan receptor chemr23. FEBS Letters. 2003;555:495–499. doi: 10.1016/s0014-5793(03)01312-7. [DOI] [PubMed] [Google Scholar]
- 8.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Methner A, Hermey G, Schinke B, Hermans-Borgmeyer I. A novel g protein-coupled receptor with homology to neuropeptide and chemoattractant receptors expressed during bone development. Biochem Biophys Res Commun. 1997;233:336–342. doi: 10.1006/bbrc.1997.6455. [DOI] [PubMed] [Google Scholar]
- 10.Owman C, Lolait SJ, Santen S, Olde B. Molecular cloning and tissue distribution of cdna encoding a novel chemoattractant-like receptor. Biochem Biophys Res Commun. 1997;241:390–394. doi: 10.1006/bbrc.1997.7822. [DOI] [PubMed] [Google Scholar]
- 11.Fan P, Kyaw H, Su K, Zeng Z, Augustus M, Carter KC, Li Y. Cloning and characterization of a novel human chemokine receptor. Biochem Biophys Res Commun. 1998;243:264–268. doi: 10.1006/bbrc.1997.7981. [DOI] [PubMed] [Google Scholar]
- 12.Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, Song CH, Galli SJ, Butcher EC. Mast cell-expressed orphan receptor ccrl2 binds chemerin and is required for optimal induction of ige-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205:2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martensson UE, Owman C, Olde B. Genomic organization and promoter analysis of the gene encoding the mouse chemoattractant-like receptor, cmklr1. Gene. 2004;328:167–176. doi: 10.1016/j.gene.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The c-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 15.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y, Du XY, Zhao L, Morser J, Leung LL. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, chem157s, which functions as a signaling molecule in glioblastoma. J Biol Chem. 2011;286:39510–39519. doi: 10.1074/jbc.M111.258921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Yamaguchi Y, Sharif S, Du XY, Song JJ, Lee DM, Recht LD, Robinson WH, Morser J, Leung LL. Chemerin158k protein is the dominant chemerin isoform in synovial and cerebrospinal fluids but not in plasma. J Biol Chem. 2011;286:39520–39527. doi: 10.1074/jbc.M111.258954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through chemr23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du XY, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL. Regulation of chemerin bioactivity by plasma carboxypeptidase n, carboxypeptidase b (activated thrombin-activable fibrinolysis inhibitor), and platelets. J Biol Chem. 2009;284:751–758. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillabert A, Wittamer V, Bondue B, Godot V, Imbault V, Parmentier M, Communi D. Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J Leukoc Biol. 2008;84:1530–1538. doi: 10.1189/jlb.0508322. [DOI] [PubMed] [Google Scholar]
- 21.Zhou JX, Liao D, Zhang S, Cheng N, He HQ, Ye RD. Chemerin c9 peptide induces receptor internalization through a clathrin-independent pathway. Acta Pharmacol Sin. 2014;35:653–663. doi: 10.1038/aps.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimamura K, Matsuda M, Miyamoto Y, Yoshimoto R, Seo T, Tokita S. Identification of a stable chemerin analog with potent activity toward chemr23. Peptides. 2009;30:1529–1538. doi: 10.1016/j.peptides.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin e1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin a(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol. 2013;33:1320–1328. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (chemr23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 27.Rourke JL, Muruganandan S, Dranse HJ, McMullen NM, Sinal CJ. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J Endocrinol. 2014;222:201–215. doi: 10.1530/JOE-14-0069. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann TN, Leick M, Ewers S, Diefenbacher A, Schraufstatter I, Honczarenko M, Burger M. Human b cells express the orphan chemokine receptor cram-a/b in a maturation-stage-dependent and ccl5-modulated manner. Immunology. 2008;125:252–262. doi: 10.1111/j.1365-2567.2008.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leick M, Catusse J, Follo M, Nibbs RJ, Hartmann TN, Veelken H, Burger M. Ccl19 is a specific ligand of the constitutively recycling atypical human chemokine receptor cram-b. Immunology. 2010;129:536–546. doi: 10.1111/j.1365-2567.2009.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Della Chiesa M, Sivori S, Castriconi R, Marcenaro E, Moretta A. Pathogen- induced private conversations between natural killer and dendritic cells. Trends Microbiol. 2005;13:128–136. doi: 10.1016/j.tim.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of nk and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 32.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to hiv-1 infection in caucasian individuals bearing mutant alleles of the ccr-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 33.Yamawaki H, Kameshima S, Usui T, Okada M, Hara Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem Biophys Res Commun. 2012;423:152–157. doi: 10.1016/j.bbrc.2012.05.103. [DOI] [PubMed] [Google Scholar]
- 34.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 35.Parlee SD, Ernst MC, Muruganandan S, Sinal CJ, Goralski KB. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor- {alpha}. Endocrinology. 2010;151:2590–2602. doi: 10.1210/en.2009-0794. [DOI] [PubMed] [Google Scholar]
- 36.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 37.Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S. Chemerin--a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007;362:1013–1018. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 38.Lorincz H, Katko M, Harangi M, Somodi S, Gaal K, Fulop P, Paragh G, Seres I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin Endocrinol (Oxf) 2014;81:370–377. doi: 10.1111/cen.12363. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3t3-l1 adipocytes. FEBS Lett. 2008;582:573–578. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, Lee JW. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol (Oxf) 2012;77:47–50. doi: 10.1111/j.1365-2265.2011.04217.x. [DOI] [PubMed] [Google Scholar]
- 41.Chamberland JP, Berman RL, Aronis KN, Mantzoros CS. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation, and lacks day/night variation in humans. Eur J Endocrinol. 2013;169:453–462. doi: 10.1530/EJE-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi M, Okimura Y, Iguchi G, Nishizawa H, Yamamoto M, Suda K, Kitazawa R, Fujimoto W, Takahashi K, Zolotaryov FN, Hong KS, Kiyonari H, Abe T, Kaji H, Kitazawa S, Kasuga M, Chihara K, Takahashi Y. Chemerin regulates beta-cell function in mice. Sci Rep. 2011;1:123. doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept. 2009;154:102–106. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Muruganandan S, Roman AA, Sinal CJ. Role of chemerin/cmklr1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res. 2010;25:222–234. doi: 10.1359/jbmr.091106. [DOI] [PubMed] [Google Scholar]
- 45.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: A review. Fertil Steril. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: Implications for female fertility and obesity. Reproduction. 2005;130:583–597. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 47.Reverchon M, Cornuau M, Rame C, Guerif F, Royere D, Dupont J. Chemerin inhibits igf-1-induced progesterone and estradiol secretion in human granulosa cells. Hum Reprod. 2012;27:1790–1800. doi: 10.1093/humrep/des089. [DOI] [PubMed] [Google Scholar]
- 48.Reverchon M, Bertoldo MJ, Rame C, Froment P, Dupont J. Chemerin (rarres2) decreases in vitro granulosa cell steroidogenesis and blocks oocyte meiotic progression in bovine species. Biol Reprod. 2014;90:102. doi: 10.1095/biolreprod.113.117044. [DOI] [PubMed] [Google Scholar]
- 49.Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, Morrison S, Carless M, Dyer TD, Cole SA, Goring HH, Moses EK, Walder K, Cawthorne MA, Blangero J, Jowett JB. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pachynski RK, Zabel BA, Kohrt HE, Tejeda NM, Monnier J, Swanson CD, Holzer AK, Gentles AJ, Sperinde GV, Edalati A, Hadeiba HA, Alizadeh AA, Butcher EC. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012;209:1427–1435. doi: 10.1084/jem.20112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y, Luo S, Wang G, Peng Z, Zeng W, Tan S, Xi Y, Fan J. Downregulation of tazarotene induced gene-2 (tig2) in skin squamous cell carcinoma. Eur J Dermatol. 2008;18:638–641. doi: 10.1684/ejd.2008.0511. [DOI] [PubMed] [Google Scholar]
- 52.Lin W, Chen YL, Jiang L, Chen JK. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clinical Laboratory. 2011;57:879–885. [PubMed] [Google Scholar]
- 53.Wang N, Wang QJ, Feng YY, Shang W, Cai M. Overexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongue. Clin Oral Investig. 2014;18:997–1004. doi: 10.1007/s00784-013-1046-8. [DOI] [PubMed] [Google Scholar]
- 54.Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen JD, Parmentier M, De Pita O, Girolomoni G, Sozzani S. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima H, Nakajima K, Nagano Y, Yamamoto M, Tarutani M, Takahashi M, Takahashi Y, Sano S. Circulating level of chemerin is upregulated in psoriasis. J Dermatol Sci. 2010;60:45–47. doi: 10.1016/j.jdermsci.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Schultz S, Saalbach A, Heiker JT, Meier R, Zellmann T, Simon JC, Beck-Sickinger AG. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in c-terminal rearrangement. Biochem J. 2013;452:271–280. doi: 10.1042/BJ20121880. [DOI] [PubMed] [Google Scholar]
- 57.Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72:342–348. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 58.Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse chemr23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 59.Kaneko K, Miyabe Y, Takayasu A, Fukuda S, Miyabe C, Ebisawa M, Yokoyama W, Watanabe K, Imai T, Muramoto K, Terashima Y, Sugihara T, Matsushima K, Miyasaka N, Nanki T. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R158. doi: 10.1186/ar3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ha YJ, Kang EJ, Song JS, Park YB, Lee SK, Choi ST. Plasma chemerin levels in rheumatoid arthritis are correlated with disease activity rather than obesity. Joint Bone Spine. 2014;81:189–190. doi: 10.1016/j.jbspin.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Dessein PH, Tsang L, Woodiwiss AJ, Norton GR, Solomon A. Circulating concentrations of the novel adipokine chemerin are associated with cardiovascular disease risk in rheumatoid arthritis. J Rheumatol. 2014;41:1746–1754. doi: 10.3899/jrheum.140122. [DOI] [PubMed] [Google Scholar]
- 62.Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, Ciupinska-Kajor M, Berdowska A, Wozniak-Grygiel E, Buldak R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2010;45:235–242. doi: 10.3109/00365520903443852. [DOI] [PubMed] [Google Scholar]
- 63.Klusek-Oksiuta M, Bialokoz-Kalinowska I, Tarasow E, Wojtkowska M, Werpachowska I, Lebensztejn DM. Chemerin as a novel non-invasive serum marker of intrahepatic lipid content in obese children. Italian Journal of Pediatrics. 2014:40. doi: 10.1186/s13052-014-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 65.Schipper HS, Nuboer R, Prop S, van den Ham HJ, de Boer FK, Kesmir C, Mombers IM, van Bekkum KA, Woudstra J, Kieft JH, Hoefer IE, de Jager W, Prakken B, van Summeren M, Kalkhoven E. Systemic inflammation in childhood obesity: Circulating inflammatory mediators and activated cd14++ monocytes. Diabetologia. 2012;55:2800–2810. doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- 66.Landgraf K, Friebe D, Ullrich T, Kratzsch J, Dittrich K, Herberth G, Adams V, Kiess W, Erbs S, Korner A. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab. 2012;97:E556–564. doi: 10.1210/jc.2011-2937. [DOI] [PubMed] [Google Scholar]
- 67.Chakaroun R, Raschpichler M, Kloting N, Oberbach A, Flehmig G, Kern M, Schon MR, Shang E, Lohmann T, Dressler M, Fasshauer M, Stumvoll M, Bluher M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61:706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Chu SH, Lee MK, Ahn KY, Im JA, Park MS, Lee DC, Jeon JY, Lee JW. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS One. 2012;7:e34710. doi: 10.1371/journal.pone.0034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:159–163. doi: 10.1016/j.diabres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr. International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, International Association for the Study of O. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 71.Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a caucasian population - a pilot study. Biomedical Papers-Olomouc. 2008;152:217–221. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- 72.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Internal Medicine. 2011;50:1093–1097. doi: 10.2169/internalmedicine.50.5025. [DOI] [PubMed] [Google Scholar]
- 73.Kostopoulos CG, Spiroglou SG, Varakis JN, Apostolakis E, Papadaki HH. Chemerin and cmklr1 expression in human arteries and periadventitial fat: A possible role for local chemerin in atherosclerosis? BMC Cardiovasc Disord. 2014;14:56. doi: 10.1186/1471-2261-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, Tostes RC. Chemerin reduces vascular nitric oxide/cgmp signalling in rat aorta: A link to vascular dysfunction in obesity? Clin Sci (Lond) 2014;127:111–122. doi: 10.1042/CS20130286. [DOI] [PubMed] [Google Scholar]
- 75.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul Pept. 2011;168:69–72. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Wu WK, Liu X, To KF, Chen GG, Yu J, Ng EK. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: A clinical and experimental study. Peptides. 2014;51:131–138. doi: 10.1016/j.peptides.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Anders HJ, Baumann M, Tripepi G, Mallamaci F. Immunity in arterial hypertension: Associations or causalities? Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duhig KE, Shennan AH. Recent advances in the diagnosis and management of pre-eclampsia. F1000Prime Rep. 2015;7:24. doi: 10.12703/P7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Schultz S, Beck-Sickinger AG. Chemerin and vaspin: Possible targets to treat obesity? ChemMedChem. 2013;8:549–559. doi: 10.1002/cmdc.201200448. [DOI] [PubMed] [Google Scholar]
- 81.Kulig P, Kantyka T, Zabel BA, Banas M, Chyra A, Stefanska A, Tu H, Allen SJ, Handel TM, Kozik A, Potempa J, Butcher EC, Cichy J. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J Immunol. 2011;187:1403–1410. doi: 10.4049/jimmunol.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulig P, Zabel BA, Dubin G, Allen SJ, Ohyama T, Potempa J, Handel TM, Butcher EC, Cichy J. Staphylococcus aureus-derived staphopain b, a potent cysteine protease activator of plasma chemerin. The Journal of Immunology. 2007;178:3713–3720. doi: 10.4049/jimmunol.178.6.3713. [DOI] [PubMed] [Google Scholar]