Abstract
Background/Aims
We previously demonstrated that anthocyanin-rich bilberry extract (ARBE) inhibits IFN-γ-induced signalling and downstream effects in human monocytic cells and ameliorates disease activity in ulcerative colitis (UC) patients. Here, we studied the molecular mechanisms of ARBE-mediated effects in vitro and by analysing colonic tissue and serum samples of UC patients treated with an oral anthocyanin-rich bilberry preparation during an open label clinical trial.
Methods
Colon specimens obtained during an open pilot study using ARBE for the treatment of mild-to-moderate UC were analyzed by immunohistochemistry. Cytokine levels in patients’ serum were quantified by ELISA. Cell culture experiments were performed using THP-1 monocytic cells.
Results
ARBE treatment inhibited the expression of IFN-γ-receptor 2 in human THP-1 monocytic cells. Colon biopsies of UC patients who responded to the 6-week long ARBE treatment revealed reduced amounts of the pro-inflammatory cytokines IFN-γ and TNF-α. Levels of phosphorylated (activated) p65-NF-κB were reduced in these patients. Further, patients with successful ARBE treatment featured enhanced levels of Th17-cell specific cytokine IL-22 and immunoregulatory cytokine IL-10 as well as reduced serum levels of TNF-α and MCP-1, but enhanced levels of IL-17A, in contrast to patients that did not reach remission after ARBE treatment.
Conclusions
Our data suggest a molecular mechanism underlying the anti-inflammatory effects of ARBE treatment in UC patients by modulating T-cell cytokine signalling and inhibiting IFN-γ signal transduction. These data are of particular interest, since ARBE is a promising therapeutic approach for the treatment of IBD.
Introduction
Phenols are plant-derived molecules with anti-inflammatory, anti-oxidant, anti-carcinogenic, anti-adipogenic and neuroprotective properties [1,2]. Chemically, they consist of one or more (polyphenols) aromatic ring(s) with at least one hydroxyl group attached. Based on their chemical structure, they are classified into two groups, flavonoids and non-flavonoids. Anthocyanidins form a crucial sub-class of dietary flavonoids and are widespread in fruits and flowers where they account for the blue, purple and red colours. In these plant-derived forms they are commonly conjugated to sugars or organic acids and consequently named anthocyanins [1,2]. Our regular diet contains a variety of natural phenols. Especially berries, red wines, leafy and root vegetables and certain whole grain cereals comprise relative high amounts of anthocyanins. Due to their health-promoting and protective characteristics substantial interest in phenols has emerged lately [3]. Multiple epidemiological studies demonstrated that polyphenol-rich foods prevented diseases like coronary heart disease, certain types of cancer and inflammatory diseases [4,5,6]. The benefits of polyphenols were originally attributed to their anti-oxidant properties. Nevertheless, additional mechanisms such as direct interference with receptor-regulated signalling pathways and gene expression have been postulated [7,8,9].
Ulcerative colitis (UC), a sub-form of inflammatory bowel disease (IBD), is characterized by a chronic and relapsing immune-mediated inflammation of the colon initiated by a dys-regulated immune response to commensal intestinal microbiota and environmental factors in the genetically susceptible host [10,11,12]. Prevalence and incidence of IBD have increased in western countries as well as in less developed countries where lower incidence rates were reported previously [13]. The fact that numerous IBD patients are not satisfactorily treated with the established treatment options or suffer from therapy-related side effects aggravates the burden of IBD [14,15]. Thus, there is a need for more effective, well-tolerated and safe therapeutic options.
Bilberries (Vaccinium myrtillus L.)–closely related to blueberries (Vaccinium corymbosum)–have one of the highest natural anthocyanin content [16]. In a previous study [17] we have confirmed the finding that anthocyanin-rich extracts reduce inflammatory gene expression in vitro [16,18,19]. Moreover, it has been demonstrated that bilberry ingestion and subsequently anthocyanin intake attenuates the severity of experimental colitis and diminishes pro-inflammatory cytokine serum levels in animal models [20,21,22,23]. Based on these findings, our group conducted an open label pilot study in patients with mild to moderate UC (approved by the local ethics committee (EK-1733), trial not registered) [24]. In addition to their standard medication, patients were treated with an anthocyanin-rich bilberry preparation. After 6 weeks, endoscopic and histologic disease activity and fecal calprotectin levels were significantly reduced in the study participants, therefore anthocyanins represent a potential therapeutic option in IBD. In this study, we aimed to further investigate the molecular processes underlying the protective properties of anthocyanins. On one hand, we conducted in vitro experiments with human monocytic THP-1 cells. On the other hand, we further analysed colon biopsies and serum samples from UC patients who had participated in the above discussed open label pilot study by Biedermann et al. [24] focussing on the expression of T-cell derived cytokines.
Materials and Methods
Reagents and Antibodies
All reagents were of analytical grade and obtained commercially. Monoclonal rabbit anti-human phospho-STAT1 (Tyr701; D4A7), polyclonal rabbit anti-human STAT1 were obtained from Cell Signaling Technologies (Danvers, MA, United States).
Human recombinant IFN-γ was obtained from Sigma (Sigma-Aldrich, St. Louis, MO, United States). The anthocyanin-rich bilberry extract (ARBE) was manufactured by Kaden Biochemicals, Symrise GmbH & Co (Holzminden, Germany) and was allocated as a powder (25% anthocyanin content). This powder was dissolved in ddH2O to establish a stock suspension of 10 mg ARBE/ml. Due to sedimentation, the stock suspension was homogenized by strong agitation during 30 minutes prior to each usage. A detailed analysis of the ARBE powder can be found in S1 File.
Patient Samples
Intestinal tissue specimens were taken during the open label bilberry ingestion pilot study by Biedermann et al. [24]. 13 patients with current mild to moderate UC underwent a first sigmoidoscopy with biopsy taking 7 days prior to and 11 patients underwent a second sigmoidoscopy at the last day of the six-week bilberry intake period as described in the aforementioned study [24]. Patient’s serum was taken at the study visits 7 days prior to the beginning of the bilberry treatment (baseline = week 1) and from there on in a 2-week interval (i.e. week 3, week 5 and at the last day of the bilberry intake at week 7). Serum was frozen at -80°C and used for ELISA experiments.
THP-1 Cell Culture, Vector Transduction, Phosphatase Inhibition and Stimulation Protocols
Human monocytic THP-1 cells (DSMZ no. ACC 16, DSMZ, Braunschweig, Germany) were cultured in RPMI 1640 medium (Life technologies, Gibco, Carlsbad, CA, United States) supplemented with 10% fetal calf serum (FCS) at an approximate density of 0.5 to 1 x 106 cells/ml. Cells were maintained in a 5% CO2 and 95% humidified incubator at 37°C. For experiments without siRNA transfection, cells were seeded in 1 ml of FCS-free RPMI 1640 medium + 1% Penicillin/Streptomycin per well at a density of 1 to 1.5 x 106 cells/ml at least 6 hours prior to treatment.
PTPN2 siRNA transfection was performed using 100 pmol of a gene specific siRNA oligonucleotide (Life technologies) and the Amaxa nuclefector system (Lonza, Walkersville, MD, United States) according to the manufacturer’s instructions. After transfection, THP-1 cells were cultured in a 12-well plate for 48 hours prior to treatment.
Pre-treatment with ARBE solution (composed of ARBE stock suspension and FCS-free RPMI 1640 medium + 1% Penicillin/Streptomycin) with a final concentration of 10 μg/ml was conducted 20 min before stimulation. Then, IFN-γ was applied in a previously validated concentration of 100 ng/ml ([25] and S1 Fig) for either 30 min (Western blot experiments) or 24 h (qPCR experiments).
Inhibition of cellular phosphatases was induced by Na3VO4 (Sigma-Aldrich, S6508) according to manufacturer’s recommendations and Gordon J.A. [26]. Na3VO4 in a final concentration of 0.5 mM was added to the THP-1 cells simultaneously with ARBE pre-treatment.
Preparation of Whole Cell Lysates
THP-1 cells were washed twice with phosphate buffered saline (PBS) and lysed in M-PER Mammalian protein extraction reagent (Pierce Biotechnology, Rockford, IL, United States) containing protease inhibitors (Roche, Basel, Switzerland) for 45 min. After centrifugation (10 min at 13,000 g), cell lysate supernatants were assayed for protein content using a NanoDrop spectrophotometer (NanoDrop ND1000; Pierce Biotechnology).
Western Blotting
Each lysate was mixed with loading buffer (NuPAGE® 4x LDS Sample Buffer (Life technologies), 500mM dithiothreitol and boiled for 5 min at 95°C. Separation of the proteins was performed with SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the proteins were transferred onto nitrocellulose membranes (Millipore, Billerica, MA, United States).
Membranes were blocked during 1 h with blocking solution (3% milk powder (C. Roth GmbH+Co. KG, Karlsruhe, Germany) and 1% bovine serum albumin (BSA) (GE Healthcare, PAA Laboratories GmbH, Pasching, Austria) in washing buffer (Tris buffered saline containing 1% Tween 20). Primary antibody was diluted in blocking solution (1:1000 for all experiments). Membranes were incubated in primary antibody solution overnight at 4°C and then washed with washing buffer for 30 min. Horseradish peroxidase (HRP)-labelled secondary anti-mouse- or anti-rabbit-IgG-antibody (1:5000; sc-2005, sc-2004; Santa Cruz Biotechnologies, Inc., Dallas, TX, United States) in blocking solution was added for 1 h and membranes were washed again for 30 min. Immunoreactive proteins were detected using an enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL, United States), and exposure on X-ray films (GE Healthcare, Little Chalfont, UK). Films were scanned and intensity of the protein bands determined using NIH ImageJ software. Densitometry values from proteins were normalized to the corresponding values from non-treated controls. For STAT1, densitometry values from phosphorylated proteins were additionally normalized to the values from the corresponding total proteins.
RNA Isolation and Complementary DNA Synthesis
THP-1 cells were washed with ice-cold phosphate buffered saline (PBS) and disrupted in RLT buffer (Qiagen, Venlo, The Netherlands) and 1 M dithiothreitol solution. Total RNA was isolated using RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. RNA concentration was measured by absorbance at 260 nm (NanoDrop ND1000). Complementary DNA (cDNA) was synthesised using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States) following the manufacturer’s recommendations.
Real-time Polymerase Chain Reaction (PCR)
Real-time PCR was performed using FAST qPCR Master Mix for Taqman Assays (life technologies) on a Fast 7900HT Real-Time PCR system using SDS Software (life technologies). Measurements were performed in triplicate, human β-actin was used as endogenous control, and results were analyzed by ΔΔCT method. The real-time PCR contained 45 cycles consisting of a denaturing (95°C, 1 sec) and an annealing/extending (60°C, 20 sec) step. Gene expression assays were all obtained from life technologies.
Immunohistochemistry
Immunohistochemistral procedures were performed using colon biopsies of eleven study participants with UC. The biopsies were taken in the context of the bilberry study by Biedermann et al. [24]. Additionally, colon biopsies from healthy controls (n = 4), from UC patients with active colitis (n = 1–2) and from UC patients with colitis in remission under conventional therapy (n = 1–2) were investigated. A peroxide-based method on the formalin-fixed and paraffin-embedded tissue specimens was applied. First, the tissue was deparaffinised with HistoClear® (Chemie Brunschwig AG, Basel, Switzerland) and rehydrated in descending concentrations of ethanol (100%, 96%, 70%), double-distilled water and PBS pH 7.2. In order to retrieve the target proteins and to avoid cross-linking reactions the biopsies were boiled in a citrate buffer solution (10mM, pH 6.0, DAKO, Glostrup, Denmark) in a 98°C hot water bath for 30 min. The specimens were cooled down at room temperature and buffer traces were removed by washing the tissue twice in PBS. For inactivation of the numerous endogenous peroxidases present in intestinal tissue the specimens were incubated in a 0.9% H2O2 solution for 15 min at room temperature. After another two washing steps in PBS the specimens were blocked with 1–3% BSA in PBS for 1 h in a humidified chamber at room temperature. Subsequently, the tissue was incubated with polyclonal rabbit anti-human IFN-γ (H-145) IgG (dilution 1:300; concentration 200 μg/ml; sc-8308, Santa Cruz Biotechnology), polyclonal Rabbit anti-human IFN-γ R1 (dilution 1:100; concentration 0.5 mg/ml; HPA029213, Sigma-Aldrich), polyclonal Rabbit anti-human IFN-γ R2 (dilution 1:20; concentration 0.5 mg/ml; HPA001535, Sigma-Aldrich) polyclonal rabbit anti-human Stat1 (dilution 1:400; #9172S, Cell Signaling), monoclonal mouse anti-human TNF-α (52B83) IgG1 (dilution 1:100; concentration 100μg/ml; sc-52746, Santa Cruz Biotechnology), monoclonal rabbit anti-human phospho-NF-κB p65 (Ser536; 93H1) IgG (dilution 1:100; #3033S, Cell Signaling), monoclonal rat anti-human IL10 (JES3-12G8) IgG2a (dilution 1:100, concentration 0.5 mg/ml; Thermo Fisher, Pierce Biotechnology), monoclonal mouse anti-human IL17A (aa1-75, 4K5F6) IgG2b,k (application 7 μg/ml, concentration 0.5 mg/ml; LS-B8323; LifeSpan BioSciences, Inc., Seattle, WA, United States), or polyclonal rabbit anti-human IL22 (dilution 1:500, concentration 1.0 mg/ml; NB-100-737, Novus Biologicals, Littleton, CO, United States), overnight at 4°C in a humidified chamber. After two rinsing steps in PBS the tissue was incubated with corresponding secondary antibodies (ImmPRESSTM HRP anti-mouse (MP-7402)/anti-rabbit (MP-7401) Ig (peroxidase) polymer detection kit from Vector laboratories, Inc., Burlingame, CA, United States; goat anti-rat IgG-HRP, sc-2006, Santa Cruz Biotechnology) for 1 h at room temperature in a wet chamber. After another two washing steps in PBS we used the 3,3′-diaminobenzidine (DAB) method according to the manufacturer’s instructions (ImmPACT DAB peroxidase (HRP) Substrate, SK-4105, Vector laboratories) for visualization and subsequently rinsed the tissue again in PBS. For counterstaining Mayer’s hemalaun solution (Cantonal Pharmacy, Zurich, Switzerland) was applied and the tissue was then dehydrated in ascending concentrations of ethanol and Histoclear®. The results of the staining were analyzed using an AxioCam MRc5 (Zeiss, Jena, Germany) on a Zeiss Axiophot microscope (Zeiss) with Axio Vision Release 4.7.2 software (Zeiss). For quantification, we performed blinded scoring and rated with no (-), few (+), moderate (++) and intense (+++) staining.
Enzyme-linked Immunosorbent Assay (ELISA)
The participant’s serum was stored at -80°C. ELISA kits detecting human TNF-α (PK-EL-63707), human MCP-1 (PK-EL-64006), human IL-10 (PK-EL-62006), human IL-13 (PK-EL-62306) and human IL-17A (PK-EL-62716) were obtained from Promokine (Heidelberg, Germany), human IFN-γ ELISA kit (SEH00380A) was purchased at Qiagen. IL-22 was detected with an ELISA set (DY-782-05) from R&D Systems, Inc. (Minneapolis, MN, United States). Assays were carried out following the manufacturer’s instructions using a sample volume of 50 and 100 μl, respectively. Absorbance at stated wavelengths was detected on a BioTek-Synergy II Multi-Mode Reader using Gen5.1.11 Software. All measurements were performed in duplicate.
Statistical Analysis
Data are presented as means ± SEM for a series of n experiments. Data are expressed as relative values of the respective control. Statistical analysis was performed by analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. p values < 0.05 were considered significant.
Results
ARBE prevents the IFN-γ-induced expression of IFN-γ receptor 2 in vitro
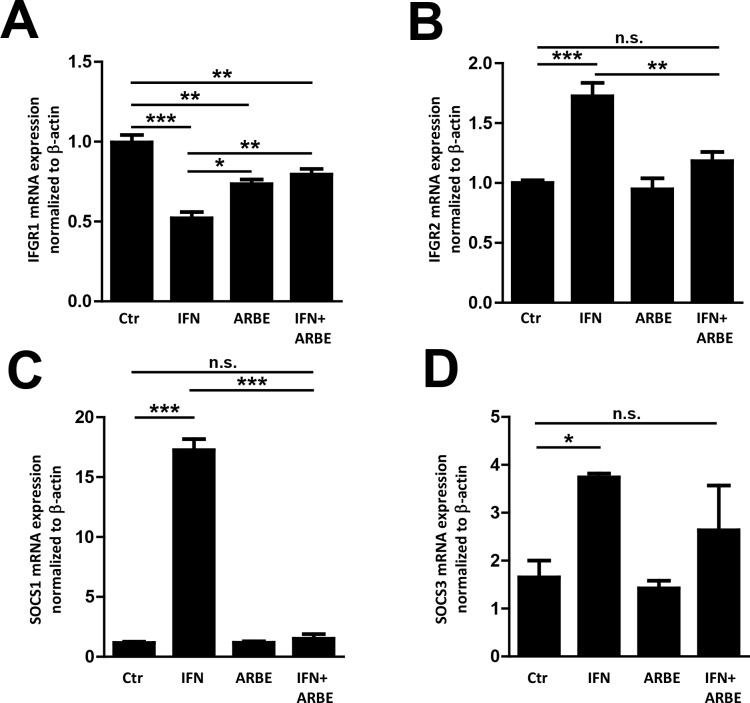
To study the molecular mechanisms of ARBE-induced reduction of IFN-γ signalling–mainly STAT1 phosphorylation–as well as cytokine expression and secretion [17], human THP-1 cells were treated with IFN-γ (100 ng/ml) and/or ARBE (10 μg/ml). As expected, IFN-γ treatment reduced the expression of IFN-γ receptor 1 (IFN-γ R1), but enhanced levels of IFN-γ R2. Interestingly, co-treatment with ARBE diminished the effect of IFN-γ treatment on the mRNA expression of IFN-γ R1 (albeit its expression was still below the level in untreated cells) and completely prevented IFN-γ-mediated induction of IFN-γ R2 (Fig 1A and 1B). (Raw data for all experiments are shown in S2 File.) This is of particular interest, since IFN-γ R1 is important for binding of IFN-γ to its receptor and IFN-γ R2 mediates signal transduction via the intracellular JAK-STAT pathway activation after IFN-γ binding. The IFN-γ induced JAK-STAT pathway mainly involves STAT1 phosphorylation, resulting in target gene expression [27,28].
Fig 1. ARBE prevents the induction of IFN-γ receptor 2 (IFGR2) expression by IFN-γ.
Administration of 100 ng/ml IFN-γ to THP-1 cells provoked a significant reduction of IFN-γ receptor 1 (IFGR1) that is crucial for binding of IFN-γ to its receptor (A). On the other hand, IFGR2 that mediates intracellular signal transduction was significantly induced upon the stimulation with IFN-γ (B). Interestingly, these effects were suppressed when cells were co-treated with 10 μg/ml ARBE (IFN+ARBE). IFGR2 expression in untreated control cells (Ctr) was comparable to co-treated cells (IFN+ARBE) (B). The expression of SOCS1 and 3 was induced by IFN-γ stimulation while co-treatment with ARBE led to reduced expression of SOCS1 and 3 (C+D). Significant results are marked as follows: * = p<0.05; ** = p<0.01; *** = p<0.001; n.s. = not significant. Error bars depict SEM. Ctr = control, IFN = IFN-γ.
We next assessed whether ARBE co-treatment might also impact STAT1 phosphorylation by affecting the expression of SOCS1 and 3. As anticipated, IFN-γ treatment induced mRNA levels of SOCS1 as well as of SOCS3. However, the IFN-γ-mediated induction of SOCS1 mRNA was completely abrogated in ARBE co-treated cells. A similar trend was also observed for SOCS3 expression (Fig 1C and 1D).
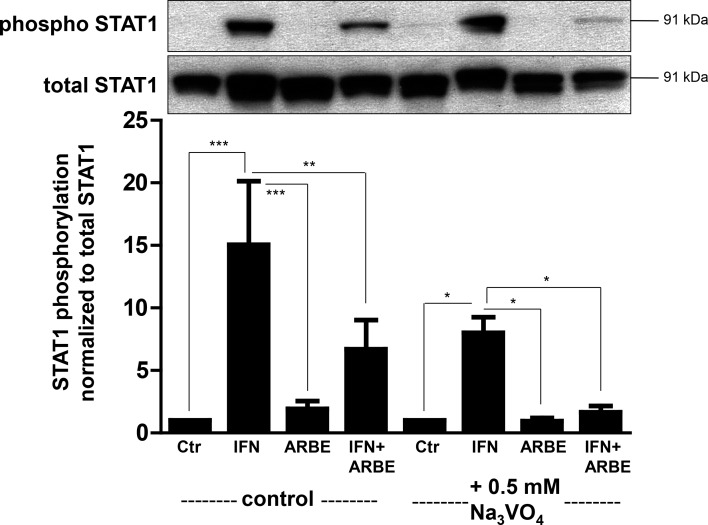
A further mechanism of STAT1 regulation is via protein tyrosine phosphatase (PTP) activity. To assess whether ARBE might inhibit phosphorylation of STAT1 via activation of PTPs, we used the PTP inhibitor Na3VO4. However, PTP inhibition had no impact on the ARBE-mediated inhibition of IFN-γ-induced STAT1 phosphorylation (Fig 2, S2 Fig). Confirmatory, siRNA-induced knock-down of protein tyrosine phosphatase non-receptor type 2 (PTPN2), the nuclear phosphatase of STAT1, did not affect the ARBE-mediated inhibition of the IFN-γ-induced effects on STAT1 phosphorylation either (S3 Fig), suggesting that PTPs do not play a role for the ARBE-mediated inhibitory effects on IFN-γ-induced STAT1 phosphorylation.
Fig 2. Inhibition of protein tyrosine phosphatases (PTPs) in THP-1 cells does not prevent ARBE-mediated reduction of IFN-γ-induced STAT1 phosphorylation.
Application of 0.5mM Na3VO4 (PTP inhibitor) did not abrogate ARBE-induced reduction of IFN-γ-mediated STAT1 phosphorylation suggesting that ARBE does not activate PTPs. Asterisks denote significant differences (* = p<0.05; ** = p<0.01; *** = p<0.001). N = 3. Error bars depict SEM. Ctr = control, IFN = IFN-γ.
IFN-γ and IFN-γ R2 expression in colon tissue decreases during ARBE treatment in vivo
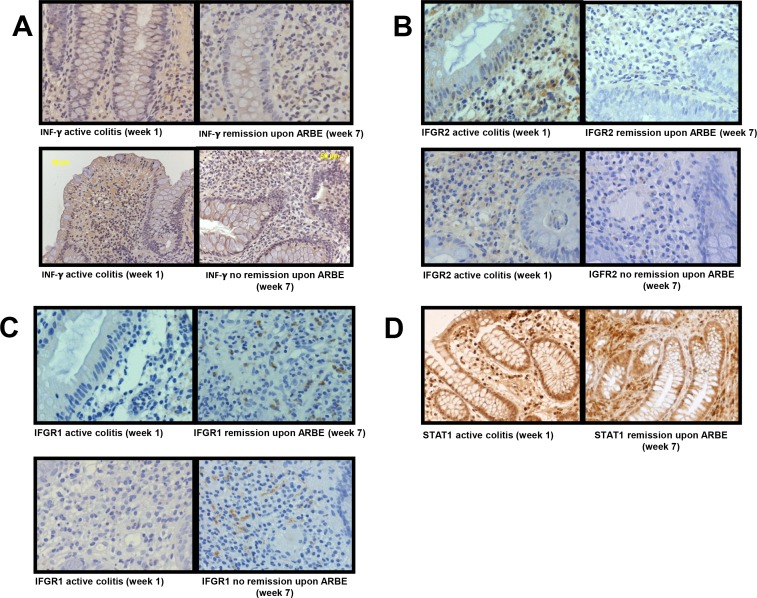
To further elucidate the anti-inflammatory properties of ARBE in the context of human disease, we performed immunohistochemical analysis of several biological markers in human colonic biopsy specimens from 11 UC patients who had completed the bilberry ingestion study of Biedermann et al. [24]. For each patient one biopsy before and one after 6 weeks of daily bilberry intake was analysed. Additionally, several cytokine concentrations were measured in the participants’ serum by the ELISA method.
Since our previous findings revealed an impressive reduction of IFN-γ-related cytokine expression/secretion upon ARBE treatment in vitro [17], we focused on the investigation of the IFN-γ-JAK/STAT-pathway in this study. Analysis of IFN-γ staining revealed an–especially submucosal–diminution of IFN-γ expression at the end of the bilberry intake period in patients in remission, whereas no alteration was detectable in patients without clinical remission (Fig 3A, S4A Fig, S1 Table). In healthy control specimens, IFN-γ expression was generally low or not evident (S5A Fig, S1 Table). On the other hand, IFN-γ serum concentrations–that were generally very low–were not affected by ARBE treatment (S6A Fig). In addition, there was no correlation between the IFN-γ serum concentrations and the clinical activity index (CAI).
Fig 3. Reduced IFGR2 expression in colon tissue after 6 weeks of bilberry ingestion.
Representative pictures from colon biopsies taken before (week 1) and after six weeks of daily bilberry treatment (week 7) from patients reaching remission or not. (A) shows specific staining for IFN-γ where a decline of IFN-γ expression was registered for patients in remission only. A marked decline in IFGR2 expression was provoked by ARBE ingestion not dependent on the remission status (B). A moderate enhancement in IFGR1 expression was detectable, both in patients that reached remission upon ARBE treatment as well as those that did not (C). Expression of total STAT1 (especially in the L. propria mucosae) decreases in patients reaching remission after 6 weeks of daily bilberry ingestion (D).
At begin of the study, IFN-γ R2 expression was readily detectable in submucosal and intestinal epithelial cells. Consistent with our cell culture data, six weeks anthocyanin intake reduced IFN-γ R2 expression in the colon of the study participants to levels comparable to healthy controls. Interestingly, this reduction was observed in all patients, independent of the remission status (Fig 3B, S4B Fig, S1 Table). On the contrary, consistently high IFN-γ R2 expression was observed in the active colitis and colitis in remission control biopsies (S5B Fig, S1 Table).
On the other hand, levels of IFN-γ R1 were low in intestinal biopsies taken before start of the ARBE regimen (and in active colitis control biopsies). However, anthocyanin ingestion resulted in submucosal accumulation of IFN-γ R1 positive cells–comparable to healthy controls–, disregarding the remission status (Fig 3C, S4C Fig, S1 Table). Though, control specimens from patients with colitis in remission upon conventional therapy did not show an accumulation of IFN-γ R1 positive cells (S5C Fig, S1 Table).
We next analysed the expression of total STAT1 in the colon biopsies. Coherently with the decline of IFN-γ expression, the number of total STAT1 expressing cells decreased to healthy control levels during the study course in patients reaching remission (Fig 3D, S4D and S5D Figs, S1 Table).
TNF-α expression in colon tissue declines depending remission status
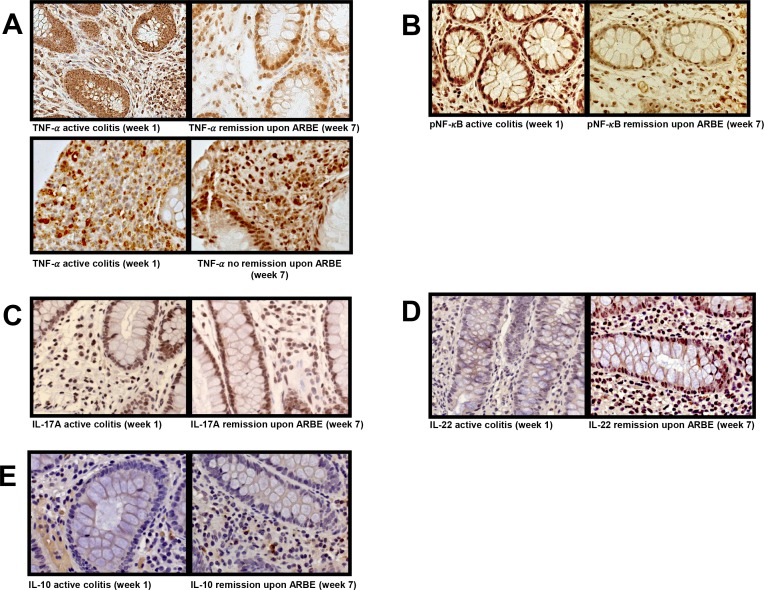
Since our previous in vitro studies demonstrated that ARBE exerts pro- and anti-inflammatory properties in response to TNF-α in human immune cells [17], we further investigated the impact of dietary anthocyanin intake on TNF-α levels in colon tissue and serum. In patients reaching remission, there was a decline in TNF-α expression to levels similar as in healthy controls after six weeks of bilberry treatment, while in patients not reaching remission, no reduction in TNF-α expression was observed (Fig 4A, S7A Fig, S1 Table). As expected, TNF-α levels in the active colitis control specimen were elevated, whereas they were lowered in a biopsy from a UC patient in remission upon conventional therapy (S8A Fig, S1 Table). This indicates that the reduction in TNF-α levels is dependent on remission status and not a direct result from ARBE treatment. A similar trend was found in serum concentrations where TNF-α decreased in patients in remission (n = 3; from 555 pg/ml down to 90 pg/ml), whereas they stayed stable when remission was not achieved (n = 2; from 3041.72 pg/ml to 2967 pg/ml) (S6B Fig).
Fig 4. A decrease in TNF-α expression is registered in patients reaching remission only.
Images illustrate representative pictures from colon biopsies taken before (week 1) and after (week 7) ARBE treatment period. TNF-α expression regressed in patients that reached remission, whereas expression levels remained stable when no remission was reached upon ARBE treatment (A). phospho-p65-NF-κB expression at the end of the study was reduced in some but not all patients reaching remission under ARBE treatment (B). Anthocyanins had no definite effect on the expression of IL-17A in colon tissue (C). Yet, IL-17A expression levels were constantly higher than in healthy control colon tissue. An increase in colonic IL-22 expression was detectable within the study course in patients reaching remission (D). IL-10 expression levels increase moderately during the study course (E), nearly approaching healthy control levels.
TNF-α causes phosphorylation (activation) of the intracellular transcription factor NF-κB which is the major signal transducer of TNF-α [29]. Therefore, we next performed a phospho-p65-NF-κB staining (Fig 4B, S7B Fig, S1 Table). Reduction of phospho-p65-NF-κB positive cells was observed in some patients reaching remission, yet levels were still above healthy control levels and comparable to the levels in colitis in remission upon conventional therapy (S8B Fig, S1 Table).
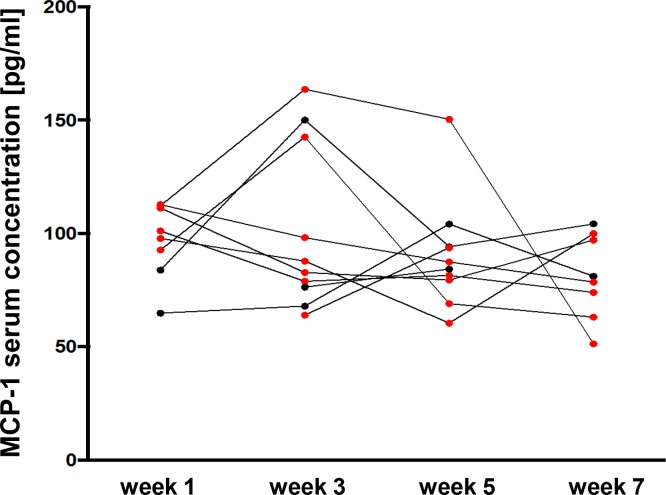
Bilberry intake reduced MCP-1 serum levels
Next, we analysed serum levels of the pro-inflammatory cytokine MCP-1 (Fig 5). Overall, patients being in remission (n = 6) featured a decline in MCP-1 serum levels at the end of the study (105 pg/ml down to 77 pg/ml), while patients not reaching remission (n = 2) at week 7 even revealed increased MCP-1 serum levels (74 pg/ml vs. 93 pg/ml). Remarkably, the two patients with infliximab treatment before study inclusion started with relative high MCP-1 levels, showed a transient rise and finally ended with the lowest MCP-1 concentrations among all the participants.
Fig 5. Serum concentrations of the pro-inflammatory cytokine MCP-1 declined during the study course.
Analysis of MCP-1 serum levels was performed at the beginning of the bilberry intake (week 1) and subsequently in intervals of 2 weeks (week 3, week 5 and week 7). Red dots are derived from patients that reached remission (n = 7) at the end of the study whereas black dots belong to patients that did not reach remission (n = 3). Overall, a decline in MCP-1 serum levels at the end of the study was registered only in patients reaching remission.
Bilberry intake reduced Th17-specific cytokine protein expression while their secretion was not affected
We next studied the impact of the daily bilberry intake with respect to the protein expression of the typical Th17 cytokines IL-17A and IL-22 in the colon. As expected, UC patients featured elevated IL-17A expression compared to healthy control colon tissue (Fig 4C, S7C and S9A Figs, S1 Table). ARBE treatment did not result in a definite change in IL-17A expression, whilst IL-17A serum levels increased when remission was reached (n = 5; 0,53 pg/ml vs 0,8 pg/ml) or remained stable in patients without remission (n = 2) (S6C Fig).
IL-22 expression was enhanced under ARBE treatment when remission was reached. Yet, active colitis and colitis in remission upon conventional therapy also featured elevated IL-22 expression levels compared to healthy controls (Fig 4D, S7D and S9B Figs, S1 Table). Serum IL-22 levels were rather stable during the study period (S6D Fig).
Bilberry ingestion enhanced IL-10 expression
We next addressed the impact of ARBE-enriched diet on IL-10 and IL-13. IL-10 colon expression either increased slightly or remained unaltered, yet levels remained under the healthy control levels (Fig 4E, S7E and S9C Figs, S1 Table). Although, IL-10 serum levels were generally very low, an increase of the IL-10 serum concentrations was detected in 5 of 8 patients (S6E Fig).
For IL-13, only serum concentrations were measured. Results were inconclusive since only 3 patients featured concentrations above the detection limits (S6F Fig).
Discussion
In this investigation on the biomolecular mechanisms underlying the anti-inflammatory properties of ARBE in UC patients, we found that ARBE suppresses IFN-γ-induced expression of IFN-γ R2 in human monocytic cells (THP-1) and reduces expression of IFN-γ R2 in the intestine of UC patients. Colon biopsies of UC patients undergoing successful ARBE treatment further revealed a modified composition of T-cell-derived cytokines.
There is increasing evidence for the attenuating potential of polyphenols on IBD disease severity in humans and in experimental colitis in mice/rats [20,21,22,24]. Here, we analysed study material of the first non-controlled clinical intervention study investigating the efficacy of an oral anthocyanin-rich bilberry preparation in 11 patients with mild to moderate UC performed by Biedermann et al. [24]. This study reported significant beneficial effects on the inflammatory disease activity. The aim of our investigations was to gain insights into the cytokine expression of the colon and the serum cytokine levels during that 6-week long study period, to ultimately permit conclusions on the molecular mechanisms of the applied bilberry-derived anthocyanins.
The cytokine profile between UC and CD shows some characteristic differences. Based on the levels of T cell-derived cytokines in IBD mucosa, it has traditionally been postulated that CD is driven by an abnormal Th1 response, while UC has been thought to be a Th2-mediated disease. Accordingly, higher amounts of IFN-γ –a Th1-derived cytokine–have been reported in CD mucosa compared to UC whereas UC has been associated with elevated IL-5 and IL-13 levels possibly derived from Th2 cells. However, this traditional view was to some extent rejected when increasing evidence for the crucial role of Th17 cells emerged [30,31]. Th17 cells are involved in the inflammation of both, UC and CD, and their presence in the colon is associated with excessive expression levels of Th17 cytokines such as IL-17A, IL-17F and IL-22 [32,33].
In this study we investigated IFN-γ in context of UC–which seems contradictory at first sight–since we found a remarkable inhibition of IFN-γ-derived cytokine expression and secretion upon ARBE application in our preceding cell culture experiments [17]. Additionally, IFN-γ remains an important cytokine in UC pathogenesis and elevated IFN-γ expression correlates with disease activity [34,35,36].
IFN-γ effects are mainly elicited through the JAK/STAT pathway [28]. Here, we found that ARBE inhibits the IFN-γ-induced expression of IFN-γ R2, which is the signal transducing part of the IFN-γ receptor. Reduced levels of this molecule might well explain, how ARBE can act in an anti-inflammatory manner by inhibiting IFN-γ-induced pro-inflammatory effects. Additionally, we found that ARBE does not promote expression of SOCS family proteins that are responsible for the negative feedback mechanism of STAT activation [7].
The importance of TNF-α in the pathogenesis of both, UC and CD, is fortified by the impressive results of clinical trials targeting TNF-α [37]. Moreover, TNF-α levels correlate with disease severity [31,38,39]. In this study, we demonstrated that daily bilberry ingestion provoked a reduction in IFN-γ and TNF-α levels in the intestinal tissue in those patients who reached remission while the levels did not decrease in those participants without remission. Since reduction of IFN-γ and TNF-α was only observed in patients reaching remission after the trial period, but not in patients consuming bilberry extract without reaching remission, this effect might be the results of reduced disease activity and not a direct effect of the ARBE ingestion. This is further strengthened since patients in remission after conventional therapy show a similar reduction of IFN-γ and TNF-α. Further, the serum levels of these cytokines were not affected in a conclusive way. As ingestion of anthocyanin-rich bilberry extract resulted in reduced expression of IFN-γ R2 and STAT1 not depending on the remission status, these effects are likely to be mediated directly by ARBE treatment.
As described above, a specific subset of Th cells–Th17 cells–has been reported to play a key pathogenic role in inflammatory conditions, including IBD. Th17 cells are characterized by their ability to produce high amounts of IL-17A, IL-17F, IL-22, IL-10 and other cytokines crucial for host defence against extracellular bacteria and fungi. In IBD, the intestinal mucosa is characterized by a massive infiltration of Th17 cells and consequently reveals excessive amounts of Th17-related cytokines [33,40]. However, neutralization of IL-17A (by antibody treatment or genetic knockdown) leads to exacerbated intestinal inflammation in the dextran sulphate sodium (DSS) colitis model [41,42]. Therefore, tissue-protective properties of Th17 cytokines are assumed. In our study, we observed an increase in IL-17A and IL-22 levels in the intestinal tissue and/or serum after the bilberry intake period. This is of particular interest and well in line with results from a clinical proof-of-concept trial using the anti-IL-17A monoclonal antibody secukinumab. Here, inhibition of IL-17A by secukinumab has been demonstrated to cause fatal effects in IBD patients [43]. All in all, more and more data suggest that Th17 cytokines IL-17 and IL-22 might be rather protective for the intestine than deleterious. In our study, we have analysed the effects of ARBE treatment in UC patients only and in our knowledge no investigations on ARBE in CD patients have been conducted so far. Yet, there is increasing evidence of the involvement of Th17 cells and their cytokines in the pathogenesis of CD suggesting common molecular mechanisms in these two disease entities [44,45]. Therefore, a potential benefit of ARBE in the treatment of CD patients remains to be assessed.
The regulatory T-cell (Treg) cytokine IL-10 is a crucial immunosuppressive cytokine produced by a variety of leukocytes and non-hematopoietic cells and features a central role in the regulation of intestinal mucosal homeostasis and prevention of IBD [46,47]. However, according to the literature inflamed intestinal tissue comprises increased numbers of mononuclear IL-10 producing cells [48,49] accompanied by increased IL-10 serum levels [50]. Our findings were in good accordance with these data, since we found an increase in IL-10 expression in patients being in remission after treatment with ARBE. The induction of IL-10 might be an important additional mechanism how ARBE exert anti-inflammatory effects in vivo.
In summary, we provide the first translational human study investigating the molecular mechanisms of action of ARBE in UC patients. Our findings are based on an open label human pilot study and therefore only in vitro data provided controlled results. On the one hand, ARBE directly down-regulates IFN-γ-induced effects by inhibiting IFN-γ R2 expression. On the other hand, ARBE seems to alter T-cell subsets in the intestine of UC patients. The small number of participants impedes conclusive statements about the role of ARBE in UC disease control and is a major drawback of this study. Yet, our findings are of importance for further clinical use of anthocyanins and/or ARBE, since they demonstrated one–but probably not the only—molecular mechanism how ARBE selectively modifies crucial immune mechanisms in the intestine. In conclusion, our data as well as the previous clinical trial data by Biedermann et al. [24] reinforce the possible therapeutic use of ARBE in UC patients and warrant further prospective, randomized and controlled clinical studies. Given the impressive reduction of IFN-γ induced signalling cascades upon ARBE treatment, and the importance of IFN-γ in CD pathogenesis, our study further provides evidence that ARBE treatment might also be a promising approach for the treatment of CD patients.
Supporting Information
We performed a concentration-action-curve stimulating THP-1 cells with 0 ng/ml, 1 ng/ml, 10 ng/ml, 100 ng/ml or 1000 ng/ml IFN-γ +/- 10 μg/ml ARBE. Detection of phospho-STAT1 is optimal with application of 100 ng/ml IFN-γ.
(TIFF)
THP-1 cells were stimulated either with 10 μg/ml ARBE, IFN-γ and/or 0.5mM or 5mM Na3VO4 (PTP inhibitor). PTP inhibition did not abrogate ARBE-induced reduction of IFN-γ-mediated STAT1 phosphorylation. Ctr = control, IFN = IFN-γ, ACE = ARBE, I+A = IFN-γ + ARBE, p-STAT1 = phospho-STAT1, tSTAT1 = total STAT1.
(TIFF)
For PTPN2 knockdown, THP-1 cells were transfected with 100 pmol PTPN2 siRNA 36 h before stimulation. Pre-stimulation with10 μg/ml ARBE lasted 20 min and subsequent stimulation with 100 ng/ml IFN-γ (IFN) lasted 30 min. Untreated cells served as control group (Ctr). In THP-1 cells with PTPN2 knockdown co-stimulation with IFN+ARBE still provoked reduced STAT1 phosphorylation. Yet, these results were not significant.
(TIFF)
Representative pictures demonstrate colon biopsies before (week 1) and after (week 7) ARBE treatment either for patients reaching remission or not. A represents IFN-γ staining, B staining specific for IFGR2, C IFGR1 staining and D shows total STAT1 expression. A more detailed view is shown in Fig 3.
(TIFF)
Pictures demonstrate representative sections of control colon biopsies derived from UC patients with active colitis, UC patients in remission under conventional therapy or from healthy persons. Low IFN-γ expression in healthy control colon tissue is detectable (A). IGFR2 expression is elevated in active colitis and colitis in remission control colon, whereas low expression is detectable in healthy controls (B). IGFR1 expression is hardly detectable in active colitis and colitis in remission controls, whereas IGFR1 expression is detectable in healthy control biopsies (C). For STAT1 expression low to moderate results were found in healthy control colon specimens (D).
(TIFF)
Serum levels of different cytokines were measured each at week 1, week 3, week 5 and week 7 of the study course. Red dots represent results from patients that reached remission, black dots belong to patients that did not attain clinical remission. ARBE treatment did not affect the generally very low IFN-γ serum levels (n = 9) (A). TNF-α serum levels (only 5 participants featured serum concentrations above the detection level) decreased in patients reaching remission (n = 3; from 555 pg/ml down to 90 pg/ml), whereas they stayed stable when remission was not achieved (n = 2; from 3041.72 pg/ml to 2967 pg/ml) (B). IL-17A concentrations increased when remission was reached (n = 5; 0,53 pg/ml vs 0,8 pg/ml) or remained stable in patients without remission (n = 2) (C). IL-22 serum concentrations were stable during the ARBE treatment period not depending on the remission status (n = 11) (D). IL-10 concentrations were very low. Yet, a minimal increase was detectable in 5 of 8 patients (E). IL-13 measurement was inconclusive since concentrations above the detection threshold were present in only 3 patients (F).
(TIFF)
Representative pictures demonstrate colon biopsies before (week 1) and after (week 7) ARBE treatment either for patients reaching remission or not. Staining is specific for TNF-α (A), phospho-p65-NF-κB (B), IL-17A (C), IL-22 (D) and IL-10 (E), respectively. A more detailed view is shown in Fig 4.
(TIFF)
These representative pictures from control colon biopsies are derived from UC patients with active colitis or UC patients in remission under conventional therapy and healthy controls, respectively. As expected, TNF-α expression in UC patients with active colitis is elevated, whereas its expression is lowered when remission is attained. Healthy control colon depicts low TNF-α expression (A). phospho-p65-NF-κB expression is low in active colitis and colitis in remission control biopsies, while nearly no expression could be detected in healthy controls (B).
(TIFF)
Pictures demonstrate representative sections of control colon biopsies derived from UC patients with active colitis, UC patients in remission under conventional therapy or from healthy controls. IL-17A expression in healthy colon is barely detectable (A). IL-22 expression levels are elevated in active colitis control as well as in colitis in remission under conventional therapy biopsies, whereas healthy controls feature lower IL-22 expression (B). IL-10 expression in healthy controls is readily detectable (C).
(TIFF)
(PDF)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by research grants from the Swiss National Science Foundation to MS (Grant No. 314730-146204 and Grant No. CRSII3_154488/1) and the Swiss IBD Cohort (Grant No. 3347CO-108792) to GR. The funding institutions had no role in study design and data interpretation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crozier A, Jaganath IB, Clifford MN (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 26: 1001–1043. 10.1039/b802662a [DOI] [PubMed] [Google Scholar]
- 2.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI (2013) Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24: 1415–1422. 10.1016/j.jnutbio.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, et al. (2014) Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 88: 1803–1853. 10.1007/s00204-014-1330-7 [DOI] [PubMed] [Google Scholar]
- 4.Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130: 2073S–2085S. [DOI] [PubMed] [Google Scholar]
- 5.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. (2012) Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 95: 740–751. 10.3945/ajcn.111.023457 [DOI] [PubMed] [Google Scholar]
- 6.Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, et al. (2012) Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 96: 781–788. [DOI] [PubMed] [Google Scholar]
- 7.Serra D, Paixão J, Nunes C, Dinis TC, Almeida LM (2013) Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: comparison with 5-aminosalicylic acid. PLoS One 8: e73001 10.1371/journal.pone.0073001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D (2010) Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr 5: 343–353. 10.1007/s12263-010-0171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Gao S, Jiang W, Luo C, Xu M, Bohlin L, et al. (2014) Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration. Int J Mol Sci 15: 16226–16245. 10.3390/ijms150916226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434. [DOI] [PubMed] [Google Scholar]
- 11.Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307–317. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho JH (2008) The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8: 458–466. 10.1038/nri2340 [DOI] [PubMed] [Google Scholar]
- 13.Bernstein CN, Shanahan F (2008) Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut 57: 1185–1191. 10.1136/gut.2007.122143 [DOI] [PubMed] [Google Scholar]
- 14.Feagan BG, Macdonald JK (2012) Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 10: CD000544 10.1002/14651858.CD000544.pub3 [DOI] [PubMed] [Google Scholar]
- 15.Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK (2012) Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 9: CD000478 10.1002/14651858.CD000478.pub3 [DOI] [PubMed] [Google Scholar]
- 16.W C (2011) Bilberry (Vaccinium myrtillus L.) In: SCM C, editor. 2nd edition. ed. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects.: Boca Raton (FL): CRC Press; pp. Available: http://www.ncbi.nlm.nih.gov/books/NBK92770/. [PubMed] [Google Scholar]
- 17.Roth S, Spalinger MR, Müller I, Lang S, Rogler G, Scharl M (2014) Bilberry-Derived Anthocyanins Prevent IFN-γ-Induced Pro-Inflammatory Signalling and Cytokine Secretion in Human THP-1 Monocytic Cells. Digestion 90: 179–189. 10.1159/000366055 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Uto T, Tanigawa S, Kumamoto T, Fujii M, Hou DX (2008) Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr Cancer 60 Suppl 1: 43–50. 10.1080/01635580802381279 [DOI] [PubMed] [Google Scholar]
- 19.Triebel S, Trieu HL, Richling E (2012) Modulation of inflammatory gene expression by a bilberry (Vaccinium myrtillus L.) extract and single anthocyanins considering their limited stability under cell culture conditions. J Agric Food Chem 60: 8902–8910. 10.1021/jf3028842 [DOI] [PubMed] [Google Scholar]
- 20.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G (2008) Probiotics and blueberry attenuate the severity of dextran sulfate sodium (DSS)-induced colitis. Dig Dis Sci 53: 2464–2473. 10.1007/s10620-007-0174-x [DOI] [PubMed] [Google Scholar]
- 21.Piberger H, Oehme A, Hofmann C, Dreiseitel A, Sand PG, Obermeier F, et al. (2011) Bilberries and their anthocyanins ameliorate experimental colitis. Mol Nutr Food Res 55: 1724–1729. 10.1002/mnfr.201100380 [DOI] [PubMed] [Google Scholar]
- 22.Wu LH, Xu ZL, Dong D, He SA, Yu H (2011) Protective Effect of Anthocyanins Extract from Blueberry on TNBS-Induced IBD Model of Mice. Evid Based Complement Alternat Med 2011: 525462 10.1093/ecam/neq040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E (2011) Comparative Study of Berberis vulgaris Fruit Extract and Berberine Chloride Effects on Acetic Acid-Induced Colitis in Rats. Iran J Pharm Res 10: 97–104. [PMC free article] [PubMed] [Google Scholar]
- 24.Biedermann L, Mwinyi J, Scharl M, Frei P, Zeitz J, Kullak-Ublick GA, et al. (2013) Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—an open pilot study. J Crohns Colitis 7: 271–279. 10.1016/j.crohns.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 25.Spalinger MR, Lang S, Weber A, Frei P, Fried M, Rogler G, et al. (2013) Loss of protein tyrosine phosphatase nonreceptor type 22 regulates interferon-gamma-induced signaling in human monocytes. Gastroenterology 144: 978–988 e910. 10.1053/j.gastro.2013.01.048 [DOI] [PubMed] [Google Scholar]
- 26.Gordon JA (1991) Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol 201: 477–482. [DOI] [PubMed] [Google Scholar]
- 27.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD (1998) How cells respond to interferons. Annu Rev Biochem 67: 227–264. [DOI] [PubMed] [Google Scholar]
- 28.Blouin CM, Lamaze C (2013) Interferon Gamma Receptor: The Beginning of the Journey. Front Immunol 4: 267 10.3389/fimmu.2013.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288. [DOI] [PubMed] [Google Scholar]
- 30.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13: 3–10. 10.1016/j.autrev.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK (2008) Role of cytokines in inflammatory bowel disease. World J Gastroenterol 14: 4280–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannon P, Reinisch W (2012) Interleukin 13 and its role in gut defence and inflammation. Gut 61: 1765–1773. 10.1136/gutjnl-2012-303461 [DOI] [PubMed] [Google Scholar]
- 33.Gálvez J (2014) Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 2014: 928461 10.1155/2014/928461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coskun M, Salem M, Pedersen J, Nielsen OH (2013) Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 76: 1–8. 10.1016/j.phrs.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J (2011) TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine 56: 633–640. 10.1016/j.cyto.2011.08.036 [DOI] [PubMed] [Google Scholar]
- 36.Rismo R, Olsen T, Cui G, Christiansen I, Florholmen J, Goll R (2012) Mucosal cytokine gene expression profiles as biomarkers of response to infliximab in ulcerative colitis. Scand J Gastroenterol 47: 538–547. 10.3109/00365521.2012.667146 [DOI] [PubMed] [Google Scholar]
- 37.MacDonald TT, Biancheri P, Sarra M, Monteleone G (2012) What's the next best cytokine target in IBD? Inflamm Bowel Dis 18: 2180–2189. 10.1002/ibd.22967 [DOI] [PubMed] [Google Scholar]
- 38.Bamias G, Martin C, Marini M, Hoang S, Mishina M, Ross WG, et al. (2003) Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol 171: 4868–4874. [DOI] [PubMed] [Google Scholar]
- 39.Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, et al. (1996) Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol 16: 144–150. [DOI] [PubMed] [Google Scholar]
- 40.Zenewicz LA, Antov A, Flavell RA (2009) CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med 15: 199–207. 10.1016/j.molmed.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 41.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y (2004) Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 110: 55–62. [DOI] [PubMed] [Google Scholar]
- 42.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. (2008) Regulation of inflammatory responses by IL-17F. J Exp Med 205: 1063–1075. 10.1084/jem.20071978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. (2012) Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61: 1693–1700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brand S (2009) Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58: 1152–1167. 10.1136/gut.2008.163667 [DOI] [PubMed] [Google Scholar]
- 45.Neurath MF (2007) IL-23: a master regulator in Crohn disease. Nat Med 13: 26–28. [DOI] [PubMed] [Google Scholar]
- 46.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. (2014) Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40: 706–719. 10.1016/j.immuni.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marlow GJ, van Gent D, Ferguson LR (2013) Why interleukin-10 supplementation does not work in Crohn's disease patients. World J Gastroenterol 19: 3931–3941. 10.3748/wjg.v19.i25.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsay JO, Hodgson HJ (2001) Review article: the immunoregulatory cytokine interleukin-10—a therapy for Crohn's disease? Aliment Pharmacol Ther 15: 1709–1716. [DOI] [PubMed] [Google Scholar]
- 49.Braat H, Peppelenbosch MP, Hommes DW (2003) Interleukin-10-based therapy for inflammatory bowel disease. Expert Opin Biol Ther 3: 725–731. [DOI] [PubMed] [Google Scholar]
- 50.Kucharzik T, Stoll R, Lügering N, Domschke W (1995) Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol 100: 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We performed a concentration-action-curve stimulating THP-1 cells with 0 ng/ml, 1 ng/ml, 10 ng/ml, 100 ng/ml or 1000 ng/ml IFN-γ +/- 10 μg/ml ARBE. Detection of phospho-STAT1 is optimal with application of 100 ng/ml IFN-γ.
(TIFF)
THP-1 cells were stimulated either with 10 μg/ml ARBE, IFN-γ and/or 0.5mM or 5mM Na3VO4 (PTP inhibitor). PTP inhibition did not abrogate ARBE-induced reduction of IFN-γ-mediated STAT1 phosphorylation. Ctr = control, IFN = IFN-γ, ACE = ARBE, I+A = IFN-γ + ARBE, p-STAT1 = phospho-STAT1, tSTAT1 = total STAT1.
(TIFF)
For PTPN2 knockdown, THP-1 cells were transfected with 100 pmol PTPN2 siRNA 36 h before stimulation. Pre-stimulation with10 μg/ml ARBE lasted 20 min and subsequent stimulation with 100 ng/ml IFN-γ (IFN) lasted 30 min. Untreated cells served as control group (Ctr). In THP-1 cells with PTPN2 knockdown co-stimulation with IFN+ARBE still provoked reduced STAT1 phosphorylation. Yet, these results were not significant.
(TIFF)
Representative pictures demonstrate colon biopsies before (week 1) and after (week 7) ARBE treatment either for patients reaching remission or not. A represents IFN-γ staining, B staining specific for IFGR2, C IFGR1 staining and D shows total STAT1 expression. A more detailed view is shown in Fig 3.
(TIFF)
Pictures demonstrate representative sections of control colon biopsies derived from UC patients with active colitis, UC patients in remission under conventional therapy or from healthy persons. Low IFN-γ expression in healthy control colon tissue is detectable (A). IGFR2 expression is elevated in active colitis and colitis in remission control colon, whereas low expression is detectable in healthy controls (B). IGFR1 expression is hardly detectable in active colitis and colitis in remission controls, whereas IGFR1 expression is detectable in healthy control biopsies (C). For STAT1 expression low to moderate results were found in healthy control colon specimens (D).
(TIFF)
Serum levels of different cytokines were measured each at week 1, week 3, week 5 and week 7 of the study course. Red dots represent results from patients that reached remission, black dots belong to patients that did not attain clinical remission. ARBE treatment did not affect the generally very low IFN-γ serum levels (n = 9) (A). TNF-α serum levels (only 5 participants featured serum concentrations above the detection level) decreased in patients reaching remission (n = 3; from 555 pg/ml down to 90 pg/ml), whereas they stayed stable when remission was not achieved (n = 2; from 3041.72 pg/ml to 2967 pg/ml) (B). IL-17A concentrations increased when remission was reached (n = 5; 0,53 pg/ml vs 0,8 pg/ml) or remained stable in patients without remission (n = 2) (C). IL-22 serum concentrations were stable during the ARBE treatment period not depending on the remission status (n = 11) (D). IL-10 concentrations were very low. Yet, a minimal increase was detectable in 5 of 8 patients (E). IL-13 measurement was inconclusive since concentrations above the detection threshold were present in only 3 patients (F).
(TIFF)
Representative pictures demonstrate colon biopsies before (week 1) and after (week 7) ARBE treatment either for patients reaching remission or not. Staining is specific for TNF-α (A), phospho-p65-NF-κB (B), IL-17A (C), IL-22 (D) and IL-10 (E), respectively. A more detailed view is shown in Fig 4.
(TIFF)
These representative pictures from control colon biopsies are derived from UC patients with active colitis or UC patients in remission under conventional therapy and healthy controls, respectively. As expected, TNF-α expression in UC patients with active colitis is elevated, whereas its expression is lowered when remission is attained. Healthy control colon depicts low TNF-α expression (A). phospho-p65-NF-κB expression is low in active colitis and colitis in remission control biopsies, while nearly no expression could be detected in healthy controls (B).
(TIFF)
Pictures demonstrate representative sections of control colon biopsies derived from UC patients with active colitis, UC patients in remission under conventional therapy or from healthy controls. IL-17A expression in healthy colon is barely detectable (A). IL-22 expression levels are elevated in active colitis control as well as in colitis in remission under conventional therapy biopsies, whereas healthy controls feature lower IL-22 expression (B). IL-10 expression in healthy controls is readily detectable (C).
(TIFF)
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.